Phosphoproteomic Analysis Reveals Impairment of Rice Germination by Chloramphenicol
Abstract
1. Introduction
2. Results
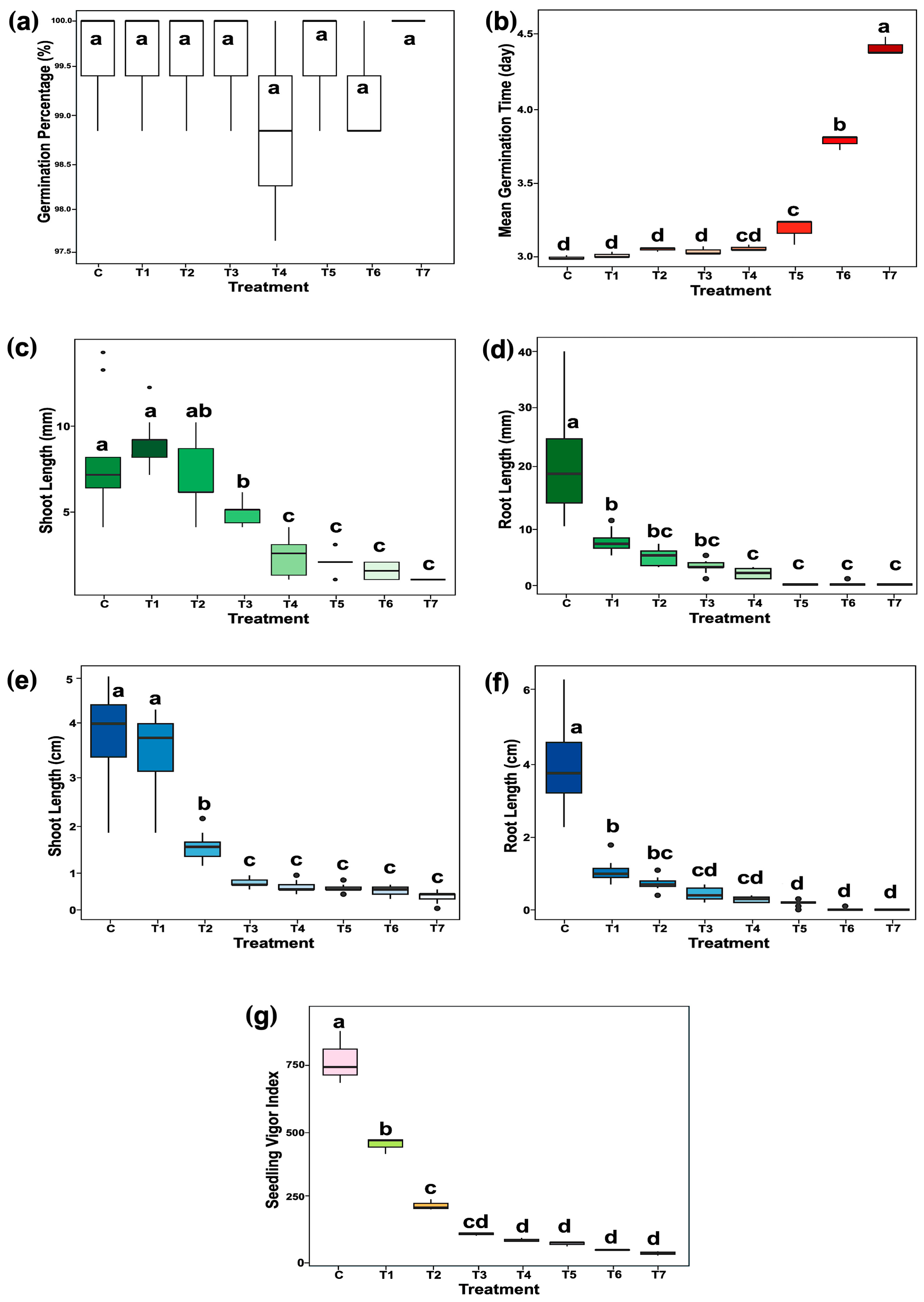
2.1. The Effect of Chloramphenicol (CAM) on Seed Germination
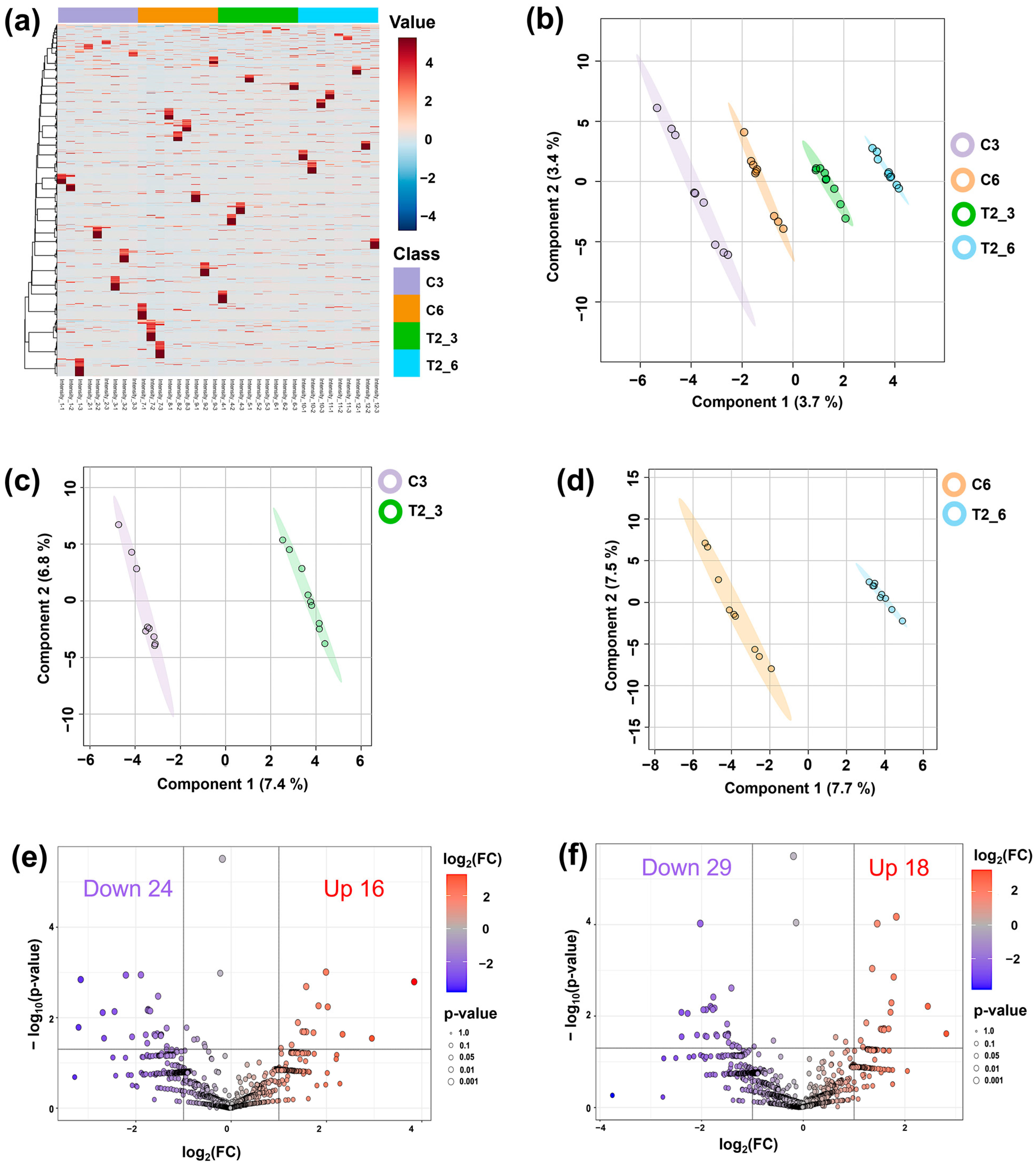
2.2. The Effect of CAM on the Phosphoprotein Profiles of Germinating Seeds
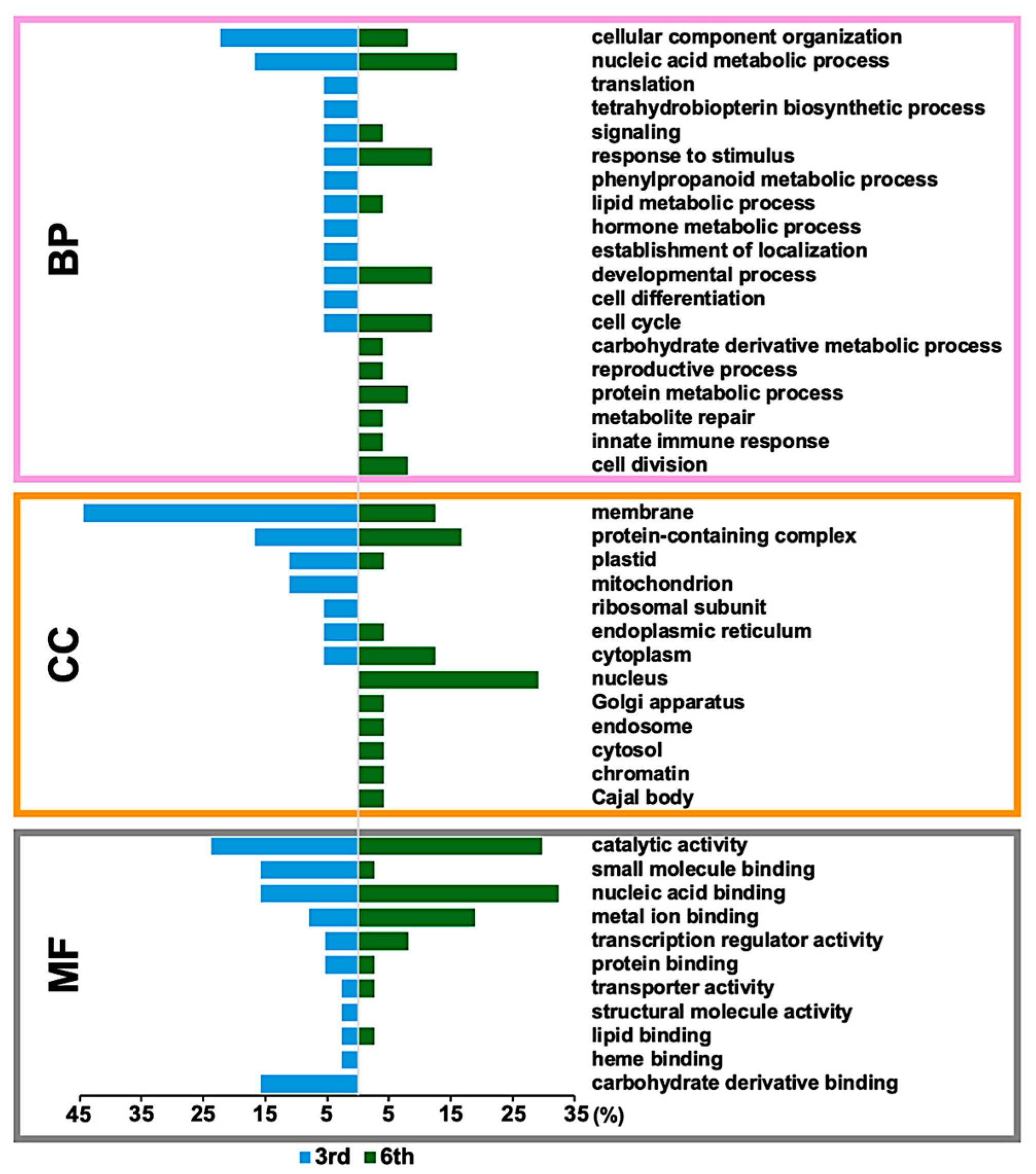
2.3. Enrichment Analysis of Identified CAM-Responsive Phosphoproteins
3. Discussion
3.1. CAM-Induced Physiological and Phosphoproteomic Changes During Germination
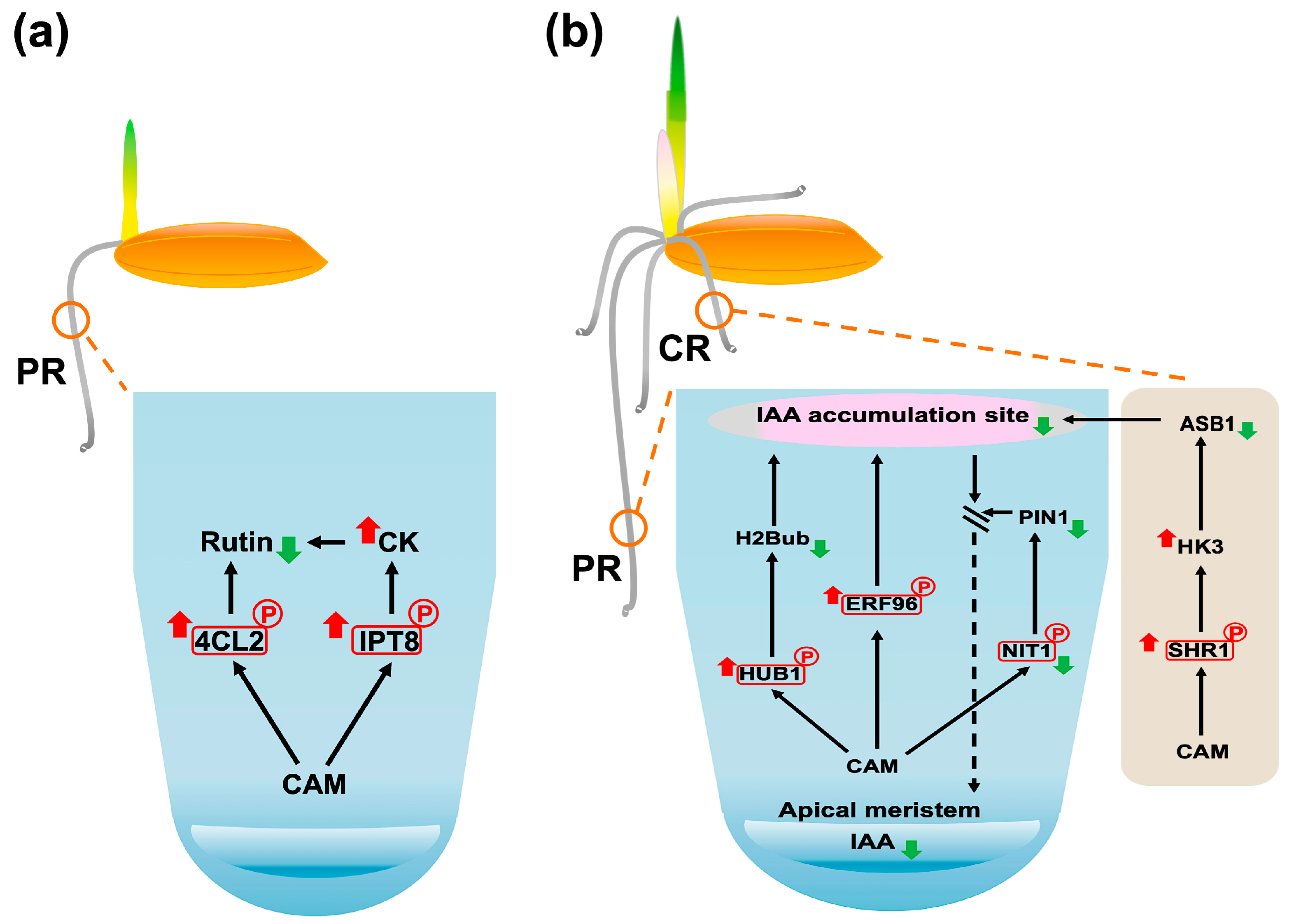
3.2. The Impact of CAM Exposure on the 3-Day Germination Stage in Rice
3.2.1. CAM Treatment Disrupts Phragmoplast-Based Cytokinesis
3.2.2. CAM Disrupts GPI Anchor Protein Maturation
3.2.3. CAM Treatment Enhances Mitochondrial CK Biosynthesis
3.2.4. CAM Exposure Disrupts Nitric Oxide Biosynthesis
3.2.5. CAM Treatment Reduces Flavonoid Metabolic Pathway
3.3. The Impact of CAM Exposure on the 6-Day Early Seedling Establishment Stage in Rice
3.3.1. CAM Enhances Nuclear Export of SHR
3.3.2. CAM Exposure Distributs Histone Modification
3.3.3. CAM Treatment Suppresses AP2/ERF-Mediated Stress Tolerance
3.3.4. CAM Exposure Influence Pre-mRNA Splicing
3.3.5. CAM Exposure Reduce ABA-Mediated Stress Response
3.3.6. CAM Exposure Impairs Castasterone Biosynthesis
3.3.7. CAM Exposure Increases Deaminated Glutathione
3.3.8. CAM Exposure Impairs DNA Replication
3.4. CAM Inhibits Root System Development During Germination
3.4.1. CAM Inhibits Primary Root Formation at Germination Stage
3.4.2. CAM Inhibits Primary Root Formation at Early Seedling Establishment Stage
3.4.3. CAM Inhibits Crown Root Formation at Early Seedling Establishment Stage
4. Materials and Methods
4.1. Plant Material Preparation
4.2. Physiological Analysis of Rice Germination
4.3. Total Protein Extraction
4.4. Label-Free Quantitative Phosphoproteomics Analysis Using LC-MS/MS
4.5. Quantification and Identification of Phosphoproteins
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominic, N.; Cenggoro, T.W.; Pardamean, B. Systematic literature review: Accelerate the rice production for global food security. AIP Conf. Proc. 2023, 2594, 80001. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/3t945q76s/tx31sh59w/np195824g/wasde0725.pdf (accessed on 24 July 2025).
- Co, H.C.; Boosarawongse, R. Forecasting Thailand’s rice export: Statistical techniques vs. artificial neural networks. Comput. Ind. Eng. 2007, 53, 610–627. [Google Scholar] [CrossRef]
- Thai Rice Exporters Association. Available online: http://www.thairiceexporters.or.th/statistic_2025.html (accessed on 24 July 2025).
- Wangcharoen, W.; Phanchaisri, C.; Daengpok, W.; Phuttawong, R.; Hangsoongnern, T.; Phanchaisri, B. Consumer acceptance test and some related properties of selected KDML 105 rice mutants. J. Food Sci. Technol. 2016, 53, 3550–3556. [Google Scholar] [CrossRef]
- He, D.; Yang, P. Proteomics of rice seed germination. Front. Plant Sci. 2013, 4, 246. [Google Scholar] [CrossRef]
- Hazra, A.; Das, S. The molecular and metabolic events behind different germination stages of rice seeds: A metabolomics perspective. JSFA Rep. 2024, 4, 118–134. [Google Scholar] [CrossRef]
- Yang, P.; Li, X.; Wang, X.; Chen, H.; Chen, F.; Shen, S. Proteomic analysis of rice (Oryza sativa) seeds during germination. Proteomics 2007, 7, 3358–3368. [Google Scholar] [CrossRef]
- Weber, M.J.; DeMoss, J.A. Inhibition of the peptide bond synthesizing cycle by chloramphenicol. J. Bacteriol. 1969, 97, 1099–1105. [Google Scholar] [CrossRef]
- Pan, M.; Wong, C.K.C.; Chu, L.M. Distribution of antibiotics in wastewater-irrigated soils and their accumulation in vegetable crops in the Pearl River Delta, Southern China. J. Agric. Food Chem. 2014, 62, 11062–11069. [Google Scholar] [CrossRef]
- Nguyen, L.M.; Nguyen, N.T.T.; Nguyen, T.T.T.; Nguyen, T.T.; Nguyen, D.T.C.; Tran, T.V. Occurrence, toxicity and adsorptive removal of the chloramphenicol antibiotic in water: A review. Environ. Chem. Lett. 2022, 20, 1929–1963. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Wu, Z.H.; Liu, J.L. Ecotoxicity of chloramphenicol and Hg acting on the root elongation of crops in North China. Int. J. Environ. Res. 2011, 5, 909–916. [Google Scholar] [CrossRef]
- Sutcliffe, J.F. New evidence for a relationship between ion absorption and protein turnover in plant cells. Nature 1960, 188, 294–297. [Google Scholar] [CrossRef]
- Önder, N. Comparative studies on water uptake and water permeability of potato tissue under the effect of indoleacetic acid, chloramphenicol and actinomycin D. Physiol. Plant. 1974, 31, 1–3. [Google Scholar] [CrossRef]
- Rehman, A.U.; Kodru, S.; Vass, I. Chloramphenicol mediates superoxide production in photosystem II and enhances its photodamage in isolated membrane particles. Front. Plant Sci. 2016, 7, 479. [Google Scholar] [CrossRef]
- Srivastava, B.I.S.; Meredith, W.O.S. Mechanism of action of gibberellic acid: Inhibition of α-amylase development during germination of barley by chloramphenicol and its reversal by gibberellic acid. Can. J. Bot. 1962, 40, 1257–1265. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Phytotoxicity of veterinary antibiotics to seed germination and root elongation of crops. Ecotoxicol. Environ. Saf. 2016, 126, 228–237. [Google Scholar] [CrossRef]
- Bellino, A.; Lofrano, G.; Carotenuto, M.; Libralato, G.; Baldantoni, D. Antibiotic effects on seed germination and root development of tomato (Solanum lycopersicum L.). Ecotoxicol. Environ. Saf. 2018, 148, 135–141. [Google Scholar] [CrossRef]
- Noodén, L.D.; Thimann, K.V. Inhibition of protein synthesis and of auxin-induced growth by chloramphenicol. Plant Physiol. 1965, 40, 193–201. [Google Scholar] [CrossRef]
- Zhang, W.J.; Zhou, Y.; Zhang, Y.; Su, Y.H.; Xu, T. Protein phosphorylation: A molecular switch in plant signaling. Cell Rep. 2023, 42, 112729. [Google Scholar] [CrossRef]
- Han, C.; Wang, K.; Yang, P. Gel-based comparative phosphoproteomic analysis on rice embryo during germination. Plant Cell Physiol. 2014, 55, 1376–1394. [Google Scholar] [CrossRef]
- Li, M.; Yin, X.; Sakata, K.; Yang, P.; Komatsu, S. Proteomic analysis of phosphoproteins in the rice nucleus during the early stage of seed germination. J. Proteome Res. 2015, 14, 2884–2896. [Google Scholar] [CrossRef]
- Frankland, B.; Smith, H. Temperature and other factors affecting chloramphenicol stimulation of the germination of light-sensitive lettuce seeds. Planta 1967, 77, 354–366. [Google Scholar] [CrossRef]
- Li, R.; Phaonakrop, N.; Lohmaneeratana, K.; Roytrakul, S.; Thamchaipenet, A. Phosphoproteomic insights into the regulation of root length in rice (Oryza sativa L. cv. KDML 105): Uncovering key events and pathways involving phosphorylated proteins. PeerJ 2025, 13, e19361. [Google Scholar] [CrossRef] [PubMed]
- Pramer, D. The movement of chloramphenicol and streptomycin in broad bean and tomato plants. Ann. Bot. 1954, 18, 463–470. [Google Scholar] [CrossRef]
- Han, C.; Yang, P.; Sakata, K.; Komatsu, S. Quantitative proteomics reveals the role of protein phosphorylation in rice embryos during early stages of germination. J. Proteome Res. 2014, 13, 1766–1782. [Google Scholar] [CrossRef]
- Krupnova, T.; Sasabe, M.; Ghebreghiorghis, L.; Gruber, C.W.; Hamada, T.; Dehmel, V.; Strompen, G.; Stierhof, Y.-D.; Lukowitz, W.; Kemmerling, B.; et al. Microtubule-associated kinase-like protein RUNKEL needed for cell plate expansion in Arabidopsis cytokinesis. Curr. Biol. 2009, 19, 518–523. [Google Scholar] [CrossRef]
- Krupnova, T.; Stierhof, Y.D.; Hiller, U.; Strompen, G.; Müller, S. The microtubule-associated kinase-like protein RUNKEL functions in somatic and syncytial cytokinesis. Plant J. 2013, 74, 781–791. [Google Scholar] [CrossRef]
- Takahashi, Y.; Soyano, T.; Kosetsu, K.; Sasabe, M.; Machida, Y. HINKEL kinesin, ANP MAPKKKs and MKK6/ANQ MAPKK, which phosphorylates and activates MPK4 MAPK, constitute a pathway that is required for cytokinesis in Arabidopsis thaliana. Plant Cell Physiol. 2010, 51, 1766–1776. [Google Scholar] [CrossRef]
- Preuss, F.; Chatterjee, D.; Mathea, S.; Shrestha, S.; St-Germain, J.; Saha, M.; Kannan, N.; Raught, B.; Rottapel, R.; Knapp, S. Nucleotide binding, evolutionary insights, and interaction partners of the pseudokinase Unc-51-like kinase 4. Structure 2020, 28, 1184–1196.e1186. [Google Scholar] [CrossRef]
- Fujita, M.; Maeda, Y.; Ra, M.; Yamaguchi, Y.; Taguchi, R.; Kinoshita, T. GPI glycan remodeling by PGAP5 regulates transport of GPI-anchored proteins from the ER to the Golgi. Cell 2009, 139, 352–365. [Google Scholar] [CrossRef]
- Komath, S.S.; Fujita, M.; Hart, G.W.; Ferguson, M.A.; Kinoshita, T. Glycosylphosphatidylinositol Anchors. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., Schnaar, R.L., Seeberger, P.H., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2022; pp. 195–210. [Google Scholar]
- Fujita, M.; Umemura, M.; Yoko-o, T.; Jigami, Y. PER1 Is required for GPI-phospholipase A2 activity and involved in lipid remodeling of GPI-anchored proteins. Mol. Biol. Cell. 2006, 17, 5253–5264. [Google Scholar] [CrossRef]
- Bernat-Silvestre, C.; Ma, Y.; Johnson, K.; Ferrando, A.; Aniento, F.; Marcote, M.J. Characterization of Arabidopsis post-glycosylphosphatidylinositol attachment to proteins phospholipase 3 like genes. Front. Plant Sci. 2022, 13, 817915. [Google Scholar] [CrossRef]
- Gillmor, C.S.; Lukowitz, W.; Brininstool, G.; Sedbrook, J.C.; Hamann, T.; Poindexter, P.; Somerville, C. Glycosylphosphatidylinositol-anchored proteins are required for cell wall synthesis and morphogenesis in Arabidopsis. Plant Cell 2005, 17, 1128–1140. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, Y.; Gao, C.; Mei, J.; Wang, S.; Ma, J.; Yang, H.; Cao, S.; Wang, Y.; Zhang, F.; et al. Glycosylphosphatidylinositol anchor lipid remodeling directs proteins to the plasma membrane and governs cell wall mechanics. Plant Cell 2022, 34, 4778–4794. [Google Scholar] [CrossRef]
- Vazquez, H.M.; Vionnet, C.; Roubaty, C.; Conzelmann, A. Cdc1 removes the ethanolamine phosphate of the first mannose of GPI anchors and thereby facilitates the integration of GPI proteins into the yeast cell wall. Mol. Biol. Cell 2014, 25, 3375–3388. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, S.K.; Singh, M.; Singh, M.P.; Chinnusamy, V. Genome wide identification and characterization of Isopentenyl transferase (IPT) gene family associated with cytokinin synthesis in rice. Plant Physiol. Rep. 2024, 29, 207–225. [Google Scholar] [CrossRef]
- Sun, J.; Niu, Q.-W.; Tarkowski, P.; Zheng, B.; Tarkowska, D.; Sandberg, G.r.; Chua, N.-H.; Zuo, J. The Arabidopsis AtIPT8/PGA22 gene encodes an isopentenyl transferase that Is involved in de novo dytokinin biosynthesis. Plant Physiol. 2003, 131, 167–176. [Google Scholar] [CrossRef]
- Sakano, Y.; Okada, Y.; Matsunaga, A.; Suwama, T.; Kaneko, T.; Ito, K.; Noguchi, H.; Abe, I. Molecular cloning, expression, and characterization of adenylate isopentenyltransferase from hop (Humulus lupulus L.). Phytochemistry 2004, 65, 2439–2446. [Google Scholar] [CrossRef]
- Takei, K.; Sakakibara, H.; Sugiyama, T. Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 26405–26410. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, W.; Chan, Z.; Wu, Y. Endogenous cytokinin overproduction modulates ROS homeostasis and decreases salt stress resistance in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 1004. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Weir, N.R.; Hill, K.; Zhang, W.; Kim, H.J.; Shiu, S.H.; Schaller, G.E.; Kieber, J.J. Characterization of genes involved in cytokinin signaling and metabolism from rice. Plant Physiol. 2012, 158, 1666–1684. [Google Scholar] [CrossRef]
- Chun, Y.; Fang, J.; Savelieva, E.M.; Lomin, S.N.; Shang, J.; Sun, Y.; Zhao, J.; Kumar, A.; Yuan, S.; Yao, X.; et al. The cytokinin receptor OHK4/OsHK4 regulates inflorescence architecture in rice via an IDEAL PLANT ARCHITECTURE1/WEALTHY FARMER’S PANICLE-mediated positive feedback circuit. Plant Cell 2023, 36, 40–64. [Google Scholar] [CrossRef]
- Eichwald, T.; da Silva, L.B.; Staats Pires, A.C.; Niero, L.; Schnorrenberger, E.; Filho, C.C.; Espíndola, G.; Huang, W.L.; Guillemin, G.J.; Abdenur, J.E.; et al. Tetrahydrobiopterin: Beyond its traditional role as a cofactor. Antioxidants 2023, 12, 1037. [Google Scholar] [CrossRef]
- Naponelli, V.; Noiriel, A.; Ziemak, M.J.; Beverley, S.M.; Lye, L.-F.; Plume, A.M.; Botella, J.R.; Loizeau, K.; Ravanel, S.p.; Rébeillé, F.; et al. Phylogenomic and runctional analysis of pterin-4a-carbinolamine dehydratase family (COG2154) proteins in plants and microorganisms. Plant Physiol. 2008, 146, 1515–1527. [Google Scholar] [CrossRef]
- Berka, V.; Yeh, H.-C.; Gao, D.; Kiran, F.; Tsai, A.-L. Redox function of tetrahydrobiopterin and effect of L-arginine on oxygen binding in endothelial nitric oxide synthase. Biochemistry 2004, 43, 13137–13148. [Google Scholar] [CrossRef]
- Kim, H.K.; Ha, S.H.; Han, J. Potential therapeutic applications of tetrahydrobiopterin: From inherited hyperphenylalaninemia to mitochondrial diseases. Ann. N. Y. Acad. Sci. 2010, 1201, 177–182. [Google Scholar] [CrossRef]
- Peres da Rocha Oliveiros Marciano, D.; Toledo Ramos, F.; Neiva Alvim, M.; Ronaldo Magalhaes, J.; Giovanni Costa França, M. Nitric oxide reduces the stress effects of aluminum on the process of germination and early root growth of rice. J. Plant Nutr. Soil Sci. 2010, 173, 885–891. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, C.; Zhong, C.; Zhang, J.; Wu, L.; Jin, Q.; Ma, Q. Nitric oxide synthase-mediated early nitric oxide burst alleviates water stress-induced oxidative damage in ammonium-supplied rice roots. BMC Plant Biol. 2019, 19, 108. [Google Scholar] [CrossRef]
- Teng, Z.; Zheng, Q.; Peng, Y.; Li, Y.; Meng, S.; Liu, B.; Peng, Y.; Duan, M.; Yuan, D.; Zhang, J.; et al. Nitrate reductase–dependent nitric oxide production mediates nitrate-conferred salt tolerance in rice seedlings. Plant Physiol. 2025, 197, kiaf080. [Google Scholar] [CrossRef]
- Li, Y.; Kim, J.I.; Pysh, L.; Chapple, C. Four isoforms of Arabidopsis 4-coumarate:CoA ligase have overlapping yet distinct roles in phenylpropanoid metabolism. Plant Physiol. 2015, 169, 2409–2421. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Feng, S.; Zou, W.; Guo, K.; Fan, C.; Si, S.; Peng, L. Analysis of five rice 4-coumarate:coenzyme A ligase enzyme activity and stress response for potential roles in lignin and flavonoid biosynthesis in rice. Biochem. Biophys. Res. Commun. 2013, 430, 1151–1156. [Google Scholar] [CrossRef]
- Li, M.; Guo, L.; Wang, Y.; Li, Y.; Jiang, X.; Liu, Y.; Xie, D.-Y.; Gao, L.; Xia, T. Molecular and biochemical characterization of two 4-coumarate: CoA ligase genes in tea plant (Camellia sinensis). Plant Mol. Biol. 2022, 109, 579–593. [Google Scholar] [CrossRef]
- Singh, A.; Gupta, R.; Pandey, R. Rice seed priming with picomolar rutin enhances rhizospheric Bacillus subtilis CIM colonization and plant growth. PLoS ONE 2016, 11, e0146013. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse physiological roles of flavonoids in plant environmental stress responses and tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef]
- Ismail, H.; Dragišic Maksimovic, J.; Maksimovic, V.; Shabala, L.; Živanovic, B.D.; Tian, Y.; Jacobsen, S.E.; Shabala, S. Rutin, a flavonoid with antioxidant activity, improves plant salinity tolerance by regulating K+ retention and Na+ exclusion from leaf mesophyll in quinoa and broad beans. Funct. Plant Biol. 2015, 43, 75–86. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konakci, C. Profiling of rutin-mediated alleviation of cadmium-induced oxidative stress in Zygophyllum fabago. Environ. Toxicol. 2015, 30, 816–835. [Google Scholar] [CrossRef]
- Quoc, C.D.; Lan, A.B.; Ngoc, T.N.; Le Hong, T.; Thanh, T.T.; Tran, G.-B.; Hoa, S.P.; Hung, T.N.; Ngoc, T.N.H.; Cong, H.N.; et al. Variations in some metabolic compounds in the roots of rice varieties (Oryza sativa L.) with different salinity tolerance under salinity stress during the seedling stage. Plant Physiol. Rep. 2024, 29, 660–677. [Google Scholar] [CrossRef]
- Tang, Y.H.; Liu, F.; Mao, K.Q.; Xing, H.C.; Chen, J.R.; Guo, Q.Q. Cloning and characterization of the key 4-coumarate CoA ligase genes in Boehmeria nivea. S. Afr. J. Bot. 2018, 116, 123–130. [Google Scholar] [CrossRef]
- Singh, A.; Gupta, R.; Pandey, R. Exogenous application of rutin and gallic acid regulate antioxidants and alleviate reactive oxygen generation in Oryza sativa L. Physiol. Mol. Biol. Plants 2017, 23, 301–309. [Google Scholar] [CrossRef]
- Lucas, M.; Swarup, R.; Paponov, I.A.; Swarup, K.; Casimiro, I.; Lake, D.; Peret, B.; Zappala, S.; Mairhofer, S.; Whitworth, M.; et al. SHORT-ROOT regulates primary, lateral, and adventitious root development in Arabidopsis. Plant Physiol. 2010, 155, 384–398. [Google Scholar] [CrossRef]
- Montiel, G.g.; Gantet, P.; Jay-Allemand, C.; Breton, C. Transcription factor networks. pathways to the knowledge of root development. Plant Physiol. 2004, 136, 3478–3485. [Google Scholar] [CrossRef]
- Gallagher, K.L.; Paquette, A.J.; Nakajima, K.; Benfey, P.N. Mechanisms regulating SHORT-ROOT intercellular movement. Curr. Biol. 2004, 14, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Levesque, M.P.; Vernoux, T.; Jung, J.W.; Paquette, A.J.; Gallagher, K.L.; Wang, J.Y.; Blilou, I.; Scheres, B.; Benfey, P.N. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science 2007, 316, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lee, C.M.; Hayashi, T.; Price, S.; Divol, F.; Henry, S.; Pauluzzi, G.; Perin, C.; Gallagher, K.L. A plausible mechanism, based upon Short-Root movement, for regulating the number of cortex cell layers in roots. Proc. Natl. Acad. Sci. USA 2014, 111, 16184–16189. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, N.; Zhang, T.; Zhang, Q.; Du, D.; Chen, X.; Lu, X.; Zhang, Y.; Zhu, M.; Liu, M.; et al. SHORT-ROOT 1 is critical to cell division and tracheary element development in rice roots. Plant J. 2021, 105, 1179–1191. [Google Scholar] [CrossRef]
- Hu, M.; Pei, B.L.; Zhang, L.F.; Li, Y.Z. Histone H2B monoubiquitination is involved in regulating the dynamics of microtubules during the defense response to Verticillium dahliae toxins in Arabidopsis. Plant Physiol. 2014, 164, 1857–1865. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Y.; Zhang, F.; Li, H.; Ma, H.; Wu, W.; Ding, Y. OsbZIP27 coordinates with OsHUB1 and OsHUB2 to modulate drought tolerance in rice. J. Genet. Genom. 2025, 52, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, E.; Xue, G.; Zhang, C.; Yang, Y.; Ding, Y. OsHUB2 inhibits function of OsTrx1 in heading date in rice. Plant J. 2022, 110, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Fleury, D.; Himanen, K.; Cnops, G.; Nelissen, H.; Boccardi, T.M.; Maere, S.; Beemster, G.T.S.; Neyt, P.; Anami, S.; Robles, P.; et al. The Arabidopsis thaliana homolog of Yeast BRE1 has a function in cell cycle regulation during early leaf and root growth. Plant Cell 2007, 19, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Li, X.; Wang, Z.; Ding, M.; Sun, Y.; Dong, F.; Chen, F.; Liu, L.; Doughty, J.; Li, Y.; et al. Histone H2B monoubiquitination mediated by HISTONE MONOUBIQUITINATION1 and HISTONE MONOUBIQUITINATION2 is involved in anther development by regulating tapetum degradation-related genes in rice. Plant Physiol. 2015, 168, 1389–1405. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Grubb, L.E.; Talasila, M.; Rodriguez Gallo, M.C.; Mehta, D.; Scandola, S.; Uhrig, R.G. B4 Raf-like MAPKKK RAF24 regulates Arabidopsis thaliana flowering time through HISTONE MONO-UBIQUITINATION 2. New Phytol. 2025, 247, 897–915. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Shen, Y.; Chen, Y.; Wang, Y.; Cai, X.; Yang, J.; Jia, B.; Dong, W.; Chen, X.; Sun, X. Osa-miR1320 targets the ERF transcription factor OsERF096 to regulate cold tolerance via JA-mediated signaling. Plant Physiol. 2022, 189, 2500–2516. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef]
- Wani, A.B.; Noor, W.; Pandit, A.; Husaini, A.M. Upregulated expression of MYB4, DREB1 and AP37 transcription factors modulates cold stress response in high-altitude Himalayan rice via time-dependent ROS regulation. Mol. Biol. Rep. 2025, 52, 417. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, L.; Li, A.; Guo, J.; Wang, H.; Qi, H.; Li, M.; Yang, P.; Song, S. An AP2/ERF transcription factor confers chilling tolerance in rice. Sci. Adv. 2024, 10, eado4788. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Moon, B.C.; Kim, J.K.; Kim, C.Y.; Kim, M.C.; Kim, I.H.; Park, C.Y.; Kim, J.C.; Park, B.O.; Koo, S.C.; et al. BWMK1, a rice mitogen-activated protein kinase, locates in the nucleus and mediates pathogenesis-related gene expression by activation of a transcription factor. Plant Physiol. 2003, 132, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ju, H.; Zhou, G.; Zhu, C.; Erb, M.; Wang, X.; Wang, P.; Lou, Y. An EAR-motif-containing ERF transcription factor affects herbivore-induced signaling, defense and resistance in rice. Plant J. 2011, 68, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, Y.; Wang, L.-X.; Fan, T.; Zhang, J.; Chen, M.-X.; Liu, Y.-G. Phylogeny and conservation of plant U2A/U2A’, a core splicing component in U2 spliceosomal complex. Planta 2021, 255, 25. [Google Scholar] [CrossRef]
- Caspary, F.; Séraphin, B. The yeast U2A′/U2B″ complex is required for pre-spliceosome formation. EMBO J. 1998, 17, 6348–6358. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, X.; Li, C.; Ma, X.; Zhao, X.; Zhao, X.; Zhang, P.; Zhu, X. A U2 snRNP-specific protein, U2A′, is involved in stress response and drug resistance in Cryptococcus deneoformans. Biochimie 2024, 220, 179–187. [Google Scholar] [CrossRef]
- Bae, M.S.; Cho, E.J.; Choi, E.-Y.; Park, O.K. Analysis of the Arabidopsis nuclear proteome and its response to cold stress. Plant J. 2003, 36, 652–663. [Google Scholar] [CrossRef]
- Chen, L.; Weinmeister, R.; Kralovicova, J.; Eperon, L.P.; Vorechovsky, I.; Hudson, A.J.; Eperon, I.C. Stoichiometries of U2AF35, U2AF65 and U2 snRNP reveal new early spliceosome assembly pathways. Nucleic Acids Res. 2016, 45, 2051–2067. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, Y.; Yao, J.; Huang, H.; Qian, B.; Liu, X.; Chen, Y.; Pang, J.; Zhan, X.; Zhu, J.-K.; et al. Modulation of plant development and chilling stress responses by alternative splicing events under control of the spliceosome protein SmEb in Arabidopsis. Plant Cell Environ. 2022, 45, 2762–2779. [Google Scholar] [CrossRef]
- Tang, N.; Ma, S.; Zong, W.; Yang, N.; Lv, Y.; Yan, C.; Guo, Z.; Li, J.; Li, X.; Xiang, Y.; et al. MODD mediates deactivation and degradation of OsbZIP46 to negatively regulate ABA signaling and drought resistance in rice. Plant Cell 2016, 28, 2161–2177. [Google Scholar] [CrossRef]
- Perez, J.M.; Chen, Y.; Xiao, T.S.; Abbott, D.W. Phosphorylation of the E3 ubiquitin protein ligase ITCH diminishes binding to its cognate E2 ubiquitin ligase. J. Biol. Chem. 2018, 293, 1100–1105. [Google Scholar] [CrossRef]
- Kanerva, K.; Uronen, R.-L.; Blom, T.; Li, S.; Bittman, R.; Lappalainen, P.; Peränen, J.; Raposo, G.; Ikonen, E. LDL cholesterol recycles to the plasma membrane via a Rab8a-Myosin5b-Actin-Dependent membrane transport route. Dev. Cell 2013, 27, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Sobajima, T.; Yoshimura, S.I.; Maeda, T.; Miyata, H.; Miyoshi, E.; Harada, A. The Rab11-binding protein RELCH/KIAA1468 controls intracellular cholesterol distribution. J. Cell Biol. 2018, 217, 1777–1796. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Gibbs, H.C.; Yeh, A.T.; Griffing, L.R. The sterol trafficking pathway in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 616631. [Google Scholar] [CrossRef]
- Joo, S.-H.; Kim, T.-W.; Son, S.-H.; Lee, W.S.; Yokota, T.; Kim, S.-K. Biosynthesis of a cholesterol-derived brassinosteroid, 28-norcastasterone, in Arabidopsis thaliana. J. Exp. Bot. 2011, 63, 1823–1833. [Google Scholar] [CrossRef]
- Kim, B.K.; Fujioka, S.; Takatsuto, S.; Tsujimoto, M.; Choe, S. Castasterone is a likely end product of brassinosteroid biosynthetic pathway in rice. Biochem. Biophys. Res. Commun. 2008, 374, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Saka, H. The promotive effect of brassinolide on lamina joint-cell elongation, germination and seedling growth under low-temperature stress in rice (Oryza sativa L.). Plant Prod. Sci. 2001, 4, 210–214. [Google Scholar] [CrossRef]
- Jantapo, K.; Wimonchaijit, W.; Wang, W.; Chaiwanon, J. Supraoptimal brassinosteroid levels inhibit root growth by reducing root meristem and cell elongation in rice. Plants 2021, 10, 1962. [Google Scholar] [CrossRef]
- Peracchi, A.; Veiga-da-Cunha, M.; Kuhara, T.; Ellens, K.W.; Paczia, N.; Stroobant, V.; Seliga, A.K.; Marlaire, S.; Jaisson, S.; Bommer, G.T.; et al. Nit1 is a metabolite repair enzyme that hydrolyzes deaminated glutathione. Proc. Natl. Acad. Sci. USA 2017, 114, e3233–e3242. [Google Scholar] [CrossRef]
- Niehaus, T.D.; Patterson, J.A.; Alexander, D.C.; Folz, J.S.; Pyc, M.; MacTavish, B.S.; Bruner, S.D.; Mullen, R.T.; Fiehn, O.; Hanson, A.D. The metabolite repair enzyme Nit1 is a dual-targeted amidase that disposes of damaged glutathione in Arabidopsis. Biochem. J. 2019, 476, 683–697. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, M.; Teng, Y.; Jia, S.; Yu, D.; Wei, T.; Chen, C.; Song, W. Overexpression of the glutathione peroxidase 5 (RcGPX5) gene from Rhodiola crenulata increases drought tolerance in Salvia miltiorrhiza. Front. Plant Sci. 2018, 9, 1950. [Google Scholar] [CrossRef]
- Song, M.; Fan, X.; Chen, J.; Qu, H.; Luo, L.; Xu, G. OsNAR2.1 interaction with OsNIT1 and OsNIT2 functions in root-growth responses to nitrate and ammonium. Plant Physiol. 2020, 183, 289–303. [Google Scholar] [CrossRef]
- Forsburg, S.L. Eukaryotic MCM proteins: Beyond replication initiation. Microbiol. Mol. Biol. Rev. 2004, 68, 109–131. [Google Scholar] [CrossRef]
- van Deursen, F.; Sengupta, S.; De Piccoli, G.; Sanchez-Diaz, A.; Labib, K. Mcm10 associates with the loaded DNA helicase at replication origins and defines a novel step in its activation. EMBO J. 2012, 31, 2195–2206. [Google Scholar] [CrossRef]
- Masai, H.; Taniyama, C.; Ogino, K.; Matsui, E.; Kakusho, N.; Matsumoto, S.; Kim, J.M.; Ishii, A.; Tanaka, T.; Kobayashi, T.; et al. Phosphorylation of MCM4 by Cdc7 kinase facilitates its interaction with Cdc45 on the chromatin. J. Biol. Chem. 2006, 281, 39249–39261. [Google Scholar] [CrossRef] [PubMed]
- Herridge, R.P.; Day, R.C.; Macknight, R.C. The role of the MCM2-7 helicase complex during Arabidopsis seed development. Plant Mol. Biol. 2014, 86, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, D.-E.; Lee, H.-S.; Kim, S.-K.; Lee, W.S.; Kim, S.-H.; Kim, M.-W. Influence of auxins, cytokinins, and nitrogen on production of rutin from callus and adventitious roots of the white mulberry tree (Morusalba L.). Plant Cell Tiss. Organ Cult. 2011, 105, 9–19. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, P.; Bai, J.; Wu, L.; Di, D.-W.; Liu, H.-Q.; Li, J.-J.; Liu, Y.-L.; Khaskheli, A.J.; Zhao, C.-M.; et al. Function of histone H2B monoubiquitination in transcriptional regulation of auxin biosynthesis in Arabidopsis. Commun. Biol. 2021, 4, 206. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Chen, Y.; Wang, Y.; Shen, Y.; Yang, J.; Jia, B.; Sun, X.; Sun, M. A comprehensive investigation of the regulatory roles of OsERF096, an AP2/ERF transcription factor, in rice cold stress response. Plant Cell Rep. 2023, 42, 2011–2022. [Google Scholar] [CrossRef]
- Rebouillat, J.; Dievart, A.; Verdeil, J.L.; Escoute, J.; Giese, G.; Breitler, J.C.; Gantet, P.; Espeout, S.; Guiderdoni, E.; Périn, C. Molecular genetics of rice root development. Rice 2009, 2, 15–34. [Google Scholar] [CrossRef]
- Mao, C.; He, J.; Liu, L.; Deng, Q.; Yao, X.; Liu, C.; Qiao, Y.; Li, P.; Ming, F. OsNAC2 integrates auxin and cytokinin pathways to modulate rice root development. Plant Biotechnol. J. 2020, 18, 429–442. [Google Scholar] [CrossRef]
- Gao, S.; Fang, J.; Xu, F.; Wang, W.; Sun, X.; Chu, J.; Cai, B.; Feng, Y.; Chu, C. CYTOKININ OXIDASE/DEHYDROGENASE4 integrates cytokinin and auxin signaling to control rice crown root formation. Plant Physiol. 2014, 165, 1035–1046. [Google Scholar] [CrossRef]
- Salvi, E.; Di Mambro, R.; Pacifici, E.; Dello Ioio, R.; Costantino, P.; Moubayidin, L.; Sabatini, S. SCARECROW and SHORTROOT control the auxin/cytokinin balance necessary for embryonic stem cell niche specification. Plant Signal Behav. 2018, 13, e1507402. [Google Scholar]
- Md Zali, A.Z.; Ja’afar, Y.; Paramisparan, K.; Ismail, S.I.; Saad, N.; Mohd Hata, E.; Md Hatta, M.A.; Ismail, M.R.; Yusof, M.T.; Zulperi, D. First report of Burkholderia gladioli causing bacterial panicle blight of rice in Malaysia. Plant Dis. 2023, 107, 551. [Google Scholar] [CrossRef]
- Debta, H.; Kunhamu, T.K.; Petrík, P.; Fleischer, P.; Jisha, K.C. Effect of hydropriming and osmopriming on the germination and seedling vigor of the East Indian Sandalwood (Santalum album L.). Forests 2023, 14, 1076. [Google Scholar] [CrossRef]
- Ros, C.; Bell, R.W.; White, P.F. Seedling vigour and the early growth of transplanted rice (Oryza sativa). Plant Soil 2003, 252, 325–337. [Google Scholar] [CrossRef]
- Chen, Y.; Gin, J.W.; Wang, Y.; de Raad, M.; Tan, S.; Hillson, N.J.; Northen, T.R.; Adams, P.D.; Petzold, C.J. Alkaline-SDS cell lysis of microbes with acetone protein precipitation for proteomic sample preparation in 96-well plate format. PLoS ONE 2023, 18, e0288102. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhang, H.; Wu, Z.; Wang, Y.; Liu, H.; Wu, X.; Wang, W. Modified TCA/acetone precipitation of plant proteins for proteomic analysis. PLoS ONE 2018, 13, e0202238. [Google Scholar] [CrossRef]
- Waterborg, J.H.; Matthews, H.R. Basic Protein and Peptide Protocols; Humana Press: Berlin, Germany, 1994; pp. 1–4. [Google Scholar]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Blom, N.; Sicheritz-Pontén, T.; Gupta, R.; Gammeltoft, S.; Brunak, S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. PROTEOMICS 2004, 4, 1633–1649. [Google Scholar] [CrossRef]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Adler, P.; Vilo, J.; Peterson, H. g:Profiler—Interoperable web service for functional enrichment analysis and gene identifier mapping (2023 update). Nucleic Acids Res. 2023, 51, W207–W212. [Google Scholar] [CrossRef]
- Ødum, M.T.; Teufel, F.; Thumuluri, V.; Almagro Armenteros, J.J.; Johansen, A.R.; Winther, O.; Nielsen, H. DeepLoc 2.1: Multi-label membrane protein type prediction using protein language models. Nucleic Acids Res. 2024, 52, W215–W220. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Santos, A.; von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016, 44, D380–D384. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Villanueva, R.A.M.; Chen, Z.J. ggplot2: Elegant graphics for data analysis. Meas. Interdiscip. Res. Perspect. 2019, 17, 160–167. [Google Scholar] [CrossRef]

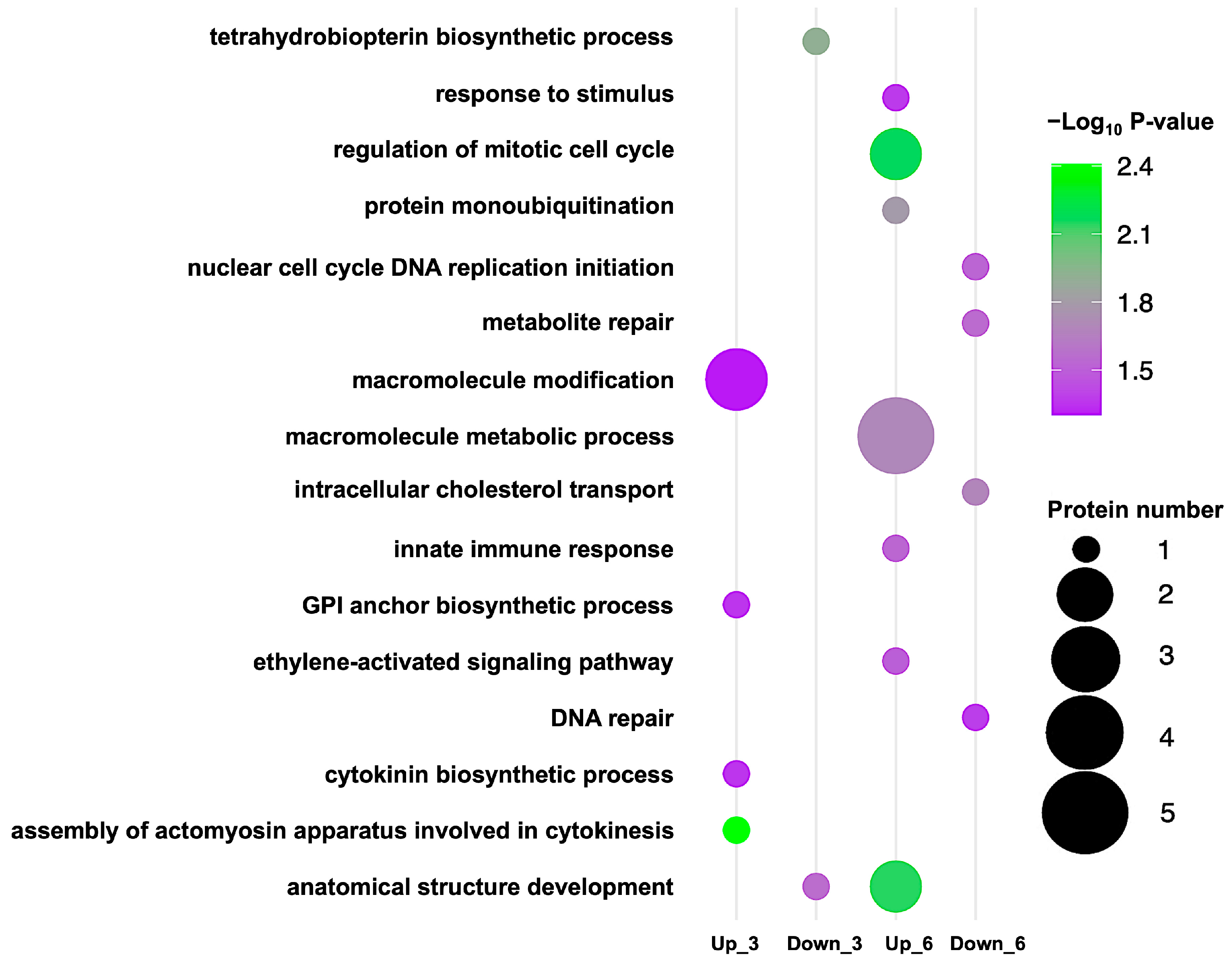
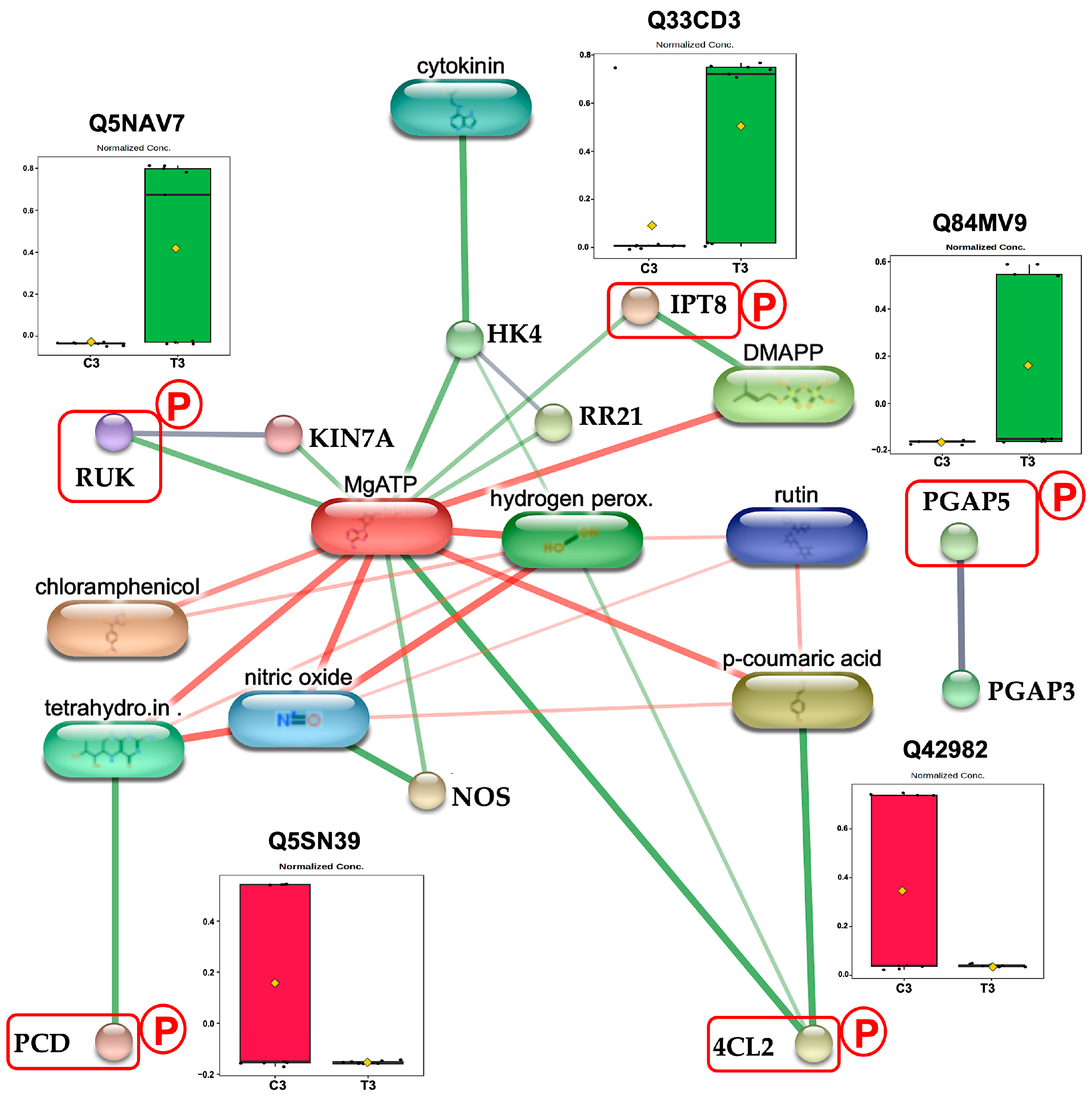

| Term Name | Term ID | −log10 (p-Value) | Count | Uniprot ID | Subcellular Localization |
|---|---|---|---|---|---|
| Upregulation at 3-day stage | |||||
| Assembly of actomyosin apparatus involved in cytokinesis | GO:0000912 | 2.408 | 1 | Q5NAV7 | Cytoplasm, Nucleus |
| GPI anchor biosynthetic process | GO:0006506 | 1.355 | 1 | Q84MV9 | Endoplasmic reticulum |
| Cytokinin biosynthetic process | GO:0009691 | 1.355 | 1 | Q33CD3 | Mitochondrion |
| Macromolecule modification | GO:0043412 | 1.302 | 3 | Q5NAV7 | Nucleus |
| Q84MV9 | Endoplasmic reticulum | ||||
| Q33CD3 | Mitochondrion | ||||
| Downregulation at 3-day stage | |||||
| Tetrahydrobiopterin biosynthetic process | GO:0006729 | 1.904 | 1 | Q5SN39 | Mitochondrion |
| Anatomical structure development | GO:0032989 | 1.558 | 1 | Q42982 | Cytoplasm |
| Upregulation at 6-day stage | |||||
| Regulation of mitotic cell cycle | GO:0007346 | 2.161 | 2 | Q8H2X8 | Nucleus |
| Q7XU27 | Nucleus | ||||
| Anatomical structure development | GO:0048366 | 2.148 | 2 | Q8H2X8 | Nucleus |
| Q7XU27 | Nucleus | ||||
| Protein monoubiquitination | GO:0006513 | 1.792 | 1 | Q7XU27 | Nucleus |
| Macromolecule metabolic process | GO:0043170 | 1.696 | 5 | Q8H2X8 | Nucleus |
| Q7XU27 | Nucleus | ||||
| Q651A5 | Nucleus | ||||
| Q6EUK2 | Nucleus | ||||
| A0A0P0WTE8 | Cytoplasm | ||||
| Innate immune response | GO:0045087 | 1.532 | 1 | Q7XU27 | Nucleus |
| Ethylene-activated signaling pathway | GO:0009873 | 1.497 | 1 | Q651A5 | Nucleus |
| Response to stimulus | GO:0050896 | 1.337 | 1 | Q7XU27 | Nucleus |
| Downregulation at 6-day stage | |||||
| Intracellular cholesterol transport | GO:0032367 | 1.679 | 1 | Q5QMW8 | Cytoplasm |
| Metabolite repair | GO:0110051 | 1.554 | 1 | Q2QQ94 | Cytoplasm |
| Nuclear cell cycle DNA replication initiation | GO:1902315 | 1.525 | 1 | Q5JKB0 | Nucleus |
| DNA repair | GO:0006281 | 1.379 | 1 | Q5JKB0 | Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Phaonakrop, N.; Roytrakul, S.; Lohmaneeratana, K.; Thamchaipenet, A. Phosphoproteomic Analysis Reveals Impairment of Rice Germination by Chloramphenicol. Plants 2025, 14, 2845. https://doi.org/10.3390/plants14182845
Li R, Phaonakrop N, Roytrakul S, Lohmaneeratana K, Thamchaipenet A. Phosphoproteomic Analysis Reveals Impairment of Rice Germination by Chloramphenicol. Plants. 2025; 14(18):2845. https://doi.org/10.3390/plants14182845
Chicago/Turabian StyleLi, Rui, Narumon Phaonakrop, Sittiruk Roytrakul, Karan Lohmaneeratana, and Arinthip Thamchaipenet. 2025. "Phosphoproteomic Analysis Reveals Impairment of Rice Germination by Chloramphenicol" Plants 14, no. 18: 2845. https://doi.org/10.3390/plants14182845
APA StyleLi, R., Phaonakrop, N., Roytrakul, S., Lohmaneeratana, K., & Thamchaipenet, A. (2025). Phosphoproteomic Analysis Reveals Impairment of Rice Germination by Chloramphenicol. Plants, 14(18), 2845. https://doi.org/10.3390/plants14182845







