Annual Dynamics of Endogenous Hormones Reveal the Mechanism of Off-Season Flowering in Macadamia
Abstract
1. Introduction
2. Results
2.1. Phenological Observation of Macadamia
2.2. Dynamics of Endogenous Hormones in Macadamia
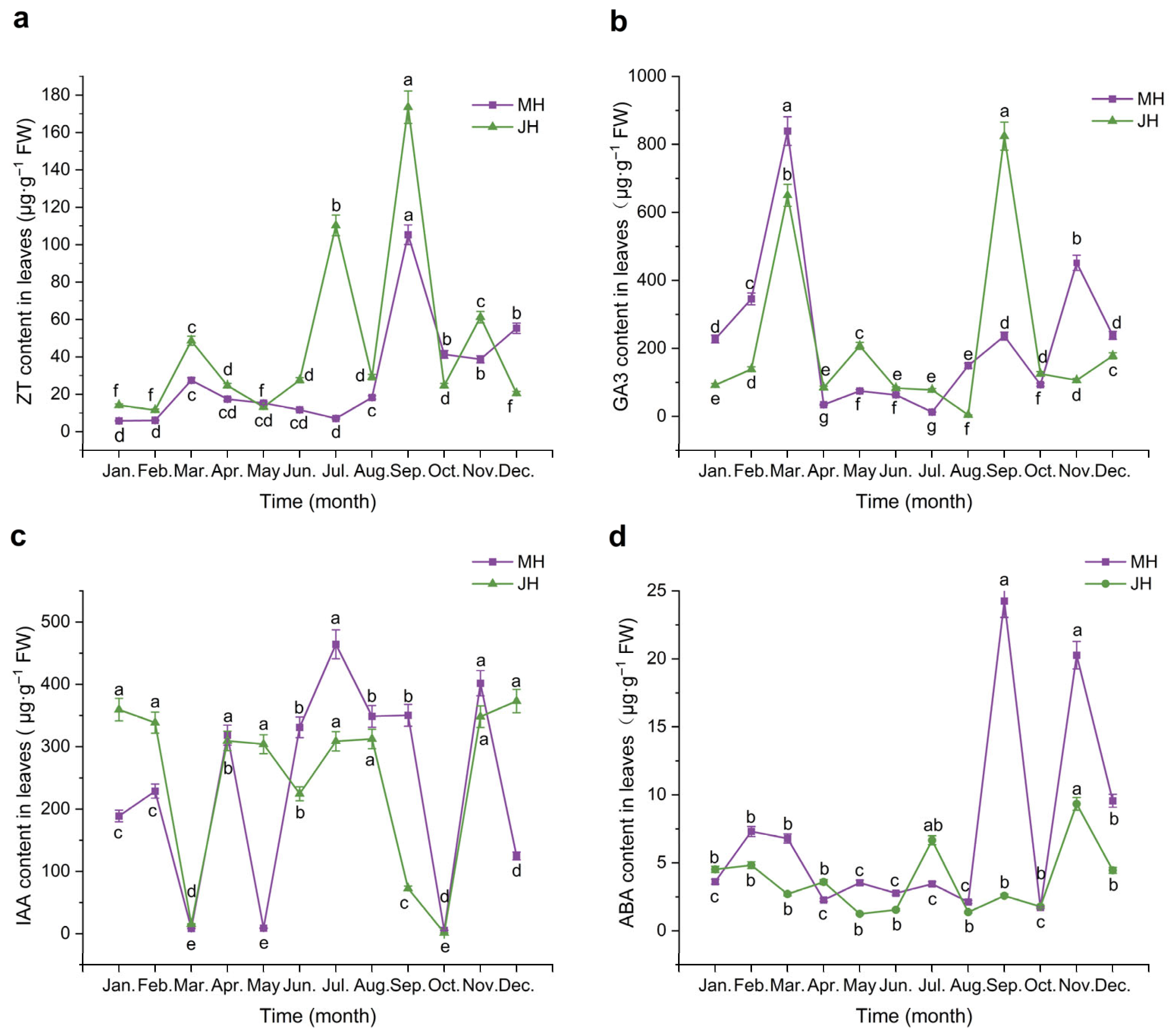
2.2.1. Dynamic Changes of Endogenous Hormones in Leaves
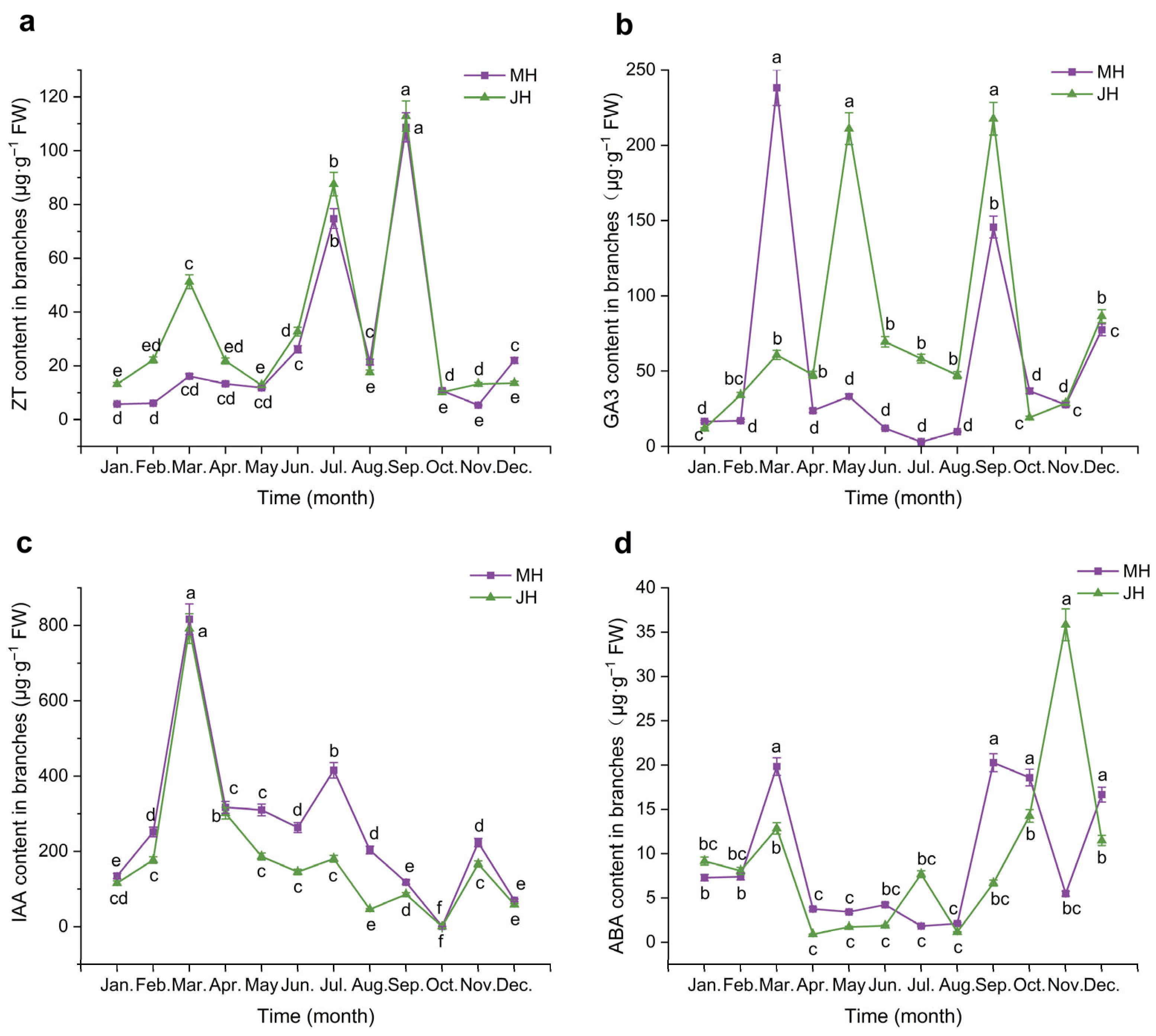
2.2.2. Dynamic Changes of Endogenous Hormones in Branches
2.3. Dynamic Changes in the Endogenous Hormone Balance Ratios in Macadamia
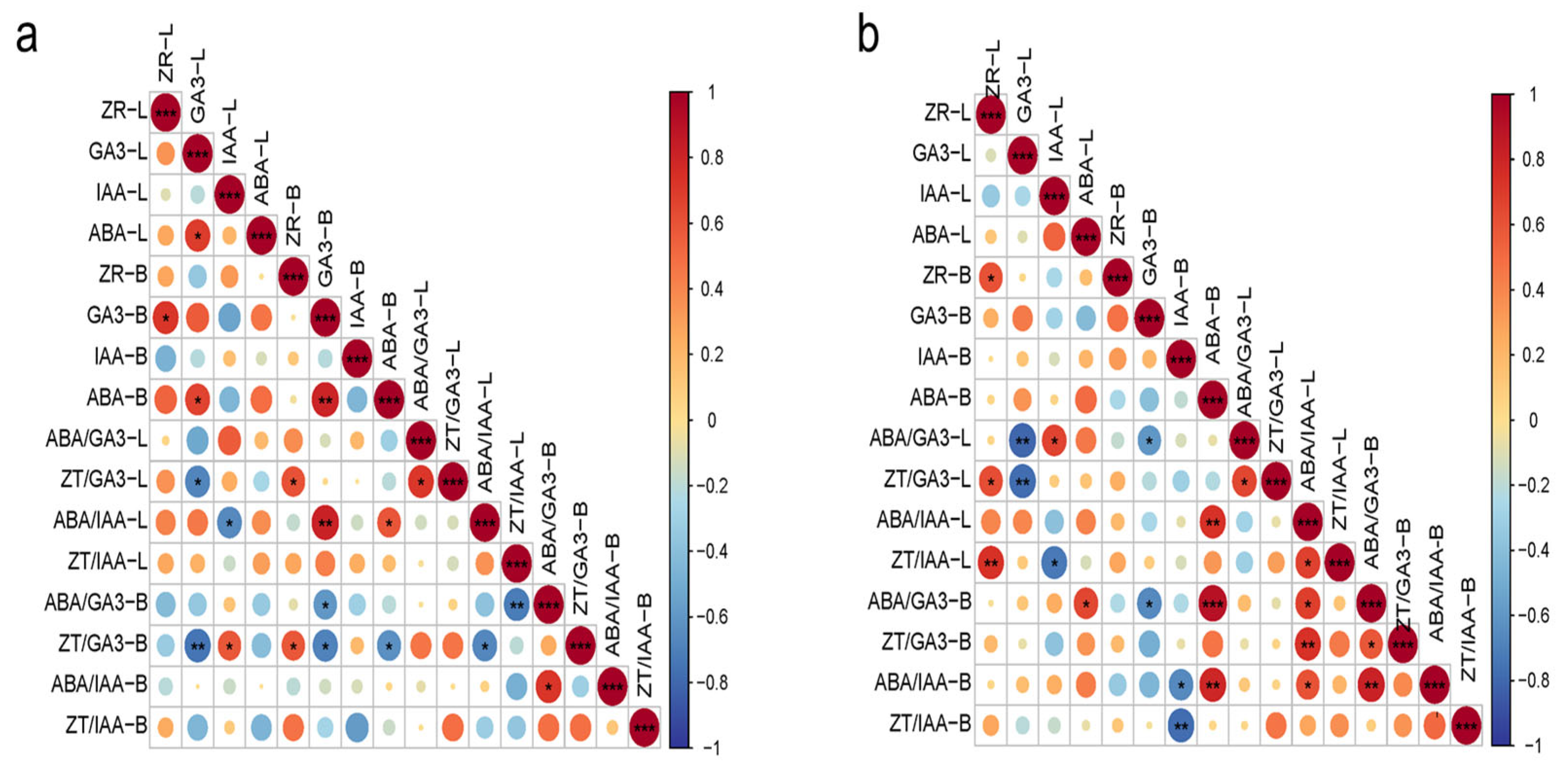
2.4. Correlation Analysis of Endogenous Hormones and Their Ratios in Macadamia
3. Discussion
3.1. Relationship Between Endogenous Hormone Dynamics and Off-Season Flowering in Macadamia
3.2. Relationship Between Endogenous Hormone Balance Ratios in Macadamia and Off-Season Flowering
3.3. Synergistic Effects of Endogenous Hormones in Macadamia and the Mechanism of Flowering Regulation
4. Materials and Methods
4.1. Site Description
4.2. Field Experiment Layout and Sample Collection
4.3. Hormone Extraction and Quantification
4.3.1. Instruments and Chemicals
4.3.2. Sample Preparation Method
4.3.3. Measurement Method
- (1)
- Preparation of Standard Solutions
- (2)
- Chromatographic Conditions
4.3.4. Method Validation for HPLC Analysis
4.3.5. Determination of Endogenous Hormone Content
4.4. Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rahman, A.; Wang, S.; Yan, J.S.; Xu, H.R. Intact macadamia nut quality assessment using near infrared spectroscopy and multivariate analysis. J. Food Compos. Anal. 2021, 102, 104033. [Google Scholar] [CrossRef]
- Appleby, N.; Edwards, D.; Batley, J. New technologies for ultrahigh throughput genotyping in plants. Methods Mol. Biol. 2009, 513, 19–39. [Google Scholar] [CrossRef]
- Malvestiti, R.; Borges, L.d.S.; Weimann, E.; Junior, E.P.d.S.; Levada-Pires, A.C.; Dermargos, A.; Lambertucci, R.H.; Hatanaka, E. The effect of macadamia oil intake on muscular inflammation and oxidative profile kinetics after exhaustive exercise. Eur. J. Lipid Sci. Technol. 2017, 119, 1600382. [Google Scholar] [CrossRef]
- He, X.Y.; Tao, L.; Liu, J.; Ni, S.B. Overview and development trends of the global macadamia industry. South China Fruits 2015, 44, 151–155. [Google Scholar] [CrossRef]
- Sedgley, M.; Olesen, T. Flowering and fruit-set in macadamias (Macadamia integrifolia): Studies on temporal patterns and sources of yield fluctuation. Sci. Hortic. 1992, 50, 107–118. [Google Scholar]
- Trueman, S.J. The reproductive biology of macadamia. Sci. Hortic. 2013, 150, 354–359. [Google Scholar] [CrossRef]
- Ning, Y.; Chen, Y.C.; Yue, H.; He, S.L.; He, X.Y. Investigation of flowering rhythm and preliminary study on flowering period regulation in macadamia in Yunnan. Trop. Agric. Sci. Technol. 2024, 47, 1–5. [Google Scholar] [CrossRef]
- Maple, R.; Zhu, P.; Hepworth, J.; Wang, J.-W.; Dean, C. Flowering time: From physiology, through genetics to mechanism. Plant Physiol. 2024, 195, 190–212. [Google Scholar] [CrossRef]
- Yuan, C.; Ahmad, S.; Cheng, T.; Wang, J.; Pan, H.; Zhao, L.; Zhang, Q. Red to far-red light ratio modulates hormonal and genetic control of axillary bud outgrowth in Chrysanthemum. Int. J. Mol. Sci. 2018, 19, 1590. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Maness, N.; Ferguson, L.; Deng, W.; Zhang, L. Role of plant hormones in flowering and exogenous hormone application in fruit/nut trees: A review of pecans. Fruit Res. 2021, 1, 15. [Google Scholar] [CrossRef]
- Chen, X.; Qi, S.; Zhang, D.; Li, Y.; An, N.; Zhao, C.; Zhao, J.; Shah, K.; Han, M.; Xing, L. Comparative RNA-sequencing–based transcriptome profiling of buds from profusely flowering ‘Qinguan’ and weakly flowering ‘Nagafu no. 2’ apple varieties reveals novel insights into the regulatory mechanisms underlying floral induction. BMC Plant Biol. 2018, 18, 370. [Google Scholar] [CrossRef]
- Gangwar, S.; Singh, V.P.; Tripathi, D.K.; Chauhan, D.K.; Prasad, S.M.; Maurya, J.N. Plant responses to metal stress: The emerging role of plant growth hormones in toxicity alleviation. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Rasool, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 215–248. [Google Scholar] [CrossRef]
- Okada, O.; Ueda, J.; Komaki, M.K.; Bell, C.J.; Shimura, Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 1991, 3, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Dar, N.A.; Amin, I.; Wani, W.; Wani, S.A.; Shikari, A.B.; Wani, S.H.; Masoodi, K.Z. Abscisic acid: A key regulator of abiotic stress tolerance in plants. Plant Gene 2017, 11, 106–111. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, H.B.; Du, L.Q.; Zou, M.H.; Lu, C.Z.; Luo, L.F.; Zhang, H.Z. Effect of spraying gibberellin on the flower bud formation of Macadamia (Macadamia integrifolia). J. Fruit Sci. 2008, 25, 203–208. [Google Scholar] [CrossRef]
- Zhang, M.; Han, M.; Ma, F.; Shu, H. Effect of bending on the dynamic changes of endogenous hormones in shoot terminals of ‘Fuji’ and ‘Gala’ apple trees. Acta Physiol. Plant. 2015, 37, 76. [Google Scholar] [CrossRef]
- Cao, S.Y.; Zhang, J.C.; Wei, L.H. Changes in endogenous hormones during floral bud development in apple. J. Fruit Sci. 2000, 17, 244–248. [Google Scholar] [CrossRef]
- Mutasa-Göttgens, E.; Hedden, P. Gibberellin as a factor in floral regulatory networks. J. Exp. Bot. 2009, 60, 1979–1989. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2001, 13, 2539–2551. [Google Scholar] [CrossRef]
- Zwack, P.J.; Rashotte, A.M. Interactions between cytokinin signalling and abiotic stress responses. J. Exp. Bot. 2015, 66, 4863–4871. [Google Scholar] [CrossRef]
- Ramirez, J.; Hoad, G.V. Cytokinin application induces flowering on defoliated pear spurs: Evidence from Zeatin experiments. J. Exp. Bot. 2008, 59, 3215–3222. [Google Scholar] [CrossRef]
- Takei, K.; Ueda, N.; Aoki, K.; Kuromori, T.; Hirayama, T.; Shinozaki, K.; Yamaya, T.; Sakakibara, H. AtIPT3 is a key determinant of nitrate-dependent cytokinin biosynthesis in Arabidopsis. Plant Cell Physiol. 2004, 45, 1053–1062. [Google Scholar] [CrossRef]
- Pérez-Rojas, M.; Marsch-Martínez, N. The Role of Cytokinins during the Development of Flower and Fruit Traits in Strawberry. Plants 2023, 12, 3672. [Google Scholar] [CrossRef]
- Leibfried, A.; To, J.P.C.; Busch, W.; Stehling, S.; Kehle, A.; Demar, M.; Kieber, J.J.; Lohmann, J.U. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 2005, 438, 1172–1175. [Google Scholar] [CrossRef]
- Hwang, I.; Sheen, J.; Müller, B. Cytokinin signaling networks. Annu. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef]
- Richter, R.; Behringer, C.; Zourelidou, M.; Schwechheimer, C. Convergence of auxin and gibberellin signaling on regulation of GNC and GNL in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 13192–13197. [Google Scholar] [CrossRef] [PubMed]
- Garmendia, A.; Gómez-Cadenas, A.; Arbona, V. Gibberellic Acid in Citrus spp. Flowering and Fruiting: A Systematic Review. PLoS ONE 2019, 14, e0223147. [Google Scholar] [CrossRef]
- Lee, J.O.; Park, H.M.; Lee, H.I. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates LEAFY. Plant J. 2008, 55, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, H.; Lee, J.H.; Amasino, R.M. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J. 2003, 35, 613–623. [Google Scholar] [CrossRef]
- Zeng, H.; Du, L.Q.; Zou, M.H.; Lu, C.Z.; Luo, L.F.; Zhang, H.Z. Changes in endogenous hormone levels during floral bud differentiation in macadamia. J. Anhui Agric. Sci. 2008, 36, 14949–14953. [Google Scholar] [CrossRef]
- Zhang, Q.; Gong, M.; Xu, X.; Li, H.; Deng, W. Roles of auxin in the growth, development, and stress tolerance of horticultural plants. Cells 2022, 11, 2761. [Google Scholar] [CrossRef]
- Jung, C. Flowering time regulation: Agrochemical control of flowering. Nat. Plants 2017, 3, 17045. [Google Scholar] [CrossRef] [PubMed]
- Brunoud, G.; Wells, D.M.; Oliva, M.; Larrieu, A.; Mirabet, V.; Burrow, A.H.; Beeckman, T.; Kepinski, S.; Traas, J.; Bennett, M.J.; et al. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 2012, 482, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-F.; Chen, P.; Lv, J.; Chen, L.; Sun, Y.-H. Transcriptomic analysis of topping-induced axillary shoot outgrowth in Nicotiana tabacum. Gene 2018, 646, 169–180. [Google Scholar] [CrossRef]
- Riboni, M.; Galbiati, M.; Tonelli, C.; Conti, L. GIGANTEA enables drought escape response via abscisic acid-dependent activation of the florigens and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1. Plant Physiol. 2013, 162, 1706–1719. [Google Scholar] [CrossRef]
- Pan, W.; Wang, X.; Zhang, D. ABA and Bud Dormancy in Perennials: Current Knowledge and Future Perspectives. Genes 2021, 12, 1635. [Google Scholar] [CrossRef]
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L. Implications of Abscisic Acid in the Drought Stress Response: Molecular Pathways and Regulatory Networks. Agronomy 2020, 10, 1323. [Google Scholar] [CrossRef]
- Shu, K.; Zhou, W.; Chen, F.; Luo, X.; Yang, W. Abscisic acid and gibberellins antagonistically mediate plant development and abiotic stress responses. Front. Plant Sci. 2018, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, D.; Fan, S.; Du, L.; Shen, Y.; Xing, L.; Li, Y.; Ma, J.; Han, M. Effect of exogenous GA3 and its inhibitor paclobutrazol on floral formation, endogenous hormones, and flowering-associated genes in ‘Fuji’ apple. Plant Physiol. Biochem. 2016, 107, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Falavigna, V.d.S.; Guitton, B.; Costes, E.; Andrés, F. I want to (bud) break free: The potential role of DAM and SVP-like genes in regulating dormancy cycle in temperate fruit trees. Front. Plant Sci. 2019, 9, 1990. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Altmann, M.; Altmann, S.; Rodriguez, P.A.; Weller, B.; Vergara, L.E.; Palme, J.; la Rosa, N.M.-D.; Sauer, M.; Wenig, M.; Villaécija-Aguilar, J.A.; et al. Extensive signal integration by the phytohormone protein network. Nature. 2020, 583, 271–276. [Google Scholar] [CrossRef]
- Guo, W.; Chen, Y.; Wu, X.; Cao, X.; Deng, X. Advances in Citrus Flowering: A Review. Front. Plant Sci. 2022, 13, 868831. [Google Scholar] [CrossRef]
- Davis, S.J. Integrating hormones into the floral-transition pathway of Arabidopsis thaliana. Plant Cell Environ. 2009, 32, 1201–1210. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Zhang, Y. GhGASA10-1 promotes cell elongation in cotton fiber development through IAA- and GA3-induced signaling. BMC Plant Biol. 2021, 21, 532. [Google Scholar] [CrossRef]
- Shah, K.; Zhu, X.; Zhang, T.; Chen, J.; Chen, J.; Qin, Y. Transcriptome Analysis Reveals Sugar and Hormone Signaling Pathways Mediating Flower Induction in Pitaya (Hylocereus polyrhizus). Int. J. Mol. Sci. 2025, 26, 1250. [Google Scholar] [CrossRef] [PubMed]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Gibberellins and abscisic acid signal crosstalk: Living and developing under unfavorable conditions. Plant Cell Rep. 2013, 32, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, S.; Hardtke, C.S. Hormone Signalling Crosstalk in Plant Growth Regulation. Curr. Biol. 2011, 21, R365–R373. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W.; El-Kereamy, A.; Zhu, T.; Beatty, P.H.; Good, A.G.; Bi, Y.-M.; Rothstein, S.J.; Copenhaver, G.P. The APETALA-2-like transcription factor OsAP2-39 controls key interactions between abscisic acid and gibberellin in rice. PLoS Genet. 2010, 6, e1001098. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Liu, X. Hormone biosynthesis and metabolism members of the 2OGD superfamily are involved in berry development and respond to MeJA and ABA treatment in Vitis vinifera L. BMC Plant Biol. 2022, 22, 427. [Google Scholar] [CrossRef]
- Diopan, V.; Adam, V.; Havel, L.; Kizek, R. Phytohormones as important biologically active molecules—Their simple simultaneous detection. Molecules 2009, 14, 1825–1839. [Google Scholar] [CrossRef]
- Dobrev, P.I.; Kamínek, M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J. Chromatogr. A 2002, 950, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.; Sandberg, G. Quantitative analysis of indole-3-acetic acid metabolites in Arabidopsis. Plant Physiol. 2001, 127, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography–mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef] [PubMed]


| Sampling Site | Initial Flowering Period | Peak Flowering Period | Fruit Enlargement Period | Fruit Oil Accumulation Period | Maturity and Harvest | Floral Bud Dormancy Break Period |
|---|---|---|---|---|---|---|
| MH | Oct–Nov | Jan | Feb–Apr | May–Jul | Aug | Aug–Sep |
| JH | Jan–Feb | Mar | Apr–May | Jun–Aug | Sep | Oct–Dec |
| Time (min) | Acetonitrile (%) | Methanol (%) | 0.1% Phosphoric Acid (%) |
|---|---|---|---|
| 3 | 5 | 5 | 90 |
| 10 | 12 | 12 | 76 |
| 20 | 22 | 22 | 56 |
| 30 | 20 | 20 | 60 |
| 35 | 5 | 5 | 90 |
| 40 | 5 | 5 | 90 |
| Component | Regression Equation | Correlation Coefficient | Linear Range (μg/mL) |
|---|---|---|---|
| ZT | y = 43,743x + 249,867 | 0.999 | 0.203–208.077 |
| GA3 | y = 9769.6x + 85,522 | 0.996 | 0.202–206.848 |
| IAA | y = 75,525x + 17,728 | 0.999 | 0.202–206.848 |
| ABA | y = 18,902x – 18,636 | 0.999 | 0.204–209.715 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, Y.; Chen, Y.; He, X.; Yang, T.; Yue, H. Annual Dynamics of Endogenous Hormones Reveal the Mechanism of Off-Season Flowering in Macadamia. Plants 2025, 14, 2637. https://doi.org/10.3390/plants14172637
Ning Y, Chen Y, He X, Yang T, Yue H. Annual Dynamics of Endogenous Hormones Reveal the Mechanism of Off-Season Flowering in Macadamia. Plants. 2025; 14(17):2637. https://doi.org/10.3390/plants14172637
Chicago/Turabian StyleNing, Ya, Yuchun Chen, Xiyong He, Tingmei Yang, and Hai Yue. 2025. "Annual Dynamics of Endogenous Hormones Reveal the Mechanism of Off-Season Flowering in Macadamia" Plants 14, no. 17: 2637. https://doi.org/10.3390/plants14172637
APA StyleNing, Y., Chen, Y., He, X., Yang, T., & Yue, H. (2025). Annual Dynamics of Endogenous Hormones Reveal the Mechanism of Off-Season Flowering in Macadamia. Plants, 14(17), 2637. https://doi.org/10.3390/plants14172637





