Effect of Electrical Conductivity of Nutrient Solution and Light Spectra on the Main Phytochemical Content of Sonchus tenerrimus L. Under Wild and Controlled Environments
Abstract
1. Introduction
2. Results
2.1. Effect of Salinity and Light on Growth Parameters in Cultivated S. tenerrimus
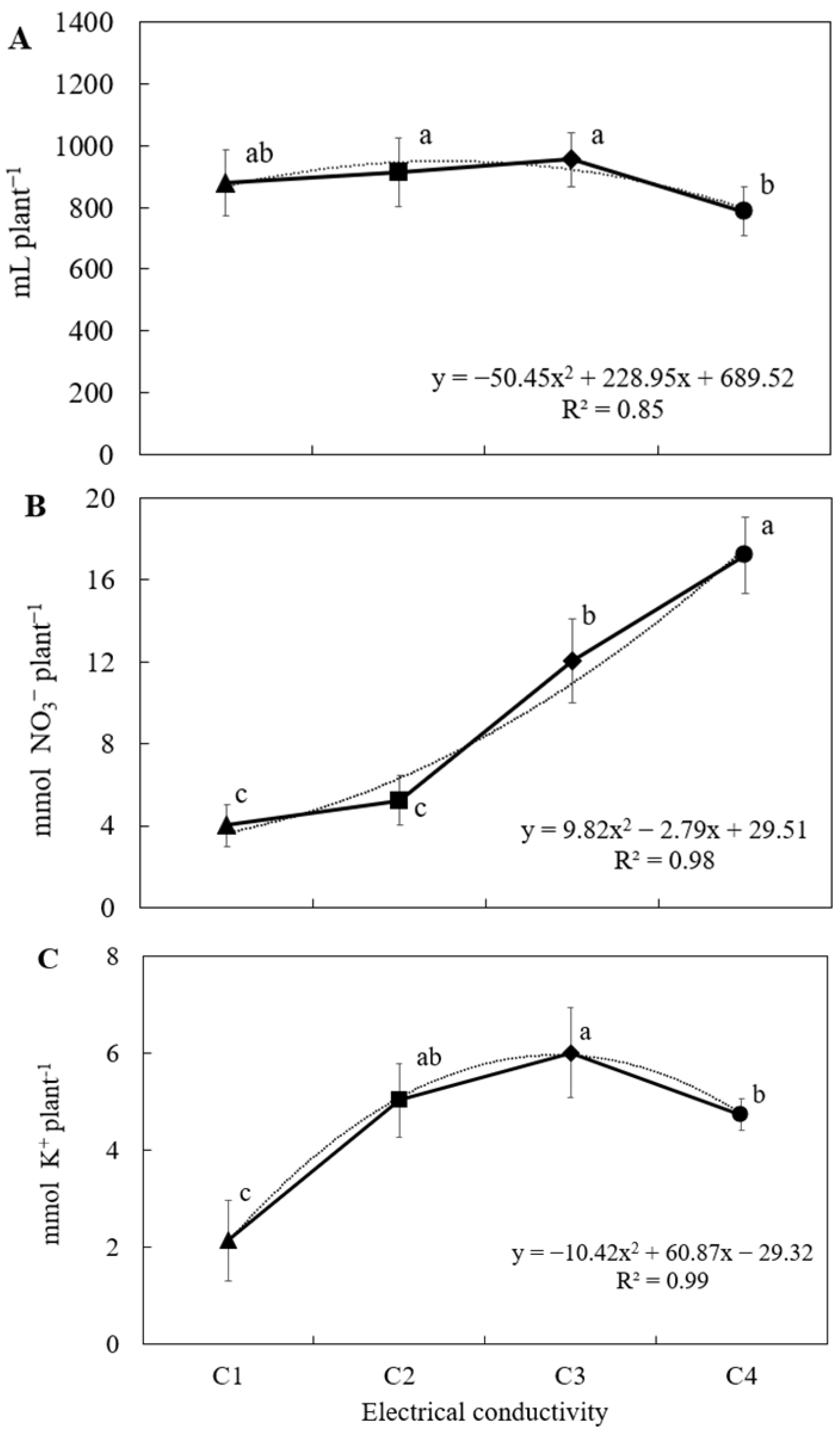
2.1.1. Fertigation Parameters
2.1.2. Fertigation Uptake and Growth Parameters
2.2. Phytochemical Characterization
2.2.1. Moisture
2.2.2. Total Carotenoids
2.2.3. Vitamin C
2.2.4. Total Phenols and Flavonoids
2.2.5. Antioxidant Activity
2.3. Principal Component Analysis (PCA)
3. Discussion
3.1. Effect of Salinity and Light on Growth Parameters in Cultivated S. tenerrimus
3.1.1. Fertigation Parameters
3.1.2. Fertigation Uptake and Growth Parameters
3.2. Total Carotenoids
3.3. Vitamin C
3.4. Total Phenols and Flavonoids
3.5. Antioxidant Activity
3.6. Principal Component Analysis
4. Materials and Methods
4.1. Solvents and Reagents
4.2. Samples and Growth Conditions
4.3. Total Carotenoids
4.4. Extraction and Quantification of Vitamin C
4.5. Extraction of Phenolic Compounds
4.6. Determination of the Total Phenolic Content
4.7. Determination of Total Flavonoid Content
4.8. Determination of the Antioxidant Activity
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ge, X.J.; Lin, Y.R. Flora of China; Science Press: Beijing, China; MBG Press: St. Louis, MI, USA, 1999; pp. 60–126. [Google Scholar]
- Afolayan, A.J.; Jimoh, F.O. Nutritional quality of some wild leafy vegetables in South Africa. Int. J. Food Sci. Nutr. 2009, 60, 424–431. [Google Scholar] [CrossRef]
- Yang, S.L.; Liu, X.K.; Wu, H.; Wang, H.B.; Qing, C. Steroidal saponins and cytotoxicity of the wild edible vegetable-Smilacina atropurpurea. Steroids 2009, 74, 7–12. [Google Scholar] [CrossRef]
- Cambiea, R.C.; Ferguson, L.R. Potential functional foods in the traditional Maori diet. Mutat. Res.-Fundam. Mol. 2003, 523–524, 109–117. [Google Scholar] [CrossRef]
- Cardoso, F.V.; Soncini, R.; Giusti-Paiva, A. Anxiolytic-Like Effect of Sonchus oleraceus L. in Mice. J. Ethnopharmacol. 2009, 124, 325–327. [Google Scholar] [CrossRef] [PubMed]
- El, S.N.; Karakaya, S. Radical scavenging and iron-chelating activities of some greens used as traditional dishes in Mediterranean diet. Int. J. Food Sci. Nutr. 2009, 55, 67–74. [Google Scholar] [CrossRef]
- Guil Guerrero, J.L.; Giménez Martıínez, J.J.; Torija Isasa, M.E. Mineral nutrient composition of edible wild plants. J. Food Compos. Anal. 1998, 11, 322–328. [Google Scholar] [CrossRef]
- Giner, R.M.; Ubeda, A.; Just, M.J.; Serrano, A.; Manez, S.; Rios, J. A chemotaxonomic survey of Sonchus subgenus Sonchus. Biochem. Syst. Ecol. 1993, 21, 617–620. [Google Scholar] [CrossRef]
- Botella, M.Á.; Hellín, P.; Hernández, V.; Dabauza, M.; Robledo, A.; Sánchez, A.; Fenoll, J.; Flores, P. Chemical composition of wild collected and cultivated edible plants (Sonchus oleraceus L. and Sonchus tenerrimus L.). Plants 2024, 13, 269. [Google Scholar] [CrossRef]
- Ferrón-Carrillo, F.; Guil-Guerrero, J.L.; González-Fernández, M.J.; Lyashenko, S.; Battafarano, F.; Cunha-Chiamolera, T.P.L.; Urrestarazu, M. LED enhances plant performance and both carotenoids and nitrates profiles in lettuce. Plant Foods Hum. Nutr. 2021, 76, 210–218. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.A.; Cunha-Chiamolera, T.P.L.; Chileh-Chelh, T.; Carmona-Fernández, M.; Urrestarazu, M.; Guil-Guerrero, J.L. Growth parameters, phytochemicals, and antitumor activity of wild and cultivated ice plants (Mesembryanthemum crystallinum L.). Food Sci. Nutr. 2024, 12, 6548–6562. [Google Scholar] [CrossRef]
- Cunha-Chiamolera, T.P.L.; Chileh-Chelh, T.; Urrestarazu, M.; Ezzaitouni, M.; López-Ruiz, R.; Gallón-Bedoya, M.; Rincón-Cervera, M.Á.; Guil-Guerrero, J.L. Crop productivity, phytochemicals, and bioactivities of wild and grown in controlled environment slender amaranth (Amaranthus viridis L.). Agronomy 2024, 14, 2038. [Google Scholar] [CrossRef]
- Delian, E.; Chira, A.; Bădulescu, L.; Chira, L. Insights into microgreens physiology. Sci. Pap. Ser. B Hortic. 2015, 59, 447–454. [Google Scholar]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A Review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Ying, Q.; Kong, Y.; Jones-Baumgardt, C.; Zheng, Y. Responses of yield and appearance quality of four brassicaceae microgreens to varied blue light proportion in red and blue light-emitting diodes lighting. Sci. Hortic. 2020, 259, 108857. [Google Scholar] [CrossRef]
- Chileh-Chelh, T.; Cunha-Chiamolera, T.P.L.; Urrestarazu, M.; Ezzaitouni, M.; López-Ruiz, R.; Nájera, C.; Rincón-Cervera, M.Á.; Guil-Guerrero, J.L. London Rocket (Sisymbrium irio L.) as healthy green: Bioactive compounds and bioactivity of plants grown in wild and controlled environments. Molecules 2025, 30, 31. [Google Scholar] [CrossRef]
- El Gendy, A.E.N.G.; Mohamed, N.A.; Sarker, T.C.; Hassan, E.M.; Garaa, A.H.; Elshamy, A.I.; Abd-ElGawad, A.M. Chemical composition, antioxidant, and cytotoxic activity of essential oils in the above-ground parts of Sonchus oleraceus L. Plants 2024, 13, 1712. [Google Scholar] [CrossRef] [PubMed]
- Al-Massarani, S.M.; El Gamal, A.A.; Alam, P.; Al-Sheddi, E.S.; Al-Oqail, M.M.; Farshori, N.N. Isolation, biological evaluation and validated HPTLC-quantification of the marker constituent of the edible Saudi plant Sisymbrium irio L. Saudi Pharm. J. 2017, 25, 750–759. [Google Scholar] [CrossRef]
- Peçanha, D.A.; Peña, J.A.M.; Freitas, M.S.M.; Chourak, Y.; Urrestarazu, M. Effect of light spectra on stem cutting rooting and lavender growth. Acta Sci. Agron. 2023, 45, e58864. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Carrasco, G. Soilless Culture and Hydroponics; Mundi-Prensa: Madrid, Spain, 2023; ISBN 13: 9788484767664. [Google Scholar]
- Brendel, O. The relationship between plant growth and water consumption: A history from the classical four elements to modern stable isotopes. Ann. For. Sci. 2021, 78, 47. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W. Plant Nutrition of Greenhouse Crops; Springer: Dordrecht, The Netherland, 2009. [Google Scholar]
- Gallegos-Cedillo, V.M.; Urrestarazu, M.; Álvaro, J.E. Influence of salinity on transport of nitrates and potassium by means of the xylem sap content between roots and shoots in young tomato plants. J. Soil Sci. Plant Nutr. 2016, 16, 991–998. [Google Scholar] [CrossRef]
- Mass, E.V.; Hoffman, G.J. Crop salt tolerance-current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Arnon, D.I.; Johnson, C.M. Influence of hydrogen ion concentration on the growth of higher plants under controlled conditions. Plant Physiol. 1942, 17, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Truog, E. Lime in relation to availability of plant nutrients. Soil Sci. 1948, 65, 1–8. [Google Scholar] [CrossRef]
- Ferrón-Carrillo, F.; Cunha-Chiamolera, T.P.L.; Urrestarazu, M. Effect of ammonium nitrogen on pepper grown under soilless culture. J. Plant Nutr. 2022, 45, 113–122. [Google Scholar] [CrossRef]
- Adams, P. Nutrition of greenhouse vegetables in NFT and hydroponic systems. Acta Hortic. 1994, 361, 245–257. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the role of red:blue led lights on resource use efficiency and nutritional properties of indoor grown sweet Basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Spalholz, H.; Perkins-Veazie, P.; Hernández, R. Impact of sun-simulated white light and varied blue:red spectrums on the growth, morphology, development, and phytochemical content of green- and red-leaf lettuce at different growth stages. Sci. Hortic. 2020, 264, 109195. [Google Scholar] [CrossRef]
- Nájera, C.; Urrestarazu, M. Effect of the intensity and spectral quality of LED light on yield and nitrate accumulation in vegetables. HortScience 2019, 54, 1745–1750. [Google Scholar] [CrossRef]
- Monego, D.L.; da Rosa, M.B.; do Nascimento, P.C. Applications of computational chemistry to the study of the antiradical activity of carotenoids: A review. Food Chem. 2017, 217, 37–44. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E. Genetic and environmental factors affecting plant lutein/zeaxanthin. Agro Food Ind. Hi-Tech 2008, 19, 44–46. [Google Scholar]
- Kaczor, A.; Pacia, M.Z. Impact of stress factors on carotenoid composition, structures, and bioavailability in microbial sources. In Carotenoids: Nutrition, Analysis and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 241–260. [Google Scholar] [CrossRef]
- López-Berenguer, C.; Martínez-Ballesta, M.C.; García-Viguera, C.; Carvajal, M. Leaf water balance mediated by aquaporins under salt stress and associated glucosinolate synthesis in broccoli. Plant Sci. 2008, 174, 321–328. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves the quality of red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Vieira-Stefen, D.L.; Leolato, L.S.; Medeiros-Grindi, D.; Nerling, D. Revisiting carotenoids and their role in plant stress response: From biosynthesis to plant signaling mechanisms during stress. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 207–232. [Google Scholar]
- Zhang, H.; Tu, Y.; Kang, J.; Song, W.; Zheng, L. Blue light dosage affects photosynthesis, chlorophyll, and antioxidant properties of Mesembryanthemum crystallinum. Photosynthetica 2021, 59, 547–556. [Google Scholar] [CrossRef]
- He, J.; Koh, D.J.Q.; Qin, L. LED spectral quality and NaCl salinity interact to affect growth, photosynthesis and phytochemical production of Mesembryanthemum crystallinum. Funct. Plant Biol. 2022, 49, 483–495. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; Pinto, M.C. Vitamin C in plants: From functions to biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free. Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.R.; Gama, F.; Salazar, M.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; et al. Wild vs cultivated halophytes: Nutritional and functional differences. Food Chem. 2020, 333, 127536. [Google Scholar] [CrossRef]
- Medyouni, I.; Zouaoui, R.; Rubio, E.; Serino, S.; Ahmed, H.B.; Bertin, N. Effects of water deficit on leaves and fruit quality during the development period in tomato plant. Food Sci. Nutr. 2021, 9, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Sami, A.; Han, S.; Haider, M.Z.; Khizar, R.; Ali, Q.; Shafiq, M.; Tabassum, J.; Khalid, M.N.; Javed, M.A.; Sajid, M.; et al. Genetics aspect of vitamin C (Ascorbic Acid) biosynthesis and signaling pathways in fruits and vegetables crops. Funct. Integr. Genom. 2024, 24, 73. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Kundu, S.; Mohapatra, S.S.; Sinha, S.; Khoshru, B.; Keswani, C.; Mitra, D. An insight into the role of phenolics in abiotic stress tolerance in plants: Current perspective for sustainable environment. J. Pure Appl. Microbiol. 2024, 18, 64–79. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Baskar, V.; Venkatesh, R.; Ramalingam, S. Flavonoids (antioxidants systems) in higher plants and their response to stresses. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer International Publishing: Cham, Switzerland, 2018; pp. 253–268. [Google Scholar] [CrossRef]
- Feng, S.; Yao, Y.T.; Wang, B.B.; Li, Y.M.; Li, L.; Bao, A.K. Flavonoids are involved in salt tolerance through ROS scavenging in the halophyte Atriplex canescens. Plant Cell Rep. 2024, 43, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Verdaguer, D.; Jansen, M.A.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Hao, J.; Lou, P.; Han, Y.; Zheng, L.; Lu, J.; Chen, Z.; Ni, J.; Yang, Y.; Xu, M. Ultraviolet-B irradiation increases antioxidant capacity of Pakchoi (Brassica rapa L.) by inducing flavonoid biosynthesis. Plants 2022, 11, 766. [Google Scholar] [CrossRef]
- Calvo, M.M.; Martín-Diana, A.B.; Rico, D.; López-Caballero, M.E.; Martínez-Álvarez, O. Antioxidant, antihypertensive, hypoglycaemic and nootropic activity of a polyphenolic extract from the halophyte ice plant (Mesembryanthemum crystallinum). Foods 2022, 11, 1581. [Google Scholar] [CrossRef]
- Lima, A.R.; Gama, F.; Castañeda-Loaiza, V.; Costa, C.; Schüler, L.M.; Santos, T.; Salazar, M.; Nunes, C.; Cruz, R.M.S.; Varela, J.; et al. Nutritional and functional evaluation of Inula crithmoides and Mesembryanthemum nodiflorum grown in different salinities for human consumption. Molecules 2021, 26, 4543. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Cybulska, I.; Zembrzuska, J.; Brudecki, G.; Hedegaard-Thomsen, M. Optimizing methods to characterize caffeic, ferulic, and chlorogenic acids in Salicornia sinus-persica and Salicornia bigelovii extracts by tandem mass spectrometry (LC-MS/MS). BioResources 2021, 16, 5508–5523. [Google Scholar] [CrossRef]
- Preciado-Rangel, P.; Rueda-Puente, E.O.; Valdez-Aguilar, L.A.; Murillo-Amador, B. Electrical conductivity of nutrient solution and their effect on bioactive compounds and yield of bell pepper (Capsicum annuum L.). Trop. Subtrop. Agroecosystems 2021, 24, 1–12. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Pitsikoulaki, G.; Stamatakis, A.; Chrysargyris, A. Ammonium to total nitrogen ratio interactive effects with salinity application on Solanum lycopersicum growth, physiology, and fruit storage in a closed hydroponic system. Agronomy 2022, 12, 386. [Google Scholar] [CrossRef]
- de Oliveira, F.A.; de Oliveira, M.K.T.; Dos Santos, S.T.; Costa, M.J.V. Production and quality of purple kohlrabi under nutrient solutions of different electrical conductivities. Rev. Bras. Eng. Agríc. Ambient. 2024, 28, e270704. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Changes of carotenoid content in carrots after application of pulsed electric field treatments. LWT 2021, 147, 111408. [Google Scholar] [CrossRef]
- Kappel, N.; Boros, I.F.; Ravelombola, F.S.; Sipos, L. EC sensitivity of hydroponically-grown lettuce (Lactuca sativa L.) types in terms of nitrate accumulation. Agriculture 2021, 11, 315. [Google Scholar] [CrossRef]
- Sago, Y.; Shigemura, A. Quantitative nutrient management reduces nitrate accumulation in hydroponic butterhead lettuces grown under artificial lighting. HortScience 2018, 53, 963–967. [Google Scholar] [CrossRef]
- Peinado-Torrubia, P.; Álvarez, R.; Lucas, M.; Rosales, M.A. Nitrogen assimilation and photorespiration become more efficient under chloride nutrition as a beneficial macronutrient. Front. Plant Sci. 2023, 13, 1058774. [Google Scholar] [CrossRef]
- Whyte, J.N. Biochemical composition and energy content of six species of phytoplankton used in mariculture of bivalves. Aquaculture 1987, 60, 231–241. [Google Scholar] [CrossRef]
- Volden, J.; Bengtsson, G.B.; Wicklund, T. Glucosinolates, L-ascorbic acid, total phenols, anthocyanins, antioxidant capacities and colour in cauliflower (Brassica oleracea L. ssp. botrytis); effects of long-term freezer storage. Food Chem. 2009, 112, 967–976. [Google Scholar] [CrossRef]
- Lyashenko, S.; Fabrikov, D.; González-Fernández, M.J.; Gómez-Mercado, F.; Ruiz, R.L.; Fedorov, A.; Guil-Guerrero, J.L. Phenolic composition and in vitro antiproliferative activity of Borago spp. seed extracts on HT-29 cancer cells. Food Biosci. 2021, 42, 101043. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of flavonoid-rich extracts of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, C.; Straver, N. Nutrient Solutions for Vegetables and Flower Grow in Water Substrates, 10th ed.; Proefstation voor tuinbouw onder glas te Naaldiwjk: Naaldldwjk, The Netherlands, 1994; Volume 8, p. 45. [Google Scholar]




| Samples/Codes | Moisture g 100 g−1 | Total Carotenoids mg 100 g FW−1 | Vitamin C mg 100 g−1 FW | TPC mg GAE 100 g FW −1 | TFC mg QE 100 g g FW −1 | DPPH mmol TE 100 g DW−1 | ABTS mmol TE 100 g DW −1 |
|---|---|---|---|---|---|---|---|
| Wild | |||||||
| WA | 85.6 ± 0.6 bc | 61.5 ± 8.9 a | 69.3 ± 5.2 d | 358.8 ± 1.9 c | 119.5 ± 4.6 de | 1.8 ± 0.1 cde | 1.1 ± 0.0 de |
| WD | 87.6 ± 0.5 a | 45.3 ± 2.9 cd | 53.2 ± 8.8 f | 430.6 ± 10.2 a | 286.5 ± 9.6 a | 2.7 ± 0.1 ab | 0.9 ± 0.0 def |
| WT | 86.7 ± 0.5 abc | 59.6 ± 9.9 ab | 89.4 ± 2.1 bc | 250.9 ± 6.4 f | 180.1 ± 8.3 b | 2.0 ± 0.1 cd | 1.3 ± 0.1 de |
| WU | 87.0 ± 0.6 ab | 49.3 ± 3.7 bc | 54.0 ± 8.6 f | 360.7 ± 5.1 c | 139.7 ± 9.5 c | 2.1 ± 0.1 bcd | 0.8 ± 0.1 ef |
| Cultivated | |||||||
| Saline treatments | |||||||
| C1 (1.2 dS m−1) | 85.2 ± 0.3 c | 35.9 ± 1.0 de | 66.8 ± 4.3 de | 361.3 ± 6.5 c | 169.7 ± 6.6 b | 1.9 ± 0.3 cd | 2.5 ± 0.2 bc |
| C2 (1.8 dS m−1) | 85.3 ± 0.9 c | 38.7 ± 2.5 cde | 73.4 ± 1.4 d | 370.3 ± 15.2 bc | 166.2 ± 6.3 b | 1.5 ± 0.2 de | 2.9 ± 0.0 a |
| C3 (2.4 dS m−1) | 82.4 ± 0.5 d | 40.1 ± 4.4 cde | 97.0 ± 1.2 ab | 372.5 ± 14.4 bc | 122.1 ± 9.1 de | 2.2 ± 0.4 bc | 3.0 ± 0.1 a |
| C4 (3.0 dS m−1) | 82.5 ± 0.3 d | 48.2 ± 7.4 bc | 105.1 ± 3.6 a | 380.8 ± 6.0 b | 117.3 ± 3.5 de | 2.1 ± 0.7 bcd | 2.6 ± 0.1 ab |
| Light treatments | |||||||
| L1 (L18 NS1) | 85.3 ± 0.8 c | 32.0 ± 6.6 e | 57.8 ± 1.3 ef | 284.7 ± 2.5 e | 126.1 ± 6.8 cd | 3.0 ± 0.4 a | 2.1 ± 0.5 c |
| L2 (L18 AP67) | 86.6 ± 0.0 abc | 34.9 ± 3.3 de | 67.5 ± 1.4 de | 305.6 ± 12.9 d | 108.9 ± 6.3 e | 1.9 ± 0.0 cd | 1.1 ± 0.3 de |
| L3 (L18 NS12) | 83.3 ± 1.7 d | 31.6 ± 0.7 e | 85.5 ± 5.9 c | 200.6 ± 3.4 g | 109.2 ± 11.6 e | 1.2 ± 0.3 e | 1.6 ± 0.2 d |
| L4 (L18 T8) | 86.0 ± 0.8 abc | 44.7 ± 1.5 cd | 70.5 ± 0.8 d | 197.0 ± 7.5 g | 72.1 ± 5.1 f | 2.4 ± 0.0 abc | 0.6 ± 0.0 f |
| Sample Code | Status | Collection Site of Seeds and Cultivation Conditions | EC 1 (dS m−1) | Lamp Type/Natural Light |
|---|---|---|---|---|
| WA | Wild | Almería, Almería (36.833252, −2.458631) | 4.5 | Natural light |
| WD | Wild | Aguadulce, Almería (36.809383, −2.574194) | 3.0 | Natural light |
| WT | Wild | El Toyo, Almería (36.836655, −2.318960) | 4.2 | Natural light |
| WU | Wild | University Campus, Almería, (36.828918, −2.405527) | 2.2 | Natural light |
| C1 | Cultivated | University Campus of Almería, Growth chamber | 1.2 | L18 T8 Roblan® |
| C2 | Cultivated | University Campus of Almería, Growth chamber | 1.8 | L18 T8 Roblan® |
| C3 | Cultivated | University Campus of Almería, Growth chamber | 2.4 | L18 T8 Roblan® |
| C4 | Cultivated | University Campus of Almería, Growth chamber | 3.0 | L18 T8 Roblan® |
| L1 | Cultivated | University Campus of Almería, Growth chamber | 2.0 | L18 NS1 Valoya® |
| L2 | Cultivated | University Campus of Almería, Growth chamber | 2.0 | L18 AP67 Valoya® |
| L3 | Cultivated | University Campus of Almería, Growth chamber | 2.0 | L18 NS12 Valoya® |
| L4 | Cultivated | University Campus of Almería, Growth chamber | 2.0 | L18 T8 Roblan® |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha-Chiamolera, T.P.L.; Chileh-Chelh, T.; Ezzaitouni, M.; Guil-Guerrero, J.L.; Urrestarazu, M. Effect of Electrical Conductivity of Nutrient Solution and Light Spectra on the Main Phytochemical Content of Sonchus tenerrimus L. Under Wild and Controlled Environments. Plants 2025, 14, 2811. https://doi.org/10.3390/plants14172811
Cunha-Chiamolera TPL, Chileh-Chelh T, Ezzaitouni M, Guil-Guerrero JL, Urrestarazu M. Effect of Electrical Conductivity of Nutrient Solution and Light Spectra on the Main Phytochemical Content of Sonchus tenerrimus L. Under Wild and Controlled Environments. Plants. 2025; 14(17):2811. https://doi.org/10.3390/plants14172811
Chicago/Turabian StyleCunha-Chiamolera, Tatiana P. L., Tarik Chileh-Chelh, Mohamed Ezzaitouni, José Luis Guil-Guerrero, and Miguel Urrestarazu. 2025. "Effect of Electrical Conductivity of Nutrient Solution and Light Spectra on the Main Phytochemical Content of Sonchus tenerrimus L. Under Wild and Controlled Environments" Plants 14, no. 17: 2811. https://doi.org/10.3390/plants14172811
APA StyleCunha-Chiamolera, T. P. L., Chileh-Chelh, T., Ezzaitouni, M., Guil-Guerrero, J. L., & Urrestarazu, M. (2025). Effect of Electrical Conductivity of Nutrient Solution and Light Spectra on the Main Phytochemical Content of Sonchus tenerrimus L. Under Wild and Controlled Environments. Plants, 14(17), 2811. https://doi.org/10.3390/plants14172811







