PsnMYB30 Enhances Salt and Drought Stress Tolerance in Transgenic Tobacco
Abstract
1. Introduction
2. Methods and Materials
2.1. Plant Materials
2.2. Sequence Analysis of PsnMYB30
2.3. Cis-Elements Analysis of Promoter Sequence
2.4. Spatiotemporal Expression Analysis of PsnMYB30
2.5. Subcellular Localisation of PsnMYB30
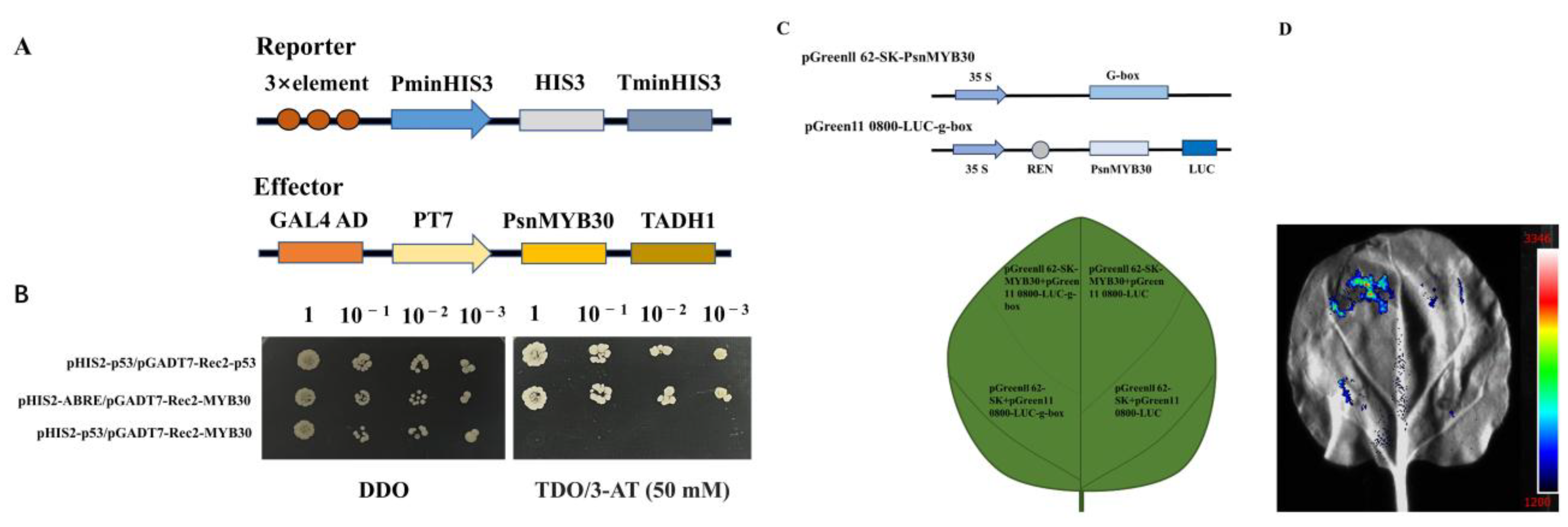
2.6. Yeast Hybrid Assay and Dual-Luciferase Reporter Assay
2.7. Acquisition of Transgenic Tobacco
2.8. Histochemical Staining and Physiological Measurement
3. Results
3.1. Expression Pattern of MYB Family Genes in Poplar Roots Under Salt Stress
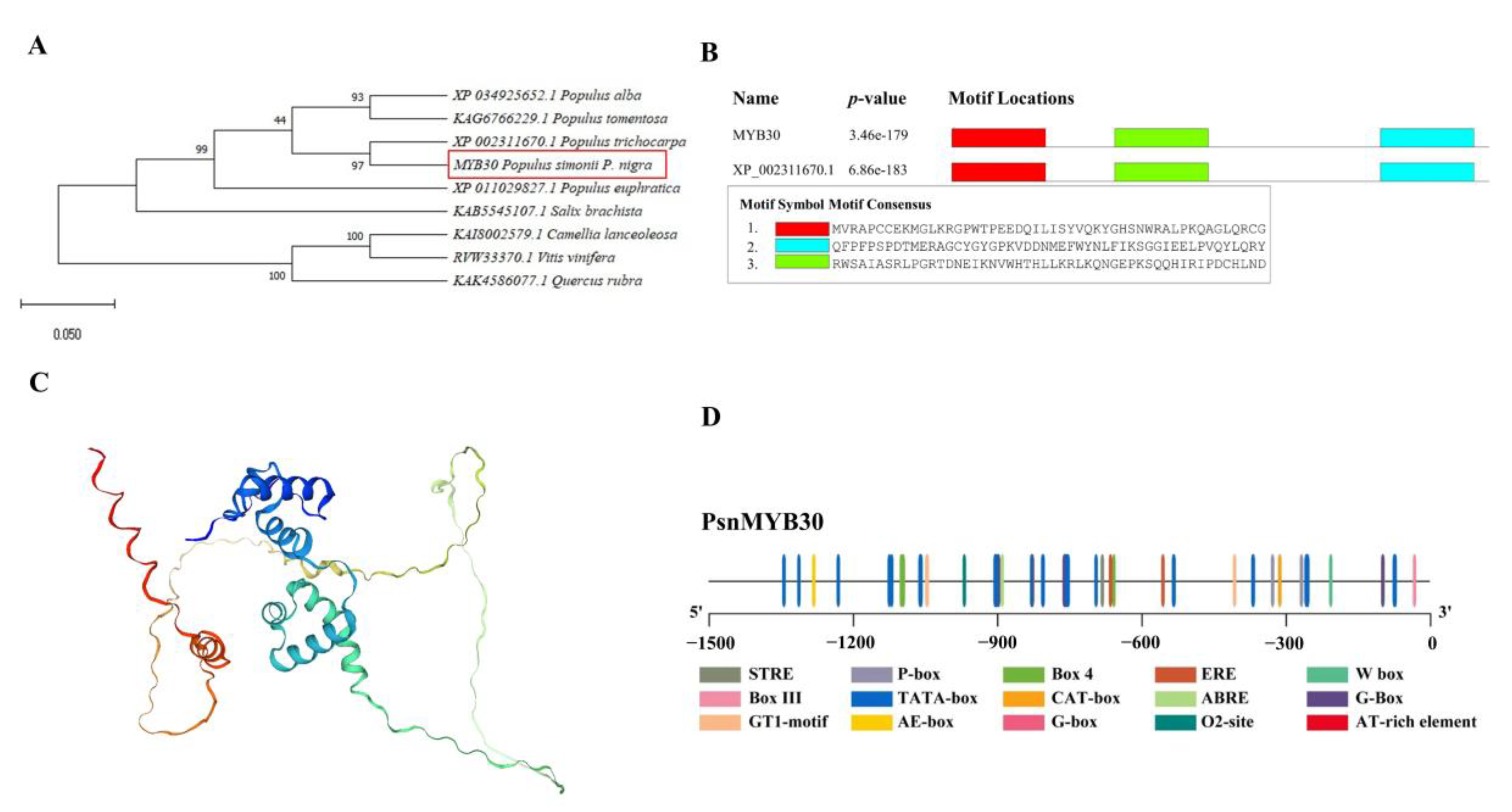
3.2. PsnMYB30 Sequence Analysis
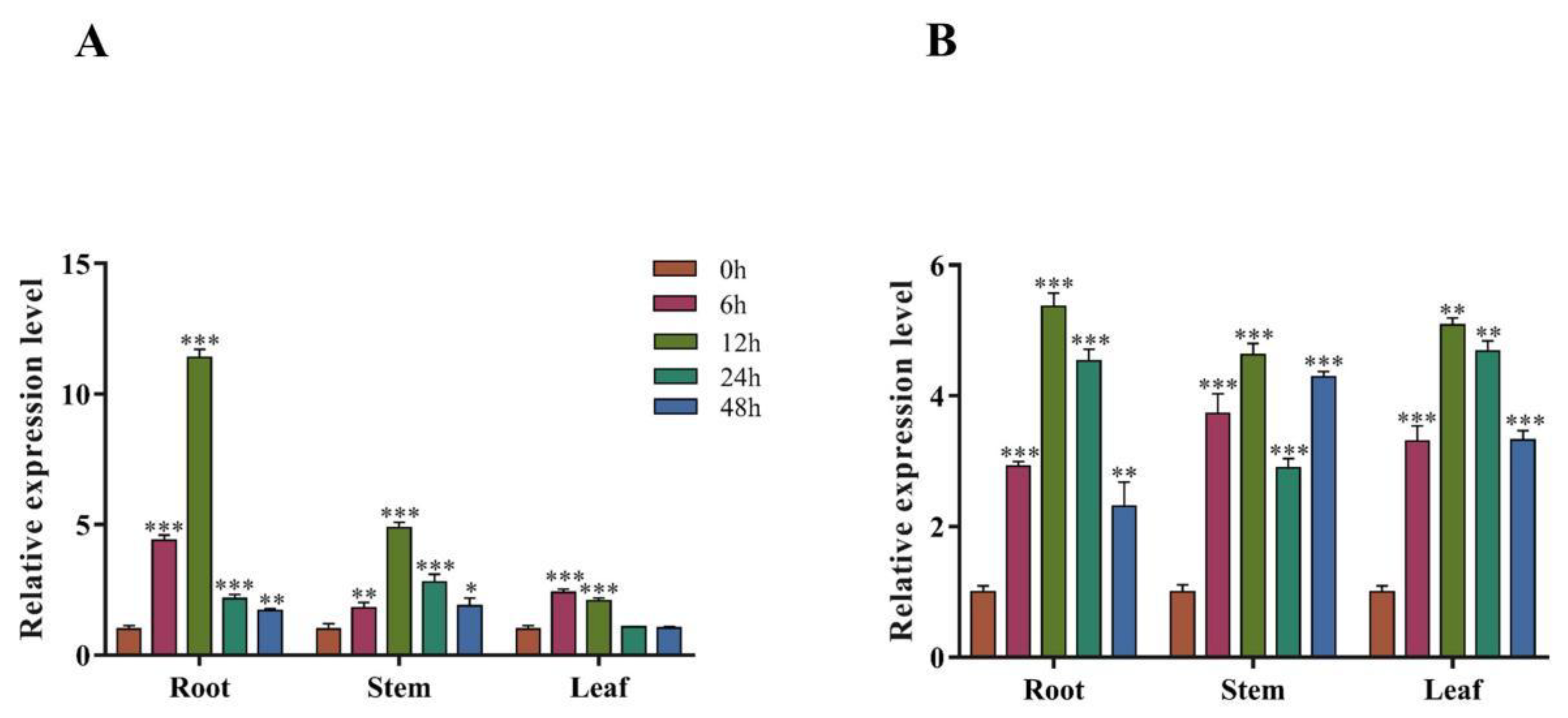
3.3. Spatiotemporal Expression Pattern of PsnMYB30 in Poplar
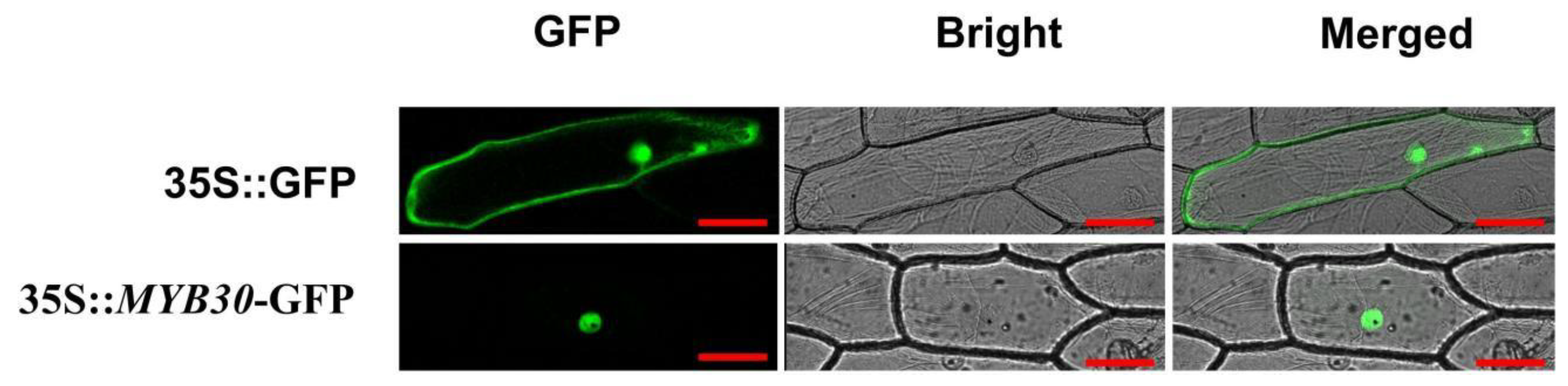
3.4. Subcellular Localisation of PsnMYB30 Protein
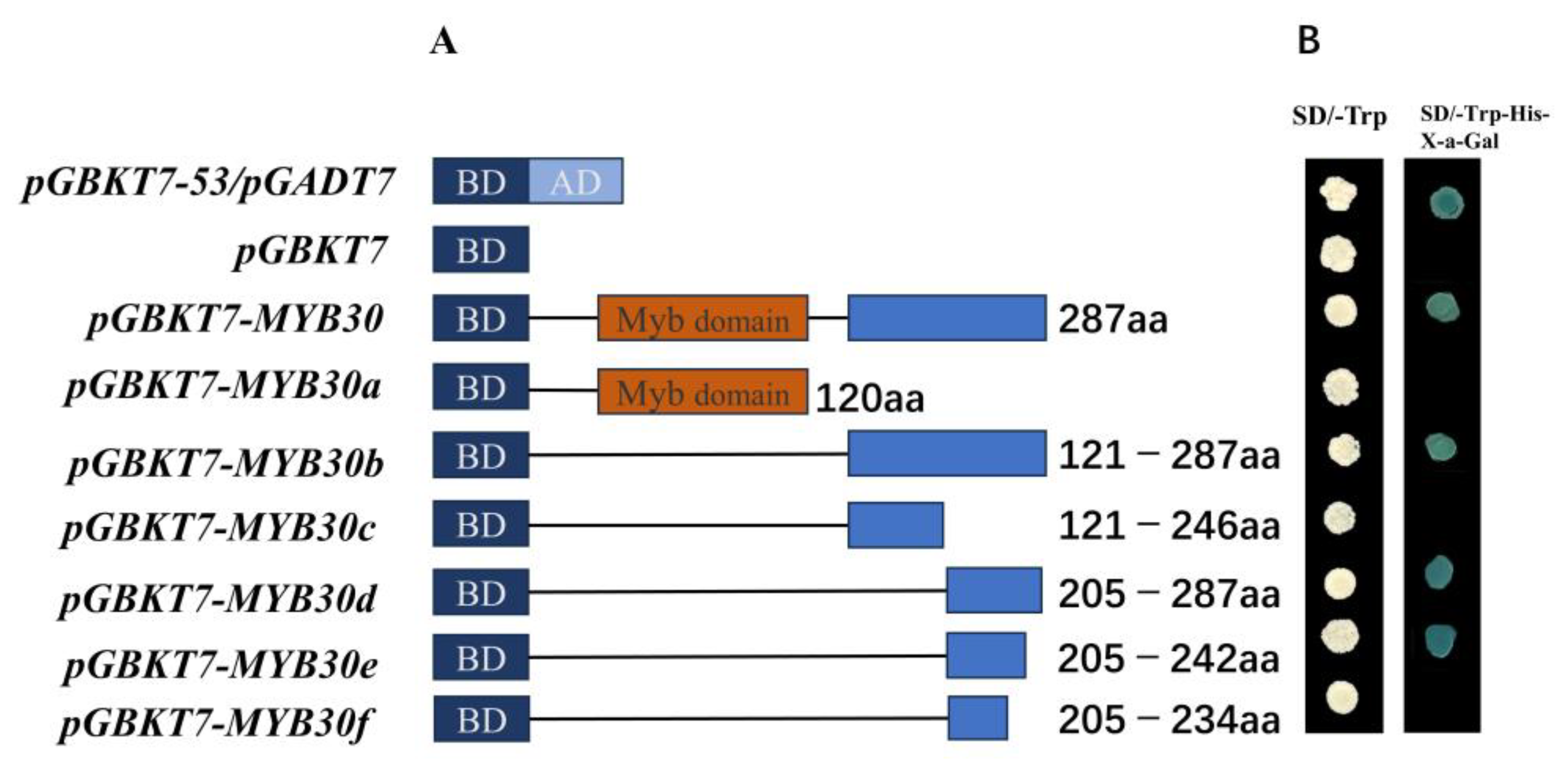
3.5. Transcriptional Activation Activity of PsnMYB30
3.6. Specific Binding of PsnMYB30 to G-Box
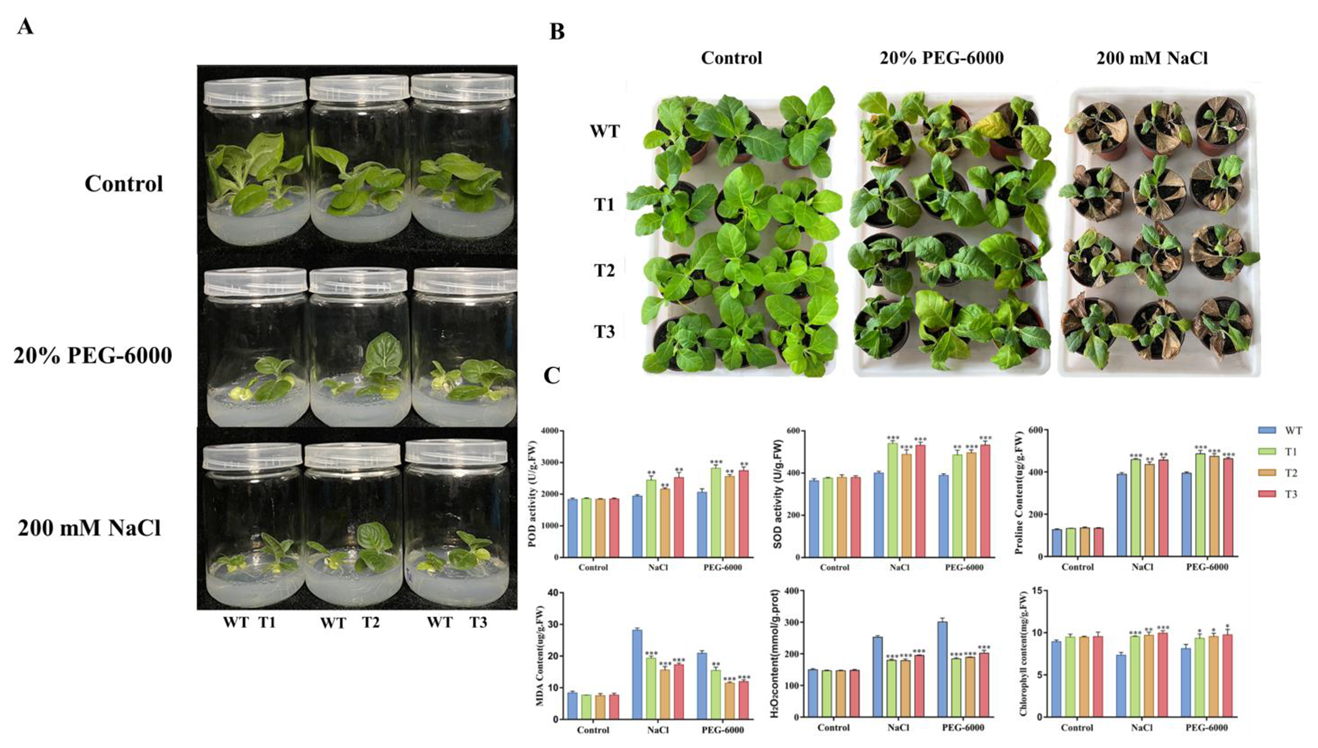
3.7. Morphological Features of Transgenic Tobacco Under Stress Conditions
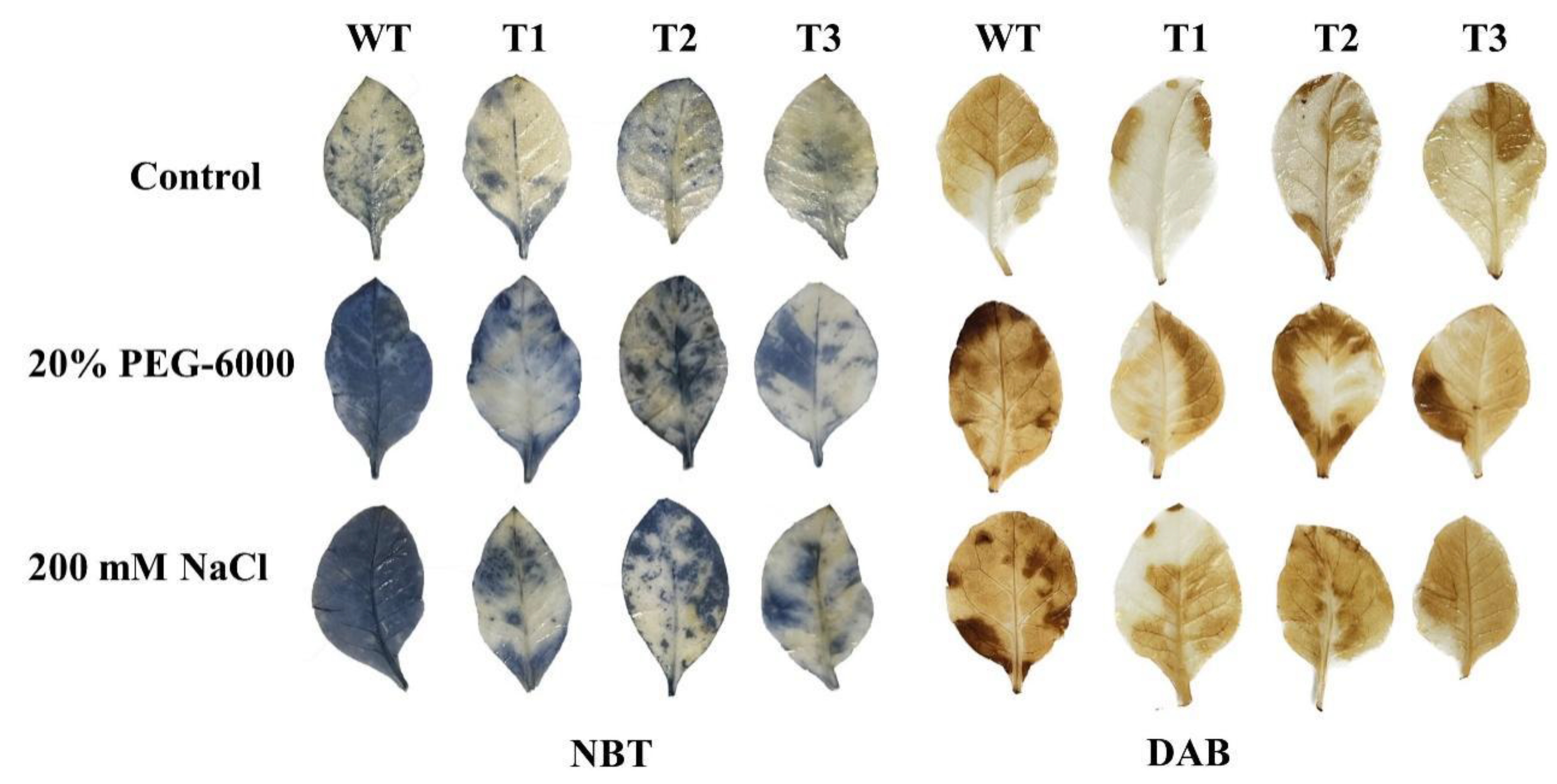
3.8. Histochemical Staining of Transgenic Tobacco
3.9. Physiological Changes in Transgenic Tobacco Under Stress Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Y.; Lee, J.H.; Poindexter, M.R.; Shao, Y.; Liu, W.; Lenaghan, S.C.; Ahkami, A.H.; Blumwald, E.; Stewart, C.N., Jr. Rational design and testing of abiotic stress-inducible synthetic promoters from poplar cis-regulatory elements. Plant Biotechnol. J. 2021, 19, 1354–1369. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Movahedi, A.; Xu, C.; Sun, W.; Li, L.; Wang, P.; Li, D.; Zhuge, Q. Overexpression of PtHMGR enhances drought and salt tolerance of poplar. Ann. Bot. 2020, 125, 785–803. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Ma, S.L.; Bai, L.P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012, 39, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Khoso, M.A.; Hussain, A.; Ritonga, F.N.; Ali, Q.; Channa, M.M.; Alshegaihi, R.M.; Meng, Q.; Ali, M.; Zaman, W.; Brohi, R.D.; et al. WRKY transcription factors (TFs): Molecular switches to regulate drought, temperature, and salinity stresses in plants. Front. Plant Sci. 2022, 13, 1039329. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Millard, P.S.; Kragelund, B.B.; Burow, M. R2R3 MYB Transcription Factors—Functions outside the DNA-Binding Domain. Trends Plant Sci. 2019, 24, 934–946. [Google Scholar] [CrossRef]
- Ding, Z.J.; Yan, J.Y.; Li, C.X.; Li, G.X.; Wu, Y.R.; Zheng, S.J. Transcription factor WRKY46 modulates the development of Arabidopsis lateral roots in osmotic/salt stress conditions via regulation of ABA signaling and auxin homeostasis. Plant J. Cell Mol. Biol. 2015, 84, 56–69. [Google Scholar] [CrossRef]
- Long, L.; Yang, W.W.; Liao, P.; Guo, Y.W.; Kumar, A.; Gao, W. Transcriptome analysis reveals differentially expressed ERF transcription factors associated with salt response in cotton. Plant Sci. Int. J. Exp. Plant Biol. 2019, 281, 72–81. [Google Scholar] [CrossRef]
- Lindemose, S.; O’Shea, C.; Jensen, M.K.; Skriver, K. Structure, function and networks of transcription factors involved in abiotic stress responses. Int. J. Mol. Sci. 2013, 14, 5842–5878. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, J.; Zhang, L.; Jin, H.; Fang, S. Genome-wide identification of bHLH transcription factors and their response to salt stress in Cyclocarya paliurus. Front. Plant Sci. 2023, 14, 1117246. [Google Scholar] [CrossRef]
- Jiang, X.; Li, S.; Ding, A.; Zhang, Z.; Liu, Q. The Novel Rose MYB Transcription Factor RhMYB96 Enhances Salt Tolerance in Transgenic Arabidopsis. Plant Mol. Biol. Rep. 2018, 36, 406–417. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niu, Y.; Zheng, Y. Multiple Functions of MYB Transcription Factors in Abiotic Stress Responses. Int. J. Mol. Sci. 2021, 22, 6125. [Google Scholar] [CrossRef]
- Tang, Y.; Bao, X.; Zhi, Y.; Wu, Q.; Guo, Y.; Yin, X.; Zeng, L.; Li, J.; Zhang, J.; He, W.; et al. Overexpression of a MYB Family Gene, OsMYB6, Increases Drought and Salinity Stress Tolerance in Transgenic Rice. Front. Plant Sci. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, W.; Jia, Y.; Wang, M.; Xia, G. The wheat TaGBF1 gene is involved in the blue-light response and salt tolerance. Plant J. Cell Mol. Biol. 2015, 84, 1219–1230. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, Y.; Liang, Y.; Chen, L.; Chen, W.; Cheng, B. Expression of the maize MYB transcription factor ZmMYB3R enhances drought and salt stress tolerance in transgenic plants. Plant Physiol. Biochem. 2019, 137, 179–188. [Google Scholar] [CrossRef]
- Wang, L.; Lu, W.; Ran, L.; Dou, L.; Yao, S.; Hu, J.; Fan, D.; Li, C.; Luo, K. R2R3-MYB transcription factor MYB6 promotes anthocyanin and proanthocyanidin biosynthesis but inhibits secondary cell wall formation in Populus tomentosa. Plant J. Cell Mol. Biol. 2019, 99, 733–751. [Google Scholar] [CrossRef]
- Chen, K.; Song, M.; Guo, Y.; Liu, L.; Xue, H.; Dai, H.; Zhang, Z. MdMYB46 could enhance salt and osmotic stress tolerance in apple by directly activating stress-responsive signals. Plant Biotechnol. J. 2019, 17, 2341–2355. [Google Scholar] [CrossRef]
- Zhu, N.; Cheng, S.; Liu, X.; Du, H.; Dai, M.; Zhou, D.X.; Yang, W.; Zhao, Y. The R2R3-type MYB gene OsMYB91 has a function in coordinating plant growth and salt stress tolerance in rice. Plant Sci. Int. J. Exp. Plant Biol. 2015, 236, 146–156. [Google Scholar] [CrossRef]
- Zhao, K.; Cheng, Z.; Guo, Q.; Yao, W.; Liu, H.; Zhou, B.; Jiang, T. Characterization of the Poplar R2R3-MYB Gene Family and Over-Expression of PsnMYB108 Confers Salt Tolerance in Transgenic Tobacco. Front. Plant Sci. 2020, 11, 571881. [Google Scholar] [CrossRef]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, Y.; Zang, W.; Li, X.; Wang, C.; Wang, R.; Jiang, T.; Zhou, B.; Yao, W. Ectopic Expression of PsnNAC090 Enhances Salt and Osmotic Tolerance in Transgenic Tobacco. Int. J. Mol. Sci. 2023, 24, 8985. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wang, S.; Zhou, B.; Jiang, T. Transgenic poplar overexpressing the endogenous transcription factor ERF76 gene improves salinity tolerance. Tree Physiol. 2016, 36, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, X.; Zhao, K.; Yao, W.; Li, R.; Zhou, B.; Jiang, T. Over-Expression of ERF38 Gene Enhances Salt and Osmotic Tolerance in Transgenic Poplar. Front. Plant Sci. 2019, 10, 1375. [Google Scholar] [CrossRef] [PubMed]
- Tene, T.M.; Eker, T.; Canci, H.; Babacan, U.; Cengiz, M.F.; Toker, C. Star of biochemical traits of heat-tolerant and heat-sensitive Phaseolus genotypes in coping with heat stress. Plant Growth Regul. 2025. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Sharoni, A.M.; Kikuchi, S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front. Microbiol. 2013, 4, 248. [Google Scholar] [CrossRef]
- Xu, L.; Liu, P.; Li, X.; Mi, Q.; Zheng, Q.; Xing, J.; Yang, W.; Zhou, H.; Cao, P.; Gao, Q.; et al. NtERF283 positively regulates water deficit tolerance in tobacco (Nicotianatabacum L.) by enhancing antioxidant capacity. Plant Physiol. Biochem. 2024, 207, 108413. [Google Scholar] [CrossRef]
- Du, H.; Yang, S.S.; Liang, Z.; Feng, B.R.; Liu, L.; Huang, Y.B.; Tang, Y.X. Genome-wide analysis of the MYB transcription factor superfamily in soybean. BMC Plant Biol. 2012, 12, 106. [Google Scholar] [CrossRef]
- Ma, R.; Liu, B.; Geng, X.; Ding, X.; Yan, N.; Sun, X.; Wang, W.; Sun, X.; Zheng, C. Biological Function and Stress Response Mechanism of MYB Transcription Factor Family Genes. J. Plant Growth Regul. 2023, 42, 83–95. [Google Scholar] [CrossRef]
- Chen, S. A Review on Plant Responses to Salt Stress and Their Mechanisms of Salt Resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Chen, Y.; Huang, M.; Zhu, S. Comprehensive Genome-Wide Analyses of Poplar R2R3-MYB Transcription Factors and Tissue-Specific Expression Patterns under Drought Stress. Int. J. Mol. Sci. 2023, 24, 5389. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.H.C.D. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Li, W.; Yuan, H.; Chen, H.; Bo, C.; Ma, Q.; Cai, R. Mutation of ZmWRKY86 confers enhanced salt stress tolerance in maize. Plant Physiol. Biochem. 2021, 167, 840–850. [Google Scholar] [CrossRef]
- Theerawitaya, C.; Tisarum, R.; Samphumphuang, T.; Takabe, T.; Cha-Um, S. Expression levels of the Na(+)/K(+) transporter OsHKT2;1 and vacuolar Na(+)/H(+) exchanger OsNHX1, Na enrichment, maintaining the photosynthetic abilities and growth performances of indica rice seedlings under salt stress. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2020, 26, 513–523. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, D.; Li, W.; Cao, X.; Ma, F.; Wang, Q.; Zhan, X.; Hu, T. Overexpression of a tomato AP2/ERF transcription factor SlERF.B1 increases sensitivity to salt and drought stresses. Sci. Hortic. 2022, 304, 111332. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, Z.; Fan, G.; Yao, W.; Li, W.; Chen, S.; Jiang, T. Functional analysis of PagNAC045 transcription factor that improves salt and ABA tolerance in transgenic tobacco. BMC Plant Biol. 2022, 22, 261. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How Does Proline Treatment Promote Salt Stress Tolerance During Crop Plant Development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Qin, L.; Wang, L.; Guo, Y.; Li, Y.; Ümüt, H.; Wang, Y. An ERF transcription factor from Tamarix hispida, ThCRF1, can adjust osmotic potential and reactive oxygen species scavenging capability to improve salt tolerance. Plant Sci. Int. J. Exp. Plant Biol. 2017, 265, 154–166. [Google Scholar] [CrossRef]
- Li, Y.; Xian, X.; Guo, L.; Zhang, J.; Gan, C.; Wang, Z.; Li, H.; Li, X.; Yuan, X.; Zhang, N.J.E.; et al. CsbZIP50 binds to the G-box/ABRE motif in CsRD29A promoter to enhance drought tolerance in cucumber. Environ. Exp. Bot. 2022, 199, 104884. [Google Scholar] [CrossRef]
- Ahmad, A.; Niwa, Y.; Goto, S.; Ogawa, T.; Shimizu, M.; Suzuki, A.; Kobayashi, K.; Kobayashi, H. bHLH106 Integrates Functions of Multiple Genes through Their G-Box to Confer Salt Tolerance on Arabidopsis. PLoS ONE 2015, 10, e0126872. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ally, M.S.; Wang, R.; Yao, W.; Jiang, T.; Liu, H. PsnMYB30 Enhances Salt and Drought Stress Tolerance in Transgenic Tobacco. Plants 2025, 14, 2681. https://doi.org/10.3390/plants14172681
Wang Y, Ally MS, Wang R, Yao W, Jiang T, Liu H. PsnMYB30 Enhances Salt and Drought Stress Tolerance in Transgenic Tobacco. Plants. 2025; 14(17):2681. https://doi.org/10.3390/plants14172681
Chicago/Turabian StyleWang, Yuting, Msangi Shamsia Ally, Ruiqi Wang, Wenjing Yao, Tingbo Jiang, and Huanzhen Liu. 2025. "PsnMYB30 Enhances Salt and Drought Stress Tolerance in Transgenic Tobacco" Plants 14, no. 17: 2681. https://doi.org/10.3390/plants14172681
APA StyleWang, Y., Ally, M. S., Wang, R., Yao, W., Jiang, T., & Liu, H. (2025). PsnMYB30 Enhances Salt and Drought Stress Tolerance in Transgenic Tobacco. Plants, 14(17), 2681. https://doi.org/10.3390/plants14172681






