Pilot Study of Genetic Diversity and Structure in Elite Germplasm of Hibiscus syriacus
Abstract
1. Introduction
2. Results
2.1. Polymorphism Detection of SSR and ISSR Markers
2.2. Evaluation of Genetic Parameters of 46 Elite H. syriacus Varieties Using the Polymorphic SSR and ISSR Markers
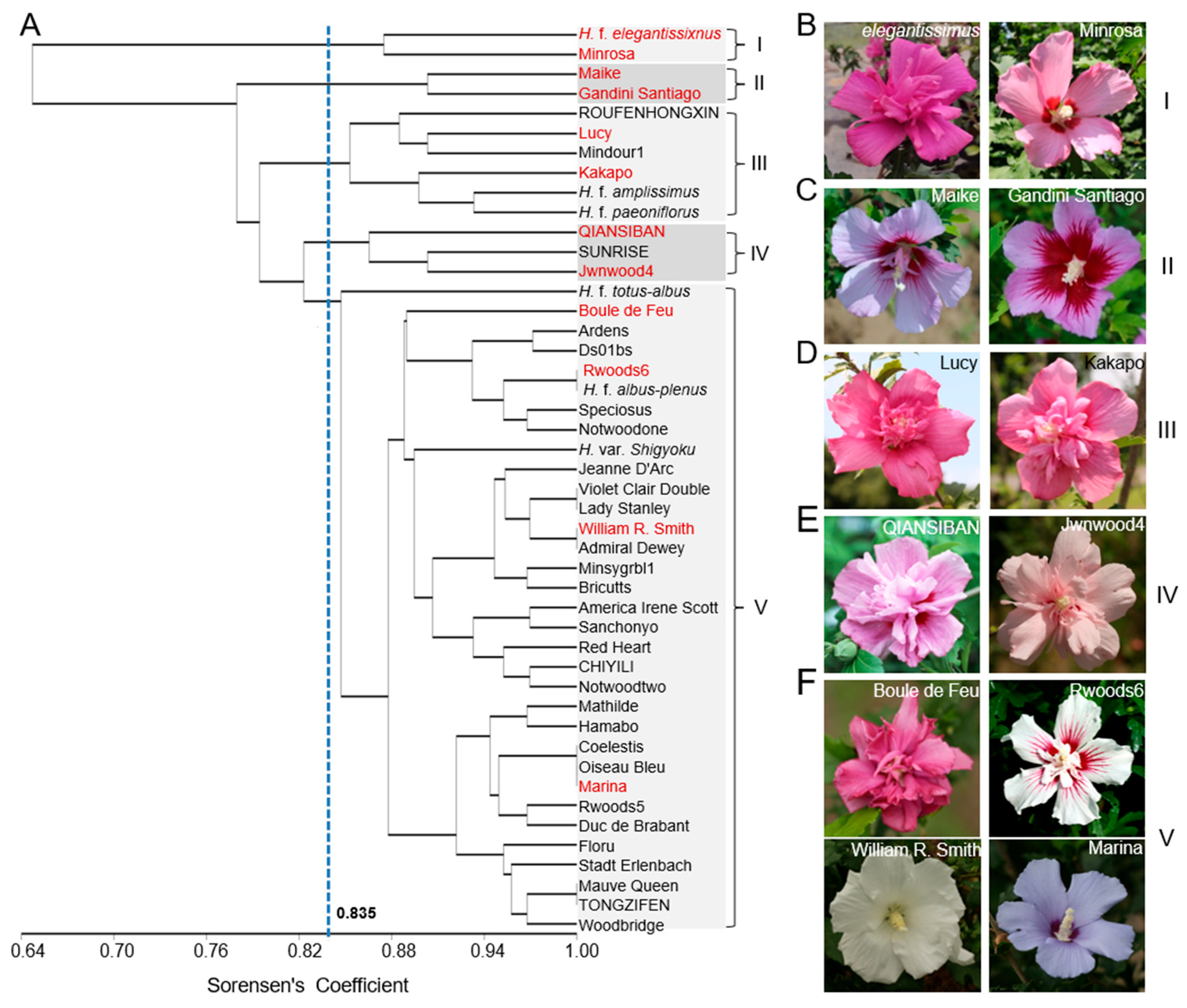
2.3. Phylogenetic Analysis of 46 Elite H. syriacus Varieties Based on the SSR Markers
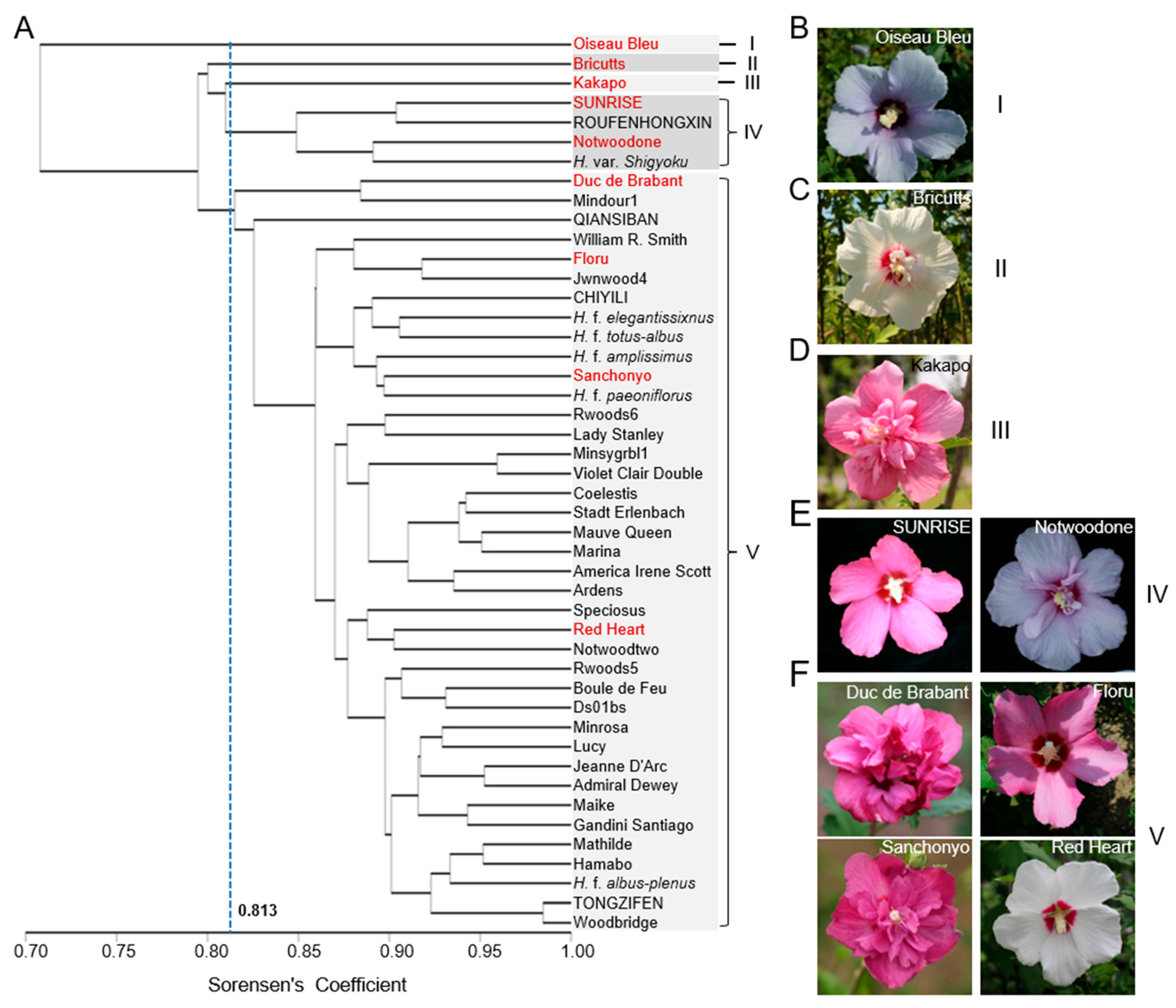
2.4. Phylogenetic Analysis of 46 Elite H. syriacus Varieties Based on the ISSR Markers
2.5. Phylogenetic Analysis of 46 Elite H. syriacus Varieties Based on the Combined SSR and ISSR Markers
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. DNA Extraction
4.3. PCR Amplification
4.4. Statistical Analysis of Data
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ljubojević, M.; Pušić, M. Review on ornamental Rose of Sharon (Hibiscus syriacus L.): Assessment of decorativeness, invasiveness and ecosystem services in public green areas. In Sustainable Practices in Horticulture and Landscape Architecture; Ostojić, J., Cig, A., Eds.; iKSAD Publishing House: Ankara, Turkey, 2022; pp. 71–122. [Google Scholar]
- Li, S.Z. Compendium of Materia Medica; originally completed 1578; People’s Medical Publishing House: Beijing, China, 1957; reprint. [Google Scholar]
- Wang, X.; Li, L.; Liu, C.; Zhang, M.; Wen, Y. An integrated metabolome and transcriptome analysis of the Hibiscus syriacus L. petals reveal the molecular mechanisms of anthocyanin accumulation. Front. Genet. 2022, 13, 995748. [Google Scholar] [CrossRef] [PubMed]
- Welsh, S.L. Utah Flora: Malvaceae. Great Basin Nat. 1980, 40, 27–37. [Google Scholar]
- Maganha, E.G.; da Costa Halmenschlager, R.; Rosa, R.M.; Henriques, J.A.P.; de Paula Ramos, A.L.L.; Saffi, J. Pharmacological evidences for the extracts and secondary metabolites from plants of the genus Hibiscus. Food Chem. 2010, 118, 1–10. [Google Scholar] [CrossRef]
- Bates, D.M. NotesonthecultivatedMalvaceae.1.Hibiscus. Baileya 1965, 13, 57–130. [Google Scholar]
- Van Laere, K.; Van Huylenbroeck, J.M.; Van Bockstaele, E. Interspecific hybridization between Hibiscus syriacus, Hibiscus sinosyriacus and Hibiscus paramutabilis. Euphytica 2007, 155, 271–283. [Google Scholar] [CrossRef]
- Yang, S.; Chen, J.; Lui, X.; Lin, C.; Cheng, Y.; Fang, R. Nutritional and Functional Compositions and Nutritional Quality of Edible Petals of Various Lines of Hibiscus syriacus L. Food Sci. 2018, 39, 213–219. (In Chinese) [Google Scholar]
- Park, Y.; Kwon, S.; Jang, Y.L.; Lee, D.; Yang, S.; Eo, H.J.; Park, G.H.; Kwon, H. Nutritional composition and phytochemical screening in different parts of Hibiscus syriacus L. Food Sci. Nutr. 2022, 10, 3034–3042. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Mishra, S.; Singh, A.; Srivastava, D.; Singh, S.; Arya, V. Hibiscus syriacus L.: A Critical Review of Medicinal Utility & Phytopharmacology with Mechanistic Approach. J. Phytopharm. 2022, 11, 204–210. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, X.; Jiang, Y.; Chen, C.; Chen, J.; Zhang, J.; Wen, Y. Combined Morphological and Palynological Classification for Hibiscus syriacus L. (Malvaceae): Construction of the Diagnostic Classification Framework and Implications of Pollen Morphological Variation on Fruiting. Agronomy 2023, 13, 828. [Google Scholar] [CrossRef]
- Xiao, F. The Research on Classification of 27 Hibiscus Cultivars. Ph.D. Thesis, Central South University of Forestry and Technology, Changsha, China, 2019. (In Chinese). [Google Scholar]
- Van Huylenbroeck, J.; De Riek, J.; De Loose, M. Genetic relationships among Hibiscus syriacus, Hibiscus sinosyriacus and Hibiscus paramutabilis revealed by AFLP, morphology and ploidy analysis. Genet. Resour. Crop Evol. 2000, 47, 335–343. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Tian, L.; Li, M.; Liu, Y.; Liu, D. Analysis the genetic diversity of Hibiscus syriacus germplasm resources by SRAP. J. Sichuan Agric. Univ. 2020, 38, 52–57. (In Chinese) [Google Scholar]
- Gao, Y. SSR of Forest Tree Fine Varieties Identification and EST-SSR Primer Development of Hibiscus. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2020. [Google Scholar]
- Dou, X.; Chen, J.Q.; Dong, Z.K.; Liu, H.; Duan, Z.H. Genetic diversity of 41 germplasm resources of Hibiscus syriacus with AFLP analysis. Shandong For. Sci. Technol. 2021, 4, 1–4. (In Chinese) [Google Scholar]
- Zhao, Q. The Application of ISSR Markers in Plant Genetic Analysis—Take the Case of Phalaenopsis, Canarium and Hibiscus. Master’s Thesis, Shantou University, Shantou, China, 2005. [Google Scholar]
- Nei, M. Analysis of Gene Diversity in Subdivided Populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Mu, K.; Zhang, J.; Zhao, Z.; Shan, S.; Xue, X.; Zhu, L.; Xu, L.; Li, H. Genetic diversity analysis of Phaseolus vulgaris based on SSR and ISSR markers. Mol. Plant Breed. 2022, 20, 7161–7173. (In Chinese) [Google Scholar]
- Amiteye, S. Basic concepts and methodologies of DNA marker systems in plant molecular breeding. Heliyon 2021, 7, e08093. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.M. Characterization, costs, cues and future perspectives of phenotypic plasticity. Ann. Bot. 2022, 130, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Li, L.; Yang, X.; Li, X. Comparison of genetic diversity of twelve Elymus species using ISSR and SSR markers. Sci. Agric. Sin. 2005, 38, 1522–1527. (In Chinese) [Google Scholar]
- Dong, H.; Ji, K.; Hou, B.; Zhao, H. Genetic relatives analysis of 41 Loropetalum chinense var. rubrum cultivars by ISSR markers. ACTA Hortic. Sin. 2014, 41, 365–374. [Google Scholar]
- Yang, F.; Li, Y.-J.; Li, X.-M.; Yong, X.-P.; Ran, K.; Chen, L.; Qin, H.-Y.; Ran, M.-L. Analysis of genetic diversity in radish (Raphanus sativus L.) with different bolting-resistance based on ISSR and SSR. Southwest China J. Agric. Sci. 2019, 32, 1708–1716. [Google Scholar]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy: The Principles and Practice of Numerical Classification; W.H. Freeman: San Francisco, CA, USA, 1973; pp. 230–234. [Google Scholar]
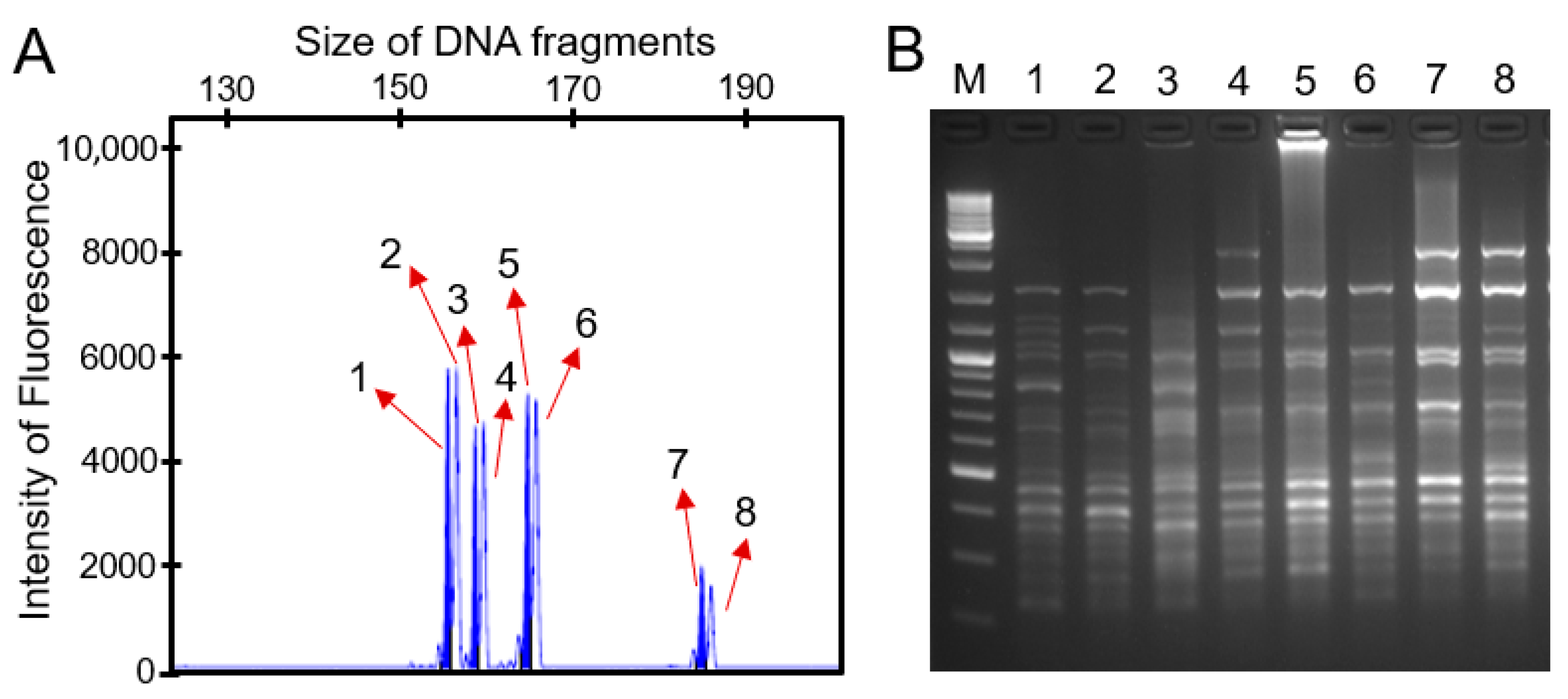

| Primer Name | Num. of Amplified Bands | Num. of Nonpolymorphic Bands | Num. of Polymorphic Bands | Polymorphic Ratio (%) |
|---|---|---|---|---|
| HP0001 | 2 | 1 | 1 | 50.00 |
| HP0002 | 1 | 1 | 0 | 0.00 |
| HP0003 | 8 | 0 | 8 | 100.00 |
| HP0010 | 2 | 2 | 0 | 0.00 |
| HP0011 | 3 | 0 | 3 | 100.00 |
| HP0012 | 4 | 0 | 4 | 100.00 |
| HP0015 | 5 | 0 | 5 | 100.00 |
| HP0018 | 4 | 0 | 4 | 100.00 |
| HP0038 | 3 | 0 | 3 | 100.00 |
| HP0055 | 1 | 1 | 0 | 0.00 |
| Total | 33 | 5 | 28 | |
| Average | 3.3 | 0.5 | 2.8 | 84.85 |
| Primer Name | Sequence | Num. of Amplified Bands | Num. of Nonpolymorphic Bands | Num. of Polymorphic Bands | Polymorphic Ratio (%) |
|---|---|---|---|---|---|
| UBC809 | AGA GAG AGA GAG AGA GG | 20 | 8 | 12 | 60.00 |
| UBC827 | ACA CAC ACA CAC ACA CG | 16 | 5 | 11 | 68.75 |
| UBC836 | AGA GAG AGA GAG AGA GYA | 20 | 5 | 15 | 75.00 |
| UBC840 | GAG AGA GAG AGA GAG AYT | 20 | 3 | 17 | 85.00 |
| UBC842 | GAG AGA GAG AGA GAG AYG | 19 | 5 | 14 | 73.68 |
| Total | 95 | 26 | 69 | ||
| Average | 19 | 5.2 | 13.8 | 72.63 |
| Marker | Item | Number of Primers | Na (Observed Number of Alleles) | Ne (Effective Number of Alleles) | H (Nei’s Gene Diversity Index) | I (Shannon Information Index) |
|---|---|---|---|---|---|---|
| SSR | Mean value | 10 | 1.818 | 1.251 | 0.163 | 0.267 |
| Standard deviation | 0.068 | 0.053 | 0.028 | 0.040 | ||
| ISSR | Mean value | 5 | 1.674 | 1.321 | 0.197 | 0.305 |
| Standard deviation | 0.053 | 0.035 | 0.019 | 0.027 |
| No. | Variety Name | Flower Pattern | Color | Eye Spot (Y/N) | Country |
|---|---|---|---|---|---|
| 1 | H. syriacus “Woodbridge” | Single | Deep Pink | Y | UK |
| 2 | H. syriacus “Notwoodtwo” | Semi-double | White | N | UK |
| 3 | H. syriacus f. paeoniflorus | Double | Pink | Y | China |
| 4 | H. syriacus TONGZIFEN | Single | Orchid Pink | Y | China |
| 5 | H. syriacus “Gandini Santiago” | Semi-double/Single | Mauve Pink | Y | Netherlands |
| 6 | H. syriacus “MINDOUR 1” | Double | Purple-Red | Y | France |
| 7 | H. syriacus “Red Heart” | Single | White | Y | unknown |
| 8 | H. syriacus “Jwnwood4” | Semi-double | Pink | Y | UK |
| 9 | H. syriacus “Duc de Brabant” | Double | Rose-Purple | Y | unknown |
| 10 | H. syriacus var. Shigyoku | Double | Purple | Y | Japan |
| 11 | H. syriacus f. amplissimus | Double | Pink-Purple | Y | China |
| 12 | H. syriacus “Bricutts” | Semi-double | White | Y | UK |
| 13 | H. syriacus ROUFENHONGXIN | Double | Pink | Y | China |
| 14 | H. syriacus f. totus-albus | Single | White | N | China |
| 15 | H. syriacus “Notwoodone” | Semi-double | Lavender | Y | UK |
| 16 | H. syriacus f. albus-plenus | Double | White | Y | China |
| 17 | H. syriacus “Ds01bs” | Double | Blue-Purple | Y | US |
| 18 | H. syriacus QIANSIBAN | Double | Pink | Y | China |
| 19 | H. syriacus “Ardens” | Double | LilacPurple | Y | unknown |
| 20 | H. syriacus “Admiral Dewey” | Double | White | N | unknown |
| 21 | H. syriacus “Boule de Feu” | Double | RosyRed | Y | unknown |
| 22 | H. syriacus “Hamabo” | Single | BlushPink | Y | unknown |
| 23 | H. syriacus “Lady Stanley” | Double | Pink | Y | unknown |
| 24 | H. syriacus “Rwoods5” | Double | Red-Purple | Y | UK |
| 25 | H. syriacus ‘Maike’ | Semi-double | Pink-Purple | Y | unknown |
| 26 | H. syriacus “Marina” | Single | Blue | Y | Netherlands |
| 27 | H. syriacus “Mathilde” | Single | Pink | Y | Netherlands |
| 28 | H. syriacus “Mauve Queen” | Single | Red-Purple | Y | US |
| 29 | H. syriacus “Oiseau Bleu” | Single | Sky Blue | Y | France |
| 30 | H. syriacus CHIYILI | Single | Red-Purple | Y | Japan |
| 31 | H. syriacus “Sanchonyo” | Double | Red-Purple | Y | unknown |
| 32 | H. syriacus “Jeanne D’Arc” | Double | White | N | unknown |
| 33 | H. syriacus RUNRISE | Single | Red | Y | Japan |
| 34 | H. syriacus “William R. Smith” | Single/Semi-double | White | N | US |
| 35 | H. syriacus “Rwoods6” | Semi-double | White | Y | UK |
| 36 | H. syriacus “America Irene Scott” | Double | Pink | Y | US |
| 37 | H. syriacus “Stadt Erlenbach” | Semi-double/Single | Lilac | Y | unknown |
| 38 | H. syriacus “Floru” | Single | Red-Purple | Y | France |
| 39 | H. syriacus “Violet Clair Double” | Double | Purple | Y | Unknown |
| 40 | H. syriacus “Minsygrbl1” | Single | Blue | Y | France |
| 41 | H. syriacus “Speciosus” | Double | White | Y | Unknown |
| 42 | H. syriacus “Kakapo” | Double | Pink | Y | Unknown |
| 43 | H. syriacus “Lucy” | Double | Purple | N | Unknown |
| 44 | H. syriacus “Coelestis” | Single | Pink | Y | Unknown |
| 45 | H. syriacus “Minrosa” | Single | Pink | Y | Unknown |
| 46 | H. syriacus f. elegantissixnus | Double | Pink | Y | China |
| Name | Repeat | Upstream (5′–3′) | Downstream (5′–3′) |
|---|---|---|---|
| HP0001 | (TCA)5 | TGCCGGAACAAAGGACTCTC | GAATCGCAGGTGGTGGAGAA |
| HP0002 | (GAT)6 | CACGCCCTCCAGGAATCTAC | TTCTCAGGTAATGCGGCTGG |
| HP0003 | (CTT)6 | ACGGAAGCAAAATCGTTGTCt | TGCTGGAACTTCTGTCGGAC |
| HP0010 | (CAG)5 | CAACAGTTGCAGCAGTCACC | GACTGTTGCTGCACCATTGG |
| HP0011 | (CAC)5 | CACCACCAATGTCGATGGGA | ACTTGCAGATGGAGGTTGGG |
| HP0012 | (CAG)5 | ACCAGAAGAGCTTGGGATGC | AGTGATGCCATTGAGTCTTGGT |
| HP0015 | (AAT)5 | GAGGCAGCTTCAAGTTTGGC | CCGGGCCTAAGTTCCCATTT |
| HP0018 | (GAG)5 | TCGAGTGGGAGGAAGTGGAT | GAACAAAACCTCCCACCCCA |
| HP0038 | (AG)6 | AGAAGAACGCAAGGAGAGGA | TGGAGAACCAGGTCCAGACA |
| HP0055 | (AT)6 | CTTCCTTACAGCACGAGCCT | CCCCCACTAGGCCGGATATA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Yan, W.; Zhang, C. Pilot Study of Genetic Diversity and Structure in Elite Germplasm of Hibiscus syriacus. Plants 2025, 14, 2909. https://doi.org/10.3390/plants14182909
Gao Y, Yan W, Zhang C. Pilot Study of Genetic Diversity and Structure in Elite Germplasm of Hibiscus syriacus. Plants. 2025; 14(18):2909. https://doi.org/10.3390/plants14182909
Chicago/Turabian StyleGao, Yan, Wei Yan, and Chunying Zhang. 2025. "Pilot Study of Genetic Diversity and Structure in Elite Germplasm of Hibiscus syriacus" Plants 14, no. 18: 2909. https://doi.org/10.3390/plants14182909
APA StyleGao, Y., Yan, W., & Zhang, C. (2025). Pilot Study of Genetic Diversity and Structure in Elite Germplasm of Hibiscus syriacus. Plants, 14(18), 2909. https://doi.org/10.3390/plants14182909






