Effects of Seawater Polyphenols from Gongolaria usneoides on Photosynthesis and Biochemical Compounds of the Invasive Alien Species Rugulopteryx okamurae (Phaeophyceae, Heterokontophyta)
Abstract
1. Introduction
2. Results
2.1. Toxicity and Recovery Tests
2.1.1. Optimal Quantum Yield (Fv/Fm) and Electron Transport Rate (ETR)
2.1.2. Polyphenols and Antioxidant Activity
2.1.3. Internal Carbon, Nitrogen, and Sulfur
3. Discussion
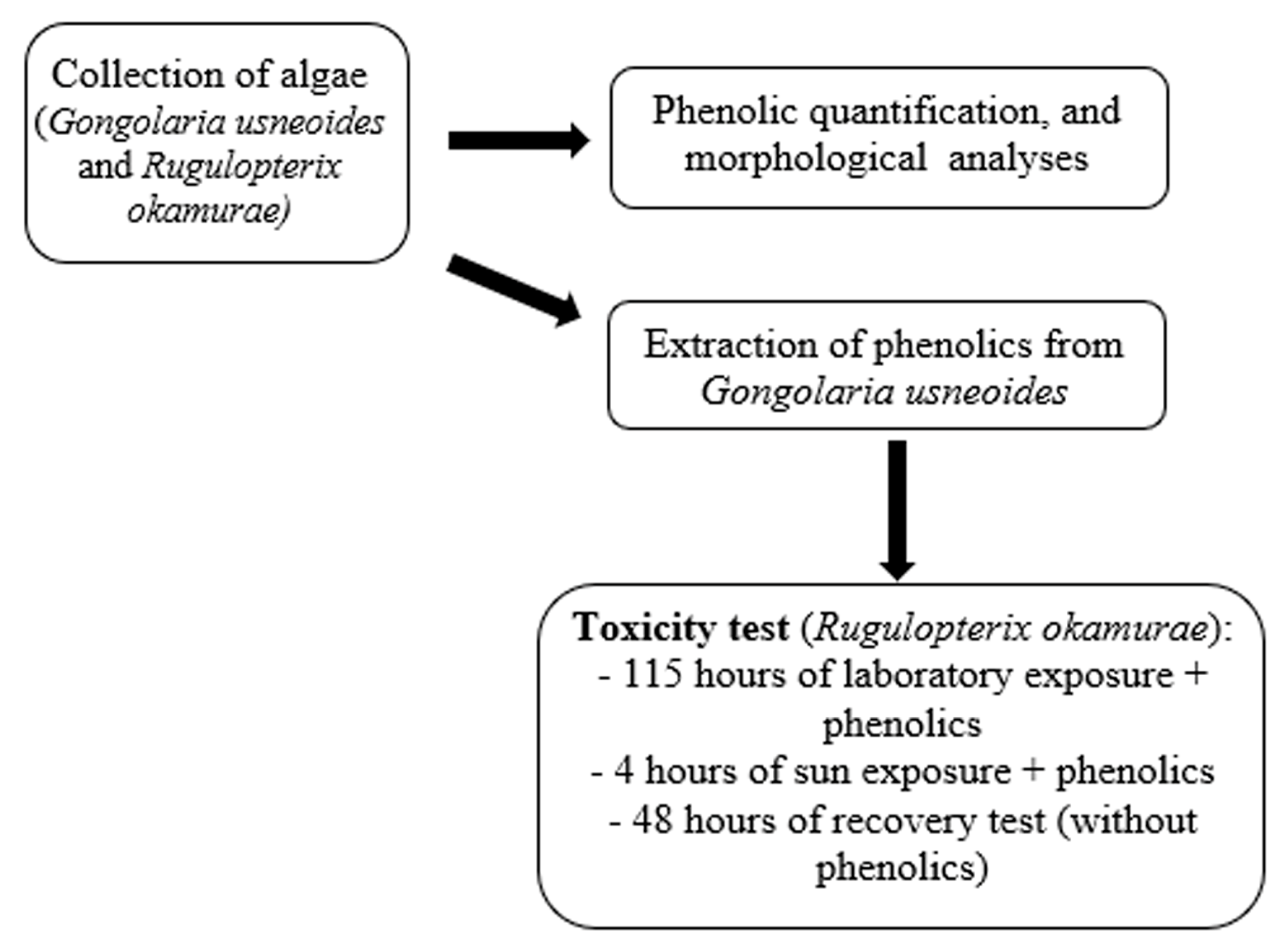
4. Materials and Methods
4.1. Collection of Biological Material
4.2. Characterization of Biological Material
4.3. Obtaining Signaled Water and Exudation of Phenolic Compounds
4.4. Toxicity and Recovery Tests
4.5. In Situ Measurements of Effective Quantum Yield of PSII
4.6. Total Phenols
4.7. Total Antioxidant Activity
4.8. Total Internal Carbon, Nitrogen, and Sulfur
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotas, J.; Gomes, L.; Pacheco, D.; Pereira, L. Ecosystem services provided by seaweeds. Hydrobiology 2023, 2, 75–96. [Google Scholar] [CrossRef]
- Clavero, M.; García-Berthou, E. Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 2005, 20, 110. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef]
- Bacher, S.; Blackburn, T.M.; Essl, F.; Genovesi, P.; Heikkilä, J.; Jeschke, J.M.; Jones, G.; Keller, R.; Kenis, M.; Kueffer, C.; et al. Socio-economic impact classification of alien taxa (SEICAT). Methods Ecol. Evol. 2018, 9, 159–168. [Google Scholar] [CrossRef]
- Pysek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Faria, J.; Prestes, A.C.; Moreu, I.; Cacabelos, E.; Martins, G.M. Dramatic changes in the structure of shallow-water marine benthic communities following the invasion by Rugulopteryx okamurae (Dictyotales, Ochrophyta) in Azores (NE Atlantic). Mar. Pollut. Bull. 2022, 175, 113358. [Google Scholar] [CrossRef]
- Cope, R.C.; Ross, J.V.; Wittmann, T.A.; Watts, M.J.; Cassey, P. Predicting the risk of biological invasions using environmental similarity and transport network connectedness. Risk Anal. 2019, 39, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, A.; Linden, O. Maritime Traffic Effects on Biodiversity in the Mediterranean Sea. Volume 1: Review of Impacts, Priority Areas and Mitigation Measures; IUCN Centre for Mediterranean Cooperation, IUCN: Gland, Switzerland, 2008; Volume 1.
- Endrina, N.; Rasero, J.C.; Konovessis, D. Risk analysis for RoPax vessels: A case of study for the Strait of Gibraltar. Ocean Eng. 2018, 151, 141–151. [Google Scholar] [CrossRef]
- Verlaque, M.; Steen, F.; De Clerck, O. Rugulopteryx (Dictyotales, Phaeophyceae), a genus recently introduced to the Mediterranean. Phycologia 2009, 48, 536–542. [Google Scholar] [CrossRef]
- El Aamri, F.; Idhalla, M.; Tamsouri, M.N. Ocurrence of the invasive brown seaweed Rugulopteryx okamurae (EY Dawson) IK Wang, WJ Lee and HS Kim (Dictyotales, Phaeophyta) in Morocco (Mediterranean Sea). Mediterr. Aquac. J. 2018, 1, 92–96. [Google Scholar]
- Ocaña, O.; Afonso-Carrillo, J.; Ballesteros, E. Massive proliferation of a dictyotalean species (Phaeophyceae, Ochrophyta) through the Strait of Gibraltar. Rev. Acad. Canar. Cienc. 2016, 28, 165–170. [Google Scholar]
- Sempere-Valverde, J.; Ostalé-Valriberas, E.; Maestre, M.; Aranda, R.G.; Bazairi, H.; Espinosa, F. Impacts of the non-indigenous seaweed Rugulopteryx okamurae on a Mediterranean coralligenous community (Strait of Gibraltar): The role of long-term monitoring. Ecol. Indic. 2012, 121, 107135. [Google Scholar] [CrossRef]
- Viard, F.; Comtet, T. Applications of DNA-based methods for the study of biological invasions. In Biological Invasions in Changing Ecosystems–Vectors, Ecological Impacts, Management and Predictions; De Gruyter Open: Berlin, Germany, 2015. [Google Scholar]
- García-Gómez, J.C.; Sempere-Valverde, J.; González, A.R.; Martínez-Chacón, M.; Olaya-Ponzone, L.; Sánchez-Moyano, E.; Ostalé-Valriberas, E.; Megina, C. From exotic to invasive in record time: The extreme impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in the strait of Gibraltar. Sci. Total Environ. 2020, 704, 135408. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien—Invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Vilà, M.; Basnou, C.; Pyšek, P.; Josefsson, M.; Genovesi, P.; Gollasch, S.; Nentwig, W.; Olenin, S.; Roques, A.; Roy, D.; et al. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front. Ecol. Environ. 2010, 8, 135–144. [Google Scholar] [CrossRef]
- García-Gómez, J.C.; Sempere-Valverde, J.; Ostalé-Valriberas, E.; Martínez, M.; Olaya-Ponzone, L.; González, A.R.; Espinosa, F.; Sánchez-Moyano, E.; Megina, C.; Parada, J.A. Rugulopteryx okamurae (E.Y. Dawson) I.K. Hwang, W.J. Lee & H.S. Kim (Dictyotales, Ochrophyta), alga exótica “explosiva” en El Estrecho de Gibraltar. Observaciones preliminares de su distribución e impacto. Rev. Est. Campogibraltareños 2018, 49, 97–113. [Google Scholar]
- García-Gómez, J.C.; Florido, M.; Olaya-Ponzone, L.; Rada, J.R.D.; Donázar-Aramendía, I.; Chacón, M.; Quintero, J.J.; Magariño, S.; Megina, C. Monitoring extreme impacts of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in El Estrecho Natural Park (Biosphere Reserve). Showing radical changes in the underwater seascape. Front. Ecol. Evol. 2021, 9, 639161. [Google Scholar] [CrossRef]
- Mogollón, S.L.; Zilio, M.I.; Buitrago, E.M.; Caraballo, M.Á.; Ñiguez, R. Economic impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) along the Andalusian coastline: The case of Tarifa, Spain. Wetl. Ecol. Manag. 2024, 32, 19–32. [Google Scholar] [CrossRef]
- Martínez, M.A.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Maximiliano, J.E.; Rodríguez, J.L.; Martínez-Larrañaga, M.R.; Anadón, A.; Peterio, C.; Rubiño, S.; et al. Brown marine algae Gongolaria baccata extract protects Caco-2 cells from oxidative stress induced by tert-butyl hydroperoxide. Food Chem. Toxicol. 2021, 156, 112460. [Google Scholar] [CrossRef] [PubMed]
- Pérès, J.M.; Picard, J. Recueil des Travaux de la Station Marine d’Endoume. In Nouveau Manuel de Bionomie Benthique de la Mer Méditerranée; Station Marine d’Endoume: Marseille, France, 1964; Volume 31, pp. 1–137. [Google Scholar]
- Montesanto, B.; Panayiotidis, P. The Cystoseira spp. communities from the Aegean Sea (NE Mediterranean). Mediterr. Mar. Sci. 2001, 2, 57–67. [Google Scholar] [CrossRef]
- Sala, E.; Ballesteros, E.; Dendrinos, P.; Di Franco, A.; Ferretti, F.; Foley, D.; Fraschetti, S.; Friedlander, A.; Garrabou, J.; Güçlüsoy, H.; et al. The structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PLoS ONE 2012, 7, e32742. [Google Scholar] [CrossRef]
- Salomidi, M.; Giakoumi, S.; Gerakaris, V.; Issaris, Y.; Sini, M.; Tsiamis, K. Setting an ecological baseline prior to the bottom-up establishment of a marine protected area in Santorini Island, Aegean Sea. Mediterr. Mar. Sci. 2016, 17, 720–737. [Google Scholar] [CrossRef]
- Lardi, P.I.; Varkitzi, I.; Tsiamis, K.; Orfanidis, S.; Koutsoubas, D.; Falace, A.; Salomidi, M. Conditions, with a view to enhancing restoration potential in the Eastern Mediterranean. Bot. Mar. 2022, 65, 279–287. [Google Scholar] [CrossRef]
- Craigie, J.T.; McLachlan, J. Excretion of colored ultraviolet-absorbing substances by marine algae. Can. J. Bot. 1964, 42, 23–33. [Google Scholar] [CrossRef]
- Ragan, M.A.; Jensen, A. Quantitative studies on brown algal phenols. II. Seasonal variation in polyphenol content of Ascophyllum nodosum (L.) Le Jol. and Fucus vesiculosus (L.). J. Exp. Mar. Biol. Ecol. 1978, 34, 245–258. [Google Scholar] [CrossRef]
- Celis-Plá, P.; Korbee, N.; Gómez-Garreta, A.; Figueroa, F.L. Seasonal acclimation patterns in the intretidal macroalga Cystoseira tamariscifolia (Ochrophyta). Sci. Mar. 2014, 78, 377–388. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef]
- Abdala-Díaz, R.T.; Cabello-Pasini, A.; Pérez-Rodríguez, E.; Álvarez, R.C.; Figueroa, F.L. Daily and seasonal variations of optimum quantum yield and phenolic compounds in Cystoseira tamariscifolia (Phaeophyta). Mar. Biol. 2006, 148, 459–465. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Bouzon, Z.L.; Hall-Spencer, Z.L.; Schmidt, E.C.; Korbee, N.; Figueroa, F.L. Seasonal biochemical and photophysiological responses in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta) collected from Southern Spain. Mar. Environ. Res. 2016, 115, 89–97. [Google Scholar] [CrossRef]
- Amsler, C.D.; Fairhead, V.A. Defensive and sensory chemical ecology of brown algae. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2005; Volume 43, pp. 1–91. [Google Scholar]
- Swanson, A.K.; Druehl, L.D. Induction, exudation and the UV protective role of kelp phlorotannins. Aquat. Bot. 2002, 73, 241–253. [Google Scholar] [CrossRef]
- Santos, S.A.; Félix, R.; Pais, A.C.; Rocha, S.M.; Silvestre, A.J. The quest for phenolic compounds from macroalgae: A review of extraction and identification methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Nagayama, K.; Shibata, T.; Fujimoto, K.; Honjo, T.; Nakamura, T. Algicidal effect of phlorotannins from the brown alga Ecklonia kurome on red tide microalgae. Aquaculture 2003, 218, 601–611. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Hall-Spencer, J.M.; Horta, P.; Milazzo, M.; Korbee, N.; Cornwall, C.E.; Figueroa, F.L. Macroalga responses to ocean acidification depend on nutrient and light levels. Front. Mar. Sci. 2015, 2, 26. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Martínez, B.; Korbee, N.; Hall-Spencer, J.M.; Figueroa, F.L. Photoprotective responses in a brown macroalgae Cystoseira tamariscifolia to increases in CO2 and temperature. Mar. Environ. Res. 2017, 130, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Celis-Plá, P.S.; Martínez, B.; Korbee, N.; Hall-Spencer, J.M.; Figueroa, F.L. Ecophysiological responses to elevated CO2 and temperature in Cystoseira tamariscifolia (Phaeophyceae). Clim. Change 2017, 142, 67–81. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Brown, M.T.; Santillán-Sarmiento, A.; Korbee, N.; Sáez, C.; Figueroa, F.L. Cooper and nutrient excess mediates differential physiological and metabolic stress responses in the Brown macroalga Cystoseira tamariscifolia. Mar. Pollut. Bull. 2018, 128, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Cetrulo, G.L.; Hay, M.E. Activated chemical defenses in tropical versus temperate seaweeds. Mar. Ecol. Prog. Ser. 2000, 207, 243–253. [Google Scholar] [CrossRef]
- Steinberg, P.D.; van Altena, I. Tolerance of marine invertebrate herbivores to brown algal phlorotannins in temperate Australasia. Ecol. Monog. 1992, 62, 189–222. [Google Scholar] [CrossRef]
- Targett, N.M.; Arnold, T.M. Predicting the effects of brown algal phlorotannins on marine herbivores in tropical and temperate oceans. J. Phycol. 1998, 34, 195. [Google Scholar] [CrossRef]
- Gómez, I.; Español, S.; Véliz, K.; Huovinen, P. Spatial distribution of phlorotannins and its relationship with photosynthetic UV tolerance and allocation of storage carbohydrates in blades of the kelp Lessonia spicata. Mar. Biol. 2016, 163, 110. [Google Scholar] [CrossRef]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 1997. [Google Scholar]
- Abdala-Díaz, R.T.; Cabello-Pasini, A.; Márquez-Garrido, E.; Figueroa, F.L. Intra-thallus variation of phenolic compounds, antioxidant activity, and phenolsulphatase activity in Cystoseira tamariscifolia (Phaeophyceae) from southern Spain. Cienc. Mar. 2014, 40, 01–09. [Google Scholar] [CrossRef]
- Castillo, A.; Celeiro, M.; Lores, M.; Grgić, K.; Banožić, M.; Jerković, I.; Jokić, S. Bioprospecting of targeted phenolic compounds of Dictyota dichotoma, Gongolaria barbata, Ericaria amentacea, Sargassum hornschuchii and Ellisolandia elongata from the Adriatic Sea extracted by two green methods. Mar. Drugs 2023, 21, 97. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, R.N.; Mellinas, C.; Jiménez, A.; Bordehore, C.; Garrigós, M.C. Invasive seaweed Rugulopteryx okamurae: A potential source of bioactive compounds with antioxidant activity. Antioxidants 2024, 13, 1298. [Google Scholar] [CrossRef]
- Carlson, D.J.; Carlson, M.L. Reassessment of exudation by fucoid macroalgae. Limnol. Oceanogr. 1984, 29, 1077–1084. [Google Scholar] [CrossRef]
- Gran, A.; Movilla, J.I.; Ballesteros, E.; Sales, M.; Bolado, I.; Galobart, C.; Cefalì, M.E. Assessing the expansion and success of a restored population of Gongolaria barbata (Stackhouse) Kuntze (Fucales, Phaeophyceae) using high-precision positioning tools and size distribution frequencies. Mediterr. Mar. Sci. 2022, 23, 907–916. [Google Scholar] [CrossRef]
- Marin, O.; Spinu, A. Assessing the Ecological Status of Gongolaria barbata (Stackhouse) Kuntze (Fucales, Ochrophyta) Habitat Along the Romanian Black Sea Coast-A Source of Multiple Ecosystem Services. Ann. Acad. Rom. Sci. Ser. Biol. Sci. 2023, 12, 42–56. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Bioactive significance of fucoxanthin and its effective extraction. Biocatal. Agric. Biotechnol. 2020, 26, 101639. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef] [PubMed]
- Vizcaíno, A.J.; Sáez, M.I.; Galafat, A.; Galindo-Melero, R.; Perera, E.; Casal-Porras, I.; Zubía, E.; Vega, J.; Figueroa, F.L.; Martinéz, T.F.; et al. Effects of feeding European seabass (Dicentrarchus labrax) juveniles with crude, hydrolysed and fermented biomass of the invasive macroalga Rugulopteryx okamurae (Ochrophyta). Aquacul. Rep. 2024, 34, 101877. [Google Scholar] [CrossRef]
- Wang, H.; Ki, J.S. Molecular identification, differential expression and protective roles of iron/manganese superoxide dismutases in the green algae Closterium ehrenbergii against metal stress. Eur. J. Protistol. 2020, 74, 125689. [Google Scholar] [CrossRef]
- Schreiber, U.; Endo, T.; Mi, H.; Asada, K. Quenching analysis of chlorophyll fluorescence by the saturation pulse method: Particular aspects relating to the study of eukaryotic algae and cyanobacteria. Plant Cell Physiol. 1995, 36, 873–882. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Conde-Alvarez, R.; Gómez, I. Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynth. Res. 2003, 75, 259–275. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Domínguez-González, B.; Korbee, N. Vulnerability and acclimation to increased UVB radiation in three intertidal macroalgae of different morpho-functional groups. Mar. Environ. Res. 2014, 97, 30–38. [Google Scholar] [CrossRef]
- Eilers, P.H.C.; Peeters, J.C.H. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Modell. 1988, 42, 199–215. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Agostini, S.; Desjobert, J.M.; Pergent, G. Distribution of phenolic compounds in the seagrass Posidonia oceanica. Phytochemistry 1998, 48, 611–617. [Google Scholar] [CrossRef]
- Dumay, O.; Costa, J.; Desjobert, J.; Pergent, G. Variations in the concentration of phenolic compounds in the seagrass under conditions of competition. Phytochemistry 2004, 65, 3211–3220. [Google Scholar] [CrossRef] [PubMed]
- Astudillo-Pascual, M.; Domínguez, I.; Aguilera, P.A.; Frenich, A.G. New Phenolic Compounds in Posidonia oceanica seagrass: A comprehensive array using high resolution mass spectrometry. Plants 2021, 10, 864. [Google Scholar] [CrossRef] [PubMed]
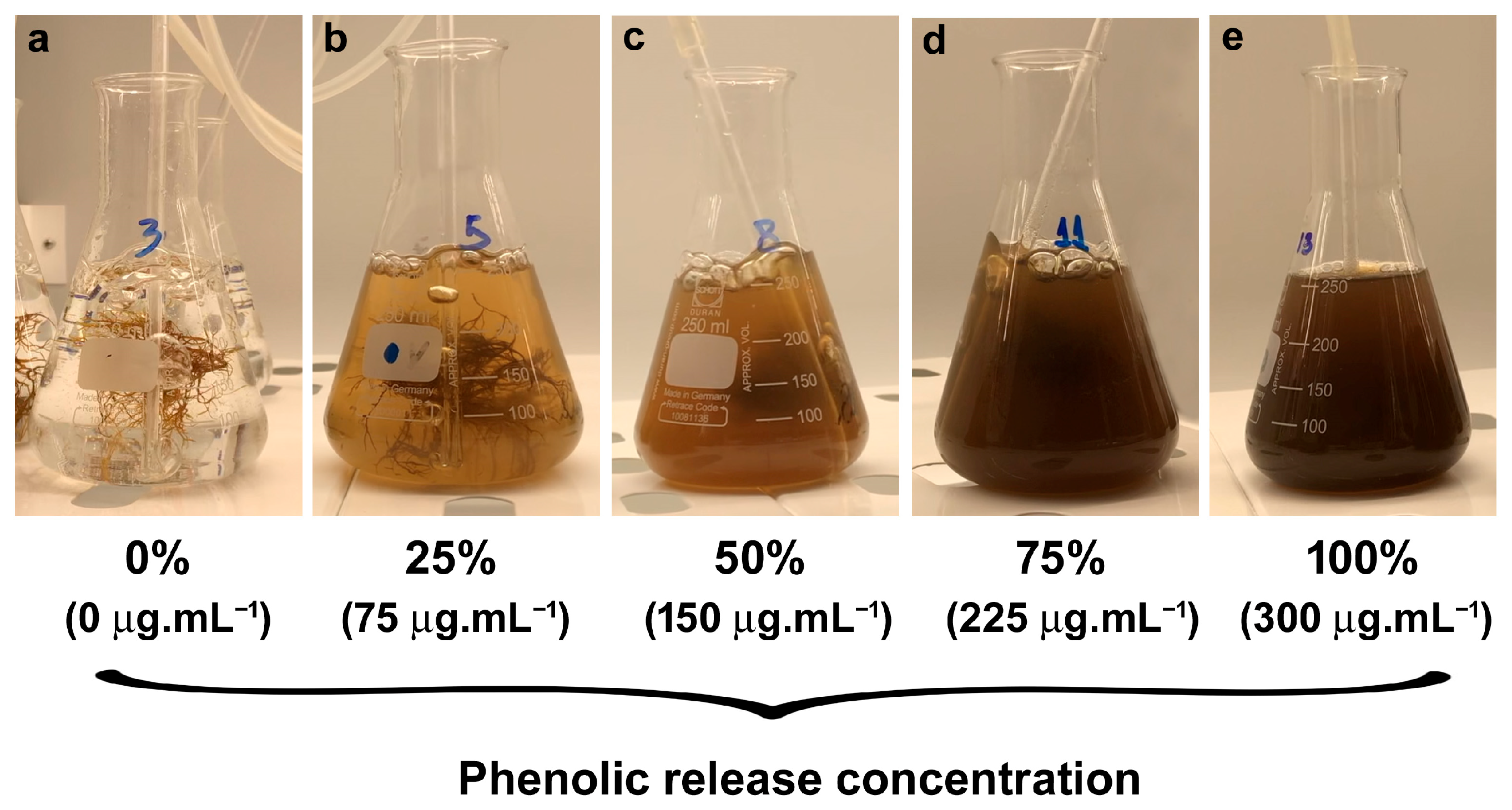
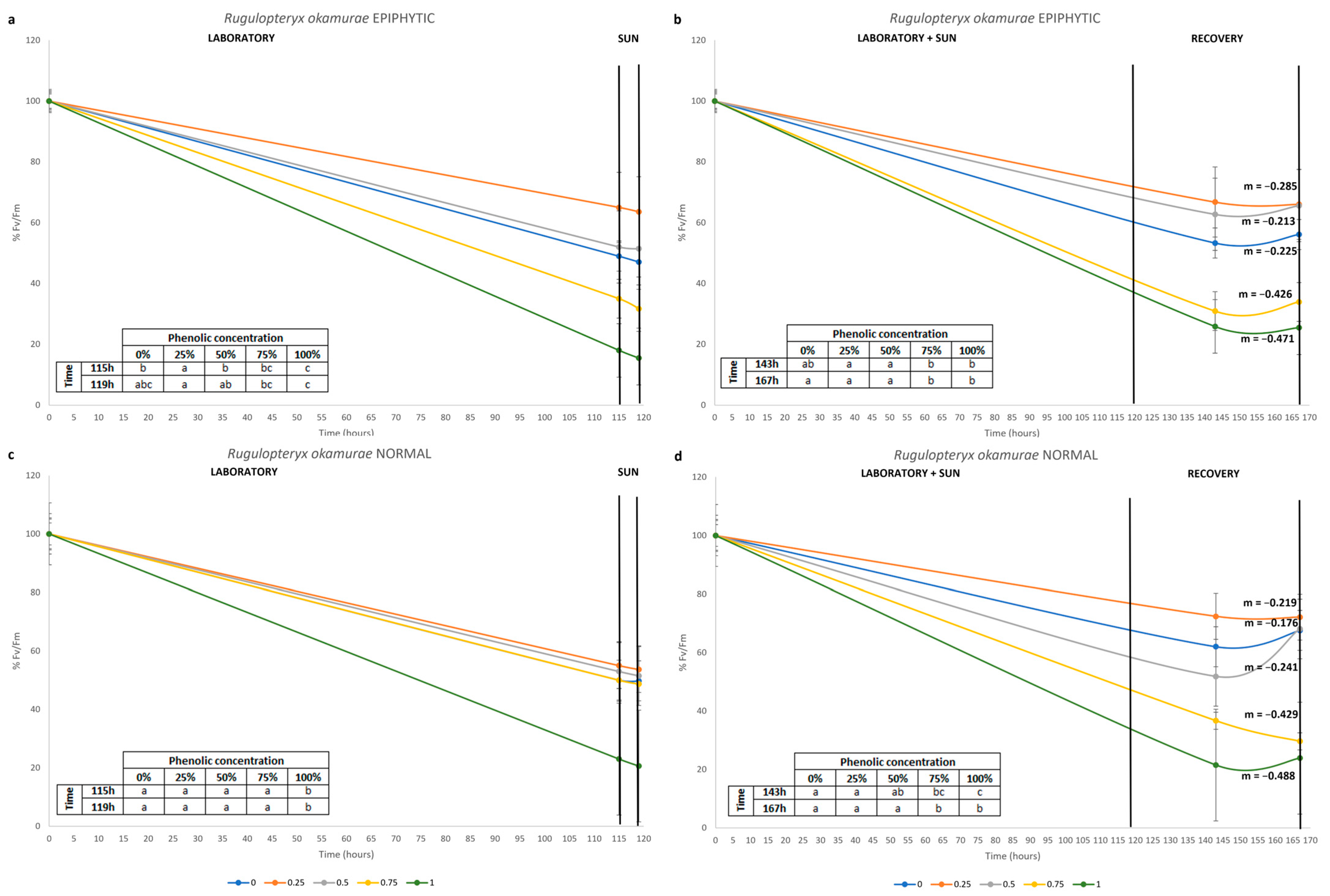
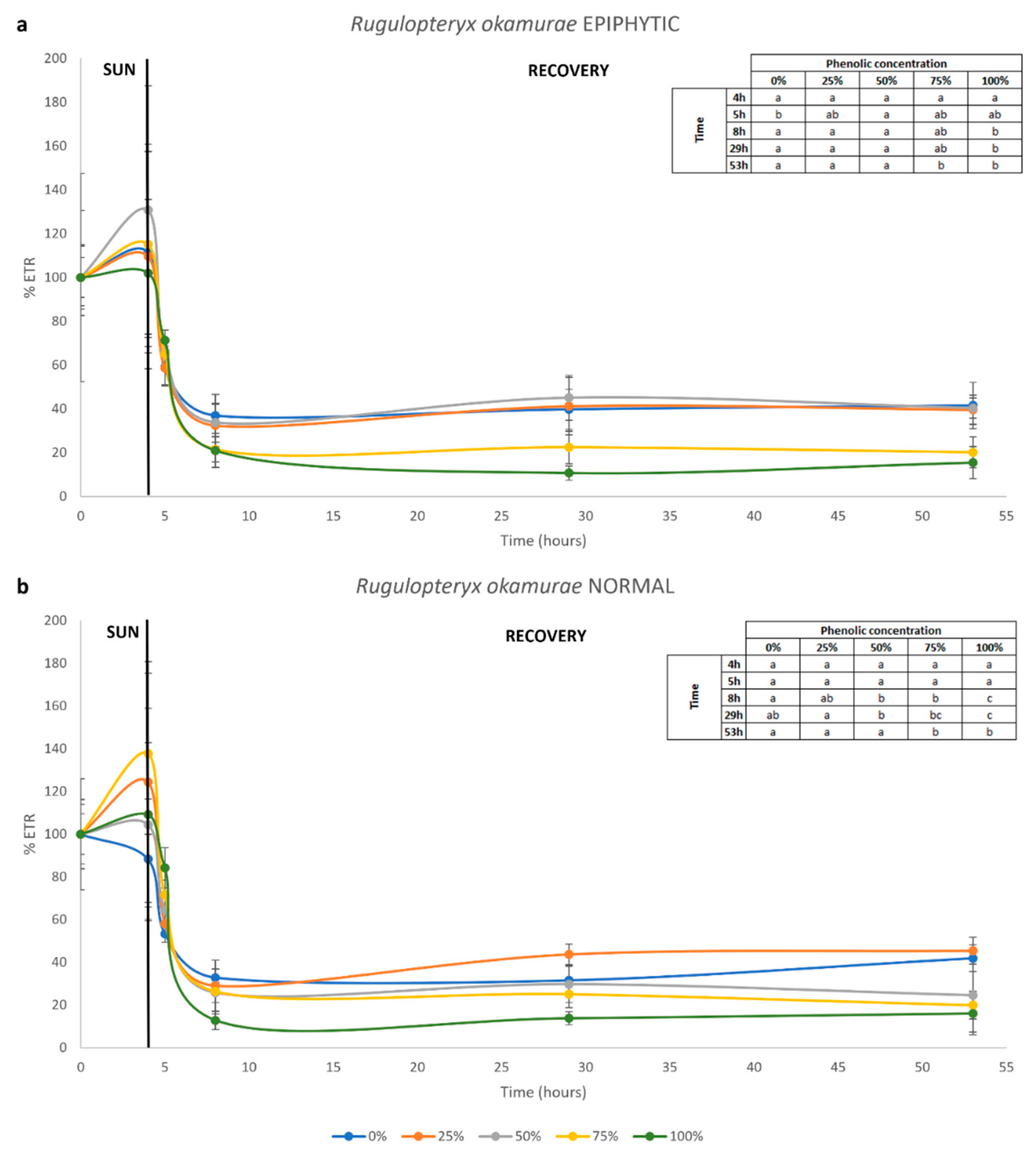
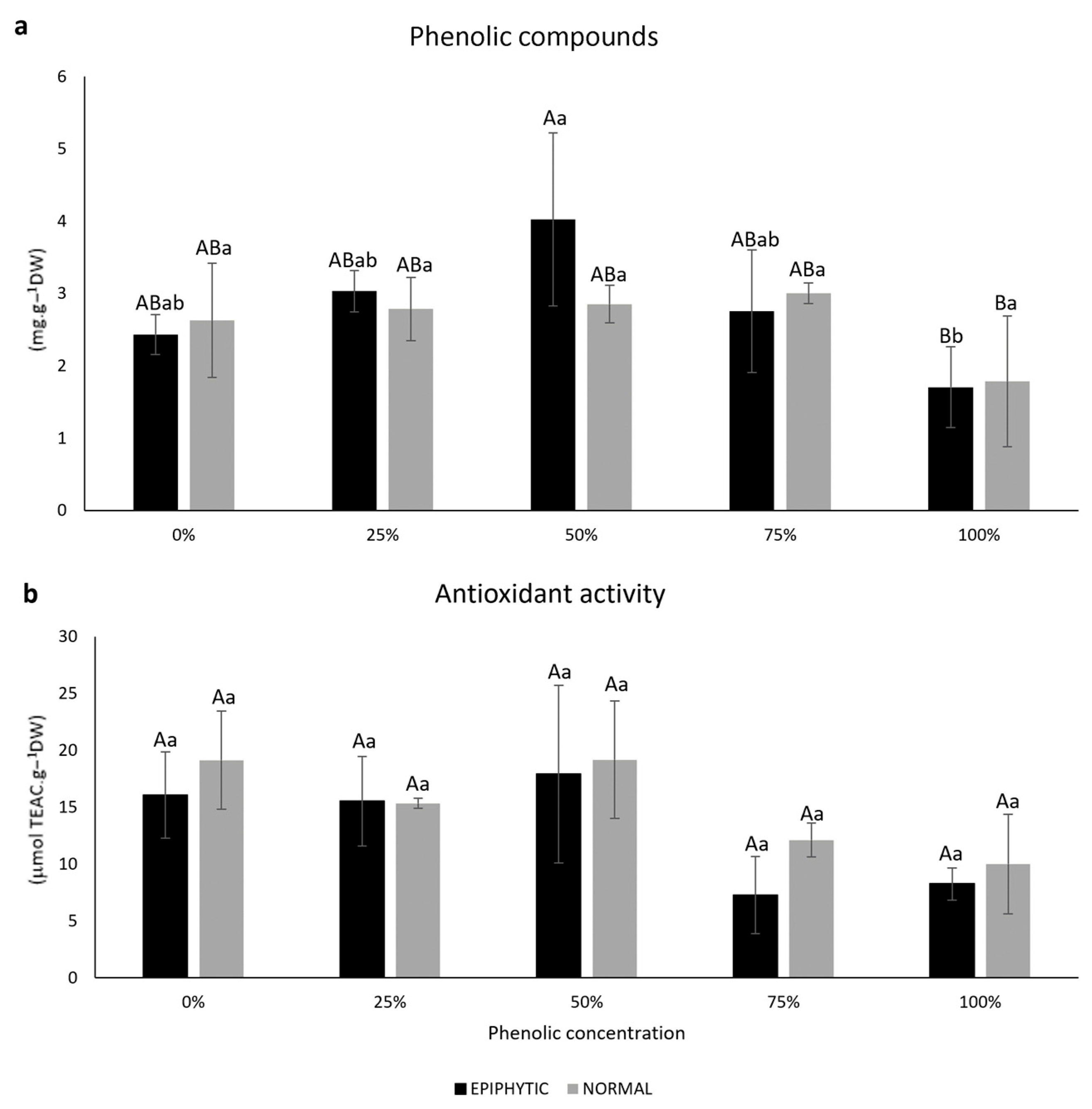



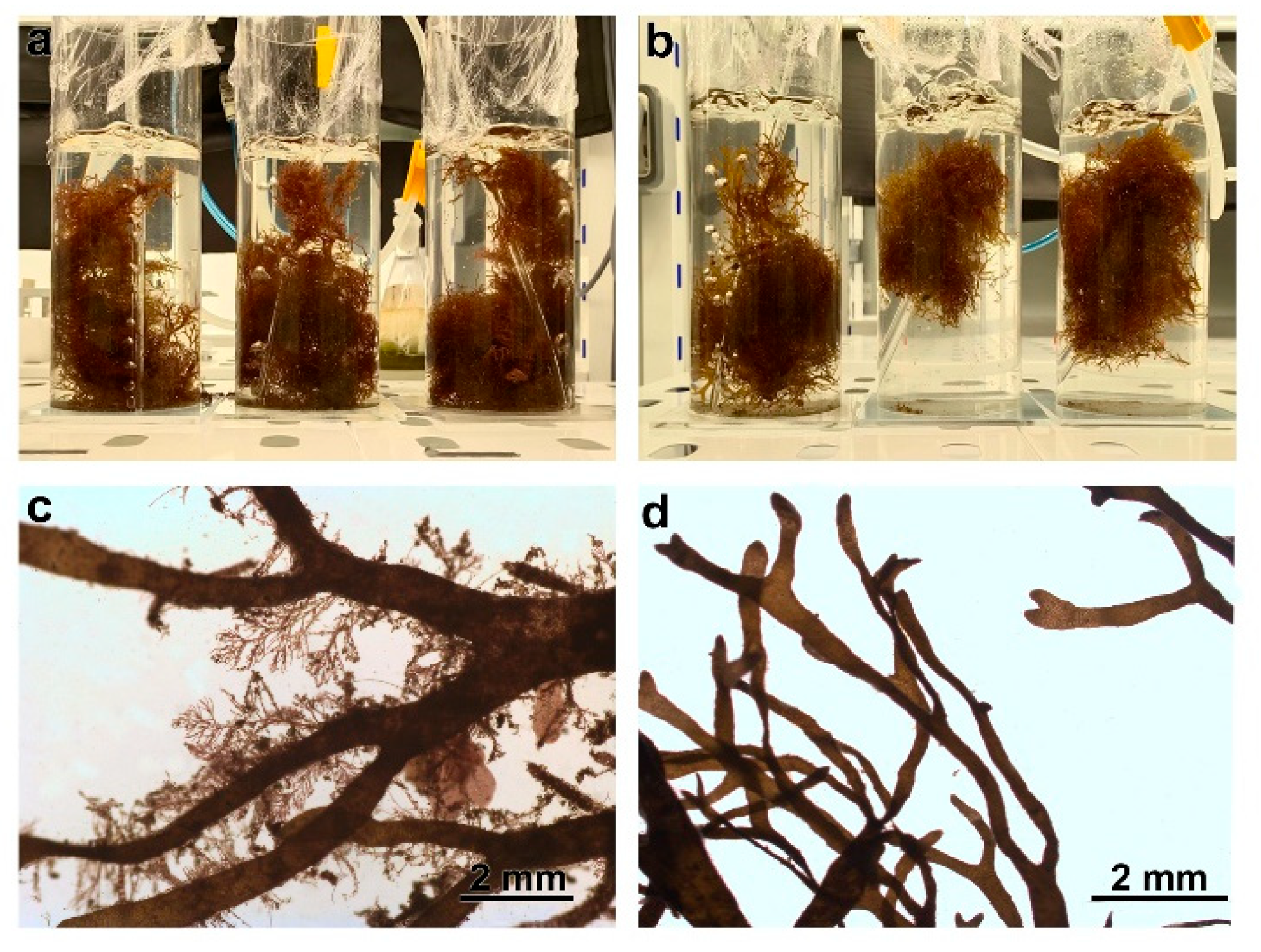
| Phenolic Concentration (Water) | Laboratory PAR | In Situ ETR | Fv/Fm | |
|---|---|---|---|---|
| Epiphytic | 0% | 190 | 80.70 ± 6.62 a | 0.331 ± 0.03 b |
| 25% | 125 | 55.08 ± 4.44 b | 0.445 ± 0.07 a | |
| 50% | 54 | 23.70 ± 2.96 c | 0.347 ± 0.07 b | |
| 75% | 45 | 16.09 ± 4.69 c | 0.235 ± 0.04 c | |
| 100% | 18 | 7.19 ± 0.86 d | 0.121 ± 0.06 d | |
| Normal | 0% | 190 | 78.75 ± 9.41 a | 0.367 ± 0.05 a |
| 25% | 125 | 52.32 ± 6.12 b | 0.382 ± 0.05 a | |
| 50% | 54 | 23.87 ± 2.52 c | 0.355 ± 0.06 a | |
| 75% | 45 | 14.64 ± 5.43 d | 0.337 ± 0.02 a | |
| 100% | 18 | 5.57 ± 2.37 e | 0.156 ± 0.02 b |
| C:N | C:S | N:S | ||||
|---|---|---|---|---|---|---|
| Phenolic Concentration | Epiphytic | Normal | Epiphytic | Normal | Epiphytic | Normal |
| 0% | 16.18 | 18.65 | 25.36 | 84.94 | 1.57 | 4.64 |
| 25% | 12.09 | 12.27 | 59.76 | 59.01 | 4.96 | 4.81 |
| 50% | 11.19 | 11.54 | 126.10 | 44.23 | 11.27 | 3.82 |
| 75% | 11.36 | 10.05 | 70.03 | 51.19 | 6.16 | 5.10 |
| 100% | 10.47 | 11.03 | 87.37 | 64.97 | 8.42 | 5.89 |
| Species | Internal Phenolic Concentration (mg g−1 DW) | |
|---|---|---|
| G. usneoides | Apical | 5.98 ± 0.32 b |
| Intermediate | 6.41 ± 0.98 b | |
| Basal | 14.03 ± 0.90 a | |
| R. okamurae | Epiphytic | 1.57 ± 0.12 |
| Normal | 1.32 ± 0.11 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, D.T.; Alarcón, F.G.; Alarcón, M.G.; Celis-Plá, P.S.M.; Figueroa, F.L. Effects of Seawater Polyphenols from Gongolaria usneoides on Photosynthesis and Biochemical Compounds of the Invasive Alien Species Rugulopteryx okamurae (Phaeophyceae, Heterokontophyta). Plants 2025, 14, 2594. https://doi.org/10.3390/plants14162594
Pereira DT, Alarcón FG, Alarcón MG, Celis-Plá PSM, Figueroa FL. Effects of Seawater Polyphenols from Gongolaria usneoides on Photosynthesis and Biochemical Compounds of the Invasive Alien Species Rugulopteryx okamurae (Phaeophyceae, Heterokontophyta). Plants. 2025; 14(16):2594. https://doi.org/10.3390/plants14162594
Chicago/Turabian StylePereira, Débora Tomazi, Fernando García Alarcón, Manolo García Alarcón, Paula S. M. Celis-Plá, and Félix L. Figueroa. 2025. "Effects of Seawater Polyphenols from Gongolaria usneoides on Photosynthesis and Biochemical Compounds of the Invasive Alien Species Rugulopteryx okamurae (Phaeophyceae, Heterokontophyta)" Plants 14, no. 16: 2594. https://doi.org/10.3390/plants14162594
APA StylePereira, D. T., Alarcón, F. G., Alarcón, M. G., Celis-Plá, P. S. M., & Figueroa, F. L. (2025). Effects of Seawater Polyphenols from Gongolaria usneoides on Photosynthesis and Biochemical Compounds of the Invasive Alien Species Rugulopteryx okamurae (Phaeophyceae, Heterokontophyta). Plants, 14(16), 2594. https://doi.org/10.3390/plants14162594







