Genome-Wide Identification and Expression Profiling of Pyruvate Kinase Genes in Litchi Under Calcium-Magnesium Foliar Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup, Field Treatment, and Sample Collection
2.2. Determination of Soluble Sugar, Peel Coloration, and Metabolic Activities
2.3. Hormonal Changes Analyzed Using Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. PK Gene Family Identification and 3D Protein Modeling
2.5. Analysis of Motif Identification, Domain, Gene Structure, and Phylogenetic Study
2.6. Ka/Ks Value Calculation, Chromosomal Location, and Subcellular Localization
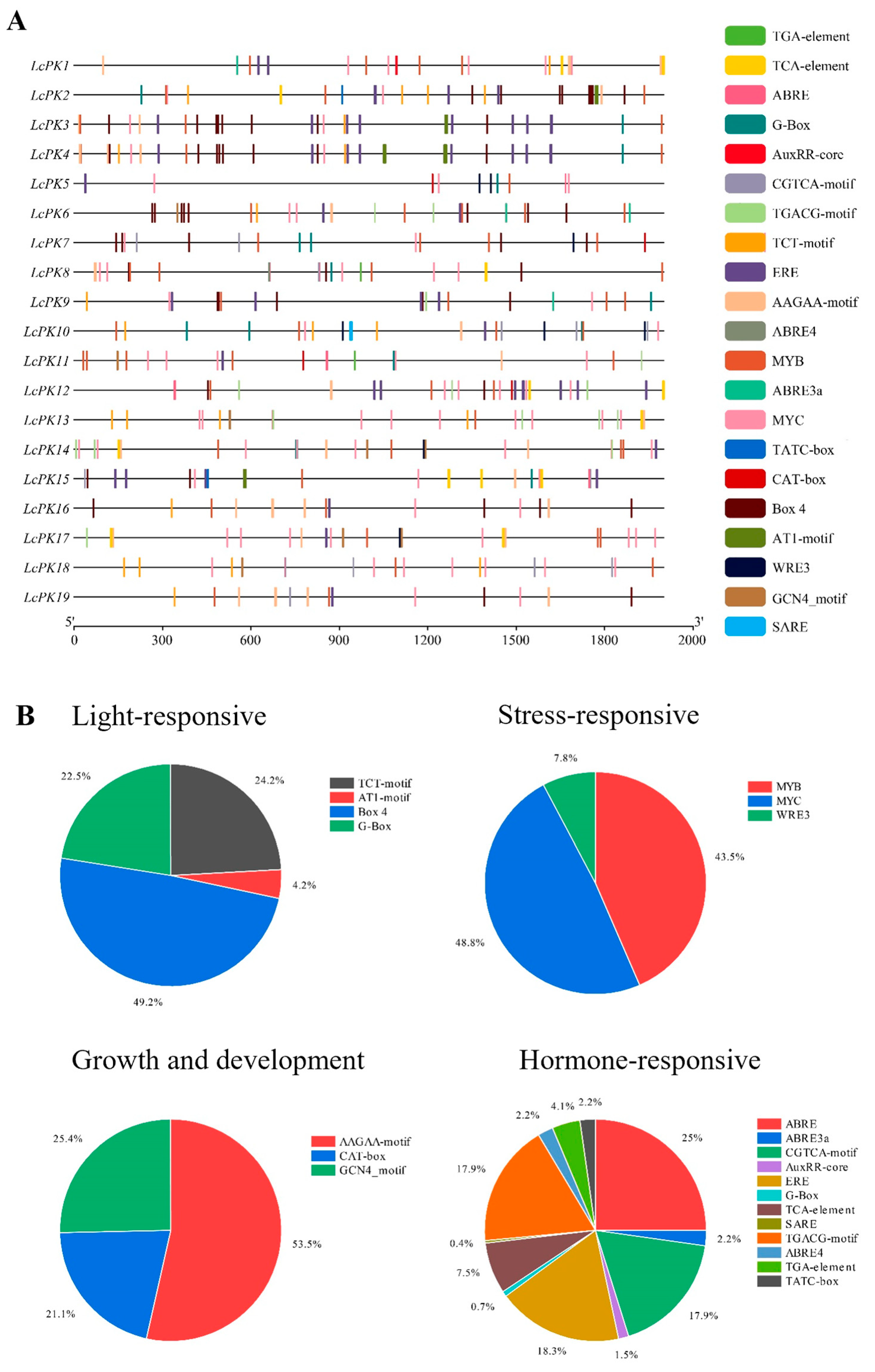
2.7. Gene Duplication, Synteny Analysis, and Cis-Acting Component Analysis
2.8. Expression Analysis of LcPK Genes
2.9. qRT-PCR Analysis
2.10. Statistical Analysis
3. Results
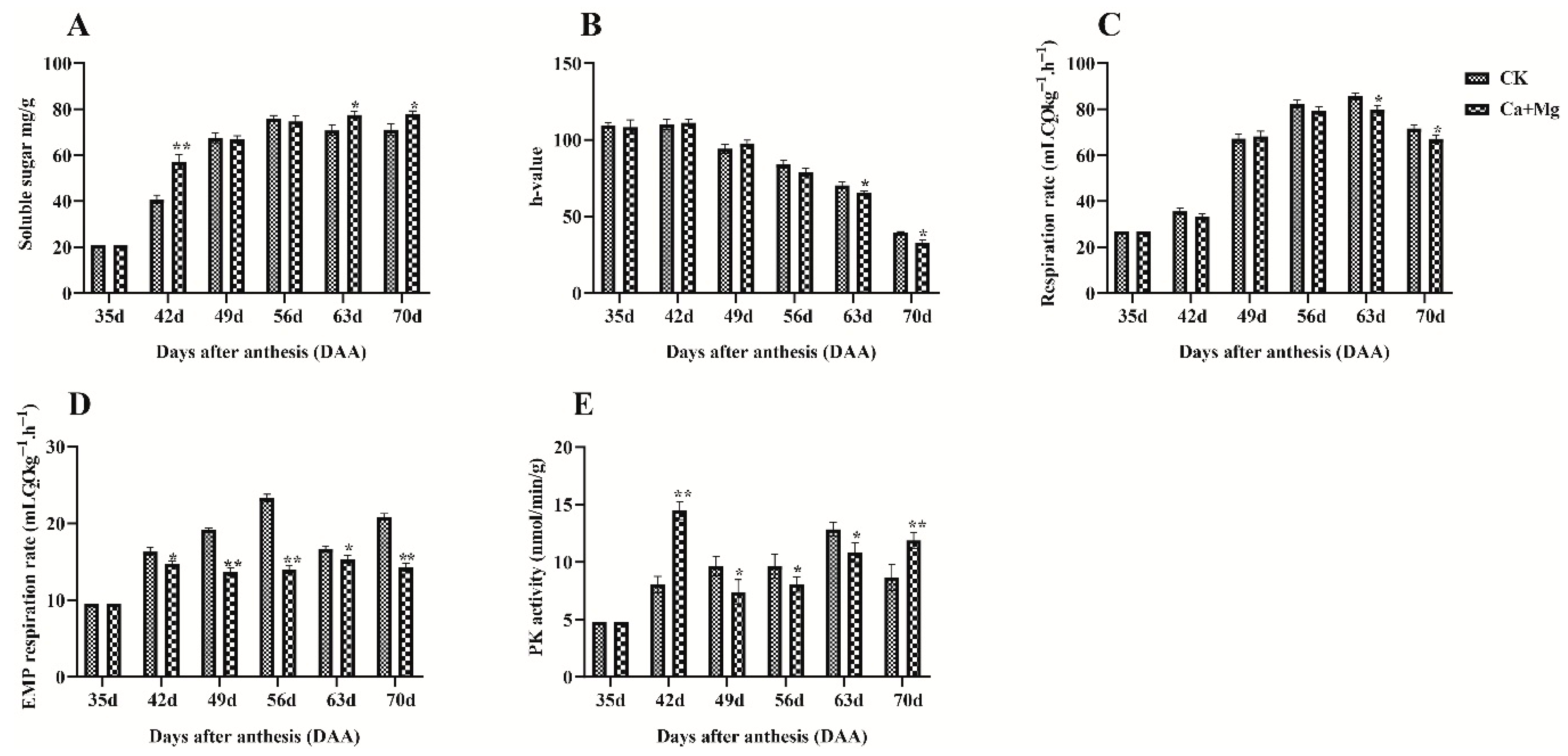
3.1. Effects of the Treatment on Soluble Sugar Content, Peel Coloration, and Metabolic Activities
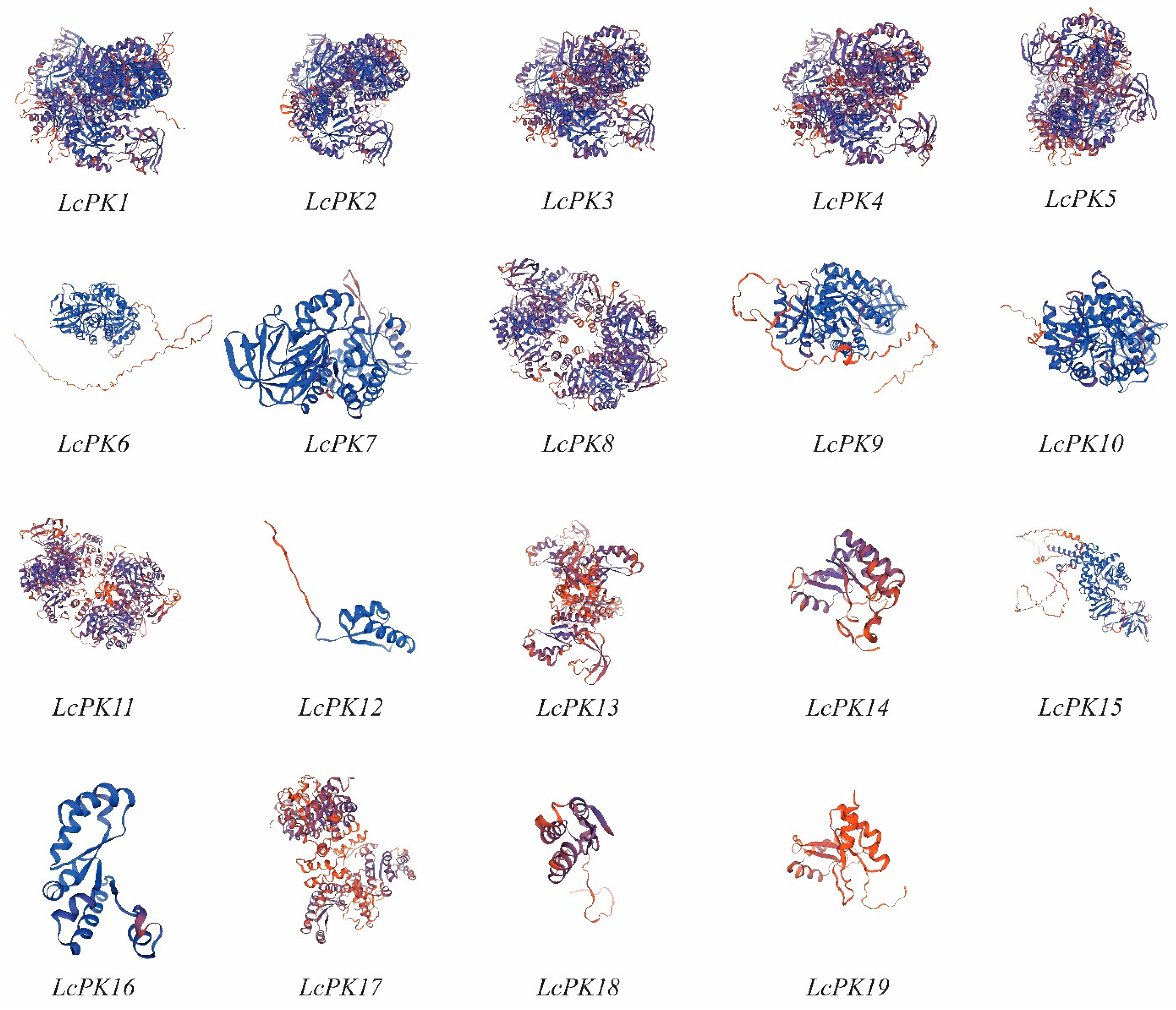
3.2. Genome-Wide Investigation and 3D Structures of the PK Gene Family
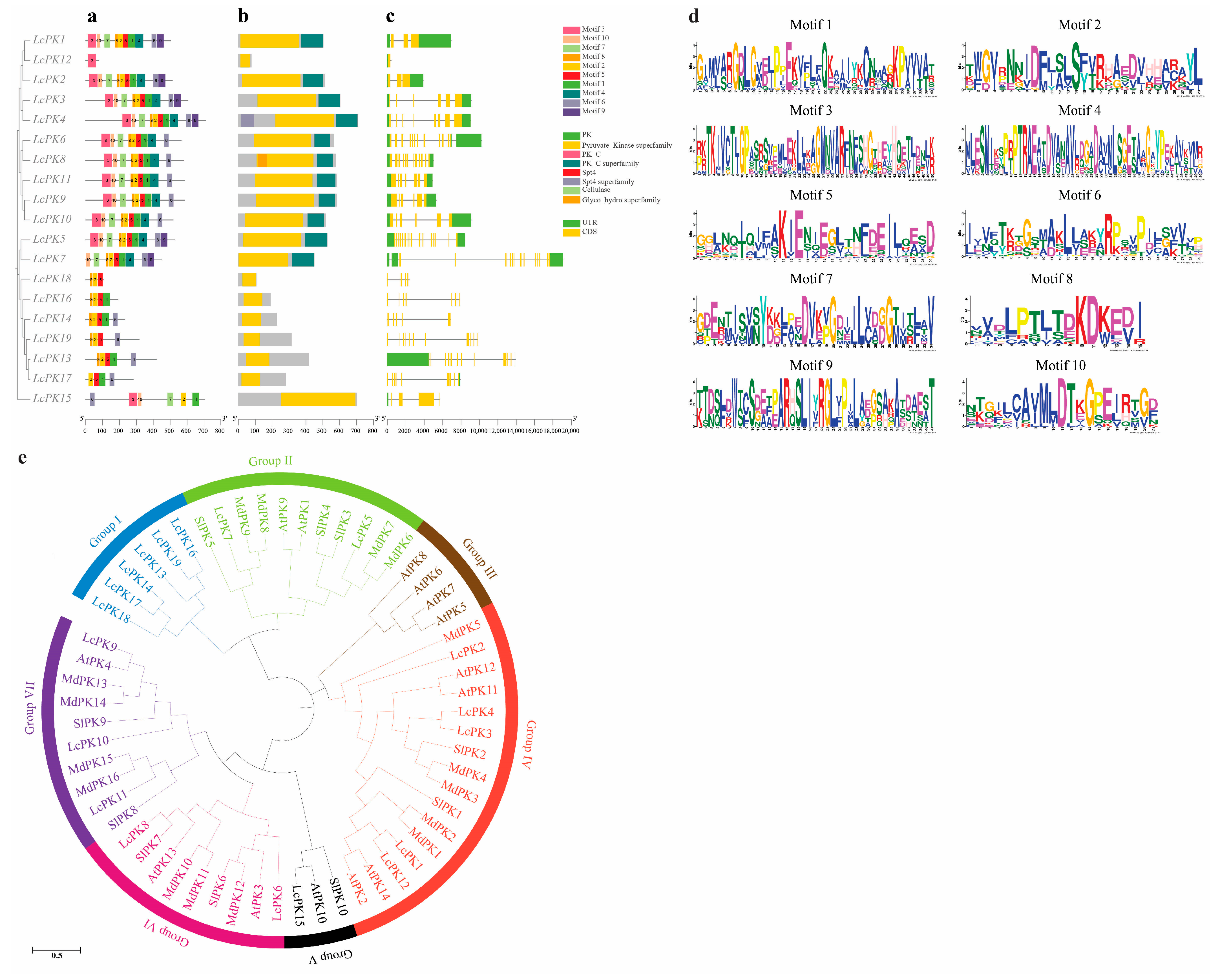
3.3. Conserved Protein Motif Analysis, Gene Structure, and Phylogenetic Analysis
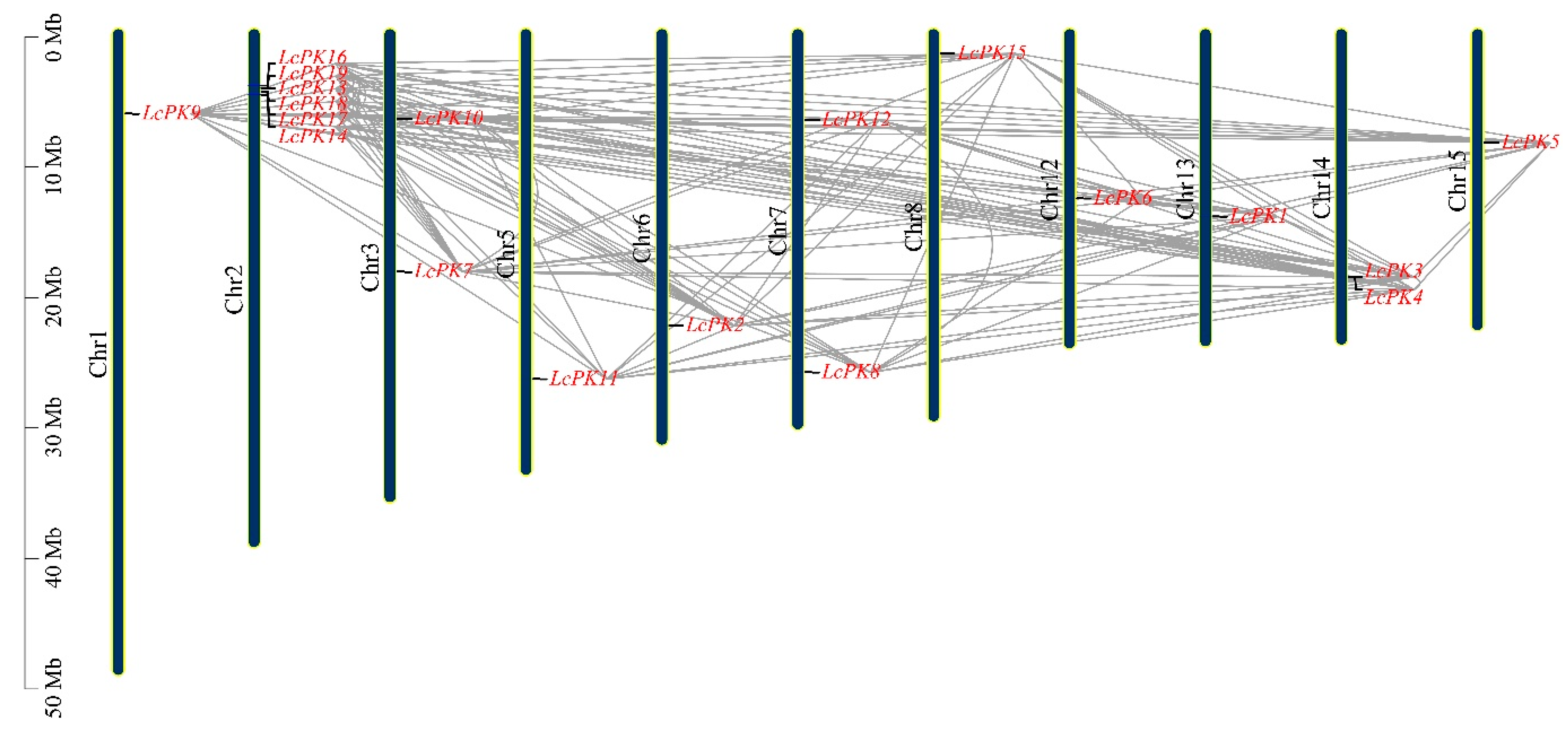
3.4. Ka/Ks Value Calculation, Chromosomal Location, Subcellular Distribution, and Gene Duplication Analysis
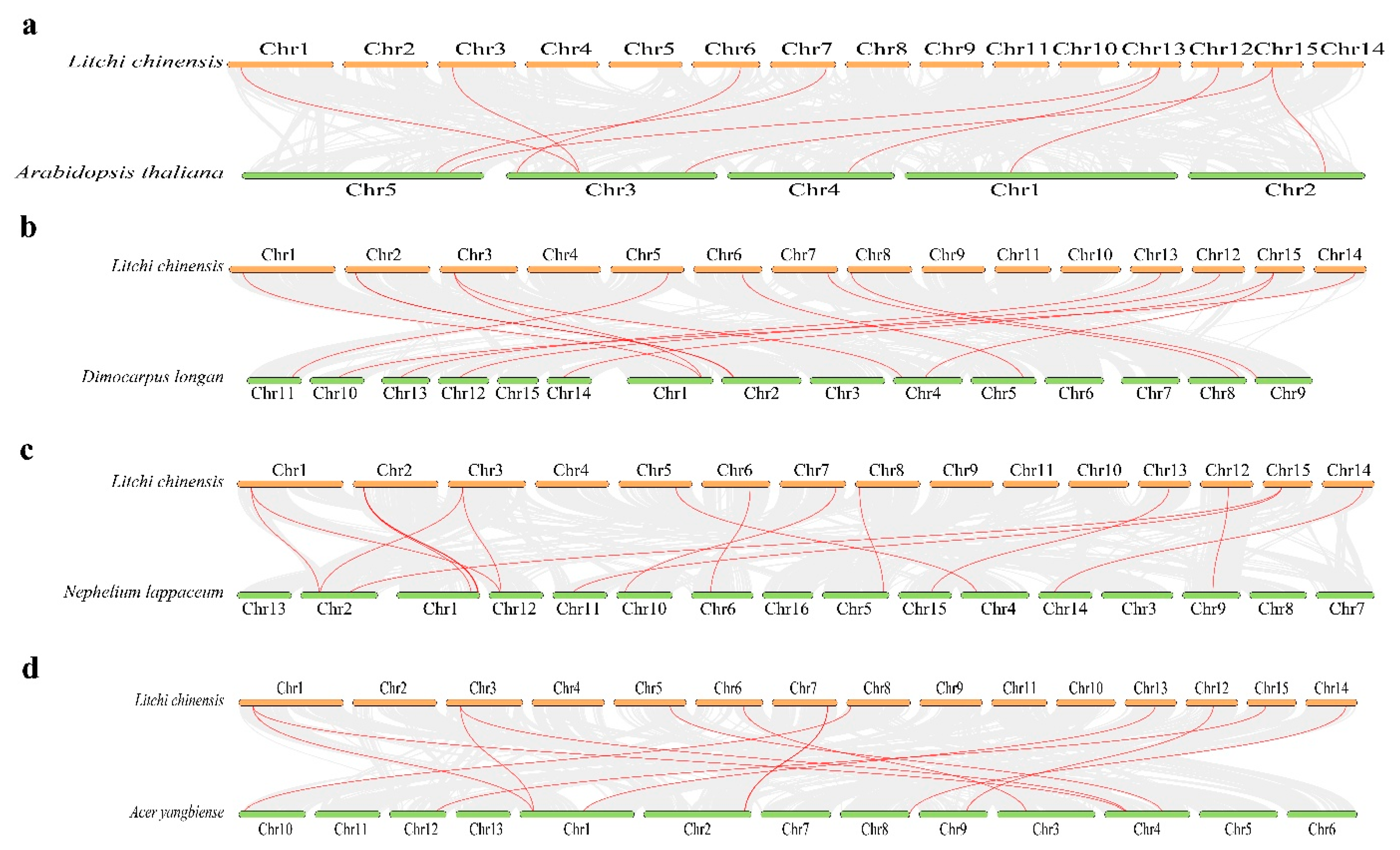
3.5. Synteny Analysis and Cis-Acting Component Analysis
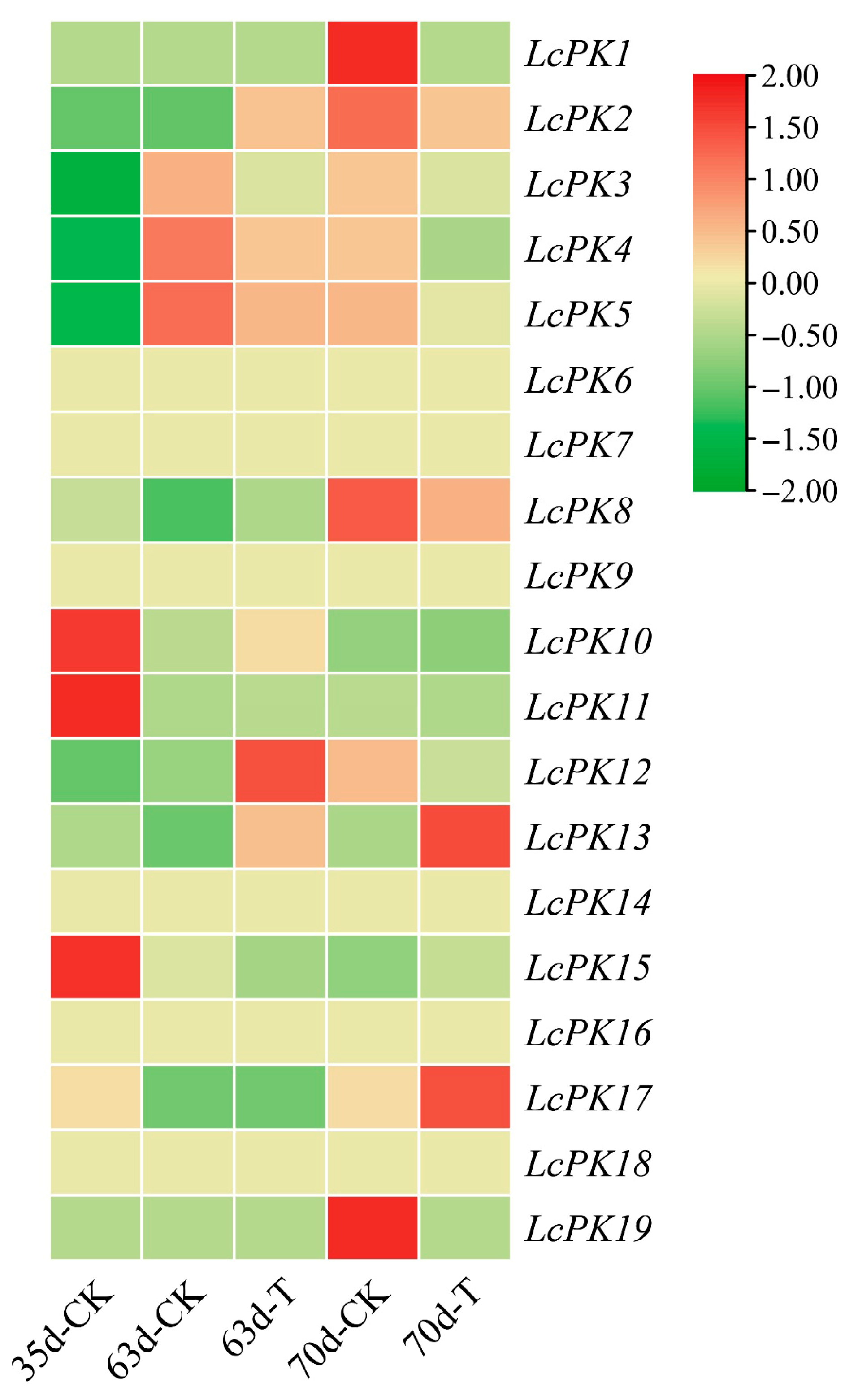
3.6. Expression Patterns of LcPK Genes
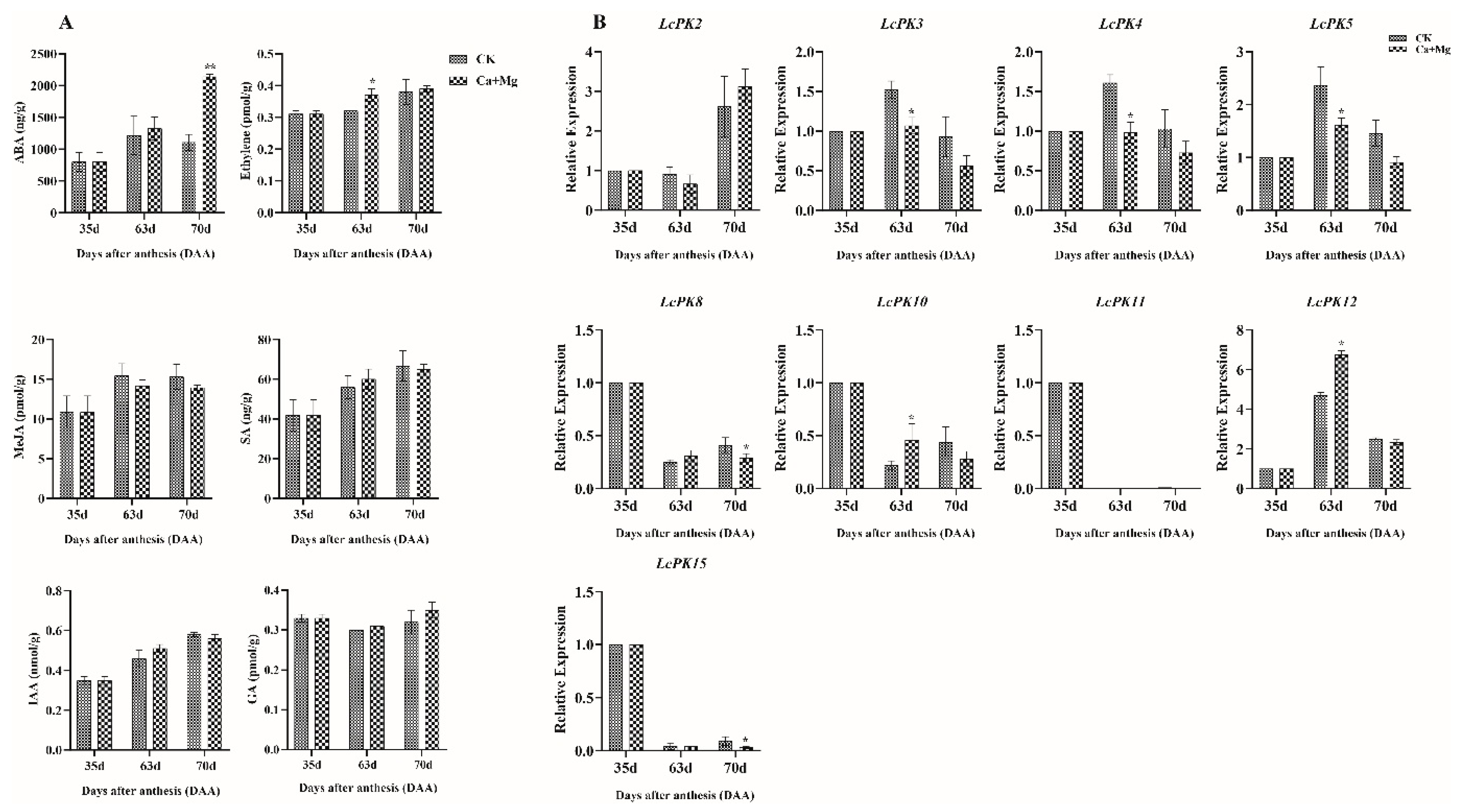
3.7. Regulation of LcPK Genes by Phytohormones During Litchi Ripening
4. Discussion
4.1. Effect of Ca+Mg Treatment on Sugar Content, Peel Coloration, and Metabolic Activity
4.2. Bioinformatics Analysis of PK Gene Family
4.3. Correlation Between Hormonal Regulation and LcPK Gene Expression Analysis Overcomes Sugar-Receding Process
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, D.; Deng, Y.; Xi, P.; Zhu, Z.; Kong, X.; Wan, L.; Situ, J.; Li, M.; Gao, L.; Jiang, Z. Biological activity of pterostilbene against Peronophythora litchii, the litchi downy blight pathogen. Postharvest Biol. Technol. 2018, 144, 29–35. [Google Scholar] [CrossRef]
- Zou, S.-C.; Zhuo, M.-G.; Abbas, F.; Hu, G.-B.; Wang, H.-C.; Huang, X.-M. Transcription factor LcNAC002 coregulates chlorophyll degradation and anthocyanin biosynthesis in litchi. Plant Physiol. 2023, 192, 1913–1927. [Google Scholar] [CrossRef]
- Wang, H.; Huang, H.; Huang, X.; Hu, Z. Sugar and acid compositions in the arils of Litchi chinensis Sonn.: Cultivar differences and evidence for the absence of succinic acid. J. Hortic. Sci. Biotechnol. 2006, 81, 57–62. [Google Scholar] [CrossRef]
- Sajjad, M.; Tahir, H.; Ma, W.; Shaopu, S.; Farooq, M.A.; Haq, M.Z.U.; Sajad, S.; Zhou, K. Effects of Foliar Ca and Mg Nutrients on the Respiration of ‘Feizixiao’ Litchi Pulp and Identification of Differential Expression Genes Associated with Respiration. Agronomy 2024, 14, 1347. [Google Scholar] [CrossRef]
- Yang, B.; Chen, M.; Zhan, C.; Liu, K.; Cheng, Y.; Xie, T.; Zhu, P.; He, Y.; Zeng, P.; Tang, H. Identification of OsPK5 involved in rice glycolytic metabolism and GA/ABA balance for improving seed germination via genome-wide association study. J. Exp. Bot. 2022, 73, 3446–3461. [Google Scholar] [CrossRef] [PubMed]
- Le, X.H.; Lee, C.-P.; Millar, A.H. The mitochondrial pyruvate carrier (MPC) complex mediates one of three pyruvate-supplying pathways that sustain Arabidopsis respiratory metabolism. Plant Cell 2021, 33, 2776–2793. [Google Scholar] [CrossRef]
- Hsu, M.-C.; Hung, W.-C. Pyruvate kinase M2 fuels multiple aspects of cancer cells: From cellular metabolism, transcriptional regulation to extracellular signaling. Mol. Cancer 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Van Dongen, J.T.; Gupta, K.J.; Ramírez-Aguilar, S.J.; Araújo, W.L.; Nunes-Nesi, A.; Fernie, A.R. Regulation of respiration in plants: A role for alternative metabolic pathways. J. Plant Physiol. 2011, 168, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Plaxton, W.C. Multifaceted functions of post-translational enzyme modifications in the control of plant glycolysis. Curr. Opin. Plant Biol. 2020, 55, 28–37. [Google Scholar] [CrossRef]
- Andre, C.; Benning, C. Arabidopsis seedlings deficient in a plastidic pyruvate kinase are unable to utilize seed storage compounds for germination and establishment. Plant Physiol. 2007, 145, 1670–1680. [Google Scholar] [CrossRef]
- The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [CrossRef]
- Oliver, S.N.; Lunn, J.E.; Urbanczyk-Wochniak, E.; Lytovchenko, A.; van Dongen, J.T.; Faix, B.; Schmälzlin, E.; Fernie, A.R.; Geigenberger, P. Decreased expression of cytosolic pyruvate kinase in potato tubers leads to a decline in pyruvate resulting in an in vivo repression of the alternative oxidase. Plant Physiol. 2008, 148, 1640–1654. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.-Y. Cotton cytosolic pyruvate kinase GhPK6 participates in fast fiber elongation regulation in a ROS-mediated manner. Planta 2016, 244, 915–926. [Google Scholar] [CrossRef]
- Turner, W.L.; Knowles, V.L.; Plaxton, W.C. Cytosolic pyruvate kinase: Subunit composition, activity, and amount in developing castor and soybean seeds, and biochemical characterization of the purified castor seed enzyme. Planta 2005, 222, 1051–1062. [Google Scholar] [CrossRef]
- Baud, S.; Wuillème, S.; Dubreucq, B.; De Almeida, A.; Vuagnat, C.; Lepiniec, L.; Miquel, M.; Rochat, C. Function of plastidial pyruvate kinases in seeds of Arabidopsis thaliana. Plant J. 2007, 52, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Wulfert, S.; Schilasky, S.; Krueger, S. Transcriptional and biochemical characterization of cytosolic pyruvate kinases in Arabidopsis thaliana. Plants 2020, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Behal, R.; Oliver, D.J. Disruption of plE2, the gene for the E2 subunit of the plastid pyruvate dehydrogenase complex, in Arabidopsis causes an early embryo lethal phenotype. Plant Mol. Biol. 2003, 52, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Ruuska, S.A.; Girke, T.; Benning, C.; Ohlrogge, J.B. Contrapuntal networks of gene expression during Arabidopsis seed filling. Plant Cell 2002, 14, 1191–1206. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, W.; Luo, L.; Pang, J.; Rong, W.; He, C. Downregulation of OsPK1, a cytosolic pyruvate kinase, by T-DNA insertion causes dwarfism and panicle enclosure in rice. Planta 2012, 235, 25–38. [Google Scholar] [CrossRef]
- Hu, L.; Tu, B.; Yang, W.; Yuan, H.; Li, J.; Guo, L.; Zheng, L.; Chen, W.; Zhu, X.; Wang, Y. Mitochondria-associated pyruvate kinase complexes regulate grain filling in rice. Plant Physiol. 2020, 183, 1073–1087. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Li, X.; Li, C.; Sun, J.; Jackson, A. Dynamic changes of enzymes involved in sugar and organic acid level modification during blueberry fruit maturation. Food Chem. 2020, 309, 125617. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- De Freitas, S.T.; Jiang, C.-Z.; Mitcham, E.J. Mechanisms involved in calcium deficiency development in tomato fruit in response to gibberellins. J. Plant Growth Regul. 2012, 31, 221–234. [Google Scholar] [CrossRef]
- Wei, J.; Wen, X.; Tang, L. Effect of methyl jasmonic acid on peach fruit ripening progress. Sci. Hortic. 2017, 220, 206–213. [Google Scholar] [CrossRef]
- Darpreet Kour, D.K.; Arti Sharma, A.S.; Wali, V.; Parshant Bakshi, P.B.; Ambika Bandhari, A.B. Effect of regulated irrigation at delayed intervals and calcium sprays on yield and quality of litchi (Litchi chinensis Sonn.) cv. Dehradun. Indian J. Ecol. 2016, 43, 603–610. [Google Scholar]
- Shui, X.; Wang, W.; Ma, W.; Yang, C.; Zhou, K. Mechanism by which high foliar calcium contents inhibit sugar accumulation in feizixiao lychee pulp. Horticulturae 2022, 8, 1044. [Google Scholar] [CrossRef]
- Tian, S.; Zhou, X.; Gong, H.; Ma, X.; Zhang, F. Orthogonal test design for optimization of the extraction of polysaccharide from Paeonia sinjiangensis KY Pan. Pharmacogn. Mag. 2011, 7, 4. [Google Scholar] [CrossRef]
- Sivakumar, D.; Korsten, L. Fruit quality and physiological responses of litchi cultivar McLean’s Red to 1-methylcyclopropene pre-treatment and controlled atmosphere storage conditions. LWT-Food Sci. Technol. 2010, 43, 942–948. [Google Scholar] [CrossRef]
- Huang, L.; Tao, S.; Zhu, Y.; Pan, Y.; Zhang, Z.; Yu, Z.; Chen, Y. Regulation of Embden–Meyerhof–Parnas (EMP) Pathway and Tricarboxylic Acid (TCA) Cycle Concerning Aberrant Chilling Injury Behavior in Postharvest Papaya (Carica papaya L.). Int. J. Mol. Sci. 2023, 24, 13898. [Google Scholar] [CrossRef]
- Patel, M.S.; Nemeria, N.S.; Furey, W.; Jordan, F. The pyruvate dehydrogenase complexes: Structure-based function and regulation. J. Biol. Chem. 2014, 289, 16615–16623. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.; Zeng, Z.; Wu, F.; Feng, J.; Liu, B.; Mai, Y.; Chu, X.; Wei, W.; Li, X. SapBase: A central portal for functional and comparative genomics of Sapindaceae species. J. Integr. Plant Biol. 2024, 66, 1561–1570. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Watson, J.D.; Thornton, J.M. ProFunc: A server for predicting protein function from 3D structure. Nucleic Acids Res. 2005, 33, W89–W93. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Paterson, A.H. MCScanX-transposed: Detecting transposed gene duplications based on multiple colinearity scans. Bioinformatics 2013, 29, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Ersahin, S.S.; Ersahin, A. Endometrial injury concurrent with hysteroscopy increases the expression of Leukaemia inhibitory factor: A preliminary study. Reprod. Biol. Endocrinol. 2022, 20, 1–6. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef]
- Ravi, B.; Foyer, C.H.; Pandey, G.K. The integration of reactive oxygen species (ROS) and calcium signalling in abiotic stress responses. Plant Cell Environ. 2023, 46, 1985–2006. [Google Scholar] [CrossRef]
- Esfandiari, E.; Shokrpour, M.; Alavi-Kia, S. Effect of Mg deficiency on antioxidant enzymes activities and lipid peroxidation. J. Agric. Sci. 2010, 2, 131–136. [Google Scholar] [CrossRef]
- De Freitas, S.T.; Mitcham, E.I. 3 factors involved in fruit calcium deficiency disorders. Hortic. Rev. 2012, 40, 107–146. [Google Scholar]
- Liebisch, F.; Max, J.F.; Heine, G.; Horst, W.J. Blossom-end rot and fruit cracking of tomato grown in net-covered greenhouses in Central Thailand can partly be corrected by calcium and boron sprays. J. Plant Nutr. Soil Sci. 2009, 172, 140–150. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, B.; Bozdar, B.; Chachar, S.; Rai, M.; Li, J.; Li, Y.; Hayat, F.; Chachar, Z.; Tu, P. The power of magnesium: Unlocking the potential for increased yield, quality, and stress tolerance of horticultural crops. Front. Plant Sci. 2023, 14, 1285512. [Google Scholar] [CrossRef]
- Saleem, S.; Anayat, R.; Mushtaq, F.; Mustafa, I.; Khan, S.R.; Farwah, S.; Hussain, S. Effect of foliar application of calcium and magnesium on growth and yield of tomato (Solanum lycopersicum L.) variety Marglobe. Int. J. Chem. Stud. 2019, 7, 1555–1561. [Google Scholar]
- Luo, T.; Shuai, L.; Lai, T.; Liao, L.; Li, J.; Duan, Z.; Xue, X.; Han, D.; Wu, Z. Up-regulated glycolysis, TCA, fermentation and energy metabolism promoted the sugar receding in ‘Shixia’longan (Dimocarpus longan Lour.) pulp. Sci. Hortic. 2021, 281, 109998. [Google Scholar] [CrossRef]
- Liaquat, M.; Ahmad, S.; Khan, A.S.; Ahmed, R. Reduction in fruit rot and enhancement in fruit quality of kinnow mandarin by calcium chloride application. Pakistan J. Agric. Sci. 2019, 56, 367–376. [Google Scholar]
- Zhou, X.; Su, Y.; Zhang, R.; Zhou, K. Effects of K, Ca and Mg applied in foliar nutrients on pericarp’s coloring of Litchi chinensis Sonn. cv. Feizixiao. Southwest China J. Agric. Sci. 2015, 28, 1713–1718. [Google Scholar]
- Kader, A.A. Postharvest Technology of Horticultural Crops; University of California Agriculture and Natural Resources: Davis, CA, USA, 2002; Volume 3311. [Google Scholar]
- Farag, K.M.; Nagy, N.M. Effect of pre-and post-harvest calcium and magnesium compounds and their combination treatments on “Anna” apple fruit quality and shelf life. J. Hortic. Sci. Ornam. Plants 2012, 4, 155–168. [Google Scholar]
- Cronje, R.B. Effect of fruit development, maturity and harvesting of litchi (Litchi chinensis Sonn.) on postharvest fruit quality. Stewart Postharvest Rev. 2008, 4, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.J.; Yuan, M.L.; Zhou, K.B. Effects of magnesium foliar application on pericarp coloring of Litchi chinensis Sonn. cv. Feizixiao and contents of potassium, calcium and magnesium in pericarp. J. South. Agric. 2017, 48, 854–860. [Google Scholar]
- Zhang, Y.-L.; Cui, Q.-L.; Wang, Y.; Shi, F.; Fan, H.; Zhang, Y.-Q.; Lai, S.-T.; Li, Z.-H.; Li, L.; Sun, Y.-K. Effect of edible carboxymethyl chitosan-gelatin based coating on the quality and nutritional properties of different sweet cherry cultivars during postharvest storage. Coatings 2021, 11, 396. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Moreno, D.A.; Romero, L. Pyruvate kinase activity as an indicator of the level of K+, Mg2+, and Ca2+ in leaves and fruits of the cucumber: The role of potassium fertilization. J. Agric. Food Chem. 1999, 47, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, S.; Grimm, H.; Boschek, C.; Vaupel, P.; Eigenbrodt, E. Pyruvate kinase type M2: A crossroad in the tumor metabolome. Br. J. Nutr. 2002, 87, S23–S29. [Google Scholar] [CrossRef]
- Yaghobi, M.; Heidari, P. Genome-wide analysis of aquaporin gene family in Triticum turgidum and its expression profile in response to salt stress. Genes 2023, 14, 202. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Shen, X.; Xia, Z.; Zhou, X.; Chen, X.; Lu, C.; Wang, W. Genome-wide survey of the phosphofructokinase family in cassava and functional characterization in response to oxygen-deficient stress. BMC Plant Biol. 2021, 21, 1–15. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, M.; Tang, Y.; Han, J.; Gui, Y.; Ge, J.; Jiang, S.; Dai, Q.; Zhang, W.; Lin, M. Modular assembly of ordered hydrophilic proteins improve salinity tolerance in Escherichia coli. Int. J. Mol. Sci. 2021, 22, 4482. [Google Scholar] [CrossRef]
- Maqsood, Q.; Sumrin, A.; Ali, Q.; Hussain, N.; Malook, S.U.; Ali, D. In-silico analysis of ribosome inactivating protein (RIP) of the Cucurbitaceae family. AMB Express 2024, 14, 61. [Google Scholar] [CrossRef]
- Numan, M.; Bukhari, S.A.; Rehman, M.-U.; Mustafa, G.; Sadia, B.; Yin, Y. Phylogenetic analyses, protein modeling and active site prediction of two pathogenesis related (PR2 and PR3) genes from bread wheat. PLoS ONE 2021, 16, e0257392. [Google Scholar] [CrossRef]
- Pražnikar, J.; Tomić, M.; Turk, D. Validation and quality assessment of macromolecular structures using complex network analysis. Sci. Rep. 2019, 9, 1678. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, T.; Dror, I.; Mathelier, A.; Wasserman, W.W.; Gordân, R.; Rohs, R. TFBSshape: A motif database for DNA shape features of transcription factor binding sites. Nucleic Acids Res. 2014, 42, D148–D155. [Google Scholar] [CrossRef]
- Liu, Y.; Do, S.; Huynh, H.; Li, J.-X.; Liu, Y.-G.; Du, Z.-Y.; Chen, M.-X. Importance of pre-mRNA splicing and its study tools in plants. Adv. Biotech. 2024, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yang, S.; Li, G.; Wan, S. Genome-wide identification and characterization of CDPK gene family in cultivated peanut (Arachis hypogaea L.) reveal their potential roles in response to Ca deficiency. Cells 2023, 12, 2676. [Google Scholar] [CrossRef]
- Su, P.S.; Li, J.; Zang, D.; Wang, Z.; Wu, Y.; Chi, S.; Sun, F.; Niu, Y.; Hua, X.; Yan, J. Genome-wide evolutionary analysis of TKL_CTR1-DRK-2 gene family and functional characterization reveals that TaCTR1 positively regulates flowering time in wheat. BMC Genom. 2024, 25, 474. [Google Scholar] [CrossRef] [PubMed]
- Lü, H.; Li, J.; Huang, Y.; Zhang, M.; Zhang, S.; Wu, J. Genome-wide identification, expression and functional analysis of the phosphofructokinase gene family in Chinese white pear (Pyrus bretschneideri). Gene 2019, 702, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Chen, L.; Ahmad, S.; Cai, Y.; Duan, Y.; Li, X.; Liu, Y.; Jiao, G.; Xie, L.; Hu, S. Genome-wide analysis and functional characterization of pyruvate kinase (PK) gene family modulating rice yield and quality. Int. J. Mol. Sci. 2022, 23, 15357. [Google Scholar] [CrossRef]
- Hillis, D.M.; Huelsenbeck, J.P.; Cunningham, C.W. Application and accuracy of molecular phylogenies. Science 1994, 264, 671–677. [Google Scholar] [CrossRef]
- Kondrashov, F.A.; Rogozin, I.B.; Wolf, Y.I.; Koonin, E.V. Selection in the evolution of gene duplications. Genome Biol. 2002, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Conant, G.C.; Wolfe, K.H. Turning a hobby into a job: How duplicated genes find new functions. Nat. Rev. Genet. 2008, 9, 938–950. [Google Scholar] [CrossRef]
- Khan, N.; Ke, H.; Hu, C.-m.; Naseri, E.; Haider, M.S.; Ayaz, A.; Amjad Khan, W.; Wang, J.; Hou, X. Genome-Wide Identification, Evolution, and Transcriptional Profiling of PP2C Gene Family in Brassica rapa. BioMed Res. Int. 2019, 2019, 2965035. [Google Scholar] [CrossRef]
- Islam, S.; Sajib, S.D.; Jui, Z.S.; Arabia, S.; Islam, T.; Ghosh, A. Genome-wide identification of glutathione S-transferase gene family in pepper, its classification, and expression profiling under different anatomical and environmental conditions. Sci. Rep. 2019, 9, 9101. [Google Scholar] [CrossRef]
- Valentini, G.; Chiarelli, L.; Fortin, R.; Speranza, M.L.; Galizzi, A.; Mattevi, A. The allosteric regulation of pyruvate kinase: A site-directed mutagenesis study. J. Biol. Chem. 2000, 275, 18145–18152. [Google Scholar] [CrossRef]
- Plaxton, W.C. The organization and regulation of plant glycolysis. Annu. Rev. Plant Biol. 1996, 47, 185–214. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Zhang, Y.; Li, W.; Wang, C.; Xu, R.; Dai, H.; Zhang, L. Characterization of the pyruvate kinase gene family in soybean and identification of a putative salt responsive gene GmPK21. BMC Genom. 2024, 25, 88. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic-and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ge, C.; Ling, Y.; Mo, F.; Yang, M.; Jiang, L.; Chen, Q.; Lin, Y.; Sun, B.; Zhang, Y. ABA and sucrose co-regulate strawberry fruit ripening and show inhibition of glycolysis. Mol. Genet. Genom. 2020, 295, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Piotrowska-Niczyporuk, A. Biosynthetic pathways of hormones in plants. Metabolites 2023, 13, 884. [Google Scholar] [CrossRef]
- Barry, C.S.; Giovannoni, J.J. Ethylene and fruit ripening. J. Plant Growth Regul. 2007, 26, 143–159. [Google Scholar] [CrossRef]
- Seymour, G.B.; Østergaard, L.; Chapman, N.H.; Knapp, S.; Martin, C. Fruit development and ripening. Annu. Rev. Plant Biol. 2013, 64, 219–241. [Google Scholar] [CrossRef]
- Jia, H.-F.; Chai, Y.-M.; Li, C.-L.; Lu, D.; Luo, J.-J.; Qin, L.; Shen, Y.-Y. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 2011, 157, 188–199. [Google Scholar] [CrossRef]
- Yang, N.; Zhou, Y.; Wang, Z.; Zhang, Z.; Xi, Z.; Wang, X. Emerging roles of brassinosteroids and light in anthocyanin biosynthesis and ripeness of climacteric and non-climacteric fruits. Crit. Rev. Food Sci. Nutr. 2023, 63, 4541–4553. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, B.; Leng, P. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J. Exp. Bot. 2009, 60, 1579–1588. [Google Scholar] [CrossRef]
- Ueda, H.; Ito, T.; Inoue, R.; Masuda, Y.; Nagashima, Y.; Kozuka, T.; Kusaba, M. Genetic interaction among phytochrome, ethylene and abscisic acid signaling during dark-induced senescence in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 564. [Google Scholar] [CrossRef]
- Umezawa, T.; Sugiyama, N.; Mizoguchi, M.; Hayashi, S.; Myouga, F.; Yamaguchi-Shinozaki, K.; Ishihama, Y.; Hirayama, T.; Shinozaki, K. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 17588–17593. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Hazra, S.; Datta, R.; Chattopadhyay, S. Transcriptome analysis of Arabidopsis mutants suggests a crosstalk between ABA, ethylene and GSH against combined cold and osmotic stress. Sci. Rep. 2016, 6, 36867. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Hu, W.; Guan, Y.; Ji, Y.; Yang, X.; Hou, M. Respiratory metabolism and quality in postharvest sweet cherries (Prunus avium L.) in response to high CO2 treatment. J. Food Process. Preserv. 2022, 46, e16879. [Google Scholar] [CrossRef]
- Millar, A.H.; Hoefnagel, M.H.; Day, D.A.; Wiskich, J.T. Specificity of the organic acid activation of alternative oxidase in plant mitochondria. Plant Physiol. 1996, 111, 613–618. [Google Scholar] [CrossRef]
- Ambasht, P.; Kayastha, A.M. Plant pyruvate kinase. Biol. Plant 2002, 45, 1–10. [Google Scholar] [CrossRef]
- Zheng, Q.; Song, J.; Campbell-Palmer, L.; Thompson, K.; Li, L.; Walker, B.; Cui, Y.; Li, X. A proteomic investigation of apple fruit during ripening and in response to ethylene treatment. J. Proteom. 2013, 93, 276–294. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Zhang, F.; Wang, H.; Zhang, C.; Zhang, S.; Zhang, J.; Xu, N.; Zhou, X.; Zhong, H.; Wu, X. Identification of trihelix transcription factors in grapevine and expression dynamics in response to biotic stress and hormone treatment. Physiol. Mol. Plant Pathol. 2025, 137, 102628. [Google Scholar] [CrossRef]

| Transcript ID | Given Name | Chr. | Strand | CDS (bp) | PL (A.A) | PMW (kDa) | pI | GRAVY |
|---|---|---|---|---|---|---|---|---|
| LITCHI024313.m1 | LcPK1 | 13 | Forward | 1512 | 503 | 54.61 | 6.73 | −0.023 |
| LITCHI003418.m1 | LcPK2 | 6 | Forward | 1542 | 513 | 55.35 | 6.35 | 0.070 |
| LITCHI006026.m1 | LcPK3 | 14 | Reverse | 1815 | 604 | 65.68 | 8.45 | 0.072 |
| LITCHI006031.m1 | LcPK4 | 14 | Reverse | 2130 | 709 | 77.42 | 6.83 | −0.032 |
| LITCHI018757.m1 | LcPK5 | 15 | Reverse | 1584 | 527 | 57.47 | 7.14 | 0.026 |
| LITCHI020380.m1 | LcPK6 | 12 | Forward | 1698 | 565 | 62.30 | 6.66 | −0.174 |
| LITCHI027188.m1 | LcPK7 | 3 | Forward | 1353 | 450 | 49.32 | 6.77 | −0.008 |
| LITCHI009466.m1 | LcPK8 | 7 | Forward | 1737 | 578 | 63.98 | 6.34 | −0.210 |
| LITCHI014910.m1 | LcPK9 | 1 | Forward | 1755 | 584 | 64.08 | 5.47 | −0.101 |
| LITCHI026147.m1 | LcPK10 | 3 | Reverse | 1557 | 518 | 57.26 | 5.59 | 0.025 |
| LITCHI001406.m1 | LcPK11 | 5 | Forward | 1755 | 584 | 64.84 | 5.93 | −0.141 |
| LITCHI008504.m1 | LcPK12 | 7 | Reverse | 237 | 78 | 86.10 | 8.69 | −0.262 |
| LITCHI012288.m1 | LcPK13 | 2 | Reverse | 1254 | 417 | 46.75 | 6.08 | −0.313 |
| LITCHI012309.m1 | LcPK14 | 2 | Forward | 693 | 230 | 25.77 | 5.43 | −0.174 |
| LITCHI010067.m1 | LcPK15 | 8 | Forward | 2106 | 702 | 77.50 | 7.84 | −0.172 |
| LITCHI012284.m1 | LcPK16 | 2 | Forward | 576 | 191 | 21.51 | 7.65 | −0.175 |
| LITCHI012308.m1 | LcPK17 | 2 | Forward | 849 | 282 | 31.85 | 6.41 | −0.350 |
| LITCHI012302.m1 | LcPK18 | 2 | Reverse | 324 | 107 | 12.00 | 5.00 | 0.036 |
| LITCHI012287.m1 | LcPK19 | 2 | Reverse | 951 | 316 | 35.62 | 5.06 | −0.198 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajjad, M.; Jiao, J.; Tahir, H.; Wei, L.; Ma, W.; Zeeshan Ul Haq, M.; Farooq, M.A.; Zhou, K. Genome-Wide Identification and Expression Profiling of Pyruvate Kinase Genes in Litchi Under Calcium-Magnesium Foliar Treatment. Plants 2025, 14, 2764. https://doi.org/10.3390/plants14172764
Sajjad M, Jiao J, Tahir H, Wei L, Ma W, Zeeshan Ul Haq M, Farooq MA, Zhou K. Genome-Wide Identification and Expression Profiling of Pyruvate Kinase Genes in Litchi Under Calcium-Magnesium Foliar Treatment. Plants. 2025; 14(17):2764. https://doi.org/10.3390/plants14172764
Chicago/Turabian StyleSajjad, Muhammad, Jiabing Jiao, Hassam Tahir, Ling Wei, Wuqiang Ma, Muhammad Zeeshan Ul Haq, Muhammad Amir Farooq, and Kaibing Zhou. 2025. "Genome-Wide Identification and Expression Profiling of Pyruvate Kinase Genes in Litchi Under Calcium-Magnesium Foliar Treatment" Plants 14, no. 17: 2764. https://doi.org/10.3390/plants14172764
APA StyleSajjad, M., Jiao, J., Tahir, H., Wei, L., Ma, W., Zeeshan Ul Haq, M., Farooq, M. A., & Zhou, K. (2025). Genome-Wide Identification and Expression Profiling of Pyruvate Kinase Genes in Litchi Under Calcium-Magnesium Foliar Treatment. Plants, 14(17), 2764. https://doi.org/10.3390/plants14172764







