Cadmium Contamination in Asian Rice (Oryza sativa L.): Mechanistic Insights from Soil Sources to Grain Accumulation and Mitigation Strategies
Abstract
1. Introduction
- An overview of the primary sources of Cd contamination in agricultural ecosystems;
- A summary of the effects of Cd contamination on rice, including its migration, uptake, and accumulation within the soil–rice system;
- A synopsis of the prevailing remediation technologies and management strategies for mitigating Cd contamination.
2. Cd Contamination in Soil and Its Sources
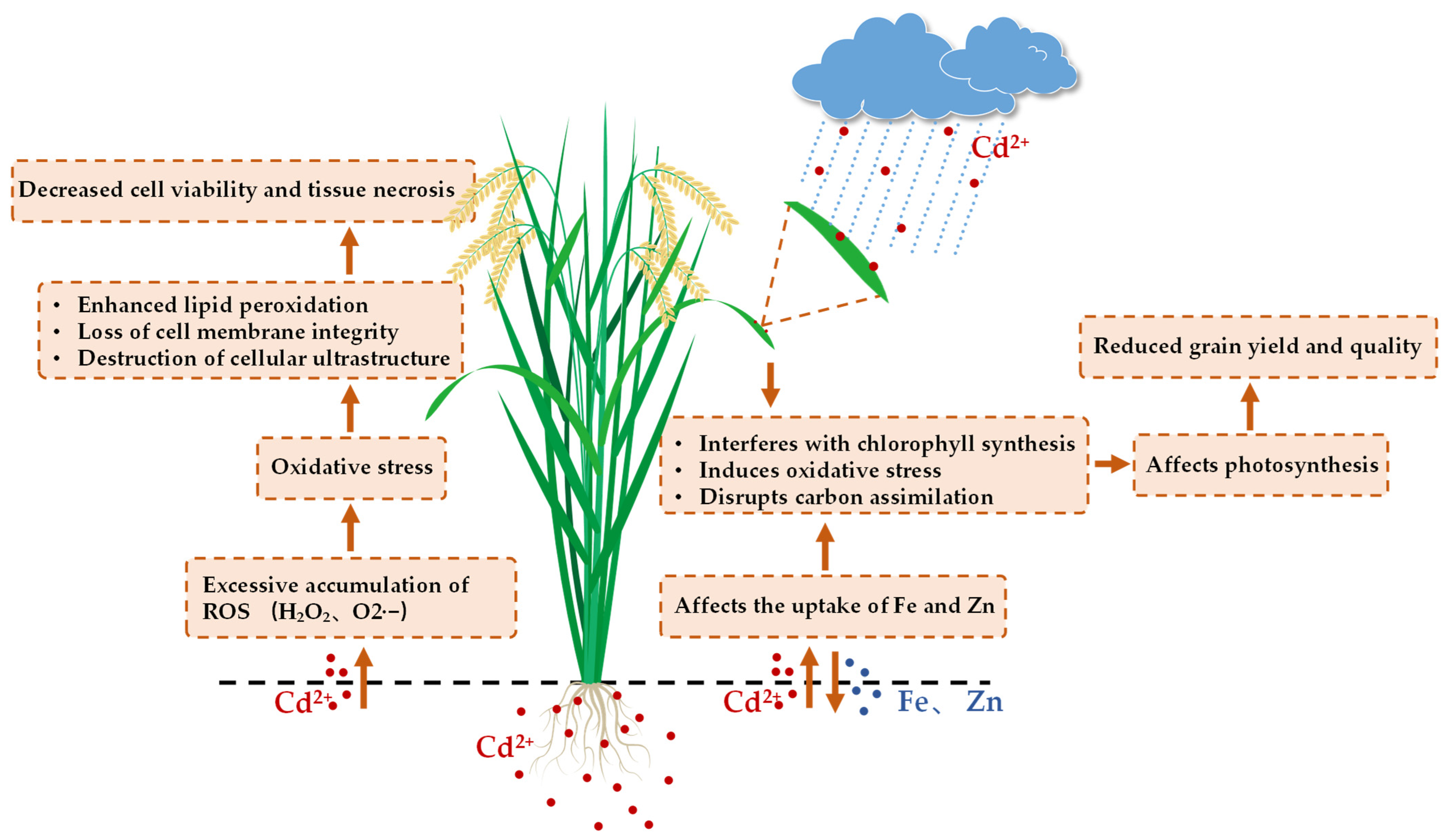
3. Toxic Effects of Cd in Rice
4. Cd Absorption and Transportation Pathways in Rice
4.1. Root Uptake
4.2. Foliar Uptake
4.3. Xylem-Mediated Transport (Translocation from Roots to Shoots)
4.3.1. Cell Wall Fixation and Vacuolar Compartmentalization
4.3.2. Xylem-Mediated Transport
4.4. Phloem Transport (Grain Translocation)
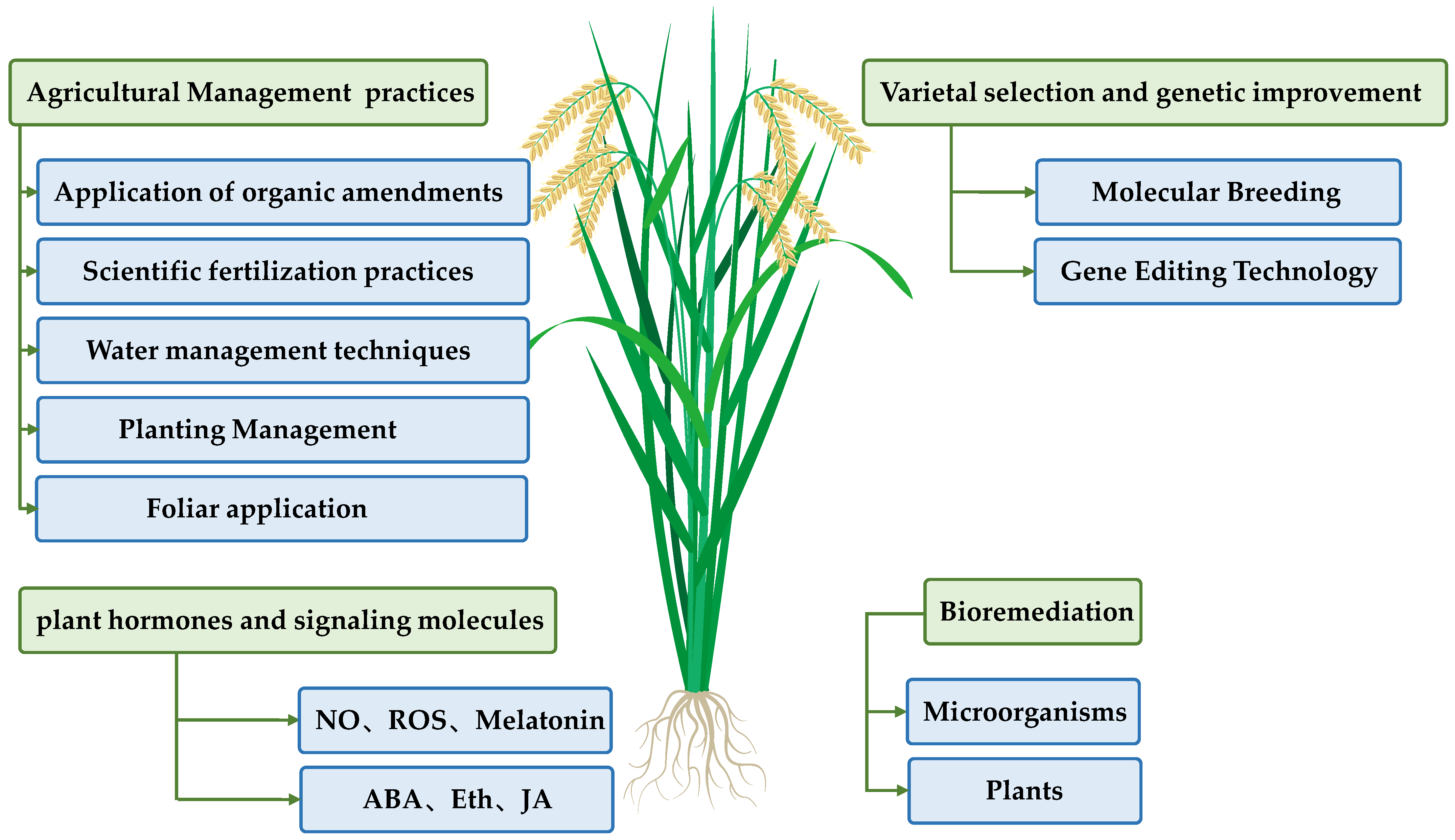
5. Integrated Strategies for Cd Pollution Control in Rice
5.1. Agricultural Management Practices
- (1)
- Application of organic amendments, including biochar [134], compost [56], and crop residues [133,149], and inorganic amendments such as lime [135], zeolite [136], and bentonite [137]. These amendments minimize the bioavailability of Cd in soil through mechanisms such as adsorption, complexation, and transformation, effectively decreasing Cd uptake and accumulation in rice and other crops. According to Liu et al. [138], liming decreased bioavailable Cd content in soil and Cd concentration in aboveground plant tissues by 19.2–29.4% and 29.3–36.3%, respectively. However, the long-term effects of these ameliorants and the changes in soil microbial communities still require further evaluation. Additionally, the application rate and effectiveness of ameliorants are influenced by various factors such as soil type and environmental conditions, and therefore need to be adjusted and optimized based on specific circumstances.
- (2)
- Targeted fertilization practices also contribute remarkably to mitigate Cd pollution. Rational application of fertilizers such as urea [139], phosphate [140], sulfur [141], selenium [142], and silicon [143] improves soil conditions, enhances crop stress resistance, and reduces Cd accumulation. Zhou et al. [150] observed that applying urea in the ratio of 30% at tillering, 40% at panicle initiation, and 30% at heading lowered Cd content in brown rice by 40.7% compared to that achieved by conventional fertilization.
- (3)
- Water management techniques, including intermittent irrigation, continuous flooding, and alternate wetting and drying, modulate soil redox status, promote the formation of iron plaques, and inhibit Cd activation and mobility, thereby reducing plant Cd uptake [32,144,151,152]. However, these water management practices may be limited by water availability and cost in arid or water-scarce regions. Furthermore, the adaptability of water management varies across different regions and needs to be flexibly adjusted based on local water resource conditions.
- (4)
- Crop rotation and fallowing effectively reduce the bioavailability of Cd in soil. As reported previously, following 2–3 years of rotation, Cd levels in brown rice decreased to below safety thresholds, with reduction levels ranging from 37% to 73% [145,146]. Although crop rotation can effectively reduce the bioavailability of cadmium in the soil, its implementation is still limited by factors such as arable land area, crop variety selection, and climate conditions. In some regions, the lack of suitable alternative crops or the long growing cycle of rice may limit the effectiveness of crop rotation.
- (5)
- Foliar application of metal chelates (e.g., iron chelates), nonmetallic compounds, or organic foliar agents is a novel approach to alleviate Cd-induced toxicity. These treatments enhance chlorophyll content, improve photosynthesis, enrich antioxidant defense systems, and decrease cell membrane permeability, ultimately increasing plant tolerance to heavy metals [147,148,153,154]. For instance, Wang et al. [147] demonstrated that spraying 50 mg/L iron chelate reduced the content of Cd in brown rice by 29% and increased the enzymatic activities of POD and SOD by 54.4% and 51.6%, respectively, facilitating ROS scavenging and decreasing oxidative stress. However, the application of foliar ameliorants often requires multiple sprays, which increases production costs. Moreover, the effectiveness of ameliorants varies significantly across different rice varieties and other crops, and frequent use may also lead to secondary environmental pollution.
5.2. Varietal Selection and Genetic Improvement
5.3. Regulation of Cd Tolerance Through Plant Hormones and Signaling Molecules
5.4. Bioremediation
6. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cd | Cadmium |
| PSII | Photosystem II |
| Rubisco | Ribulose-1,5-bisphosphate carboxylase/oxygenase |
| ROS | Reactive oxygen species |
| O2− | Superoxide anions |
| H2O2 | Hydrogen peroxide |
| POD | Peroxidase |
| SOD | Superoxide dismutase |
| KO | Knockout |
| NO | Nitric oxide |
References
- Shi, T.; Zhang, Y.; Gong, Y.; Ma, J.; Wei, H.; Wu, X.; Zhao, L.; Hou, H. Status of cadmium accumulation in agricultural soils across China (1975–2016): From temporal and spatial variations to risk assessment. Chemosphere 2019, 230, 136–143. [Google Scholar] [CrossRef]
- Yuan, Z.; Luo, T.; Liu, X.; Hua, H.; Zhuang, Y.; Zhang, X.; Zhang, L.; Zhang, Y.; Xu, W.; Ren, J. Tracing anthropogenic cadmium emissions: From sources to pollution. Sci. Total Environ. 2019, 676, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, M.Z.; Usman, M.; He, Z.L.; Yang, X. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci. Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef]
- Aoshima, K. Itai-itai disease: Renal tubular osteomalacia induced by environmental exposure to cadmium—Historical review and perspectives. Soil Sci. Plant Nutr. 2016, 62, 319–326. [Google Scholar] [CrossRef]
- Thakur, N.; Mehta, P.; Kaur, N.; Deshwal, A. A Review on the Effects of Cadmium Toxicity on Living Beings. J. Pharm. Res. Int. 2021, 33, 300–305. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Hao, H.; Cui, J.; Huang, L.; Liang, Q. The association between cadmium exposure and the risk of chronic obstructive pulmonary disease: A systematic review and meta-analysis. J. Hazard. Mater. 2024, 469, 133828. [Google Scholar] [CrossRef]
- Baba, H.; Tsuneyama, K.; Yazaki, M.; Nagata, K.; Minamisaka, T.; Tsuda, T.; Nomoto, K.; Hayashi, S.; Miwa, S.; Nakajima, T.; et al. The liver in itai-itai disease (chronic cadmium poisoning): Pathological features and metallothionein expression. Mod. Pathol. 2013, 26, 1228–1234. [Google Scholar] [CrossRef]
- Satarug, S.; Moore, M.R. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ. Health Perspect. 2004, 112, 1099–1103. [Google Scholar] [CrossRef]
- Zeng, D.; Tian, Z.; Rao, Y.; Dong, G.; Yang, Y.; Huang, L.; Leng, Y.; Xu, J.; Sun, C.; Zhang, G.; et al. Rational design of high-yield and superior-quality rice. Nat. Plants 2017, 3, 17031. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. China Statistical Yearbook 2024; China Statistics Press: Beijing, China, 2024. [Google Scholar]
- Zhang, J.; Wang, Z.; Du, W.; Huang, F.; Zhang, B.; Wang, H. Differential Association of Wheat and Rice Consumption With Overweight/Obesity in Chinese Adults: China Health and Nutrition Survey 1991–2015. Front. Nutr. 2022, 9, 808301. [Google Scholar] [CrossRef]
- Chen, H.; Tang, Z.; Wang, P.; Zhao, F.J. Geographical variations of cadmium and arsenic concentrations and arsenic speciation in Chinese rice. Environ. Pollut. 2018, 238, 482–490. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Draft and Proposed Draft Maximum Levels for Cadmium: Comments at Step 6 and at Step 3; Codex Alimentarius Commission: The Hague, The Netherlands, 2005. [Google Scholar]
- Tang, H.; Li, T.; Yu, H.; Zhang, X. Cadmium accumulation characteristics and removal potentials of high cadmium accumulating rice line grown in cadmium-contaminated soils. Environ. Sci. Pollut. Res. Int. 2016, 23, 15351–15357. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yang, L.; Chen, Y.; Jing, H.; Wu, P.; Yang, W. Selection of rice and maize varieties with low cadmium accumulation and derivation of soil environmental thresholds in karst. Ecotoxicol. Environ. Saf. 2022, 247, 114244. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jiang, Z.; Li, X.; Liu, H.; Li, N.; Wei, S. Mitigation of rice cadmium (Cd) accumulation by joint application of organic amendments and selenium (Se) in high-Cd-contaminated soils. Chemosphere 2020, 241, 125106. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Ashraf, M.N.; Shafeeq Ur, R.; Abbas, A.; Li, J.; Farooq, M. Cadmium stress in paddy fields: Effects of soil conditions and remediation strategies. Sci. Total Environ. 2021, 754, 142188. [Google Scholar] [CrossRef]
- Liu, X.; Tian, G.; Jiang, D.; Zhang, C.; Kong, L. Cadmium (Cd) distribution and contamination in Chinese paddy soils on national scale. Environ. Sci. Pollut. Res. Int. 2016, 23, 17941–17952. [Google Scholar] [CrossRef]
- Shi, J.; Du, P.; Luo, H.; Wu, H.; Zhang, Y.; Chen, J.; Wu, M.; Xu, G.; Gao, H. Soil contamination with cadmium and potential risk around various mines in China during 2000–2020. J. Environ. Manag. 2022, 310, 114509. [Google Scholar] [CrossRef]
- Fan, H.; Tang, S.; Long, J.; He, R.; Xiao, Z.; Hou, H.; Peng, P. Effects of exogenous chloride ions on the migration and transformation of Cd in a soil-rice system. Front. Environ. Sci. 2024, 12, 1403989. [Google Scholar] [CrossRef]
- Rahimi, M.; Bertalan-Balázs, B.; Adelinia, A.; Ebrahimi, E.; Ojani, M. Impact assessment of Zeolite, Ca-bentonite and Biochar amendments on Cd bioavailability and fractions in polluted calcareous soils. Environ. Earth Sci. 2024, 83, 506. [Google Scholar] [CrossRef]
- Xu, D.; Shen, Z.; Dou, C.; Dou, Z.; Li, Y.; Gao, Y.; Sun, Q. Effects of soil properties on heavy metal bioavailability and accumulation in crop grains under different farmland use patterns. Sci. Rep. 2022, 12, 9211. [Google Scholar] [CrossRef]
- Zhong, S.; Li, X.; Li, F.; Huang, Y.; Liu, T.; Yin, H.; Qiao, J.; Chen, G.; Huang, F. Cadmium uptake and transport processes in rice revealed by stable isotope fractionation and Cd-related gene expression. Sci. Total Environ. 2022, 806, 150633. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Zhou, J.; Cui, H.; Liang, J.; Liu, Q.; Zhou, J. Nodes play a major role in cadmium (Cd) storage and redistribution in low-Cd-accumulating rice (Oryza sativa L.) cultivars. Sci. Total Environ. 2023, 859, 160436. [Google Scholar] [CrossRef]
- Luo, J.S.; Huang, J.; Zeng, D.L.; Peng, J.S.; Zhang, G.B.; Ma, H.L.; Guan, Y.; Yi, H.Y.; Fu, Y.L.; Han, B.; et al. A defensin-like protein drives cadmium efflux and allocation in rice. Nat. Commun. 2018, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, D.; Xue, R.; Long, J.; Lin, X.; Lin, Y.; Jia, L.; Zeng, R.; Song, Y. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J. Hazard. Mater. 2019, 367, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, M.; Hendrix, S.; Kyndt, T.; Demeestere, K.; Vandamme, D.; Cuypers, A. Short-term effects of cadmium on leaf growth and nutrient transport in rice plants. Plant Sci. 2021, 313, 111054. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-Ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. Int. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Wang, T.; Fu, Y.; Song, S.; Li, Y.; Yang, H.; Bai, L.; Li, L. Xizi 3: A new rice variety with stable low-cadmium-accumulation characteristics. Mol. Breed. 2025, 45, 21. [Google Scholar] [CrossRef]
- Sun, L.; Wang, R.; Tang, W.; Chen, Y.; Zhou, J.; Ma, H.; Li, S.; Deng, H.; Han, L.; Chen, Y.; et al. Robust identification of low-Cd rice varieties by boosting the genotypic effect of grain Cd accumulation in combination with marker-assisted selection. J. Hazard. Mater. 2022, 424, 127703. [Google Scholar] [CrossRef]
- Li, Y.; Tao, H.; Cao, H.; Wan, X.; Liao, X. Achieving synergistic benefits through integrated governance of cultivated cadmium contamination via multistakeholder collaboration. Nat. Commun. 2024, 15, 9817. [Google Scholar] [CrossRef]
- Honma, T.; Ohba, H.; Kaneko-Kadokura, A.; Makino, T.; Nakamura, K.; Katou, H. Optimal Soil Eh, pH, and Water Management for Simultaneously Minimizing Arsenic and Cadmium Concentrations in Rice Grains. Environ. Sci. Technol. 2016, 50, 4178–4185. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, L.; Zhao, T.; Dahlgren, R.A.; Xu, J. Fertilizer application alters cadmium and selenium bioavailability in soil-rice system with high geological background levels. Environ. Pollut. 2024, 350, 124033. [Google Scholar] [CrossRef]
- Tang, X.; Li, Q.; Wu, M.; Lin, L.; Scholz, M. Review of remediation practices regarding cadmium-enriched farmland soil with particular reference to China. J. Environ. Manag. 2016, 181, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xi, J.; Ke, J.; Wang, Y.; Chen, X.; Zhang, Z.; Lin, Y. Deciphering soil amendments and actinomycetes for remediation of cadmium (Cd) contaminated farmland. Ecotoxicol. Environ. Saf. 2023, 249, 114388. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Y. Use of clay to remediate cadmium contaminated soil under different water management regimes. Ecotoxicol. Environ. Saf. 2017, 141, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Dong, W.; Wei, Z.; Wu, Q.; Qiu, R.; Gao, T.; Guo, Y.; Li, Z.; Li, M.; Chen, Y. Effects of Straw Return on Mitigating Cadmium-Contaminated Soils. Soil Use Manag. 2025, 41, e70085. [Google Scholar] [CrossRef]
- Xia, X.; Ji, J.; Yang, Z.; Han, H.; Huang, C.; Li, Y.; Zhang, W. Cadmium risk in the soil-plant system caused by weathering of carbonate bedrock. Chemosphere 2020, 254, 126799. [Google Scholar] [CrossRef]
- Li, C.; Yang, Z.; Yu, T.; Jiang, Z.; Huang, Q.; Yang, Y.; Liu, X.; Ma, X.; Li, B.; Lin, K.; et al. Cadmium accumulation in paddy soils affected by geological weathering and mining: Spatial distribution patterns, bioaccumulation prediction, and safe land usage. J. Hazard. Mater. 2023, 460, 132483. [Google Scholar] [CrossRef]
- Mason, E.; Edmonds, M.; McConnell, J.R. Volatile trace metals deposited in ice as soluble volcanic aerosols during the 17.7 ka eruptions of Mt Takahe, West Antarctic Rift. Front. Earth Sci. 2022, 10, 1002366. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, Q.; Lin, Q.; Zeng, C.; Zhong, C. Cadmium source identification in soils and high-risk regions predicted by geographical detector method. Environ. Pollut. 2020, 263, 114338. [Google Scholar] [CrossRef]
- Wang, L.; Cui, X.; Cheng, H.; Chen, F.; Wang, J.; Zhao, X.; Lin, C.; Pu, X. A review of soil cadmium contamination in China including a health risk assessment. Environ. Sci. Pollut. Res. Int. 2015, 22, 16441–16452. [Google Scholar] [CrossRef]
- Tong, Y.; Gao, J.; Yue, T.; Zhang, X.; Liu, J.; Bai, J. Distribution, chemical fractionation, and potential environmental risks of Hg, Cr, Cd, Pb, and As in wastes from ultra-low emission coal-fired industrial boilers in China. J. Hazard. Mater. 2023, 446, 130606. [Google Scholar] [CrossRef]
- Mar, S.S.; Okazaki, M. Investigation of Cd contents in several phosphate rocks used for the production of fertilizer. Microchem. J. 2012, 104, 17–21. [Google Scholar] [CrossRef]
- Gong, X.; Chen, Z.; Luo, Z. Spatial distribution, temporal variation, and sources of heavy metal pollution in groundwater of a century-old nonferrous metal mining and smelting area in China. Environ. Monit. Assess. 2014, 186, 9101–9116. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xue, M.; Xu, Z.; Huang, C. Health risk assessment of heavy metals (Cr, Ni, Cu, Zn, Cd, Pb) in circumjacent soil of a factory for recycling waste electrical and electronic equipment. J. Mater. Cycles Waste Manag. 2013, 15, 556–563. [Google Scholar] [CrossRef]
- Samrane, K.; Bouhaouss, A. Cadmium in Phosphorous Fertilizers: Balance and Trends. RASAYAN J. Chem. 2022, 15, 2103–2117. [Google Scholar] [CrossRef]
- Khadim, M.U.; Murtaza, G.; Farooqi, Z.U.R.; Hussain, T.; Mahmood, N.; Hussain, S. An Application of Rock Phosphate Increased Soil Cadmium Contamination and Hampered the Morphophysiological Growth of Brassica campestris L. J. Soil Sci. Plant Nutr. 2023, 23, 4583–4595. [Google Scholar] [CrossRef]
- Fu, Y.; Li, F.; Guo, S.; Zhao, M. Cadmium concentration and its typical input and output fluxes in agricultural soil downstream of a heavy metal sewage irrigation area. J. Hazard. Mater. 2021, 412, 125203. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, W.; Yang, X.; Liu, C.; Zhou, Y. Input-output balance of cadmium in typical agriculture soils with historical sewage irrigation in China. J. Environ. Manag. 2020, 276, 111298. [Google Scholar] [CrossRef]
- Qadir, M.; Ghafoor, A.; Murtaza, G. Cadmium Concentration in Vegetables Grown on Urban Soils Irrigated with Untreated Municipal Sewage. Environ. Dev. Sustain. 2000, 2, 13–21. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Dai, M.; Diao, X.; Dai, S.; Fang, T.; Dong, X. Input of Cd from agriculture phosphate fertilizer application in China during 2006–2016. Sci. Total. Environ. 2020, 698, 134149. [Google Scholar] [CrossRef]
- Owoade, K.O.; Hopke, P.K.; Olise, F.S.; Ogundele, L.T.; Fawole, O.G.; Olaniyi, B.H.; Jegede, O.O.; Ayoola, M.A.; Bashiru, M.I. Chemical compositions and source identification of particulate matter (PM2.5 and PM2.5–10) from a scrap iron and steel smelting industry along the Ife–Ibadan highway, Nigeria. Atmos. Pollut. Res. 2015, 6, 107–119. [Google Scholar] [CrossRef]
- Ou, J.; Zheng, L.; Tang, Q.; Liu, M.; Zhang, S. Source analysis of heavy metals in atmospheric particulate matter in a mining city. Environ. Geochem. Health 2022, 44, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Z.U.; Khan, S.; Brusseau, M.L.; Shah, M.T. Lead and cadmium contamination and exposure risk assessment via consumption of vegetables grown in agricultural soils of five-selected regions of Pakistan. Chemosphere 2017, 168, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, S.; Saha, S.; Ferdush, J.; Tusher, T.R.; Abu-Sharif, M.; Alam, M.F.; Balks, M.R.; Parveen, Z. Cadmium contamination in agricultural soils of Bangladesh and management by application of organic amendments: Evaluation of field assessment and pot experiments. Environ. Geochem. Health 2021, 43, 3557–3582. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Jiao, X.; Chen, Y.; Liang, H.; Liang, W.; Liu, C. Knockout of OsHMA3 in an indica rice increases cadmium sensitivity and inhibits plant growth. Plant Growth Regul. 2024, 103, 635–646. [Google Scholar] [CrossRef]
- Lan, Z.; He, Q.; Zhang, M.; Liu, H.; Fang, L.; Nie, J. Assessing the Effects of Cadmium Stress on the Growth, Physiological Characteristics, and Metabolic Profiling of Rice (Oryza sativa L.) Using HPLC-QTOF/MS. Chemosensors 2023, 11, 558. [Google Scholar] [CrossRef]
- Huybrechts, M.; Hendrix, S.; Bertels, J.; Beemster, G.T.S.; Vandamme, D.; Cuypers, A. Spatial analysis of the rice leaf growth zone under controlled and cadmium-exposed conditions. Environ. Exp. Bot. 2020, 177, 104120. [Google Scholar] [CrossRef]
- Pooja Parmar, N.K.V.S. Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot. Stud. 2013, 54, 45. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; Li, K.; Wu, M.; Zhang, R.; Zhang, L.; Chen, G. Photosynthetic responses of Oryza sativa L. seedlings to cadmium stress: Physiological, biochemical and ultrastructural analyses. Biometals 2014, 27, 389–401. [Google Scholar] [CrossRef]
- Pagliano, C.; Raviolo, M.; Dalla Vecchia, F.; Gabbrielli, R.; Gonnelli, C.; Rascio, N.; Barbato, R.; La Rocca, N. Evidence for PSII donor-side damage and photoinhibition induced by cadmium treatment on rice (Oryza sativa L.). J. Photochem. Photobiol. B 2006, 84, 70–78. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Rajpoot, R.; Pandey, P.; Rani, A.; Dubey, R.S. Cadmium Alters Mitochondrial Membrane Potential, Inhibits Electron Transport Chain Activity and Induces Callose Deposition in Rice Seedlings. J. Plant Growth Regul. 2017, 37, 335–344. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Wang, W.; Zhu, L. Photosynthetic mechanisms of carbon fixation reduction in rice by cadmium and polycyclic aromatic hydrocarbons. Environ. Pollut. 2024, 344, 123436. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Wu, J.; Wang, W.; Zhu, L. Insights into molecular mechanism underlying carbon fixation inhibition of rice induced by cadmium. Front. Environ. Sci. Eng. 2025, 19, 96. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, P.; Liu, D.; Wang, X.; Lu, S.; Liu, Z.; Yang, M.; Deng, T.; Chen, L.; Qi, H.; et al. OsPLDalpha1 mediates cadmium stress response in rice by regulating reactive oxygen species accumulation and lipid remodeling. J. Hazard. Mater. 2024, 479, 135702. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.A.; Prity, S.A.; Das, U.; Akther, M.S.; Sajib, S.A.; Reza, M.A.; Kabir, A.H. Silicon induces phytochelatin and ROS scavengers facilitating cadmium detoxification in rice. Plant Biol. 2020, 22, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Lu, Y.; Li, F.; Zeng, W.; Shen, L.; Yu, R.; Li, J. Microbial extracellular polymeric substances alleviate cadmium toxicity in rice (Oryza sativa L.) by regulating cadmium uptake, subcellular distribution and triggering the expression of stress-related genes. Ecotoxicol. Environ. Saf. 2023, 257, 114958. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Hessini, K.; Yu, F.; Ahmad, P. Zinc oxide nanoparticles alleviates the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system. Ecotoxicol. Environ. Saf. 2021, 220, 112401. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, T.; Liu, H.; Zhou, F.; Zhang, J.; Zhang, M.; Liu, X.; Shi, W.; Cao, T. Nano-selenium controlled cadmium accumulation and improved photosynthesis in indica rice cultivated in lead and cadmium combined paddy soils. J. Environ. Sci. 2021, 103, 336–346. [Google Scholar] [CrossRef]
- Wang, S.; Wang, F.; Gao, S. Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ. Sci. Pollut. Res. Int. 2015, 22, 2837–2845. [Google Scholar] [CrossRef]
- Chen, K.; Yu, B.; Xue, W.; Sun, Y.; Zhang, C.; Gao, X.; Zhou, X.; Deng, Y.; Yang, J.; Zhang, B. Citric Acid Inhibits Cd Absorption and Transportation by Improving the Antagonism of Essential Elements in Rice Organs. Toxics 2024, 12, 431. [Google Scholar] [CrossRef]
- Xue, W.; Zhang, X.; Zhang, C.; Wang, C.; Huang, Y.; Liu, Z. Mitigating the toxicity of reactive oxygen species induced by cadmium via restoring citrate valve and improving the stability of enzyme structure in rice. Chemosphere 2023, 327, 138511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Xiao, W.; Chen, D.; Hu, J.; Gao, N.; Huang, M.; Ye, X. Comparative transcriptomic analysis reveals the important process in two rice cultivars with differences in cadmium accumulation. Ecotoxicol. Environ. Saf. 2023, 252, 114629. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Cheng, D.; Yang, Y.; Zhang, G.; Qin, M.; Chen, J.; Chen, Y.; Jiang, M. Co-expression network analysis of the transcriptomes of rice roots exposed to various cadmium stresses reveals universal cadmium-responsive genes. BMC Plant Biol. 2017, 17, 194. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.Y.; Li, Y.; Yan, J.; Peng, S.Y.; Wei, S.J.; Yin, Y.; Li, K.T.; Cheng, X. Root cell wall remodeling: A way for exopolysaccharides to mitigate cadmium toxicity in rice seedling. J. Hazard. Mater. 2023, 443, 130186. [Google Scholar] [CrossRef]
- Dong, Q.; Wu, Y.; Li, B.; Chen, X.; Peng, L.; Sahito, Z.A.; Li, H.; Chen, Y.; Tao, Q.; Xu, Q.; et al. Multiple insights into lignin-mediated cadmium detoxification in rice (Oryza sativa). J. Hazard. Mater. 2023, 458, 131931. [Google Scholar] [CrossRef]
- Guo, L.; Chen, A.; He, N.; Yang, D.; Liu, M. Exogenous silicon alleviates cadmium toxicity in rice seedlings in relation to Cd distribution and ultrastructure changes. J. Soils Sediments 2017, 18, 1691–1700. [Google Scholar] [CrossRef]
- Chang, J.D.; Huang, S.; Yamaji, N.; Zhang, W.; Ma, J.F.; Zhao, F.J. OsNRAMP1 transporter contributes to cadmium and manganese uptake in rice. Plant Cell Environ. 2020, 43, 2476–2491. [Google Scholar] [CrossRef]
- Yu, E.; Yamaji, N.; Mao, C.; Wang, H.; Ma, J.F. Lateral roots but not root hairs contribute to high uptake of manganese and cadmium in rice. J. Exp. Bot. 2021, 72, 7219–7228. [Google Scholar] [CrossRef]
- Tang, L.; Mao, B.; Li, Y.; Lv, Q.; Zhang, L.; Chen, C.; He, H.; Wang, W.; Zeng, X.; Shao, Y.; et al. Knockout of OsNramp5 using the CRISPR/Cas9 system produces low Cd-accumulating indica rice without compromising yield. Sci. Rep. 2017, 7, 14438. [Google Scholar] [CrossRef]
- Shahzad, M.; Peng, D.; Khan, A.; Ayyaz, A.; Askri, S.M.H.; Naz, S.; Huang, B.; Zhang, G. Sufficient manganese supply is necessary for OsNramp5 knockout rice plants to ensure normal growth and less Cd uptake. Ecotoxicol. Environ. Saf. 2024, 288, 117386. [Google Scholar] [CrossRef]
- Yang, C.-H.; Zhang, Y.; Huang, C.-F. Reduction in cadmium accumulation in japonica rice grains by CRISPR/Cas9-mediated editing of OsNRAMP5. J. Integr. Agric. 2019, 18, 688–697. [Google Scholar] [CrossRef]
- Nakanishi, H.; Ogawa, I.; Ishimaru, Y.; Mori, S.; Nishizawa, N.K. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci. Plant Nutr. 2006, 52, 464–469. [Google Scholar] [CrossRef]
- Yan, H.; Xu, W.; Xie, J.; Gao, Y.; Wu, L.; Sun, L.; Feng, L.; Chen, X.; Zhang, T.; Dai, C.; et al. Variation of a major facilitator superfamily gene contributes to differential cadmium accumulation between rice subspecies. Nat. Commun. 2019, 10, 2562. [Google Scholar] [CrossRef]
- Tan, L.; Qu, M.; Zhu, Y.; Peng, C.; Wang, J.; Gao, D.; Chen, C. ZINC TRANSPORTER5 and ZINC TRANSPORTER9 Function Synergistically in Zinc/Cadmium Uptake. Plant Physiol. 2020, 183, 1235–1249. [Google Scholar] [CrossRef]
- Lim, S.D.; Hwang, J.G.; Han, A.R.; Park, Y.C.; Lee, C.; Ok, Y.S.; Jang, C.S. Positive regulation of rice RING E3 ligase OsHIR1 in arsenic and cadmium uptakes. Plant Mol. Biol. 2014, 85, 365–379. [Google Scholar] [CrossRef]
- Tian, J.; Wang, L.; Hui, S.; Yang, D.; He, Y.; Yuan, M. Cadmium accumulation regulated by a rice heavy-metal importer is harmful for host plant and leaf bacteria. J. Adv. Res. 2023, 45, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef]
- Tiwari, M.; Sharma, D.; Dwivedi, S.; Singh, M.; Tripathi, R.D.; Trivedi, P.K. Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ. 2014, 37, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, L.; Ma, Y.; Hu, R.; Wang, J.; Li, W.; Dong, J.; Yang, T.; Zhou, L.; Chen, J.; et al. OsNRAMP2 facilitates Cd efflux from vacuoles and contributes to the difference in grain Cd accumulation between japonica and indica rice. Crop J. 2023, 11, 417–426. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, M.; Chen, M.; Lin, X.; Xu, P.; Cao, Z. Mutation of OsNRAMP5 reduces cadmium xylem and phloem transport in rice plants and its physiological mechanism. Environ. Pollut. 2024, 341, 122928. [Google Scholar] [CrossRef]
- Li, M.Z.; Hu, D.W.; Liu, X.Q.; Zhang, R.; Liu, H.; Tang, Z.; Zhao, F.J.; Huang, X.Y. The OsZIP2 transporter is involved in root-to-shoot translocation and intervascular transfer of cadmium in rice. Plant Cell Environ. 2024, 47, 3865–3881. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Liang, S.; Qiao, K.; Wang, F.; Zhang, Y.; Chai, T. Co-expression of multiple heavy metal transporters changes the translocation, accumulation, and potential oxidative stress of Cd and Zn in rice (Oryza sativa). J. Hazard. Mater. 2019, 380, 120853. [Google Scholar] [CrossRef]
- Tan, L.; Zhu, Y.; Fan, T.; Peng, C.; Wang, J.; Sun, L.; Chen, C. OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem. Biophys. Res. Commun. 2019, 512, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zeng, M.; Wang, J.; Zeng, Z.; Dai, J.; Xie, Z.; Yang, Y.; Tian, L.; Chen, L.; Li, D. A Node-Expressed Transporter OsCCX2 Is Involved in Grain Cadmium Accumulation of Rice. Front. Plant Sci. 2018, 9, 476. [Google Scholar] [CrossRef]
- Tang, L.; Dong, J.; Tan, L.; Ji, Z.; Li, Y.; Sun, Y.; Chen, C.; Lv, Q.; Mao, B.; Hu, Y.; et al. Overexpression of OsLCT2, a Low-Affinity Cation Transporter Gene, Reduces Cadmium Accumulation in Shoots and Grains of Rice. Rice 2021, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Qi, Z.; Wang, Y.; Chen, S.; Yan, J.; Qiu, H.; Yu, Y.; Fang, Z.; Wang, J.; Gong, J. An endophytic fungus interacts with the defensin-like protein OsCAL1 to regulate cadmium allocation in rice. Mol. Plant 2024, 17, 312–324. [Google Scholar] [CrossRef]
- Uraguchi, S.; Kamiya, T.; Sakamoto, T.; Kasai, K.; Sato, Y.; Nagamura, Y.; Yoshida, A.; Kyozuka, J.; Ishikawa, S.; Fujiwara, T. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc. Natl. Acad. Sci. USA 2011, 108, 20959–20964. [Google Scholar] [CrossRef]
- Gu, T.Y.; Qi, Z.A.; Chen, S.Y.; Yan, J.; Fang, Z.J.; Wang, J.M.; Gong, J.M. Dual-function DEFENSIN 8 mediates phloem cadmium unloading and accumulation in rice grains. Plant Physiol. 2023, 191, 515–527. [Google Scholar] [CrossRef]
- Yamaji, N.; Xia, J.; Mitani-Ueno, N.; Yokosho, K.; Feng Ma, J. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol. 2013, 162, 927–939. [Google Scholar] [CrossRef]
- Satoh-Nagasawa, N.; Mori, M.; Nakazawa, N.; Kawamoto, T.; Nagato, Y.; Sakurai, K.; Takahashi, H.; Watanabe, A.; Akagi, H. Mutations in rice (Oryza sativa) heavy metal ATPase 2 (OsHMA2) restrict the translocation of zinc and cadmium. Plant Cell Physiol. 2012, 53, 213–224. [Google Scholar] [CrossRef]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.; Takahashi, H.; et al. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fu, S.; Huang, J.; Li, L.; Long, Y.; Wei, Q.; Wang, Z.; Chen, Z.; Xia, J. The tonoplast-localized transporter OsABCC9 is involved in cadmium tolerance and accumulation in rice. Plant Sci. 2021, 307, 110894. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Zhan, J.; Meng, L.; Chen, Y.; Chen, D.; Zhang, M.; He, H.; Chen, J.; Ye, G. The Cation/H+ exchanger OsCAX2 is involved in cadmium tolerance and accumulation through vacuolar sequestration in rice. bioRxiv 2022. [Google Scholar] [CrossRef]
- Liu, P.; Sun, L.; Zhang, Y.; Tan, Y.; Zhu, Y.; Peng, C.; Wang, J.; Yan, H.; Mao, D.; Liang, G.; et al. The metal tolerance protein OsMTP11 facilitates cadmium sequestration in the vacuoles of leaf vascular cells for restricting its translocation into rice grains. Mol. Plant 2024, 17, 1733–1752. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Senoura, T.; Shimo, H.; Ishikawa, S.; Arao, T.; Nakanishi, H.; Nishizawa, N.K. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J. Exp. Bot. 2011, 62, 4843–4850. [Google Scholar] [CrossRef]
- Xia, R.; Zhou, J.; Sun, Y.; Zeng, Z.; Liu, H.; Cui, H.; Yan, J.; Kou, L.; Hu, K.; Zhang, H.; et al. Stable Isotope Ratios Trace the Rice Uptake of Cadmium from Atmospheric Deposition via Leaves and Roots. Environ. Sci. Technol. 2023, 57, 16873–16883. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Z.; Peng, J.; Fei, J.; Yu, P.; Wang, M.; Tan, Y.; Huang, Y.; Zhran, M.; Fahmy, A. The contribution of atmospheric deposition of cadmium and lead to their accumulation in rice grains. Plant Soil 2022, 477, 373–387. [Google Scholar] [CrossRef]
- Xia, R.; Zhou, J.; Zeng, Z.; Sun, Y.; Cui, H.; Liu, H.; Zhou, J. Cadmium Isotope Fractionations Induced by Foliar and Root Uptake for Rice Exposed to Atmospheric Particles: Implications for Environmental Source Tracing. Environ. Sci. Technol. Lett. 2023, 10, 1096–1102. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, R.; Landis, J.D.; Sun, Y.; Zeng, Z.; Zhou, J. Isotope Evidence for Rice Accumulation of Newly Deposited and Soil Legacy Cadmium: A Three-Year Field Study. Environ. Sci. Technol. 2024, 58, 17283–17294. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, Z.; Zhao, Y.; Huang, Z.; Fei, J.; Han, Y.; Wang, M.; Yu, P.; Peng, J.; Huang, Y.; et al. Foliar uptake, accumulation, and distribution of cadmium in rice (Oryza sativa L.) at different stages in wet deposition conditions. Environ. Pollut. 2022, 306, 119390. [Google Scholar] [CrossRef]
- Uraguchi, S.; Mori, S.; Kuramata, M.; Kawasaki, A.; Arao, T.; Ishikawa, S. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 2009, 60, 2677–2688. [Google Scholar] [CrossRef]
- Fujimaki, S.; Suzui, N.; Ishioka, N.S.; Kawachi, N.; Ito, S.; Chino, M.; Nakamura, S. Tracing cadmium from culture to spikelet: Noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice plant. Plant Physiol. 2010, 152, 1796–1806. [Google Scholar] [CrossRef]
- Wei, W.; Peng, H.; Xie, Y.; Wang, X.; Huang, R.; Chen, H.; Ji, X. The role of silicon in cadmium alleviation by rice root cell wall retention and vacuole compartmentalization under different durations of Cd exposure. Ecotoxicol. Environ. Saf. 2021, 226, 112810. [Google Scholar] [CrossRef]
- Yang, H.; Yu, H.; Wu, Y.; Huang, H.; Zhang, X.; Ye, D.; Wang, Y.; Zheng, Z.; Li, T. Nitric oxide amplifies cadmium binding in root cell wall of a high cadmium-accumulating rice (Oryza sativa L.) line by promoting hemicellulose synthesis and pectin demethylesterification. Ecotoxicol. Environ. Saf. 2022, 234, 113404. [Google Scholar] [CrossRef]
- Wang, L.; Wu, K.; Liu, Z.; Li, Z.; Shen, J.; Wu, Z.; Liu, H.; You, L.; Yang, G.; Rensing, C.; et al. Selenite reduced uptake/translocation of cadmium via regulation of assembles and interactions of pectins, hemicelluloses, lignins, callose and Casparian strips in rice roots. J. Hazard. Mater. 2023, 448, 130812. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Guo, R.; Jiang, Q.; Chen, C.Z.; Gao, Y.Q.; Jiang, M.M.; Shen, R.F.; Zhu, X.F.; Huang, J. Brassinosteroid decreases cadmium accumulation via regulating gibberellic acid accumulation and Cd fixation capacity of root cell wall in rice (Oryza sativa). J. Hazard. Mater. 2024, 469, 133862. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, L.; Tang, Z.; Huang, X.Y.; Ma, J.F.; Zhao, F.J. Producing cadmium-free Indica rice by overexpressing OsHMA3. Environ. Int. 2019, 126, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yu, H.; Wang, S.; Bayouli, I.T.; Huang, H.; Ye, D.; Zhang, X.; Liu, T.; Wang, Y.; Zheng, Z.; et al. Root radial apoplastic transport contributes to shoot cadmium accumulation in a high cadmium-accumulating rice line. J. Hazard. Mater. 2023, 460, 132276. [Google Scholar] [CrossRef]
- Tanaka, K.; Fujimaki, S.; Fujiwara, T.; Yoneyama, T.; Hayashi, H. Quantitative estimation of the contribution of the phloem in cadmium transport to grains in rice plants (Oryza sativa L.). Soil Sci. Plant Nutr. 2007, 53, 72–77. [Google Scholar] [CrossRef]
- Guan, M.; Xia, Y.; Zhang, W.; Chen, M.; Cao, Z. A Review of Reducing Cadmium Pollution in the Rice–Soil System in China. Foods 2025, 14, 1747. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Ayub, A.; Hussain, S.; Waraich, E.A.; El-Esawi, M.A.; Ishfaq, M.; Ahmad, M.; Ali, N.; Maqsood, M.F. Cadmium Toxicity in Plants: Recent Progress on Morpho-physiological Effects and Remediation Strategies. J. Soil Sci. Plant Nutr. 2021, 22, 212–269. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Xu, K.; Wang, J. Cadmium in Cereal Crops: Uptake and Transport Mechanisms and Minimizing Strategies. J. Agric. Food Chem. 2022, 70, 5961–5974. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, Z.; Zhang, L.; Sun, D.; Li, X. The Status and Research Progress of Cadmium Pollution in Rice- (Oryza sativa L.) and Wheat- (Triticum aestivum L.) Cropping Systems in China: A Critical Review. Toxics 2022, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Du, R.; Wang, X. Genetic Regulation Mechanism of Cadmium Accumulation and Its Utilization in Rice Breeding. Int. J. Mol. Sci. 2023, 24, 1247. [Google Scholar] [CrossRef]
- Peera Sheikh Kulsum, P.G.; Khanam, R.; Das, S.; Nayak, A.K.; Tack, F.M.G.; Meers, E.; Vithanage, M.; Shahid, M.; Kumar, A.; Chakraborty, S.; et al. A state-of-the-art review on cadmium uptake, toxicity, and tolerance in rice: From physiological response to remediation process. Environ. Res. 2023, 220, 115098. [Google Scholar] [CrossRef]
- Asgher, M.; Khan, M.I.R.; Anjum, N.A.; Khan, N.A. Minimising toxicity of cadmium in plants—Role of plant growth regulators. Protoplasma 2014, 252, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, J.; Ding, Z.; Xiong, F.; Liu, X.; Tian, J.; Wu, N. Cadmium-absorptive Bacillus vietnamensis 151-6 reduces the grain cadmium accumulation in rice (Oryza sativa L.): Potential for cadmium bioremediation. Ecotoxicol. Environ. Saf. 2023, 254, 114760. [Google Scholar] [CrossRef]
- Cao, H.W.; Zhao, Y.N.; Liu, X.S.; Rono, J.K.; Yang, Z.M. A metal chaperone OsHIPP16 detoxifies cadmium by repressing its accumulation in rice crops. Environ. Pollut. 2022, 311, 120058. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Mou, R.; Cao, Z.; Xu, P.; Wu, X.; Zhu, Z.; Chen, M. Characterization of cadmium-resistant bacteria and their potential for reducing accumulation of cadmium in rice grains. Sci. Total Environ. 2016, 569–570, 97–104. [Google Scholar] [CrossRef]
- Long, H.Y.; Feng, G.F.; Fang, J. In-Situ remediation of cadmium contamination in paddy fields: From rhizosphere soil to rice kernel. Environ. Geochem. Health 2024, 46, 404. [Google Scholar] [CrossRef]
- Huang, A.; Feng, J.; Guo, W.; Li, Z.; Hwang, J.-Y.; Su, X.; Mo, W.; Sun, W.; Wang, D.; Ma, S.; et al. Utilization of local rich banana straw bioresource to solve Cd2+ pollution problem in major non-ferrous metal production areas of Southwest China. Mater. Today Sustain. 2024, 25, 100670. [Google Scholar] [CrossRef]
- Van Poucke, R.; Ainsworth, J.; Maeseele, M.; Ok, Y.S.; Meers, E.; Tack, F.M.G. Chemical stabilization of Cd-contaminated soil using biochar. Appl. Geochem. 2018, 88, 122–130. [Google Scholar] [CrossRef]
- Tian, T.; Yu, L.; Feng, R.; Yao, C.; Gong, L.; Xiao, H.; Liu, L.; Li, F. Unveiling the combined effects of water management and lime on remediation of Cd-contaminated soils with improved soil quality. J. Environ. Chem. Eng. 2024, 12, 114778. [Google Scholar] [CrossRef]
- Xu, H.; Ou, Z.; Li, W.; Hu, T.; Zhang, Y.; Xu, H.; Wang, J.; Li, Y. Cadmium(II) adsorption by recyclable Zeolite-Loaded Hydrogel: Extension to the removal of Cadmium(II) from contaminated soil. Chem. Eng. J. 2024, 492, 151842. [Google Scholar] [CrossRef]
- Guan, X.; Bai, J.; Yuan, X.; Wang, H.; Li, Y.; Zhao, Y. Remediation of Cadmium Pollution in Paddy Soil by FeMg-LDH/bentonite Mixed with Compost and Effect on Soil Microorganisms. J. Soil Sci. Plant Nutr. 2025, 25, 2346–2357. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Jia, L.; Chen, Y.; Zhao, P.; Zhu, S.; Yang, S.; Long, G. Lime-assisted cultivation of Erigeron breviscapus: Enhancing plant biomass production and scutellarin content in cadmium-contaminated soil. J. Soils Sediments 2024, 24, 3422–3433. [Google Scholar] [CrossRef]
- Deng, X.; Chen, Y.; Yang, Y.; Lu, L.; Yuan, X.; Zeng, H.; Zeng, Q. Cadmium accumulation in rice (Oryza sativa L.) alleviated by basal alkaline fertilizers followed by topdressing of manganese fertilizer. Environ. Pollut. 2020, 262, 114289. [Google Scholar] [CrossRef]
- Jia, S.; Zhao, X.; Huang, J.; Yao, X.; Xie, F. Phosphorus Alleviates Cadmium Damage by Reducing Cadmium Accumulation and Enhancing Antioxidant Enzymes at the Vegetative Phase in Soybean. Agronomy 2025, 15, 637. [Google Scholar] [CrossRef]
- Cao, Z.Z.; Qin, M.L.; Lin, X.Y.; Zhu, Z.W.; Chen, M.X. Sulfur supply reduces cadmium uptake and translocation in rice grains (Oryza sativa L.) by enhancing iron plaque formation, cadmium chelation and vacuolar sequestration. Environ. Pollut. 2018, 238, 76–84. [Google Scholar] [CrossRef]
- Huang, P.; Yang, W.; Li, Q.; Liao, Q.; Si, M.; Shi, M.; Yang, Z. A novel slow-release selenium approach for cadmium reduction and selenium enrichment in rice (Oryza sativa L.). Chemosphere 2023, 342, 140183. [Google Scholar] [CrossRef]
- Liu, X.; Yin, L.; Deng, X.; Gong, D.; Du, S.; Wang, S.; Zhang, Z. Combined application of silicon and nitric oxide jointly alleviated cadmium accumulation and toxicity in maize. J. Hazard. Mater. 2020, 395, 122679. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Luo, L.; Wang, P.; You, R. Effect of Water Management under Different Soil Conditions on Cadmium and Arsenic Accumulation in Rice. Agronomy 2023, 13, 2472. [Google Scholar] [CrossRef]
- Li, M.; Chen, L.; Yang, Y.; Wu, S.; Zhang, Q.; Deng, X.; Luo, S.; Zeng, Q. Analysis of the phytoremediation potential, rice safety, and economic benefits of light to moderate Cd-contaminated farmland in oilseed rape-rice rotation with straw removal: A three-year field trial. Environ. Res. 2024, 263, 120280. [Google Scholar] [CrossRef]
- Deng, X.; Wu, S.; Yang, Y.; Qin, Y.; Huang, Q.; Wu, W.; Rong, X.; Zeng, Q. A rice-chicory rotation pattern ensures safe grain production and phytoremediation of cadmium-contaminated paddy fields: A four-year field experiment in southern China. Chemosphere 2023, 322, 138192. [Google Scholar] [CrossRef]
- Wang, X.; Deng, S.; Zhou, Y.; Long, J.; Ding, D.; Du, H.; Lei, M.; Chen, C.; Tie, B.Q. Application of different foliar iron fertilizers for enhancing the growth and antioxidant capacity of rice and minimizing cadmium accumulation. Environ. Sci. Pollut. Res. Int. 2021, 28, 7828–7839. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Zheng, J.; Shen, Z.; Xu, X. Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2019, 167, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Lan, Y.; Zhan, Y.; Li, Y.; Jiang, J.; Li, Y.; Zhang, L.; Fan, X. Preparation of porous biochar from fusarium wilt-infected banana straw for remediation of cadmium pollution in water bodies. Sci. Rep. 2024, 14, 13821. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, H.; Xu, C.; Zheng, S.; Wu, M.; Zhang, Q.; Liao, Y.; Zhu, H.; Zhu, Q.; Huang, D. Nitrogen application practices to reduce cadmium concentration in rice (Oryza sativa L.) grains. Environ. Sci. Pollut. Res. Int. 2022, 29, 50530–50539. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Q.; Xiao, X.Y.; Guo, Z.H.; Xie, Y.H.; Zhu, H.W.; Peng, C.; Liang, Y.Q. Release of cadmium in contaminated paddy soil amended with NPK fertilizer and lime under water management. Ecotoxicol. Environ. Saf. 2018, 159, 38–45. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.Q.; Huang, S.Y.; Kong, L.X.; Zhuang, Z.; Wang, Q.; Li, H.F.; Wan, Y.N. The risks of sulfur addition on cadmium accumulation in paddy rice under different water-management conditions. J. Environ. Sci. 2022, 118, 101–111. [Google Scholar] [CrossRef]
- Sarwar, M.J.; Zahir, Z.A.; Asghar, H.N.; Shabaan, M.; Ayyub, M. Co-application of organic amendments and Cd-tolerant rhizobacteria for suppression of cadmium uptake and regulation of antioxidants in tomato. Chemosphere 2023, 327, 138478. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, Q.; Zhang, Z.; Xu, J.; Yang, J.; Wong, M.H. Variations in cadmium accumulation among rice cultivars and types and the selection of cultivars for reducing cadmium in the diet. J. Sci. Food Agric. 2004, 85, 147–153. [Google Scholar] [CrossRef]
- Kibria, K.Q.; Islam, M.A.; Hoque, S.; Siddique, M.A.B.; Hossain, M.Z.; Islam, M.A. Variations in cadmium accumulation among amon rice cultivars in Bangladesh and associated human health risks. Environ. Sci. Pollut. Res. Int. 2022, 29, 39888–39902. [Google Scholar] [CrossRef]
- Wang, K.; Yan, T.Z.; Xu, S.L.; Yan, X.; Zhou, Q.F.; Zhao, X.H.; Li, Y.F.; Wu, Z.X.; Qin, P.; Fu, C.J.; et al. Validating a segment on chromosome 7 of japonica for establishing low-cadmium accumulating indica rice variety. Sci. Rep. 2021, 11, 6053. [Google Scholar] [CrossRef]
- Jegadeesan, S.; Yu, K.; Poysa, V.; Gawalko, E.; Morrison, M.J.; Shi, C.; Cober, E. Mapping and validation of simple sequence repeat markers linked to a major gene controlling seed cadmium accumulation in soybean [Glycine max (L.) Merr]. Theor. Appl. Genet. 2010, 121, 283–294. [Google Scholar] [CrossRef]
- Pan, X.; Li, Y.; Liu, W.; Liu, S.; Min, J.; Xiong, H.; Dong, Z.; Duan, Y.; Yu, Y.; Li, X. QTL mapping and candidate gene analysis of cadmium accumulation in polished rice by genome-wide association study. Sci. Rep. 2020, 10, 11791. [Google Scholar] [CrossRef]
- Cao, Z.Z.; Lin, X.Y.; Yang, Y.J.; Guan, M.Y.; Xu, P.; Chen, M.X. Gene identification and transcriptome analysis of low cadmium accumulation rice mutant (lcd1) in response to cadmium stress using MutMap and RNA-seq. BMC Plant Biol. 2019, 19, 250. [Google Scholar] [CrossRef]
- Ishikawa, S.; Ishimaru, Y.; Igura, M.; Kuramata, M.; Abe, T.; Senoura, T.; Hase, Y.; Arao, T.; Nishizawa, N.K.; Nakanishi, H. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc. Natl. Acad. Sci. USA 2012, 109, 19166–19171. [Google Scholar] [CrossRef]
- Meng, Y.; Jing, H.; Huang, J.; Shen, R.; Zhu, X. The Role of Nitric Oxide Signaling in Plant Responses to Cadmium Stress. Int. J. Mol. Sci. 2022, 23, 6901. [Google Scholar] [CrossRef]
- Lu, L.; Chen, X.; Chen, J.; Zhang, Z.; Zhang, Z.; Sun, Y.; Wang, Y.; Xie, S.; Ma, Y.; Song, Y.; et al. MicroRNA-encoded regulatory peptides modulate cadmium tolerance and accumulation in rice. Plant Cell Environ. 2024, 47, 1452–1470. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ji, J.; Harris-Shultz, K.R.; Wang, H.; Wang, H.; Abd-Allah, E.F.; Luo, Y.; Hu, X. The Dynamic Changes of the Plasma Membrane Proteins and the Protective Roles of Nitric Oxide in Rice Subjected to Heavy Metal Cadmium Stress. Front. Plant Sci. 2016, 7, 190. [Google Scholar] [CrossRef]
- Ali, E.; Hussain, N.; Shamsi, I.H.; Jabeen, Z.; Siddiqui, M.H.; Jiang, L.X. Role of jasmonic acid in improving tolerance of rapeseed (Brassica napus L.) to Cd toxicity. J. Zhejiang Univ. Sci. B 2018, 19, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Huang, J.; Jing, H.; Wu, Q.; Shen, R.; Zhu, X. Exogenous abscisic acid alleviates Cd toxicity in Arabidopsis thaliana by inhibiting Cd uptake, translocation and accumulation, and promoting Cd chelation and efflux. Plant Sci. 2022, 325, 111464. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Song, F.; Xia, J.; Wang, R. A Glucuronic Acid-Producing Endophyte Pseudomonas sp. MCS15 Reduces Cadmium Uptake in Rice by Inhibition of Ethylene Biosynthesis. Front Plant Sci. 2022, 13, 876545. [Google Scholar] [CrossRef]
- Rolon-Cardenas, G.A.; Arvizu-Gomez, J.L.; Soria-Guerra, R.E.; Pacheco-Aguilar, J.R.; Alatorre-Cobos, F.; Hernandez-Morales, A. The role of auxins and auxin-producing bacteria in the tolerance and accumulation of cadmium by plants. Environ. Geochem. Health 2022, 44, 3743–3764. [Google Scholar] [CrossRef]
- Farooq, H.; Asghar, H.N.; Khan, M.Y.; Saleem, M.; Zahir, Z.A. Auxin-mediated growth of rice in cadmium-contaminated soil. Turk. J. Agric. For. 2015, 39, 272–276. [Google Scholar] [CrossRef]
- Jiang, M.; Dai, S.; Wang, B.; Xie, Z.; Li, J.; Wang, L.; Li, S.; Tan, Y.; Tian, B.; Shu, Q.; et al. Gold nanoparticles synthesized using melatonin suppress cadmium uptake and alleviate its toxicity in rice. Environ. Sci. Nano 2021, 8, 1042–1056. [Google Scholar] [CrossRef]
- Chen, J.; Qin, H.; Zhang, B.; Mao, W.; Lou, L.; Shen, C.; Mao, J.; Lin, Q. Development of melatonin nano-delivery systems to reduce cadmium accumulation in rice (Oryza sativa L.) seedlings: Insights from photosynthetic efficiency, antioxidative response and gene expression. Environ. Exp. Bot. 2022, 196, 104822. [Google Scholar] [CrossRef]
- Munir, R.; Yasin, M.U.; Afzal, M.; Jan, M.; Muhammad, S.; Jan, N.; Nana, C.; Munir, F.; Iqbal, H.; Tawab, F.; et al. Melatonin alleviated cadmium accumulation and toxicity by modulating phytohormonal balance and antioxidant metabolism in rice. Chemosphere 2024, 346, 140590. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, H.; Li, Y.; Bao, Q.; Huang, Y. Metabolic regulation mechanism of melatonin for reducing cadmium accumulation and improving quality in rice. Food Chem. 2024, 455, 139857. [Google Scholar] [CrossRef]
- Siripornadulsil, S.; Siripornadulsil, W. Cadmium-tolerant bacteria reduce the uptake of cadmium in rice: Potential for microbial bioremediation. Ecotoxicol. Environ. Saf. 2013, 94, 94–103. [Google Scholar] [CrossRef]
- Treesubsuntorn, C.; Dhurakit, P.; Khaksar, G.; Thiravetyan, P. Effect of microorganisms on reducing cadmium uptake and toxicity in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. Int. 2018, 25, 25690–25701. [Google Scholar] [CrossRef] [PubMed]
- Suksabye, P.; Pimthong, A.; Dhurakit, P.; Mekvichitsaeng, P.; Thiravetyan, P. Effect of biochars and microorganisms on cadmium accumulation in rice grains grown in Cd-contaminated soil. Environ. Sci. Pollut. Res. Int. 2016, 23, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Sehrish, A.K.; Alomrani, S.O.; Zhang, L.; Waseem, M.; Noureen, S.; Ullah, I.; Tabassam, R.; Abbas, G.; Ali, S. Combined application of biochar and metal-tolerant bacteria alleviates cadmium toxicity by modulating the antioxidant defense mechanism and physicochemical attributes in rice (Oryza sativa L.) grown in cadmium-contaminated soil. Plant Stress 2024, 11, 100348. [Google Scholar] [CrossRef]
- Hammami, H.; Parsa, M.; Mohassel, M.H.; Rahimi, S.; Mijani, S. Weeds ability to phytoremediate cadmium-contaminated soil. Int. J. Phytoremediat. 2016, 18, 48–53. [Google Scholar] [CrossRef]
- Vural, A. Trace element accumulation behavior, ability, and propensity of Taraxacum officinale F.H. Wigg (Dandelion). Environ. Sci. Pollut. Res. Int. 2024, 31, 16667–16684. [Google Scholar] [CrossRef]
- Tefhanie, G.H.M.; Bantoto-Kinamot, V. Sargassum, Padina and Turbinaria as bioindicators of cadmium in Bais Bay, Negros Oriental. Palawan Sci. 2021, 13, 90–98. [Google Scholar] [CrossRef]
- Wan, Y.; Peng, M.; Wang, Y.P. Assessment of heavy metal concentrations in roadside soils and plants around the Dexing copper mine: Implications for environmental management and remediation. Environ. Monit. Assess. 2024, 196, 251. [Google Scholar] [CrossRef]
- Ariyachandra, S.P.; Alwis, I.S.; Wimalasiri, E.M. Phytoremediation Potential of Heavy Metals by Cyperus rotundus. Rev. Agric. Sci. 2023, 11, 20–35. [Google Scholar] [CrossRef]
- Gao, H.; Zhai, S.; Sun, Z.; Liu, J.; Tong, C. Differences in biomass and silica content in typical plant communities with ecotones in the Min River estuary of southeast China. PeerJ 2019, 7, e7218. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Zhang, S.; Gao, F.; Sun, X.; Ren, X. Interactive effect of 24-epibrassinolide and silicon on the alleviation of cadmium toxicity in rice (Oryza sativa L.) plants. Environ. Technol. 2023, 45, 4725–4736. [Google Scholar] [CrossRef]
- Ma, C.; Ci, K.; Zhu, J.; Sun, Z.; Liu, Z.; Li, X.; Zhu, Y.; Tang, C.; Wang, P.; Liu, Z. Impacts of exogenous mineral silicon on cadmium migration and transformation in the soil-rice system and on soil health. Sci. Total Environ. 2021, 759, 143501. [Google Scholar] [CrossRef]
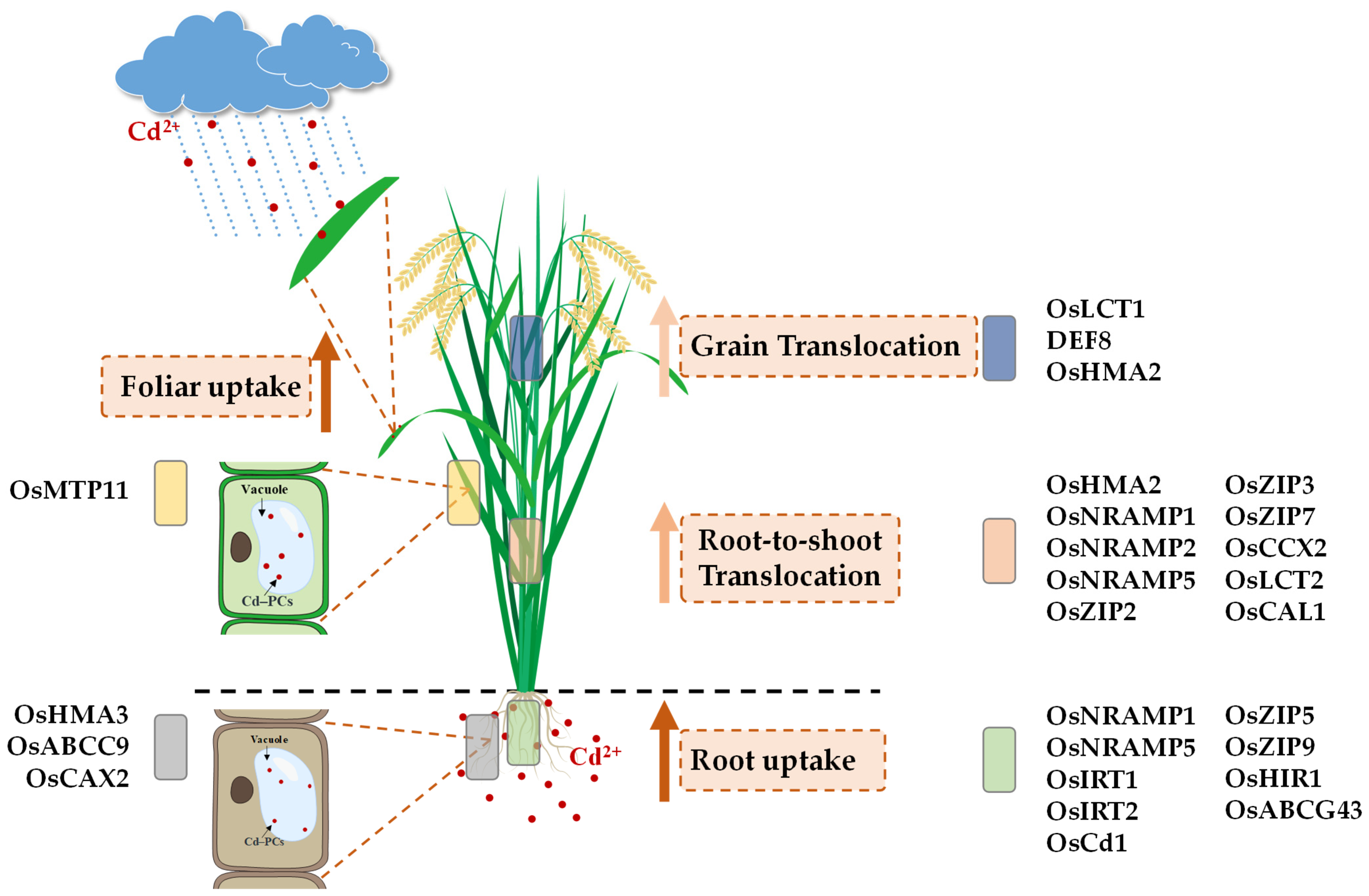
| Proteins | Family/Protein Product | Normal Substrates (Physiological Role) | Transportation | References |
|---|---|---|---|---|
| Root Uptake | ||||
| OsNRAMP1 | Natural resistance-associated macrophage protein | Mn2+, Fe2+ | Mediates Cd uptake from soil into root cells | [79] |
| OsNRAMP5 | Natural resistance-associated macrophage protein | Mn2+, Fe2+ | Facilitates Cd uptake into rice roots from soil | [80,81,82,83] |
| OsIRT1 | Iron-regulated metal transporter; Fe(II) transporter gene | Zn2+, Fe2+ | Mediates Cd influx into root cells from soil | [84] |
| OsIRT2 | Iron-regulated metal transporter; Fe(II) transporter gene | Fe2+ | Mediates Cd uptake from soil into root cells | [84] |
| OsCd1 | Major facilitator superfamily domain-containing protein | Unknown | Mediates Cd absorption in roots | [85] |
| OsZIP5 | ZRT- and IRT-like protein; zinc transporter | Zn2+ | Mediates Cd uptake from soil solution into root cells | [86] |
| OsZIP9 | ZRT- and IRT-like protein; zinc transporter | Zn2+ | Mediates Cd uptake from soil into rice root cells | [86] |
| OsHIR1 | RING finger protein; heavy metal induced RING E3 ubiquitin ligase 1 | Not a transporter | Enhances Cd uptake into roots through modulation of transporter activity | [87] |
| OsABCG43 | ATP binding cassette (ABC)-type transporter | Unknown | Mediates Cd influx into roots from environment or xylem | [88] |
| Xylem-Mediated Transport | ||||
| OsHMA2 | Heavy metal ATPase 2 | Zn2+ | Facilitates root-to-shoot Cd transport through xylem | [89] |
| OsNRAMP1 | Natural resistance-associated macrophage protein | Mn2+, Fe2+ | Facilitates Cd loading into xylem for root-to-shoot translocation | [90] |
| OsNRAMP2 | Natural resistance-associated macrophage protein | Fe2+ | Mediates vacuolar Cd efflux and facilitates Cd translocation | [91] |
| OsNRAMP5 | Natural resistance-associated macrophage protein | Mn2+, Fe2+ | Affects Cd translocation via xylem | [92] |
| OsZIP2 | ZRT- and IRT-like protein; zinc transporter | Zn2+, Mn2+ | Transports root-absorbed Cd to aboveground tissues via the xylem | [93] |
| OsZIP3 | ZRT- and IRT-like protein; zinc transporter | Zn2+ | Mediates Cd translocation from roots to shoots in rice | [94] |
| OsZIP7 | ZRT- and IRT-like protein; zinc transporter | Zn2+, Fe2+ | Promotes Cd xylem loading and inter-node transfer to grains | [95] |
| OsCCX2 | Na+/Ca2+ exchanger | Ca2+, Na+, Fe2+ | Enhances Cd transport to grains via xylem loading | [96] |
| OsLCT2 | Low-affinity Cation Transporter 2 | Unknown | Limits Cd xylem loading and root-to-shoot transport | [97] |
| OsCAL1 | Cell wall localized defensin protein | Unknown | Mediates Cd efflux to the apoplast | [98] |
| Phloem Transport | ||||
| OsLCT1 | Low-affinity Cation Transporter 1 | K+, Mg2+, Ca2+, Mn2+ | Promotes Cd phloem loading and transport to grains | [99] |
| DEF8 | Defensin-like protein 8 | Not a transporter | Facilitates Cd unloading from the phloem into grains | [100] |
| OsHMA2 | Heavy metal ATPase 2 | Zn2+ | Promotes the phloem loading and transport of Cd to developing grains | [101,102] |
| Vacuolar Cd Compartmentalization | ||||
| OsHMA3 | Heavy metal ATPase 3 | Zn2+ | Sequesters Cd into vacuoles to restrict its translocation to shoots | [103] |
| OsABCC9 | C-type ABC transporter | GSH, PC–Metal Complexes | Limits the transport from roots to stems | [104] |
| OsCAX2 | Cation/H+ Exchanger | Ca2+ | Limits the transport from roots to stems | [105] |
| OsMTP11 | Metal tolerance protein | Mn2+ | Promotes vacuolar Cd sequestration in leaf vasculature, limiting transfer to grains | [106] |
| Type of Strategy | Main Mechanisms and Advantages | Limitations | Representative Measures/Progress | References |
|---|---|---|---|---|
| Agricultural management | Regulating soil pH, organic matter, moisture, and nutrients to reduce Cd bioavailability. | Effect influenced by soil type and climate, requiring continuous input. | Lime/biochar/organic fertilizer, crop rotation, and irrigation management. | [17,28,122,123,124] |
| Varietal improvement | Breeding or genetically modifying low-Cd-accumulating varieties to block Cd entry into grains at the source. | Long breeding cycle with the need to balance adaptability and yield. | Molecular breeding with OsHMA3/OsNRAMP5 and QTL pyramiding. | [125,126,127] |
| Plant hormones/signaling molecules | Enhancing antioxidant capacity and regulating transporters to alleviate Cd toxicity. | Mostly short-term exogenous treatments with limited field application. | Exogenous melatonin, brassinosteroids, NO, etc. | [28,124,128] |
| Bioremediation | Adsorbing/immobilizing Cd to reduce its bioavailability in soil. | Long remediation cycle with limited economic and field feasibility. | Intercropping with hyperaccumulator plants and inoculation with Cd-adsorbing bacteria. | [17,129,130,131,132] |
| Strategies | Treatment | Results | References |
|---|---|---|---|
| Soil amendment | |||
| Organic amendments | Biochar and vermicompost (5 t/hm2 biochar + 5 t/hm2 vermicompost) | Cd (plant concentration) was significantly reduced by 72% | [56] |
| Biochar (adsorption) | Cd2+ adsorption capacity was maximum at 244.43 mg/g | [133] | |
| Biochar (4%) | Cd (soil solution) was significantly reduced by 67% | [134] | |
| Inorganic amendments | Moisture and lime (2% lime) | Suppress bioavailability and toxicity of soil Cd | [135] |
| zeolite@cellulose-poly (acrylamide) hydrogel (2.5 w/w) | The bioavailable and total Cd concentrations were reduced by 59.38% and 1.75%, respectively | [136] | |
| FeMg-LDH/Bentonite to compost (3:7, 1:1, 7:3) | Reduce soil cadmium bioavailability and plant cadmium concentration. | [137] | |
| Lime (CdL5, CdL15 and CdL20) | Reduced soil available Cd by 19.2–29.4% and shoot Cd by 29.3–36.3% | [138] | |
| Scientific fertilization practices | |||
| Compound fertilizer | Compound fertilizer (600 kg/ha) | Cd (grain Cd content) decreased by 26.46–56.53% | [139] |
| Phosphate | Phosphate (0, 0.05 and 0.5 mmol/L) | Boost antioxidant defense and reduce Cd toxicity in soybeans | [140] |
| Na2SO4 | Na2SO4 (2.64 and 5.28 mM) | Cd (grain Cd content) decreased by 23.5% and 39.5%, respectively | [141] |
| Selenium fertilizer | Soil Se content (0.25, 0.375, 0.50, 0.75 and 1.00 mg·kg−1) | Cd (grain Cd content) was reduced by 48.4%~82.89% | [142] |
| silicon and nitric oxide | Si and NO (100 μM Sodium nitroprusside + 3293.3 kg/ha K2SiO3) | Cd (grain Cd content) decreased by 66% | [143] |
| Water management techniques | |||
| Intermittent irrigation | Three-day flooding and five-day drainage | Significant reduction in grain Cd content. | [32] |
| Long-term flooding | A 3–5 cm deep water layer maintained on the soil surface until rice harvest | Cd content in rice husk and grains was significantly reduced by 90.2% | [144] |
| Crop rotation and fallowing | |||
| Rotation | An oilseed rape-rice rotation | In the second year, the minimum grain Cd concentrations of the two varieties were 0.10 and 0.11 mg kg−1, respectively | [145] |
| A rice–chicory rotation | Effectively reduce cadmium accumulation in subsequent rice crops | [146] | |
| Foliar ameliorant | |||
| Foliar iron fertilization | Foliar iron fertilization (20, 50 and 100 mg/L) | The 50 mg/L treatment reduced grain Cd concentration by 29.0% | [147] |
| Foliar application fulvic acid | fulvic acid (0.5 g/L) | Significantly alleviated Cd-induced toxicity symptoms in lettuce | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wu, B.; Zhou, L.; Liu, K.; You, A.; Zha, W. Cadmium Contamination in Asian Rice (Oryza sativa L.): Mechanistic Insights from Soil Sources to Grain Accumulation and Mitigation Strategies. Plants 2025, 14, 2844. https://doi.org/10.3390/plants14182844
Wang J, Wu B, Zhou L, Liu K, You A, Zha W. Cadmium Contamination in Asian Rice (Oryza sativa L.): Mechanistic Insights from Soil Sources to Grain Accumulation and Mitigation Strategies. Plants. 2025; 14(18):2844. https://doi.org/10.3390/plants14182844
Chicago/Turabian StyleWang, Jing, Bian Wu, Lei Zhou, Kai Liu, Aiqing You, and Wenjun Zha. 2025. "Cadmium Contamination in Asian Rice (Oryza sativa L.): Mechanistic Insights from Soil Sources to Grain Accumulation and Mitigation Strategies" Plants 14, no. 18: 2844. https://doi.org/10.3390/plants14182844
APA StyleWang, J., Wu, B., Zhou, L., Liu, K., You, A., & Zha, W. (2025). Cadmium Contamination in Asian Rice (Oryza sativa L.): Mechanistic Insights from Soil Sources to Grain Accumulation and Mitigation Strategies. Plants, 14(18), 2844. https://doi.org/10.3390/plants14182844






