High Basal Expression and Dual Stress Responsiveness of Soybean (Glycine max) Resistance Gene SRC4
Abstract
1. Introduction
2. Results
2.1. Expression Pattern of SRC4
2.2. Transcriptional Regulatory Analysis of SRC4
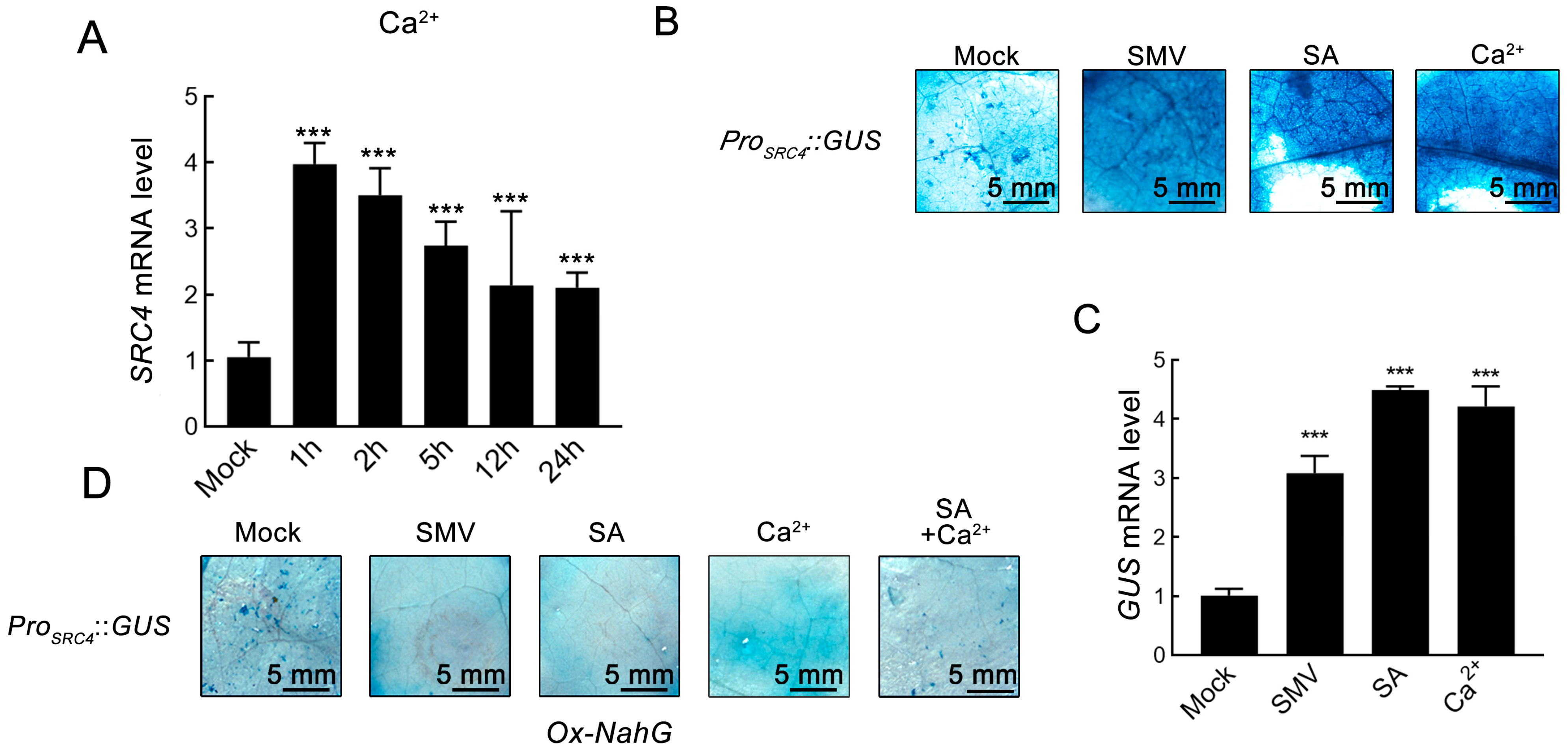
2.3. Ca2+ Signal-Dependent Regulatory Mechanism of SRC4 Expression
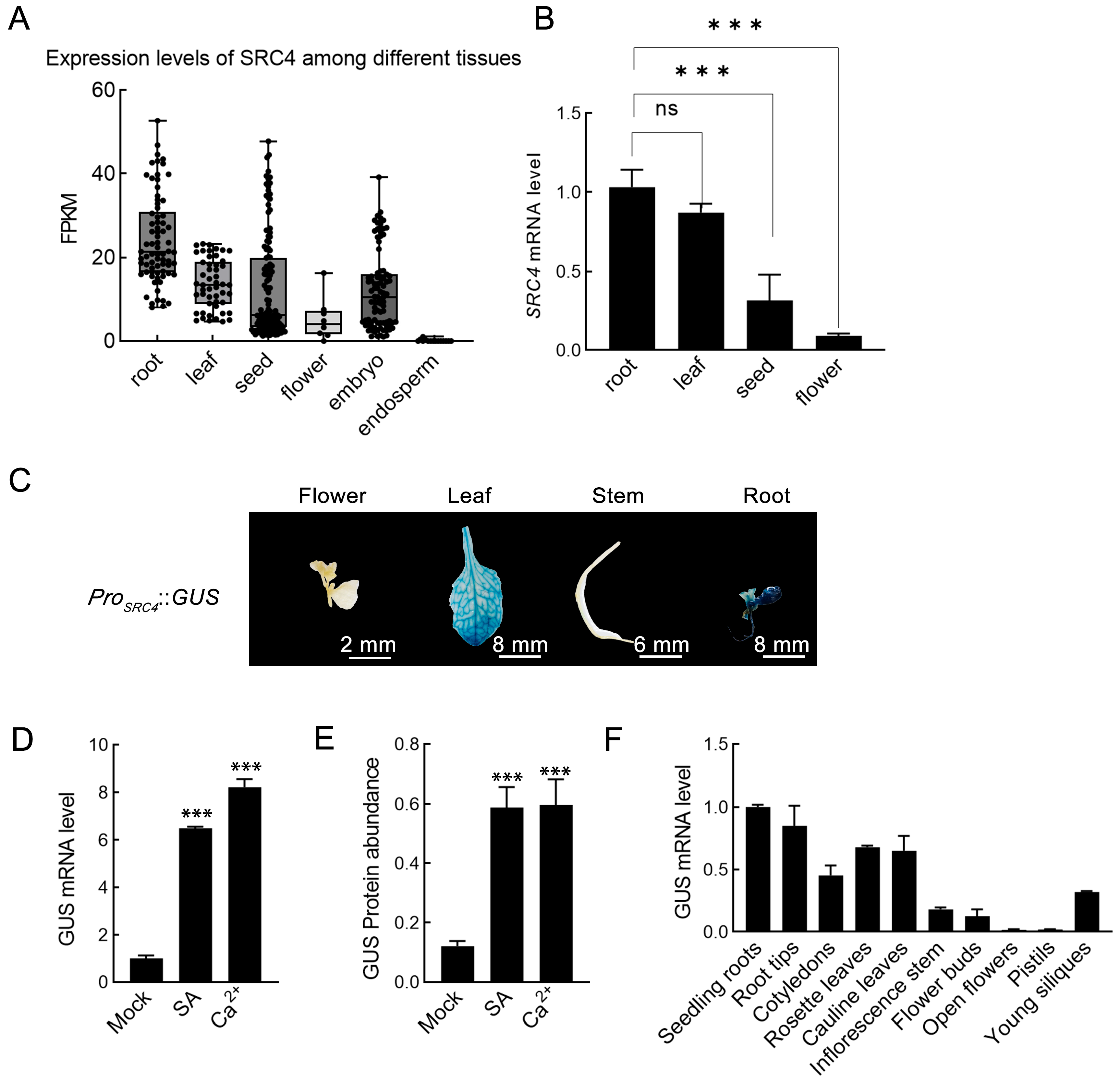
2.4. Tissue-Specific Expression Pattern of SRC4
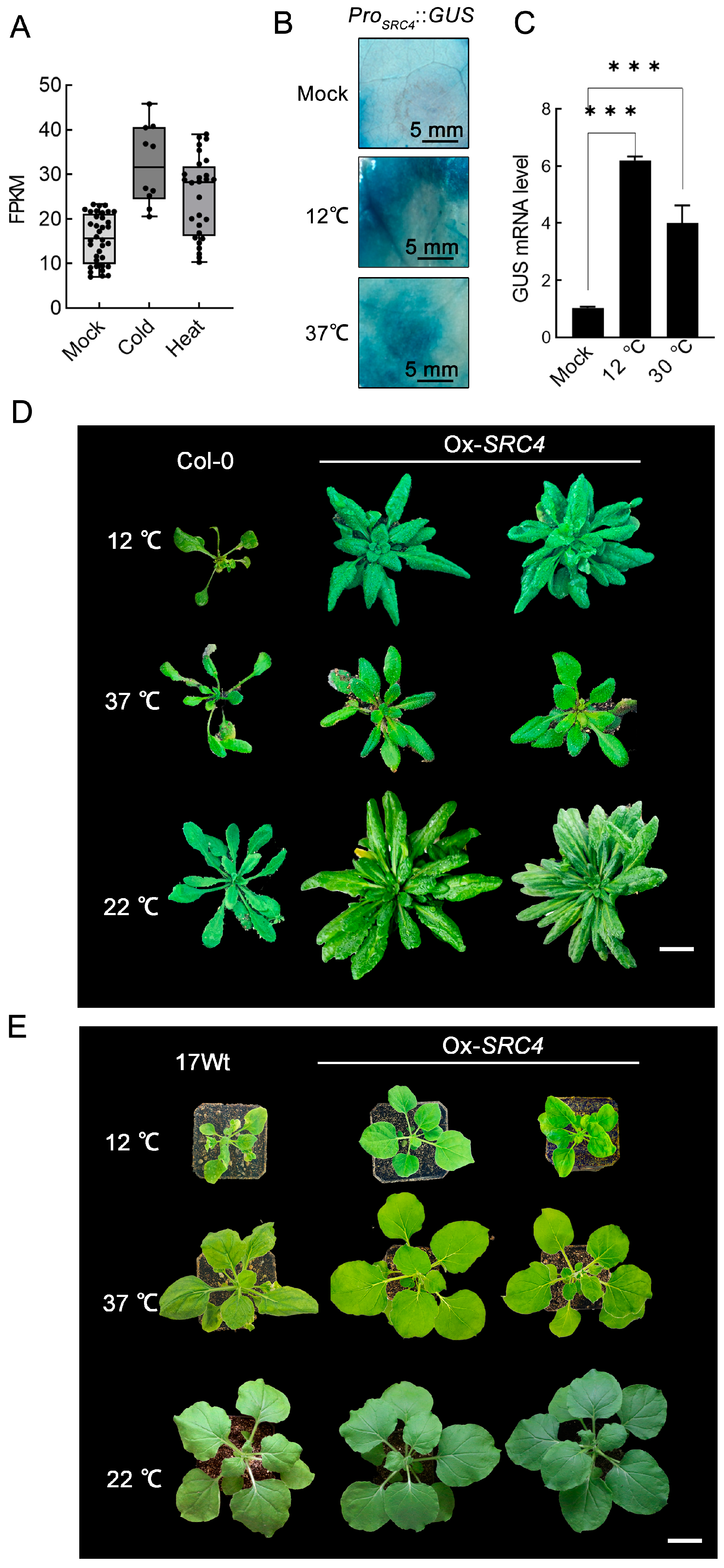
2.5. Functional Analysis of SRC4 Response to Abiotic Stress
3. Discussion
3.1. Limitations
3.2. Future Directions
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Transcriptome Data Analysis
4.3. Promoter Analysis and Vector Construction
4.4. Plant Transformation and Transgenic Line Generation
4.5. Gene Expression Analysis
4.6. GUS Activity Analysis
4.7. Stress Treatment Experiments
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Zhao, J.; Yang, H.; Weng, Q.; Zhu, C.; Liu, H.; Lu, X.; Jin, X.; Xu, M.; Huang, L.; Zhang, H.; et al. Current Status and Prospects of Wheat Stripe Rust Research. J. Integr. Agric. 2022, 21, 3706–3721. [Google Scholar]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Karmakar, S.; Molla, K.A.; Chanda, P.K.; Sarkar, S.N.; Datta, S.K.; Datta, K. Green Super Rice for Saltwater Agriculture. Nat. Biotechnol. 2024, 42, 59–66. [Google Scholar]
- Singh, R.P.; Hodson, D.P.; Huerta-Espino, J.; Jin, Y.; Bhavani, S.; Njau, P.; Herrera-Foessel, S.; Singh, P.K.; Singh, S.; Govindan, V. The Emergence of Ug99 Races of the Stem Rust Fungus Is a Threat to World Wheat Production. Annu. Rev. Phytopathol. 2011, 49, 465–481. [Google Scholar] [CrossRef]
- Pandit, M.K.; Pocock, M.J.; Kunin, W.E. Ploidy Influences Rarity and Invasiveness in Plants. J. Ecol. 2011, 99, 1108–1115. [Google Scholar] [CrossRef]
- Li, J.; Besseau, S.; Törönen, P.; Sipari, N.; Kollist, H.; Holm, L.; Palva, E.T. Defense-Related Transcription Factors WRKY70 and WRKY54 Modulate Osmotic Stress Tolerance by Regulating Stomatal Aperture in Arabidopsis. New Phytol. 2013, 200, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Zhang, Y. Plant Immunity: Danger Perception and Signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef]
- McHale, L.; Tan, X.; Koehl, P.; Michelmore, R.W. Plant NBS-LRR Proteins: Adaptable Guards. Genome Biol. 2006, 7, 212. [Google Scholar] [CrossRef]
- Wang, W.; Feng, B.; Zhou, J.M.; Tang, D. Plant Immune Signaling: Advancing on Two Frontiers. J. Integr. Plant Biol. 2020, 62, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Marone, D.; Russo, M.A.; Laidò, G.; De Leonardis, A.M.; Mastrangelo, A.M. Plant Nucleotide Binding Site-Leucine-Rich Repeat (NBS-LRR) Genes: Active Guardians in Host Defense Responses. Int. J. Mol. Sci. 2013, 14, 7302–7326. [Google Scholar] [PubMed]
- Cao, Y.; Mo, W.; Li, Y.; Xiong, Y.; Wang, H.; Zhang, Y.; Lin, M.; Zhang, L.; Li, X. Functional Characterization of NBS-LRR Genes Reveals an NBS-LRR Gene That Mediates Resistance against Fusarium Wilt. BMC Biol. 2024, 22, 59. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; von Dahl, C.C.; Preston, C.A. Volatile Signaling in Plant-Plant Interactions: “Talking Trees” in the Genomics Era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef]
- MacQueen, A.; Bergelson, J. Modulation of R-Gene Expression across Environments. J. Exp. Bot. 2016, 67, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.C.; Kozik, A.; Griego, A.; Kuang, H.; Michelmore, R.W. Genome-Wide Analysis of NBS-LRR-Encoding Genes in Arabidopsis. Plant Cell 2003, 15, 809–834. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.R.; Godiard, L.; Straube, E.; Ashfield, T.; Lewald, J.; Sattler, A.; Innes, R.W.; Dangl, J.L. Structure of the Arabidopsis RPM1 Gene Enabling Dual Specificity Disease Resistance. Science 1995, 269, 843–846. [Google Scholar] [CrossRef]
- Heil, M.; Baldwin, I.T. Fitness Costs of Induced Resistance: Emerging Experimental Support for a Slippery Concept. Trends Plant Sci. 2002, 7, 61–67. [Google Scholar] [CrossRef]
- Rate, D.N.; Cuenca, J.V.; Bowman, G.R.; Guttman, D.S.; Greenberg, J.T. The Gain-of-Function Arabidopsis acd6 Mutant Reveals Novel Regulation and Function of the Salicylic Acid Signaling Pathway in Controlling Cell Death, Defenses, and Cell Growth. Plant Cell 1999, 11, 1695–1708. [Google Scholar] [CrossRef]
- Stokes, T.L.; Kunkel, B.N.; Richards, E.J. Epigenetic Variation in Arabidopsis Disease Resistance. Genes Dev. 2002, 16, 171–182. [Google Scholar] [CrossRef]
- Oldroyd, G.E.; Staskawicz, B.J. Genetically Engineered Broad-Spectrum Disease Resistance in Tomato. Proc. Natl. Acad. Sci. USA 1998, 95, 10300–10305. [Google Scholar]
- Tian, D.; Traw, M.B.; Chen, J.Q.; Kreitman, M.; Bergelson, J. Fitness Costs of R-Gene-Mediated Resistance in Arabidopsis thaliana. Nature 2003, 423, 74–77. [Google Scholar] [CrossRef]
- Mauch, F.; Mauch-Mani, B.; Gaille, C.; Kull, B.; Haas, D.; Reimmann, C. Manipulation of Salicylate Content in Arabidopsis thaliana by the Expression of an Engineered Bacterial Salicylate Synthase. Plant J. 2001, 25, 67–77. [Google Scholar] [CrossRef]
- Heil, M. Ecological Costs of Induced Resistance. Curr. Opin. Plant Biol. 2002, 5, 345–350. [Google Scholar] [CrossRef]
- Singh, K.; Foley, R.C.; Oñate-Sánchez, L. Transcription Factors in Plant Defense and Stress Responses. Curr. Opin. Plant Biol. 2002, 5, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Somssich, I.E. Networks of WRKY Transcription Factors in Defense Signaling. Curr. Opin. Plant Biol. 2007, 10, 366–371. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Ramírez, V.; García-Andrade, J.; Flors, V.; Vera, P. The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity. PLoS Genet. 2011, 7, e1002434. [Google Scholar] [CrossRef] [PubMed]
- Dowen, R.H.; Pelizzola, M.; Schmitz, R.J.; Lister, R.; Dowen, J.M.; Nery, J.R.; Dixon, J.E.; Ecker, J.R. Widespread Dynamic DNA Methylation in Response to Biotic Stress. Proc. Natl. Acad. Sci. USA 2012, 109, E2183–E2191. [Google Scholar] [CrossRef]
- Du, L.; Ali, G.S.; Simons, K.A.; Hou, J.; Yang, T.; Reddy, A.S.N.; Poovaiah, B.W. Ca2+/Calmodulin Regulates Salicylic-Acid-Mediated Plant Immunity. Nature 2009, 457, 1154–1158. [Google Scholar] [CrossRef]
- Spoel, S.H.; Dong, X. Salicylic Acid in Plant Immunity and Beyond. Plant Cell 2012, 24, 2022–2038. [Google Scholar]
- Spoel, S.H.; Dong, X. How Do Plants Achieve Immunity? Defence Mechanisms. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar]
- Wang, L.; Tsuda, K.; Truman, W.; Sato, M.; Nguyen, L.V.; Katagiri, F.; Glazebrook, J. CBP60g and SARD1 Play Partially Redundant Critical Roles in Salicylic Acid Signaling. Plant J. 2011, 67, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Busta, L.; Zhang, Q.; Ding, P.; Jetter, R.; Zhang, Y. TGACG-BINDING FACTOR 1 (TGA1) and TGA4 Regulate Salicylic Acid and Pipecolic Acid Biosynthesis by Modulating the Expression of SYSTEMIC ACQUIRED RESISTANCE DEFICIENT 1 (SARD1) and CALMODULIN-BINDING PROTEIN 60g (CBP60g). New Phytol. 2018, 217, 344–354. [Google Scholar] [CrossRef]
- DeFalco, T.A.; Zipfel, C. Molecular Mechanisms of Early Plant Pattern-Triggered Immune Signaling. Mol. Cell 2021, 81, 3449–3467. [Google Scholar] [CrossRef]
- Tian, W.; Hou, C.; Ren, Z.; Pan, Y.; Jia, J.; Zhang, H.; Bai, F.; Zhang, P.; Zhu, H.; He, Y.; et al. A Molecular Pathway for CO2 Response in Arabidopsis Guard Cells. Nat. Commun. 2015, 6, 6057. [Google Scholar] [CrossRef]
- Wang, L.; Tsuda, K.; Sato, M.; Cohen, J.D.; Katagiri, F.; Glazebrook, J. Arabidopsis CaM Binding Protein CBP60g Contributes to MAMP-Induced SA Accumulation and Is Involved in Disease Resistance against Pseudomonas syringae. PLoS Pathog. 2009, 5, e1000301. [Google Scholar] [CrossRef]
- Roychowdhury, M.; Liang, C.; Bhadra, T.; Ayaz, A.; Edgerton, M.D.; Gurley, W.B.; Kotak, S. HSF1 and HSF2 Spatially and Temporally Regulate Anthranilate Biosynthesis and ER Body Formation in Arabidopsis. J. Exp. Bot. 2013, 64, 7047–7061. [Google Scholar]
- O’Malley, R.C.; Huang, S.C.; Song, L.; Lewsey, M.G.; Bartlett, A.; Nery, J.R.; Galli, M.; Gallavotti, A.; Ecker, J.R. Cistrome and Epicistrome Features Shape the Regulatory DNA Landscape. Cell 2016, 165, 1280–1292. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.; Gilmour, S.J.; Thomashow, M.F. Roles of CAMTA Transcription Factors and Salicylic Acid in Configuring the Low-Temperature Transcriptome and Freezing Tolerance of Arabidopsis. Plant J. 2013, 75, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Senthil-Kumar, M. Drought Stress Promotes Plant-Pathogen Interactions. Plant Signal. Behav. 2017, 12, e1082719. [Google Scholar]
- Köster, P.; DeFalco, T.A.; Zipfel, C. Ca2+ Signals in Plant Immunity. EMBO J. 2022, 41, e110741. [Google Scholar] [CrossRef]
- Jacob, P.; Kim, N.H.; Wu, F.; El-Kasmi, F.; Chi, Y.; Walton, W.G.; Furzer, O.J.; Lietzan, A.D.; Sunil, S.; Kempthorn, K.; et al. Plant “Helper” Immune Receptors Are Ca2+-Permeable Nonselective Cation Channels. Science 2021, 373, 420–425. [Google Scholar] [CrossRef]
- Chen, J.; Li, M.; Liu, L.; Chen, G.; Fu, Z.Q. ZAR1 Resistosome and Helper NLRs: Bringing in Calcium and Inducing Cell Death. Mol. Plant 2021, 14, 1234–1236. [Google Scholar] [CrossRef]
- Bi, G.; Su, M.; Li, N.; Liang, Y.; Dang, S.; Xu, J.; Hu, M.; Wang, J.; Zou, M.; Deng, Y.; et al. The ZAR1 Resistosome Is a Calcium-Permeable Channel Triggering Plant Immune Signaling. Cell 2021, 184, 3528–3541.e12. [Google Scholar] [CrossRef]
- Li, C.; Chen, M.; He, X.; Ouyang, D. A Mini-Review on Ion Fluxes That Regulate NLRP3 Inflammasome Activation. Acta Biochim. Biophys. Sin. 2021, 53, 131–139. [Google Scholar] [CrossRef]
- Saijo, Y.; Loo, E.P. Plant Immunity in Signal Integration between Biotic and Abiotic Stress Responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef]
- Huot, B.; Castroverde, C.D.M.; Velásquez, A.C.; Hubbard, E.; Pulman, J.A.; Yao, J.; Childs, K.L.; Tsuda, K.; Montgomery, B.L.; He, S.Y. Dual Impact of Elevated Temperature on Plant Defence and Bacterial Virulence in Arabidopsis. Nat. Commun. 2017, 8, 1808. [Google Scholar] [CrossRef]
- Cheng, C.; Yun, K.Y.; Ressom, H.W.; Mohanty, B.; Bajic, V.B.; Jia, Y.; Yun, S.J.; de los Reyes, B.G. An Early Response Regulatory Cluster Induced by Low Temperature and Hydrogen Peroxide in Seedlings of Chilling-Tolerant Japonica Rice. BMC Genom. 2007, 8, 175. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature Increase Reduces Global Yields of Major Crops in Four Independent Estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [PubMed]
- Dong, Q.; Wallrad, L.; Almutairi, B.O.; Kudla, J. Ca2+ Signaling in Plant Responses to Abiotic Stresses. J. Integr. Plant Biol. 2022, 64, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Noel, K.; Wolf, I.R.; Hughes, D.; Valente, G.T.; Qi, A.; Huang, Y.J.; Fitt, B.D.L.; Stotz, H.U. Transcriptomics of Temperature-Sensitive R Gene-Mediated Resistance Identifies a WAKL10 Protein Interaction Network. Sci. Rep. 2024, 14, 5023. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.S.V.; Prasad, P.V.V.; Siddique, K.H.M. Thermal Stress Impacts Reproductive Development and Grain Yield in Rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef]
- Zhang, G.F.; Wang, S.H.; You, J.; Zhang, Y.X.; Wang, Q.S.; Ding, Y.F. Effects of Relatively High Temperature at Grain-Filling Stage on Rice Grain’s Starch Viscosity Profile and Magnesium and Potassium Contents. Ying Yong Sheng Tai Xue Bao 2008, 19, 1959–1964. [Google Scholar]
- Yan, T.; Zhou, Z.; Wang, R.; Bao, D.; Li, S.; Li, A.; Yu, R.; Wuriyanghan, H. A Cluster of Atypical in Soybean Confers Broad-Spectrum Antiviral Activity. Plant Physiol. 2022, 188, 1277–1293. [Google Scholar] [CrossRef]
- Liu, G.; Fang, Y.; Liu, X.; Jiang, J.; Ding, G.; Wang, Y.; Zhao, X.; Xu, X.; Liu, M.; Wang, Y.; et al. Genome-Wide Association Study and Haplotype Analysis Reveal Novel Candidate Genes for Resistance to Powdery Mildew in Soybean. Front. Plant Sci. 2024, 15, 1369650. [Google Scholar] [CrossRef]
- Galaud, J.P.; Genin, S.; Aldon, D. Pathogen Effectors Hijack Calcium Signaling to Promote Virulence. Trends Plant Sci. 2024, 29, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Nelson, R.S. The Cell Biology of Tobacco Mosaic Virus Replication and Movement. Front. Plant Sci. 2013, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Perraki, A.; Gronnier, J.; Gouguet, P.; Boudsocq, M.; Deroubaix, A.F.; Simon, V.; German-Retana, S.; Legrand, A.; Habenstein, B.; Zipfel, C.; et al. REM1.3’s Phospho-Status Defines Its Plasma Membrane Nanodomain Organization and Activity in Restricting PVX Cell-to-Cell Movement. PLoS Pathog. 2018, 14, e1007378. [Google Scholar] [CrossRef]
- Takken, F.L.; Goverse, A. How to Build a Pathogen Detector: Structural Basis of NB-LRR Function. Curr. Opin. Plant Biol. 2012, 15, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Eulgem, T. Transcript-level Expression Control of Plant NLR Genes. Mol. Plant Pathol. 2018, 19, 1267–1281. [Google Scholar] [CrossRef]
- Kudla, J.; Batistič, O.; Hashimoto, K. Calcium Signals: The Lead Currency of Plant Information Processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef]
- Deng, Y.; Zhai, K.; Xie, Z.; Yang, D.; Zhu, X.; Liu, J.; Wang, X.; Qin, P.; Yang, Y.; Zhang, G.; et al. Epigenetic Regulation of Antagonistic Receptors Confers Rice Blast Resistance with Yield Balance. Science 2017, 355, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Pallas, V.; García, J.A. How Do Plant Viruses Induce Disease? Interactions and Interference with Host Components. J. Gen. Virol. 2011, 92, 2691–2705. [Google Scholar] [CrossRef]
- Karasov, T.L.; Kniskern, J.M.; Gao, L.; DeYoung, B.J.; Ding, J.; Dubiella, U.; Lastra, R.O.; Nallu, S.; Roux, F.; Innes, R.W.; et al. The Long-Term Maintenance of a Resistance Polymorphism through Diffuse Interactions. Nature 2014, 512, 436–440. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.; Langenbach, C.J.; Jaskiewicz, M.R. Priming for Enhanced Defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Zhang, J.; Du, X.; Wang, Q.; Chen, X.; Lv, D.; Xu, K.; Qu, S.; Zhang, Z. Expression of Pathogenesis Related Genes in Response to Salicylic Acid, Methyl Jasmonate and 1-Aminocyclopropane-1-Carboxylic Acid in Malus hupehensis (Pamp.) Rehd. BMC Res. Notes 2010, 3, 208. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Naidoo, S.; van den Berg, N. The NONEXPRESSOR OF PATHOGENESIS-RELATED GENES 1 (NPR1) and Related Family: Mechanistic Insights in Plant Disease Resistance. Front. Plant Sci. 2019, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Yin, D.; Shi, Y.; Zhou, Z.; Li, C.; Liu, P.; Jia, Y.; Wang, Y.; Liu, Z.; Yu, M.; et al. OsNPR3.3-Dependent Salicylic Acid Signaling Is Involved in Recessive Gene Xa5-Mediated Immunity to Rice Bacterial Blight. Sci. Rep. 2020, 10, 6313. [Google Scholar] [CrossRef]
- Vicente, M.R.S.; Plasencia, J. Salicylic Acid beyond Defence: Its Role in Plant Growth and Development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Shao, H.; Zheng, A.; Zhao, L.; Xu, Y. Advances in Roles of Salicylic Acid in Plant Tolerance Responses to Biotic and Abiotic Stresses. Plants 2023, 12, 3475. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, F.; Zhu, S.; Li, X. The Maize NBS-LRR Gene ZmNBS25 Enhances Disease Resistance in Rice and Arabidopsis. Front. Plant Sci. 2018, 9, 1033. [Google Scholar] [CrossRef]
- Braam, J. Regulated Expression of the Calmodulin-Related TCH Genes in Cultured Arabidopsis Cells: Induction by Calcium and Heat Shock. Proc. Natl. Acad. Sci. USA 1992, 89, 3213–3216. [Google Scholar] [CrossRef]
- Seyfferth, C.; Tsuda, K. Salicylic Acid Signal Transduction: The Initiation of Biosynthesis, Perception and Transcriptional Reprogramming. Front. Plant Sci. 2014, 5, 697. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Ding, P.; Wang, D.; Cheng, Y.T.; He, J.; Gao, M.; Xu, F.; Li, Y.; Zhu, Z.; et al. Control of Salicylic Acid Synthesis and Systemic Acquired Resistance by Two Members of a Plant-Specific Family of Transcription Factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18220–18225. [Google Scholar] [CrossRef]
- Johnson, C.; Boden, E.; Arias, J. Salicylic Acid and NPR1 Induce the Recruitment of Trans-Activating TGA Factors to a Defense Gene Promoter in Arabidopsis. Plant Cell 2003, 15, 1846–1858. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Garcia, C.M.; Finer, J.J. Identification and Validation of Promoters and Cis-Acting Regulatory Elements. Plant Sci. 2014, 217–218, 109–119. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [PubMed]
- Choi, W.G.; Toyota, M.; Kim, S.H.; Hilleary, R.; Gilroy, S. Salt Stress-Induced Ca2+ Waves Are Associated with Rapid, Long-Distance Root-to-Shoot Signaling in Plants. Proc. Natl. Acad. Sci. USA 2014, 111, 6497–6502. [Google Scholar] [CrossRef]
- Park, C.J.; Shin, R. Calcium Channels and Transporters: Roles in Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2022, 13, 964059. [Google Scholar] [CrossRef] [PubMed]
- Ranty, B.; Aldon, D.; Cotelle, V.; Galaud, J.P.; Thuleau, P.; Mazars, C. Calcium Sensors as Key Hubs in Plant Responses to Biotic and Abiotic Stresses. Front. Plant Sci. 2016, 7, 327. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and Biotic Stress Combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The Interaction of Plant Biotic and Abiotic Stresses: From Genes to the Field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef]
- Kamiya, T.; Oña, L.; Wertheim, B.; van Doorn, G.S. Coevolutionary Feedback Elevates Constitutive Immune Defence: A Protein Network Model. BMC Ecol. Evol. 2016, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene Networks Involved in Drought Stress Response and Tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, S.J.; Park, Y.J.; Kim, S.Y.; Ryu, C.M. Crossing the Kingdom Border: Human Diseases Caused by Plant Pathogens. Environ. Microbiol. 2013, 15, 2485–2495. [Google Scholar] [CrossRef]
- Torabi, S.; Seifi, S.; Geddes-McAlister, J.; Tenuta, A.; Wally, O.; Torkamaneh, D.; Eskandari, M. Soybean–SCN Battle: Novel Insight into Soybean’s Defense Strategies against Heterodera glycines. Int. J. Mol. Sci. 2023, 24, 16232. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, G.; Cheng, C.; Lei, L.; Sun, J.; Xu, Y.; Deng, K.; Han, L.; Ma, Q.; Wei, X.; et al. A Comprehensive Online Database for Exploring ∼20,000 Public Arabidopsis RNA-Seq Libraries. Mol. Plant 2020, 13, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Zeng, Y.; Shen, W.; Tuo, D.; Li, X.; Zhou, P. Nimble Cloning: A Simple, Versatile, and Efficient System for Standardized Molecular Cloning. Front. Bioeng. Biotechnol. 2019, 7, 460. [Google Scholar] [CrossRef]


| Element Position (bp) | Function Description | Element Position (bp) | Function Description |
|---|---|---|---|
| −1906~−1876 | Light-responsive element | −1144~−1114 | MYB binding site, involved in light response |
| −1905~−1875 | Abscisic acid-responsive element | −950~−920 | Light-responsive element |
| −1898~−1868 | Light-responsive element | −586~−556 | Light-responsive element |
| −1761~−1731 | Salicylic acid-responsive element | −537~−507 | MYB binding site, involved in light response |
| −1694~−1664 | Zein metabolism regulatory element | −457~−427 | Light-responsive element |
| −1645~−1615 | Conserved DNA module (CMA3) | −450~−420 | Light-responsive element |
| −1627~−1597 | Zein metabolism regulatory element | −237~−207 | Conserved DNA module, involved in light response |
| −1591~−1561 | Defense and stress-responsive element | −188~−158 | Defense and stress-responsive element |
| −1571~−1541 | Gibberellin-responsive element | −139~−109 | Light-responsive element |
| −1490~−1460 | Anaerobic induction essential element | −129~−99 | Inducible activation element |
| −1314~−1284 | Anaerobic induction essential element | −1906~−1876 | Light-responsive element |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Bao, Z.; Miao, D.; Zhou, Y.; Niu, N.; Wuriyanghan, H. High Basal Expression and Dual Stress Responsiveness of Soybean (Glycine max) Resistance Gene SRC4. Plants 2025, 14, 2820. https://doi.org/10.3390/plants14182820
Zhou Z, Bao Z, Miao D, Zhou Y, Niu N, Wuriyanghan H. High Basal Expression and Dual Stress Responsiveness of Soybean (Glycine max) Resistance Gene SRC4. Plants. 2025; 14(18):2820. https://doi.org/10.3390/plants14182820
Chicago/Turabian StyleZhou, Zikai, Zhuo Bao, Di Miao, Yuxi Zhou, Niu Niu, and Hada Wuriyanghan. 2025. "High Basal Expression and Dual Stress Responsiveness of Soybean (Glycine max) Resistance Gene SRC4" Plants 14, no. 18: 2820. https://doi.org/10.3390/plants14182820
APA StyleZhou, Z., Bao, Z., Miao, D., Zhou, Y., Niu, N., & Wuriyanghan, H. (2025). High Basal Expression and Dual Stress Responsiveness of Soybean (Glycine max) Resistance Gene SRC4. Plants, 14(18), 2820. https://doi.org/10.3390/plants14182820






