Climate-Resilient Crops: Integrating AI, Multi-Omics, and Advanced Phenotyping to Address Global Agricultural and Societal Challenges
Abstract
1. Introduction
2. Climate Change and Agricultural Stress
2.1. Abiotic Stress Factors
2.1.1. Drought and Water Scarcity
2.1.2. Extreme Temperature Stress
2.2. Agricultural Management Strategies and Abiotic Stresses
3. Multi-Omics Approaches for Enhancing Crop Resilience
3.1. Genomics and Genetic Engineering
3.1.1. Genome-Wide Association Studies (GWAS)
3.1.2. CRISPR and Gene Editing for Stress Tolerance
3.2. Transcriptomics: Gene Expression Under Stress
3.2.1. RNA Sequencing (RNA-Seq) for Identifying Stress-Responsive Genes
3.2.2. Regulatory Networks in Plant Stress Adaptation
3.3. Proteomics and Metabolomics
3.3.1. Stress-Induced Changes in Protein Expression
3.3.2. Metabolic Pathways Involved in Plant Defense
3.4. Epigenomics and Environmental Adaptation
3.4.1. Role of DNA Methylation in Stress Memory
3.4.2. Transgenerational Adaptation Through Epigenetic Modifications
4. Field-Based Phenomics: Advanced Trait Monitoring
4.1. High-Throughput Screening Technologies
4.2. Integrating Multi-Omics and Phenomics
5. The Role of AI and ML
5.1. AI in Data Analysis and Prediction
5.2. AI in Precision Agriculture
5.3. AI and Automated Crop Management
6. Plant-Associated Microbiomes and Sustainable Agriculture
6.1. Microbiome Engineering for Stress Tolerance
6.1.1. Role of Beneficial Microbes in Drought and Salinity Resistance
6.1.2. Synthetic Microbial Communities for Crop Resilience
6.2. Bioinoculants and Soil Health Improvement
6.2.1. Enhancing Nutrient Uptake and Root Development
6.2.2. Imaging Methods for Evaluation of Root System Architecture
6.2.3. Reducing Dependency on Chemical Fertilizers
7. Societal, Social, and Economic Impacts of Climate-Resilient Crops
7.1. Global Food Security and Hunger Reduction
7.2. Integrating Climate-Agriculture Education
8. Future Directions
8.1. Integrating AI, Multi-Omics, and Phenomics in Crop Breeding
8.2. Investment in Climate-Smart Agricultural Technologies
8.3. Strengthening Global Collaborations and Research Initiatives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Standards | Topic | |
|---|---|---|
| Climate Change | Biotechnology | |
| NGSS [341] |
|
|
| AFNR [342] |
|
|
References
- Toromade, A.S.; Soyombo, D.A.; Kupa, E.; Ijomah, T.I. Reviewing the impact of climate change on global food security: Challenges and solutions. Int. J. Appl. Res. Soc. Sci. 2024, 6, 1403–1416. [Google Scholar] [CrossRef]
- FAO. The Impact of Disasters and Crises on Agriculture and Food Security: 2021; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/30c0d98d-1c21-48ef-b5d9-8d988e6fa6f2/content (accessed on 12 April 2025).
- United Nations. World Population Prospects 2022: Summary of Results; Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2022; Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf (accessed on 12 April 2025).
- EPA. Climate Change Impacts on Agriculture and Food Supply. Available online: https://www.epa.gov/climateimpacts/climate-change-impacts-agriculture-and-food-supply (accessed on 12 April 2025).
- Lobell, D.B.; Gourdji, S.M. The influence of climate change on global crop productivity. Plant Physiol. 2012, 160, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Walthall, C.L. Meeting global food needs: Realizing the potential via genetics × environment × management interactions. Agron. J. 2015, 107, 1215–1226. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Borlaug, N.E. Impacts of breeding on international collaborative wheat improvement. J. Agric. Sci. 2006, 144, 3–17. [Google Scholar] [CrossRef]
- Kamilaris, A.; Prenafeta-Boldú, F.X. Deep learning in agriculture: A state-of-the-art review. Comput. Electron. Agric. 2018, 147, 70–90. [Google Scholar] [CrossRef]
- Ferentinos, K.P. Deep learning models for plant disease detection and diagnosis. Comput. Electron. Agric. 2018, 145, 311–318. [Google Scholar] [CrossRef]
- Varshney, R.K.; Bohra, A.; Yu, J.; Graner, A.; Zhang, Q.; Sorrells, M.E. Designing future crops: Genomics-assisted breeding comes of age. Trends Plant Sci. 2021, 26, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.P.; Hellin, J.; Govaerts, B.; Kosina, P.; Sonder, K.; Hobbs, P.; Braun, H. Global crop improvement networks to bridge technology gaps. J. Exp. Bot. 2012, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Bohra, A.; Roorkiwal, M.; Barmukh, R.; Cowling, W.A.; Chitikineni, A.; Lam, H.M.; Hickey, L.T.; Croser, J.S.; Bayer, P.E.; et al. Fast-forward breeding for a food-secure world. Trends Genet. 2021, 37, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Thingujam, D.; Tan, Z.; Wang, Y.; Pajerowska-Mukhtar, K.M.; Mukhtar, M.S. PRIMER Cells: Immune Hotspots in Plants. Trends Plant Sci. 2025, in press. [CrossRef]
- Araus, J.L.; Serret, M.D.; Edmeades, G.O. Phenotyping maize for adaptation to drought. Front. Physiol. 2012, 3, 305. [Google Scholar] [CrossRef]
- Thingujam, D.; Liu, J.; Majeed, A.; Mukhtar, M.S. Plant-Microbiome Dynamics through Spatial Metatranscriptomics and Network Biology. Trends Plant Sci. 2024, 29, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar]
- The Intergovernmental Working Group on Drought. The Blue Paper: Drought Risks, Resilience and Restoration; UNCCD: Bonn, Germany, 2022; Available online: https://www.unccd.int/resources/all-resources (accessed on 12 May 2025).
- Furtak, K.; Wolińska, A. The impact of extreme weather events as a consequence of climate change on the soil moisture and on the quality of the soil environment and agriculture—A review. Catena 2023, 231, 107378. [Google Scholar] [CrossRef]
- Tabari, H.; Hosseinzadehtalaei, P.; Thiery, W.; Willems, P. Amplified drought and flood risk under future socioeconomic and climatic change. Earth’s Future 2021, 9, e2021EF002295. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Res. Environ. Sustain. 2021, 2021, 100032. [Google Scholar] [CrossRef]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water scarcity in agriculture: An overview of causes, impacts and approaches for reducing the risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and Biotic Stress Combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Kodadinne Narayana, N.; Wijewardana, C.; Alsajri, F.A.; Reddy, K.R.; Stetina, S.R.; Bheemanahalli, R. Resilience of soybean genotypes to drought stress during the early vegetative stage. Sci. Rep. 2024, 14, 17365. [Google Scholar] [CrossRef]
- Poudel, S.; Adhikari, B.; Dhillon, J.; Reddy, K.R.; Stetina, S.R.; Bheemanahalli, R. Quantifying the physiological, yield, and quality plasticity of southern USA soybeans under heat stress. Plant Stress 2023, 9, 100195. [Google Scholar] [CrossRef]
- Vennam, R.R.; Poudel, S.; Ramamoorthy, P.; Samiappan, S.; Reddy, K.R.; Bheemanahalli, R. Impact of soil moisture stress during the silk emergence and grain-filling in maize. Physiol. Plant. 2023, 175, e14029. [Google Scholar] [CrossRef]
- Bista, M.K.; Adhikari, B.; Sankarapillai, L.V.; Pieralisi, B.; Reddy, K.R.; Jenkins, J.; Bheemanahalli, R. Drought and heat stress induce differential physiological and agronomic trait responses in cotton. Ind. Crops Prod. 2024, 222, 119540. [Google Scholar] [CrossRef]
- Leng, G.; Hall, J. Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci. Total Environ. 2019, 654, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Tijjani, S.B.; Giri, S.; Woznicki, S.A. Quantifying the potential impacts of climate change on irrigation demand, crop yields, and green water scarcity in the New Jersey coastal plain. Sci. Total Environ. 2022, 838, 156538. [Google Scholar] [CrossRef]
- Poudel, S.; Vennam, R.V.; Sankarapillai, L.V.; Liu, J.; Reddy, K.R.; Wijewardane, N.K.; Mukhtar, M.S.; Bheemanahalli, R. Negative synergistic effects of drought and heat during flowering and seed setting in soybean. Environ. Exp. Bot. 2024, 222, 105769. [Google Scholar] [CrossRef]
- Chakravaram, A.; Sankarapillai, L.V.; Poudel, S.; Bheemanahalli, R. Interactive effects of drought and high night temperature on physiology and yield components of cowpea (Vigna unguiculata (L.) Walp.). J. Agric. Food Res. 2025, 21, 101844. [Google Scholar] [CrossRef]
- Heino, M.; Kinnunen, P.; Anderson, W.; Ray, D.K.; Puma, M.J.; Varis, O.; Siebert, S.; Kummu, M. Increased probability of hot and dry weather extremes during the growing season threatens global crop yields. Sci. Rep. 2023, 13, 3583. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Ramamoorthy, P.; Poudel, S.; Samiappan, S.; Wijewardane, N.; Reddy, K.R. Effects of drought and heat stresses during reproductive stage on pollen germination, yield, and leaf reflectance properties in maize (Zea mays L.). Plant Direct 2022, 6, e434. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Sunoj, V.S.J.; Saripalli, G.; Prasad, P.V.V.; Balyan, H.S.; Gupta, P.K.; Grant, N.; Gill, K.S.; Jagadish, S.V.K. Quantifying the impact of heat stress on pollen germination, seed set, and grain filling in spring wheat. Crop Sci. 2019, 59, 684–696. [Google Scholar] [CrossRef]
- Poudel, S.; Vennam, R.R.; Shrestha, A.; Reddy, K.R.; Wijewardane, N.K.; Reddy, K.N.; Bheemanahalli, R. Resilience of soybean cultivars to drought stress during flowering and early-seed setting stages. Sci. Rep. 2023, 13, 1277. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef]
- Mansfield, T.A. Environmental physiology of plants. In: Fitter, A.H.; Hay, R.K.M. Ann. Bot. 2002, 89, 650. [Google Scholar] [CrossRef][Green Version]
- Nievola, C.C.; Carvalho, C.P.; Carvalho, V.; Rodrigues, E. Rapid responses of plants to temperature changes. Temperature 2017, 4, 371–405. [Google Scholar] [CrossRef]
- Hincha, D.K.; Zuther, E. Introduction: Plant cold acclimation and freezing tolerance. Methods Mol. Biol. 2014, 1166, 1–6. [Google Scholar] [PubMed]
- Guy, C.L. Cold acclimation and freezing stress tolerance: Role of protein metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 187–223. [Google Scholar] [CrossRef]
- Moghimi, N.; Desai, J.S.; Bheemanahalli, R.; Impa, S.M.; Vennapusa, A.R.; Sebela, D.; Perumal, R.; Doherty, C.J. New candidate loci and marker genes on chromosome 7 for improved chilling tolerance in sorghum. J. Exp. Bot. 2019, 70, 3357–3371. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, Z.; Kong, X.; Khan, A.; Ullah, N.; Zhang, X. Plant coping with cold stress: Molecular and physiological adaptive mechanisms with future perspectives. Cells 2025, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Martini, L.F.; Roma-Burgos, N.; Tseng, T.; Fipke, M.V.; Noldin, J.A.; de Avila, L.A. Acclimation to cold stress reduces injury from low temperature and bispyribac-sodium on rice. Pest Manag. Sci. 2021, 77, 4016–4025. [Google Scholar] [CrossRef]
- Adhikari, L.; Makaju, S.O.; Lindstrom, O.M.; Missaoui, A.M. Mapping Freezing Tolerance QTL in Alfalfa: Based on Indoor Phenotyping. BMC Plant Biol. 2021, 21, 403. [Google Scholar] [CrossRef]
- Goering, R.; Larsen, S.; Tan, J.; Whelan, J.; Makarevitch, I. QTL mapping of seedling tolerance to exposure to low temperature in the maize IBM RIL population. PLoS ONE 2021, 16, e0254437. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extremes 2015, 10, 4–10. [Google Scholar]
- Jagadish, S.; Craufurd, P.; Wheeler, T. High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Aiqing, S.; Somayanda, I.; Sebastian, S.V.; Singh, K.; Gill, K.; Prasad, P.V.V.; Jagadish, S.V.K. Heat stress during flowering affects time of day of flowering, seed set and grain quality in spring wheat. Crop Sci. 2018, 58, 380–392. [Google Scholar] [CrossRef]
- Morice, C.P.; Kennedy, J.J.; Rayner, N.A.; Winn, J.P.; Hogan, E.; Killick, R.E.; Dunn, R.J.H.; Osborn, T.J.; Jones, P.D.; Simpson, I.R. An updated assessment of near-surface temperature change from 1850: The HadCRUT5 dataset. J. Geophys. Res. Atmos. 2020, 126, e2019JD032361. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.; et al. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosyst. 2020, 155, 211–234. [Google Scholar] [CrossRef]
- Zhao, G.; Xu, H.; Zhang, P.; Su, X.; Zhao, H. Effects of 2,4-epibrassinolide on photosynthesis and Rubisco activase gene expression in Triticum aestivum L. seedlings under a combination of drought and heat stress. Plant Growth Regul. 2017, 81, 377–384. [Google Scholar] [CrossRef]
- Sankarapillai, L.V.; Adhikari, B.; Bista, M.K.; Shrestha, A.; Stetina, S.R.; Reddy, K.R.; Bheemanahalli, R. High night temperature disrupts the assimilate utilization and yield potential in soybean. Plant Stress 2025, 16, 100826. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, Y.; Sun, J.; Mao, F.; Yao, Q.; Li, B.; Wang, Y.; Gao, Y.; Dong, X.; Liao, S.; et al. From the Floret to the Canopy: High Temperature Tolerance during Flowering. Plant Commun. 2023, 4, 100629. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Bheemanahalli, R.; Jagadish, S.V.K. Field crops and the fear of heat stress—Opportunities, challenges and future directions. Field Crops Res. 2017, 200, 114–121. [Google Scholar] [CrossRef]
- Lohani, N.; Singh, M.B.; Bhalla, P.L. High temperature susceptibility of sexual reproduction in crop plants. J. Exp. Bot. 2019, 71, 555–568. [Google Scholar] [CrossRef]
- Impa, S.M.; Raju, B.; Hein, N.T.; Sandhu, J.; Prasad, P.V.V.; Walia, H.; Jagadish, S.V.K. High night temperature effects on wheat and rice: Current status and way forward. Plant Cell Environ. 2021, 44, 2049–2065. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Feng, B.; Zhang, C.; Yang, Y.; Yang, X.; Chen, T.; Zhao, X.; Zhang, X.; Jin, Q.; Tao, L. Heat stress is more damaging to superior spikelets than inferiors of rice (Oryza sativa L.) due to their different organ temperatures. Front. Plant Sci. 2016, 7, 1637. [Google Scholar] [CrossRef] [PubMed]
- Bheemanahalli, R.; Sathishraj, R.; Manoharan, M.; Sumanth, H.N.; Muthurajan, R.; Ishimaru, T.; Krishna, J.S.V. Is early morning flowering an effective trait to minimize heat stress damage during flowering in rice? Field Crops Res. 2017, 203, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Wang, C.; Hao, H.; Song, W.; Guo, X. Maize Anthesis-Silking Interval Estimation via Image Detection under Field Rail-Based Phenotyping Platform. Agronomy 2024, 14, 1723. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Fei, K.; Wang, R.; He, J.; Fu, L.; Shao, S.; Li, K.; Zhu, K.; Zhang, W.; et al. Physiological mechanism underlying the effect of high temperature during anthesis on spikelet-opening of photo-thermo-sensitive genic male sterile rice lines. Sci. Rep. 2020, 10, 2210. [Google Scholar] [CrossRef]
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.S.V.; Prasad, P.V.V.; Siddique, K.H.M. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M. The Heat Is On: Maize Pollen Development after a Heat Wave. Plant Physiol. 2019, 181, 387–388. [Google Scholar] [CrossRef]
- Choi, S.; Harvey, J.T.; Leskovar, D.I. Solid humic substance enhanced spinach abiotic stress tolerance under combined drought, salinity, and daily heat stress. Plant Stress 2024, 13, 100544. [Google Scholar] [CrossRef]
- Jiang, Q.; Lou, R.; Madramootoo, C.A.; Qi, Z.; Xue, L.; Bukovsky, M.; He, Y. Potential contribution of water management practices under intensive crop production to climate-change-associated global warming. J. Clean. Prod. 2024, 470, 143230. [Google Scholar] [CrossRef]
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.-H.; Li, W.-J. Soil salinity and drought tolerance: An evaluation of plant growth, productivity, microbial diversity, and amelioration strategies. Plant Stress 2024, 11, 100319. [Google Scholar] [CrossRef]
- Singh, G.; Davis, M.; Nelson, K.A.; Kaur, G. Drainage water management, woodchip bioreactor, and saturated riparian buffer as stacked conservation practices for improving crop yields and water quality. Environ. Technol. Innov. 2024, 36, 103779. [Google Scholar] [CrossRef]
- Hashimi, R.; Huang, Q.; Dewi, R.K.; Nishiwaki, J.; Komatsuzaki, M. No-tillage and rye cover crop systems improve soil water retention by increasing soil organic carbon in Andosols under humid subtropical climate. Soil Tillage Res. 2023, 234, 105861. [Google Scholar] [CrossRef]
- Liang, S.; Sun, N.; Meersmans, J.; Longdoz, B.; Colinet, G.; Xu, M.; Wu, L. Impacts of climate change on crop production and soil carbon stock in a continuous wheat cropping system in southeast England. Agric. Ecosyst. Environ. 2024, 365, 108909. [Google Scholar] [CrossRef]
- Locatelli, J.L.; Del Grosso, S.; Santos, R.S.; Hong, M.; Gurung, R.; Stewart, C.E.; Cherubin, M.R.; Bayer, C.; Cerri, C.E. Modeling Soil Organic Matter Changes under Crop Diversification Strategies and Climate Change Scenarios in the Brazilian Cerrado. Agric. Ecosyst. Environ. 2025, 379, 109334. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, W.; Yao, R.; Feng, Y.; Wang, X.; Xie, H.; Feng, Y.; Yang, J. Biochar and Hydrochar Application Influence Soil Ammonia Volatilization and the Dissolved Organic Matter in Salt-Affected Soils. Sci. Total Environ. 2024, 926, 171845. [Google Scholar] [CrossRef]
- Taweengern, K.; Thapsamut, T.; Khaobang, C.; Areeprasert, C.; Aramrak, S. Circular utilization of sugarcane bagasse for water and nutrient retention in two-type of sugarcane cultivation soil by biochar and hydrochar addition. Fuel 2025, 392, 134870. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, L.; Trakal, L.; Wang, S.; Shaheen, S.M.; Rinklebe, J.; Chen, Q. Pyrolytic and hydrothermal carbonization affect the transformation of phosphorus fractions in the biochar and hydrochar derived from organic materials: A meta-analysis study. Sci. Total Environ. 2024, 906, 167418. [Google Scholar] [CrossRef] [PubMed]
- Bona, D.; Bertoldi, D.; Borgonovo, G.; Mazzini, S.; Ravasi, S.; Silvestri, S.; Zaccone, C.; Giannetta, B.; Tambone, F. Evaluating the Potential of Hydrochar as a Soil Amendment. Waste Manag. 2023, 159, 75–83. [Google Scholar] [CrossRef]
- Muema, F.M.; Richardson, Y.; Keita, A.; Sawadogo, M. An interdisciplinary overview on biochar production engineering and its agronomic applications. Biomass Bioenergy 2024, 190, 107416. [Google Scholar] [CrossRef]
- Al-Nuaimy, M.N.M.; Azizi, N.; Nural, Y.; Yabalak, E. Recent advances in environmental and agricultural applications of hydrochars: A review. Environ. Res. 2024, 250, 117923. [Google Scholar] [CrossRef]
- Yao, H.; Cheng, Y.; Kong, Q.; Wang, X.; Rong, Z.; Quan, Y.; You, X.; Zheng, H.; Li, Y. Variation in Microbial Communities and Network Ecological Clusters Driven by Soil Organic Carbon in an Inshore Saline Soil Amended with Hydrochar in Yellow River Delta, China. Environ. Res. 2025, 264, 120369. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Benavente, V.; Jansson, S.; Mašek, O. Comparative characterisation and phytotoxicity assessment of biochar and hydrochar derived from municipal wastewater microalgae biomass. Bioresour. Technol. 2023, 386, 129567. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Liu, X.; Huang, Y.; Cai, A.; Xu, H.; Ran, J.; Xiao, J.; Zhang, W. A global dataset of biochar application effects on crop yield, soil properties, and greenhouse gas emissions. Sci. Data 2024, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Haruna, S.I. Effects of cover crop on selected abiotic and biotic soil health indicators. Environ. Chall. 2024, 17, 101045. [Google Scholar] [CrossRef]
- Klopp, H.W.; Blanco-Canqui, H.; Jasa, P.; Slater, G.; Ferguson, R.B. Lessons about Soil Health and Corn Yield after a Decade of Cover Crop and Corn Residue Management. Field Crops Res. 2025, 326, 109860. [Google Scholar] [CrossRef]
- Connell, R.K.; James, T.Y.; Blesh, J. A legume-grass cover crop builds mineral-associated organic matter across variable agricultural soils. Soil Biol. Biochem. 2025, 203, 109726. [Google Scholar] [CrossRef]
- Scarlato, M.; Rieppi, M.; Alliaume, F.; Illarze, G.; Bajsa, N.; Bertoni, P.; Bianchi, F.J.; Echeverriborda, G.; Galván, G.; de Souza, M.G.; et al. Towards the Development of Cover Crop-Reduced Tillage Systems without Herbicides and Synthetic Fertilizers in Onion Cultivation: Promising but Challenges Remain. Soil Tillage Res. 2024, 240, 106061. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, L.; Jacinthe, P.A.; Ren, W. Global synthesis of cover crop impacts on main crop yield. Field Crops Res. 2024, 310, 109343. [Google Scholar] [CrossRef]
- Sui, X.; Bao, X.; Xie, H.; Ba, X.; Yu, Y.; Yang, Y.; He, H.; Liang, C.; Zhang, X. Contrasting Seasonal Effects of Legume and Grass Cover Crops as Living Mulch on the Soil Microbial Community and Nutrient Metabolic Limitations. Agric. Ecosyst. Environ. 2025, 380, 109374. [Google Scholar] [CrossRef]
- Rauber, L.R.; Reinert, D.J.; Gubiani, P.I.; Loss, A. Structure and water infiltration in an Ultisol affected by cover crops and seasonality. Soil Tillage Res. 2025, 247, 106366. [Google Scholar] [CrossRef]
- Ali, W.; Gao, R.; Zhao, T.; Chen, J.; He, Y.; Luo, T.; Hussain, S. Fall/winter cover crop roots change soil hydrology to control the drought status of subsequent season summer maize in Ultisol. Soil Tillage Res. 2024, 236, 105948. [Google Scholar] [CrossRef]
- Yu, Y.; Loiskandl, W.; Kaul, H.-P.; Himmelbauer, M.; Wei, W.; Chen, L.; Bodner, G. Estimation of runoff mitigation by morphologically different cover crop root systems. J. Hydrol. 2016, 538, 667–676. [Google Scholar] [CrossRef]
- Mackie, K.A.; Schmidt, H.P.; Müller, T.; Kandeler, E. Cover crops influence soil microorganisms and phytoextraction of copper from a moderately contaminated vineyard. Sci. Total Environ. 2014, 500–501, 34–43. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; De Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-wide association studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Bajgain, P.; Zhang, X.; Anderson, J.A. Genome-Wide Association Study of Yield Component Traits in Intermediate Wheatgrass and Implications in Genomic Selection and Breeding. G3 2019, 9, 2429–2439. [Google Scholar] [CrossRef]
- Ma, J.; Cao, Y. Genetic Dissection of Grain Yield of Maize and Yield-Related Traits through Association Mapping and Genomic Prediction. Front. Plant Sci. 2021, 12, 690059. [Google Scholar] [CrossRef]
- Sukumaran, S.; Reynolds, M.P.; Sansaloni, C. Genome-wide association analyses identify QTL hotspots for yield and component traits in durum wheat grown under yield potential, drought, and heat stress environments. Front. Plant Sci. 2018, 9, 81. [Google Scholar] [CrossRef]
- Yasir, M.; He, S.; Sun, G.; Geng, X.; Pan, Z.; Gong, W.; Jia, Y.; Du, X. A genome-wide association study revealed key SNPs/genes associated with salinity stress tolerance in upland cotton. Genes 2019, 10, 829. [Google Scholar] [CrossRef]
- Devate, N.B.; Krishna, H.; Parmeshwarappa, S.K.; Manjunath, K.K.; Chauhan, D.; Singh, S.; Singh, J.B.; Kumar, M.; Patil, R.; Khan, H.; et al. Genome-Wide Association Mapping for Component Traits of Drought and Heat Tolerance in Wheat. Front. Plant Sci. 2022, 13, 943033. [Google Scholar] [CrossRef]
- Chen, S.; Dang, D.; Liu, Y.; Ji, S.; Zheng, H.; Zhao, C.; Dong, X.; Li, C.; Guan, Y.; Zhang, A.; et al. Genome-wide association study presents insights into the genetic architecture of drought tolerance in maize seedlings under field water-deficit conditions. Front. Plant Sci. 2023, 14, 1165582. [Google Scholar] [CrossRef]
- Li, C.; Guo, J.; Wang, D.; Chen, X.; Guan, H.; Li, Y.; Zhang, D.; Liu, X.; He, G.; Wang, T.; et al. Genomic insight into changes of root architecture under drought stress in maize. Plant Cell Environ. 2023, 46, 1860–1872. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.G.; Zhang, X.; Beyene, Y.; Semagn, K.; Olsen, M.; Prasanna, B.M.; Buckler, E.S. Genome-wide association for plant height and flowering time across 15 tropical maize populations under managed drought stress and well-watered conditions in Sub-Saharan Africa. Crop Sci. 2016, 56, 2365–2378. [Google Scholar] [CrossRef]
- Warraich, A.S.; Krishnamurthy, S.L.; Sooch, B.S.; Vinaykumar, N.M.; Dushyanthkumar, B.M.; Bose, J.; Sharma, P.C. Rice GWAS reveals key genomic regions essential for salinity tolerance at reproductive stage. Acta Physiol. Plant. 2020, 42, 134. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, A.; Mithra, S.A.; Krishnamurthy, S.L.; Parida, S.K.; Jain, S.; Tiwari, K.K.; Kumar, P.; Rao, A.R.; Sharma, S.K.; et al. Genome-Wide Association Mapping of Salinity Tolerance in Rice (Oryza sativa). DNA Res. 2015, 22, 133–145. [Google Scholar] [CrossRef]
- Haak, D.C.; Fukao, T.; Grene, R.; Hua, Z.; Ivanov, R.; Perrella, G.; Li, S. Multilevel regulation of abiotic stress responses in plants. Front. Plant Sci. 2017, 8, 1564. [Google Scholar] [CrossRef] [PubMed]
- Thoen, M.P.; Davila Olivas, N.H.; Kloth, K.J.; Coolen, S.; Huang, P.P.; Aarts, M.G.; Bac-Molenaar, J.A.; Bakker, J.; Bouwmeester, H.J.; Broekgaarden, C.; et al. Genetic Architecture of Plant Stress Resistance: Multi-Trait Genome-Wide Association Mapping. New Phytol. 2017, 213, 1346–1362. [Google Scholar] [CrossRef]
- Gantait, S.; Sarkar, S.; Verma, S.K. Marker-assisted selection for abiotic stress tolerance in crop plants. In Molecular Plant Abiotic Stress: Biology and Biotechnolog; Wiley: Hoboken, NJ, USA, 2019; pp. 335–368. [Google Scholar]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Kim, D.; Alptekin, B.; Budak, H. CRISPR/Cas9 genome editing in wheat. Funct. Integr. Genom. 2018, 18, 31–41. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Y.; Lv, P.; Antwi-Boasiako, A.; Begum, N.; Zhao, T.; Zhao, J. NAC transcription factor GmNAC12 improved drought stress tolerance in soybean. Int. J. Mol. Sci. 2022, 23, 12029. [Google Scholar] [CrossRef]
- Bo, W.; Zhaohui, Z.; Huanhuan, Z.; Xia, W.; Binglin, L.; Lijia, Y.; Xiangyan, H.; Deshui, Y.; Xuelian, Z.; Chunguo, W.; et al. Targeted Mutagenesis of NAC Transcription Factor Gene, OsNAC041, Leading to Salt Sensitivity in Rice. Rice Sci. 2019, 26, 98–108. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, Y.G.; Zhao, K. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef]
- Liu, D.; Chen, X.; Liu, J.; Ye, J.; Guo, Z. The rice ERF transcription factor OsERF922 negatively regulates resistance to Magnaporthe oryzae and salt tolerance. J. Exp. Bot. 2012, 63, 3899–3911. [Google Scholar] [CrossRef] [PubMed]
- Távora, F.T.; Meunier, A.C.; Vernet, A.; Portefaix, M.; Milazzo, J.; Adreit, H.; Tharreau, D.; Franco, O.L.; Mehta, A. CRISPR/Cas9-Targeted Knockout of Rice Susceptibility Genes OsDjA2 and OsERF104 Reveals Alternative Sources of Resistance to Pyricularia oryzae. Rice Sci. 2022, 29, 535–544. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Wang, F.; Li, T.; Chen, Z.; Kong, D.; Bi, J.; Zhang, F.; Luo, X.; Wang, J.; et al. Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Mol. Breed. 2019, 39, 47. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Kim, E.G.; Jang, Y.H.; Jan, R.; Farooq, M.; Ubaidillah, M.; Kim, K.M. Applications of CRISPR/Cas9 as New Strategies for Short Breeding to Drought Gene in Rice. Front. Plant Sci. 2022, 13, 850441. [Google Scholar] [CrossRef]
- Shen, L.; Wang, C.; Fu, Y.; Wang, J.; Liu, Q.; Zhang, X.; Yan, C.; Qian, Q.; Wang, K. QTL Editing Confers Opposing Yield Performance in Different Rice Varieties. J. Integr. Plant Biol. 2018, 60, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Jian, C.; Cheng, X.; Chen, B.; Mei, F.; Li, F.; Zhang, Y.; Li, S.; Du, L.; Li, T.; et al. The Wheat ABA Receptor Gene TaPYL1-1B Contributes to Drought Tolerance and Grain Yield by Increasing Water-Use Efficiency. Plant Biotechnol. J. 2022, 20, 846–861. [Google Scholar] [CrossRef]
- Lyzenga, W.J.; Pozniak, C.J.; Kagale, S. Advanced domestication: Harnessing the precision of gene editing in crop breeding. Plant Biotechnol. J. 2021, 19, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Rahim, A.A.; Uzair, M.; Rehman, N.; Fiaz, S.; Attia, K.A.; Abushady, A.M. CRISPR/Cas9 mediated TaRPK1 root architecture gene mutagenesis confers enhanced wheat yield. J. King Saud Univ. Sci. 2024, 36, 103063. [Google Scholar] [CrossRef]
- Li, X.; Xu, S.; Fuhrmann-Aoyagi, M.B.; Yuan, S.; Iwama, T.; Kobayashi, M.; Miura, K. CRISPR/Cas9 technique for temperature, drought, and salinity stress responses. Curr. Issues Mol. Biol. 2022, 44, 2664–2682. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Kumar, S.; Seem, K.; Kumar, S.; Mohapatra, T. RNA-seq analysis reveals the genes/pathways responsible for genetic plasticity of rice to varying environmental conditions on direct-sowing and transplanting. Sci. Rep. 2022, 12, 2241. [Google Scholar] [CrossRef]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional factors regulate plant stress responses through mediating secondary metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef]
- Chen, W.; Yao, Q.; Patil, G.B.; Agarwal, G.; Deshmukh, R.K.; Lin, L.; Wang, B.; Wang, Y.; Prince, S.J.; Song, L.; et al. Identification and Comparative Analysis of Differential Gene Expression in Soybean Leaf Tissue under Drought and Flooding Stress Revealed by RNA-Seq. Front. Plant Sci. 2016, 7, 1044. [Google Scholar] [CrossRef]
- Seok, H.Y.; Nguyen, L.V.; Nguyen, D.V.; Lee, S.Y.; Moon, Y.H. Investigation of a novel salt stress-responsive pathway mediated by Arabidopsis DEAD-box RNA helicase gene AtRH17 using RNA-Seq analysis. Int. J. Mol. Sci. 2020, 21, 1595. [Google Scholar] [CrossRef]
- Guo, X.L.; Yuan, S.N.; Zhang, H.N.; Zhang, Y.Y.; Zhang, Y.J.; Wang, G.Y.; Li, Y.Q.; Li, G.L. Heat-Response Patterns of the Heat Shock Transcription Factor Family in Advanced Development Stages of Wheat (Triticum aestivum L.) and Thermotolerance-Regulation by TaHsfA2-10. BMC Plant Biol. 2020, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Badia-i-Mompel, P.; Wessels, L.; Müller-Dott, S.; Trimbour, R.; Ramirez Flores, R.O.; Argelaguet, R.; Saez-Rodriguez, J. Gene regulatory network inference in the era of single-cell multi-omics. Nat. Rev. Genet. 2023, 24, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Mishra, B.K.; Liu, J.; Mohan, B.; Thingujam, D.; Pajerowska-Mukhtar, K.M.; Mukhtar, M.S. Network biology analyses and dynamic modeling of gene regulatory networks under drought stress reveal major transcriptional regulators in Arabidopsis. Int. J. Mol. Sci. 2023, 24, 7349. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Song, Y.; Li, S.; Zhang, L.; Zou, C.; Yu, D. The role of WRKY transcription factors in plant abiotic stresses. Biochim. Biophys. Acta Gene Regul. Mech. 2012, 1819, 120–128. [Google Scholar] [CrossRef]
- Mohan, B.; Thingujam, D.; Pajerowska-Mukhtar, K.M.; Mukhtar, M.S. EccDNA in plant-stress and biotechnological solutions in agriculture. Trends Biotechnol. 2024, 42, 1588–1591. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, F.; Zhou, B. The characters of non-coding RNAs and their biological roles in plant development and abiotic stress response. Int. J. Mol. Sci. 2022, 23, 4124. [Google Scholar] [CrossRef]
- Yang, H.; Cui, Y.; Feng, Y.; Hu, Y.; Liu, L.; Duan, L. Long non-coding RNAs of plants in response to abiotic stresses and their regulating roles in promoting environmental adaptation. Cells 2023, 12, 729. [Google Scholar] [CrossRef]
- Jha, U.C.; Nayyar, H.; Jha, R.; Khurshid, M.; Zhou, M.; Mantri, N.; Siddique, K.H. Long non-coding RNAs: Emerging players regulating plant abiotic stress response and adaptation. BMC Plant Biol. 2020, 20, 466. [Google Scholar] [CrossRef] [PubMed]
- Kosová, K.; Vítámvás, P.; Prášil, I.T.; Renaut, J. Plant proteome changes under abiotic stress—Contribution of proteomics studies to understanding plant stress response. J. Proteom. 2011, 74, 1301–1322. [Google Scholar] [CrossRef]
- Balasubramani, S.; Gayathiri, E.; Arya, S.K.; Gurusamy, D.; Prakash, P.; Appu, M.; Moola, A.K. Role of heat shock proteins in abiotic and biotic stress response in plants. In Defense-Related Proteins in Plants; Academic Press: Cambridge, MA, USA, 2024; pp. 299–332. [Google Scholar]
- Ali, M.K.; Azhar, A.; Salam, E.; Galani, S. Differential expression of molecular chaperon (Hsp70) and antioxidant enzymes: Inducing thermotolerance in rice (Oryza sativa L.). Pak. J. Bot. 2017, 49, 229–238. [Google Scholar]
- Zhou, Y.; Li, H.; Chen, H.; Yang, X.; Yu, T.; Wang, Y.; Wang, Y.; Jiang, K.; Wang, Y.; Chen, Z.; et al. Proteomic Investigation of Molecular Mechanisms in Response to PEG-Induced Drought Stress in Soybean Roots. Plants 2022, 11, 1173. [Google Scholar] [CrossRef]
- Latef, A.A.; Jan, S.; Abd-Allah, E.F.; Rashid, B.; John, R.; Ahmad, P. Soybean under abiotic stress: Proteomic approach. In Plant-Environment Interaction: Responses and Approaches to Mitigate Stress; Wiley: Hoboken, NJ, USA, 2016; pp. 28–42. [Google Scholar]
- Sharma, M.; Sidhu, A.K.; Samota, M.K.; Gupta, M.; Koli, P.; Choudhary, M. Post-translational modifications in histones and their role in abiotic stress tolerance in plants. Proteomes 2023, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, A.; Yadav, N.; Yadav, D.K. Current perspectives of ubiquitination and SUMOylation in abiotic stress tolerance in plants. Front. Plant Sci. 2022, 13, 993194. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, E.; Borner, G.H.H. Spatial proteomics: A powerful discovery tool for cell biology. Nat. Rev. Mol. Cell Biol. 2019, 20, 285–302. [Google Scholar] [CrossRef]
- Mohan, B.; Thingujam, D.; Pajerowska-Mukhtar, K.M. Cytotrap: An innovative approach for protein-protein interaction studies for cytoplasmic proteins. Methods Mol. Biol. 2023, 2690, 9–22. [Google Scholar]
- Yang, X.; Jiang, Z.; He, J.; Shen, L. iTRAQ-Based Quantitative Proteomics Unveils Protein Dynamics in the Root of Solanum melongena L. under Waterlogging Stress Conditions. Life 2023, 13, 1399. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Bharat, J.S.; Kumar, A.; Jaitak, V. Recent Advancement in Mass Based Plant Metabolomics: Techniques, Tools, and Analytical Approaches. Phytochem. Rev. 2024, 1–38, in press. [Google Scholar] [CrossRef]
- Misra, B.B. New Software Tools, Databases, and Resources in Metabolomics: Updates from 2020. Metabolomics 2021, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Fujita, M. Roles of osmolytes in plant adaptation to drought and salinity. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 37–68. [Google Scholar]
- Jabeen, M.; Jabeen, A.; Ahmad, M. Role of compatible solutes in alleviating effect of abiotic stress in plants. Int. Res. J. Educ. Innov. 2022, 3, 141–153. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Anti-Oxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Saleem, S.; Ul Mushtaq, N.; Shah, W.H.; Rasool, A.; Hakeem, K.R.; Ul Rehman, R. Beneficial role of phytochemicals in oxidative stress mitigation in plants. In Antioxidant Defense in Plants: Molecular Basis of Regulation; Springer: Singapore, 2022; pp. 435–451. [Google Scholar]
- Sharma, A.; Kohli, S.K.; Khanna, K.; Ramakrishnan, M.; Kumar, V.; Bhardwaj, R.; Brestic, M.; Skalicky, M.; Landi, M.; Zheng, B. Salicylic acid: A phenolic molecule with multiple roles in salt-stressed plants. J. Plant Growth Regul. 2023, 42, 4581–4605. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, J. Chloroplasts at the crossroad of photosynthesis, pathogen infection and plant defense. Int. J. Mol. Sci. 2018, 19, 3900. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Z.; Wang, F.; Jia, W.; Xu, Z. Combined transcriptomic and metabolomic analyses uncover rearranged gene expression and metabolite metabolism in tobacco during cold acclimation. Sci. Rep. 2020, 10, 5242. [Google Scholar] [CrossRef]
- Kumar, M.; Rani, K. Epigenomics in Stress Tolerance of Plants under the Climate Change. Mol. Biol. Rep. 2023, 50, 6201–6216. [Google Scholar] [CrossRef]
- Pandian, K.; Matsui, M.; Hankemeier, T.; Ali, A.; Okubo-Kurihara, E. Advances in Single-Cell Metabolomics to Unravel Cellular Heterogeneity in Plant Biology. Plant Physiol. 2023, 193, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, R.; Zhao, Q. Multi-Omics Techniques in Genetic Studies and Breeding of Forest Plants. Forests 2023, 14, 1196. [Google Scholar] [CrossRef]
- Wei, W.; Wang, L.F.; Tao, J.J.; Zhang, W.K.; Chen, S.Y.; Song, Q.; Zhang, J.S. The Comprehensive Regulatory Network in Seed Oil Biosynthesis. J. Integr. Plant Biol. 2025, 67, 649–668. [Google Scholar] [CrossRef]
- Ashapkin, V.V.; Kutueva, L.I.; Aleksandrushkina, N.I.; Vanyushin, B.F. Epigenetic mechanisms of plant adaptation to biotic and abiotic stresses. Int. J. Mol. Sci. 2020, 21, 7457. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Seki, M. Epigenetic memory for stress response and adaptation in plants. Plant Cell Physiol. 2014, 55, 1859–1863. [Google Scholar] [CrossRef]
- Vaissière, T.; Sawan, C.; Herceg, Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat. Res. Rev. Mutat. Res. 2008, 659, 40–48. [Google Scholar] [CrossRef]
- Sun, M.; Yang, Z.; Liu, L.; Duan, L. DNA methylation in plant responses and adaption to abiotic stresses. Int. J. Mol. Sci. 2022, 23, 6910. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Roychoudhury, A. Epigenetic regulation during salinity and drought stress in plants: Histone modifications and DNA methylation. Plant Gene 2017, 11, 199–204. [Google Scholar] [CrossRef]
- Choi, J.Y.; Purugganan, M.D. Evolutionary epigenomics of retrotransposon-mediated methylation spreading in rice. Mol. Biol. Evol. 2018, 35, 365–382. [Google Scholar] [CrossRef]
- Kleinmanns, J.A.; Schubert, D. Polycomb and Trithorax group protein-mediated control of stress responses in plants. Biol. Chem. 2014, 395, 1291–1300. [Google Scholar] [CrossRef]
- Boyko, A.; Blevins, T.; Yao, Y.; Golubov, A.; Bilichak, A.; Ilnytskyy, Y.; Hollander, J.; Meins, F., Jr.; Kovalchuk, I. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-like proteins. PLoS ONE 2010, 5, e9514. [Google Scholar] [CrossRef]
- Elkelish, A.; Alqudah, A.M.; Alhudhaibi, A.M.; Alqahtani, H.; Börner, A.; Thabet, S.G. Inherited endurance: Deciphering genetic associations of transgenerational and intergenerational heat stress memory in barley. Plant Mol. Biol. 2025, 115, 42. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Vu, N.T.; Cheong, J.J. Transcriptional stress memory and transgenerational inheritance of drought tolerance in plants. Int. J. Mol. Sci. 2022, 23, 12918. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, L.; Huang, R.; Song, F.; Li, L.; Li, P.; Fang, Y.; Lu, W.; Lv, C.; Quan, M.; et al. Local Diversity of Drought Resistance and Resilience in Populus tomentosa Correlates with the Variation of DNA Methylation. Plant Cell Environ. 2023, 46, 479–497. [Google Scholar] [CrossRef]
- Ahtisham, M.; Obaid, Z. Harnessing epigenetic mechanisms for crop resilience: A comprehensive review of plant responses to biotic and abiotic stresses. Plant Biol. 2024, 1, 100007. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, L.; Xia, H.; Wei, H.; Lou, Q.; Li, M.; Li, T.; Luo, L. Transgenerational epimutations induced by multi-generation drought imposition mediate rice plant’s adaptation to drought condition. Sci. Rep. 2017, 7, 39843. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Walias, F.J.; García, M.; Moreno, M.; Giannoukos, I.; González, N.; Sanz-García, E.; Necira, K.; Canto, T.; Tenllado, F. Transgenerational tolerance to salt and osmotic stresses induced by plant virus infection. Int. J. Mol. Sci. 2022, 23, 12497. [Google Scholar] [CrossRef]
- Rashid, M.M.; Vaishnav, A.; Verma, R.K.; Sharma, P.; Suprasanna, P.; Gaur, R.K. Epigenetic regulation of salinity stress responses in cereals. Mol. Biol. Rep. 2022, 49, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Chen, Z.J. Transgenerational epigenetic inheritance during plant evolution and breeding. Trends Plant Sci. 2024, in press. [CrossRef]
- Miranda, M.C.d.C.; Aono, A.H.; Fagundes, T.G.; Arduini, G.M.; Pinheiro, J.B. High-throughput phenotyping and machine learning techniques in soybean breeding: Exploring the potential of aerial imaging and vegetation indices. Agron. J. 2025, 117, e70012. [Google Scholar] [CrossRef]
- Xie, C.; Yang, C. A Review on Plant High-Throughput Phenotyping Traits Using UAV-Based Sensors. Comput. Electron. Agric. 2020, 175, 105589. [Google Scholar] [CrossRef]
- Mohammadi, S.; Uhlen, A.K.; Aamot, H.U.; Dieseth, J.A.; Shafiee, S. Integrating UAV-based multispectral remote sensing and machine learning for detection and classification of chocolate spot disease in faba bean. Crop Sci. 2025, 65, e21454. [Google Scholar] [CrossRef]
- Nguyen, P.; Badenhorst, P.E.; Shi, F.; Spangenberg, G.C.; Smith, K.F.; Daetwyler, H.D. Design of an Unmanned Ground Vehicle and LiDAR Pipeline for the High-Throughput Phenotyping of Biomass in Perennial Ryegrass. Remote Sens. 2020, 13, 20. [Google Scholar] [CrossRef]
- Pugh, N.A.; Young, A.; Emendack, Y.; Sanchez, J.; Xin, Z.; Hayes, C. High-throughput phenotyping of stay-green in a sorghum breeding program using unmanned aerial vehicles and machine learning. Plant Phenomics J. 2025, 8, e70014. [Google Scholar] [CrossRef]
- Volpato, L.; Pinto, F.; González-Pérez, L.; Thompson, I.G.; Borém, A.; Reynolds, M.; Gérard, B.; Molero, G.; Rodrigues, F.A. High Throughput Field Phenotyping for Plant Height Using UAV-Based RGB Imagery in Wheat Breeding Lines: Feasibility and Validation. Front. Plant Sci. 2021, 12, 591587. [Google Scholar] [CrossRef] [PubMed]
- Sagan, V.; Maimaitijiang, M.; Sidike, P.; Eblimit, K.; Peterson, K.T.; Hartling, S.; Esposito, F.; Khanal, K.; Newcomb, M.; Pauli, D.; et al. UAV-Based High Resolution Thermal Imaging for Vegetation Monitoring and Plant Phenotyping Using ICI 8640 P, FLIR Vue Pro R 640, and Thermomap Cameras. Remote Sens. 2019, 11, 330. [Google Scholar] [CrossRef]
- Qin, J.; Monje, O.; Nugent, M.R.; Finn, J.R.; O’Rourke, A.E.; Wilson, K.D.; Fritsche, R.F.; Baek, I.; Chan, D.E.; Kim, M.S. A hyperspectral plant health monitoring system for space crop production. Front. Plant Sci. 2023, 14, 1133505. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, A.; Yang, C.; Anderson, J.A. Aerial Hyperspectral Imagery and Deep Neural Networks for High-Throughput Yield Phenotyping in Wheat. Comput. Electron. Agric. 2020, 172, 105299. [Google Scholar] [CrossRef]
- Eladhim Zidi, F.A.; Abdelkrim, O. Hyperspectral imaging using deep learning in wheat diseases: (Review). In Proceedings of the 8th International Conference on Image and Signal Processing and their Applications (ISPA), Biskra, Algeria, 21–22 April 2024. [Google Scholar]
- Ma, Z.; Rayhana, R.; Feng, K.; Liu, Z.; Xiao, G.; Ruan, Y.; Sangha, J.S. A review on sensing technologies for high-throughput plant phenotyping. IEEE Open J. Instrum. Meas. 2022, 1, 9500121. [Google Scholar] [CrossRef]
- Camacho, D.M.; Collins, K.M.; Powers, R.K.; Costello, J.C.; Collins, J.J. Next-generation machine learning for biological networks. Cell 2018, 173, 1581–1592. [Google Scholar] [CrossRef]
- Libbrecht, M.W.; Noble, W.S. Machine learning applications in genetics and genomics. Nat. Rev. Genet. 2015, 16, 321–332. [Google Scholar] [CrossRef] [PubMed]
- McMurray, B.; Hollich, G. Core computational principles of language acquisition: Can statistical learning do the job? Introduction to special section. Dev. Sci. 2009, 12, 365–368. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Gao, P.; Zhao, H.; Luo, Z.; Lin, Y.; Feng, W.; Li, Y.; Kong, F.; Li, X.; Fang, C.; Wang, X. SoyDNGP: A web-accessible deep learning framework for genomic prediction in soybean breeding. Brief. Bioinform. 2023, 24, bbad284. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Y.; Lu, Q.; Zhang, R.; Gui, J.; Liu, X.; Yue, Z. RiceSNP-BST: A deep learning framework for predicting biotic stress-associated SNPs in rice. Brief. Bioinform. 2024, 25, bbae099. [Google Scholar] [CrossRef]
- Lu, Q.; Xu, J.; Zhang, R.; Liu, H.; Wang, M.; Liu, X.; Yue, Z.; Gao, Y. RiceSNP-ABST: A deep learning approach to identify abiotic stress-associated single nucleotide polymorphisms in rice. Brief. Bioinform. 2024, 26, bbae702. [Google Scholar] [CrossRef]
- Ma, W.; Qiu, Z.; Song, J.; Li, J.; Cheng, Q.; Zhai, J.; Ma, C. A deep convolutional neural network approach for predicting phenotypes from genotypes. Planta 2018, 248, 1307–1318. [Google Scholar] [CrossRef]
- Wang, K.; Abid, M.A.; Rasheed, A.; Crossa, J.; Hearne, S.; Li, H. DNNGP, a deep neural network-based method for genomic prediction using multi-omics data in plants. Mol. Plant 2023, 16, 279–293. [Google Scholar] [CrossRef]
- Labani, M.; Beheshti, A.; O’Brien, T.A. GENet: A Graph-Based Model Leveraging Histone Marks and Transcription Factors for Enhanced Gene Expression Prediction. Genes 2024, 15, 938. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, Y.; Wang, X.; Ma, Y.; Li, P.; Yang, Z.; Zhang, X.; Xu, C.; Xu, S. Incorporation of Parental Phenotypic Data into Multi-Omic Models Improves Prediction of Yield-Related Traits in Hybrid Rice. Plant Biotechnol. J. 2021, 19, 261–272. [Google Scholar] [CrossRef]
- Fernandes, I.K.; Vieira, C.C.; Dias, K.O.; Fernandes, S.B. Using Machine Learning to Combine Genetic and Environmental Data for Maize Grain Yield Predictions across Multi-Environment Trials. Theor. Appl. Genet. 2024, 137, 189. [Google Scholar] [CrossRef]
- Farooq, M.A.; Gao, S.; Hassan, M.A.; Huang, Z.; Rasheed, A.; Hearne, S.; Prasanna, B.; Li, X.; Li, H. Artificial intelligence in plant breeding. Trends Genet. 2024, in press. [CrossRef]
- Azrai, M.; Aqil, M.; Andayani, N.N.; Efendi, R.; Suarni; Suwardi; Jihad, M.; Zainuddin, B.; Salim; Bahtiar, M.; et al. Optimizing ensembles machine learning, genetic algorithms, and multivariate modeling for enhanced prediction of maize yield and stress tolerance index. Front. Sustain. Food Syst. 2024, 8, 1334421. [Google Scholar] [CrossRef]
- Vieira, R.A.; Nogueira, A.P.O.; Fritsche-Neto, R. Optimizing the selection of quantitative traits in plant breeding using simulation. Front. Plant Sci. 2025, 16, 1495662. [Google Scholar] [CrossRef]
- Yoosefzadeh Najafabadi, M.; Hesami, M.; Eskandari, M. Machine Learning-Assisted Approaches in Modernized Plant Breeding Programs. Genes 2023, 14, 777. [Google Scholar] [CrossRef] [PubMed]
- Elbasi, E.; Mostafa, N.; Zaki, C.; AlArnaout, Z.; Topcu, A.E.; Saker, L. Optimizing Agricultural Data Analysis Techniques through AI-Powered Decision-Making Processes. Appl. Sci. 2024, 14, 8018. [Google Scholar] [CrossRef]
- Hachimi, C.E.; Belaqziz, S.; Khabba, S.; Sebbar, B.; Dhiba, D.; Chehbouni, A. Smart Weather Data Management Based on Artificial Intelligence and Big Data Analytics for Precision Agriculture. Agriculture 2023, 13, 95. [Google Scholar] [CrossRef]
- Sharma, K.; Shivandu, S.K. Integrating artificial intelligence and Internet of Things (IoT) for enhanced crop monitoring and management in precision agriculture. Sens. Int. 2024, 5, 100292. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, A.; Tselykh, A.; Bozhenyuk, A.; Choudhury, T.; Alomar, M.A.; Sánchez-Chero, M. Artificial intelligence and internet of things oriented sustainable precision farming: Towards modern agriculture. Open Life Sci. 2023, 18, 20220713. [Google Scholar] [CrossRef]
- Muhammed, D.; Ahvar, E.; Ahvar, S.; Trocan, M.; Montpetit, M.J.; Ehsani, R. Artificial Intelligence of Things (AIoT) for smart agriculture: A review of architectures, technologies and solutions. J. Netw. Comput. Appl. 2024, 228, 103905. [Google Scholar] [CrossRef]
- Munasinghe, I.; Perera, A.; Deo, R.C. A Comprehensive Review of UAV-UGV Collaboration: Advancements and Challenges. J. Sens. Actuator Netw. 2024, 13, 81. [Google Scholar] [CrossRef]
- Adewusi, A.O.; Asuzu, O.F.; Olorunsogo, T.; Adaga, E.; Daraojimba, D.O. AI in precision agriculture: A review of technologies for sustainable farming practices. World J. Adv. Res. Rev. 2024, 21, 2276–2285. [Google Scholar] [CrossRef]
- Linaza, M.T.; Posada, J.; Bund, J.; Eisert, P.; Quartulli, M.; Döllner, J.; Pagani, A.; Olaizola, I.G.; Barriguinha, A.; Moysiadis, T.; et al. Data-Driven Artificial Intelligence Applications for Sustainable Precision Agriculture. Agronomy 2021, 11, 1227. [Google Scholar] [CrossRef]
- Gul, D.; Banday, R.U.Z. Transforming crop management through advanced AI and machine learning: Insights into innovative strategies for sustainable agriculture. AI Comput. Sci. Robot. Technol. 2024, 3. [Google Scholar] [CrossRef]
- Ye, K.; Hu, G.; Tong, Z.; Xu, Y.; Zheng, J. Key Intelligent Pesticide Prescription Spraying Technologies for the Control of Pests, Diseases, and Weeds: A Review. Agriculture 2025, 15, 81. [Google Scholar] [CrossRef]
- Abdullah, H.M.; Mohana, N.T.; Khan, B.M.; Ahmed, S.M.; Hossain, M.; Islam, K.S.; Redoy, M.H.; Ferdush, J.; Bhuiyan, M.A.H.B.; Hossain, M.M.; et al. Present and future scopes and challenges of plant pest and disease (P&D) monitoring: Remote sensing, image processing, and artificial intelligence perspectives. Remote Sens. Appl. Soc. Environ. 2023, 32, 100996. [Google Scholar]
- Jafar, A.; Bibi, N.; Naqvi, R.A.; Sadeghi-Niaraki, A.; Jeong, D. Revolutionizing agriculture with artificial intelligence: Plant disease detection methods, applications, and their limitations. Front. Plant Sci. 2024, 15, 1356260. [Google Scholar] [CrossRef]
- Linardatos, P.; Papastefanopoulos, V.; Kotsiantis, S. Explainable AI: A review of machine learning interpretability methods. Entropy 2021, 23, 18. [Google Scholar] [CrossRef]
- Oğuztürk, G.E. AI-Driven Irrigation Systems for Sustainable Water Management: A Systematic Review and Meta-Analytical Insights. Smart Agric. Technol. 2025, 11, 100982. [Google Scholar] [CrossRef]
- Kaya, C. Optimizing crop production with plant phenomics through high-throughput phenotyping and AI in controlled environments. Food Energy Secur. 2025, 14, e70050. [Google Scholar] [CrossRef]
- Kamilaris, A.; Prenafeta-Boldú, F.X. A Review of the Use of Convolutional Neural Networks in Agriculture. J. Agric. Sci. 2018, 156, 312–322. [Google Scholar] [CrossRef]
- Thingujam, D.; Majeed, A.; Sivarathri, B.S.; Narayana, N.K.; Bista, M.K.; Cowart, K.E.; Knight, A.J.; Pajerowska-Mukhtar, K.M.; Bheemanahalli, R.; Mukhtar, M.S. The impact of soybean genotypes on rhizosphere microbial dynamics and nodulation efficiency. Int. J. Mol. Sci. 2025, 26, 2878. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Shaukat, M.; Ashraf, M.; Zhu, C.; Jin, Q.; Zhang, J. Salinity stress in arid and semi-arid climates: Effects and management in field crops. In Climate Change and Agriculture; IntechOpen: London, UK, 2019. [Google Scholar]
- Kaundal, A.; Srivastava, A.K.; Yadav, D. Editorial: The role of the microbiome in plant and soil health in a changing climate. Front. Plant Sci. 2024, 15, 1491438. [Google Scholar] [CrossRef]
- Li, Y.; Ye, W.; Wang, M.; Yan, X. Climate change and drought: A risk assessment of crop-yield impacts. Clim. Res. 2009, 39, 31–46. [Google Scholar] [CrossRef]
- Gond, S.; Torres, M.; Bergen, M.; Helsel, Z.; White, J., Jr. Induction of salt tolerance and up-regulation of aquaporin genes in tropical corn by rhizobacterium Pantoea agglomerans. Lett. Appl. Microbiol. 2015, 60, 392–399. [Google Scholar] [CrossRef]
- Marulanda, A.; Azcón, R.; Chaumont, F.; Ruiz-Lozano, J.M.; Aroca, R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 2010, 232, 533–543. [Google Scholar] [CrossRef]
- Albureikan, M.O.I. Rhizosphere microorganisms with different strategies and mechanisms to enhance plant growth in the occurrence of different environmental stress factors. J. Pure Appl. Microbiol. 2023, 17, 3. [Google Scholar] [CrossRef]
- Chakraborty, U.; Roy, S.; Chakraborty, A.P.; Dey, P.; Chakraborty, B. Plant growth promotion and amelioration of salinity stress in crop plants by a salt-tolerant bacterium. Recent Res. Sci. Technol. 2011, 3, 11. [Google Scholar]
- Duan, B.; Li, L.; Chen, G.; Su-Zhou, C.; Li, Y.; Merkeryan, H.; Liu, W.; Liu, X. 1-Aminocyclopropane-1-carboxylate deaminase-producing plant growth-promoting rhizobacteria improve drought stress tolerance in grapevine (Vitis vinifera L.). Front. Plant Sci. 2021, 12, 706990. [Google Scholar] [CrossRef]
- Gouli, S.; Majeed, A.; Liu, J.; Moseley, D.; Mukhtar, M.S.; Ham, J.H. Microbiome structures and beneficial bacteria in soybean roots under field conditions of prolonged high temperatures and drought stress. Microorganisms 2024, 12, 2630. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A.; Upadhyaya, C.P.; Baskar, V.; Venkatesh, J.; Nookaraju, A.; Park, S.W. Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J. Plant Growth Regul. 2013, 32, 245–258. [Google Scholar] [CrossRef]
- Morcillo, R.J.L.; Manzanera, M. The effects of plant-associated bacterial exopolysaccharides on plant abiotic stress tolerance. Metabolites 2021, 11, 337. [Google Scholar] [CrossRef]
- Asano, T.; Hayashi, N.; Kikuchi, S.; Ohsugi, R. CDPK-mediated abiotic stress signaling. Plant Signal. Behav. 2012, 7, 817–821. [Google Scholar] [CrossRef]
- Ghosh, D.; Sen, S.; Mohapatra, S. Drought-mitigating Pseudomonas putida GAP-P45 modulates proline turnover and oxidative status in Arabidopsis thaliana under water stress. Ann. Microbiol. 2018, 68, 579–594. [Google Scholar] [CrossRef]
- Habib, S.H.; Kausar, H.; Saud, H.M. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. Biomed. Res. Int. 2016, 2016, 6284547. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant growth-promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Neshat, M.; Abbasi, A.; Hosseinzadeh, A.; Sarikhani, M.R.; Dadashi Chavan, D.; Rasoulnia, A. Plant growth promoting bacteria (PGPR) induce antioxidant tolerance against salinity stress through biochemical and physiological mechanisms. Physiol. Mol. Biol. Plants 2022, 28, 347–361. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef] [PubMed]
- Mohan, B.; Majeed, A.; Thingujam, D.; Burton, S.S.; Cowart, K.E.; Pajerowska-Mukhtar, K.M.; Mukhtar, M.S. Amplicon sequencing analysis of submerged plant microbiome diversity and screening for ACC deaminase production by microbes. Int. J. Mol. Sci. 2024, 25, 13330. [Google Scholar] [CrossRef] [PubMed]
- Großkopf, T.; Soyer, O.S. Synthetic microbial communities. Curr. Opin. Microbiol. 2014, 18, 72–77. [Google Scholar] [CrossRef]
- Li, M.; Hu, J.; Wei, Z.; Jousset, A.; Pommier, T.; Yu, X.; Xu, Y.; Shen, Q. Synthetic microbial communities: Sandbox and blueprint for soil health enhancement. Imeta 2024, 3, e172. [Google Scholar] [CrossRef]
- Tariq, A.; Guo, S.; Farhat, F.; Shen, X. Engineering synthetic microbial communities: Diversity and applications in soil for plant resilience. Agronomy 2025, 15, 513. [Google Scholar] [CrossRef]
- Toju, H.; Peay, K.G.; Yamamichi, M.; Narisawa, K.; Hiruma, K.; Naito, K.; Fukuda, S.; Ushio, M.; Nakaoka, S.; Onoda, Y. Core microbiomes for sustainable agroecosystems. Nat. Plants 2018, 4, 247–257. [Google Scholar] [CrossRef]
- Armanhi, J.S.L.; de Souza, R.S.C.; Biazotti, B.B.; Yassitepe, J.E.C.T.; Arruda, P. Modulating drought stress response of maize by a synthetic bacterial community. Front. Microbiol. 2021, 12, 747541. [Google Scholar] [CrossRef]
- Ju, W.; Liu, L.; Fang, L.; Cui, Y.; Duan, C.; Wu, H. Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol. Environ. Saf. 2019, 167, 218–226. [Google Scholar] [CrossRef]
- Choudhury, A.R.; Choi, J.; Walitang, D.I.; Trivedi, P.; Lee, Y.; Sa, T. ACC deaminase and indole acetic acid producing endophytic bacterial co-inoculation improves physiological traits of red pepper (Capsicum annum L.) under salt stress. J. Plant Physiol. 2021, 267, 153544. [Google Scholar] [CrossRef]
- Khan, M.Y.; Nadeem, S.M.; Sohaib, M.; Waqas, M.R.; Alotaibi, F.; Ali, L.; Zahir, Z.A.; Al-Barakah, F.N. Potential of plant growth promoting bacterial consortium for improving the growth and yield of wheat under saline conditions. Front. Microbiol. 2022, 13, 958522. [Google Scholar] [CrossRef]
- Boulahouat, S.; Cherif-Silini, H.; Silini, A.; Bouket, A.C.; Luptakova, L.; Alenezi, F.N.; Belbahri, L. Biocontrol efficiency of rhizospheric Bacillus against the plant pathogen Fusarium oxysporum: A promising approach for sustainable agriculture. Microbiol. Res. 2023, 14, 892–908. [Google Scholar] [CrossRef]
- De Jensen, C.E.; Percich, J.; Graham, P. Integrated management strategies of bean root rot with Bacillus subtilis and Rhizobium in Minnesota. Field Crops Res. 2002, 74, 107–115. [Google Scholar] [CrossRef]
- Mezeal, I. Study biocontrol efficacy of Pseudomonas fluorescens and Bacillus subtilis against Rhizoctonia solani and Fusarium oxysporum causing disease in tomato (Lycopersicon esculentum L.). Indian J. Fundam. Appl. Life Sci. 2014, 4, 175–183. [Google Scholar]
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; Santos-Villalobos, S.d.l.; Orozco-Mosqueda, M.d.C.; Glick, B.R. Plant growth stimulation by microbial consortia. Agronomy 2021, 11, 219. [Google Scholar] [CrossRef]
- Sibponkrung, S.; Kondo, T.; Tanaka, K.; Tittabutr, P.; Boonkerd, N.; Yoshida, K.-i.; Teaumroong, N. Co-inoculation of Bacillus velezensis strain S141 and Bradyrhizobium strains promotes nodule growth and nitrogen fixation. Microorganisms 2020, 8, 678. [Google Scholar] [CrossRef]
- Petrillo, C.; Vitale, E.; Ambrosino, P.; Arena, C.; Isticato, R. Plant growth-promoting bacterial consortia as a strategy to alleviate drought stress in Spinacia oleracea. Microorganisms 2022, 10, 1798. [Google Scholar] [CrossRef]
- Szopa, D.; Pstrowska, K.; Witek-Krowiak, A. Chitosan-Coated Alginate Matrices with Protein-Based Biostimulants: A Controlled-Release System for Sustainable Agriculture. Materials 2025, 18, 591. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, N.; Vassileva, M.; Martos, V.; Garcia del Moral, L.F.; Kowalska, J.; Tylkowski, B.; Malusá, E. Formulation of microbial inoculants by encapsulation in natural polysaccharides: Focus on beneficial properties of carrier additives and derivatives. Front. Plant Sci. 2020, 11, 270. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef]
- Beringer, J.; Brewin, N.; Johnston, A.; Schulman, H.; Hopwood, D. The Rhizobium-legume symbiosis. Proc. R. Soc. Lond. B Biol. Sci. 1979, 204, 219–233. [Google Scholar]
- Di Benedetto, N.A.; Corbo, M.R.; Campaniello, D.; Cataldi, M.P.; Bevilacqua, A.; Sinigaglia, M.; Flagella, Z. The role of plant growth promoting bacteria in improving nitrogen use efficiency for sustainable crop production: A focus on wheat. AIMS Microbiol. 2017, 3, 413–432. [Google Scholar] [CrossRef]
- Amri, M.; Rjeibi, M.R.; Gatrouni, M.; Mateus, D.M.; Asses, N.; Pinho, H.J.; Abbes, C. Isolation, identification, and characterization of phosphate-solubilizing bacteria from Tunisian soils. Microorganisms 2023, 11, 783. [Google Scholar] [CrossRef]
- Chen, X.; Yang, C.; Palta, J.A.; Li, Y.; Fan, X. An Enterobacter cloacae strain NG-33 that can solubilize phosphate and promote maize growth. Front. Microbiol. 2022, 13, 1047313. [Google Scholar] [CrossRef]
- Kumawat, K.; Sharma, P.; Singh, I.; Sirari, A.; Gill, B. Co-existence of Leclercia adecarboxylata (LSE-1) and Bradyrhizobium sp. (LSBR-3) in nodule niche for multifaceted effects and profitability in soybean production. World J. Microbiol. Biotechnol. 2019, 35, 172. [Google Scholar] [CrossRef]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects—A review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.; Verma, J.P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol. Res. 2014, 169, 337–347. [Google Scholar] [CrossRef]
- Kang, S.-M.; Waqas, M.; Shahzad, R.; You, Y.-H.; Asaf, S.; Khan, M.A.; Lee, K.-E.; Joo, G.-J.; Kim, S.-J.; Lee, I.-J. Isolation and characterization of a novel silicate-solubilizing bacterial strain Burkholderia eburnea CS4-2 that promotes growth of japonica rice (Oryza sativa L. cv. Dongjin). Soil Sci. Plant Nutr. 2017, 63, 233–241. [Google Scholar]
- Sheng, X.F.; Zhao, F.; He, L.Y.; Qiu, G.; Chen, L. Isolation and characterization of silicate mineral-solubilizing Bacillus globisporus Q12 from the surfaces of weathered feldspar. Can. J. Microbiol. 2008, 54, 1064–1068. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Ramos-Solano, B.; Lucas García, J.A.; Garcia-Villaraco, A.; Algar, E.; Garcia-Cristobal, J.; Gutierrez Mañero, F.J. Siderophore and chitinase producing isolates from the rhizosphere of Nicotiana glauca Graham enhance growth and induce systemic resistance in Solanum lycopersicum L. Plant Soil 2010, 334, 189–197. Plant Soil 2010, 334, 189–197. [Google Scholar] [CrossRef]
- Dutta, S.; Podile, A.R. Plant growth promoting rhizobacteria (PGPR): The bugs to debug the root zone. Crit. Rev. Microbiol. 2010, 36, 232–244. [Google Scholar] [CrossRef]
- Vega, N.W.O. A review on beneficial effects of rhizosphere bacteria on soil nutrient availability and plant nutrient uptake. Rev. Fac. Nac. Agron. Medellín 2007, 60, 3621–3643. [Google Scholar]
- Abadin, Z.u.; Yasin, M.; Faisal, M. Bacterial-mediated selenium biofortification of Triticum aestivum: Strategy for improvement in selenium phytoremediation and biofortification. In Agriculturally Important Microbes for Sustainable Agriculture; Springer: Singapore, 2017; Volume 1, pp. 299–315. [Google Scholar]
- Eswayah, A.S.; Smith, T.J.; Gardiner, P.H. Microbial transformations of selenium species of relevance to bioremediation. Appl. Environ. Microbiol. 2016, 82, 4848–4859. [Google Scholar] [CrossRef]
- Bottini, R.; Cassán, F.; Piccoli, P. Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl. Microbiol. Biotechnol. 2004, 65, 497–503. [Google Scholar] [CrossRef]
- Park, S.; Kim, A.-L.; Hong, Y.-K.; Shin, J.-H.; Joo, S.-H. A highly efficient auxin-producing bacterial strain and its effect on plant growth. J. Genet. Eng. Biotechnol. 2021, 19, 179. [Google Scholar] [CrossRef]
- Idris, E.E.; Iglesias, D.J.; Talon, M.; Borriss, R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant Microbe Interact. 2007, 20, 619–626. [Google Scholar] [CrossRef]
- Mikiciuk, G.; Miller, T.; Kisiel, A.; Cembrowska-Lech, D.; Mikiciuk, M.; Łobodzińska, A.; Bokszczanin, K. Harnessing beneficial microbes for drought tolerance: A review of ecological and agricultural innovations. Agriculture 2024, 14, 2228. [Google Scholar] [CrossRef]
- Uzma, M.; Iqbal, A.; Hasnain, S. Drought tolerance induction and growth promotion by indole acetic acid producing Pseudomonas aeruginosa in Vigna radiata. PLoS ONE 2022, 17, e0262932. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef]
- Hirooka, K.; Kunikane, S.; Matsuoka, H.; Yoshida, K.-I.; Kumamoto, K.; Tojo, S.; Fujita, Y. Dual regulation of the Bacillus subtilis regulon comprising the lmrAB and yxaGH operons and yxaF gene by two transcriptional repressors, LmrA and YxaF, in response to flavonoids. J. Bacteriol. 2007, 189, 5170–5182. [Google Scholar] [CrossRef]
- Neal, A.L.; Ahmad, S.; Gordon-Weeks, R.; Ton, J. Benzoxazinoids in root exudates of maize attract Pseudomonas putida to the rhizosphere. PLoS ONE 2012, 7, e35498. [Google Scholar] [CrossRef]
- Van Bastelaere, E.; De Mot, R.; Michiels, K.; Vanderleyden, J. Differential gene expression in Azospirillum spp. by plant root exudates: Analysis of protein profiles by two-dimensional polyacrylamide gel electrophoresis. FEMS Microbiol. Lett. 1993, 112, 335–341. [Google Scholar] [CrossRef]
- Van Bastelaere, E.; Lambrecht, M.; Vermeiren, H.; Van Dommelen, A.; Keijers, V.; Proost, P.; Vanderleyden, J. Characterization of a sugar-binding protein from Azospirillum brasilense mediating chemotaxis to and uptake of sugars. Mol. Microbiol. 1999, 32, 703–714. [Google Scholar] [CrossRef]
- Lynch, J. Root architecture and plant productivity. Plant Physiol. 1995, 109, 7. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.A.; Pound, M.P.; Bennett, M.J.; Wells, D.M. Uncovering the hidden half of plants using new advances in root phenotyping. Curr. Opin. Biotechnol. 2019, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wasson, A.P.; Nagel, K.A.; Tracy, S.; Watt, M. Beyond digging: Noninvasive root and rhizosphere phenotyping. Trends Plant Sci. 2020, 25, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, S.; Hassan, M.A.; Xia, X.; York, L.M.; Rasheed, A.; He, Z. Root system architecture in cereals: Progress, challenges and perspective. Plant J. 2022, 110, 23–42. [Google Scholar] [CrossRef]
- Weihs, B.J.; Heuschele, D.-J.; Tang, Z.; York, L.M.; Zhang, Z.; Xu, Z. The state of the art in root system architecture image analysis using artificial intelligence: A review. Plant Phenomics 2024, 6, 0178. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Shovelomics: High throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 2011, 341, 75–87. [Google Scholar] [CrossRef]
- Fang, H.; Zi-wen, X.; Hai-gang, L.; Yan, G.; Bao-guo, L.; Yun-ling, L.; Yun-tao, M. Image-based root phenotyping for field-grown crops: An example under maize/soybean intercropping. J. Integr. Agric. 2022, 21, 1606–1619. [Google Scholar] [CrossRef]
- Xu, Z.; York, L.M.; Seethepalli, A.; Bucciarelli, B.; Cheng, H.; Samac, D.A. Objective phenotyping of root system architecture using image augmentation and machine learning in alfalfa (Medicago sativa L.). Plant Phenomics 2022, 2022, 9834127. [Google Scholar] [CrossRef]
- McGrail, R.K.; Van Sanford, D.A.; McNear, D.H. Trait-based root phenotyping as a necessary tool for crop selection and improvement. Agronomy 2020, 10, 1328. [Google Scholar] [CrossRef]
- Wasson, A.; Rebetzke, G.; Kirkegaard, J.; Christopher, J.; Richards, R.; Watt, M. Soil coring at multiple field environments can directly quantify variation in deep root traits to select wheat genotypes for breeding. J. Exp. Bot. 2014, 65, 6231–6249. [Google Scholar] [CrossRef] [PubMed]
- Bauer, F.M.; Lärm, L.; Morandage, S.; Lobet, G.; Vanderborght, J.; Vereecken, H.; Schnepf, A. Development and validation of a deep learning based automated minirhizotron image analysis pipeline. Plant Phenomics 2022, 2022, 9817420. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, J.; Hu, P.; Zhang, W.; Ma, Y.; Yu, K.; Guo, Y.; Cao, J.; Li, H.; Li, B. Quantification of the three-dimensional root system architecture using an automated rotating imaging system. Plant Methods 2023, 19, 11. [Google Scholar] [CrossRef]
- Rellán-Álvarez, R.; Lobet, G.; Lindner, H.; Pradier, P.-L.; Sebastian, J.; Yee, M.-C.; Geng, Y.; Trontin, C.; LaRue, T.; Schrager-Lavelle, A. GLO-Roots: An imaging platform enabling multidimensional characterization of soil-grown root systems. eLife 2015, 4, e07597. [Google Scholar] [CrossRef]
- LaRue, T.; Lindner, H.; Srinivas, A.; Exposito-Alonso, M.; Lobet, G.; Dinneny, J.R. Uncovering natural variation in root system architecture and growth dynamics using a robotics-assisted phenomics platform. eLife 2022, 11, e76968. [Google Scholar] [CrossRef]
- Narisetti, N.; Henke, M.; Seiler, C.; Shi, R.; Junker, A.; Altmann, T.; Gladilin, E. Semi-automated root image analysis (saRIA). Sci. Rep. 2019, 9, 19674. [Google Scholar] [CrossRef]
- Falk, K.G.; Jubery, T.Z.; Mirnezami, S.V.; Parmley, K.A.; Sarkar, S.; Singh, A.; Ganapathysubramanian, B.; Singh, A.K. Computer vision and machine learning enabled soybean root phenotyping pipeline. Plant Methods 2020, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Piñeros, M.A.; Larson, B.G.; Shaff, J.E.; Schneider, D.J.; Falcão, A.X.; Yuan, L.; Clark, R.T.; Craft, E.J.; Davis, T.W.; Pradier, P.L. Evolving technologies for growing, imaging and analyzing 3D root system architecture of crop plants. J. Integr. Plant Biol. 2016, 58, 230–241. [Google Scholar] [CrossRef]
- Metzner, R.; Eggert, A.; van Dusschoten, D.; Pflugfelder, D.; Gerth, S.; Schurr, U.; Uhlmann, N.; Jahnke, S. Direct comparison of MRI and X-ray CT technologies for 3D imaging of root systems in soil: Potential and challenges for root trait quantification. Plant Methods 2015, 11, 17. [Google Scholar] [CrossRef]
- Mairhofer, S.; Zappala, S.; Tracy, S.; Sturrock, C.; Bennett, M.J.; Mooney, S.J.; Pridmore, T.P. Recovering complete plant root system architectures from soil via X-ray μ-computed tomography. Plant Methods 2013, 9, 8. [Google Scholar] [CrossRef]
- Belzile, F.; Seck, W.; Sanghera, P.; Han, L.; Dutilleul, P. Comparing Results from 2-D and 3-D Phenotyping Systems for Soybean Root System Architecture: A ‘Comparison of Apples and Oranges’? Plants 2024, 13, 3369. [Google Scholar] [CrossRef]
- Prince, S.; Kanda Das, N.T.; Murphy, M.; Valliyodan, B.; DeSouza, G.N.; Nguyen, H.T. Prediction of soybean root response in the field using nondestructive seedling three-dimensional root features. Plant Phenom. J. 2018, 1, 1–15. [Google Scholar] [CrossRef]
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [PubMed]
- Arora, N.K.; Mishra, J. Next generation microbe-based bioinoculants for sustainable agriculture and food security. Environ. Sustain. 2024, 7, 1–4. [Google Scholar] [CrossRef]
- Bishnoi, U. Agriculture and the dark side of chemical fertilizers. Environ. Anal. Ecol. Stud. 2018, 3, 552. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Cruz, C.; Babalola, O.O. Agricultural sustainability: Microbial biofertilizers in rhizosphere management. Agriculture 2021, 11, 163. [Google Scholar] [CrossRef]
- Jurado, M.; Suárez-Estrella, F.; López, M.; Vargas-García, M.; López-González, J.; Moreno, J. Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Bioresour. Technol. 2015, 186, 15–24. [Google Scholar] [CrossRef]
- Kamath, A.; Shukla, A.; Saiyed, T.; Bhatt, S.; Rathod, H.; Makwana, V.; Soni, D.; Banerjee, S.; Patel, D. Bioinoculants: The agrarian avengers. Symbiosis 2023, 91, 151–166. [Google Scholar] [CrossRef]
- Kontchou, C.Y.; Blondeau, R. Biodegradation of soil humic acids by Streptomyces viridosporus. Can. J. Microbiol. 1992, 38, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Melkani, S.; Oliveira-Paiva, C.; Bini, D.; Pavuluri, K.; Gatiboni, L.; Mahmud, A.; Torres, M.; McLamore, E.; Bhadha, J.H. Biofertilizer use in the United States: Definition, regulation, and prospects. Appl. Microbiol. Biotechnol. 2024, 108, 511. [Google Scholar] [CrossRef]
- Aloo, B.N.; Tripathi, V.; Makumba, B.A.; Mbega, E.R. Plant growth-promoting rhizobacterial biofertilizers for crop production: The past, present, and future. Front. Plant Sci. 2022, 13, 1002448. [Google Scholar] [CrossRef]
- Bruno, J.; Ham, J.H. Seed priming for defense priming: An innovative approach to enhance the resilience of crop plants to biotic and abiotic stresses. Plant Health Prog. 2024, 25, 228–231. [Google Scholar] [CrossRef]
- Elnahal, A.S.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Williams, G.D.; Dwyer, G.S.; Gatiboni, L.; Duckworth, O.W.; Vengosh, A. Evidence for the accumulation of toxic metal(loid)s in agricultural soils impacted from long-term application of phosphate fertilizer. Sci. Total Environ. 2024, 907, 167863. [Google Scholar] [CrossRef]
- Otekunrin, O.A. Assessing the prevalence and severity of global hunger and food insecurity: Recent dynamics and sub-Saharan Africa’s burden. Sustainability 2024, 16, 4877. [Google Scholar] [CrossRef]
- Dawson, T.P.; Perryman, A.H.; Osborne, T.M. Modelling impacts of climate change on global food security. Clim. Change 2016, 134, 429–440. [Google Scholar] [CrossRef]
- Bellemare, M.F. Rising food prices, food price volatility, and social unrest. Am. J. Agric. Econ. 2015, 97, 1–21. [Google Scholar] [CrossRef]
- Molotoks, A.; Smith, P.; Dawson, T.P. Impacts of land use, population, and climate change on global food security. Food Energy Secur. 2021, 10, e261. [Google Scholar] [CrossRef]
- Moore, M.; Wesselbaum, D. Climatic factors as drivers of migration: A review. Environ. Dev. Sustain. 2023, 25, 2955–2975. [Google Scholar] [CrossRef]
- Wiebe, K.; Lotze-Campen, H.; Sands, R.; Tabeau, A.; van der Mensbrugghe, D.; Biewald, A.; Bodirsky, B.; Islam, S.; Kavallari, A.; Mason-D’Croz, D. Climate change impacts on agriculture in 2050 under a range of plausible socioeconomic and emissions scenarios. Environ. Res. Lett. 2015, 10, 085010. [Google Scholar] [CrossRef]
- Dhankher, O.P.; Foyer, C.H. Climate resilient crops for improving global food security and safety. Plant Cell Environ. 2018, 41, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Voora, V.; Bermúdez, J.; Le, H.; Larrea, C.; Luna, E. Soybean Prices and Sustainability; ISSD: Winnipeg, MB, Canada, 2024; p. 2. [Google Scholar]
- Martignone, G.M.B.; Ghosh, B.; Papadas, D.; Behrendt, K. The rise of soybean in international commodity markets: A quantile investigation. Heliyon 2024, 10, e26157. [Google Scholar] [CrossRef] [PubMed]
- Hart, C. The economic evolution of the soybean industry. In The Soybean Genome; Springer: Cham, Switzerland, 2017; pp. 1–9. [Google Scholar]
- Mase, A.S.; Gramig, B.M.; Prokopy, L.S. Climate change beliefs, risk perceptions, and adaptation behavior among Midwestern US crop farmers. Clim. Risk Manag. 2017, 15, 8–17. [Google Scholar] [CrossRef]
- Twumasi, Y.A.; Merem, E.C.; Ning, Z.H.; Yeboah, H.B.; Loh, P.M.; Osei, J.D.; Ferchaud, V.; Anokye, M.; Dadzie, E.; Gyan, D.T.; et al. The impacts of Louisiana’s changing climate on food crop production. Agric. Sci. 2024, 15, 1195–1222. [Google Scholar] [CrossRef]
- DeDecker, J.; Malone, T.; Snapp, S.; Thelen, M.; Anderson, E.; Tollini, C.; Davis, A. The relationship between farmer demographics, social identity and tillage behavior: Evidence from Michigan soybean producers. J. Rural Stud. 2022, 89, 378–386. [Google Scholar] [CrossRef]
- Daghagh Yazd, S.; Wheeler, S.A.; Zuo, A. Key risk factors affecting farmers’ mental health: A systematic review. Int. J. Environ. Res. Public Health 2019, 16, 4849. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Cerel, J.; Kheibari, A.; Leske, S.; Watts, J. A comparison of farming- and non-farming-related suicides from the United States’ National Violent Deaths Reporting System, 2003–2016. Suicide Life Threat. Behav. 2021, 51, 504–514. [Google Scholar] [CrossRef]
- Bhat, M.A.; Mir, R.A.; Kumar, V.; Shah, A.A.; Zargar, S.M.; Rahman, S.; Jan, A.T. Mechanistic insights of CRISPR/Cas-mediated genome editing towards enhancing abiotic stress tolerance in plants. Physiol. Plant. 2021, 172, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Angessa, T.; Hill, C.B.; Zhang, X.-Q.; Telfer, P.; Westcott, S.; Li, C. Genetic solutions through breeding counteract climate change and secure barley production in Australia. Crop Des. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Tabe-Ojong, M.P.J.; Lokossou, J.C.; Gebrekidan, B.; Affognon, H.D. Adoption of climate-resilient groundnut varieties increases agricultural production, consumption, and smallholder commercialization in West Africa. Nat. Commun. 2023, 14, 5175. [Google Scholar] [CrossRef] [PubMed]
- Bartels, W.-L.; Furman, C.A.; Diehl, D.C.; Royce, F.S.; Dourte, D.R.; Ortiz, B.V.; Zierden, D.F.; Irani, T.A.; Fraisse, C.W.; Jones, J.W. Warming up to climate change: A participatory approach to engaging with agricultural stakeholders in the Southeast US. Reg. Environ. Change 2013, 13, 45–55. [Google Scholar] [CrossRef]
- Kranz, J.; Schwichow, M.; Breitenmoser, P.; Niebert, K. The (Un) political perspective on climate change in education—A systematic review. Sustainability 2022, 14, 4194. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Bryan, A. Climate change education in the context of education for sustainable development: Rationale and principles. J. Educ. Sustain. Dev. 2015, 9, 4–26. [Google Scholar] [CrossRef]
- Anderson, A. Climate change education for mitigation and adaptation. J. Educ. Sustain. Dev. 2012, 6, 191–206. [Google Scholar] [CrossRef]
- Monroe, M.C.; Plate, R.R.; Oxarart, A.; Bowers, A.; Chaves, W.A. Identifying effective climate change education strategies: A systematic review of the research. Environ. Educ. Res. 2019, 25, 791–812. [Google Scholar] [CrossRef]
- Hestness, E.; McDonald, R.C.; Breslyn, W.; McGinnis, J.R.; Mouza, C. Science teacher professional development in climate change education informed by the Next Generation Science Standards. J. Geosci. Educ. 2014, 62, 319–329. [Google Scholar] [CrossRef]
- Rousell, D.; Cutter-Mackenzie-Knowles, A. A systematic review of climate change education: Giving children and young people a ‘voice’ and a ‘hand’ in redressing climate change. Child. Geogr. 2020, 18, 191–208. [Google Scholar] [CrossRef]
- Ledley, T.S.; Rooney-Varga, J.; Niepold, F. Addressing climate change through education. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Reid, A. Climate change education and research: Possibilities and potentials versus problems and perils? Environ. Educ. Res. 2019, 25, 767–790. [Google Scholar] [CrossRef]
- NGSS Lead States. Next Generation Science Standards: For States, by States; The National Academy Press: Washington, DC, USA, 2013. [Google Scholar]
- National Council for Agricultural Education. Agriculture, Food, and Natural Resources (AFNR) Content Standards. 2015. Available online: https://live-the-council.pantheonsite.io/afnr/ (accessed on 14 May 2025).
- Chakeredza, S.; Temu, A.B.; Yaye, A.; Makungwa, S.; Saka, J.D.K. Mainstreaming climate change into agricultural education: Challenges and perspectives. J. Agric. Educ. 2009, 50, 22–29. [Google Scholar]
- Wang, H.H.; Bhattacharya, D.; Nelson, B.J. Secondary agriculture teachers’ knowledge, beliefs, and teaching practices of climate change. J. Agric. Educ. Ext. 2020, 26, 5–17. [Google Scholar] [CrossRef]
- Campbell Hibbs, A.; Kahl, D.W.; Pytlik-Zillig, L.; Champion, B.; Abdel-Monem, T.; Steffensmeier, T.R.; Hubbard, K. Agricultural producer perceptions of climate change and climate education needs for the central Great Plains. J. Ext. 2014, 52, 3FEA2. [Google Scholar] [CrossRef]
- McNeal, P.; Petcovic, H.; Reeves, P. What is motivating middle-school science teachers to teach climate change? Int. J. Sci. Educ. 2017, 39, 1069–1088. [Google Scholar] [CrossRef]
- Nation, M.T.; Feldman, A. Environmental education in the secondary science classroom: How teachers’ beliefs influence their instruction of climate change. J. Sci. Teach. Educ. 2021, 32, 481–499. [Google Scholar] [CrossRef]
- Herman, B.C.; Feldman, A.; Vernaza-Hernandez, V. Florida and Puerto Rico secondary science teachers’ knowledge and teaching of climate change science. Int. J. Sci. Math. Educ. 2017, 15, 451–471. [Google Scholar] [CrossRef]
- Wise, S.B. Climate change in the classroom: Patterns, motivations, and barriers to instruction among Colorado science teachers. J. Geosci. Educ. 2010, 58, 297–309. [Google Scholar] [CrossRef]
- Morris, C.; Arbuckle, J. The effects of collective trauma on Iowa farmers, their communities, and sustainability outcomes. Agric. Hum. Values 2024, 41, 729–748. [Google Scholar] [CrossRef]
- Meilinda, M.; Rustaman, N.Y.; Tjasyono, B. The perceptions of pre-service science teachers and science teachers about climate change. J. Pendidik. IPA Indones. 2017, 6, 292–297. [Google Scholar] [CrossRef]
- Oversby, J. Teachers’ learning about climate change education. Procedia Soc. Behav. Sci. 2015, 167, 23–27. [Google Scholar] [CrossRef]
- McKinsey; Company. Agriculture’s Connected Future: How Technology Can Yield New Growth; McKinsey Global Institute: New York, NY, USA, 2020; Available online: https://www.mckinsey.com/~/media/McKinsey/Industries/Agriculture/Our%20Insights/Agricultures%20connected%20future%20How%20technology%20can%20yield%20new%20growth/Agricultures-connected-future-How-technology-can-yield-new-growth-F.pdf (accessed on 18 August 2025).
- Zingaretti, L.M.; Gezan, S.A.; Ferrão, L.F.; Osorio, L.F.; Monfort, A.; Muñoz, P.R.; Whitaker, V.M.; Pérez-Enciso, M. Exploring Deep Learning for Complex Trait Genomic Prediction in Polyploid Outcrossing Species. Front. Plant Sci. 2020, 11, 25. [Google Scholar] [CrossRef]
- Krause, M.R.; González-Pérez, L.; Crossa, J.; Pérez-Rodríguez, P.; Montesinos-López, O.; Singh, R.P.; Dreisigacker, S.; Poland, J.; Rutkoski, J.; Sorrells, M.; et al. Hyperspectral Reflectance-Derived Relationship Matrices for Genomic Prediction of Grain Yield in Wheat. G3 Genes Genomes Genet. 2019, 9, 1231–1247. [Google Scholar] [CrossRef]
- Fiorani, F.; Schurr, U. Future scenarios for plant phenotyping. Annu. Rev. Plant Biol. 2013, 64, 267–291. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Li, Q.; Li, H.; Xia, Y.; Jackson, R.; Sun, G.; Zhou, G.; Deakin, G.; Jiang, D.; et al. CropQuant-Air: An AI-Powered System to Enable Phenotypic Analysis of Yield- and Performance-Related Traits Using Wheat Canopy Imagery Collected by Low-Cost Drones. Front. Plant Sci. 2023, 14, 1219983. [Google Scholar] [CrossRef]
- FAO. Climate-Smart Agriculture Sourcebook; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Bhatnagar, S.; Chaudhary, R.; Sharma, S.; Janjhua, Y.; Thakur, P.; Sharma, P.; Keprate, A. Exploring the Dynamics of Climate-Smart Agricultural Practices for Sustainable Resilience in a Changing Climate. Environ. Sustain. Indic. 2024, 24, 100535. [Google Scholar] [CrossRef]
- Mujeyi, A.; Mudhara, M. Economic Analysis of Climate-Smart Agriculture Technologies in Maize Production in Smallholder Farming Systems. In African Handbook of Climate Change Adaptation; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–16. [Google Scholar]
- Teklu, A.; Simane, B.; Bezabih, M. Multiple Adoption of Climate-Smart Agriculture Innovation for Agricultural Sustainability: Empirical Evidence from the Upper Blue Nile Highlands of Ethiopia. Clim. Risk Manag. 2023, 39, 100477. [Google Scholar] [CrossRef]
- Bijarniya, D.; Parihar, C.M.; Jat, R.K.; Kalvania, K.; Kakraliya, S.K.; Jat, M.L. Portfolios of Climate Smart Agriculture Practices in Smallholder Rice-Wheat System of Eastern Indo-Gangetic Plains—Crop Productivity, Resource Use Efficiency and Environmental Footprints. Agronomy 2020, 10, 1561. [Google Scholar] [CrossRef]
- Challinor, A.J.; Watson, J.; Lobell, D.B.; Howden, S.M.; Smith, D.R.; Chhetri, N. A Meta-Analysis of Crop Yield under Climate Change and Adaptation. Nat. Clim. Change 2014, 4, 287–291. [Google Scholar] [CrossRef]
- Beza, E.; Reidsma, P.; Poortvliet, M.; Belay, M.M.; Bijen, B.S.; Kooistra, L. Exploring farmers’ intentions to adopt mobile Short Message Service (SMS) for citizen science in agriculture. Comput. Electron. Agric. 2018, 151, 295–310. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Wei, P.; Zhang, B.; Gou, F.; Feng, Z.; Mao, Y.; Yang, L.; Zhang, H.; Xu, N.; et al. The CRISPR/Cas9 System Produces Specific and Homozygous Targeted Gene Editing in Rice in One Generation. Plant Biotechnol. J. 2014, 12, 797–807. [Google Scholar] [CrossRef]
- World Bank. Enabling the Business of Agriculture. 2021. Available online: https://eba.worldbank.org (accessed on 14 May 2025).
- CGIAR. One CGIAR Research Initiatives. 2021. Available online: https://www.cgiar.org (accessed on 14 May 2025).
- Fuglie, K.O.; Echeverria, R.G. The Economic Impact of CGIAR-Related Crop Technologies on Agricultural Productivity in Developing Countries, 1961–2020. World Dev. 2024, 176, 106523. [Google Scholar] [CrossRef]
- Araus, J.L.; Kefauver, S.C.; Zaman-Allah, M.; Olsen, M.S.; Cairns, J.E. Translating High-Throughput Phenotyping into Genetic Gain. Trends Plant Sci. 2018, 23, 451–466. [Google Scholar] [CrossRef]
- Li, T.; Angeles, O.; Marcaida, M., III; Manalo, E.; Manalili, M.P.; Radanielson, A.; Mohanty, S. From ORYZA2000 to ORYZA (v3): An Improved Simulation Model for Rice in Drought and Nitrogen-Deficient Environments. Agric. For. Meteorol. 2017, 237, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Martre, P.; Zhao, Z.; Ewert, F.; Maiorano, A.; Rötter, R.P.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. The Uncertainty of Crop Yield Projections Is Reduced by Improved Temperature Response Functions. Nat. Plants 2017, 3, 17102. [Google Scholar] [CrossRef] [PubMed]

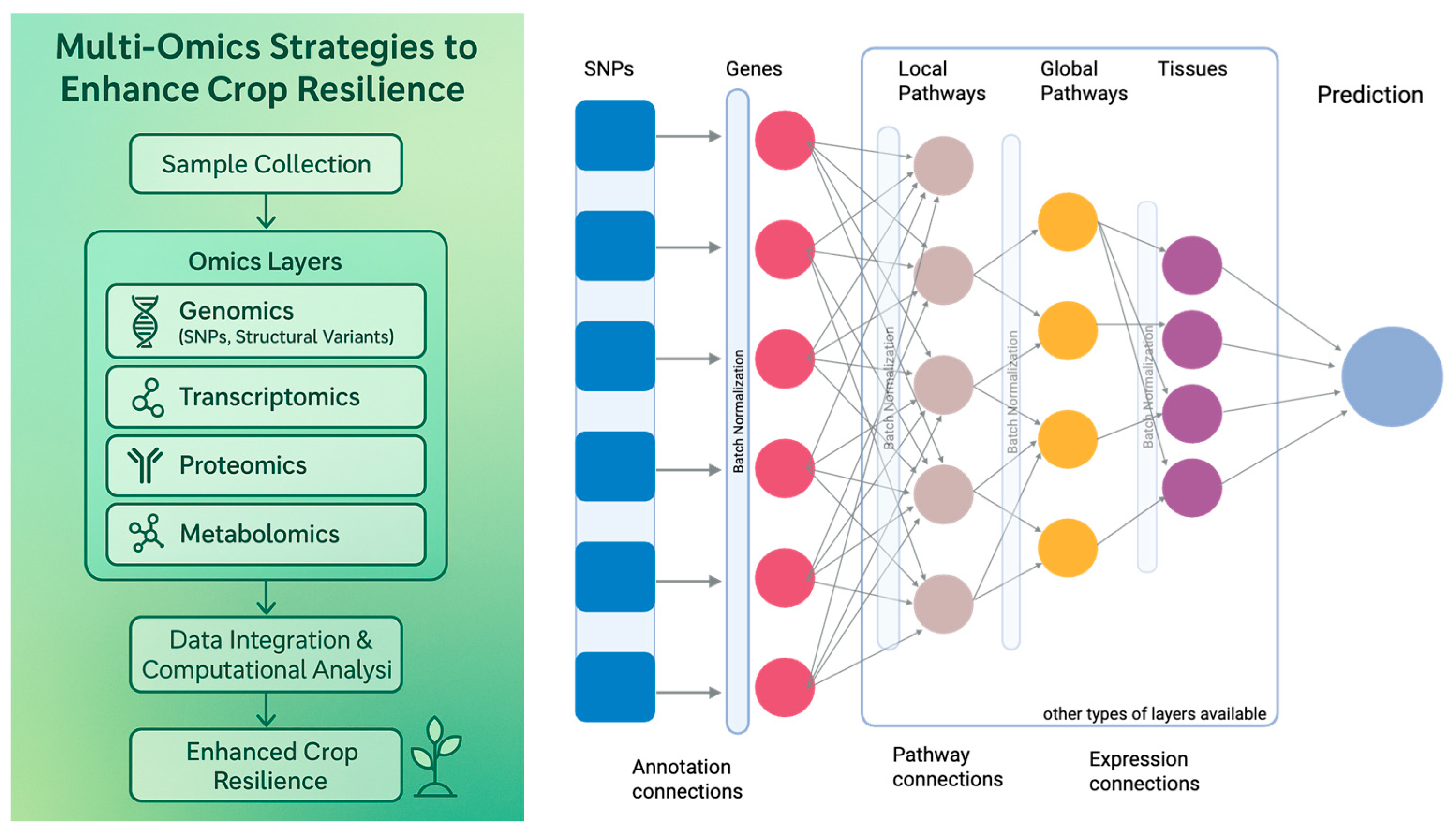
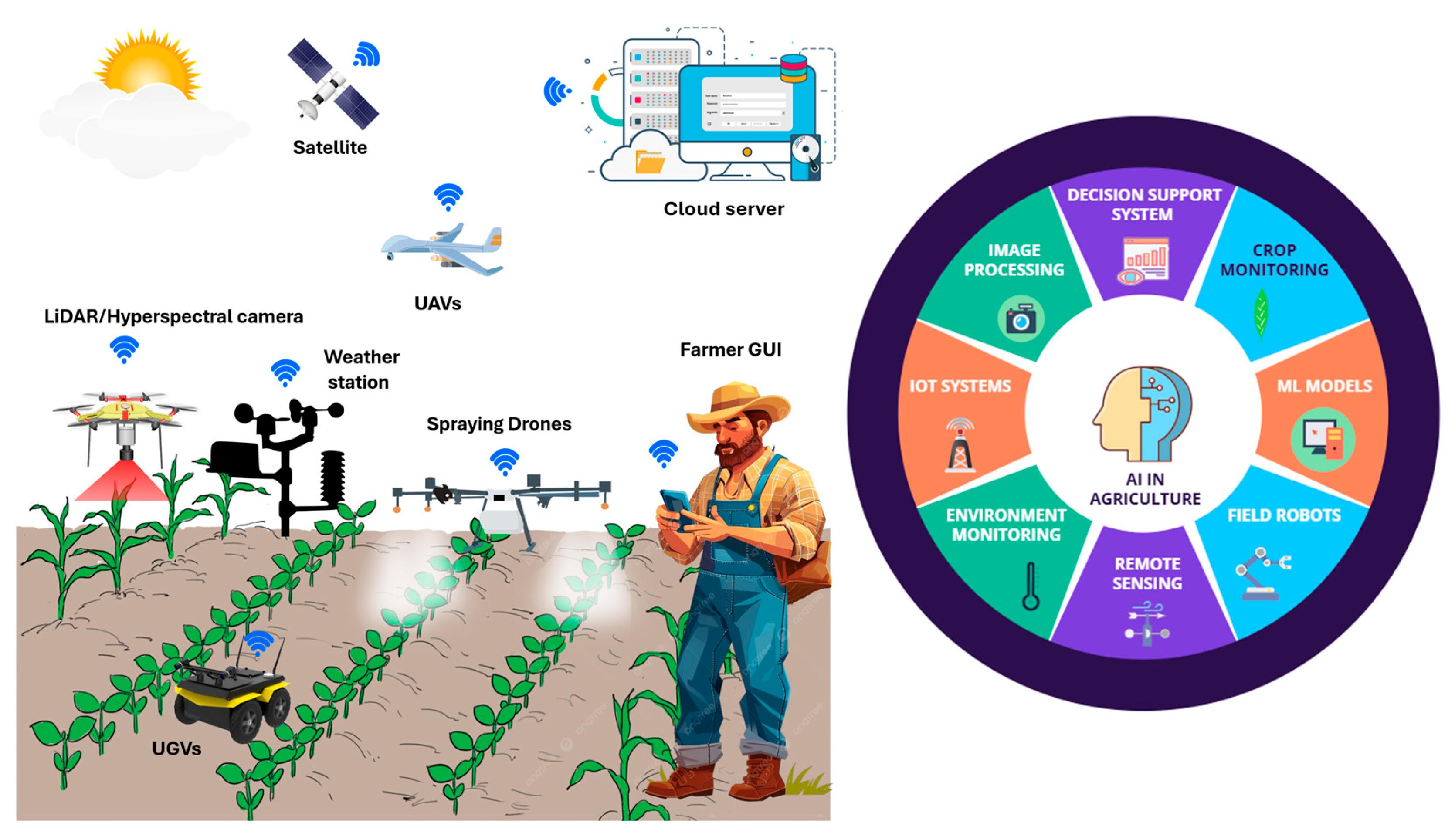
| Standards | Topic | |
|---|---|---|
| Climate Change | Biotechnology | |
| NGSS [341] |
|
|
| AFNR [342] |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thingujam, D.; Gouli, S.; Cooray, S.P.; Chandran, K.B.; Givens, S.B.; Gandhimeyyan, R.V.; Tan, Z.; Wang, Y.; Patam, K.; Greer, S.A.; et al. Climate-Resilient Crops: Integrating AI, Multi-Omics, and Advanced Phenotyping to Address Global Agricultural and Societal Challenges. Plants 2025, 14, 2699. https://doi.org/10.3390/plants14172699
Thingujam D, Gouli S, Cooray SP, Chandran KB, Givens SB, Gandhimeyyan RV, Tan Z, Wang Y, Patam K, Greer SA, et al. Climate-Resilient Crops: Integrating AI, Multi-Omics, and Advanced Phenotyping to Address Global Agricultural and Societal Challenges. Plants. 2025; 14(17):2699. https://doi.org/10.3390/plants14172699
Chicago/Turabian StyleThingujam, Doni, Sandeep Gouli, Sachin Promodh Cooray, Katie Busch Chandran, Seth Bradley Givens, Renganathan Vellaichamy Gandhimeyyan, Zhengzhi Tan, Yiqing Wang, Keerthi Patam, Sydney A. Greer, and et al. 2025. "Climate-Resilient Crops: Integrating AI, Multi-Omics, and Advanced Phenotyping to Address Global Agricultural and Societal Challenges" Plants 14, no. 17: 2699. https://doi.org/10.3390/plants14172699
APA StyleThingujam, D., Gouli, S., Cooray, S. P., Chandran, K. B., Givens, S. B., Gandhimeyyan, R. V., Tan, Z., Wang, Y., Patam, K., Greer, S. A., Acharya, R., Moseley, D. O., Osman, N., Zhang, X., Brooker, M. E., Tagert, M. L., Schafer, M. J., Jeong, C., Hoffseth, K. F., ... Mukhtar, M. S. (2025). Climate-Resilient Crops: Integrating AI, Multi-Omics, and Advanced Phenotyping to Address Global Agricultural and Societal Challenges. Plants, 14(17), 2699. https://doi.org/10.3390/plants14172699









