Arbuscular Mycorrhizal Fungi Enhance Antioxidant Defense Systems in Sugarcane Under Soil Cadmium Stress
Abstract
1. Introduction
2. Results
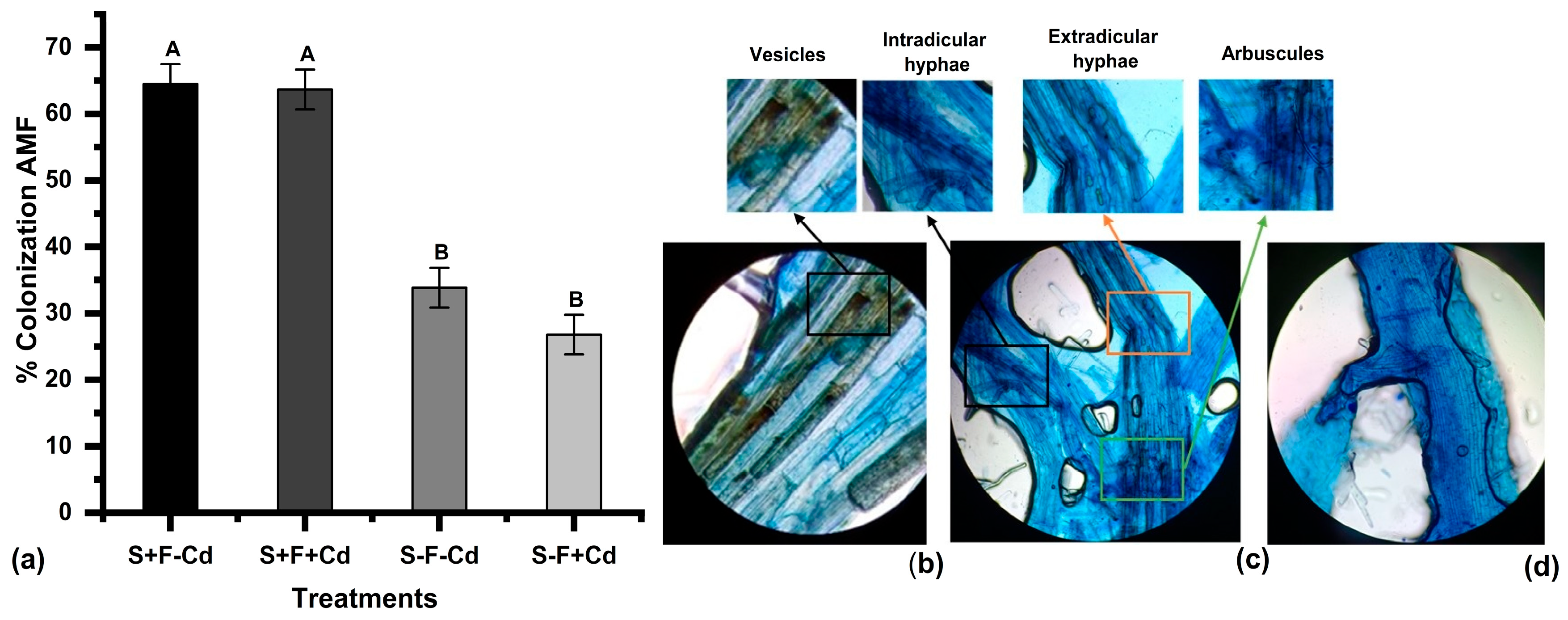
2.1. Colonization of Arbuscular Mycorrhizal Fungi and Cadmium Content
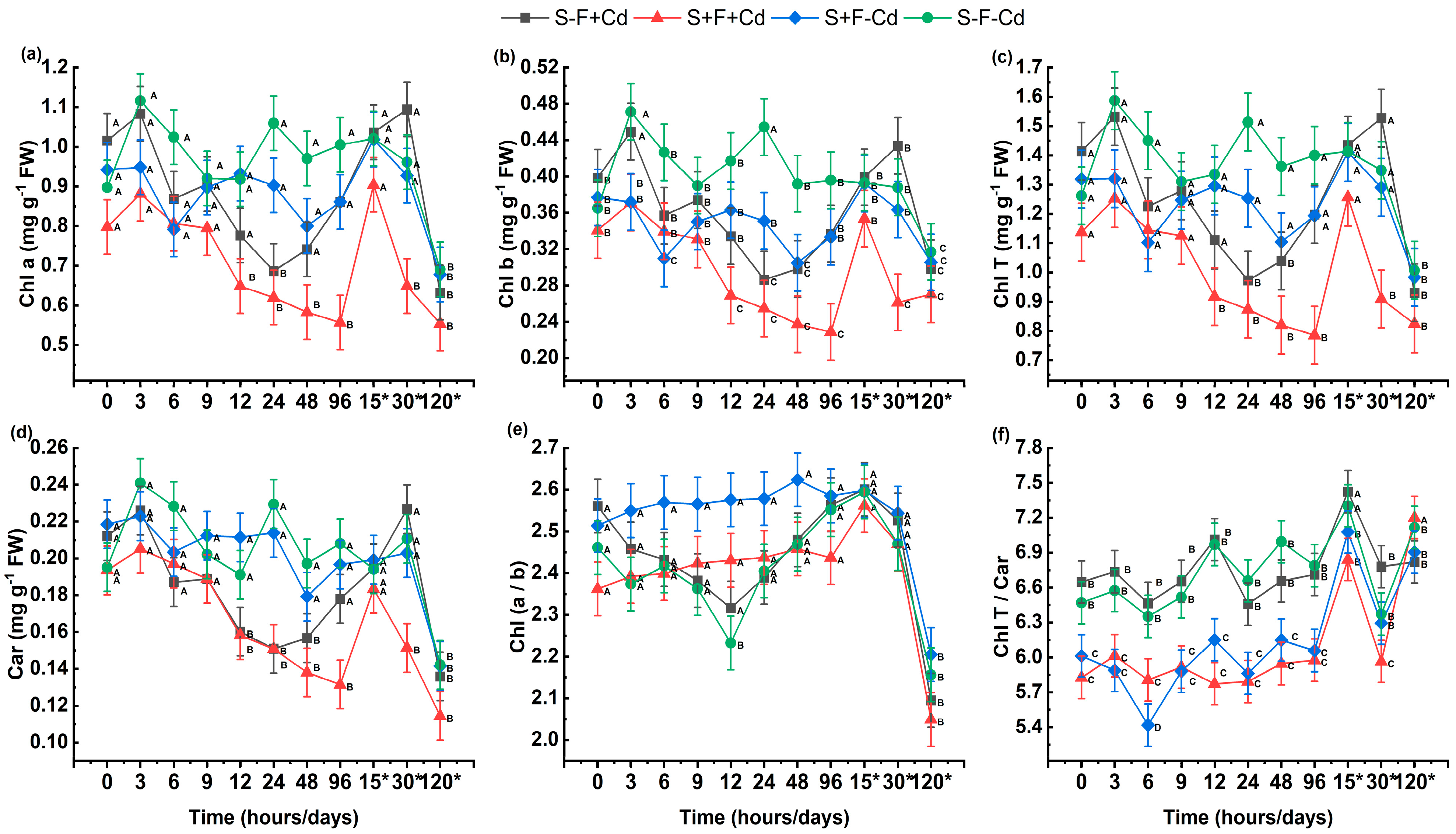
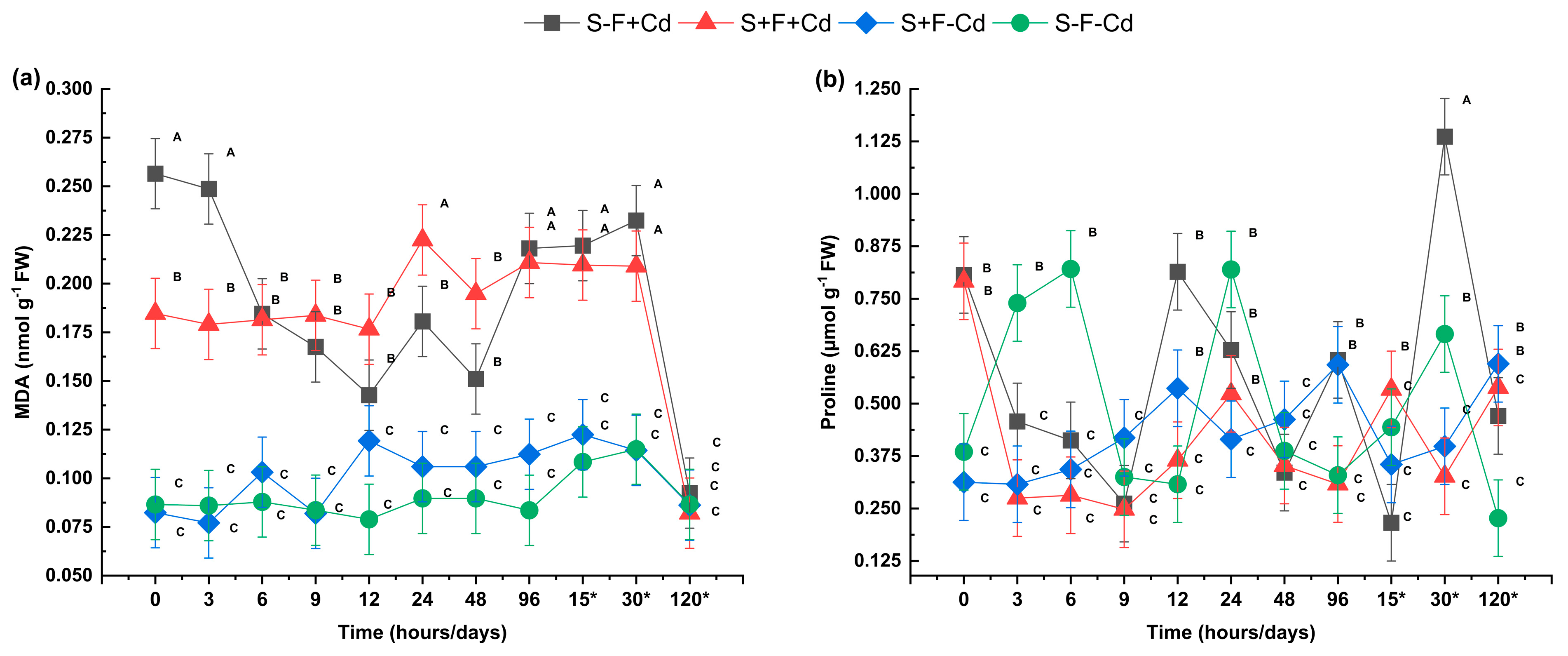
2.2. Analysis of Photosynthetic Pigments and Biochemical Stress Markers
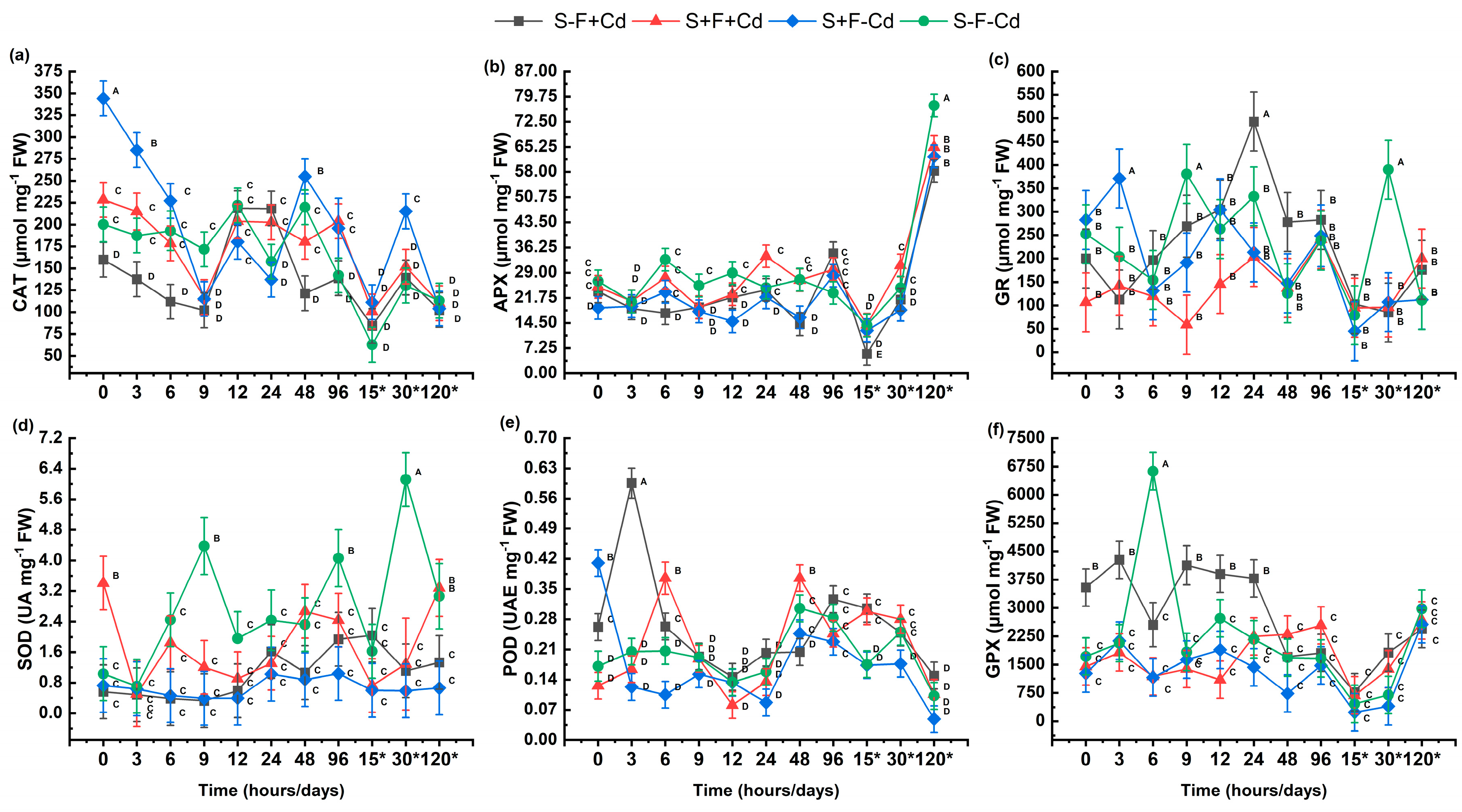
2.3. Enzymatic Antioxidant Defense System
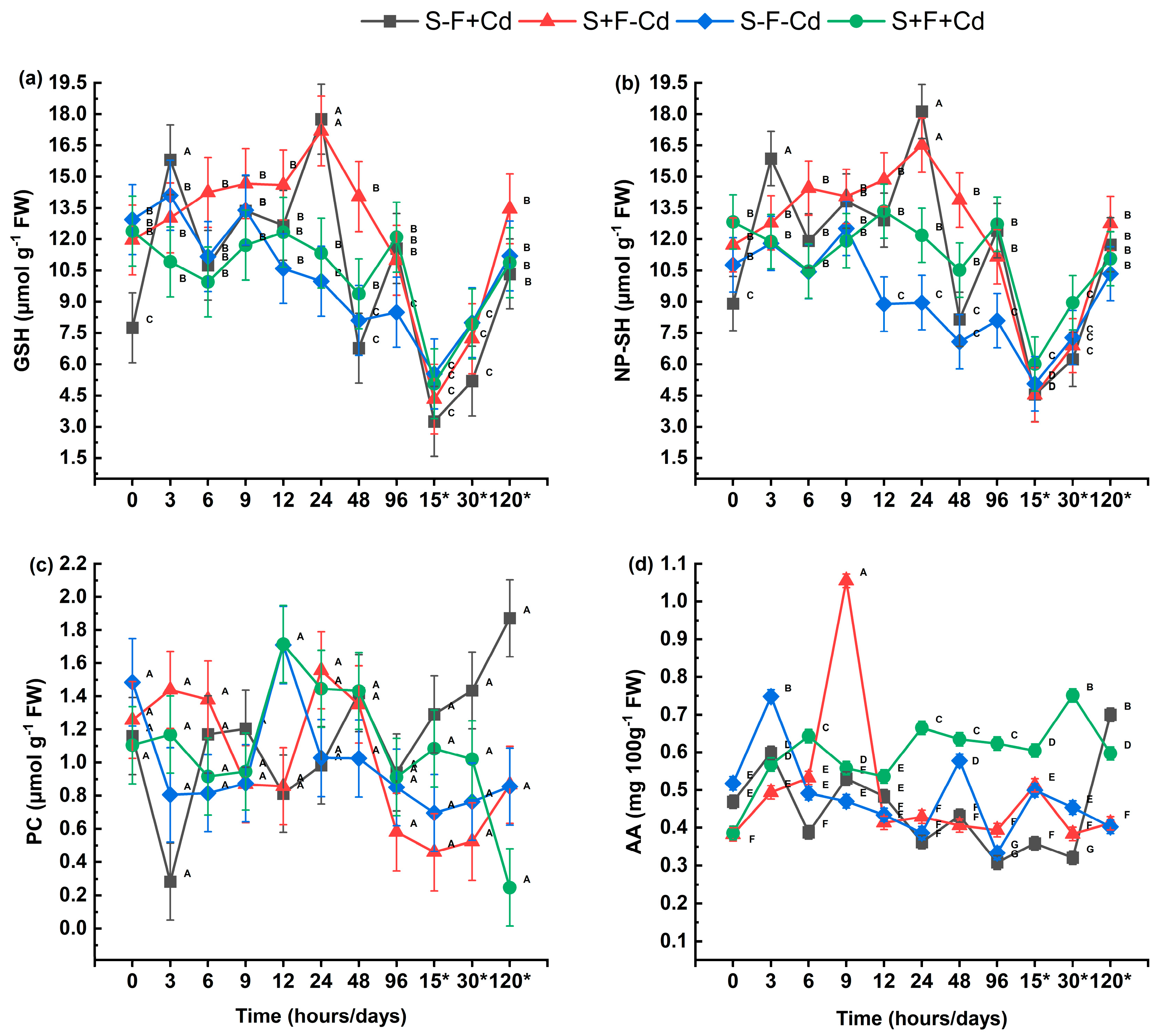
2.4. Non-Enzymatic Antioxidant Defense System
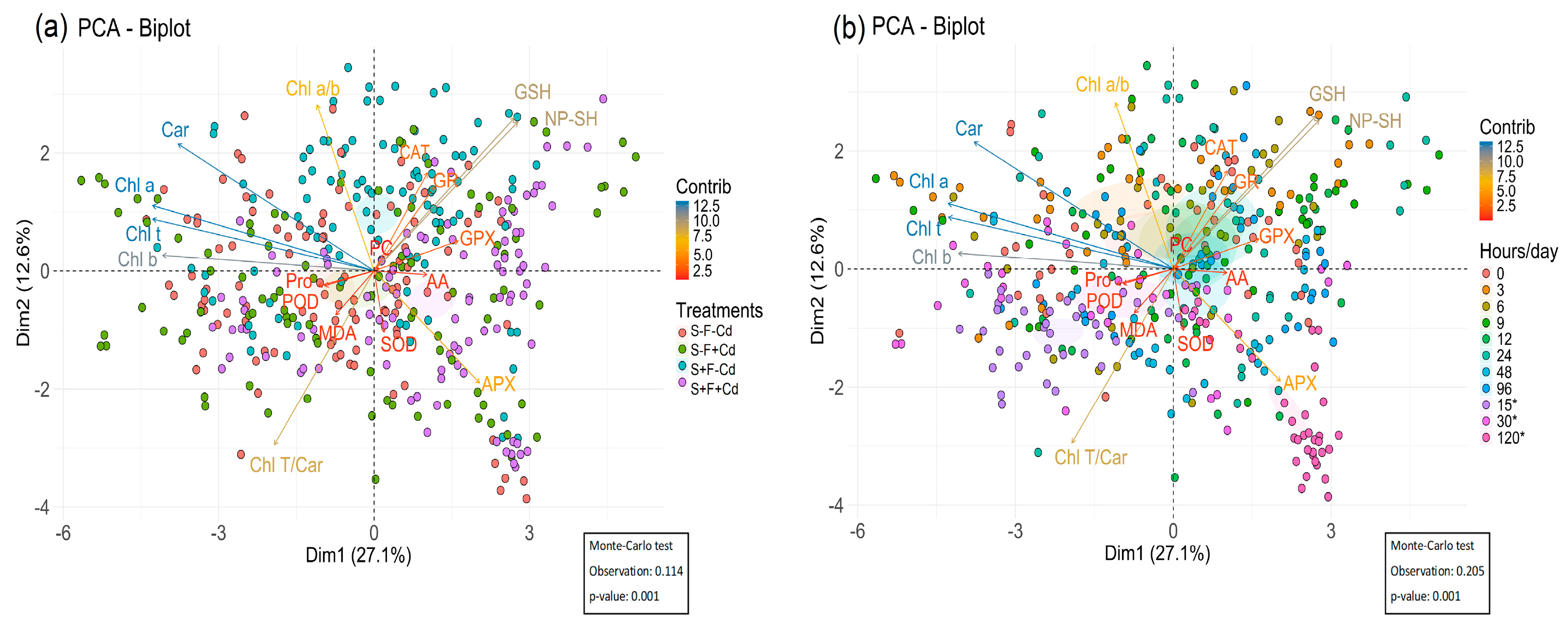
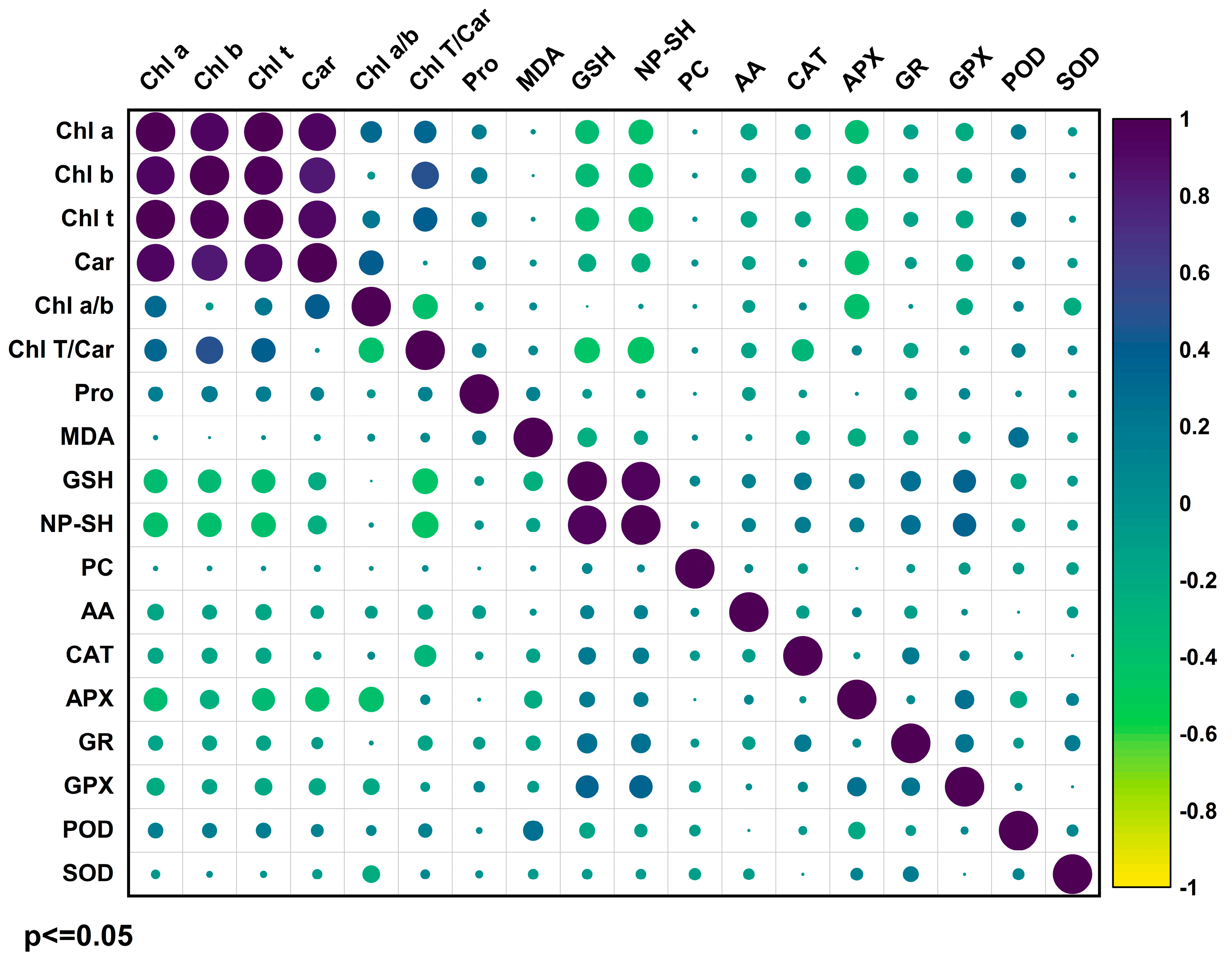
2.5. Principal Component Analysis of Photosynthetic Pigments and Antioxidant Systems in AMF-Colonized Sugarcane Under Cd Stress
3. Discussion
4. Materials and Methods
4.1. Crop Establishment and Treatments
4.2. Determination of AMF Colonization and Cadmium Content Analysis
4.3. Collection and Pretreatment of Samples for Biochemical Parameters
4.4. Analysis of Photosynthetic Pigments and Biochemical Stress Markers
4.5. Enzymatic Antioxidant Defense System
4.6. Non-Enzymatic Antioxidant Defense System
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Musa, Y.; Ridwan, I.; Ponto, H.; Ala, A.; Farid, B.M.; Widiayani, N.; Yayank, A.R. Application of Arbuscular Mycorrhizal Fungus (AMF) improves the growth of single-bud sugarcane (Saccharum officinarum L.) seedlings from different bud location. IOP Conf. Ser. Earth Envion. Sci. 2020, 486, 012122. [Google Scholar] [CrossRef]
- FAO FAOSTAT 2025. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 July 2025).
- Yousefi, Z.; Kolahi, M.; Majd, A.; Jonoubi, P. Effect of cadmium on morphometric traits, antioxidant enzyme activity and phytochelatin synthase gene expression (SoPCS) of Saccharum officinarum var. cp48-103 in vitro. Ecotoxicol. Envion. Saf. 2018, 157, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Ling, Q.; Hu, F.; Wu, J.; Yang, Z.; Qi, Y.; Li, Q. Genotypic Differences in Growth and Antioxidant Enzyme Activities Under Cadmium Stress in Sugarcane. Bull. Envion. Contam. Toxicol. 2017, 99, 607–613. [Google Scholar] [CrossRef]
- Guo, J.; Xu, L.; Su, Y.; Wang, H.; Gao, S.; Xu, J.; Que, Y. ScMT2-1-3, a metallothionein gene of sugarcane, plays an important role in the regulation of heavy metal tolerance/accumulation. Biomed. Res. Int. 2013, 2013, 904769. [Google Scholar] [CrossRef] [PubMed]
- Sereno, M.L.; Almeida, R.S.; Nishimura, D.S.; Figueira, A. Response of sugarcane to increasing concentrations of copper and cadmium and expression of metallothionein genes. J. Plant Physiol. 2007, 164, 1499–1515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Hu, Z.H.; Yan, T.X.; Lu, R.R.; Peng, C.L.; Li, S.S.; Jing, Y.-X. Arbuscular mycorrhizal fungi alleviate Cd phytotoxicity by altering Cd subcellular distribution and chemical forms in Zea mays. Ecotoxicol. Envion. Saf. 2019, 171, 352–360. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, C.; Li, L.; Mohsen, M.; Wang, T.; Wang, X.; Zhang, L.; Huang, W. Single and combined effects of microplastics and cadmium on the sea cucumber Apostichopus japonicus. Mar. Envion. Res. 2023, 186, 105927. [Google Scholar] [CrossRef]
- Bora, M.S.; Sarma, K.P. Anatomical and ultrastructural alterations in Ceratopteris pteridoides under cadmium stress: A mechanism of cadmium tolerance. Ecotoxicol. Envion. Saf. 2021, 218, 112285. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Kim, S.H.; Lee, H.S.; Marinoia, E.; Song, W.Y. Characterization of Brassica rapa metallothionein and phytochelatin synthase genes potentially involved in heavy metal detoxification. PLoS ONE 2021, 16, e0252899. [Google Scholar] [CrossRef]
- Carvalho, M.E.A.; Castro, P.R.C.; Azevedo, R.A. Hormesis in plants under Cd exposure: From toxic to beneficial element? J. Hazard. Mater. 2020, 384, 121434. [Google Scholar] [CrossRef]
- Daud, M.K.; Ali, S.; Variath, M.T.; Zhu, S.J. Differential physiological, ultramorphological and metabolic responses of cotton cultivars under cadmium stress. Chemosphere 2013, 93, 2593–2602. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Kaur, H. Response of Antioxidant Enzymes, Phytochelatins and Glutathione Production Towards Cd and Zn Stresses in Cajanus cajan (L.) Millsp. Genotypes Colonized by Arbuscular Mycorrhizal Fungi. J. Agron. Crop Sci. 2013, 199, 118–133. [Google Scholar] [CrossRef]
- Nualla-ong, A.; Phongdara, A.; Buapet, P. Copper and zinc differentially affect root glutathione accumulation and phytochelatin synthase gene expression of Rhizophora mucronata seedlings: Implications for mechanisms underlying trace metal tolerance. Ecotoxicol. Envion. Saf. 2020, 205, 111175. [Google Scholar] [CrossRef]
- Franchina, D.G.; Dostert, C.; Brenner, D. Reactive Oxygen Species: Involvement in T Cell Signaling and Metabolism. Trends Immunol. 2018, 39, 489–502. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Luo, P.; Wu, J.; Li, T.T.; Shi, P.; Ma, Q.; Di, D.W. An Overview of the Mechanisms through Which Plants Regulate ROS Homeostasis under Cadmium Stress. Antioxidants 2024, 13, 1174. [Google Scholar] [CrossRef]
- Bellini, E.; Maresca, V.; Betti, C.; Castiglione, M.R.; Fontanini, D.; Capocchi, A.; Sorce, C.; Borsò, M.; Bruno, L.; Sorbo, S.; et al. The Moss Leptodictyum riparium Counteracts Severe Cadmium Stress by Activation of Glutathione Transferase and Phytochelatin Synthase, but Slightly by Phytochelatins. Int. J. Mol. Sci. 2020, 21, 1583. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Alam, P.; Hussain, A.; Karabulut, F.; Tonny, S.H.; Cheng, S.H.; Yusuf, M.; Adil, M.F.; Sehar, S.; Alomrani, S.O.; et al. Phytochelatins: Key regulator against heavy metal toxicity in plants. Plant Stress. 2024, 11, 100355. [Google Scholar] [CrossRef]
- Bolchi, A.; Ruotolo, R.; Marchini, G.; Vurro, E.; di Toppi, L.S.; Kohler, A.; Tisserant, E.; Martin, F.; Ottonello, S. Genome-wide inventory of metal homeostasis-related gene products including a functional phytochelatin synthase in the hypogeous mycorrhizal fungus Tuber melanosporum. Fungal Genet. Biol. 2011, 48, 573–584. [Google Scholar] [CrossRef]
- Haselwandter, K.; Winkelmann, G. Ferricrocin—An ectomycorrhizal siderophore of Cenococcum geophilum. BioMetals 2002, 15, 73–77. [Google Scholar] [CrossRef]
- Morel, M.; Kohler, A.; Martin, F.; Gelhaye, E.; Rouhier, N. Comparison of the thiol-dependent antioxidant systems in the ectomycorrhizal Laccaria bicolor and the saprotrophic Phanerochaete chrysosporium. New Phytol. 2008, 180, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Iijima, T.; Higuchi, R. Use of specific phospholipid fatty acids for identifying and quantifying the external hyphae of the arbuscular mycorrhizal fungus Gigaspora rosea. Soil. Biol. Biochem. 2004, 36, 1827–1834. [Google Scholar] [CrossRef]
- Xu, Z.; Ban, Y.; Li, Z.; Chen, H.; Yang, R.; Tang, M. Arbuscular mycorrhizal fungi play a role in protecting roots of Sophora viciifolia Hance. from Pb damage associated with increased phytochelatin synthase gene expression. Environ. Sci. Pollut. Res. 2014, 21, 12671–12683. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Bhandari, P. Cadmium toxicity in crop plants and its alleviation by arbuscular mycorrhizal (AM) fungi: An overview. Plant Biosyst. 2014, 148, 609–621. [Google Scholar] [CrossRef]
- Li, W.; Chen, K.; Li, Q.; Tang, Y.; Jiang, Y.; Su, Y. Effects of Arbuscular Mycorrhizal Fungi on Alleviating Cadmium Stress in Medicago truncatula Gaertn. Plants 2023, 12, 547. [Google Scholar] [CrossRef]
- Kuang, Y.; Li, X.; Wang, Z.; Wang, X.; Wei, H.; Chen, H.; Hu, W.; Tang, M. Effects of Arbuscular Mycorrhizal Fungi on the Growth and Root Cell Ultrastructure of Eucalyptus grandis under Cadmium Stress. J. Fungi 2023, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Liu, S.; Xu, S.; Qin, S.; Lyu, D.; He, J.; Zhou, J. Arbuscular Mycorrhizal Fungi Alleviate Cadmium Phytotoxicity by Regulating Cadmium Mobility, Physiological Responses, and Gene Expression Patterns in Malus hupehensis Rehd. Int. J. Mol. Sci. 2025, 26, 1418. [Google Scholar] [CrossRef]
- Römkens, P.; Rietra, R.; Kros, H.; Cees Voogd, J.; De Vries, W. Impact of Cadmium Levels in Fertilisers on Cadmium Accumulation in Soil and Uptake by Food Crops; CABI Databases: Wageningen, The Netherlands, 2018. [Google Scholar]
- Atakan, A.; Özkaya, H.Ö. Arbuscular Mycorrhizal Fungi and Glomalin. Turk. J. Agric.—Food Sci. Technol. 2021, 9, 2371–2375. [Google Scholar] [CrossRef]
- Likus-Cieślik, J.; Józefowska, A.; Frouz, J.; Vicena, J.; Pietrzykowski, M. Relationships between soil properties, vegetation and soil biota in extremely sulfurized mine soils. Ecol. Eng. 2023, 186, 106836. [Google Scholar] [CrossRef]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular Mycorrhizal Fungi and Associated Microbiota as Plant Biostimulants: Research Strategies for the Selection of the Best Performing Inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef]
- Liu, C.L.; Gao, Z.Y.; Shang, L.G.; Yang, C.H.; Ruan, B.P.; Zeng, D.L.; Guo, L.; Zhao, F.; Huang, C.; Qian, Q. Natural variation in the promoter of OsHMA3 contributes to differential grain cadmium accumulation between Indica and Japonica rice. J. Integr. Plant Biol. 2020, 62, 314–329. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, M.; Chen, M.; Lin, X.; Xu, P.; Cao, Z. Mutation of OsNRAMP5 reduces cadmium xylem and phloem transport in rice plants and its physiological mechanism. Environ. Pollut. 2024, 341, 122928. [Google Scholar] [CrossRef]
- Song, Y.; Jin, L.; Wang, X. Cadmium absorption and transportation pathways in plants. Int. J. Phytoremed. 2017, 19, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, Y.; Li, Y.; Li, Q.; Yao, L.; Yu, J.; Chen, H.; Wu, K.; Qiu, D.; Wu, Z.; et al. Mitigating sediment cadmium contamination through combining PGPR Enterobacter ludwigii with the submerged macrophyte Vallisneria natans. J. Hazard. Mater. 2024, 473, 134662. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Wei, H.; Kuang, Y.; Chen, H.; Tang, M.; Hu, W. Arbuscular mycorrhizal fungi inoculation and exogenous indole-3-acetic acid application induce antioxidant defense response to alleviate cadmium toxicity in Broussonetia papyrifera. Pedosphere 2024, 34, 447–459. [Google Scholar] [CrossRef]
- Garg, N.; Singh, S. Arbuscular Mycorrhiza Rhizophagus irregularis and Silicon Modulate Growth, Proline Biosynthesis and Yield in Cajanus cajan L. Millsp. (pigeonpea) Genotypes Under Cadmium and Zinc Stress. J. Plant Growth Regul. 2018, 37, 46–63. [Google Scholar] [CrossRef]
- Ruley, A.T.; Sharma, N.C.; Sahi, S.V.; Singh, S.R.; Sajwan, K.S. Effects of lead and chelators on growth, photosynthetic activity and Pb uptake in Sesbania drummondii grown in soil. Environ. Pollut. 2006, 144, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Han, X.; Liang, Y.; Ghosh, A.; Chen, J.; Tang, M. The Combined Effects of Arbuscular Mycorrhizal Fungi (AMF) and Lead (Pb) Stress on Pb Accumulation, Plant Growth Parameters, Photosynthesis, and Antioxidant Enzymes in Robinia pseudoacacia L. PLoS ONE 2015, 10, e0145726. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.; Wu, J.; Li, H.; Li, Y.; Li, Z.; He, Y. Enhanced Ultraviolet B Radiation Suppresses Magnaporthe oryzae Infection and Alleviates Its Damage to the Photosynthesis of Rice Leaves. Phyton-Int. J. Exp. Bot. 2024, 93, 2613–2628. [Google Scholar] [CrossRef]
- Singh, S.; Eapen, S.; D’Souza, S.F. Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 2006, 62, 233–246. [Google Scholar] [CrossRef]
- Chen, L.; Hu, X.; Yang, W.; Xu, Z.; Zhang, D.; Gao, S. The effects of arbuscular mycorrhizal fungi on sex-specific responses to Pb pollution in Populus cathayana. Ecotoxicol. Envion. Saf. 2015, 113, 460–468. [Google Scholar] [CrossRef]
- Fan, P.; Wu, L.; Wang, Q.; Wang, Y.; Luo, H.; Song, J.; Yang, M.; Yao, H.; Chen, S. Physiological and molecular mechanisms of medicinal plants in response to cadmium stress: Current status and future perspective. J. Hazard. Mater. 2023, 450, 131008. [Google Scholar] [CrossRef]
- Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen Peroxide: Its Role in Plant Biology and Crosstalk with Signalling Networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef]
- Yang, T.; Poovaiah, B.W. Hydrogen peroxide homeostasis: Activation of plant catalase by calcium/calmodulin. Proc. Natl. Acad. Sci. USA 2002, 99, 4097–4102. [Google Scholar] [CrossRef]
- Shi, G.; Liu, C.; Cai, Q.; Liu, Q.; Hou, C. Cadmium accumulation and tolerance of two safflower cultivars in relation to photosynthesis and antioxidantive enzymes. Bull. Envion. Contam. Toxicol. 2010, 85, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Al Huqail, A.A.; Egamberdieva, D.; Wirth, S. Alleviation of cadmium stress in Solanum lycopersicum L. by arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi J. Biol. Sci. 2016, 23, 272–281. [Google Scholar] [CrossRef]
- Wang, L.; Jia, X.; Zhao, Y.; Zhang, C.Y.; Zhao, J. Effect of arbuscular mycorrhizal fungi in roots on antioxidant enzyme activity in leaves of Robinia pseudoacacia L. seedlings under elevated CO2 and Cd exposure. Environ. Pollut. 2022, 294, 118652. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Tai, R.; Cao, Y.; Ge, Y.; Chen, J.; Li, J. Genome-wide identification and comparative expression analysis of ascorbate peroxidase (APX) gene family in apple fruit under 1-methylcyclopropene (1-MCP) and ethephon treatments during ripening. Sci. Hortic. 2024, 329, 113016. [Google Scholar] [CrossRef]
- Janeczko, A.; Przywara, M.; Maslanka, R.; Raś, B.; Ziaja, K.; Kwolek-Mirek, M.; Zadrag-Tecza, R.; Bednarska, S. Redox perturbations in yeast cells lacking glutathione reductase. Fungal Genet. Biol. 2023, 167, 103810. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; Fathi Abd-Allah, E. Mycorrhizal Association and ROS in Plants. In Oxidative Damage to Plants Antioxidant Networks and Signaling; Academic Press: Cambridge, MA, USA, 2014; pp. 453–475. [Google Scholar] [CrossRef]
- Wood, J.P. Review of techniques for the in-situ sterilization of soil contaminated with Bacillus anthracis spores or other pathogens. Res. Microbiol. 2024, 175, 104175. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN18. [Google Scholar] [CrossRef]
- Aguilar-Ulloa, W.; Arce-Acuña, P.; Galiano-Murillo, F.; Torres-Cruz, T.J. Spore isolation and evaluation of inoculation methods in the production of mycorrhizae in trap crops. Rev. Tecnol. Marcha 2016, 29, 5–14. [Google Scholar] [CrossRef]
- Garg, N.; Chandel, S. Role of Arbuscular Mycorrhizal (AM) Fungi on Growth, Cadmium Uptake, Osmolyte, and Phytochelatin Synthesis in Cajanus cajan (L.) Millsp. Under NaCl and Cd Stresses. J. Plant Growth Regul. 2012, 31, 292–308. [Google Scholar] [CrossRef]
- Nishiyama, I.; Fukuda, T.; Oota, T. Genotypic Differences in Chlorophyll, Lutein, and β-Carotene Contents in the Fruits of Actinidia Species. J. Agric. Food Chem. 2005, 53, 6403–6407. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Guo, Y.; Ma, Y.; Yang, M.; Fu, R.; Sun, Y. Elevated CO2 delayed yellowing by maintaining chlorophyll biosynthesis and inhibiting chlorophyll degradation and carotenoid accumulation of postharvest broccoli. Postharvest Biol. Technol. 2022, 194, 112089. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Tran, L.S.P. Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front. Plant Sci. 2016, 7, 182842. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Anitha, R.; Mary, P.C.N.; Joseph, M.A.; Savery, R.; Sritharan, N.; Purushothaman, R.S. Differential responses of sugarcane (Saccharum officinarum L.) genotypes under salt stress condition. Plant Arch. 2015, 15, 1055–1060. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, A.; Sedlářová, M.; Pospíšil, P. Malondialdehyde enhances PsbP protein release during heat stress in Arabidopsis. Plant Physiol. Biochem. 2023, 202, 107984. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1974; pp. 673–684. [Google Scholar] [CrossRef]
- Azizollahi, Z.; Ghaderian, S.M.; Ghotbi-Ravandi, A.A. Cadmium accumulation and its effects on physiological and biochemical characters of summer savory (Satureja hortensis L.). Int. J. Phytoremed. 2019, 21, 1241–1253. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of Ascorbate Peroxidase in Spinach Chloroplasts; Its Inactivation in Ascorbate-Depleted Medium and Reactivation by Monodehydroascorbate Radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar] [CrossRef]
- dos Santos, M.L.S.; de Almeida, A.A.F.; da Silva, N.M.; Oliveira, B.R.M.; Silva, J.V.S.; Junior, J.O.S.; Ahnert, D.; Baligar, V.C. Mitigation of cadmium toxicity by zinc in juvenile cacao: Physiological, biochemical, molecular and micromorphological responses. Envion. Exp. Bot. 2020, 179, 104201. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Fijalkowski, K.L.; Kwarciak-Kozlowska, A. Phytotoxicity assay to assess sewage sludge phytoremediation rate using guaiacol peroxidase activity (GPX): A comparison of four growth substrates. J. Envion. Manag. 2020, 263, 110413. [Google Scholar] [CrossRef]
- Kireyko, A.V.; Veselova, I.A.; Shekhovtsova, T.N. Mechanisms of peroxidase oxidation of o-dianisidine, 3,3′,5,5′- tetramethylbenzidine, and o-phenylenediamine in the presence of sodium dodecyl sulfate. Russ. J. Bioorg Chem. 2006, 32, 71–77. [Google Scholar] [CrossRef]
- Neves, V.A.; Lourenço, E.J. Peroxidase from peach fruit: Thermal Stability. Braz. Arch. Biol. Technol. 1998, 41, 179–186. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Alsiary, W.A.; Madany, M.M.Y.; AbdElgawad, H. The pleiotropic role of Salinicoccus bacteria in enhancing ROS homeostasis and detoxification metabolism in soybean and oat to cope with pollution of triclosan. Plant Physiol. Biochem. 2024, 207, 108327. [Google Scholar] [CrossRef]
- Tawfik, D.S. Modification of Sulfhydryl Groups with DTNB; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Jinadasa, N.; Collins, D.; Holford, P.; Milham, P.J.; Conroy, J.P. Reactions to cadmium stress in a cadmium-tolerant variety of cabbage (Brassica oleracea L.): Is cadmium tolerance necessarily desirable in food crops? Environ. Sci. Pollut. Res. 2016, 23, 5296–5306. [Google Scholar] [CrossRef] [PubMed]
- Berthou, M.; Clarot, I.; Gouyon, J.; Steyer, D.; Monat, M.A.; Boudier, A.; Pallotta, A. Thiol sensing: From current methods to nanoscale contribution. Microchem. J. 2022, 183, 107994. [Google Scholar] [CrossRef]
- Castillo-Israel, K.A.T.; Flandez, L.E.L.; Tuaño, A.P.P.; Sartagoda, K.J.D.; Compendio, M.C.M. Vitamin C levels of selected Philippine indigenous berries as affected by fruit maturity and processing treatment. Food Prod. Process. Nutr. 2023, 5, 33. [Google Scholar] [CrossRef]
- Di Rienzo Julio Macchiavelli, R.; Casanoves, F. Modelos Mixtos en Infostat. In Manual de Usuario; Editorial Brujas: Córdoba, Argentina, 2012; p. 193. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; EISPACK; Heisterkamp, S.; Willigen, B.; Van Ranke, J.; The Comprehensive R. Linear and Nonlinear Mixed Effects Models (Package ’nlme’). 2025. Available online: https://cran.r-project.org/web/packages/nlme/nlme.pdf (accessed on 18 December 2024).
| Treatments | Soil | Leaves |
|---|---|---|
| S-F+Cd | 68.38 ± 4.55 A | 1.21 ± 0.10 A |
| S+F+Cd | 48.73 ± 4.55 B | 0.47 ± 0.10 B |
| S-F-Cd | 0.18 ± 4.55 C | 0.15 ± 0.10 B |
| S+F-Cd | 0.18 ± 4.55 C | 0.15 ± 0.10 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paladines-Beltrán, G.M.; Venegas, N.A.; Suárez, J.C. Arbuscular Mycorrhizal Fungi Enhance Antioxidant Defense Systems in Sugarcane Under Soil Cadmium Stress. Plants 2025, 14, 2916. https://doi.org/10.3390/plants14182916
Paladines-Beltrán GM, Venegas NA, Suárez JC. Arbuscular Mycorrhizal Fungi Enhance Antioxidant Defense Systems in Sugarcane Under Soil Cadmium Stress. Plants. 2025; 14(18):2916. https://doi.org/10.3390/plants14182916
Chicago/Turabian StylePaladines-Beltrán, Gloria Magaly, Nathalia Alejandra Venegas, and Juan Carlos Suárez. 2025. "Arbuscular Mycorrhizal Fungi Enhance Antioxidant Defense Systems in Sugarcane Under Soil Cadmium Stress" Plants 14, no. 18: 2916. https://doi.org/10.3390/plants14182916
APA StylePaladines-Beltrán, G. M., Venegas, N. A., & Suárez, J. C. (2025). Arbuscular Mycorrhizal Fungi Enhance Antioxidant Defense Systems in Sugarcane Under Soil Cadmium Stress. Plants, 14(18), 2916. https://doi.org/10.3390/plants14182916






