Foliar Traits Drive Chlorophyll Fluorescence Variability in Chilean Sclerophyllous Species Under Early Outplanting Stress
Abstract
1. Introduction
2. Results
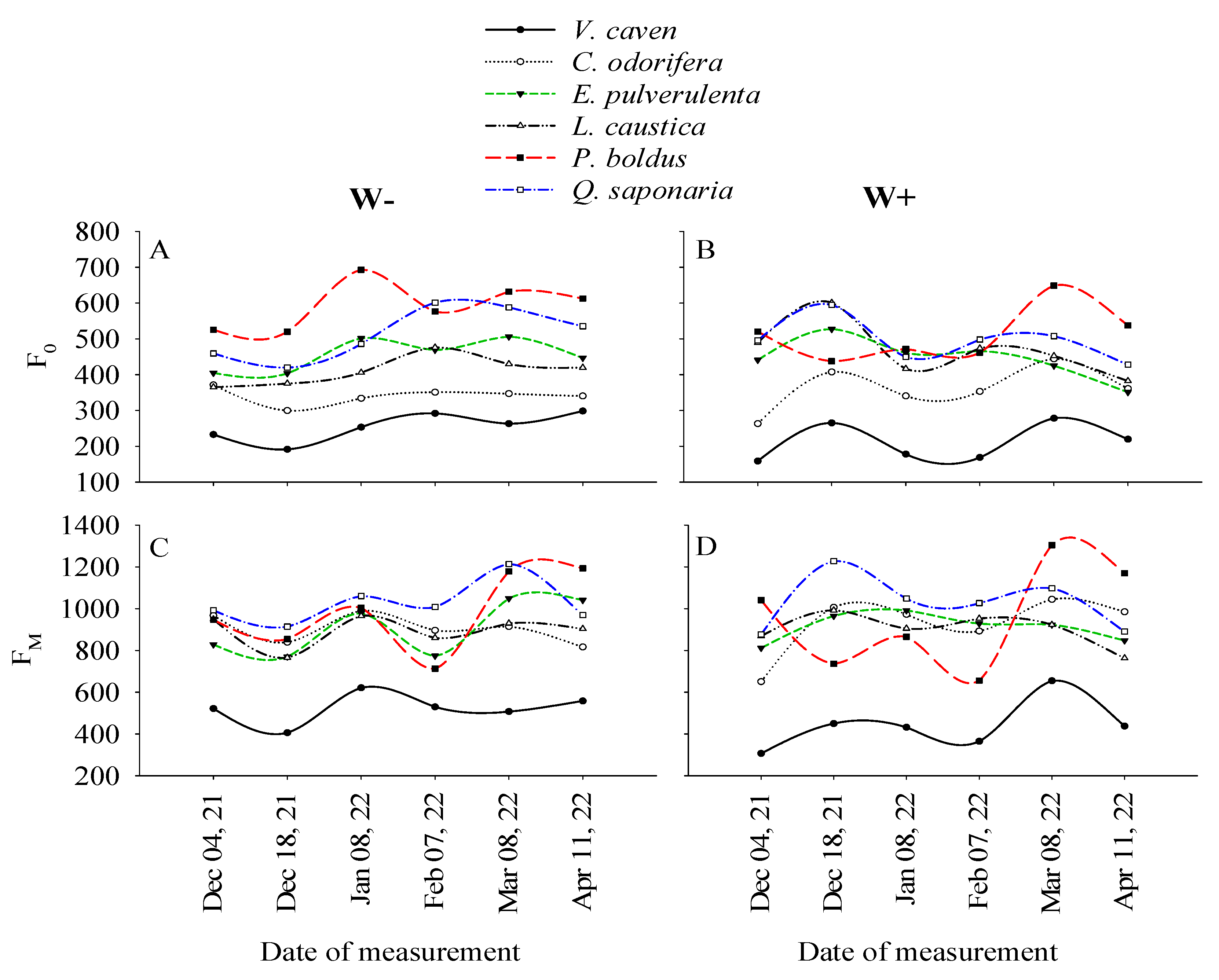
2.1. Temporal Variations in F0 and FM Associated with the Species and Watering Treatments
2.2. Responses of the Chlorophyll Fluorescence Parameters of the Different Species According to the Watering Treatment
2.3. Temporal Variations in Chlorophyll Fluorescence Parameters Among Species
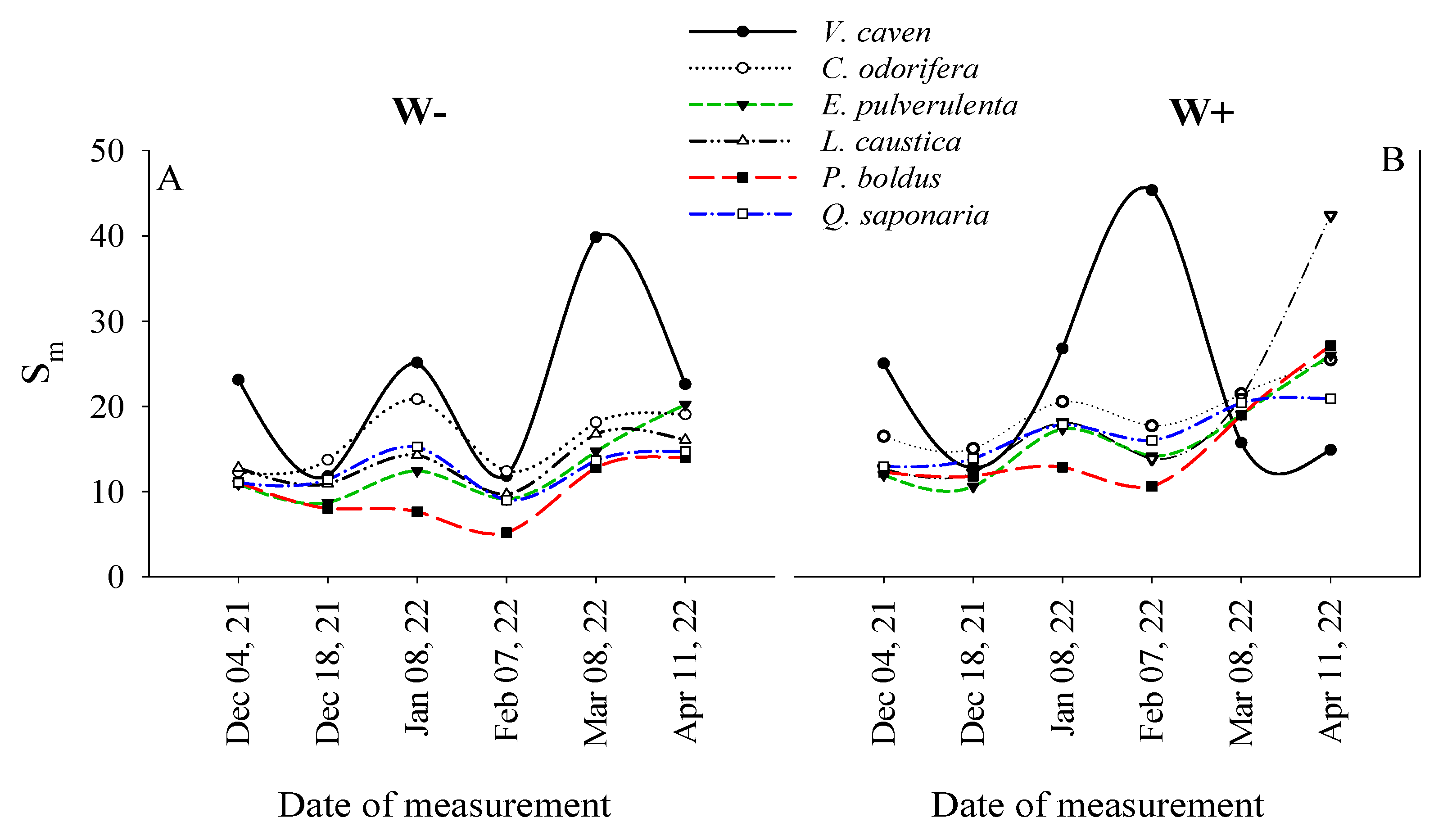
2.4. Temporal Variations in Sm Among Species and Watering Treatments
3. Discussion
3.1. Differences Among Species for Temporal Variations in Chlorophyll Fluorescence Parameters
3.2. Responses of the Chlorophyll Fluorescence Parameters of the Different Species According to the Watering Treatment
4. Materials and Methods
4.1. Sites
4.2. Species
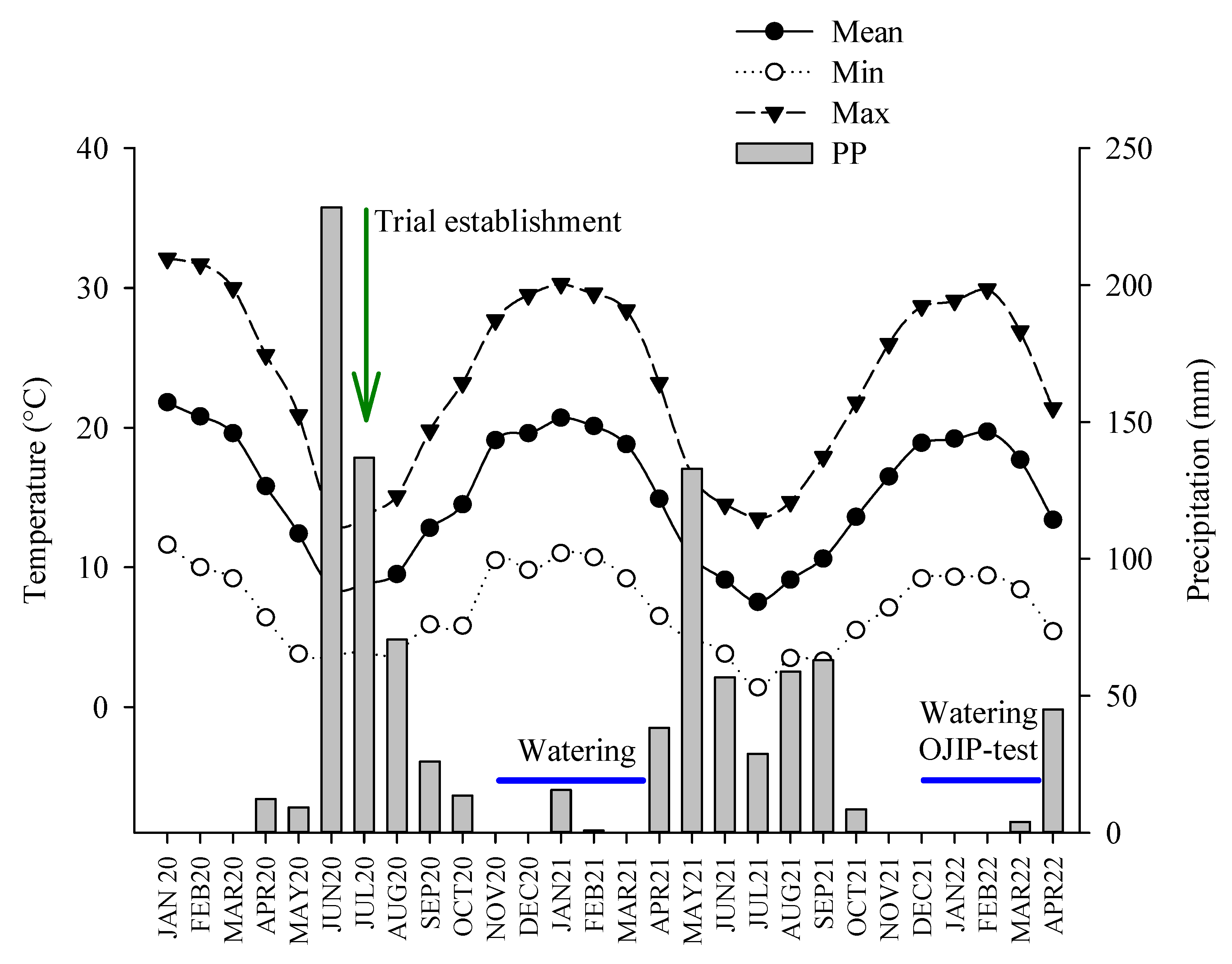
4.3. Trial Establishment and Watering Treatments
4.4. Data Collection and OJIP Tests
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| F0 | Minimal fluorescence from a dark-adapted leaf |
| FM | Maximal fluorescence from a dark-adapted leaf |
| FV/FM | Maximum quantum yield of primary PSII photochemistry |
| FV/F0 | Ratio between variable and minimal fluorescence |
| Sm | Normalized area above the OJIP transient |
| ABS/RC | Effective antenna size of an active reaction center (RC) |
| PIABS | Performance index on absorption basis |
| ψE0 | Probability that a photon trapped by the PSII reaction center enters the electron transport chain |
| ΔVIP | Efficiency with which a PSII trapped electron is transferred to final PSI acceptors |
References
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Mátyás, C. Climatic adaptation of trees: Rediscovering provenance tests. Euphytica 1996, 92, 45–54. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Strasser, R.J. The energy flux theory 35 years later: Formulations and applications. Photosynth. Res. 2013, 117, 289–320. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M. Revisiting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed]
- Ogaya, R.; Peñuelas, J. Comparative field study of Quercus ilex and Phillyrea latifolia photosynthetic response to experimental drought conditions. Environ. Exp. Bot. 2003, 50, 137–148. [Google Scholar] [CrossRef]
- Oliveira, G.; Peñuelas, J. Effects of winter cold stress on photosynthesis and photochemical efficiency of PSII of two Mediterranean Cistus albidus and Quercus ilex. Plant Ecol. 2004, 175, 179–191. [Google Scholar] [CrossRef]
- Percival, G.C. The use of chlorophyll fluorescence to identify chemical and environmental stress in leaf tissue of three oak (Quercus) species. J. Arboricult. 2005, 31, 215–227. [Google Scholar] [CrossRef]
- Baquedano, F.J.; Castillo, F.J. Comparative ecophysiological effects of drought on seedlings of the Mediterranean water saver Pinus halepensis and water-spenders Quercus coccifera and Quercus ilex. Trees Struct. Funct. 2006, 20, 689–700. [Google Scholar] [CrossRef]
- Salvatori, E.; Fusaro, L.; Manes, F. Chlorophyll fluorescence for phenotyping drought-stressed trees in a mixed deciduous forest. Ann. Bot. 2016, 6, 39–49. [Google Scholar]
- Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence parameters as early indicators of light stress in barley. J. Photochem. Photobiol. 2012, 112, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Bussotti, F.; Desotgiu, R.; Pollastrini, M.; Cascio, C. The JIP test: A tool to screen the capacity of plant adaptation to climate change. Scand. J. Forest. Res. 2010, 25, 43–50. [Google Scholar] [CrossRef]
- Giliberto, J.; Estay, H. Seasonal water stress in some chilean matorral shrubs. Bot. Gaz. 1978, 139, 236–240. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Comstock, J.P. Stress tolerance and adaptive variation in leaf absorptance and leaf angle. In The California Chaparral: Paradigms Re-Examined; Keeley, S.C., Ed.; Natural History Museum of Los Angeles Co.: Los Angeles, CA, USA, 1989; pp. 21–24. [Google Scholar]
- Myers, D.A.; Jordan, D.N.; Vogelmann, T.C. Inclination of sun and shade leaves influences chloroplast light harvesting and utilization. Physiol. Plant. 1997, 99, 395–404. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Leaf size and angle vary widely across species: What consequences for light interception? New Phytol. 2003, 158, 509–525. [Google Scholar] [CrossRef]
- Mänd, P.; Hallik, L.; Peñuelas, J.; Kull, O. Electron transport efficiency at opposite leaf sides: Effect of vertical distribution of leaf angle, structure, chlorophyll content and species in a forest canopy. Tree Physiol. 2013, 33, 202–210. [Google Scholar] [CrossRef]
- Sepulveda, M.; Bown, H.E.; Fernandez, L.B. Stomatal conductance responses of Acacia caven to seasonal patterns of water availability at different soil depths in a Mediterranean Savanna. Water 2018, 10, 1534. [Google Scholar] [CrossRef]
- Armesto, J.J.; Martίnez, J.A. Relations between vegetation structure and slope aspect in the mediterranean region of Chile. J. Ecol. 1978, 66, 881–889. [Google Scholar] [CrossRef]
- Montenegro, G.; Avila, G.; Aljaro, M.E.; Osorio, R.; Gomez, M. Chile. In Plant Pheno-Morphological Studies in Mediterranean Type Ecosystems; Orshan, G., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 347–387. [Google Scholar]
- Dreyer, E.; Epron, D.; Yog Matig, O.E. Photochemical efficiency of photosystem II in rapidly dehydrating leaves of 11 temperate and tropical tree species differing in their tolerance to drought. Ann. Sci. For. 1992, 49, 615–625. [Google Scholar] [CrossRef][Green Version]
- Scarascia-Mugnozza, G.; De Angelis, P.; Matteucci, G.; Valentini, R. Long-term exposure to elevated [CO2] in a natural Quercus ilex L. community: Net photosynthesis and photochemical efficiency of PSII at different levels of water stress. Plant Cell Environ. 1996, 19, 643–654. [Google Scholar] [CrossRef]
- Silva Dalberto, D.; Garbin Martinazzo, E.; Antonio Bacarin, M. Chlorophyll a fluorescence reveals adaptation strategies in drought stress in Ricinus communis. Braz. J. Bot. 2017, 40, 861–870. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Morales, F.; Flexas, J.; Gil-Pelegrín, E. Differential photosynthetic performance and photoprotection mechanisms of three Mediterranean evergreen oaks under severe drought stress. Funct. Plant Biol. 2009, 36, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Armesto, J.J.; Arroyo, M.T.K.; Hinojosa, F.L. The Mediterranean environment of central PM Chile. In The Physical Geography of South America; Veblen, T.T., Young, K.R., Orme, A.R., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 184–199. [Google Scholar]
- Montenegro, G.; Riveros De La Puente, F. Comparison of differential environmental responses of Colliguaya odorifera. Flora 1977, 166, 125–135. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Comstock, J. Leaf absorptance and leaf angle: Mechanisms for stress avoidance. In Plant Response to Stress; Tenhunen, J.D., Oechel, W.C., Catarino, F.M., Lange, O.L., Eds.; Springer: Berlin/Heidelberg, Germany, 1987; pp. 55–76. [Google Scholar]
- Cabello, A.; Donoso, C. Peumus boldus (Molina) Johnston. Boldo, Folo. Familia: Monimiaceae. In Las Especies Arbóreas de los Bosques Templados de Chile y Argentina. Autoecología; Donoso, C., Ed.; Marisa Cuneo Ediciones: Valdivia, Chile, 2013; pp. 510–515. [Google Scholar]
- Armesto, J.J.; Vidiella, P.E.; Jiménez, H.E. Evaluating causes and mechanisms of succession in the mediterranean regions in Chile and California. In Ecology and Biogeography of Mediterranean Ecosystems in Chile, California, and Australia; Kalin Arroyo, M.T., Zedler, P.H., Fox, M.D., Eds.; Springer: New York, NY, USA, 1995; pp. 418–434. [Google Scholar]
- Hoffmann, A.J.; Alliende, M.C. Interactions in the patterns of vegetative growth and reproduction in woody dioecious plants. Oecologia 1984, 61, 109–114. [Google Scholar] [CrossRef]
- Gotor, B. Evaluación de Parámetros Fisiológicos y de Crecimiento en Plantas de Quillaja saponaria Mol. bajo Condiciones de Déficit Hídrico. Undergraduate Thesis, Forestry Engenieer, Facultad de Ciencias Forestales, Universidad de Chile, Santiago, Chile, 2008. [Google Scholar]
- Montenegro, G. Atlas de Anatomía de Especies Vegetales Autóctonas de la Zona Central; Ediciones Universidad Católica de Chile: Santiago, Chile, 1984. [Google Scholar]
- Yáñez, M.A.; Espinoza, S.E.; Magni, C.R.; Martínez-Herrera, E. Early Growth and Physiological Acclimation to Shade and Water Restriction of Seven Sclerophyllous Species of the Mediterranean Forests of Central Chile. Plants 2024, 13, 2410. [Google Scholar] [CrossRef]
- Doll, U.; Aedo, D.; Lopez, P. Caracterización morfológica de tres procedencias de boldo (Peumus boldus) en una plantación joven de 6 años. Bosque 2005, 26, 45–54. [Google Scholar] [CrossRef]
- Valladares, F.; Pugnaire, F.I. Tradeoffs between irradiance capture and avoidance in semi-arid environments assessed with a crown architecture model. Ann. Bot. 1999, 83, 459–469. [Google Scholar] [CrossRef]
- Nikolopoulos, D.; Bresta, P.; Daliani, V.; Haghiou, V.; Darra, N.; Liati, M.; Mavrogianni, E.; Papanastasiou, A.; Porfyraki, T.; Psaroudi, V.; et al. Leaf anatomy affects optical properties and enhances photosynthetic performance under oblique light. Plant Cell Environ. 2024, 47, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Öquist, G.; Chow, W.S.; Anderson, J.M. Photoinhibition of photosynthesis represents a mechanism for the long-term regulation of photosystem II. Planta 1992, 186, 450–460. [Google Scholar] [CrossRef]
- Oukarroum, A.; Schansker, G.; Strasser, R.J. Drought stress effects on photosystem I content and photosystem II thermotolerance analyzed using Chl a fluorescence kinetics in barley varieties differing in their drought tolerance. Physiol. Plant. 2009, 137, 188–199. [Google Scholar] [CrossRef]
- Guidi, L.; Calatayud, A. Non-invasive tools to estimate stress-induced changes in photosynthetic performance in plants inhabiting Mediterranean areas. Env. Exp. Bot. 2014, 103, 42–52. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Schansker, G.; Strasser, R.J. Quantification of non-QB-reducing centers in leaves using a far-red preillumination. Photosynth. Res. 2005, 84, 145–151. [Google Scholar] [CrossRef]
- Tausz, M.; Šircelj, H.; Grill, D. The glutathione system as a stress marker in plant ecophysiology: Is a stress-response concept valid? J. Exp. Bot. 2004, 55, 1955–1962. [Google Scholar] [CrossRef]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef]
- Peña-Rojas, K.; Donoso, S.; Pacheco, C.; Riquelme, A.; Gangas, R.; Guajardo, A.; Durán, S. Respuestas morfo-fisiológicas de plantas de Lithraea caustica (Anacardiaceae) sometidas a restricción hídrica controlada. Bosque 2018, 39, 27–36. [Google Scholar] [CrossRef]
- Yang, X.; Li, R.; Jablonski, A.; Stovall, A.; Kim, J.; Yi, K.; Ma, Y.; Beverly, D.; Phillips, R.; Novick, K.; et al. Leaf angle as a leaf and canopy trait: Rejuvenating its role in ecology with new technology. Ecol. Lett. 2023, 26, 1005–1020. [Google Scholar] [CrossRef]
- Methy, M.; Damesin, C.; Rambal, S. Drought and photosystem II activity in two Mediterranean oaks. Ann. Sci. For. 1996, 53, 255–262. [Google Scholar] [CrossRef]
- Di Marco, G.A.; Massacci, A.; Gabrielli, R. Drought effects on photosynthesis and fluorescence in hard wheat cultivars grown in the field. Physiol. Plant. 1988, 74, 385–390. [Google Scholar] [CrossRef]
- Fini, A.; Ferrini, F.; Frangi, P.; Amoroso, G.; Piatti, R. Withholding irrigation during the establishment phase affected growth and physiology of Norway maple (Acer platanoides) and linden (Tilia spp.). Arboric. Urban For. 2009, 35, 241–251. [Google Scholar] [CrossRef]
- Swoczyna, T.; Kalaji, H.M.; Pietkiewicz, S.; Borowski, J.; Zaras-Januszkiewicz, E. Monitoring young urban trees tolerance to roadside conditions by application of chlorophyll fluorescence. Zesz. Probl. Postepow Nauk Roln. 2010, 545, 303–309. [Google Scholar]
- Wang, Z.X.; Chen, L.; Ai, J.; Qin, H.Y.; Liu, Y.X.; Xu, P.L.; Jiao, Z.Q.; Zhao, Y.; Zhang, Q.T. Photosynthesis and activity of photosystem II in response to drought stress in amur grape (Vitis amurensis Rupr.). Photosynthetica 2012, 50, 189–196. [Google Scholar] [CrossRef]
- Stoof, C.R.; Wesseling, J.G.; Ritsema, C.J. Effects of fire and ash on soil water retention. Geoderma 2010, 159, 276–285. [Google Scholar] [CrossRef]
- Padilla, F.M.; Pugnaire, F.I. The role of nurse plants in the restoration of degraded environments. Front. Ecol. Environ. 2006, 4, 196–202. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Zamora, R.; Gómez, J.M.; Hodar, J.A.; Castro, J.; Baraza, E. Applying plant positive interactions to reforestation in Mediterranean mountains: A meta-analysis of the use of shrubs as nurse plants. Ecol. Appl. 2004, 14, 1128–1138. [Google Scholar] [CrossRef]
- Ceacero, C.J.; Díaz-Hernández, J.L.; del Campo, A.D.; Navarro-Cerrillo, R.M. Evaluación temprana de técnicas de restauración forestal mediante fluorescencia de la clorofila y diagnóstico de vitalidad de brinzales de encina (Quercus ilex sub. ballota). Bosque 2012, 33, 191–202. [Google Scholar] [CrossRef]


| Source of Variation | F0 | FM |
|---|---|---|
| Watering (W) | 0.76 ns | 0.49 ns |
| Species (S) | 138.7 ** | 92.8 ** |
| Date (D) | 5.91 ** | 11.1 ** |
| W × S | 6.3 ** | 0.8 ns |
| W × D | 10.0 ** | 4.7 ** |
| S × D | 1.4 ns | 3.3 ** |
| W × S × D | 1.5 * | 1.7 * |
| Source of Variation | FV/FM | Sm | ABS/RC | FV/F0 | PIABS | ψE0 | ΔVIP |
|---|---|---|---|---|---|---|---|
| Watering (W) | 0.0 ns | 23.7 ** | 3.5 ns | 0.0 ns | 0.0 ns | 1.5 ns | 52.4 ** |
| Species (S) | 44.9 ** | 11.5 ** | 11.7 ** | 56.6 ** | 33.8 ** | 18.1 ** | 57.8 ** |
| Date (D) | 12.0 ** | 12.2 ** | 9.1 ** | 12.3 ** | 39.3 ** | 9.6 ** | 17.5 ** |
| W × S | 3.2 ** | 0.8 ns | 2.1 ns | 4.4 ** | 4.5 ** | 5.8 ** | 1.3 ns |
| W × D | 4.4 ** | 3.6 ** | 2.3 * | 5.3 ** | 4.9 ** | 6.0 ** | 2.9 * |
| S × D | 6.2 ** | 2.0 ** | 5.2 ** | 2.8 ** | 4.4 ** | 3.8 ** | 3.1 ** |
| W × S × D | 0.6 ns | 2.8 ** | 1.0 ns | 0.6 ns | 1.1 ns | 1.0 ns | 1.0 ns |
| Species | Life Form and Leaf Habit | Leaf Inclination Angle 1 and Characteristic of the Lamina | Leaf Absorptance (%) 2 | References |
|---|---|---|---|---|
| Q. saponaria | tree, broad-leaved evergreen | 45°, flat lamina | 78.3 | [20,21,27,28,29,30,31,32,33] |
| C. odorifera | shrub, semi-deciduous | 65°, flat lamina | 81.7 | |
| E. pulverulenta | shrub, broad-leaved evergreen | 45°, slightly rolled towards the abaxial surface | nd | |
| L. caustica | tree, broad-leaved evergreen | 45°, lamina folded upwards (V-shaped) | 87.2 | |
| V. caven | shrub, deciduous | 15°, small compound leaves | nd | |
| P. boldus | tree, broad-leaved evergreen | 45°, rolled towards the abaxial surface | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, S.; Magni, C.; Yáñez, M.; Toro, N.; Martínez-Herrera, E. Foliar Traits Drive Chlorophyll Fluorescence Variability in Chilean Sclerophyllous Species Under Early Outplanting Stress. Plants 2025, 14, 2682. https://doi.org/10.3390/plants14172682
Espinoza S, Magni C, Yáñez M, Toro N, Martínez-Herrera E. Foliar Traits Drive Chlorophyll Fluorescence Variability in Chilean Sclerophyllous Species Under Early Outplanting Stress. Plants. 2025; 14(17):2682. https://doi.org/10.3390/plants14172682
Chicago/Turabian StyleEspinoza, Sergio, Carlos Magni, Marco Yáñez, Nicole Toro, and Eduardo Martínez-Herrera. 2025. "Foliar Traits Drive Chlorophyll Fluorescence Variability in Chilean Sclerophyllous Species Under Early Outplanting Stress" Plants 14, no. 17: 2682. https://doi.org/10.3390/plants14172682
APA StyleEspinoza, S., Magni, C., Yáñez, M., Toro, N., & Martínez-Herrera, E. (2025). Foliar Traits Drive Chlorophyll Fluorescence Variability in Chilean Sclerophyllous Species Under Early Outplanting Stress. Plants, 14(17), 2682. https://doi.org/10.3390/plants14172682







