Functional Studies and Expression Characteristics of the Vacuolar Sugar Transporter CoSWEET2a in Camellia oleifera
Abstract
1. Introduction
2. Results
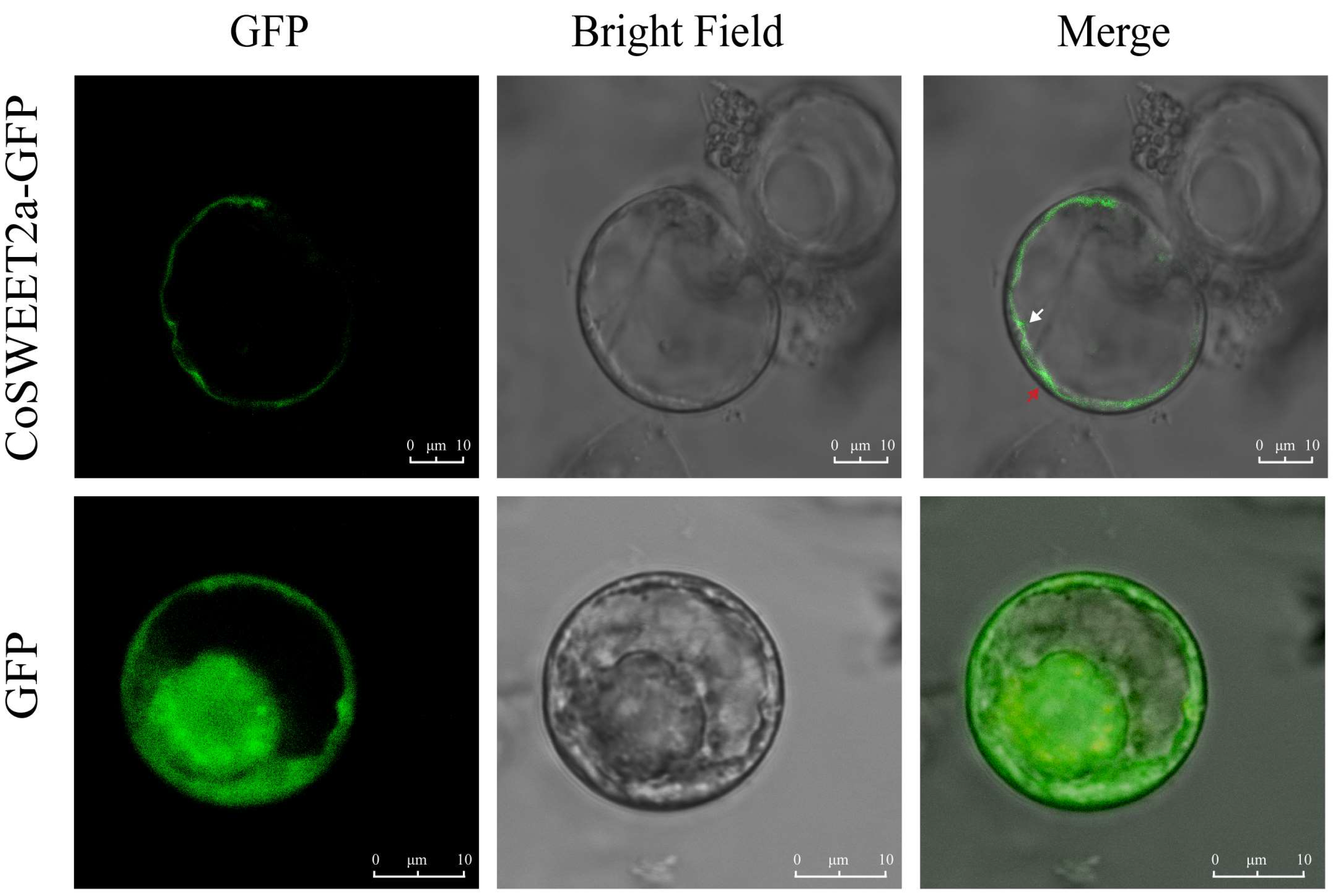
2.1. CoSWEET2a Is Localized to the Vacuolar Membrane in C. oleifera
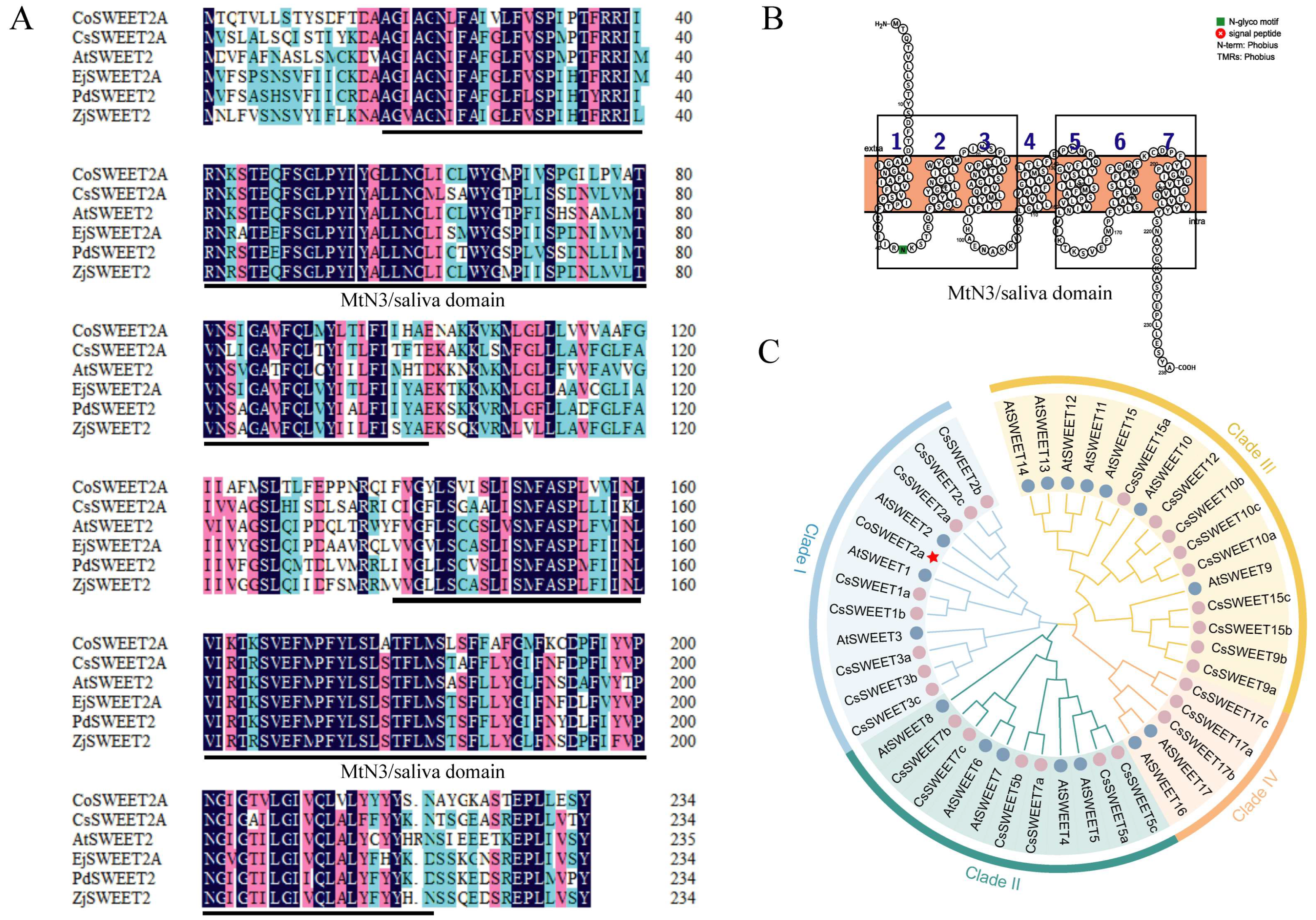
2.2. Basic Characteristics and Phylogenetic Analysis of CoSWEET2a
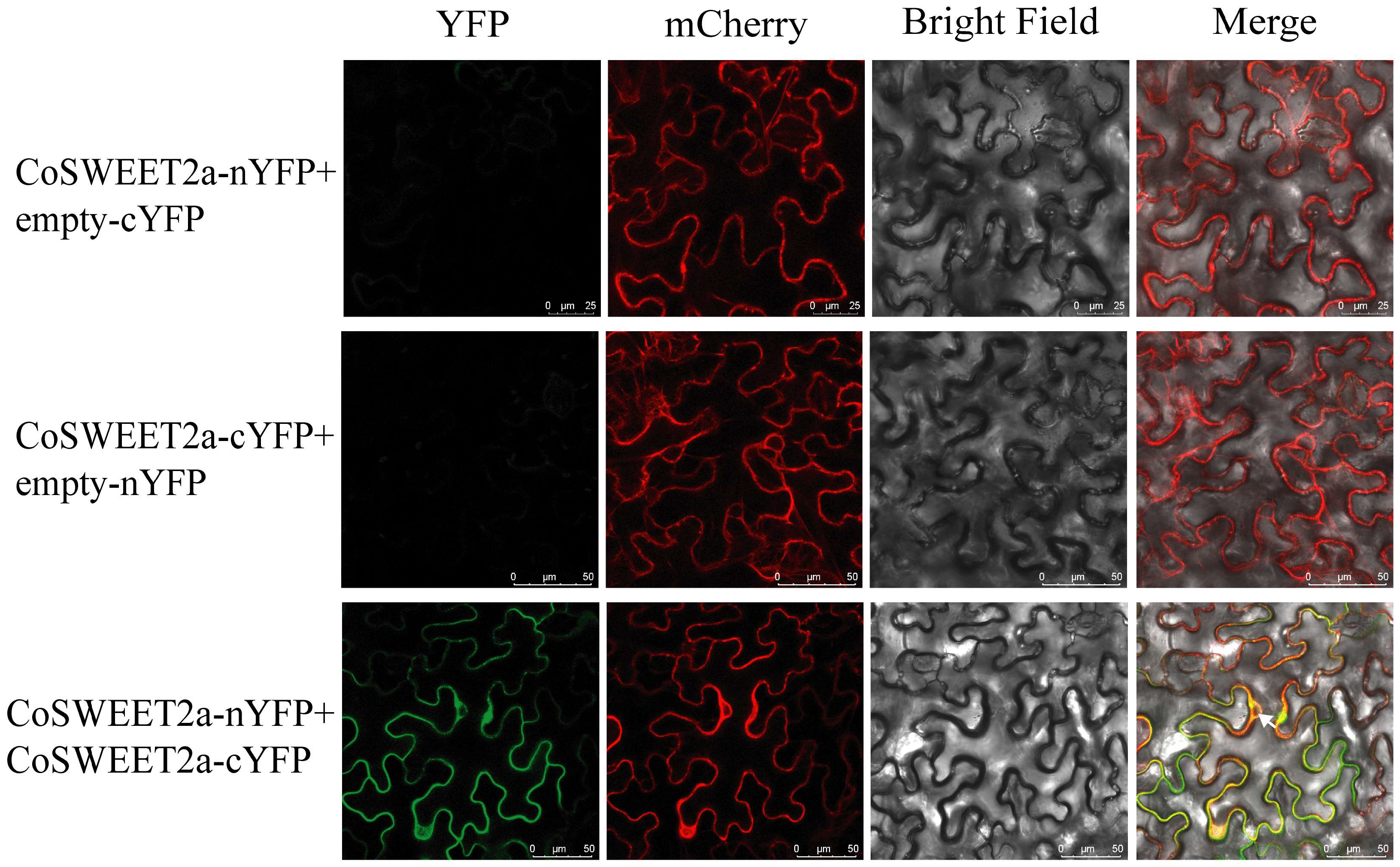
2.3. CoSWEET2a Forms Homodimers at the Vesicular Membrane
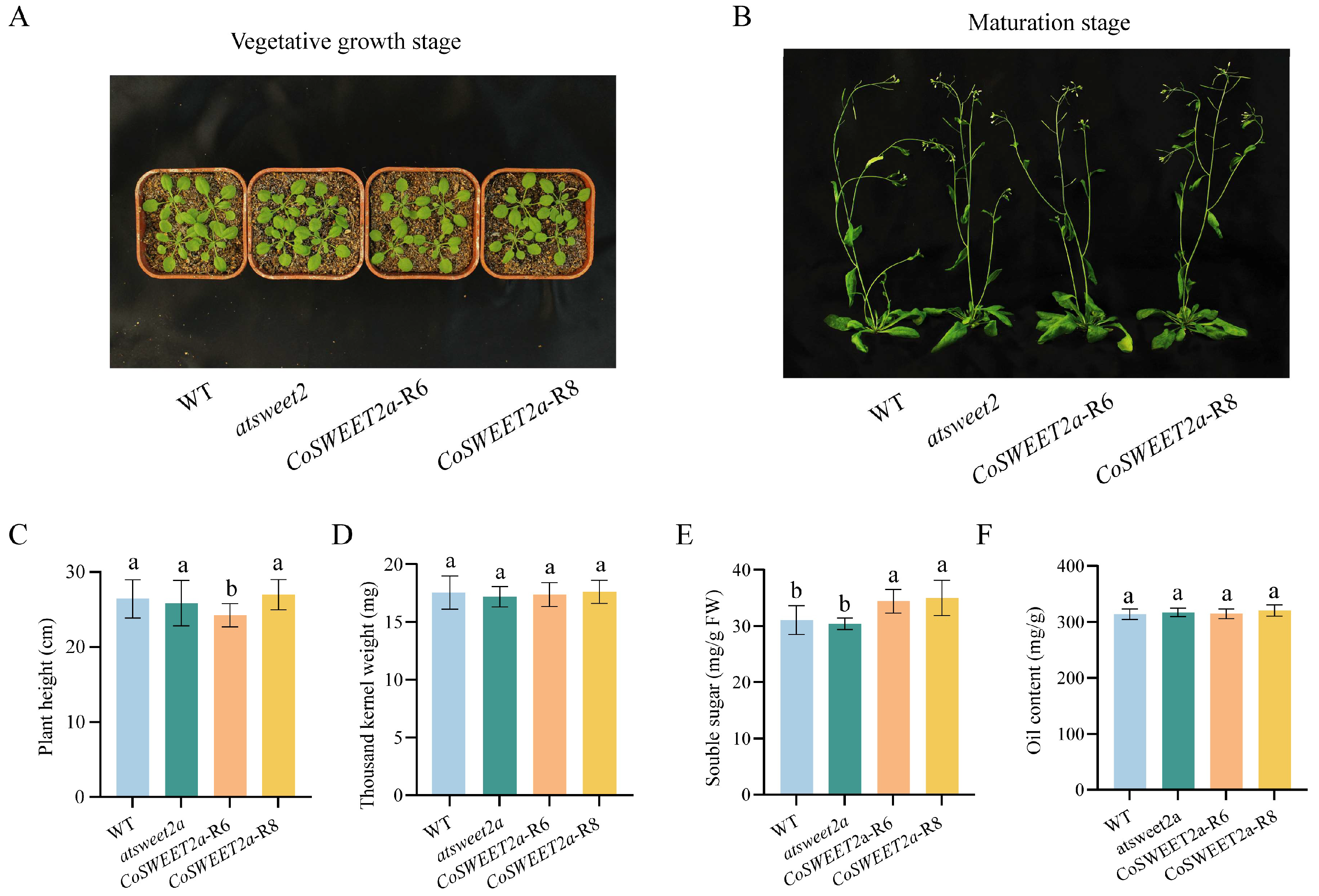
2.4. CoSWEET2a Overexpression Enhances Sugar Accumulation in Seeds
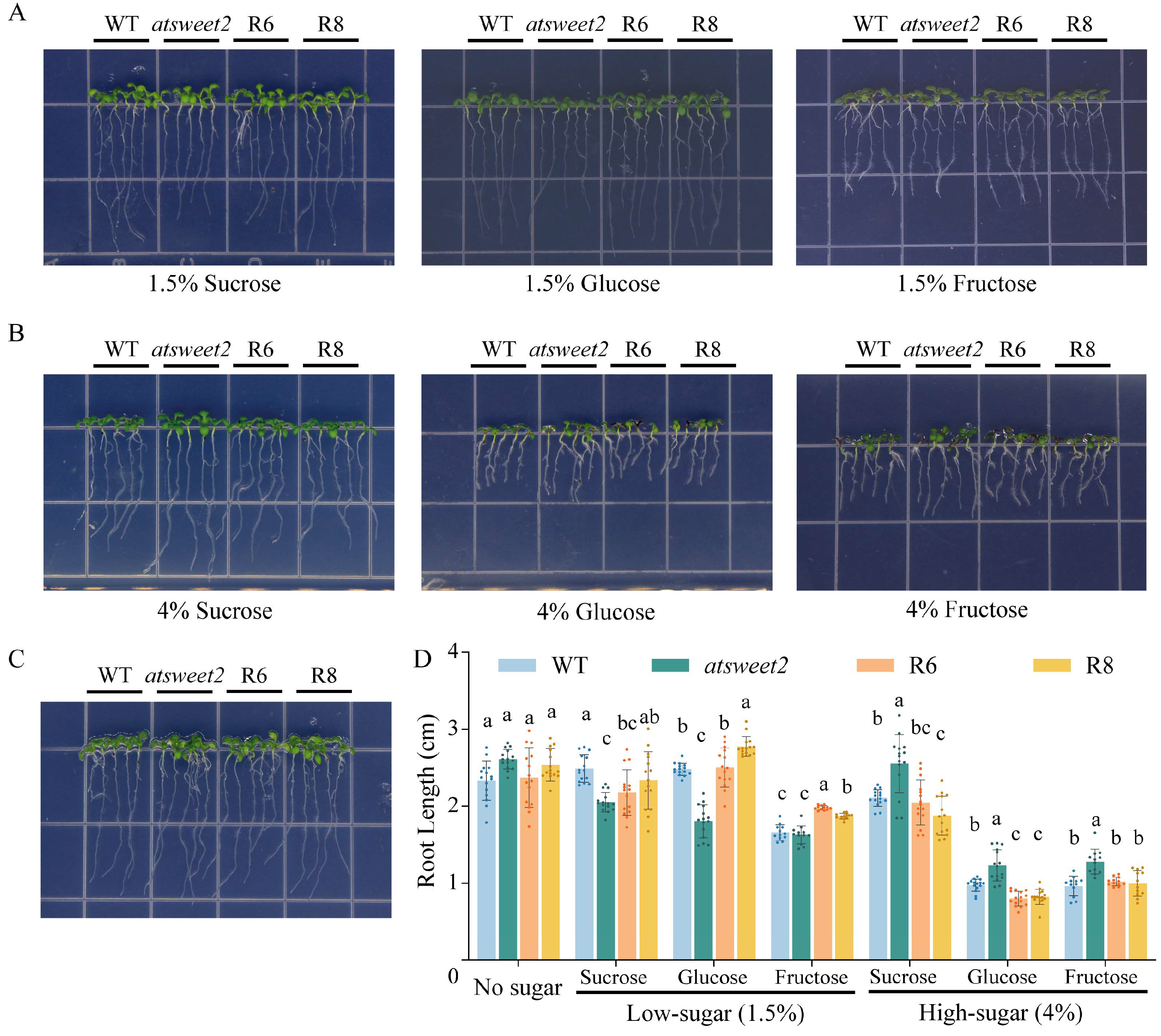
2.5. Analysis of Seedling Root Growth of Arabidopsis Lines Under Different Sugar Treatments
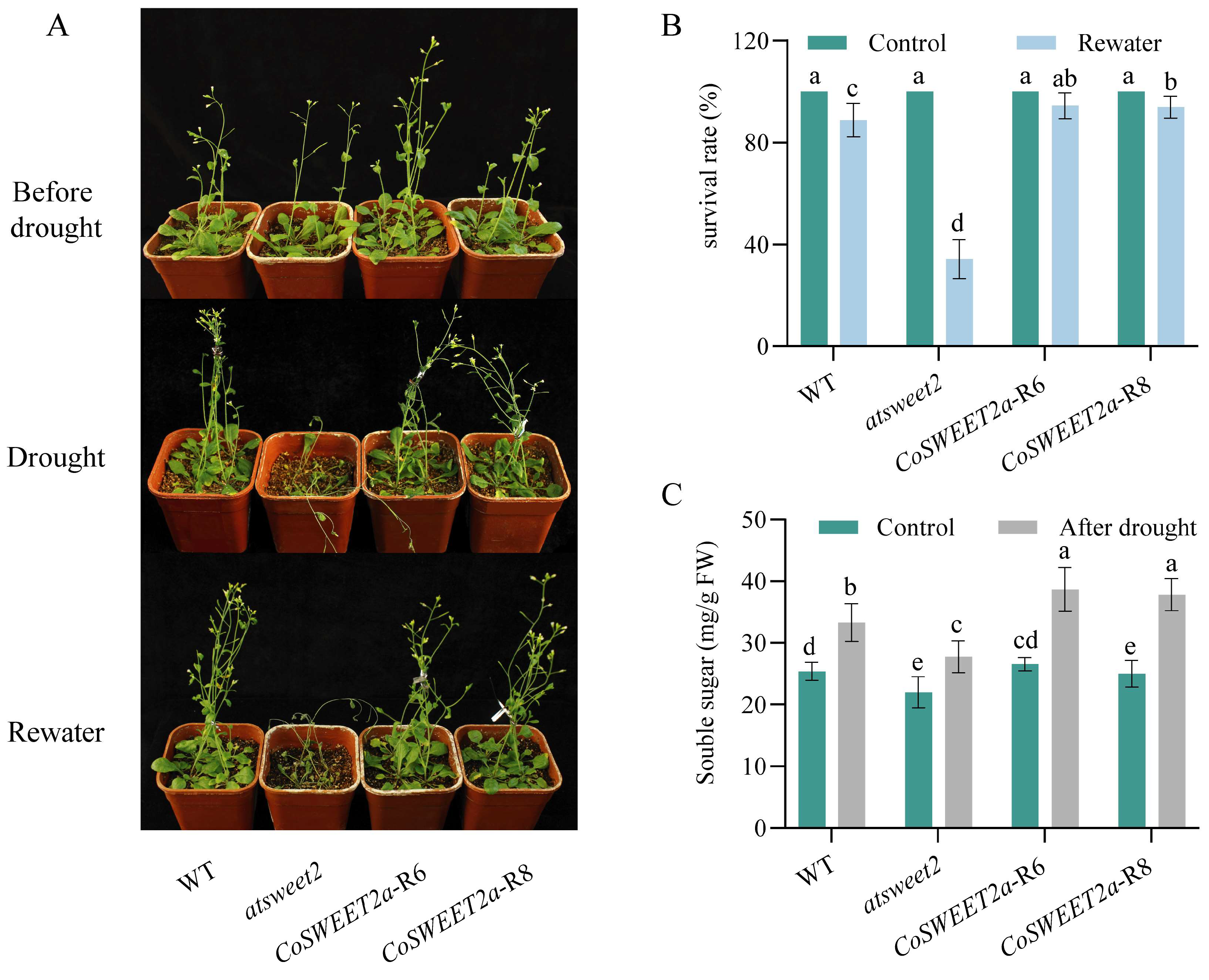
2.6. Phenotypic Observation and Analysis of Arabidopsis Strains Under Drought Treatment
2.7. Analysis of CoSWEET2a Gene Promoter Elements and Response to Different Hormones
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Stress Treatment
4.2. Cloning and Subcellular Localization of CoSWEET2a Gene
4.3. Bioinformatics Analysis
4.4. Bimolecular Fluorescence Complementation Experiments
4.5. Generation of Arabidopsis Homozygous Mutants and Restoration Lines
4.6. Phenotypic Observation and Physiological Analysis of A. thaliana
4.7. In Vitro Culture of C. oleifera Seeds Under Different Hormone Treatments
4.8. RT-qPCR Analysis of CoSWEET2a Gene
4.9. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-Sink Transport of Sugar and Regulation by Environmental Factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar Input, Metabolism, and Signaling Mediated by Invertase: Roles in Development, Yield Potential, and Response to Drought and Heat. Mol. Plant. 2010, 3, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, D.; Yang, B.; Frommer, W.B.; Eom, J.S. SWEET11 and 15 as Key Players in Seed Filling in Rice. New Phytol. 2018, 218, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, S.; Wang, J.; Yokosho, K.; Zhou, B.; Yu, Y.C.; Liu, Z.; Frommer, W.B.; Ma, J.F.; Chen, L.Q.; et al. Simultaneous Changes in Seed Size, Oil Content and Protein Content Driven by Selection of SWEET Homologues during Soybean Domestication. Natl. Sci. Rev. 2020, 7, 1776–1786. [Google Scholar] [CrossRef]
- Doidy, J.; Grace, E.; Kühn, C.; Simon-Plas, F.; Casieri, L.; Wipf, D. Sugar Transporters in Plants and in Their Interactions with Fungi. Trends Plant Sci. 2012, 17, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yokosho, K.; Guo, R.; Whelan, J.; Ruan, Y.L.; Ma, J.F.; Shou, H. The Soybean Sugar Transporter GmSWEET15 Mediates Sucrose Export from Endosperm to Early Embryo. Plant Physiol. 2019, 180, 2133–2141. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, F.; Song, S.; Yu, X.; Ren, Y.; Zhao, X.; Liu, H.; Liu, G.; Wang, Y.; He, H. CsSWEET2, a Hexose Transporter from Cucumber (Cucumis sativus L.), Affects Sugar Metabolism and Improves Cold Tolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 3886. [Google Scholar] [CrossRef]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.Q.; Guo, W.J.; Kim, J.G.; Underwood, W.; Chaudhuri, B.; et al. Sugar Transporters for Intercellular Exchange and Nutrition of Pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef]
- Chen, H.Y.; Huh, J.H.; Yu, Y.C.; Ho, L.H.; Chen, L.Q.; Tholl, D.; Frommer, W.B.; Guo, W.J. The Arabidopsis Vacuolar Sugar Transporter SWEET2 Limits Carbon Sequestration from Roots and Restricts Pythium Infection. Plant J. 2015, 83, 1046–1058. [Google Scholar] [CrossRef]
- Tao, Y.; Cheung, L.S.; Li, S.; Eom, J.-S.; Chen, L.-Q.; Xu, Y.; Perry, K.; Frommer, W.B.; Feng, L. Structure of a Eukaryotic SWEET Transporter in a Homotrimeric Complex. Nature 2015, 527, 259–263. [Google Scholar] [CrossRef]
- Lauschke, A.; Maibaum, L.; Engel, M.; Eisengräber, L.; Bayer, S.; Hackel, A.; Kühn, C. The Potato Sugar Transporter SWEET1g Affects Apoplasmic Sugar Ratio and Phloem-Mobile Tuber- and Flower-Inducing Signals. Plant Physiol. 2025, 197, kiae602. [Google Scholar] [CrossRef]
- Valifard, M.; Le Hir, R.; Müller, J.; Scheuring, D.; Neuhaus, H.E.; Pommerrenig, B. Vacuolar Fructose Transporter SWEET17 Is Critical for Root Development and Drought Tolerance. Plant Physiol. 2021, 187, 2716–2730. [Google Scholar] [CrossRef]
- Klemens, P.A.W.; Patzke, K.; Deitmer, J.; Spinner, L.; Le Hir, R.; Bellini, C.; Bedu, M.; Chardon, F.; Krapp, A.; Neuhaus, H.E. Overexpression of the Vacuolar Sugar Carrier AtSWEET16 Modifies Germination, Growth, and Stress Tolerance in Arabidopsis. Plant Physiol. 2013, 163, 1338–1352. [Google Scholar] [CrossRef]
- Wang, L.; Yao, L.; Hao, X.; Li, N.; Qian, W.; Yue, C.; Ding, C.; Zeng, J.; Yang, Y.; Wang, X. Tea Plant SWEET Transporters: Expression Profiling, Sugar Transport, and the Involvement of CsSWEET16 in Modifying Cold Tolerance in Arabidopsis. Plant Mol. Biol. 2018, 96, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, Z.; Zhou, J.; Gu, Y.; Tan, X. Comparative Study on Fruit Development and Oil Synthesis in Two Cultivars of Camellia oleifera. BMC Plant Biol. 2021, 21, 348. [Google Scholar] [CrossRef]
- Lin, P.; Wang, K.; Wang, Y.; Hu, Z.; Yan, C.; Huang, H.; Ma, X.; Cao, Y.; Long, W.; Liu, W.; et al. The Genome of Oil-Camellia and Population Genomics Analysis Provide Insights into Seed Oil Domestication. Genome Biol. 2022, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yao, X.; Ren, H.; Wang, K. Determination of Fatty Acid Composition and Metallic Element Content of Four Camellia Species Used for Edible Oil Extraction in China. J. Consum. Prot. Food Saf. 2017, 12, 165–169. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, X.; Liu, S. Risk Assessment to China’s Agricultural Drought Disaster in County Unit. Nat. Hazards 2012, 61, 785–801. [Google Scholar] [CrossRef]
- Lu, K.; Chen, C.; Zhou, J.; Yuan, J.; Lu, M.; Qiu, J.; Xiao, Z.; Tan, X. Metagenomic and Metabolomic Profiling of Rhizosphere Microbiome Adaptation to Irrigation Gradients in Camellia Oil Trees. Ind. Crop. Prod. 2025, 232, 121250. [Google Scholar] [CrossRef]
- Qu, X.; Wang, H.; Chen, M.; Liao, J.; Yuan, J.; Niu, G. Drought Stress–Induced Physiological and Metabolic Changes in Leaves of Two Oil Tea Cultivars. J. Am. Soc. Horticult. Sci. 2019, 144, 439–447. [Google Scholar] [CrossRef]
- Guo, P.R.; Wu, L.L.; Wang, Y.; Liu, D.; Li, J.A. Effects of Drought Stress on the Morphological Structure and Flower Organ Physiological Characteristics of Camellia oleifera Flower Buds. Plants 2023, 12, 2585. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, T.; Li, X.; Song, C.-P.; Zhu, J.K.; Chen, L.; Zhao, Y. Phosphorylation of SWEET Sucrose Transporters Regulates Plant Root:Shoot Ratio under Drought. Nat. Plants 2022, 8, 68–77. [Google Scholar] [CrossRef]
- Du, B.; Cao, Y.; Zhou, J.; Chen, Y.; Ye, Z.; Huang, Y.; Zhao, X.; Zou, X.; Zhang, L. Sugar Import Mediated by Sugar Transporters and Cell Wall Invertases for Seed Development in Camellia oleifera. Hortic. Res. 2024, 11, uhae133. [Google Scholar] [CrossRef]
- Du, B.; Zou, X.; Wang, Z.; Zhang, X.; Cao, Y.; Zhang, L. Genome-wide identification and expression analysis of the SWEET gene family in Camellia oleifera. Biotechnol. Bull. 2024, 40, 179–190. (In Chinese) [Google Scholar] [CrossRef]
- Cai, H.; Liang, M.; Qin, X.; Dong, R.; Wang, X.; Wang, H.; Sun, S.; Cui, X.; Yang, W.; Li, R. Tonoplast Sugar Transporters Coordinately Regulate Tomato Fruit Development and Quality. Plant Commun. 2025, 6, 101314. [Google Scholar] [CrossRef]
- Xuan, Y.H.; Hu, Y.B.; Chen, L.-Q.; Sosso, D.; Ducat, D.C.; Hou, B.-H.; Frommer, W.B. Functional Role of Oligomerization for Bacterial and Plant SWEET Sugar Transporter Family. Proc. Natl. Acad. Sci. USA 2013, 110, E3685–E3694. [Google Scholar] [CrossRef]
- Nelson, B.K.; Cai, X.; Nebenfuehr, A. A Multicolored Set of in Vivo Organelle Markers for Co-Localization Studies in Arabidopsis and Other Plants. Plant J. 2007, 51, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Das, S.; Jagadis Gupta, K.; Ranjan, A.; Foyer, C.H.; Thakur, J.K. Physiological Implications of SWEETs in Plants and Their Potential Applications in Improving Source–Sink Relationships for Enhanced Yield. Plant Biotechnol. J. 2023, 21, 1528–1541. [Google Scholar] [CrossRef]
- Xu, Y.; Tao, Y.; Cheung, L.S.; Fan, C.; Chen, L.-Q.; Xu, S.; Perry, K.; Frommer, W.B.; Feng, L. Structures of Bacterial Homologues of SWEET Transporters in Two Distinct Conformations. Nature 2014, 515, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Gwon, S.; Park, J.; Huque, A.K.M.; Cheung, L.S. The Arabidopsis SWEET1 and SWEET2 Uniporters Recognize Similar Substrates While Differing in Subcellular Localization. J. Biol. Chem. 2023, 299, 105389. [Google Scholar] [CrossRef]
- Gautam, T.; Dutta, M.; Jaiswal, V.; Zinta, G.; Gahlaut, V.; Kumar, S. Emerging Roles of SWEET Sugar Transporters in Plant Development and Abiotic Stress Responses. Cells 2022, 11, 1303. [Google Scholar] [CrossRef] [PubMed]
- Sosso, D.; Luo, D.; Li, Q.-B.; Sasse, J.; Yang, J.; Gendrot, G.; Suzuki, M.; Koch, K.E.; McCarty, D.R.; Chourey, P.S.; et al. Seed Filling in Domesticated Maize and Rice Depends on SWEET-Mediated Hexose Transport. Nat. Genet. 2015, 47, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Du, B.; Zhou, J.; Cao, Y.; Zhang, L. Camellia oleifera CoSWEET10 Is Crucial for Seed Development and Drought Resistance by Mediating Sugar Transport in Transgenic Arabidopsis. Plants 2023, 12, 2818. [Google Scholar] [CrossRef] [PubMed]
- Breia, R.; Conde, A.; Badim, H.; Fortes, A.M.; Gerós, H.; Granell, A. Plant SWEETs: From Sugar Transport to Plant–Pathogen Interaction and More Unexpected Physiological Roles. Plant Physiol. 2021, 186, 836–852. [Google Scholar] [CrossRef]
- Valifard, M.; Khan, A.; Berg, J.; Le Hir, R.; Pommerrenig, B.; Neuhaus, H.E.; Keller, I. Carbohydrate Distribution via SWEET17 Is Critical for Arabidopsis Inflorescence Branching under Drought. J. Exp. Bot. 2024, 75, 3903–3919. [Google Scholar] [CrossRef]
- He, Z.; Zhang, P.; Jia, H.; Zhang, S.; Nishawy, E.; Sun, X.; Dai, M. Regulatory Mechanisms and Breeding Strategies for Crop Drought Resistance. New Crop. 2024, 1, 100029. [Google Scholar] [CrossRef]
- Xing, M.Q.; Chen, S.H.; Zhang, X.F.; Xue, H.W. Rice OsGA2ox9 Regulates Seed GA Metabolism and Dormancy. Plant Biotechnol. J. 2023, 21, 2411–2413. [Google Scholar] [CrossRef]
- Mathan, J.; Singh, A.; Ranjan, A. Sucrose Transport in Response to Drought and Salt Stress Involves ABA-Mediated Induction of OsSWEET13 and OsSWEET15 in Rice. Physiol. Plant. 2021, 171, 620–637. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Wang, T.; Zhang, J.; Liu, W.; Fang, H.; Zhang, Z.; Peng, F.; Chen, X.; Wang, N. Abscisic Acid and Regulation of the Sugar Transporter Gene MdSWEET9b Promote Apple Sugar Accumulation. Plant Physiol. 2023, 192, 2081–2101. [Google Scholar] [CrossRef]
- Kanno, Y.; Oikawa, T.; Chiba, Y.; Ishimaru, Y.; Shimizu, T.; Sano, N.; Koshiba, T.; Kamiya, Y.; Ueda, M.; Seo, M. AtSWEET13 and AtSWEET14 Regulate Gibberellin-Mediated Physiological Processes. Nat. Commun. 2016, 7, 13245. [Google Scholar] [CrossRef]
- Morii, M.; Sugihara, A.; Takehara, S.; Kanno, Y.; Kawai, K.; Hobo, T.; Hattori, M.; Yoshimura, H.; Seo, M.; Ueguchi-Tanaka, M. The Dual Function of OsSWEET3a as a Gibberellin and Glucose Transporter Is Important for Young ShootDevelopment in Rice. Plant Cell Physiol. 2020, 61, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Ye, T.W.; Xu, X.; Yuan, D.Y.; Xiao, S.X. Callus Induction, Suspension Culture and Protoplast Isolation in Camellia oleifera. Sci. Hortic. 2021, 286, 110193. [Google Scholar] [CrossRef]
- Li, S.; Zhao, R.; Ye, T.; Guan, R.; Xu, L.; Ma, X.; Zhang, J.; Xiao, S.; Yuan, D. Isolation, Purification and PEG-Mediated Transient Expression of Mesophyll Protoplasts in Camellia oleifera. Plant Methods 2022, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, C.; Wang, M.; Li, T.; Liu, X.; Jiang, J. Plasma Membrane-Localized SlSWEET7a and SlSWEET14 Regulate Sugar Transport and Storage in Tomato Fruits. Hortic. Res. 2021, 8, 186. [Google Scholar] [CrossRef]
- Zhang, W.; Ruan, C.; Li, J.; Han, P.; Ding, J.; Liu, L.; Wu, B.; Ruan, D. Screening of reference genes in four woody-oil trees and spatio-temporal expression analysis of Actin gene. Mol. Plant Breed. 2018, 16, 4576–4582. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, J.; Du, B.; Chen, Y.; Cao, Y.; Yu, M.; Zhang, L. Integrative Physiological and Transcriptomic Analysis Reveals the Transition Mechanism of Sugar Phloem Unloading Route in Camellia oleifera Fruit. Int. J. Mol. Sci. 2022, 23, 4590. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, X.; Du, B.; Zhou, J.; Hu, J.; Cao, Y.; Zhang, L. Functional Studies and Expression Characteristics of the Vacuolar Sugar Transporter CoSWEET2a in Camellia oleifera. Plants 2025, 14, 2618. https://doi.org/10.3390/plants14172618
Zou X, Du B, Zhou J, Hu J, Cao Y, Zhang L. Functional Studies and Expression Characteristics of the Vacuolar Sugar Transporter CoSWEET2a in Camellia oleifera. Plants. 2025; 14(17):2618. https://doi.org/10.3390/plants14172618
Chicago/Turabian StyleZou, Xinhui, Bingshuai Du, Jing Zhou, Jingjing Hu, Yibo Cao, and Lingyun Zhang. 2025. "Functional Studies and Expression Characteristics of the Vacuolar Sugar Transporter CoSWEET2a in Camellia oleifera" Plants 14, no. 17: 2618. https://doi.org/10.3390/plants14172618
APA StyleZou, X., Du, B., Zhou, J., Hu, J., Cao, Y., & Zhang, L. (2025). Functional Studies and Expression Characteristics of the Vacuolar Sugar Transporter CoSWEET2a in Camellia oleifera. Plants, 14(17), 2618. https://doi.org/10.3390/plants14172618





