Facilitation in the Dry Season: Species Interactions Between a Limestone-Endemic Plant and Moss Altered by Precipitation Dynamics
Abstract
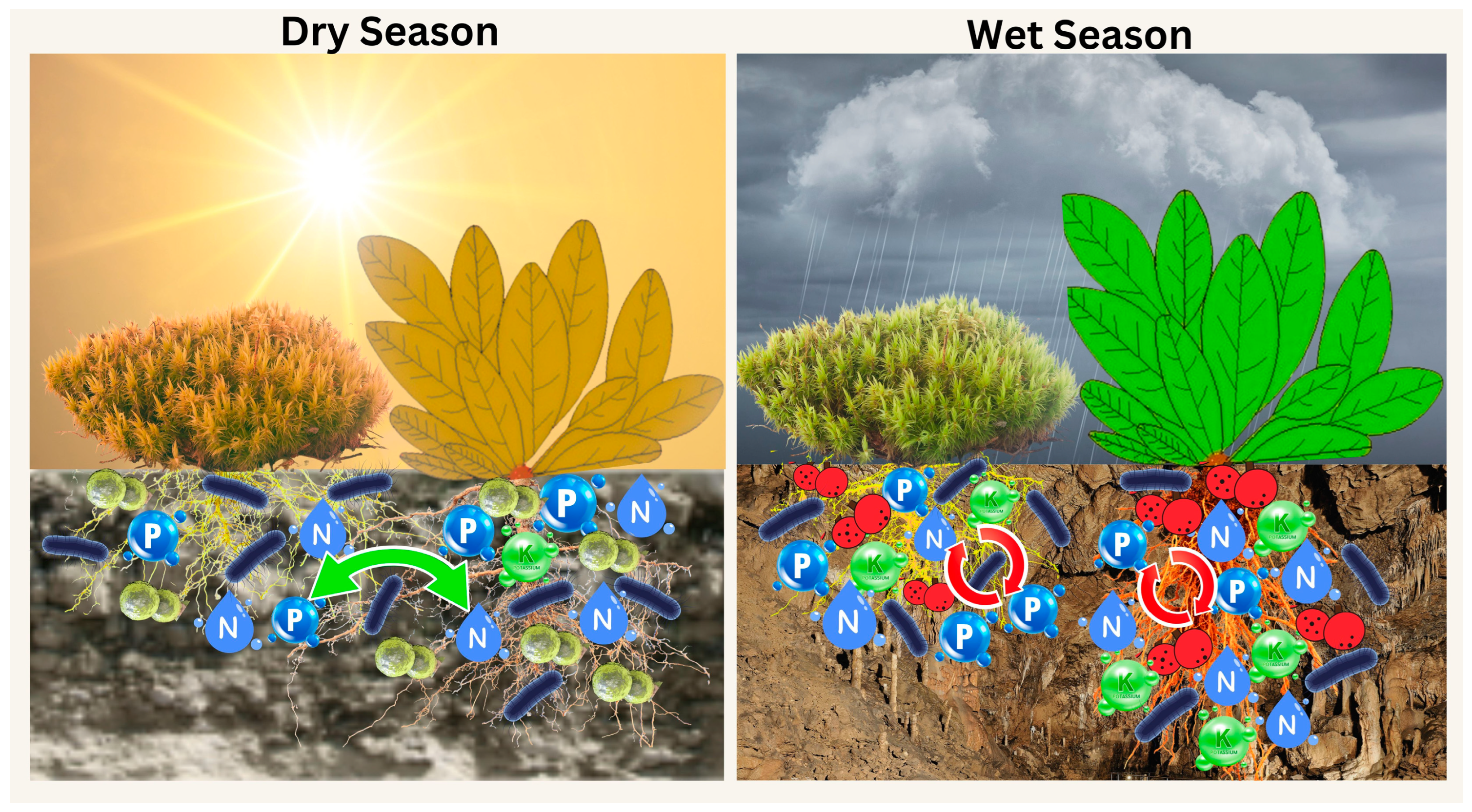
1. Introduction
2. Results
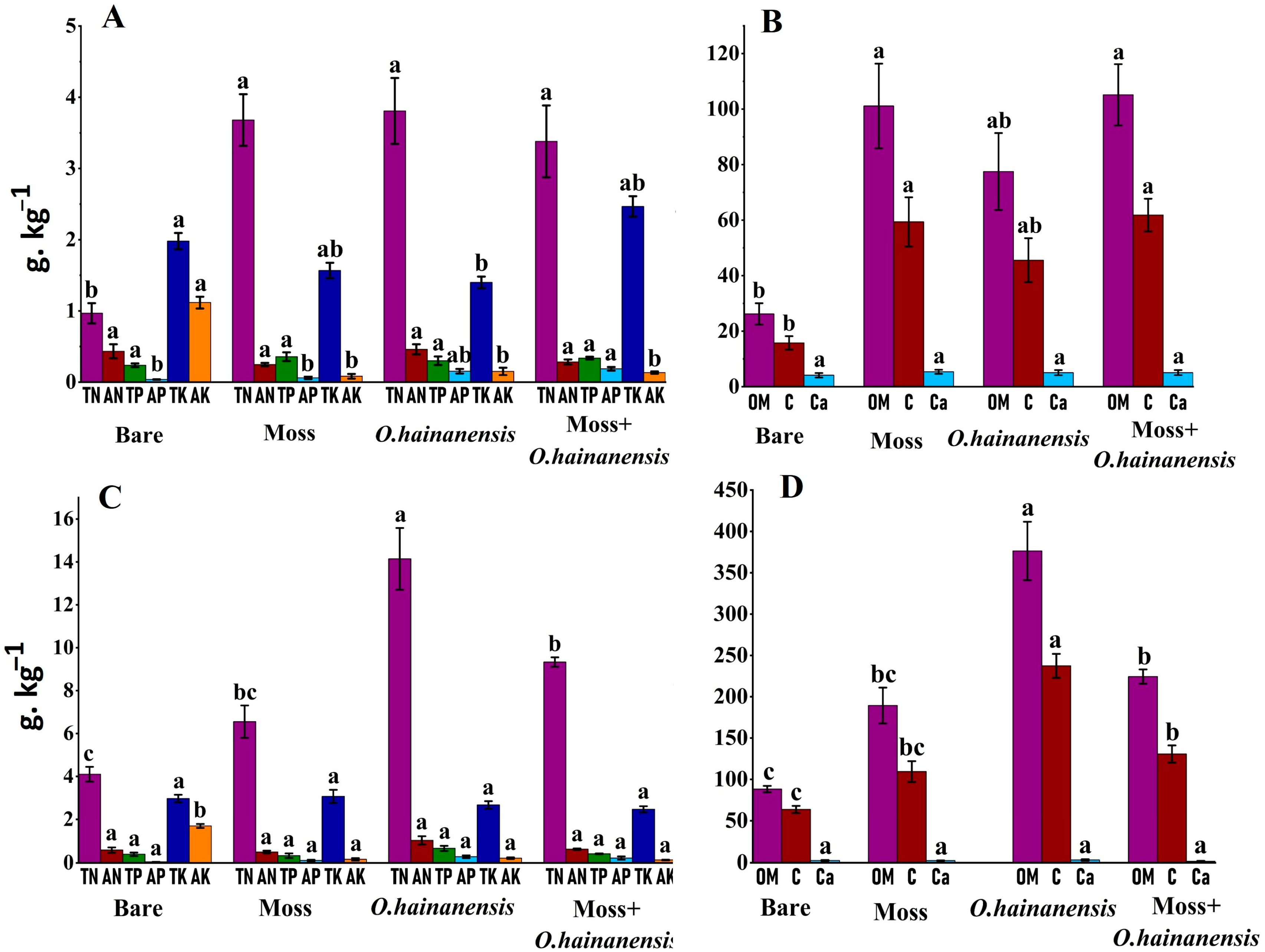
2.1. Physicochemical Parameters
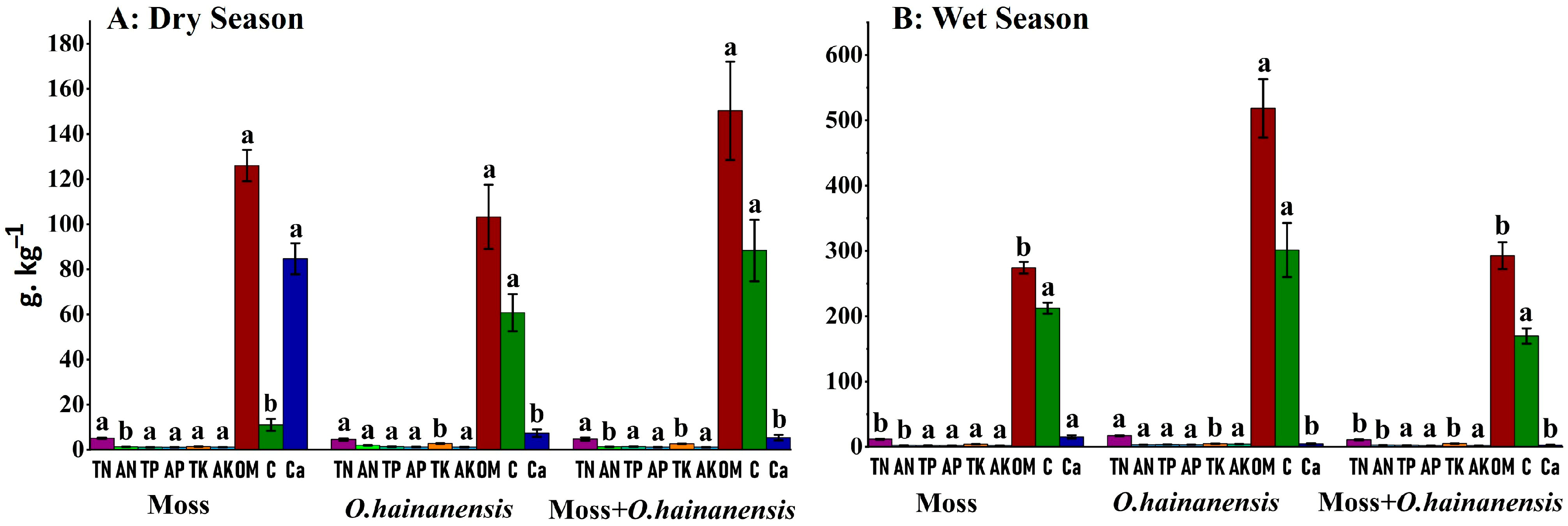
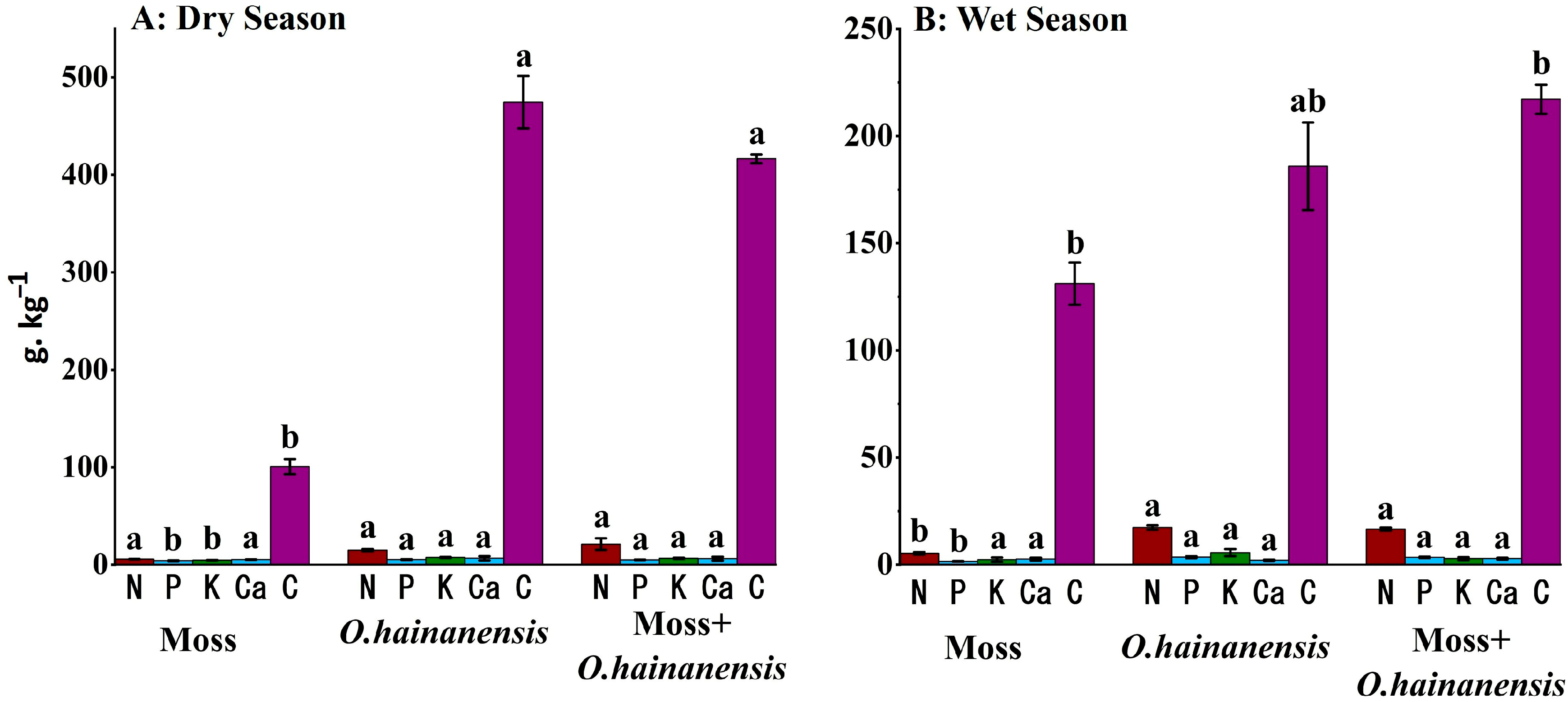
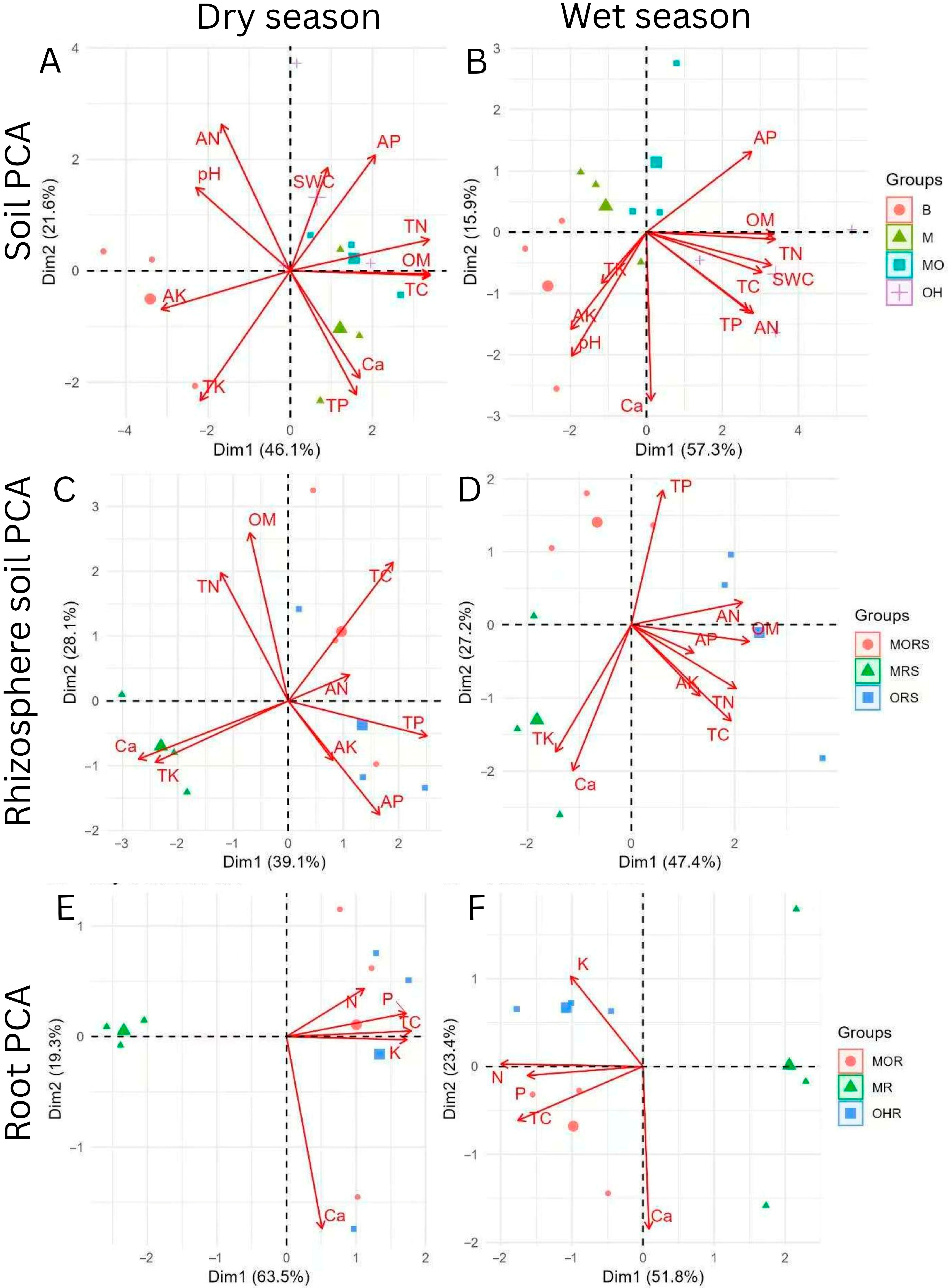
2.2. Soil, Rhizosphere Soil, and Root PCA
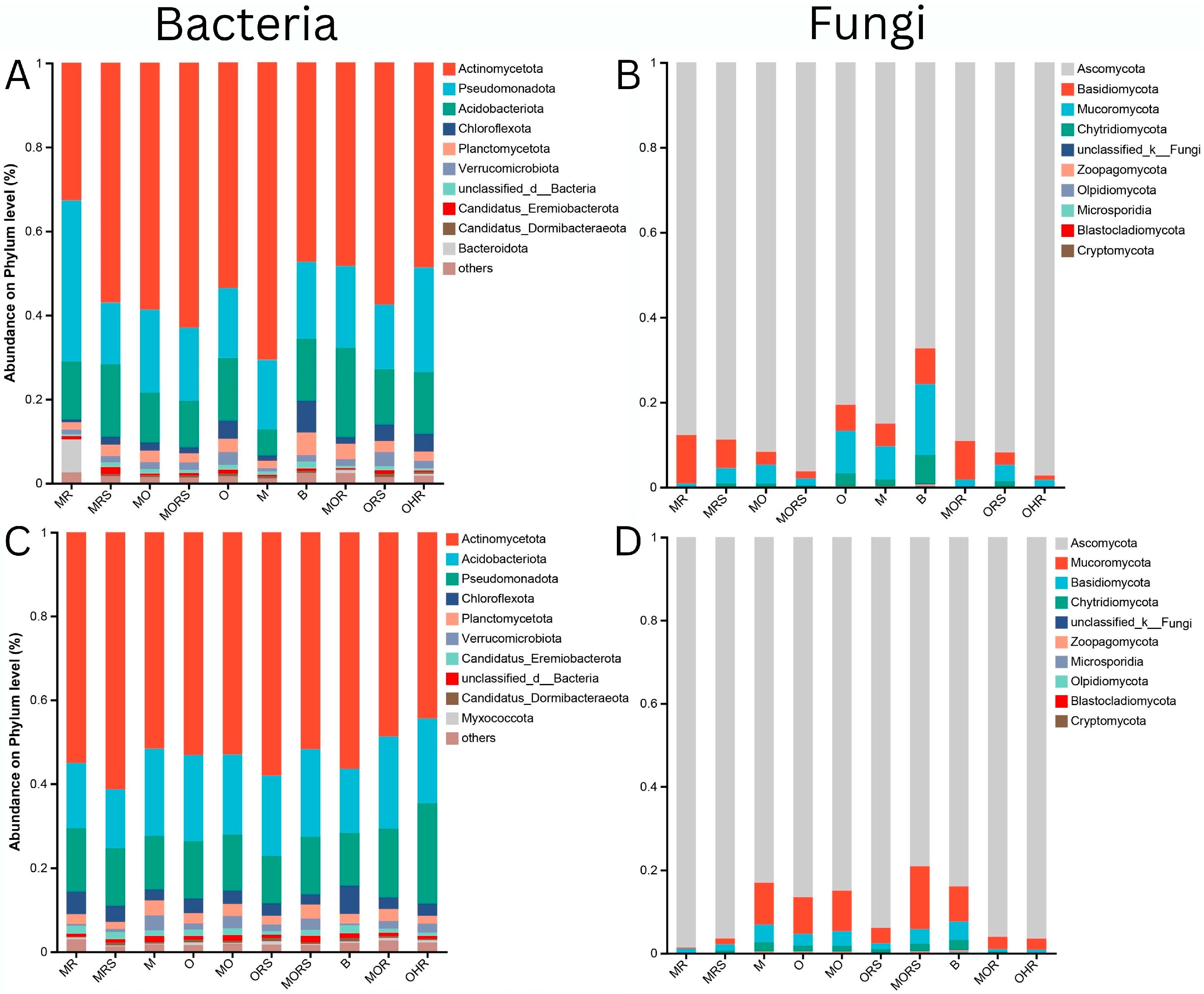
2.3. Bacterial Community
2.4. Fungal Community
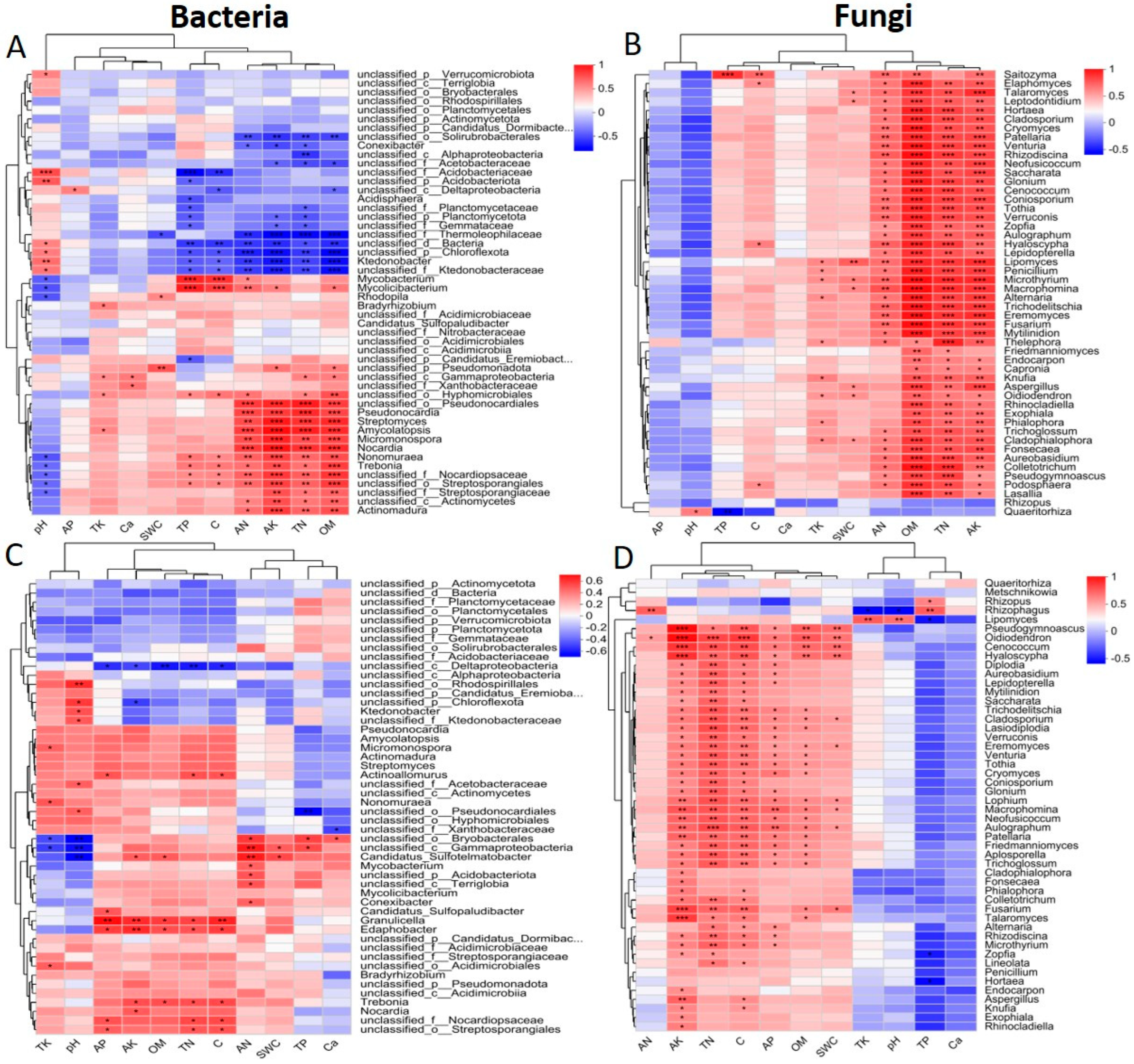
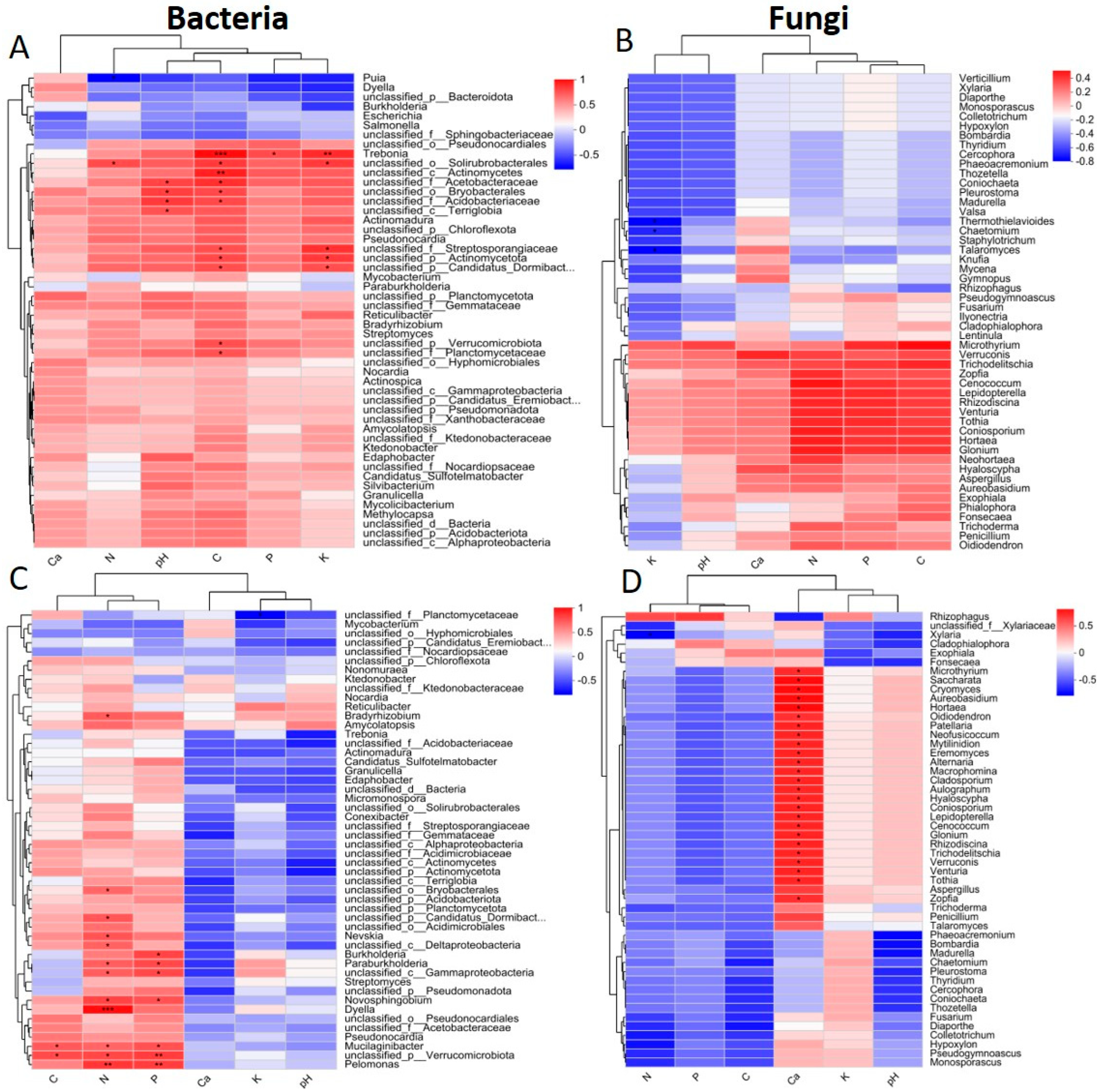
2.5. Relationship Between Microbial Communities and Soil, Rhizosphere Soil, and Root Variables
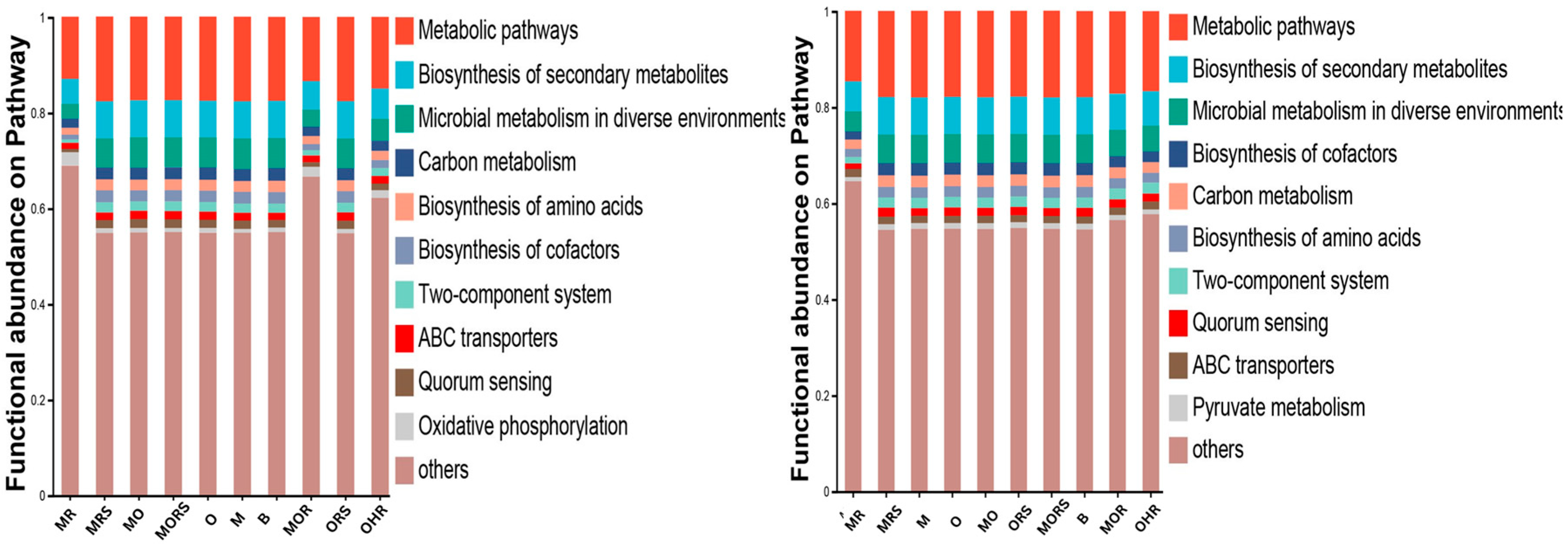
2.6. Microbial Functional Analysis
3. Discussion
3.1. Correlations Between Microbes and Physiochemical Traits
3.2. KEGG Functional Pathways
4. Materials and Methods
4.1. Study Site
4.2. Species and Sampling
4.3. Measurement of Soil, Rhizosphere Soil, and Root Variables
4.4. Microbial Diversity Analysis
4.4.1. DNA Extraction, Library Construction, and Metagenomics Sequencing
4.4.2. Sequence Quality Control
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.; Shourie, A. Climate Change Impacts on Plant–Microbe Interactions. In Climate Change and the Microbiome: Sustenance of the Ecosphere; Springer International Publishing: Cham, Switzerland, 2021; pp. 155–186. [Google Scholar]
- Cheng, Y.T.; Zhang, L.; He, S.Y. Plant-Microbe Interactions Facing Environmental Challenge. Cell Host Microbe 2019, 26, 183–192. [Google Scholar] [CrossRef]
- Zhang, X.; Myrold, D.D.; Shi, L.; Kuzyakov, Y.; Dai, H.; Thu Hoang, D.T.; Dippold, M.A.; Meng, X.; Song, X.; Li, Z.; et al. Resistance of Microbial Community and Its Functional Sensitivity in the Rhizosphere Hotspots to Drought. Soil Biol. Biochem. 2021, 161, 108360. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Grube, M.; Köberl, M. The Plant Microbiome Explored: Implications for Experimental Botany. J. Exp. Bot. 2016, 67, 995–1002. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Furtak, K. Soil–Plant–Microbe Interactions Determine Soil Biological Fertility by Altering Rhizospheric Nutrient Cycling and Biocrust Formation. Sustainability 2022, 15, 625. [Google Scholar] [CrossRef]
- LING, S.-J.; WEN, F.; REN, M.-X. Oreocharis Hainanensis (Gesneriaceae), a New Species from Karst Regions in Hainan Island, South China. Phytotaxa 2022, 538, 281–291. [Google Scholar] [CrossRef]
- Gornall, J.L.; Woodin, S.J.; Jónsdóttir, I.S.; van der Wal, R. Balancing Positive and Negative Plant Interactions: How Mosses Structure Vascular Plant Communities. Oecologia 2011, 166, 769–782. [Google Scholar] [CrossRef]
- Sohlberg, E.H.; Bliss, L.C. Responses of Ranunculus Sabinei and Papaver Radicatum to Removal of the Moss Layer in a High-Arctic Meadow. Can. J. Bot. 1987, 65, 1224–1228. [Google Scholar] [CrossRef]
- Mohan, S.M.; Sudhakar, P. Metagenomic Approaches for Studying Plant–Microbe Interactions. In Understanding the Microbiome Interactions in Agriculture and the Environment; Springer Nature: Singapore, 2022; pp. 243–254. [Google Scholar]
- Bardgett, R.D.; van der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo- and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Douda, J.; Doudová, J.; Hulík, J.; Havrdová, A.; Boublík, K. Reduced Competition Enhances Community Temporal Stability under Conditions of Increasing Environmental Stress. Ecology 2018, 99, 2207–2216. [Google Scholar] [CrossRef]
- Price, T.D.; Kirkpatrick, M. Evolutionarily Stable Range Limits Set by Interspecific Competition. Proc. R. Soc. B Biol. Sci. 2009, 276, 1429–1434. [Google Scholar] [CrossRef]
- Cornacchia, L.; van de Koppel, J.; van der Wal, D.; Wharton, G.; Puijalon, S.; Bouma, T.J. Landscapes of Facilitation: How Self-organized Patchiness of Aquatic Macrophytes Promotes Diversity in Streams. Ecology 2018, 99, 832–847. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Zamora, R.; Castro, J.; Hódar, J.A. Facilitation of Tree Saplings by Nurse Plants: Microhabitat Amelioration or Protection against Herbivores? J. Veg. Sci. 2008, 19, 161–172. [Google Scholar] [CrossRef]
- Rai, A.K.; Singh, D.P.; Prabha, R.; Kumar, M.; Sharma, L. Microbial Inoculants: Identification, Characterization, and Applications in the Field. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New Delhi, India, 2016; pp. 103–115. [Google Scholar]
- Hong, C.E.; Kim, J.U.; Lee, J.W.; Bang, K.H.; Jo, I.H. Metagenomic Analysis of Bacterial Endophyte Community Structure and Functions in Panax Ginseng at Different Ages. 3 Biotech 2019, 9, 300. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The Rhizosphere Microbiome: Significance of Plant Beneficial, Plant Pathogenic, and Human Pathogenic Microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reith, F.; Dennis, P.G.; Hamonts, K.; Powell, J.R.; Young, A.; Singh, B.K.; Bissett, A. Ecological Drivers of Soil Microbial Diversity and Soil Biological Networks in the Southern Hemisphere. Ecology 2018, 99, 583–596. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The Unseen Majority: Soil Microbes as Drivers of Plant Diversity and Productivity in Terrestrial Ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Bever, J.D.; Dickie, I.A.; Facelli, E.; Facelli, J.M.; Klironomos, J.; Moora, M.; Rillig, M.C.; Stock, W.D.; Tibbett, M.; Zobel, M. Rooting Theories of Plant Community Ecology in Microbial Interactions. Trends Ecol. Evol. 2010, 25, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Kaur, T.; Kour, D.; Rana, K.L.; Yadav, A.; Yadav, A.N. Beneficial Fungal Communities from Different Habitats and Their Roles in Plant Growth Promotion and Soil Health. Microb. Biosyst. 2020, 5, 21–47. [Google Scholar] [CrossRef]
- Keymer, D.P.; Lankau, R.A. Disruption of Plant–Soil–Microbial Relationships Influences Plant Growth. J. Ecol. 2017, 105, 816–827. [Google Scholar] [CrossRef]
- Sepp, S.-K.; Vasar, M.; Davison, J.; Oja, J.; Anslan, S.; Al-Quraishy, S.; Bahram, M.; Bueno, C.G.; Cantero, J.J.; Fabiano, E.C.; et al. Global Diversity and Distribution of Nitrogen-Fixing Bacteria in the Soil. Front. Plant Sci. 2023, 14, 1100235. [Google Scholar] [CrossRef]
- Tanvir, R.; Sheikh, A.A.; Javeed, A. Endophytic Actinomycetes in the Biosynthesis of Bioactive Metabolites: Chemical Diversity and the Role of Medicinal Plants. Stud. Nat. Prod. Chem. 2019, 60, 399–424. [Google Scholar]
- Khomutovska, N.; Jasser, I.; Isidorov, V.A. Unraveling the Role of Bacteria in Nitrogen Cycling: Insights from Leaf Litter Decomposition in the Knyszyn Forest. Forests 2024, 15, 1065. [Google Scholar] [CrossRef]
- Ulrich, D.E.M.; Sevanto, S.; Ryan, M.; Albright, M.B.N.; Johansen, R.B.; Dunbar, J.M. Plant-Microbe Interactions before Drought Influence Plant Physiological Responses to Subsequent Severe Drought. Sci. Rep. 2019, 9, 249. [Google Scholar] [CrossRef]
- Hodge, A.; Stewart, J.; Robinson, D.; Griffiths, B.S.; Fitter, A.H. Competition between Roots and Soil Micro-organisms for Nutrients from Nitrogen-rich Patches of Varying Complexity. J. Ecol. 2000, 88, 150–164. [Google Scholar] [CrossRef]
- Jiao, S.; Peng, Z.; Qi, J.; Gao, J.; Wei, G. Linking Bacterial-Fungal Relationships to Microbial Diversity and Soil Nutrient Cycling. mSystems 2021, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Jog, R.; Pandya, M.; Nareshkumar, G.; Rajkumar, S. Mechanism of Phosphate Solubilization and Antifungal Activity of Streptomyces Spp. Isolated from Wheat Roots and Rhizosphere and Their Application in Improving Plant Growth. Microbiology 2014, 160, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Hamdali, H.; Hafidi, M.; Virolle, M.J.; Ouhdouch, Y. Rock Phosphate-Solubilizing Actinomycetes: Screening for Plant Growth-Promoting Activities. World J. Microbiol. Biotechnol. 2008, 24, 2565–2575. [Google Scholar] [CrossRef]
- Ng, W.-L.; Bassler, B.L. Bacterial Quorum-Sensing Network Architectures. Annu. Rev. Genet. 2009, 43, 197–222. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate Solubilizing Bacteria and Their Role in Plant Growth Promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Schütze, E.; Klose, M.; Merten, D.; Nietzsche, S.; Senftleben, D.; Roth, M.; Kothe, E. Growth of Streptomycetes in Soil and Their Impact on Bioremediation. J. Hazard. Mater. 2014, 267, 128–135. [Google Scholar] [CrossRef]
- Nouioui, I.; Cortés-albayay, C.; Carro, L.; Castro, J.F.; Gtari, M.; Ghodhbane-Gtari, F.; Klenk, H.-P.; Tisa, L.S.; Sangal, V.; Goodfellow, M. Genomic Insights Into Plant-Growth-Promoting Potentialities of the Genus Frankia. Front. Microbiol. 2019, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, O.S.; Fernandes, A.S.; Tupy, S.M.; Ferreira, T.G.; Almeida, L.N.; Creevey, C.J.; Santana, M.F. Insights into Plant Interactions and the Biogeochemical Role of the Globally Widespread Acidobacteriota Phylum. Soil Biol. Biochem. 2024, 192, 109369. [Google Scholar] [CrossRef]
- Xu, T.; Shen, Y.; Ding, Z.; Zhu, B. Seasonal Dynamics of Microbial Communities in Rhizosphere and Bulk Soils of Two Temperate Forests. Rhizosphere 2023, 25, 100673. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global Diversity and Geography of Soil Fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.D.M.; Forn, I.; Gadelha, C.; Egan, M.J.; Bass, D.; Massana, R.; Richards, T.A. Discovery of Novel Intermediate Forms Redefines the Fungal Tree of Life. Nature 2011, 474, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Muneer, M.A.; Huang, X.; Hou, W.; Zhang, Y.; Cai, Y.; Munir, M.Z.; Wu, L.; Zheng, C. Response of Fungal Diversity, Community Composition, and Functions to Nutrients Management in Red Soil. J. Fungi 2021, 7, 554. [Google Scholar] [CrossRef]
- Islam, W.; Noman, A.; Naveed, H.; Huang, Z.; Chen, H.Y.H. Role of Environmental Factors in Shaping the Soil Microbiome. Environ. Sci. Pollut. Res. 2020, 27, 41225–41247. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate Solubilizing Microbes: Sustainable Approach for Managing Phosphorus Deficiency in Agricultural Soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Malik, A.A.; Bouskill, N.J. Drought Impacts on Microbial Trait Distribution and Feedback to Soil Carbon Cycling. Funct. Ecol. 2022, 36, 1442–1456. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Fitter, A. Are Microorganisms More Effective than Plants at Competing for Nitrogen? Trends Plant Sci. 2000, 5, 304–308. [Google Scholar] [CrossRef]
- Bever, J.D.; Platt, T.G.; Morton, E.R. Microbial Population and Community Dynamics on Plant Roots and Their Feedbacks on Plant Communities. Annu. Rev. Microbiol. 2012, 66, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the Soil Microbiome to Increase Soil Health and Plant Fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Maestre, F.T.; Callaway, R.M.; Valladares, F.; Lortie, C.J. Refining the Stress-gradient Hypothesis for Competition and Facilitation in Plant Communities. J. Ecol. 2009, 97, 199–205. [Google Scholar] [CrossRef]
- Aguilar-Trigueros, C.A.; Hempel, S.; Powell, J.R.; Anderson, I.C.; Antonovics, J.; Bergmann, J.; Cavagnaro, T.R.; Chen, B.; Hart, M.M.; Klironomos, J.; et al. Branching out: Towards a Trait-Based Understanding of Fungal Ecology. Fungal Biol. Rev. 2015, 29, 34–41. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of Maintenance of Species Diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Kong, D.; Wang, X.; Nie, J.; Niu, G. Regulation of Antibiotic Production by Signaling Molecules in Streptomyces. Front. Microbiol. 2019, 10, 2927. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Guo, P.; Wang, R.; Liu, T.; Luo, J.; Hao, B.; Wang, Y.; Guo, W. Synergistic Mechanisms of Bioorganic Fertilizer and AMF Driving Rhizosphere Bacterial Community to Improve Phytoremediation Efficiency of Multiple HMs-Contaminated Saline Soil. Sci. Total Environ. 2023, 883, 163708. [Google Scholar] [CrossRef]
- Pang, Z.; Dong, F.; Liu, Q.; Lin, W.; Hu, C.; Yuan, Z. Soil Metagenomics Reveals Effects of Continuous Sugarcane Cropping on the Structure and Functional Pathway of Rhizospheric Microbial Community. Front. Microbiol. 2021, 12, 627569. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going Back to the Roots: The Microbial Ecology of the Rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Lemieux, H.; Blier, P.U. Exploring Thermal Sensitivities and Adaptations of Oxidative Phosphorylation Pathways. Metabolites 2022, 12, 360. [Google Scholar] [CrossRef]
- Davidson, A.L.; Dassa, E.; Orelle, C.; Chen, J. Structure, Function, and Evolution of Bacterial ATP-Binding Cassette Systems. Microbiol. Mol. Biol. Rev. 2008, 72, 317–364. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum Sensing Signal–Response Systems in Gram-Negative Bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum Sensing in Bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial Diversity Drives Multifunctionality in Terrestrial Ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Chubukov, V.; Gerosa, L.; Kochanowski, K.; Sauer, U. Coordination of Microbial Metabolism. Nat. Rev. Microbiol. 2014, 12, 327–340. [Google Scholar] [CrossRef]
- Yun, Q.; Zhang, Q. Spatiotemporal Variation Characteristics of Extreme Temperature Events in Hainan Province over the Past Four Decades. Front. Environ. Sci. 2025, 13, 1510445. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, L.; Qi, S.; Chen, J. Spatiotemporal Dynamics and Driving Factors of Vegetation Greenness in Typical Tourist Region: A Case Study of Hainan Island, China. Land 2024, 13, 1687. [Google Scholar] [CrossRef]
- Schad, P. World Reference Base for Soil Resources—Its Fourth Edition and Its History. J. Plant Nutr. Soil Sci. 2023, 186, 151–163. [Google Scholar] [CrossRef]
- Chengmin, H.; Zitong, G.; Yurong, H. Elemental Geochemistry of a Soil Chronosequence on Basalt on Northern Hainan Island, China. Chin. J. Geochemistry 2004, 23, 245–254. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, B.; Hu, G. Spatial Heterogeneity of Soil Chemical Properties in a Subtropical Karst Forest, Southwest China. Sci. World J. 2014, 2014, 473651. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, Variation, and Assembly of the Root-Associated Microbiomes of Rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef]
- Feng, J.; Xu, Y.; Ma, B.; Tang, C.; Brookes, P.C.; He, Y.; Xu, J. Assembly of Root-Associated Microbiomes of Typical Rice Cultivars in Response to Lindane Pollution. Environ. Int. 2019, 131, 104975. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysi: Part 2 Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- RICHARDS, L.A. Diagnosis and Improvement of Saline and Alkali Soils. Soil Sci. 1954, 78, 154. [Google Scholar] [CrossRef]
- Peech, M. Hydrogen-Ion Activity. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1965; pp. 914–926. [Google Scholar]
- Yaseen, M.; Khan, W.R.; Bahadur, S.; Batool, F.; Khalid, F.; Ahmed, U.; Ashraf, M. Intra- and Inter-Specific Responses of Plant Functional Traits to Environmental Variables: Implications for Community Ecology in the Tropical Monsoonal Dwarf Forest on Hainan Island. Front. For. Glob. Change 2023, 6. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable Cations. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; pp. 159–165. [Google Scholar]
- Zhong, Y.; Hu, J.; Xia, Q.; Zhang, S.; Li, X.; Pan, X.; Zhao, R.; Wang, R.; Yan, W.; Shangguan, Z.; et al. Soil Microbial Mechanisms Promoting Ultrahigh Rice Yield. Soil Biol. Biochem. 2020, 143, 107741. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An Ultra-Fast Single-Node Solution for Large and Complex Metagenomics Assembly via Succinct de Bruijn Graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for Clustering the next-Generation Sequencing Data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short Oligonucleotide Alignment Program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Liu, A.; Li, Y.; Wang, Q.; Zhang, X.; Xiong, J.; Li, Y.; Lei, Y.; Sun, Y. Analysis of Microbial Diversity and Community Structure of Rhizosphere Soil of Cistanche Salsa from Different Host Plants. Front. Microbiol. 2022, 13, 971228. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, A.; Ling, S.-J.; Wei, Y.-L.; Bahadur, S.; Ren, M.-X. Facilitation in the Dry Season: Species Interactions Between a Limestone-Endemic Plant and Moss Altered by Precipitation Dynamics. Plants 2025, 14, 2588. https://doi.org/10.3390/plants14162588
Raza A, Ling S-J, Wei Y-L, Bahadur S, Ren M-X. Facilitation in the Dry Season: Species Interactions Between a Limestone-Endemic Plant and Moss Altered by Precipitation Dynamics. Plants. 2025; 14(16):2588. https://doi.org/10.3390/plants14162588
Chicago/Turabian StyleRaza, Ali, Shao-Jun Ling, Ya-Li Wei, Saraj Bahadur, and Ming-Xun Ren. 2025. "Facilitation in the Dry Season: Species Interactions Between a Limestone-Endemic Plant and Moss Altered by Precipitation Dynamics" Plants 14, no. 16: 2588. https://doi.org/10.3390/plants14162588
APA StyleRaza, A., Ling, S.-J., Wei, Y.-L., Bahadur, S., & Ren, M.-X. (2025). Facilitation in the Dry Season: Species Interactions Between a Limestone-Endemic Plant and Moss Altered by Precipitation Dynamics. Plants, 14(16), 2588. https://doi.org/10.3390/plants14162588








