Physiological Mechanisms of Exogenous ABA in Alleviating Drought Stress in Nitraria tangutorum
Abstract
1. Introduction
2. Results
2.1. Effects of Different Degrees of Drought Stress on Growth Traits and Fluorescence Characteristic in N. tangutorum Seedlings
2.1.1. Effects of Different Degrees of Drought Stress on the Plant Height and RWC of N. tangutorum Seedlings
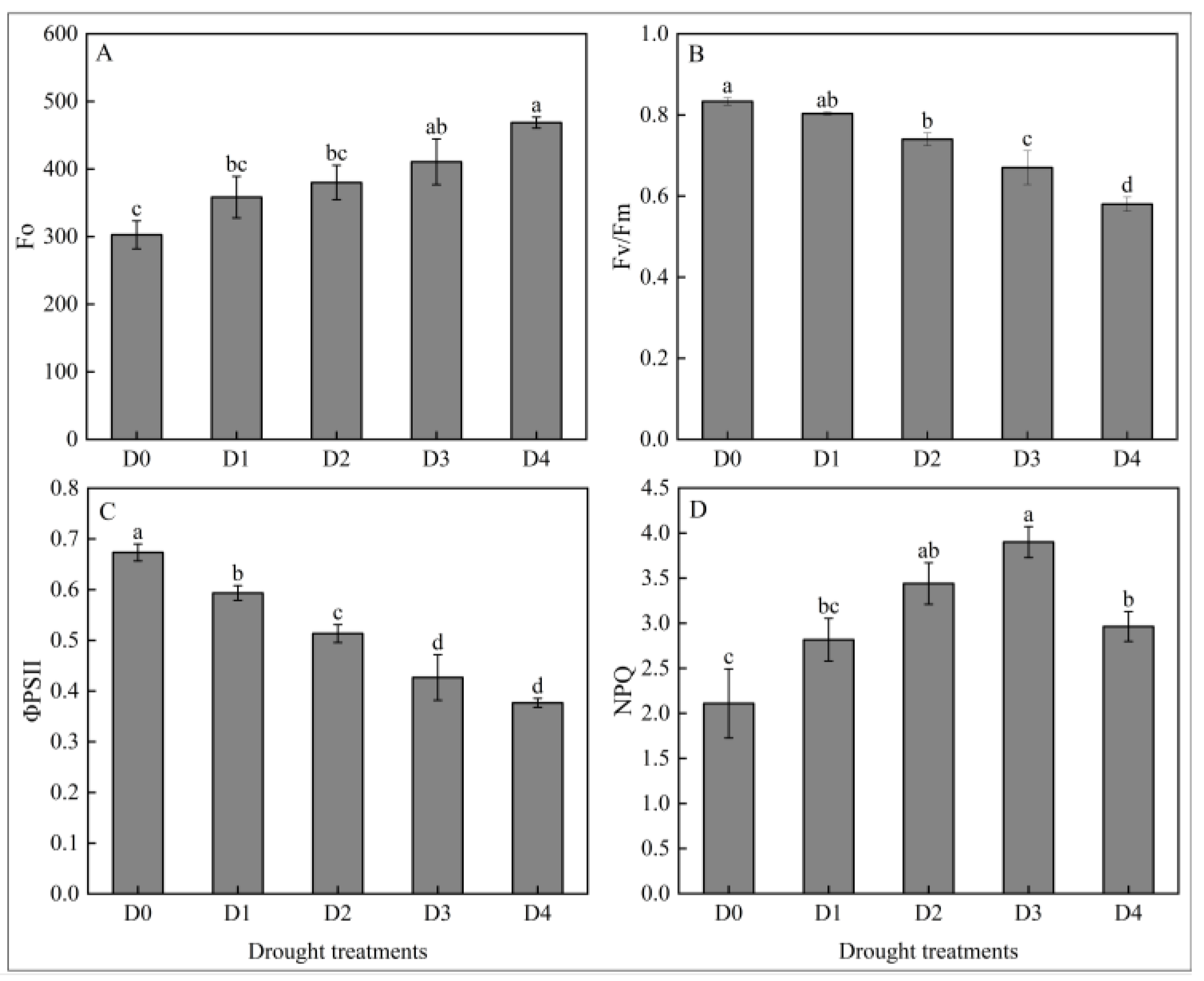
2.1.2. Effects of Different Degrees of Drought Stress on Fluorescence Characteristic in N. tangutorum Seedlings
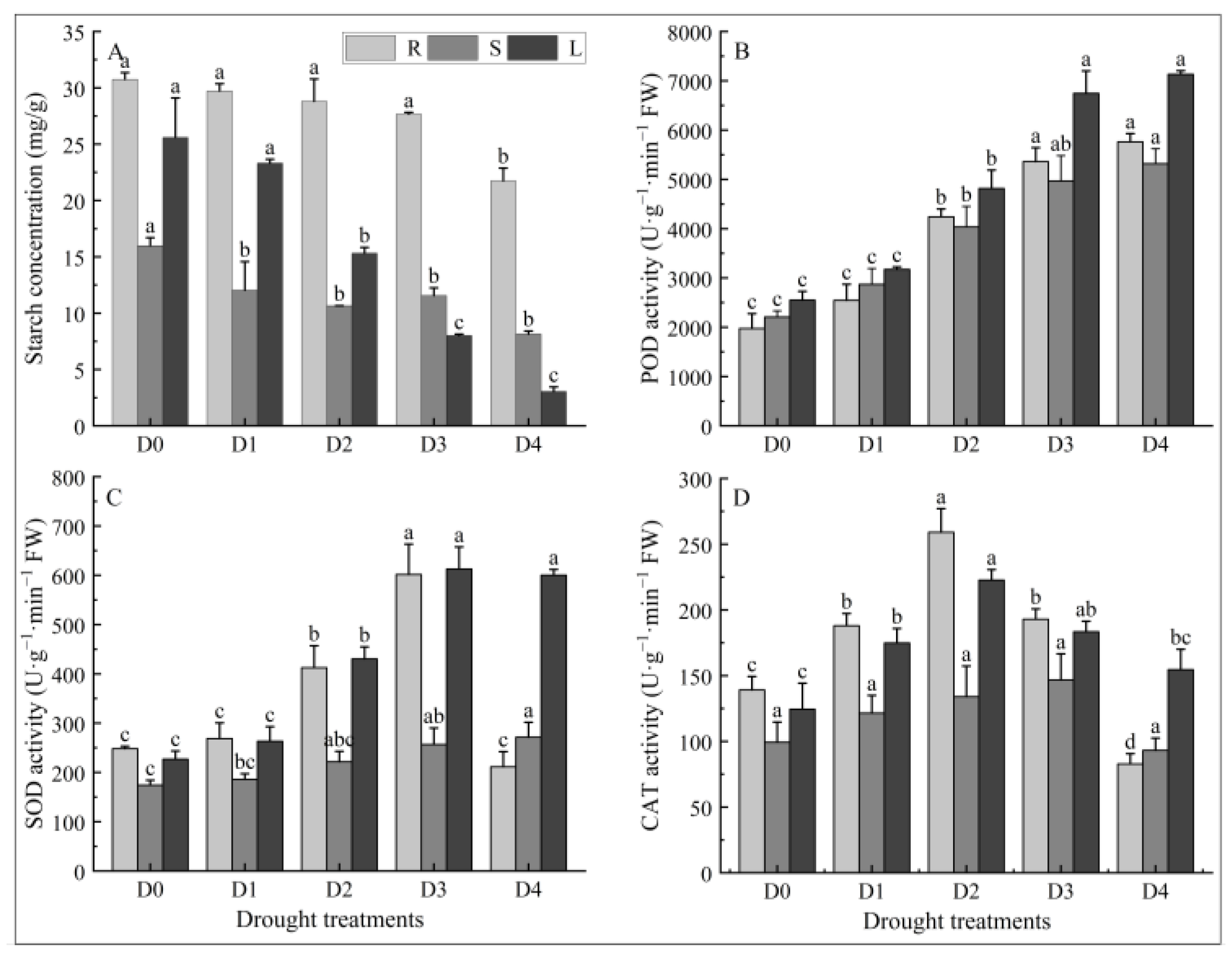
2.2. Effects of Different Degrees of Drought Stress on MDA Content in Different Tissues of N. tangutorum Seedlings
2.3. Effects of Different Degrees of Drought Stress on Osmoprotectants Content in Different Tissues of N. tangutorum Seedlings
2.4. Effects of Different Degrees of Drought Stress on Starch Content in Different Tissues of N. tangutorum Seedlings
2.5. Effects of Different Degrees of Drought Stress on Antioxidant Enzymes Activity in Different Tissues of N. tangutorum Seedlings
2.6. Effects of Exogenous ABA on Growth Traits and Fluorescence Characteristics of N. tangutorum Seedlings Under Drought Stress
2.6.1. Effects of Exogenous ABA on the Plant Height and RWC of N. tangutorum Seedlings Under Drought Stress
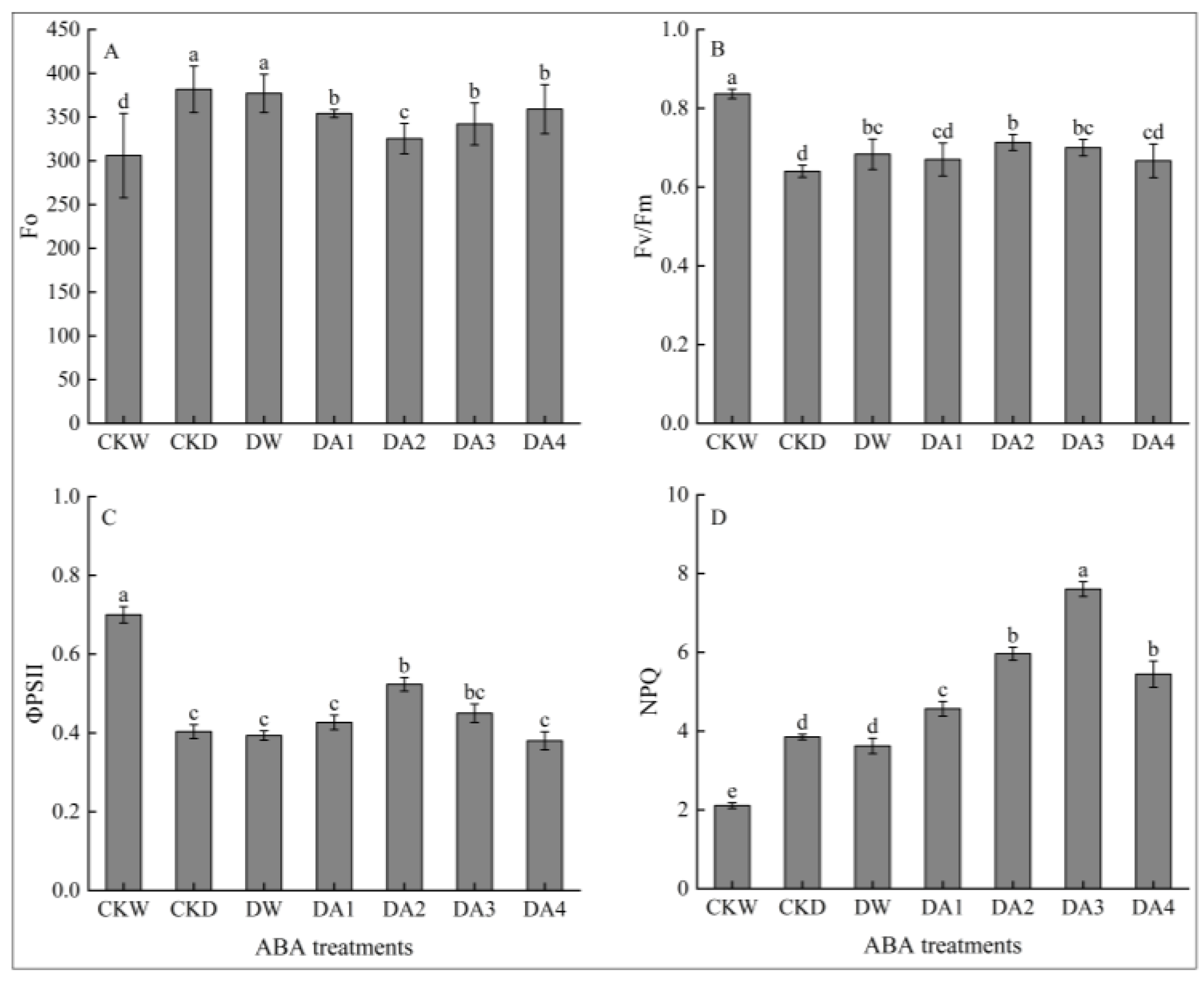
2.6.2. Effects of Exogenous ABA on Fluorescence Characteristic of N. tangutorum Seedlings Under Drought Stress
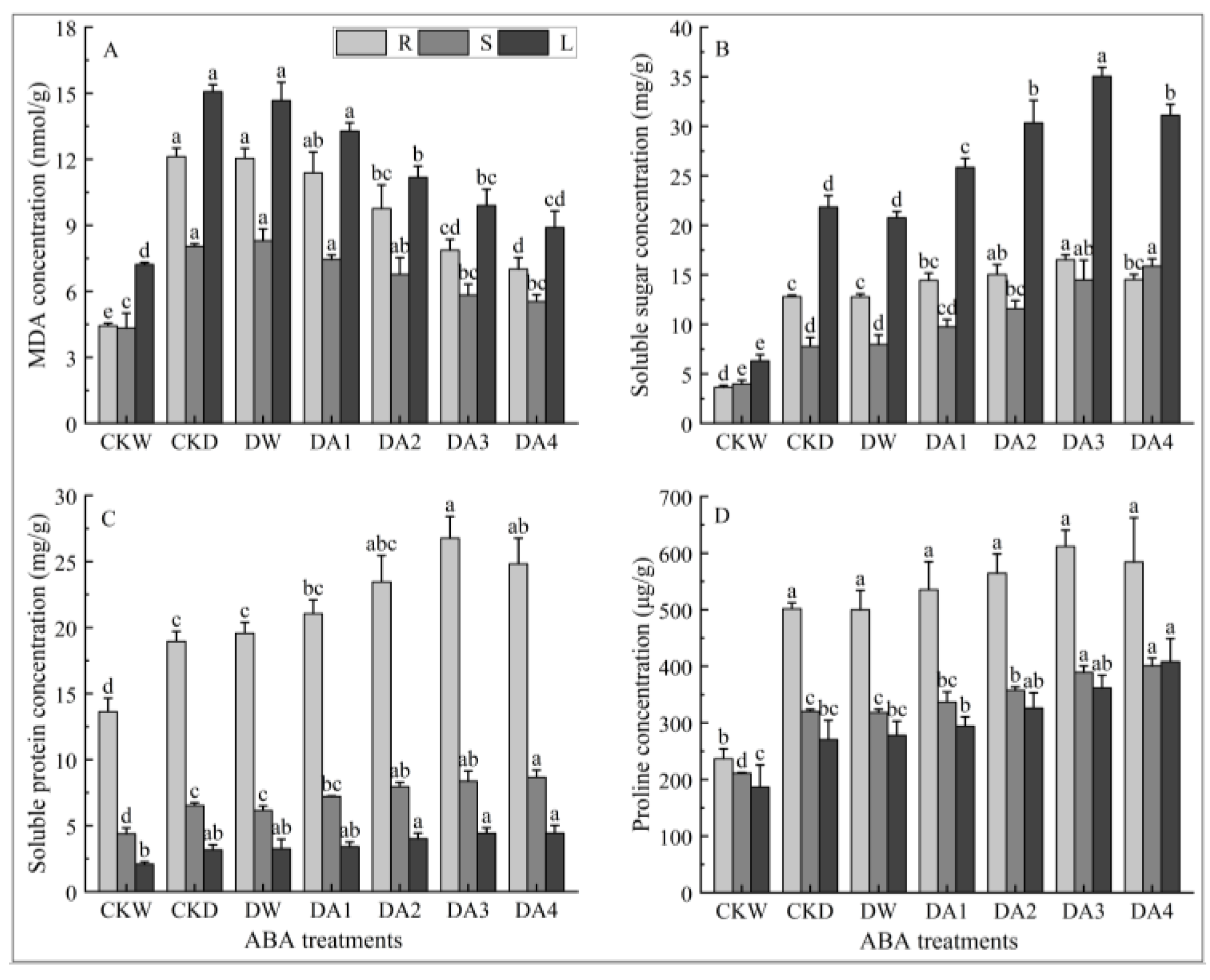
2.7. Effects of Exogenous ABA on MDA Content in Different Tissues of N. tangutorum Seedlings Under Drought Stress
2.8. Effects of Exogenous ABA on Osmolytes Content in Different Tissues of N. tangutorum Seedlings Under Drought Stress
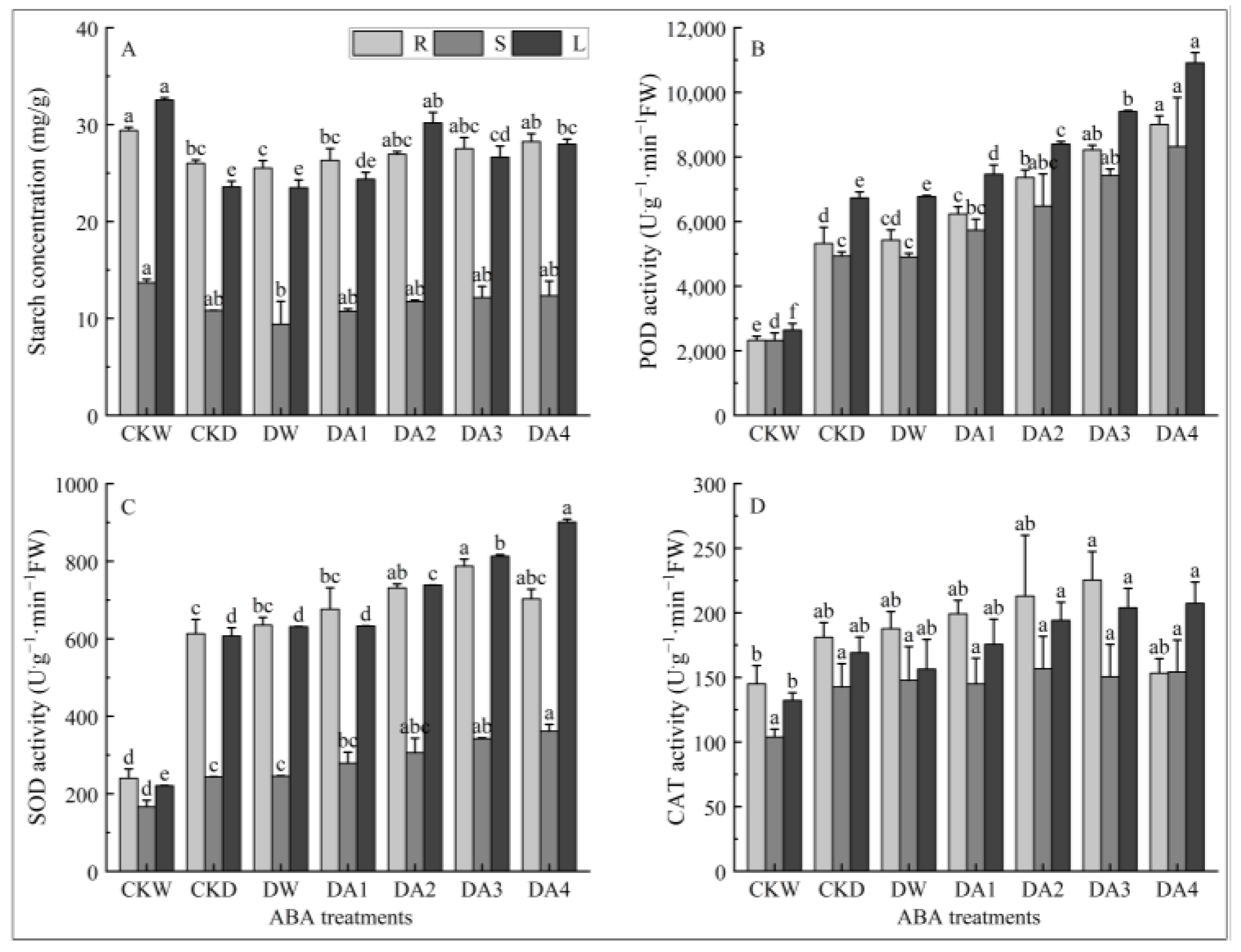
2.9. Effects of Exogenous ABA on Starch Content in Different Tissues of N. tangutorum Seedlings Under Drought Stress
2.10. Effects of Exogenous ABA on Antioxidant Enzymes Activity in Different Tissues of N. tangutorum Under Drought Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Experimental Design
4.2.1. Drought Stress Treatments
4.2.2. Abscisic Acid (ABA) Spray Treatments
4.3. Measurement of Growth Index
4.4. Measurement of Chlorophyll Fluorescence Parameters
4.5. Determination of Malondialdehyde (MDA) Content
4.6. Determination of Osmotic Adjustment Substances Content
4.6.1. Proline
4.6.2. Soluble Sugar
4.6.3. Starch
4.6.4. Soluble Protein
4.7. Determination of Antioxidant Enzyme Activity
5. Conclusions
- (1)
- Different levels of drought stress inhibited the growth, reduced the leaf relative water content, destroyed the reaction center structure of PSII, damaged the biofilm system, and led to a significant increase in the MDA content of N. tangutorum seedlings. N. tangutorum seedlings responded to drought stress by increasing the contents of osmoregulators such as soluble sugar, soluble protein, proline, and starch, as well as enhancing the activities of antioxidant enzymes such as POD, SOD, and CAT. The NPQ value, proline content in roots, and CAT activity in roots, stems, and leaves of N. tangutorum seedlings under D4 treatment were significantly lower than those under D3 treatment. It is speculated that extreme drought may cause metabolic collapse, resulting in irreversible damage to the N. tangutorum seedlings. After comprehensive analysis, D3 treatment (20–25% field capacities) was selected as the drought background of exogenous ABA treatment.
- (2)
- Different concentrations of exogenous ABA treatment of N. tangutorum seedlings under drought stress could promote the growth, increase the leaf relative water content, and alleviate the photosynthetic inhibition phenomenon of N. tangutorum seedlings under drought stress. In addition, exogenous ABA can also alleviate the drought damage of N. tangutorum seedlings under drought stress by increasing the contents of osmoregulators such as soluble sugar, soluble protein, proline, and starch, as well as enhancing the activities of antioxidant enzymes such as POD, SOD, and CAT. The comprehensive analysis showed that the exogenous ABA concentrations of 20 μM and 30 μM had the best alleviating effect on the drought damage of N. tangutorum seedlings. The determination of this concentration range is of significant guidance for the restoration, propagation, and conservation of N. tangutorum under in situ desert conditions. For example, applying this concentration of ABA during the seedling establishment stage can help seedlings better adapt to arid environments and improve establishment survival rates. Additionally, applying ABA to established N. tangutorum populations prior to extreme drought events can enhance their drought resistance, reducing growth inhibition and yield decline caused by drought.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Wei, X.; Shi, R.; Fan, Q.; An, L. Salinity-induced physiological modification in the callus from halophyte Nitraria tangutorum Bobr. J. Plant Growth Regul. 2010, 29, 465–476. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, W.Z.; Luo, W.C.; Liu, B. Species diversity and vegetation distribution in nebkhas of Nitraria tangutorum in the Desert Steppes of China. Ecol. Res. 2015, 30, 735–744. [Google Scholar] [CrossRef]
- Yang, R.M.; Suo, Y.R.; Wang, H.L. Studies on chemical constituents and pharmacological effects of Nitraria tangutorum Bobr. fruit. Nat. Product Res. Dev. 2012, 24, 985–989+1005. [Google Scholar]
- Wu, Z.B.; Deng, J.; Tian, Y.Z.; Wang, X.; Yuan, Y.Y.; Wang, B.; Zhou, Y.B. Analysis and evaluation forage quality of four Nitraria Species. J. Gansu Agric. Univ. 2017, 52, 97–100. [Google Scholar]
- Jia, B.Q.; Cai, T.J.; Gao, Z.H.; Ding, F.; Zhang, G.Z. Biomass forcast models of Nitraria Tangutorum shrub in sand dune. J. Arid Land Resour. Environ. 2002, 16, 96–99. [Google Scholar]
- Bazzaz, F.A. The response of natural ecosystems to the rising global CO2 levels. Annu. Rev. Ecol. Evol. Syst. 1990, 21, 167–196. [Google Scholar] [CrossRef]
- Smith, S.D.; Huxman, T.E.; Zitzer, S.F.; Charlet, T.N.; Housman, D.C.; Coleman, J.S.; Fenstermaker, L.K.; Seemann, J.R.; Nowak, R.S. Elevated CO2 increases productivity and invasive species success in an arid ecosystem. Nature 2000, 408, 79–82. [Google Scholar] [CrossRef]
- Anonymous. Editorial: Shifting Habitats and Biodiversity Management Under Climate Change. Nat. Clim. Change 2020, 10, 377. [Google Scholar]
- Welter, S.; Brunner, K.; Hofstraat, J.W.; De Cola, L. Electroluminescent device with reversible switching between red and green emission. Nature 2003, 421, 54–57. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. Summary for Policymakers. In Climate Change 2021—The physical science basis: Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Eds.; IPCC: Geneva, Switzerland, 2021; pp. 22–23. [Google Scholar]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Change 2013, 3, 171. [Google Scholar] [CrossRef]
- Buttler, A.; Mariotte, P.; Meisser, M.; Guillaume, T.; Signarbieux, C.; Vitra, A.; Preux, S.; Mercier, G.; Quezada, J.; Bragazza, L.; et al. Drought-induced decline of productivity in the dominant grassland species Lolium perenne L. depends on soil type and prevailing climatic conditions. Soil Biol. Biochem. 2019, 132, 47–57. [Google Scholar] [CrossRef]
- Jing, W.; Fu, B.Z.; Li, S.X.; Xing, W.; Song, W.X.; Ye, Y.N.; Hu, P.F.; Wang, T.R. Effects of exogenous melatonin on growth and physiological characteristics of Agropyron mongolicum seedlings under drought stress. Ying Yong Sheng Tai Xue Bao (J. Appl. Ecol.) 2023, 34, 2947–2957. [Google Scholar]
- Loutfy, N.; El-Tayeb, M.A.; Hassanen, A.M.; Moustafa, M.F.M.; Sakuma, Y.; Inouhe, M. Changes in the water status and osmotic solute contents in response to drought and salicylic acid treatments in four different cultivars of wheat (Triticum aestivum). J. Plant Res. 2011, 125, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Xinling, L.; Yunmei, W.; Zhiyi, L.; Honghong, D.; Jin, W.; Lijin, L.; Qunxian, D.; Xiulan, L.; Kunfu, X.; et al. 24-Epibrassinolide and nitric oxide combined to improve the drought tolerance in kiwifruit seedlings by proline pathway and nitrogen metabolism. Sci. Hortic. 2022, 297, 110929. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Akan, D.; Mainaak, M.; Bipasa, S.; Dipanwita, S.; Mondal, T.K. Influence of drought stress on cellular ultrastructure and antioxidant system in tea cultivars with different drought sensitivities. J. Environ. Biol. 2015, 36, 875–882. [Google Scholar]
- Souza, T.C.; Magalhães, P.C.; Castro, E.M.; Carneiro, N.P.; Padilha, F.A.; Júnior, C.C.G. ABA application to maize hybrids contrasting for drought tolerance: Changes in water parameters and in antioxidant enzyme activity. Plant Growth Regul. 2014, 73, 205–217. [Google Scholar] [CrossRef]
- Wei, L.; Wang, L.; Yang, Y.; Wang, P.; Guo, T.; Kang, G. Abscisic acid enhances tolerance of wheat seedlings to drought and regulates transcript levels of genes encoding ascorbate-glutathione biosynthesis. Front. Plant Sci. 2015, 6, 458. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Y.; Wang, R.; He, Y.; Ma, X.; Shen, J.; He, Z.; Lai, H. Effects of exogenous abscisic acid on the physiological and biochemical responses of Camellia oleifera seedlings under drought stress. Plants 2024, 13, 225. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, M.; Hu, J.; Zhang, X.; Wang, K.; Ashraf, M. Modulation role of abscisic acid (ABA) on growth, water relations and Glycinebetaine metabolism in two maize (Zea mays L.) cultivars under drought stress. Int. J. Mol. Sci. 2012, 13, 3189–3202. [Google Scholar] [CrossRef]
- Liu, C.; Duan, N.; Chen, X.; Li, H.; Zhao, X.; Duo, P.; Wang, J.; Li, Q. Metabolic pathways involved in the drought stress response of Nitraria tangutorum as revealed by transcriptome analysis. Forests 2022, 13, 509. [Google Scholar] [CrossRef]
- Nemat, H.; Heba, E.; Alshafei, A. Exogenous application of spermine and putrescine mitigate adversities of drought stress in wheat by protecting membranes and chloroplast ultra-structure. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2020, 26, 233–245. [Google Scholar]
- Zhang, Z.; Zhang, A.; Zhang, Y.; Zhao, J.; Wang, Y.; Zhang, L.; Zhang, S. Ectopic expression of HaPEPC1 from the desert shrub Haloxylon ammodendron confers drought stress tolerance in Arabidopsis thaliana. Plant Physiol. Biochem. PPB 2024, 208, 108536. [Google Scholar] [CrossRef]
- Fang, Y.; Guanghui, L. Metabolomic analysis of the response of Haloxylon ammodendron and Haloxylon persicum to drought. Int. J. Mol. Sci. 2023, 24, 9099. [Google Scholar] [CrossRef]
- Zhou, L.; Tian, X.; Cui, B.; Hussain, A. Physiological and biochemical responses of invasive species Cenchrus pauciflorus Benth to drought stress. Sustainability 2021, 13, 5976. [Google Scholar] [CrossRef]
- Nouri, K.; Nikbakht, A.; Haghighi, M.; Etemadi, N.; Rahimmalek, M.; Szumny, A. Screening some pine species from North America and dried zones of western Asia for drought stress tolerance in terms of nutrients status, biochemical and physiological characteristics. Front. Plant Sci. 2023, 14, 1281688. [Google Scholar] [CrossRef]
- Wei, P.; Li, H.; Wu, Y.; Zhang, C. Association of the electrical parameters and photosynthetic characteristics of the tea tree manifests its response to simulated karst drought. Plant Signal. Behav. 2024, 19, 2359258. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Yu, H.Y.; Kong, D.S.; Yan, F.; Zhang, Y.J. Effects of drought stress on growth and chlorophyll fluorescence of Lycium ruthenicum Murr. seedlings. Photosynthetica 2016, 54, 524–531. [Google Scholar] [CrossRef]
- Bandurska, H.; Niedziela, J.; Pietrowska-Borek, M.; Nuc, K.; Chadzinikolau, T.; Radzikowska, D. Regulation of proline biosynthesis and resistance to drought stress in two barley (Hordeum vulgare L.) genotypes of different origin. Plant Physiol. Biochem. 2017, 118, 427–437. [Google Scholar] [CrossRef]
- Gao, M.; Li, Y.; Chong, P.F.; Su, S.P. Physiological responses of Nitraria tangutorum from different geographic provenances under osmotic stress. Acta Prataculturae Sin. 2011, 20, 99–107. [Google Scholar]
- Chen, M.T.; Zhao, Z.; Quan, J.E. Variation of soluble protein components and contents in seedling roottips of four trees under drought stress. Acta Bot. Boreali-Occident. Sin. 2010, 30, 1157–1165. [Google Scholar]
- Ling, M. Compensatory Photosynthetic Mechanism of Mulberry Under Soil Drought Stress and Rewatering. Master’s Thesis, Northeast Forestry University, Harbin, China, 2012. [Google Scholar]
- Matthias, T.; Diana, S. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, Y.; Chen, Y.; Gao, M.; Zhao, Y.; Wu, L. Effects of Drought Stress and Rehydration on Physiological and Biochemical Properties of Four Oak Species in China. Plants 2022, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Jin, S. Enhancing drought tolerance in horticultural plants through plant hormones: A strategic coping mechanism. Front. Plant Sci. 2025, 15, 1502438. [Google Scholar] [CrossRef] [PubMed]
- Sansberro, P.A.; Mroginski, L.A.; Bottini, R. Foliar sprays with ABA promote growth of Ilex paraguariensis by alleviating diurnal water stress. Plant Growth Regul. Int. J. Nat. Synth. Regul. 2004, 42, 105–111. [Google Scholar] [CrossRef]
- Travaglia, C.; Reinoso, H.; Cohen, A.; Luna, C.; Tommasino, E.; Castillo, C.; Bottini, R. Exogenous ABA increases yield in field-grown wheat with moderate water restriction. J. Plant Growth Regul. 2010, 29, 366–374. [Google Scholar] [CrossRef]
- Chen, J. Effects of Drought Stress and Exogenous ABA Application on Growth and Rhizospheric Effects of Ginger (Zingiber officinale Roscoe). Master’s Thesis, Sichuan University, Chengdu, China, 2007. [Google Scholar]
- Xi, J.L.; Zhang, J.C.; Xi, K.P.; Yao, J.Z.; Duan, L.J.; Liu, Y.P. Effects of exogenous ABA on wheat drought resistance and yield. Crops 2014, 3, 105–108. [Google Scholar]
- Ma, X.L.; Wang, W.Y.; Zhou, H.K.; Li, W.J.; Li, J.; Li, Y.; Qiu, Q.H.; Yin, H.X. Effect of soaking exogenous abscisic acid on Onobrychis viciifolia seed germination and physiological mechanism under drought stress. Chin. J. Eco-Agric. 2024, 32, 1882–1890. [Google Scholar]
- Baker, N.R. Chlorophyll Fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Q.; Xu, Z.; Wu, B.G.; Ren, J.; Dai, W.R. Effects of exogenous ABA application on the photosynthetic characteristics of bermudagrass seedlings exposed to drought stress. Grassl. Turf 2024, 44, 99–105. [Google Scholar]
- Wang, W.Q.L.; Lin, X.Y.; Xiao, L.; Zhou, L.; Fu, X.; Qu, Y. Effects of abscisic acid spray on hormone metabolism of Camellia reticulata seedlings under drought stress. Fujian J. Agric. Sci. 2024, 39, 946–958. [Google Scholar]
- Zhang, Y.; Zhu, J.; Khan, M.; Wang, Y.; Xiao, W.; Fang, T.; Qu, J.; Xiao, P.; Li, C.; Liu, J.-H. Transcription factors ABF4 and ABR1 synergistically regulate amylase-mediated starch catabolism in drought tolerance. Plant Physiol. 2023, 191, 591–609. [Google Scholar] [CrossRef]
- Lu, S.; Su, W.; Li, H.; Guo, Z. Abscisic acid improves drought tolerance of triploid bermudagrass and involves H2O2- and NO-induced antioxidant enzyme activities. Plant Physiol. Biochem. 2009, 47, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, F.; Li, M.; Liang, D.; Zou, J. Physiological responses of kiwifruit plants to exogenous ABA under drought conditions. Plant Growth Regul. 2010, 64, 63–74. [Google Scholar] [CrossRef]
- Li, S.S. Physiological Regulation Mechanis of Exogenous ABA/NO on Drought Tolerance of Nitraria Tangutorum and Effects of Habitat on Feeding Quality of Nitraria Tangutorum. Master’s Thesis, Inner Mongolia University, Hohhot, China, 2022. [Google Scholar]
- Song, L.L.; Yu, Y.L.; Chen, H.Z.; Feng, Y.W.; Chen, S.; Zhang, H.H.; Zhou, H.J.; Meng, L.; Wang, Y. Response of photosynthetic characteristics and antioxidant system in the leaves of safflower to NaCl and NaHCO3. Plant Cell Rep. 2024, 43, 146. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yang, Z.; Zhou, C.; Geng, S.; Chen, S.; Cai, N.; Tang, J.; Chen, L.; Xu, Y. The response and evaluation of morphology, physiology, and biochemistry traits in triploid passiflora edulis sims ‘Mantianxing’ to drought stress. Plants 2024, 13, 1685. [Google Scholar] [CrossRef]
- Huang, L.J.; Ding, L.L.; Wang, W.J.; Zhao, L.L.; Zhao, X.; Wang, P.C. Effects of simulated drought stress on the growth and physiological and biochemical parameters of Paspalum wettsteinii. Acta Physiol. Plant 2023, 45, 82. [Google Scholar] [CrossRef]
- Hou, P.C.; Wang, F.F.; Luo, B.; Li, A.X.; Wang, C.; Shabala, L.; Ahmed, H.A.I.; Deng, S.R.; Zhang, H.L.; Song, P.; et al. Antioxidant enzymatic activity and osmotic adjustment as components of the drought tolerance mechanism in Carex duriuscula. Plants 2021, 10, 436. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Zhou, Q.; Wang, X.; Song, S.; Dong, S. Physiological response of soybean plants to water deficit. Front. Plant Sci. 2022, 12, 809692. [Google Scholar] [CrossRef]
- Zhou, H.M.; Li, Q.Y.; Gichuki, D.K.; Hou, Y.J.; Fan, P.G.; Gong, L.Z.; Xin, H.P. Dynamics of starch degradation and expression of related genes during chilling stress in grapevine. Hortic. Adv. 2023, 1, 2–6. [Google Scholar] [CrossRef]
- Li, H.; Li, H. Principles and Techniques of Plant Physiological Biochemical Experimental; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Sanyal, R.P.; Prashar, V.; Jawali, N.; Sunkar, R.; Misra, H.S.; Saini, A. Molecular and biochemical analysis of duplicated cytosolic CuZn superoxide dismutases of rice and in silico analysis in plants. Front. Plant Sci. 2022, 13, 864330. [Google Scholar] [CrossRef]
- Eid, M.A.M.; El-hady, M.A.A.; Abdelkader, M.A.; Abd-Elkrem, Y.M.; El-Gabry, Y.A.; El-temsah, M.E.; El-Areed, S.R.M.; Rady, M.M.; Alamer, K.H.; Alqubaie, A.I.; et al. Response in physiological traits and antioxidant capacity of two cotton cultivars under water limitations. Agronomy 2022, 12, 803. [Google Scholar] [CrossRef]
- Gou, T.; Chen, X.; Han, R.; Liu, J.; Zhu, Y.; Gong, H. Silicon can improve seed germination and ameliorate oxidative damage of bud seedlings in cucumber under salt stress. Acta Physiol. Plant. 2020, 42, 12. [Google Scholar] [CrossRef]
- Li, S.Y.; Jiang, H.L.; Wang, J.J.; Wang, Y.D.; Pan, S.G.; Tian, H.; Duan, M.Y.; Wang, S.L.; Tang, X.G.; Mo, Z.W. Responses of plant growth, physiological, gas exchange parameters of super and non-super rice to rhizosphere temperature at the tillering stage. Sci. Rep. 2019, 9, 10618. [Google Scholar] [CrossRef] [PubMed]
- Demirel, U.; Morris, W.L.; Ducreux, L.J.M.; Yavuz, C.; Asim, A.; Tindas, I.; Campbell, R.; Morris, J.A.; Verrall, S.R.; Hedley, P.E.; et al. Physiological, biochemical, and transcriptional responses to single and combined abiotic stress in stress-tolerant and stress-sensitive potato genotypes. Front. Plant Sci. 2020, 11, 169. [Google Scholar] [CrossRef]
- Qi, Z.X.; Ling, F.L.; Jia, D.S.; Cui, J.J.; Zhang, Z.A.; Xu, C.; Yu, L.T.; Guan, C.L.; Wang, Y.; Zhang, M.R.; et al. Effects of low nitrogen on seedling growth, photosynthetic characteristics and antioxidant system of rice varieties with different nitrogen efficiencies. Sci. Rep. 2023, 13, 19780. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, H.; He, C.; Li, Y. Physiological Mechanisms of Exogenous ABA in Alleviating Drought Stress in Nitraria tangutorum. Plants 2025, 14, 2643. https://doi.org/10.3390/plants14172643
Li X, Liu H, He C, Li Y. Physiological Mechanisms of Exogenous ABA in Alleviating Drought Stress in Nitraria tangutorum. Plants. 2025; 14(17):2643. https://doi.org/10.3390/plants14172643
Chicago/Turabian StyleLi, Xiaolan, Hanghang Liu, Cai He, and Yi Li. 2025. "Physiological Mechanisms of Exogenous ABA in Alleviating Drought Stress in Nitraria tangutorum" Plants 14, no. 17: 2643. https://doi.org/10.3390/plants14172643
APA StyleLi, X., Liu, H., He, C., & Li, Y. (2025). Physiological Mechanisms of Exogenous ABA in Alleviating Drought Stress in Nitraria tangutorum. Plants, 14(17), 2643. https://doi.org/10.3390/plants14172643






