Species-Specific Responses of Kiwifruit Seed Germination to Climate Change Using Classifier Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species and Seed Collection
- A. setosa: Siyuan Yakou, Yilan County (1870 m); Cuifeng, Nantou County (2250 m)
- A. deliciosa: Shengguang, Heping District, Taichung City (2500 m)
- A. rufa: Near Qingjing Farm, Nantou County (1800 m)
- A. latifolia: Beidongyan Mountain, Nantou County (1600 m); Wufeng, Taichung County (200 m); Mudan Township, Pingtung County (400 m)
2.2. Experimental Design
2.2.1. Cold Stratification Treatments
2.2.2. Temperature and Warming Treatments
- No warming (15/6 °C): This serves as the control group, representing the current or baseline climate conditions. It allows researchers to compare how seed germination performs under the current climate compared to warmer scenarios.
- Mild warming (20/10 °C): This level simulates a slight increase in temperature, reflecting a potential future climate scenario with minimal warming.
- Moderate warming (25/15 °C): This represents a more significant temperature increase, corresponding to a scenario with moderate climate warming.
- Extreme warming (30/20 °C): This level simulates a substantial temperature rise, indicative of a future climate with significant warming. It helps researchers understand how seeds might respond to more extreme temperature conditions.
2.2.3. Drought Stress Treatments
- Mild drought: 2 weeks of dry storage (RH 33%)
- Moderate drought: 4–8 weeks of dry storage
- Severe drought: ≥12 weeks of dry storage
2.3. Germination Assessment and Data Collection
- Germination conditions were maintained in a controlled-environment growth chamber (relative humidity: 70%; light/dark cycle: 12/12 h; photon irradiance: 60–80 µmol m−2 s−1).
- Watering and seed hydration conditions were standardized to prevent variation in moisture levels.
- Germination percentages were calculated as:
2.4. Classifier Modeling and Model Validation
2.4.1. Model Structure and Predictors
- Chilling Duration Model: Influence of cold stratification on germination success.
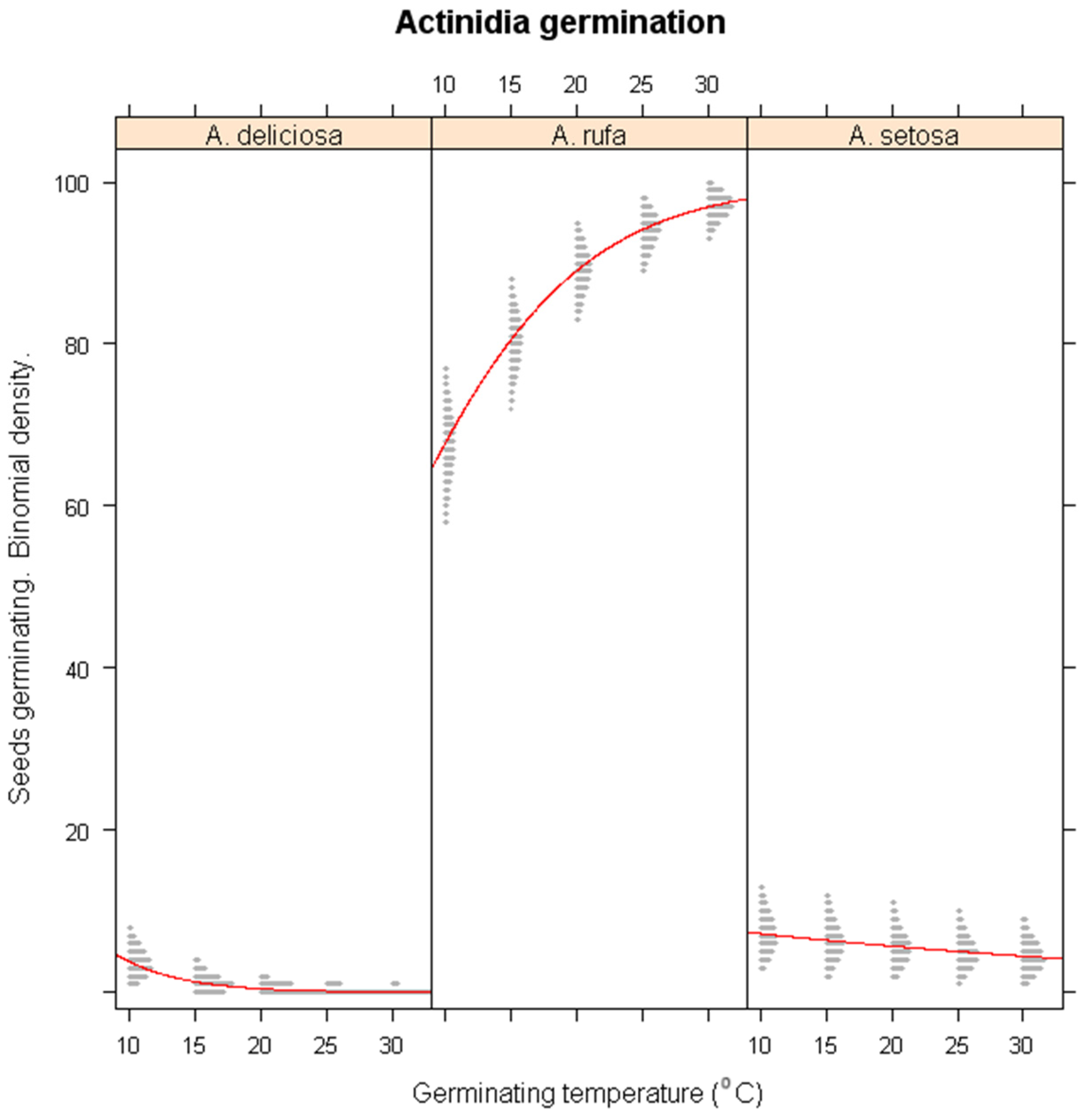
- Winter Warming Model: Effect of increasing temperature on A. setosa, A. deliciosa, and A. rufa germination.
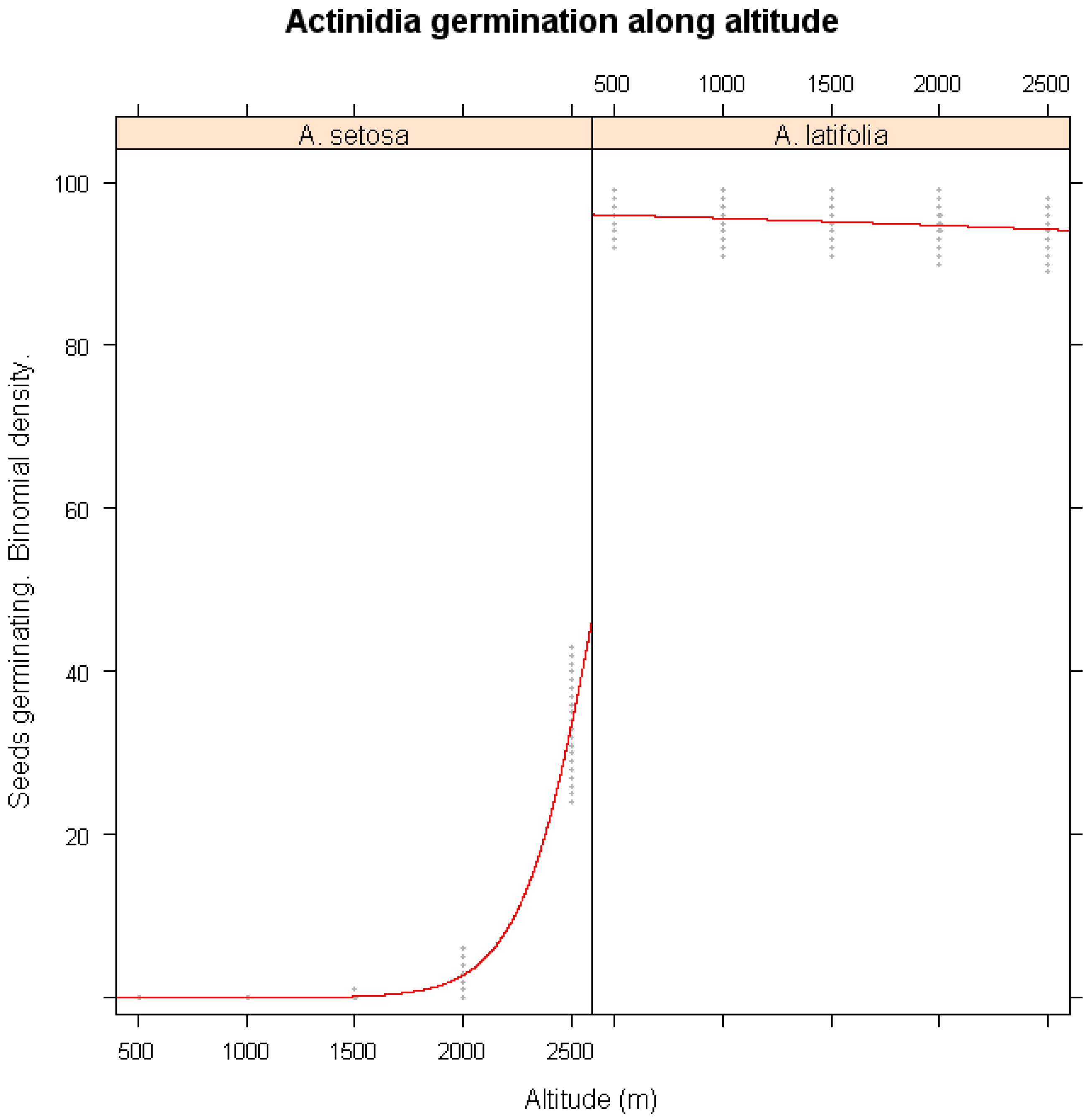
- Altitudinal Model: Relationship between germination percentage and elevation for A. setosa and A. latifolia.
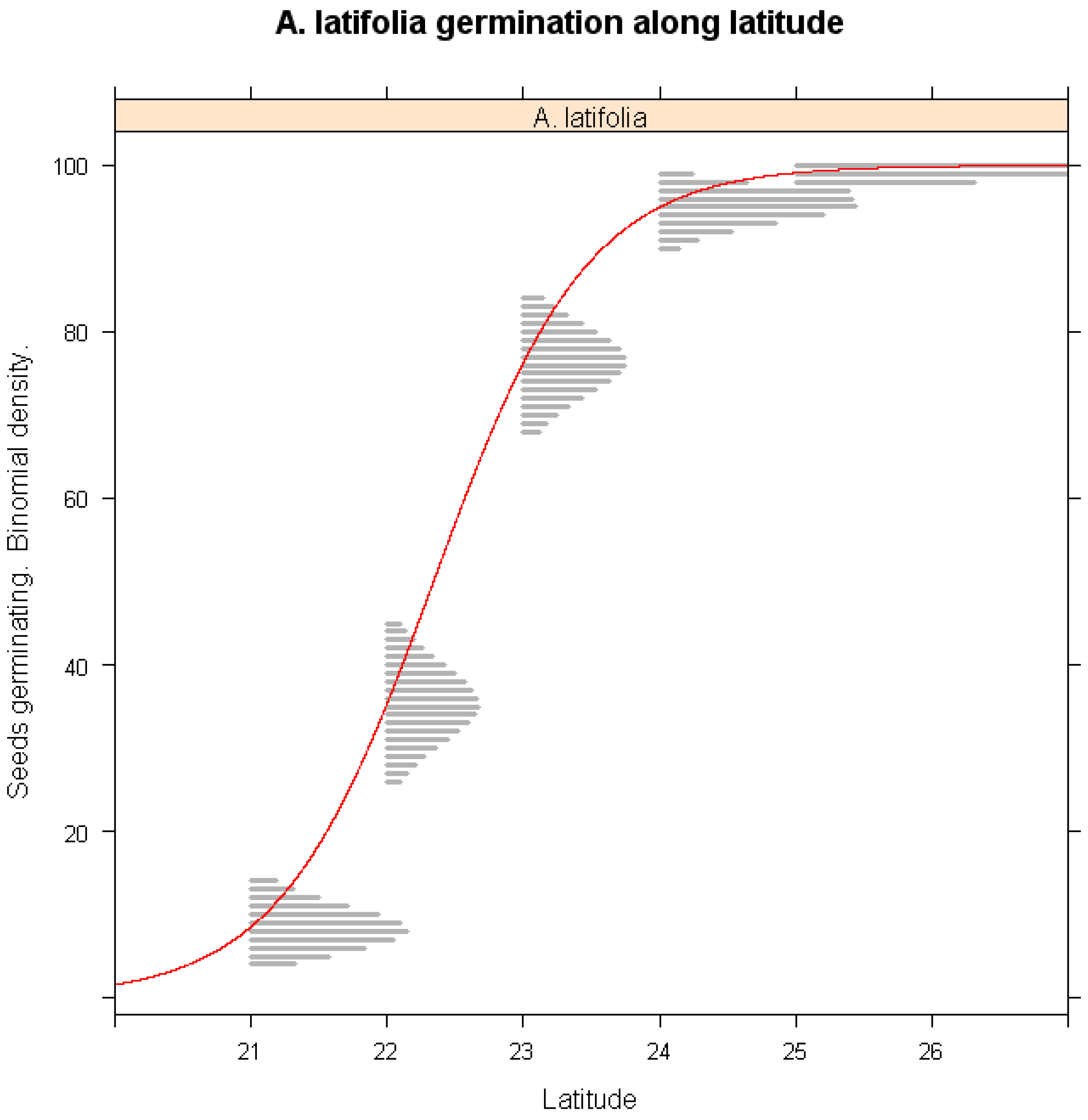
- Latitudinal Model: Impact of latitude on A. latifolia germination under warming conditions.
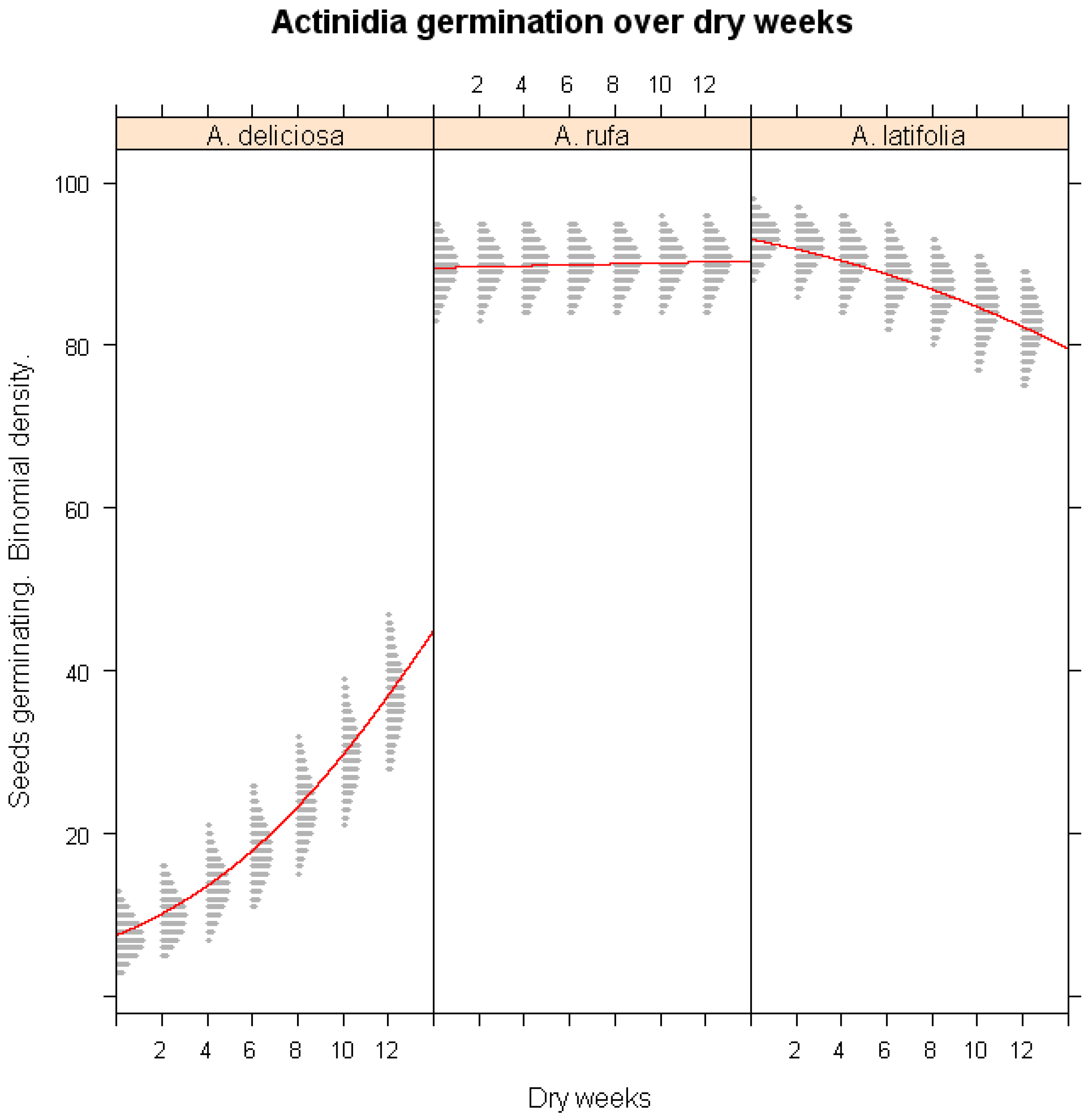
- Drought Model: Effect of dry storage duration on germination probability for A. latifolia, A. deliciosa, and A. rufa.
- Combined Stress Model: Interactive effects of warming and drought on A. deliciosa and A. rufa.
2.4.2. Model Validation with Field Data
3. Results
3.1. Seed Germination Responses Across Different Species and Elevations
3.2. Impact of Low Temperature on Germination
3.3. Effects of Winter Warming on Germination
3.4. Altitudinal and Latitudinal Variability in Germination
3.5. Influence of Winter Drought on Germination
3.6. Combined Effects of Winter Warming and Drought Stress
3.7. Validation of Model Predictions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hsieh, T.-Y.; Chiou, C.-R. Phytophenology and its applications in climate change research: Review and future perspectives. Q. J. Chin. For. 2013, 46, 391–410, (In Chinese, with English abstract). [Google Scholar]
- Hsieh, T.-Y. Impacts of Climate Change on Species, Ecosystems, and their Management Strategies. Taiwan J. For. Sci. 2016, 31, 227–255, (In Chinese, with English abstract). [Google Scholar]
- Dang, C.; Shao, Z.; Huang, X.; Zhuang, Q.; Cheng, G.; Qian, J. Climate warming-induced phenology changes dominate vegetation productivity in Northern Hemisphere ecosystems. Ecol. Indic. 2023, 151, 110326. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, D. Impacts of climate change on vegetation phenology over the Great Lakes Region of Central Asia from 1982 to 2014. Sci. Total Environ. 2022, 845, 157227. [Google Scholar] [CrossRef]
- Hsieh, T.-Y.; Yang, C.-J.; Li, F.; Chiou, C.-R. Numerical Ecology and Social Network Analysis of the Forest Community in the Lienhuachih Area of Taiwan. Diversity 2023, 15, 60. [Google Scholar] [CrossRef]
- Rathore, M.K.; Sharma, L.K. Efficacy of species distribution models (SDMs) for ecological realms to ascertain biological conservation and practices. Biodivers. Conserv. 2023, 32, 3053–3087. [Google Scholar] [CrossRef]
- Chien, C.-T.; Kuo-Huang, L.-L.; Lin, T.-P. Changes in ultrastructure and abscisic acid level, and response to applied gibberellins in Taxus mairei seeds treated with warm and cold stratification. Ann. Bot. 1998, 81, 41–47. [Google Scholar] [CrossRef]
- Thuiller, W.; Münkemüller, T.; Lavergne, S.; Mouillot, D.; Mouquet, N.; Schiffers, K.; Gravel, D. A road map for integrating eco-evolutionary processes into biodiversity models. Ecol. Lett. 2013, 16, 94–105. [Google Scholar] [CrossRef]
- Mou, S.S.; Haus, M.J.; Hayden, Z.D.; Patterson, E.L.; Saha, D. Climate-driven challenges in weed management for ornamental crop production in the United States: A review. Front. Agron. 2025, 7, 1556418. [Google Scholar] [CrossRef]
- Hudson, A.R.; Ayre, D.J.; Ooi, M.K. Physical dormancy in a changing climate. Seed Sci. Res. 2015, 25, 66–81. [Google Scholar] [CrossRef]
- Solarik, K.A.; Gravel, D.; Ameztegui, A.; Bergeron, Y.; Messier, C. Assessing tree germination resilience to global warming: A manipulative experiment using sugar maple (Acer saccharum). Seed Sci. Res. 2016, 26, 153–164. [Google Scholar] [CrossRef]
- Whitmore, T. Potential impact of climatic change on tropical rain forest seedlings and forest regeneration. Clim. Change 1998, 39, 429–438. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of seed dormancy and germination mechanisms in a changing environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef]
- Zu, K.; Chen, F.; Li, Y.; Shrestha, N.; Fang, X.; Ahmad, S.; Nabi, G.; Wang, Z. Climate change impacts flowering phenology in Gongga Mountains, Southwest China. Plant Divers. 2023, 46, 774–782. [Google Scholar] [CrossRef]
- Wang, J.Y.; Bu, Z.J.; Poschlod, P.; Yusup, S.; Zhang, J.Q.; Zhang, Z.X. Seed dormancy types and germination response of 15 plant species in temperate montane peatlands. Ecol. Evol. 2024, 14, e11671. [Google Scholar] [CrossRef]
- Lamont, B.B.; Pausas, J.G. Seed dormancy revisited: Dormancy-release pathways and environmental interactions. Funct. Ecol. 2023, 37, 1106–1125. [Google Scholar] [CrossRef]
- Giménez-Benavides, L.; Escudero, A.; García-Camacho, R.; García-Fernández, A.; Iriondo, J.; Lara-Romero, C.; Morente-López, J. How does climate change affect regeneration of Mediterranean high-mountain plants? An integration and synthesis of current knowledge. Plant Biol. 2018, 20, 50–62. [Google Scholar] [CrossRef]
- Chiou, C.-R.; Hsieh, T.-Y.; Chien, C.-C. Plant bioclimatic models in climate change research. Bot. Stud. 2015, 56, e26. [Google Scholar] [CrossRef]
- Lee-Yaw, J.A.; McCune, J.L.; Pironon, S.; Sheth, S.N. Species distribution models rarely predict the biology of real populations. Ecography 2022, 2022, e05877. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; García Molinos, J.; Mammola, S.; Bede-Fazekas, Á.; Feng, X.; Kitazawa, D.; Assis, J.; Qiu, T.; Lin, Q. Incorporating physiological knowledge into correlative species distribution models minimizes bias introduced by the choice of calibration area. Mar. Life Sci. Technol. 2024, 6, 349–362. [Google Scholar] [CrossRef]
- Ferguson, A.R. Kiwifruit: The wild and the cultivated plants. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2013; Volume 68, pp. 15–32. [Google Scholar]
- Li, H.-L. A taxonomic review of the genus Actinidia. J. Arnold Arbor. 1952, 33, 1–61. [Google Scholar] [CrossRef]
- Hsieh, T.-Y.; Ku, S.-M.; Chien, C.-T.; Liou, Y.-T. Classifier modeling and numerical taxonomy of Actinidia (Actinidiaceae) in Taiwan. Bot. Stud. 2011, 52, 337–357. [Google Scholar]
- Nee, C.C.; Tsay, T.T. Kiwifruit in Taiwan. Acta Hortic. 1991, 297, 175–182. [Google Scholar] [CrossRef]
- Chien, C.T.; Chen, S.Y.; Yang, J.C. Effect of stratification and drying on the germination and storage of Purnus campanulata seeds. Taiwan J. For. Sci. 2002, 17, 413–420. [Google Scholar]
- Chen, S.-Y.; Chou, S.-H.; Tsai, C.-C.; Hsu, W.-Y.; Baskin, C.C.; Baskin, J.M.; Chien, C.-T.; Kuo-Huang, L.-L. Effects of moist cold stratification on germination, plant growth regulators, metabolites and embryo ultrastructure in seeds of Acer morrisonense (Sapindaceae). Plant Physiol. Biochem. 2015, 94, 165–173. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Yao, H.-P. Studies on the seed germination and dormancy of Ilex rotunda, Castanopsis fargesii, Melia azedarach and Helicia rengetiensis from central Taiwan. Taiwan J. For. Sci. 2024, 39, 189–198. [Google Scholar]
- Chen, S.-Y.; Liu, C.-P.; Baskin, C.C.; Chien, C.-T. Deep complex morphophysiological dormancy in seeds of Viburnum plicatum var. formosanum (Adoxaceae) from subtropical mountains. Seed Sci. Res. 2021, 31, 236–242. [Google Scholar]
- Chen, S.-Y.; Tsai, Y.-H.; Su, H.-W.; Chien, C.-T. Seed dormancy and germination in the woody species of Berberis kawakamii, Mahonia oiwakensis, and Lonicera acuminata of the high mountains of Taiwan. Taiwan J. For. Sci. 2022, 37, 295–307. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Hsieh, T.-Y. Distribution of Indigenous Actinidia in Taiwan. Hortic. NCHU 2011, 36, 1–8, (In Chinese, with English abstract). [Google Scholar]
- Hsieh, T.-Y. Taxonomy and Distribution of Indigenous Actinidia in Taiwan. Ph.D. Thesis, National Chung-Hsing University, Taichung, Taiwan, 2011. (In Chinese, with English abstract). [Google Scholar]
- Lang, G.A. Dormancy: A new universal terminology. HortScience 1987, 22, 817–820. [Google Scholar] [CrossRef]
- Esfandiari, A.; Norling, C.; Kaji, R.; McLachlan, A.; Mathew, L.; Fleming, M.; Morgan, E.; Nadarajan, J. Variations in Seed Dormancy Occurrence and Their Classifications in Thirteen Actinidia Species. Seeds 2024, 3, 179–195. [Google Scholar] [CrossRef]
- Smith, R.L.; Toy, S.J. Effects of stratification and alternating temperatures on seed germination of chinese gooseberry Actinidia chinensis Planch. Proc. Am. Soc. Hortic. Sci. 1967, 90, 409–412. [Google Scholar]
- Lawes, G.; Anderson, D. Influence of temperature and gibberellic acid on kiwifruit (Actinidia chinensis) seed germination. N. Z. J. Exp. Agric. 1980, 8, 277–280. [Google Scholar] [CrossRef]
- Geneve, R.L. Impact of temperature on seed dormancy. HortScience 2003, 38, 336–340. [Google Scholar] [CrossRef]
- Celik, H.; Zenginbal, H.; Özcan, M. Enhancing germination of kiwifruit seeds with temperature, medium and gibberellic acid. Hortic. Sci. 2006, 33, 39–45. [Google Scholar] [CrossRef]
- Hsieh, T.Y.; Nee, C.C.; Chien, C.T. Seed germination of Taiwanese Actinidia latifolia (Gardn. and Champ.) Merr. Taiwan J. For. Sci. 2004, 19, 173–176, (In Chinese, with English abstract). [Google Scholar]
- Marris, E. The escalator effect. Nat. Rep. Clim. Change 2007, 1, 94–96. [Google Scholar] [CrossRef]


| Species | Collection Sites, Elevations, and GPS | Maximum Seed Germination (%) | Maximum Seed Germination After Cold Stratification (%) |
|---|---|---|---|
| Actinidia latifolia | Mutan, Pingtung County elevation 400 m, (22.18262, 120.85061) | 43.0 | 51.5 |
| Wufeng, Taichung County elevation 200 m, (24.07752, 120.72262) | 96.3 | 94.3 | |
| Peitungyenshan, Nantou County elevation 1600 m, (24.07688, 121.13007) | 94.8 | 94.6 | |
| Actinidia setosa | Tsuifeng, Nantou County elevation 2250 m, (24.10683, 121.19871) | 10.7 | 14.0 |
| Siyuan-Yakou, Yilan County elevation 1870 m, (24.39488, 121.35662) | 8 | 16 | |
| Actinidia rufa | Qingjing, Nantou County elevation 1800 m, (24.06300, 121.16810) | 89.3 | 90.7 |
| Actinidia deliciosa | Shengguang, Heping Dist., Taichung City elevation 2500 m, (24.36882, 121.33922) | 3.3 | 93.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, T.-Y.; Li, F.; Huang, S.-L.; Chien, C.-T. Species-Specific Responses of Kiwifruit Seed Germination to Climate Change Using Classifier Modeling. Plants 2025, 14, 2665. https://doi.org/10.3390/plants14172665
Hsieh T-Y, Li F, Huang S-L, Chien C-T. Species-Specific Responses of Kiwifruit Seed Germination to Climate Change Using Classifier Modeling. Plants. 2025; 14(17):2665. https://doi.org/10.3390/plants14172665
Chicago/Turabian StyleHsieh, Tung-Yu, Feng Li, Shih-Li Huang, and Ching-Te Chien. 2025. "Species-Specific Responses of Kiwifruit Seed Germination to Climate Change Using Classifier Modeling" Plants 14, no. 17: 2665. https://doi.org/10.3390/plants14172665
APA StyleHsieh, T.-Y., Li, F., Huang, S.-L., & Chien, C.-T. (2025). Species-Specific Responses of Kiwifruit Seed Germination to Climate Change Using Classifier Modeling. Plants, 14(17), 2665. https://doi.org/10.3390/plants14172665








