From Resistance Mechanism to Green Application: Discovery of Rutaevin as a Key Phytoalexin in Larch and Cross-Species Resource Optimization
Abstract
1. Introduction
2. Results
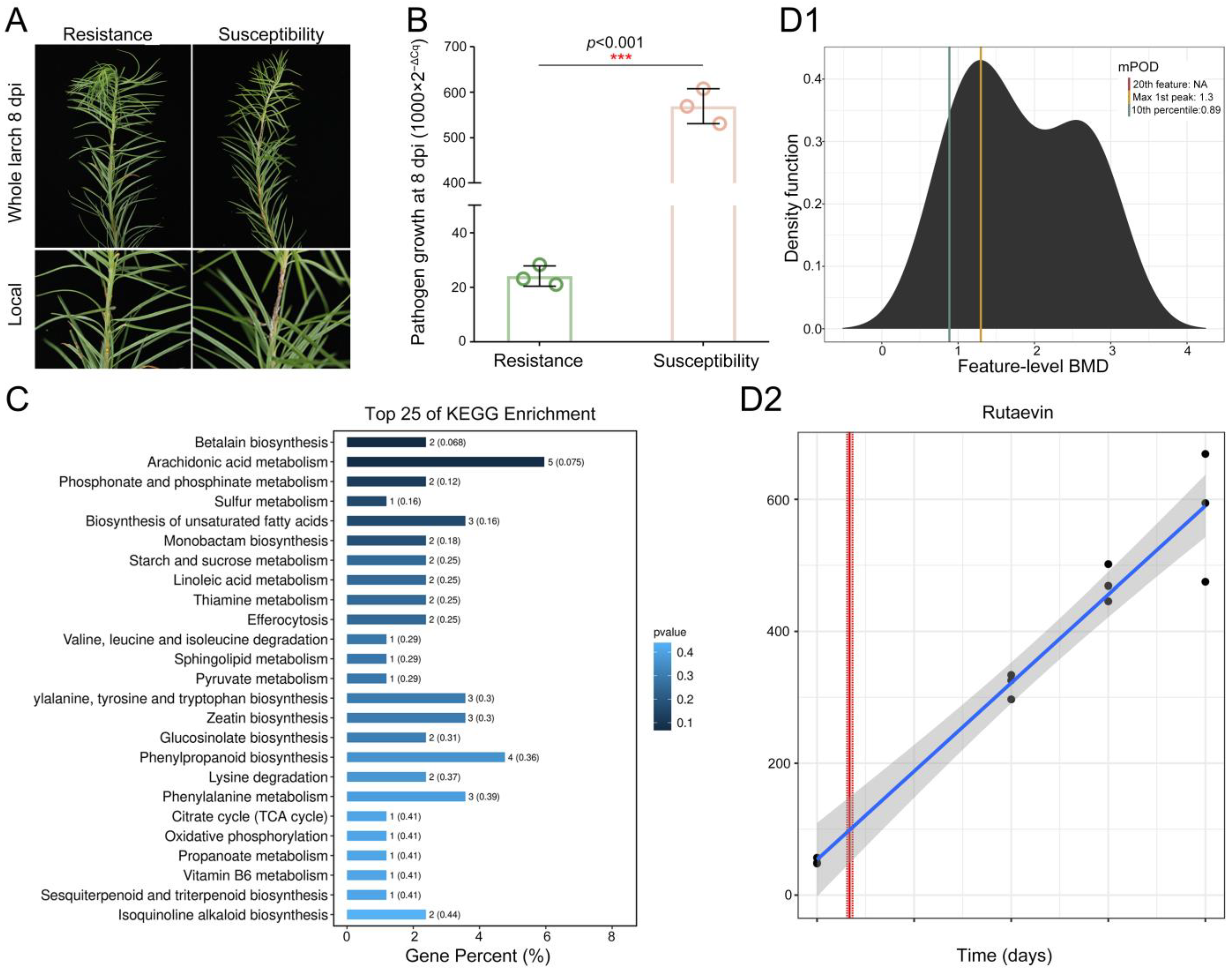
2.1. Discovery of Rutaevin as a Critical Disease-Resistant Metabolite in Larch
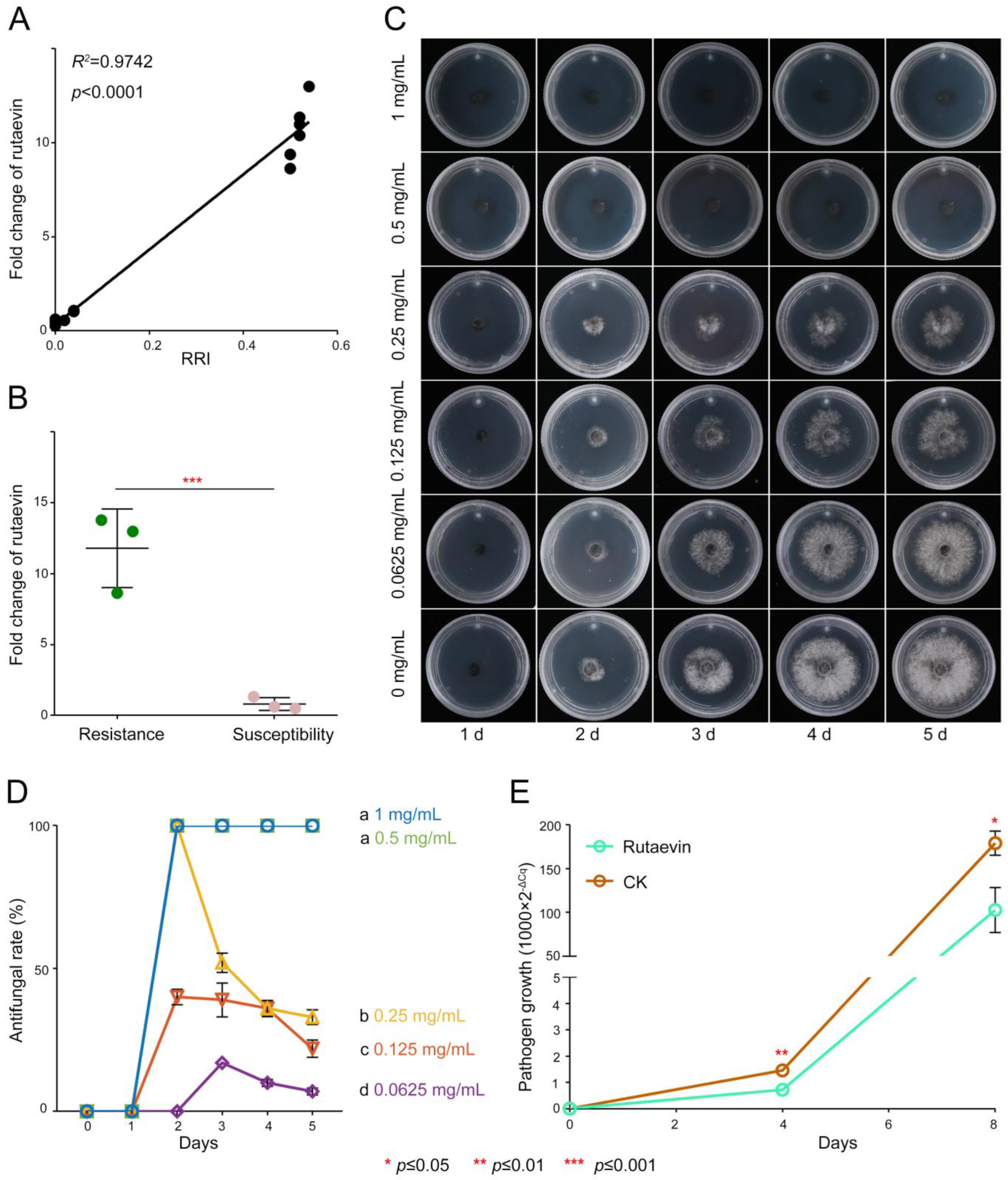
2.2. Functional Validation of Rutaevin in Larch Defense: Temporal Accumulation Dynamics and Dose-Dependent Antifungal Activity
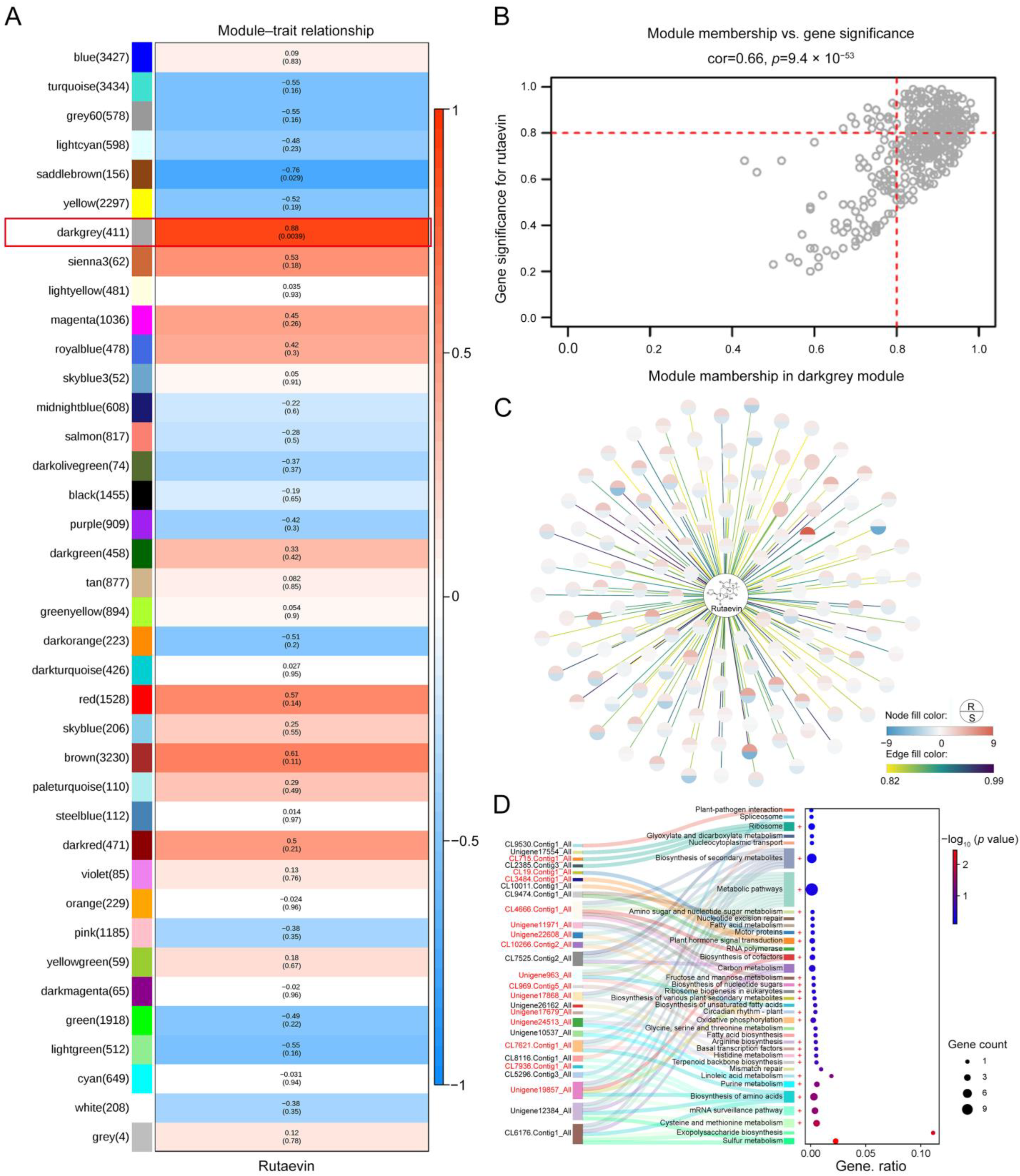
2.3. Identification of Genes Related to Rutaevin Metabolic Pathway and Derivation of Its Synthesis Mechanism
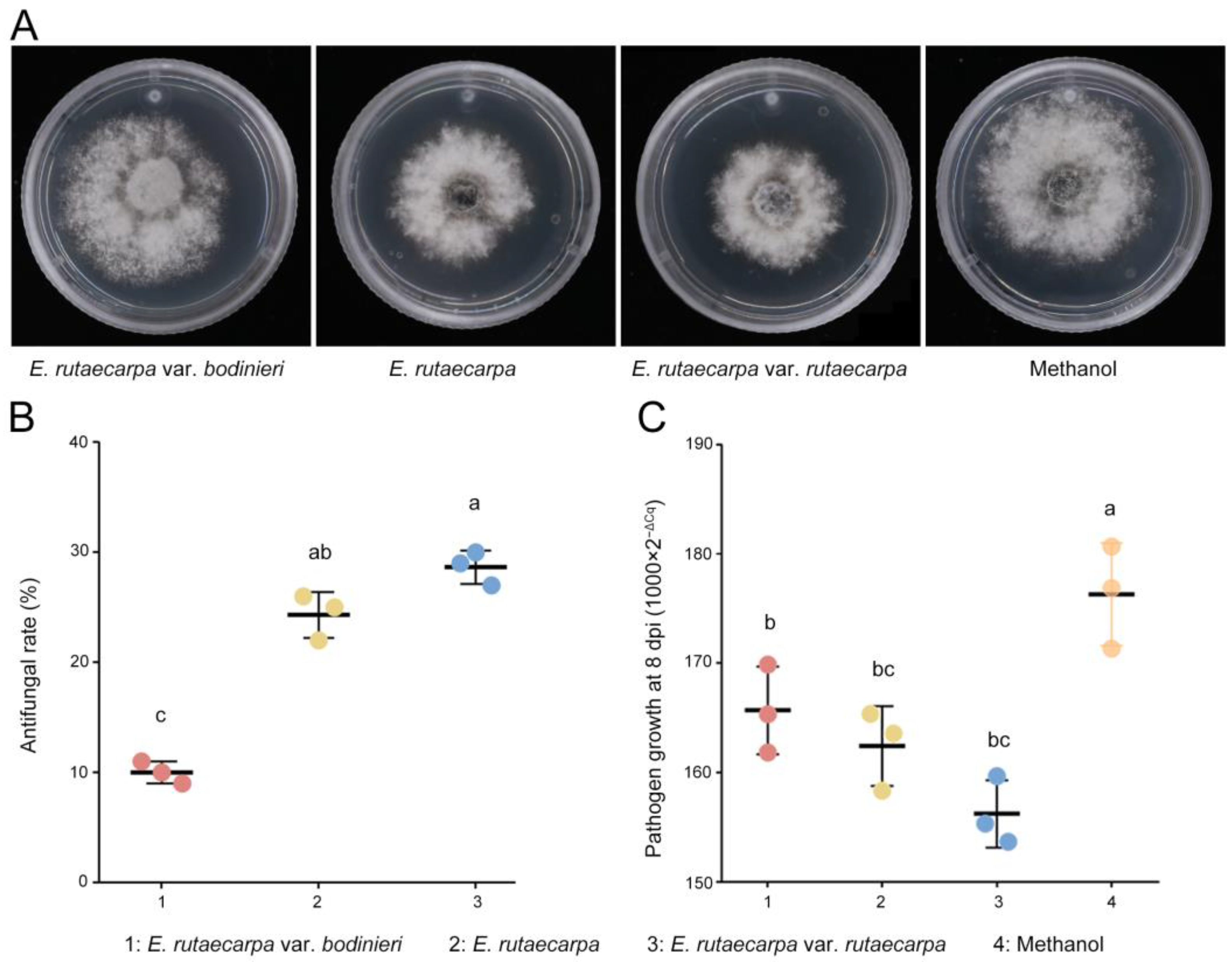
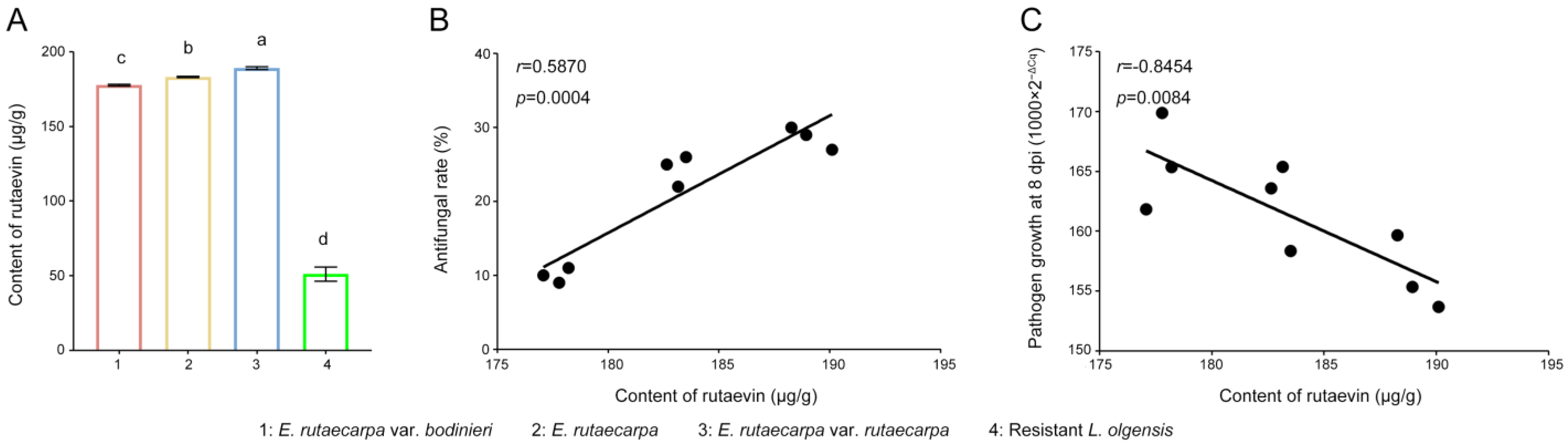
2.4. Distribution Patterns of Rutaevin Across Different Provenances and Plant Organs: Identification of Optimal Sources for Efficient Extraction and as an Effective Biocontrol Agent Against N. laricinum in Larch
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Artificial Inoculation of L. olgensis with N. laricinum
4.3. Screening of Differential Phytoalexins Using Metabolomics
4.4. Transcriptomic and Proteomic Analysis of Phytoalexin Synthesis Pathways
4.5. Assessment of Fungicidal Activity of Rutaevin and Control of Larch Shoot Blight
4.6. Validation of Antifungal Activity of Different Evodia spp. Extracts
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sato, H.; Kobayashi, H.; Iwahana, G.; Ohta, T. Endurance of larch forest ecosystems in eastern Siberia under warming trends. Ecol. Evol. 2016, 6, 5690–5704. [Google Scholar] [CrossRef]
- Wang, M.; Sui, X.; Wang, X.; Zhang, X.; Zeng, X. Soil fungal community differences in manual plantation larch forest and natural larch forest in Northeast China. Microorganisms 2024, 12, 1322. [Google Scholar] [CrossRef]
- Hattori, Y.; Ando, Y.; Nakashima, C. Taxonomical re-examination of the genus Neofusicoccum in Japan. Mycoscience 2021, 62, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Nakashima, C.; Masuya, H. Re-epitypification of Neofusicoccum laricinum. Mycoscience 2024, 65, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Ju, F.; Qi, Z.; Tan, J.; Liu, T.; Dai, T. Development of a recombinase polymerase amplification method combined with a lateral flow dipstick assay for rapid detection of the larch pathogen Neofusicoccum laricinum. Plant Dis. 2025, 109, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Bruda, E.A.; Xia, R.; Zhang, R.; Wang, H.; Yu, Q.; Hu, M.; Wang, F. Evaluation on the efficacy of farrerol in inhibiting shoot blight of larch (Neofusicoccum laricinum). Plants 2024, 13, 3004. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, W.; Liang, Y. Analysis of the potential distribution of shoot blight of larch in China based on the optimized MaxEnt and Biomod2 ensemble models. Forests 2024, 15, 1313. [Google Scholar] [CrossRef]
- Liu, Y.; Han, S.; Song, L.; Li, L.; Wang, H.; Pan, M.; Tan, J. Screening of bacterial endophytes of larch against Neofusicoccum laricinum and validation of their safety. Microbiol. Spectr. 2024, 12, e0411223. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, W.; Liang, Y. Prediction of potential suitable distribution of shoot blight of larch (Neofusicoccum laricinum) in China. Acte Ecol. Sin. 2024, 44, 3027–3037. [Google Scholar] [CrossRef]
- Lv, Q.; Zhang, S.; Lin, R.; Wang, H. Occurrence status of main forestry invasive species in China and their research trends. Plant Prot. 2022, 48, 21–38. [Google Scholar] [CrossRef]
- Wang, W. The harm and control methods of larch shoot blight. Mod. Agric. 2019, 10, 87–88. [Google Scholar] [CrossRef]
- Deshmukh, R.; Tiwari, S. Molecular interaction of charcoal rot pathogenesis in soybean: A complex interaction. Plant Cell Rep. 2021, 40, 1799–1812. [Google Scholar] [CrossRef]
- Chowdhury, S.; Basu, A.; Kundu, S. Biotrophy-necrotrophy switch in pathogen evoke differential response in resistant and susceptible sesame involving multiple signaling pathways at different phases. Sci. Rep. 2017, 7, 17251. [Google Scholar] [CrossRef] [PubMed]
- Rudd, J.J.; Kanyuka, K.; Hassani-Pak, K.; Derbyshire, M.; Andongabo, A.; Devonshire, J.; Lysenko, A.; Saqi, M.; Desai, N.M.; Powers, S.J.; et al. Transcriptome and metabolite profiling of the infection cycle of Zymoseptoria tritici on wheat reveals a biphasic interaction with plant immunity involving differential pathogen chromosomal contributions and a variation on the hemibiotrophic lifestyle definition. Plant Physiol. 2015, 167, 1158–1185. [Google Scholar] [CrossRef]
- Choi, H.W.; Lee, B.G.; Kim, N.H.; Park, Y.; Lim, C.W.; Song, H.K.; Hwang, B.K. A role for a menthone reductase in resistance against microbial pathogens in plants. Plant Physiol. 2008, 148, 383–401. [Google Scholar] [CrossRef]
- Yoshioka, M.; Adachi, A.; Sato, Y.; Doke, N.; Kondo, T.; Yoshioka, H. RNAi of the sesquiterpene cyclase gene for phytoalexin production impairs pre- and post-invasive resistance to potato blight pathogens. Mol. Plant Pathol. 2019, 20, 907–922. [Google Scholar] [CrossRef]
- Ding, L.; Xu, H.; Yi, H.; Yang, L.; Kong, Z.; Zhang, L.; Xue, S.; Jia, H.; Ma, Z. Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS ONE 2011, 6, e19008. [Google Scholar] [CrossRef]
- Xiang, C.; Pan, X.; Xiang, Y.; Zhao, H.; Li, Y.; Tian, X.; Wang, H.; Jia, T. Study on host resistance of larch shoot blight. J. Northeast For. Univ. 1995, 4, 1–9. [Google Scholar]
- Yu, W.; Wang, H.; Tian, X.; Dong, S. Study on the disease resistance mechanism of larch to larch shoot blight. For. Sci. Technol. 2006, 31, 24–27. [Google Scholar]
- Shirai, A.; Kawasaka, K.; Tsuchiya, K. Antimicrobial action of phenolic acids combined with violet 405-nm light for disinfecting pathogenic and spoilage fungi. J. Photochem. Photobiol. B Biol. 2022, 229, 112411. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, X.; Xie, Y.; Zhong, Y. o-Vanillin, a promising antifungal agent, inhibits Aspergillus flavus by disrupting the integrity of cell walls and cell membranes. Appl. Microbiol. Biotechnol. 2021, 105, 5147–5158. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Sun, M.; Wang, L.; Zou, Y.; Fu, L.; Han, C.; Li, A.; Li, L.; Zhu, C. Impact of vanillin on postharvest disease control of apple. Front. Microbiol. 2022, 13, 979737. [Google Scholar] [CrossRef]
- Yang, X.B.; Qian, P.; Yang, X.W.; Liu, J.X.; Gong, N.B.; Lv, Y. Limonoid constituents of Euodia rutaecarpa var. bodinieri and their inhibition on NO production in lipopolysaccharide-activated RAW264.7 macrophages. J. Asian Nat. Prod. Res. 2013, 15, 1130–1138. [Google Scholar] [CrossRef]
- Sheu, J.R. Pharmacological effects of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa. Cardiovasc. Drug Rev. 1999, 17, 237–245. [Google Scholar] [CrossRef]
- Li, W.; Sun, X.; Liu, B.; Zhang, L.; Fan, Z.; Ji, Y. Screening and identification of hepatotoxic component in Evodia rutaecarpa based on spectrum-effect relationship and UPLC-Q-TOFMS. Biomed. Chromatogr. 2016, 30, 1975–1983. [Google Scholar] [CrossRef]
- Cai, Q.; Wei, J.; Zhao, W.; Shi, S.; Zhang, Y.; Wei, R.; Zhang, Y.; Li, W.; Wang, Q. Toxicity of Evodiae fructus on rat liver mitochondria: The role of oxidative stress and mitochondrial permeability transition. Molecules 2014, 19, 21168–21182. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Liu, Y.; Ge, Q.; Sun, C. Cytochrome P450 mediated bioactivation of rutaevin, a bioactive and potentially hepatotoxic component of Evodia rutaecarpa. Chem. Res. Toxicol. 2020, 33, 3054–3064. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chen, X.; Chen, X.; Xia, Y.; Liu, J.; Zhou, G. Plant immune receptors interact with hemibiotrophic pathogens to activate plant immunity. Front. Microbiol. 2023, 14, 1252039. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Kong, M.; Freeman, A.; Chen, H.; Liu, F. More stories to tell: NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1, a salicylic acid receptor. Plant Cell Environ. 2021, 44, 1716–1727. [Google Scholar] [CrossRef]
- Ma, A.; Zhang, D.; Wang, G.; Wang, K.; Li, Z.; Gao, Y.; Li, H.; Bian, C.; Cheng, J.; Han, Y.; et al. Verticillium dahliae effector VDAL protects MYB6 from degradation by interacting with PUB25 and PUB26 E3 ligases to enhance Verticillium wilt resistance. Plant Cell 2021, 33, 3675–3699. [Google Scholar] [CrossRef]
- Zhang, Y.; Lubberstedt, T.; Xu, M. The genetic and molecular basis of plant resistance to pathogens. J. Genet. Genom. 2013, 40, 23–35. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Lv, J.; Yang, S.; Zhou, W.; Liu, Z.; Tan, J.; Wei, M. Microbial regulation of plant secondary metabolites: Impact, mechanisms and prospects. Microbiol. Res. 2024, 283, 127688. [Google Scholar] [CrossRef] [PubMed]
- Vela, S.; Wolf, E.S.A.; Rollins, J.A.; Cuevas, H.E.; Vermerris, W. Dual-RNA-sequencing to elucidate the interactions between sorghum and Colletotrichum sublineola. Front. Fungal Biol. 2024, 5, 1437344. [Google Scholar] [CrossRef]
- Po-Wen, C.; Singh, P.; Zimmerli, L. Priming of the Arabidopsis pattern-triggered immunity response upon infection by necrotrophic Pectobacterium carotovorum bacteria. Mol. Plant Pathol. 2013, 14, 58–70. [Google Scholar] [CrossRef]
- Kharat, K.R.; Pottathil, R. Chemically defined elicitors activate priming in tomato seedlings. Plant Signal. Behav. 2022, 17, 2095143. [Google Scholar] [CrossRef]
- Wrzesińska-Krupa, B.; Szmatoła, T.; Praczyk, T.; Obrępalska-Stęplowska, A. Transcriptome analysis indicates the involvement of herbicide-responsive and plant-pathogen interaction pathways in the development of resistance to ACCase inhibitors in Apera spica-venti. Pest Manag. Sci. 2023, 79, 1944–1962. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ahmad, D.; Zhang, X.; Zhang, Y.; Wu, L.; Jiang, P.; Ma, H. Genome-wide analysis of family-1 UDP glycosyltransferases (UGT) and identification of UGT genes for FHB resistance in wheat (Triticum aestivum L.). BMC Plant Biol. 2018, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Paulert, R.; Ascrizzi, R.; Malatesta, S.; Berni, P.; Noseda, M.D.; Mazetto de Carvalho, M.; Marchioni, I.; Pistelli, L.; Rabello Duarte, M.E.; Mariotti, L.; et al. Ulva intestinalis extract acts as biostimulant and modulates metabolites and hormone balance in basil (Ocimum basilicum L.) and parsley (Petroselinum crispum L.). Plants 2021, 10, 1391. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Yang, Y. Anthocyanin-mediated arsenic tolerance in plants. Environ. Pollut. 2022, 292, 118475. [Google Scholar] [CrossRef]
- El-Mahdy, M.T.; Ali, M.; Pisam, W.M.M.; Abeed, A.H.A. Physiological and molecular analysis of pitaya (Hylocereus polyrhizus) reveal up-regulation of secondary metabolites, nitric oxide, antioxidant defense system, and expression of responsive genes under low-temperature stress by the pre-treatment of hydrogen peroxide. Plant Physiol. Biochem. 2024, 213, 108840. [Google Scholar] [CrossRef]
- Douglas, A.E. Strategies for enhanced crop resistance to insect pests. Annu. Rev. Plant Biol. 2018, 69, 637–660. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Liu, X. The molecular diagnosis of the larch shoot blight. Plant Quar. 2009, 23, 1–4. [Google Scholar] [CrossRef]
- Hacquard, S.; Veneault-Fourrey, C.; Delaruelle, C.; Frey, P.; Martin, F.; Duplessis, S. Validation of Melampsora larici-populina reference genes for in planta RT-quantitative PCR expression profiling during time-course infection of poplar leaves. Physiol. Mol. Plant Pathol. 2011, 75, 106–112. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. MetaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Di Guida, R.; Engel, J.; Allwood, J.W.; Weber, R.J.; Jones, M.R.; Sommer, U.; Viant, M.R.; Dunn, W.B. Non-targeted UHPLC-MS metabolomic data processing methods: A comparative investigation of normalisation, missing value imputation, transformation and scaling. Metabol. Off. J. Metabol. Soc. 2016, 12, 93. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Wen, B.; Zhou, R.; Feng, Q.; Wang, Q.; Wang, J.; Liu, S. IQuant: An automated pipeline for quantitative proteomics based upon isobaric tags. Proteomics 2014, 14, 2280–2285. [Google Scholar] [CrossRef]
- Brosch, M.; Yu, L.; Hubbard, T.; Choudhary, J. Accurate and sensitive peptide identification with Mascot Percolator. J. Proteome Res. 2009, 8, 3176–3181. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Lavecchia, A.; Gramaglia, V.; Gobbetti, M. Long-term fungal inhibition by Pisum sativum flour hydrolysate during storage of wheat flour bread. Appl. Environ. Microbiol. 2015, 81, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Zhang, S.; Xia, R.; Chen, X.; Chen, J.; Wang, F.; Li, D. From Resistance Mechanism to Green Application: Discovery of Rutaevin as a Key Phytoalexin in Larch and Cross-Species Resource Optimization. Plants 2025, 14, 2947. https://doi.org/10.3390/plants14192947
Zhang R, Zhang S, Xia R, Chen X, Chen J, Wang F, Li D. From Resistance Mechanism to Green Application: Discovery of Rutaevin as a Key Phytoalexin in Larch and Cross-Species Resource Optimization. Plants. 2025; 14(19):2947. https://doi.org/10.3390/plants14192947
Chicago/Turabian StyleZhang, Ruizhi, Shuang Zhang, Rui Xia, Xinyan Chen, Jiarui Chen, Feng Wang, and Danlei Li. 2025. "From Resistance Mechanism to Green Application: Discovery of Rutaevin as a Key Phytoalexin in Larch and Cross-Species Resource Optimization" Plants 14, no. 19: 2947. https://doi.org/10.3390/plants14192947
APA StyleZhang, R., Zhang, S., Xia, R., Chen, X., Chen, J., Wang, F., & Li, D. (2025). From Resistance Mechanism to Green Application: Discovery of Rutaevin as a Key Phytoalexin in Larch and Cross-Species Resource Optimization. Plants, 14(19), 2947. https://doi.org/10.3390/plants14192947






