Kiwifruit Cross-Pollination Analysis: Characterisation of the Pollinator-Assemblage and Practices to Enhance Fruit Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Area of Study
2.2. Bee Pollination Service
2.3. Floral Visitors and Pollinators Sampling
2.4. Impact of the Attractant on Honeybee Pollination, Fruit Production, and Quality
2.5. Statistical Analyses
3. Results
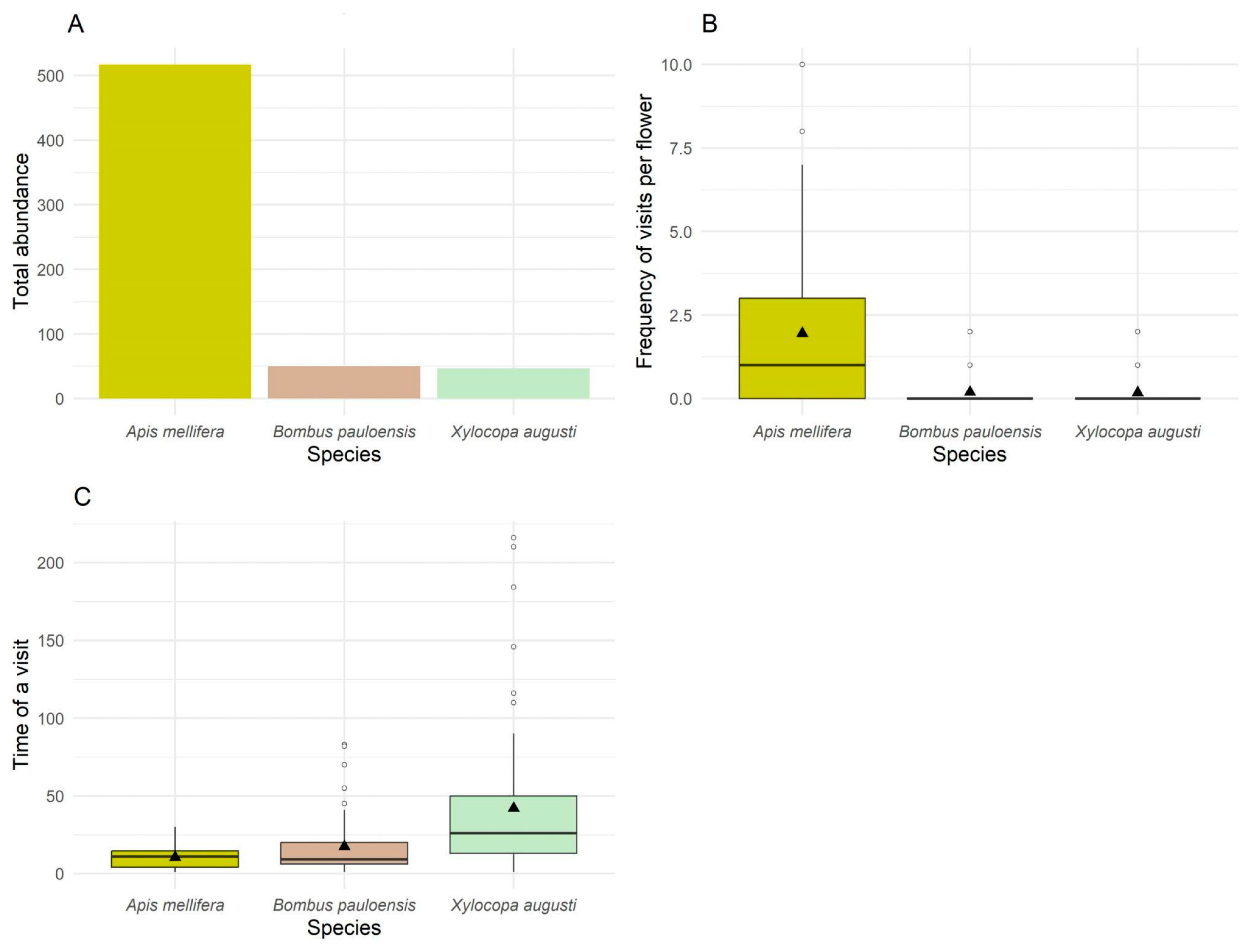
3.1. The Floral Visitors and Their Visitation Patterns
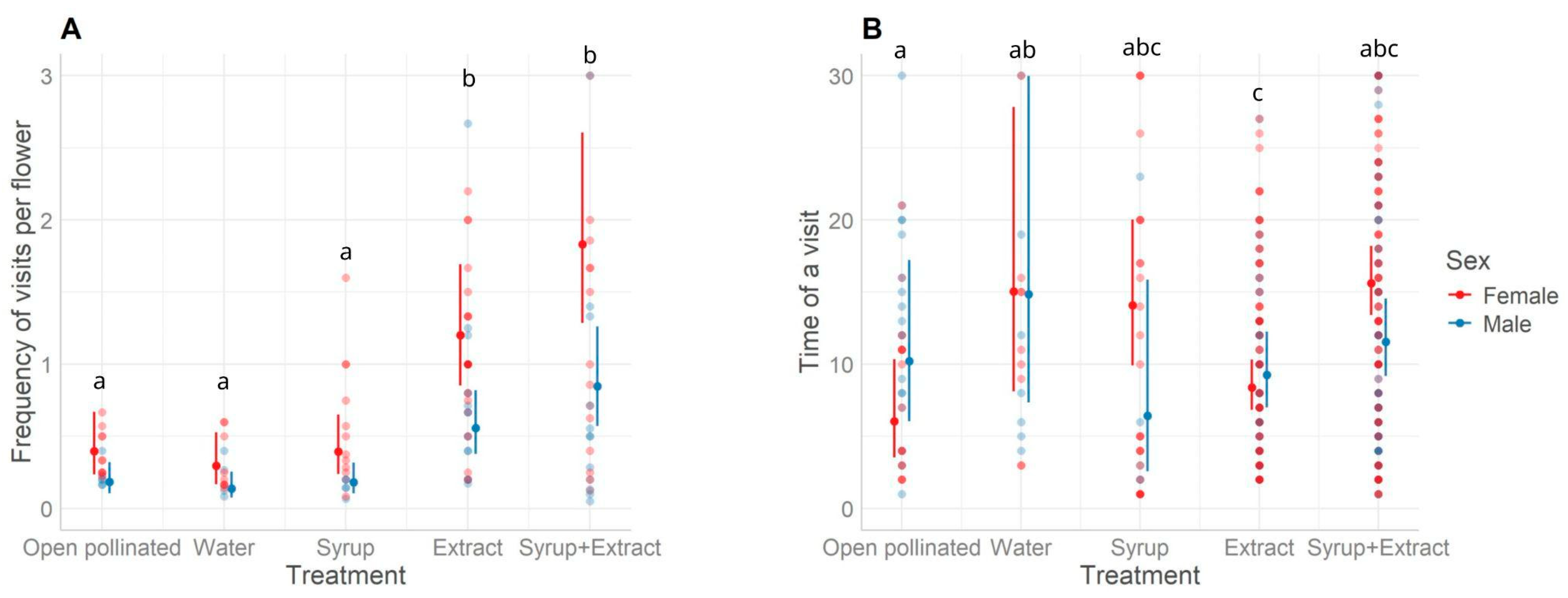
3.2. Impact of the Extract on Honeybee Pollination and Fruit Quality
4. Discussion
4.1. The Floral Visitors and Their Visitation Patterns
4.2. Impact of the Extract on Honeybee Pollination and Fruit Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godoy, C.; Dome, C. Relationship between physiological maturity and commercial maturity of kiwifruit “Hayward” growing at the South-East of Buenos Aires province (Argentina). N. Z. J. Crop Hortic. Sci. 2013, 41, 311–325. [Google Scholar] [CrossRef]
- Palmer-Jones, T.; Clinch, P.G. Observations on the pollination of Chinese gooseberries variety “Hayward”. N. Z. J. Exp. Agric. 1974, 2, 455–458. [Google Scholar] [CrossRef]
- Hopping, M.E.; Jerram, E.M. Supplementary pollination of tree fruits. I. Development of suspension media. N. Z. J. Agric. Res. 1979, 22, 509–515. [Google Scholar] [CrossRef][Green Version]
- Jay, S.C.; Jay, D.H. The effect of kiwifruit (Actinidia deliciosa A. Chev) and yellow flowered broom (Cytisus scoparius Link) pollen on the ovary development of worker honey bees (Apis mellifera L.). Apidologie 1993, 24, 557–563. [Google Scholar] [CrossRef]
- Testolin, R.; Vizzotio, G.; Costa, G. Kiwifruit pollination by wind and insects in Italy. N. Z. J. Crop Hortic. Sci. 1991, 19, 381–384. [Google Scholar] [CrossRef]
- Costa, G.; Testolin, R.; Vizzotto, G. Kiwifruit pollination: An unbiased estimate of wind and bee contribution. N. Z. J. Crop Hortic. Sci. 1993, 21, 189–195. [Google Scholar] [CrossRef]
- Vaissière, B.E.; Rodet, G.; Cousin, M.; Botella, L.; Grossa, J.P.T. Pollination effectiveness of honey bees (Hymenoptera: Apidae) in a kiwifruit orchard. J. Econ. Entomol. 1996, 89, 453–461. [Google Scholar] [CrossRef]
- Miñarro, M.; Twizell, K.W. Pollination services provided by wild insects to kiwifruit (Actinidia deliciosa). Apidologie 2015, 46, 276–285. [Google Scholar] [CrossRef]
- Delaplane, K.S.; Mayer, D.F. Crop Pollination by Bees, 1st ed.; CABI Publishing: New York, NY, USA, 2000; pp. 1–344. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, J.H.; Yuan, Y.; Wang, Y.C.; Wei, X.P.; Zhang, D.H.; Chen, Y. Effects of Apis cerana cerana pollination on the yield and quality of kiwifruit. J. Econ. Entomol. 2022, 115, 1425–1433. [Google Scholar] [CrossRef]
- King, M.J.; Ferguson, A.M. Vibratory collection of Actinidia deliciosa (kiwifruit) pollen. Ann. Bot. 1994, 74, 479–482. [Google Scholar] [CrossRef]
- De Luca, P.A.; Vallejo-Marín, M. What’s the “buzz” about? The ecology and evolutionary significance of buzz-pollination. Curr. Opin. Plant Biol. 2013, 16, 429–435. [Google Scholar] [CrossRef]
- Pritchard, D.J.; Vallejo-Marín, M. Floral vibrations by buzz-pollinating bees achieve higher frequency, velocity and acceleration than flight and defence vibrations. J. Exp. Biol. 2020, 223, jeb220541. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.; Aslam, M.; Razaq, M. Pollination ecology with special reference to insects: A review. J. Sci. Res. 2004, 4, 395–409. [Google Scholar]
- Goodwin, R.M. Kiwifruit flowers: Anther dehiscence and daily collection of pollen by honey bees. N. Z. J. Exp. Agric. 1986, 14, 449–452. [Google Scholar] [CrossRef]
- Goodwin, R.M.; Steven, D. Behavior of honeybees visiting kiwifruit flowers. N. Z. J. Crop Hortic. Sci. 1993, 21, 17–24. [Google Scholar] [CrossRef]
- Goodwin, R.M.; Houten, A.T.; Perry, J.H. Effect of staminate kiwifruit vine distribution and flower number on kiwifruit pollination. N. Z. J. Crop Hortic. Sci. 1999, 27, 63–68. [Google Scholar] [CrossRef]
- Howpage, D.; Spooner-Hart, R.N.; Vithanage, V. Influence of honey bee (Apis mellifera) on kiwifruit pollination and fruit quality under Australian conditions. N. Z. J. Crop Hortic. Sci. 2001, 29, 51–59. [Google Scholar] [CrossRef]
- Sáez, A.; Negri, P.; Viel, M.; Aizen, M.A. Pollination efficiency of artificial and bee pollination practices in kiwifruit. Sci. Hortic. 2019, 246, 1017–1021. [Google Scholar] [CrossRef]
- Goodwin, R.M.; Congdon, N.M. Recognition and attractiveness of staminate and pistillate kiwifruit flowers (Actinidia deliciosa var. deliciosa) by honey bees (Apis mellifera L.). N. Z. J. Crop Hortic. Sci. 2018, 46, 72–80. [Google Scholar] [CrossRef]
- Free, J.B. Insect Pollination of Crops, 2nd ed.; Academic Press: London, UK, 1993; pp. 1–544. [Google Scholar]
- Goodwin, R.M.; McBrydie, H.M.; Taylor, M.A. Wind and honey bee pollination of kiwifruit (Actinidia chinensis “HORT16A”). N. Z. J. Bot. 2013, 51, 229–240. [Google Scholar] [CrossRef]
- Meroi Arcerito, F.R.; De Feudis, L.L.; Amarilla, L.D.; Galetto, L.; Mitton, G.; Fernández, N.; Damiani, N.; Ramos, F.; Iglesias, A.E.; Gende, L.B.; et al. Fragrance addition improves visitation by honeybees and fruit quality in kiwifruit (Actinidia deliciosa). J. Sci. Food Agric. 2021, 101, 5082–5088. [Google Scholar] [CrossRef]
- David, M.A.; Yommi, A.; Sánchez, E.; Martinez, A.; Murillo, N.; Marcellán, O.; Palacio, M.A. Strategic use of honey bees (Apis mellifera L.) to increase the number and size of fruits in kiwifruit (Actinidia chinensis var. deliciosa). Eur. J. Agron. 2022, 133, 126420. [Google Scholar] [CrossRef]
- Farina, W.M.; Arenas, A.; Estravis-Barcala, M.C.; Palottini, F. Targeted crop pollination by training honey bees: Advances and perspectives. Front. Bee Sci. 2023, 1, 1253157. [Google Scholar] [CrossRef]
- Juárez, V.; Mantobani, J.M. La costa bonaerense: Un territorio particular. In Manual de Manejo Costero para la Provincia de Buenos Aires; Isla, F.I., Lasta, C.A., Eds.; Editorial EUDEM: Mar del Plata, Argentina, 2006; pp. 1–41. [Google Scholar]
- De Piano, F.G.; Meroi Arcerito, F.R.; De Feudis, L.D.; Basilio, A.M.; Galetto, L.; Eguaras, M.J.; Maggi, M.D. Suplemento alimenticio en colonias de abejas para la mejora del servicio de polinización de kiwi (Actinidia deliciosa Liang and Ferguson) (Actinidiaceae: Theales). Rev. Soc. Entomol. Argent. 2021, 80, 31–36. [Google Scholar] [CrossRef]
- Abbate, A.P.; Campbell, J.W.; Vinson, E.L.; Williams, G.R. The pollination and fruit quality of two kiwifruit cultivars (Actinidia chinensis var. chinensis ‘AU Golden Sunshine’ and ‘AU Gulf Coast Gold’) (Ericales: Actinidiaceae) grown in the Southeastern United States. J. Econ. Entomol. 2021, 114, 1234–1241. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biometr. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Breheny, P.; Burchett, W. Visualization of regression models using visreg. R J. 2017, 9, 56–71. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 22 May 2025).
- Delignette-Muller, M.L.; Dutang, C. Fitdistrplus: An R package for fitting distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.4. 2021. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 13 July 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; pp. 189–201. [Google Scholar]
- Nayak, R.K.; Rana, K.; Sharma, H.K.; Singh, P.; Thakur, S.; Yankit, P. Foraging behavior of bumble bees (Bombus haemorrhoidalis Smith) and honey bees (Apis mellifera L.) on kiwifruit (Actinidia deliciosa Chev.). Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2043–2051. [Google Scholar] [CrossRef]
- Lee, K.Y.; Cho, H.; Yoon, H.J.; Lee, S.; Ko, H. Comparison of the foraging activity and pollination effects of honeybees (Apis mellifera L.) and bumblebees (Bombus terrestris L.) in gold kiwi (Actinidia chinensis P.‘Haegeum’). J. Apic. 2019, 34, 295–304. [Google Scholar] [CrossRef]

| Models Tested for Pollinators | p-Value |
|---|---|
| Dependent variable: Flower frequency | |
| Family Lognormal | |
| treatment+fsex | 0.001 |
| treatment | |
| null | |
| Dependent variable: Time of a visit | |
| Family Lognormal | |
| treatment+fsex | |
| treatment | 0.001 |
| null |
| Models Tested for Quality of Fruit | p-Value |
|---|---|
| Dependent variable: Weight (g) | |
| Family Gaussian | |
| treatment | 0.001 |
| null | |
| Dependent variable: # of seeds | |
| Family Negative Binomial | |
| treatment | 0.001 |
| null | |
| Dependent variable: FSI | |
| Family Gaussian | |
| treatment | 0.001 |
| null |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meroi Arcerito, F.R.; Mazzei, M.P.; Corti, C.; Lezcano, M.B.; Fernández de Landa, G.; Fernández de Landa, M.; Iglesias, A.E.; Ramos, F.; Fernández, N.J.; Damiani, N.; et al. Kiwifruit Cross-Pollination Analysis: Characterisation of the Pollinator-Assemblage and Practices to Enhance Fruit Quality. Plants 2025, 14, 2580. https://doi.org/10.3390/plants14162580
Meroi Arcerito FR, Mazzei MP, Corti C, Lezcano MB, Fernández de Landa G, Fernández de Landa M, Iglesias AE, Ramos F, Fernández NJ, Damiani N, et al. Kiwifruit Cross-Pollination Analysis: Characterisation of the Pollinator-Assemblage and Practices to Enhance Fruit Quality. Plants. 2025; 14(16):2580. https://doi.org/10.3390/plants14162580
Chicago/Turabian StyleMeroi Arcerito, Facundo René, Mariana Paola Mazzei, Camila Corti, María Belén Lezcano, Gregorio Fernández de Landa, Mateo Fernández de Landa, Azucena Elizabeth Iglesias, Facundo Ramos, Natalia Jorgelina Fernández, Natalia Damiani, and et al. 2025. "Kiwifruit Cross-Pollination Analysis: Characterisation of the Pollinator-Assemblage and Practices to Enhance Fruit Quality" Plants 14, no. 16: 2580. https://doi.org/10.3390/plants14162580
APA StyleMeroi Arcerito, F. R., Mazzei, M. P., Corti, C., Lezcano, M. B., Fernández de Landa, G., Fernández de Landa, M., Iglesias, A. E., Ramos, F., Fernández, N. J., Damiani, N., Gende, L. B., Porrini, D. P., Maggi, M. D., & Galetto, L. (2025). Kiwifruit Cross-Pollination Analysis: Characterisation of the Pollinator-Assemblage and Practices to Enhance Fruit Quality. Plants, 14(16), 2580. https://doi.org/10.3390/plants14162580






