Rice Heat Stress Response: Physiological Changes and Molecular Regulatory Network Research Progress
Abstract
1. Introduction
2. Impact of Heat Stress on the Agronomic Performance of Rice
2.1. Impact of Heat Stress on the Vegetative Growth Stage of Rice
2.2. Effects During the Reproductive Growth Stage
3. Physiological and Biochemical Alterations Induced by Heat Stress in Rice
3.1. Membrane Damage and ROS Accumulation
3.2. Photosynthetic Impairment
3.3. Energy Metabolism Imbalance
3.4. Hormonal Imbalance
4. The Cloning of Heat-Tolerance-Related Genes in Rice
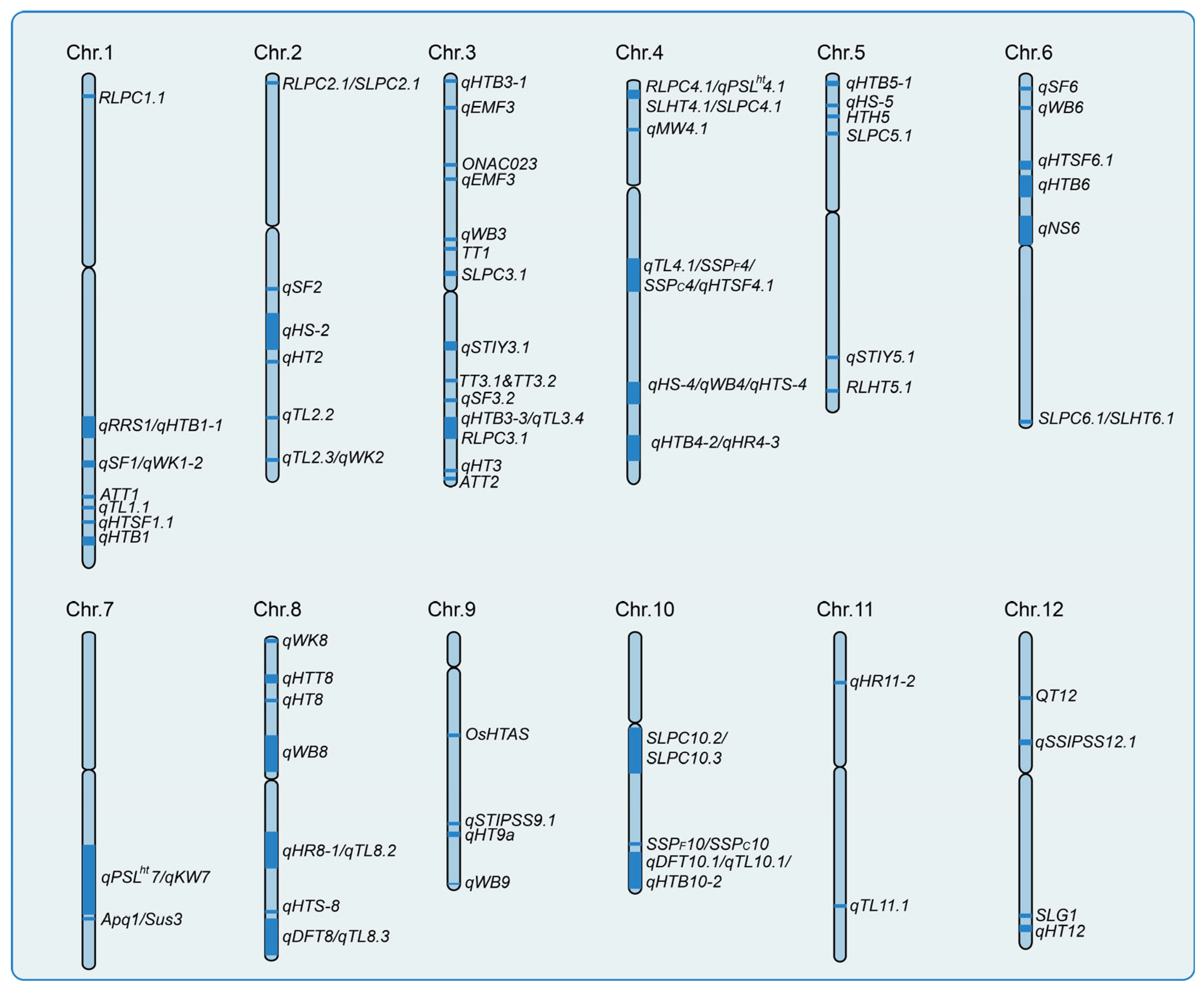
4.1. Identification of Heat Tolerance-Related QTLs in Rice
4.2. Molecular Cloning of Functional Genes Involved in Heat Tolerance in Rice
| Gene | Gene ID | Gene Characteristics | Function Period | Functional Pathway | Regulative Effect | References |
|---|---|---|---|---|---|---|
| ATT2 | Os03g0856700 | Gibberellin 20-oxidase gene | Seedling, reproductive stages | GA signaling | + | [76] |
| HTH5 | Os05g0150000 | Pyridoxal phosphate homeostasis protein | Seedling | Energy metabolism | + | [79] |
| OsMYB55 | Os05g0553400 | MYB transcription factor | Seedling | Amino acid metabolism | + | [96] |
| OsHSP1 | Os04g0107900 | Heat-stimulated protein | Seedling | Molecular chaperone | + | [97] |
| SNAC3 | Os07g0225300 | NAC transcription factor | Seedling | Antioxidant regulation | + | [98] |
| OsANN1 | Os02g0753800 | Membrane-bound protein | Seedling | Antioxidant defense | + | [99] |
| OsHTAS | Os09g0323100 | Ubiquitin ligase | Seedling | Stomatal closure | + | [100] |
| OsTOGR1 | Os03g0669000 | DEAD-box RNA helicase | Seedling | RNA helicase | + | [101] |
| OsRab7 | Os05g0516600 | Small GTPase | Seedling | Antioxidant pathways | + | [102] |
| OsHIRP1 | Os03g0302200 | E3 ubiquitin ligase | Seedling | Protein ubiquitination | + | [103] |
| OsCNGC14 | Os03g0758300 | Cyclic nucleotide-gated ion channel | Seedling | Calcium influx | + | [104] |
| OsCNGC16 | Os05g0502000 | Cyclic nucleotide-gated ion channel | Seedling | Calcium influx | + | [104] |
| OsNSUN2 | Os09g0471900 | m5C RNA methyltransferase | Seedling | RNA methylation | + | [105] |
| HTS1 | Os04g0376300 | β-ketolipoyl carrier protein reductase | Seedling | Fatty acid synthesis | + | [106] |
| TT3.1 | Os03g0706900 | E3 ubiquitin ligase | Seedling | Protein ubiquitination | + | [28,107] |
| OsSGS3 | Os12g0197500 | Gene silencing repressor | Seedling | Gene silencing | + | [108] |
| OsUGT72F1 | Os05g0215300 | UDP-glucosyltransferase | Seedling | Flavonoid metabolism | + | [109] |
| GS2 | Os02g0701300 | Growth-regulating factor | Seedling | Hydroxymethyl-glutathione synthetase | + | [110] |
| OsIAA7 | Os02g0228900 | Auxin-responsive Aux/IAA gene family member | Seedling | Auxin signaling | + | [111] |
| HTS2 | Os12g0268000 | Cytochrome P450 protein | Seedling | Serotonin biosynthesis | + | [112] |
| OsCAF1 | Os10g0412100 | Carbon catabolite repressor protein | Seedling | mRNA degradation | + | [113] |
| OsDUGT1 | Os01g0597800 | Glycosyltransferase | Seedling | Flavonoid glycosylation | + | [114] |
| OsPP91 | Os06g0698300 | Protein phosphatase | Seedling | Phosphatase activity | + | [115] |
| OsEIL5 | Os02g0574800 | Transcription factor | Seedling, reproductive stages | Gene activation | + | [115] |
| OsRGB1 | Os03g0669200 | G protein β subunit | Germination, seedling | Signaling pathways | + | [116] |
| OsGRP162 | Os12g0632000 | Glycine rich-RNA binding protein | Seedling, booting, fertilization | RNA binding | + | [117] |
| OsU2AF35a | Os09g0491756 | Splicing auxiliary factor | Seedling, reproductive stages | RNA splicing | + | [118] |
| TT1 | Os03g0387100 | α2 subunit of the 26S proteasome | Seedling, flowering, filling | Protein degradation | + | [119] |
| HSP24.1 | Os02g0758000 | Heat-stimulated protein | Seedling, Flowering, filling | Molecular chaperone | + | [119] |
| ERECTA | Os06g0203800 | Receptor kinase | Seedling, flowering | Signaling pathways | + | [120] |
| SLG1 | Os12g0588900 | Cytoplasmic tRNA 2-thiolated protein | Seedling, flowering | tRNA modification | + | [121] |
| OsNCED1 | Os02g0704000 | 9-cis-Epoxy carotenoid dioxygenase | Flowering | ABA biosynthesis | + | [122] |
| Sus3 | Os07g0616800 | Sucrose synthase | Filling | Sucrose biosynthesis | + | [123] |
| OsGRP3 | Os03g0670700 | Glycine-rich RNA-binding protein | Seedling, booting, fertilization | RNA binding | + | [117,124] |
| OsPRMT6b | Os04g0677066 | Protein arginine methyltransferase | Seed gemination, growth promotion | ABA receptor degradation | + | [125] |
| OsNTL3 | Os01g0261200 | NAC transcription factor | Seedling | ER folding | + | [126] |
| ATT1 | Os01g0883800 | Gibberellin 20-oxidase gene | Seedling, reproductive stages | GA signaling | − | [76] |
| TT3.2 | Os03g0707200 | Chloroplast precursor protein | Seedling | Chloroplast protection | − | [28,107] |
| OsARF6 | Os02g0164900 | Auxin response factor | Seedling | Auxin signaling | − | [111] |
| OsEBF1 | Os06g0605900 | F box protein | Seedling, reproductive stages | Protein degradation | − | [115] |
| SCE1 | Os10g0536000 | SUMO-conjugating enzyme E2 | Seedling, flowering, filling | Protein SUMOylation | − | [119] |
| OsFBN1 | Os09g0133600 | Ciliary protein | Seedling, flowering, fertilization | Lipid remodeling | − | [127] |
| OsMDHAR4 | Os02g0707100 | Monodehydroascorbate reductase | Seedling | ROS scavenging | − | [128] |
| OsUBP21 | Os11g0573000 | Ubiquitin-specific protease | Seedling | Ubiquitin removal | − | [129] |
| NAT1 | Os07g0590100 | C2H2 family transcription factor | Seedling, reproductive stages | Wax deposition | − | [130] |
| HTT1 | Os08g0200100 | Stearoyl-acyl carrier protein | Seedling | Lipid saturation | − | [131] |
| OsRbohB | Os09g0438000 | NADPH oxidase | Seedling | ROS accumulation | − | [132] |
| TT2 | Os03g0407400 | G protein γ subunit | Seedling, flowering | Wax biosynthesis | − | [133] |
| QT12 | Os12g0173366 | Protein transport protein Sec61 subunit beta | Grain quality and yield | ER stress | − | [134] |
| NF-YA8 | Os10g0397900 | Nuclear transcription factor | Grain quality and yield | Gene transcription | − | [134] |
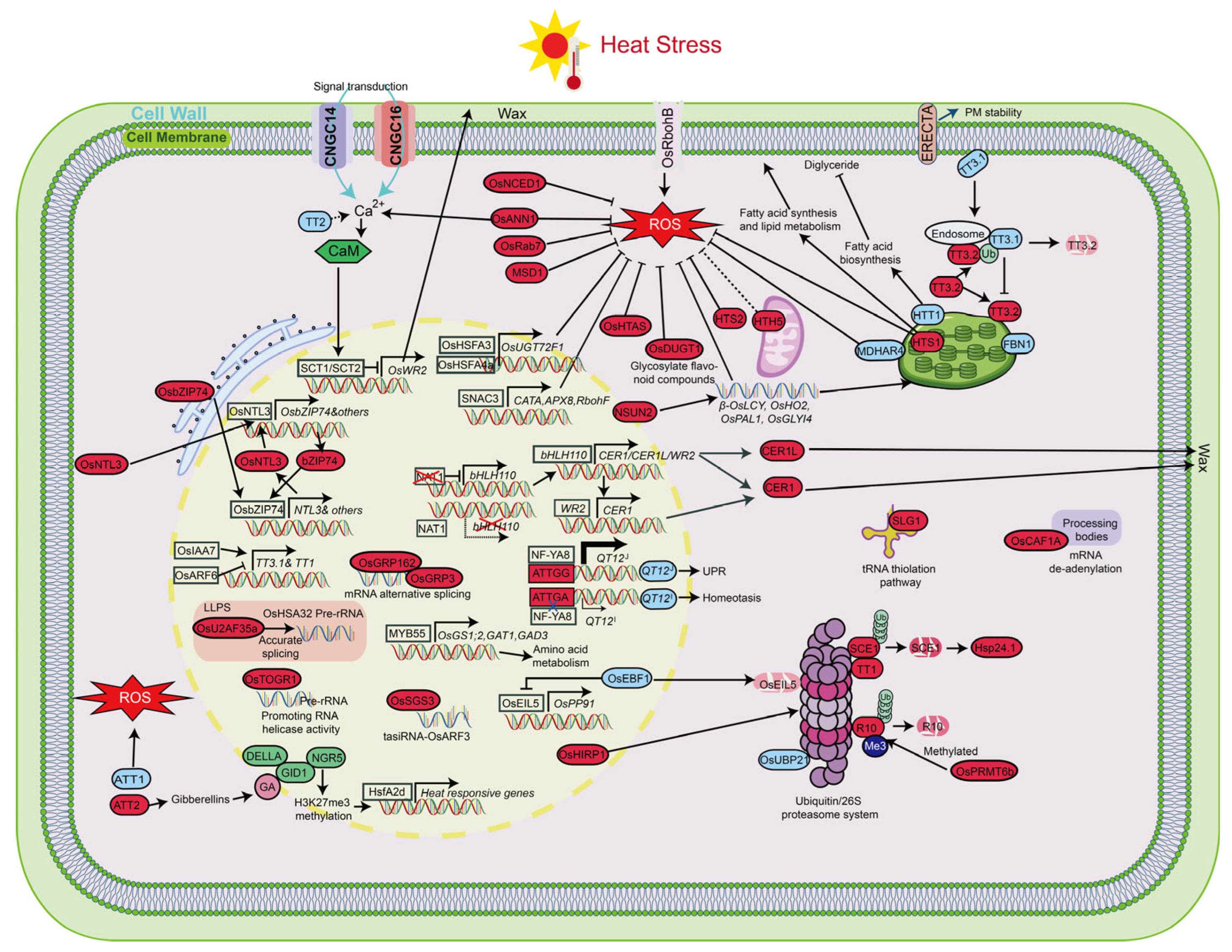
5. Molecular Regulatory Networks of Heat Stress Tolerance in Rice
5.1. Heat Stress Sensing and Plasma Membrane Integrity
5.2. ROS Accumulation and Antioxidant Regulation
5.3. Photosynthetic and Chloroplast Regulatory Mechanisms
5.4. Protein Homeostasis Regulation
5.5. Regulatory Network of Transcription Factors
5.6. RNA Metabolism Regulation and Response Mechanisms
6. Conclusions and Future Directions
6.1. Breeding Strategies Utilizing Existing Resources
6.2. Translational Challenges in Breeding Heat-Tolerant Rice
6.3. Future Research Directions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, R.; Dong, S.; Han, Z.; Li, W. Increased exposure of rice to compound drought and hot extreme events during its growing seasons in China. Ecol. Indic. 2024, 167, 112735. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Lyman, N.B.; Jagadish, K.S.; Nalley, L.L.; Dixon, B.L.; Siebenmorgen, T. Neglecting rice milling yield and quality underestimates economic losses from high-temperature stress. PLoS ONE 2013, 8, e72157. [Google Scholar] [CrossRef]
- Seth, P.; Sebastian, J. Plants and global warming: Challenges and strategies for a warming world. Plant Cell Rep. 2024, 43, 27. [Google Scholar] [CrossRef]
- Jeong, D.H.; Park, S.; Zhai, J.; Gurazada, S.G.; De Paoli, E.; Meyers, B.C.; Green, P.J. Massive analysis of rice small RNAs: Mechanistic implications of regulated microRNAs and variants for differential target RNA cleavage. Plant Cell 2011, 23, 4185–4207. [Google Scholar] [CrossRef]
- Bahuguna, R.N.; Jha, J.; Pal, M.; Shah, D.; Lawas, L.M.; Khetarpal, S.; Jagadish, K.S. Physiological and biochemical characterization of NERICA-L-44: A novel source of heat tolerance at the vegetative and reproductive stages in rice. Physiol. Plant. 2015, 154, 543–559. [Google Scholar] [CrossRef]
- Liu, J.; Hasanuzzaman, M.; Wen, H.; Zhang, J.; Peng, T.; Sun, H.; Zhao, Q. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef]
- Resentini, F.; Orozco-Arroyo, G.; Cucinotta, M.; Mendes, M.A. The impact of heat stress in plant reproduction. Front. Plant Sci. 2023, 14, 12. [Google Scholar] [CrossRef]
- Aryan, S.; Gulab, G.; Habibi, N.; Kakar, K.; Sadat, M.I.; Zahid, T.; Rashid, R.A. Phenological and physiological responses of hybrid rice under different high-temperature at seedling stage. Bull. Natl. Res. Cent. 2022, 46, 45. [Google Scholar] [CrossRef]
- Ahmad, S.; Tabassum, J.; Sheng, Z.; Lv, Y.; Chen, W.; Zeb, A.; Dong, N.; Ali, U.; Shao, G.; Wei, X.; et al. Loss-of-function of PGL10 impairs photosynthesis and tolerance to high-temperature stress in rice. Physiol. Plant. 2024, 176, e14369. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Huang, R.; Wai, H.P.; Xiong, H.; Shen, X.; He, H.; Yan, S. Mapping quantitative trait loci for heat tolerance at the booting stage using chromosomal segment substitution lines in rice. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2017, 23, 817–825. [Google Scholar] [CrossRef]
- Matsui, T.; Hasegawa, T. Effect of long anther dehiscence on seed set at high temperatures during flowering in rice (Oryza sativa L.). Sci. Rep. 2019, 9, 20363. [Google Scholar] [CrossRef]
- Sakai, H.; Cheng, W.; Chen, C.P.; Hasegawa, T.J.A.; Meteorology, F. Short-term high nighttime temperatures pose an emerging risk to rice grain failure. Agric. For. Meteorol. 2022, 314, 108779. [Google Scholar] [CrossRef]
- Wu, C.; Cui, K.; Fahad, S. Heat Stress Decreases Rice Grain Weight: Evidence and Physiological Mechanisms of Heat Effects Prior to Flowering. Int. J. Mol. Sci. 2022, 23, 10922. [Google Scholar] [CrossRef]
- Raza, Q.; Riaz, A.; Bashir, K.; Sabar, M. Reproductive tissues-specific meta-QTLs and candidate genes for development of heat-tolerant rice cultivars. Plant Mol. Biol. 2020, 104, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, S.V.; Bahuguna, R.N.; Djanaguiraman, M.; Gamuyao, R.; Prasad, P.V.; Craufurd, P.Q. Implications of high temperature and elevated CO2 on flowering time in plants. Front Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Matsui, T.; Omasa, K. Rice (Oryza sativa L.) cultivars tolerant to high temperature at flowering: Anther characteristics. Ann. Bot. 2002, 89, 683–687. [Google Scholar] [CrossRef]
- Shi, W.; Yang, J.; Kumar, R.; Zhang, X.; Impa, S.M.; Xiao, G.; Jagadish, S.V.K. Heat Stress During Gametogenesis Irreversibly Damages Female Reproductive Organ in Rice. Rice 2022, 15, 32. [Google Scholar] [CrossRef]
- Takeoka, Y.; Hiroi, K.; Kitano, H.; Wada, T.J.S.P.R. Pistil hyperplasia in rice spikelets as affected by heat stress. Sex. Plant Reprod. 1991, 4, 39–43. [Google Scholar] [CrossRef]
- Lin, G.; Yang, Y.; Chen, X.; Yu, X.; Wu, Y.; Xiong, F. Effects of high temperature during two growth stages on caryopsis development and physicochemical properties of starch in rice. Int. J. Biol. Macromol. 2020, 145, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Wu, J.; Luo, Q.; Li, J.; Zhuang, W.; Xiao, G.; Deng, Q.; Lei, D.; Bai, B. Influence of high natural field temperature during grain filling stage on the morphological structure and physicochemical properties of rice (Oryza sativa L.) starch. Food Chem. 2020, 310, 125817. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zeng, B.; Zhao, J.; Yan, S.; Wan, J.; Cao, Z. Genetic research progress: Heat tolerance in rice. Int. J. Mol. Sci. 2023, 24, 7140. [Google Scholar] [CrossRef] [PubMed]
- Finka, A.; Cuendet, A.F.; Maathuis, F.J.; Saidi, Y.; Goloubinoff, P. Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired heat tolerance. Plant Cell 2012, 24, 3333–3348. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Zou, Y.; Liu, C.; Liu, Y.; Wang, Y.; Zhang, W. Over-expression of mitochondrial heat shock protein 70 suppresses programmed cell death in rice. FEBS Lett. 2011, 585, 231–239. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, L.; Liu, J.; Du, X.; Asad, M.A.; Huang, F.; Pan, G.; Cheng, F. Relationship of ROS accumulation and superoxide dismutase isozymes in developing anther with floret fertility of rice under heat stress. Plant Physiol. Biochem. 2018, 122, 90–101. [Google Scholar] [CrossRef]
- Sailaja, B.; Subrahmanyam, D.; Neelamraju, S.; Vishnukiran, T.; Rao, Y.V.; Vijayalakshmi, P.; Voleti, S.R.; Bhadana, V.P.; Mangrauthia, S.K. Integrated physiological, biochemical, and molecular analysis identifies important traits and mechanisms associated with differential response of rice genotypes to elevated temperature. Front. Plant Sci. 2015, 6, 1044. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, L.; Liu, J.; Cao, Z.; Du, X.; Huang, F.; Pan, G.; Cheng, F. Involvement of CAT in the detoxification of HT-induced ROS burst in rice anther and its relation to pollen fertility. Plant Cell Rep. 2018, 37, 741–757. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, J.F.; Kan, Y.; Shan, J.X.; Ye, W.W.; Dong, N.Q.; Guo, T.; Xiang, Y.H.; Yang, Y.B.; Li, Y.C.; et al. A genetic module at one locus in rice protects chloroplasts to enhance heat tolerance. Science 2022, 376, 1293–1300. [Google Scholar] [CrossRef]
- Maruta, T.; Noshi, M.; Tanouchi, A.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J. Biol. Chem. 2012, 287, 11717–11729. [Google Scholar] [CrossRef] [PubMed]
- Hüve, K.; Bichele, I.; Rasulov, B.; Niinemets, U. When it is too hot for photosynthesis: Heat-induced instability of photosynthesis in relation to respiratory burst, cell permeability changes and H2O2 formation. Plant Cell Environ. 2011, 34, 113–126. [Google Scholar] [CrossRef]
- Wang, Q.L.; Chen, J.H.; He, N.Y.; Guo, F.Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef]
- Wang, X.; Xu, C.; Cai, X.; Wang, Q.; Dai, S. Heat-responsive photosynthetic and signaling pathways in plants: Insight from proteomics. Int. J. Mol. Sci. 2017, 18, 2191. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.F.; Zhou, Z.J.; Feng, X.P.; Yang, W.J.; Jiang, D.A. Two rubisco activase isoforms may play different roles in photosynthetic heat acclimation in the rice plant. Physiol. Plant. 2010, 139, 55–67. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.; Li, Y.; Tan, Y.; Kong, J.; Li, H.; Li, Y.; Zhu, Y. Overexpression of SBPase enhances photosynthesis against high temperature stress in transgenic rice plants. Plant Cell Rep. 2007, 26, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Qin-Di, D.; Gui-Hua, J.; Xiu-Neng, W.; Zun-Guang, M.; Qing-Yong, P.; Shiyun, C.; Yu-Jian, M.; Shuang-Xi, Z.; Yong-Xiang, H.; Yu, L. High temperature-mediated disturbance of carbohydrate metabolism and gene expressional regulation in rice: A review. Plant Signal. Behav. 2021, 16, 1862564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rerksiri, W.; Liu, A.; Zhou, X.; Xiong, H.; Xiang, J.; Chen, X.; Xiong, X. Transcriptome profile reveals heat response mechanism at molecular and metabolic levels in rice flag leaf. Gene 2013, 530, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Hakata, M. Atlas of rice grain filling-related metabolism under high temperature: Joint analysis of metabolome and transcriptome demonstrated inhibition of starch accumulation and induction of amino acid accumulation. Plant Cell Physiol. 2010, 51, 795–809. [Google Scholar] [CrossRef]
- Glaubitz, U.; Erban, A.; Kopka, J.; Hincha, D.K.; Zuther, E. High night temperature strongly impacts TCA cycle, amino acid and polyamine biosynthetic pathways in rice in a sensitivity-dependent manner. J. Exp. Bot. 2015, 66, 6385–6397. [Google Scholar] [CrossRef]
- Zhao, C.; Xie, J.; Li, L.; Cao, C. Comparative Transcriptomic Analysis in Paddy Rice under Storage and Identification of Differentially Regulated Genes in Response to High Temperature and Humidity. J. Agric. Food Chem. 2017, 65, 8145–8153. [Google Scholar] [CrossRef]
- Liao, J.L.; Zhou, H.W.; Zhang, H.Y.; Zhong, P.A.; Huang, Y.J. Comparative proteomic analysis of differentially expressed proteins in the early milky stage of rice grains during high temperature stress. J. Exp. Bot. 2014, 65, 655–671. [Google Scholar] [CrossRef]
- Wang, Z.M.; Li, H.X.; Liu, X.F.; He, Y.; Zeng, H.L. Reduction of pyruvate orthophosphate dikinase activity is associated with high temperature-induced chalkiness in rice grains. Plant Physiol. Biochem. PPB 2015, 89, 76–84. [Google Scholar] [CrossRef]
- Liao, J.L.; Zhou, H.W.; Peng, Q.; Zhong, P.A.; Zhang, H.Y.; He, C.; Huang, Y.J. Transcriptome changes in rice (Oryza sativa L.) in response to high night temperature stress at the early milky stage. BMC Genom. 2015, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Nakata, M.; Fukamatsu, Y.; Miyashita, T.; Hakata, M.; Kimura, R.; Nakata, Y.; Kuroda, M.; Yamaguchi, T.; Yamakawa, H. High temperature-induced expression of rice α-amylases in developing endosperm produces chalky grains. Front. Plant Sci. 2017, 8, 2089. [Google Scholar] [CrossRef]
- Yamashita, A.; Nijo, N.; Pospísil, P.; Morita, N.; Takenaka, D.; Aminaka, R.; Yamamoto, Y.; Yamamoto, Y. Quality control of photosystem II: Reactive oxygen species are responsible for the damage to photosystem II under moderate heat stress. J. Biol. Chem. 2008, 283, 28380–28391. [Google Scholar] [CrossRef] [PubMed]
- Shiraya, T.; Mori, T.; Maruyama, T.; Sasaki, M.; Takamatsu, T.; Oikawa, K.; Itoh, K.; Kaneko, K.; Ichikawa, H.; Mitsui, T. Golgi/plastid-type manganese superoxide dismutase involved in heat-stress tolerance during grain filling of rice. Plant Biotechnol. J. 2015, 13, 1251–1263. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef]
- Gautam, H.; Fatma, M.; Sehar, Z.; Iqbal, N.; Albaqami, M.; Khan, N.A. Exogenously-sourced ethylene positively modulates photosynthesis, carbohydrate metabolism, and antioxidant defense to enhance heat tolerance in rice. Int. J. Mol. Sci. 2022, 23, 1031. [Google Scholar] [CrossRef]
- Iqbal, N.; Nazar, R.; Syeed, S.; Masood, A.; Khan, N.A. Exogenously-sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. J. Exp. Bot. 2011, 62, 4955–4963. [Google Scholar] [CrossRef]
- Khan, M.I.; Khan, N.A. Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and antioxidant metabolism. Protoplasma 2014, 251, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, X.; Bürger, M.; Chory, J.; Wang, X. The role of ethylene in plant temperature stress response. Trends Plant Sci. 2023, 28, 808–824. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.S.; Yi, C.Y.; Wang, F.; Zhou, J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Hydrogen peroxide mediates abscisic acid-induced HSP70 accumulation and heat tolerance in grafted cucumber plants. Plant Cell Environ. 2014, 37, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A.; Inupakutika, M.A.; Mittler, R. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J. Exp. Bot. 2016, 67, 5381–5390. [Google Scholar] [CrossRef]
- Rezaul, I.M.; Baohua, F.; Tingting, C.; Weimeng, F.; Caixia, Z.; Longxing, T.; Guanfu, F. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol. Plant. 2019, 165, 644–663. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.J.; Chen, S.Z. Abscisic acid-induced heat tolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J. Plant Physiol. 1998, 153, 488–496. [Google Scholar] [CrossRef]
- Islam, M.R.; Feng, B.; Chen, T.; Tao, L.; Fu, G. Role of abscisic acid in thermal acclimation of plants. J. Plant Biol. 2018, 61, 255–264. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Y.; Sprague, S.A.; Kakeshpour, T.; Park, J.; Nakata, P.A.; Cheng, N.; Hirschi, K.D.; White, F.F.; Park, S. Expression of a monothiol glutaredoxin, AtGRXS17, in tomato (Solanum lycopersicum) enhances drought tolerance. Biochem. Biophys. Res. Commun. 2017, 491, 1034–1039. [Google Scholar] [CrossRef]
- Zhao, Q.; Guan, X.; Zhou, L.; Asad, M.A.; Xu, Y.; Pan, G.; Cheng, F. ABA-triggered ROS burst in rice developing anthers is critical for tapetal programmed cell death induction and heat stress-induced pollen abortion. Plant Cell Environ. 2023, 46, 1453–1471. [Google Scholar] [CrossRef]
- Qin, P.; Zhang, G.; Hu, B.; Wu, J.; Chen, W.; Ren, Z.; Liu, Y.; Xie, J.; Yuan, H.; Tu, B.; et al. Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci. Adv. 2021, 7, eabc8873. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C.; Zhang, G.; Fu, W.; Feng, B.; Chen, T.; Peng, S.; Tao, L.; Fu, G. Abscisic Acid Negatively Modulates Heat Tolerance in Rolled Leaf Rice by Increasing Leaf Temperature and Regulating Energy Homeostasis. Rice 2020, 13, 18. [Google Scholar] [CrossRef]
- Sakata, T.; Oshino, T.; Miura, S.; Tomabechi, M.; Tsunaga, Y.; Higashitani, N.; Miyazawa, Y.; Takahashi, H.; Watanabe, M.; Higashitani, A. Auxins reverse plant male sterility caused by high temperatures. Proc. Natl. Acad. Sci. USA 2010, 107, 8569–8574. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, J. Free IAA in stigmas and styles during pollen germination and pollen tube growth of Nicotiana tabacum. Physiol. Plant. 2008, 134, 202–215. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, B.; Moreno, I.; Dupláková, N.; Simon, S.; Carraro, N.; Reemmer, J.; Pěnčík, A.; Chen, X.; Tejos, R.; et al. ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat. Commun. 2012, 3, 941. [Google Scholar] [CrossRef]
- Sun, J.; Qi, L.; Li, Y.; Chu, J.; Li, C. PIF4-mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating arabidopsis hypocotyl growth. PLoS Genet. 2012, 8, e1002594. [Google Scholar] [CrossRef]
- Goda, H.; Sasaki, E.; Akiyama, K.; Maruyama-Nakashita, A.; Nakabayashi, K.; Li, W.; Ogawa, M.; Yamauchi, Y.; Preston, J.; Aoki, K.; et al. The AtGenExpress hormone and chemical treatment data set: Experimental design, data evaluation, model data analysis and data access. Plant J. Cell Mol. Biol. 2008, 55, 526–542. [Google Scholar] [CrossRef]
- Zheng, Z.; Guo, Y.; Novák, O.; Chen, W.; Ljung, K.; Noel, J.P.; Chory, J. Local auxin metabolism regulates environment-induced hypocotyl elongation. Nat. Plants 2016, 2, 16025. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, B.; Chen, T.; Zhang, X.; Tao, L.; Fu, G. Sugars, antioxidant enzymes and IAA mediate salicylic acid to prevent rice spikelet degeneration caused by heat stress. Plant Growth Regul. 2017, 83, 313–323. [Google Scholar] [CrossRef]
- Soeno, K.; Goda, H.; Ishii, T.; Ogura, T.; Tachikawa, T.; Sasaki, E.; Yoshida, S.; Fujioka, S.; Asami, T.; Shimada, Y. Auxin biosynthesis inhibitors, identified by a genomics-based approach, provide insights into auxin biosynthesis. Plant Cell Physiol. 2010, 51, 524–536. [Google Scholar] [CrossRef]
- Zhang, C.; Li, G.; Chen, T.; Feng, B.; Fu, W.; Yan, J.; Islam, M.R.; Jin, Q.; Tao, L.; Fu, G. Heat stress induces spikelet sterility in rice at anthesis through inhibition of pollen tube elongation interfering with auxin homeostasis in pollinated pistils. Rice 2018, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cui, K.; Wang, W.; Li, Q.; Fahad, S.; Hu, Q.; Huang, J.; Nie, L.; Peng, S. Heat-induced phytohormone changes are associated with disrupted early reproductive development and reduced yield in rice. Sci. Rep. 2016, 6, 34978. [Google Scholar] [CrossRef]
- Kudo, T.; Kiba, T.; Sakakibara, H. Metabolism and long-distance translocation of cytokinins. J. Integr. Plant Biol. 2010, 52, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cui, K.; Wang, W.; Li, Q.; Fahad, S.; Hu, Q.; Huang, J.; Nie, L.; Mohapatra, P.K.; Peng, S. Heat-Induced Cytokinin Transportation and Degradation Are Associated with Reduced Panicle Cytokinin Expression and Fewer Spikelets per Panicle in Rice. Front. Plant Sci. 2017, 8, 371. [Google Scholar] [CrossRef]
- Ha, S.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. Cytokinins: Metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef]
- Wu, C.; Hong, C. An in vivo GA- and ABA-responsive dual-luciferase reporter system for simultaneous detection of GA and ABA responses, hormone crosstalk, and heat-stress response in rice. Plant Biotechnol. J. 2021, 19, 1486–1488. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, C.; Chen, T.; Zhang, X.; Tao, L.; Fu, G. Salicylic acid reverses pollen abortion of rice caused by heat stress. BMC Plant Biol. 2018, 18, 245. [Google Scholar] [CrossRef]
- Pantoja-Benavides, A.D.; Garces-Varon, G.; Restrepo-Díaz, H. Foliar cytokinins or brassinosteroids applications influence the rice plant acclimatization to combined heat stress. Front. Plant Sci. 2022, 13, 983276. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.Q.; Chen, Y.X.; Ju, Y.L.; Pan, C.Y.; Shan, J.X.; Ye, W.W.; Dong, N.Q.; Kan, Y.; Yang, Y.B.; Zhao, H.Y.; et al. Fine-tuning gibberellin improves rice alkali-thermal tolerance and yield. Nature 2025, 639, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, W.; Li, J.; Wei, Y.; Qing, D.; Huang, J.; Yang, X.; Tang, M.; Zhang, Z.; Yu, J.; et al. Identifying Heat Adaptability QTLs and Candidate Genes for Grain Appearance Quality at the Flowering Stage in Rice. Rice 2025, 18, 13. [Google Scholar] [CrossRef]
- Ravikiran, K.T.; Gopala Krishnan, S.; Abhijith, K.P.; Bollinedi, H.; Nagarajan, M.; Vinod, K.K.; Bhowmick, P.K.; Pal, M.; Ellur, R.K.; Singh, A.K. Genome-Wide Association Mapping Reveals Novel Putative Gene Candidates Governing Reproductive Stage Heat Stress Tolerance in Rice. Front. Genet. 2022, 13, 876522. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Tang, H.; Cai, Y.; Zeng, B.; Zhao, J.; Tang, X.; Lu, M.; Wang, H.; Zhu, X.; Wu, X.; et al. Natural variation of HTH5 from wild rice, Oryza rufipogon Griff., is involved in conferring high-temperature tolerance at the heading stage. Plant Biotechnol. J. 2022, 20, 1591–1605. [Google Scholar] [CrossRef]
- Zhibin, C.; Xiuying, T.; Weijun, X.; Linghua, M.; Yuanyuan, N.; Yonghui, L.; Hongwei, X.; Yaohui, C. Identification and genetic effect analysis of QTL(qhth10) for heat tolerance at heading and flowering stage of rice. Mol. Plant Breed. 2019, 17, 2223–2230. [Google Scholar]
- Cao, Z.; Li, Y.; Tang, H.; Zeng, B.; Tang, X.; Long, Q.; Wu, X.; Cai, Y.; Yuan, L.; Wan, J. Fine mapping of the qHTB1-1QTL, which confers heat tolerance at the booting stage, using an Oryza rufipogon Griff. introgression line. TAG Theor. Appl. Genet. Theor. Und Angew. Genet. 2020, 133, 1161–1175. [Google Scholar] [CrossRef]
- Ye, C.; Argayoso, M.A.; Redoña, E.D.; Sierra, S.N.; Laza, M.A.; Dilla, C.J.; Mo, Y.; Thomson, M.J.; Chin, J.; Delavi, A. Mapping QTL for heat tolerance at flowering stage in rice using SNP markers. Plant Breed. 2012, 131, 33–41. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Tang, M.; Zhang, X.; Pan, Y.; Yang, X.; Gao, G.; Lv, R.; Tao, W.; Jiang, L.; et al. QTL Mapping and Identification of Candidate Genes for Heat Tolerance at the Flowering Stage in Rice. Front. Genet. 2020, 11, 621871. [Google Scholar] [CrossRef]
- Zhao, L.; Lei, J.; Huang, Y.; Zhu, S.; Chen, H.; Huang, R.; Peng, Z.; Tu, Q.; Shen, X.; Yan, S. Mapping quantitative trait loci for heat tolerance at anthesis in rice using chromosomal segment substitution lines. Breed. Sci. 2016, 66, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, T.; Yu, T.; Zhang, S.; Mao, X.; Zhao, J.; Wang, X.; Dong, J.; Liu, B. Integrating Small RNA Sequencing with QTL Mapping for Identification of miRNAs and Their Target Genes Associated with Heat Tolerance at the Flowering Stage in Rice. Front. Plant Sci. 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Shen, S.; Cheng, M.; Chen, Q. Identification of QTLs for Heat Tolerance at the Flowering Stage Using Chromosome Segment Substitution Lines in Rice. Genes 2022, 13, 2248. [Google Scholar] [CrossRef] [PubMed]
- Nubankoh, P.; Wanchana, S.; Saensuk, C.; Ruanjaichon, V.; Cheabu, S.; Vanavichit, A.; Toojinda, T.; Malumpong, C.; Arikit, S. QTL-seq reveals genomic regions associated with spikelet fertility in response to a high temperature in rice (Oryza sativa L.). Plant Cell Rep. 2020, 39, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.M.S.; Akkareddy, S.; Shanthi, P.; Latha, P. Identification of differentially expressed genes under heat stress conditions in rice (Oryza sativa L.). Mol. Biol. Rep. 2020, 47, 1935–1948. [Google Scholar] [CrossRef]
- Li, P.; Jiang, J.; Zhang, G.; Miao, S.; Lu, J.; Qian, Y.; Zhao, X.; Wang, W.; Qiu, X.; Zhang, F.; et al. Integrating GWAS and transcriptomics to identify candidate genes conferring heat tolerance in rice. Front. Plant Sci. 2022, 13, 1102938. [Google Scholar] [CrossRef]
- Stephen, K.; Aparna, K.; Beena, R.; Sah, R.P.; Jha, U.C.; Behera, S. Identification of simple sequence repeat markers linked to heat tolerance in rice using bulked segregant analysis in F(2) population of NERICA-L 44 × Uma. Front. Plant Sci. 2023, 14, 1113838. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, C.F.; Zhou, L.H.; Lin, J.; Zhao, Q.Y.; Zhu, Z.; Chen, T.; Yao, S.; Hasegawa, T.; Matsui, T.J.E. QTL mapping of dehiscence length at the basal part of thecae related to heat tolerance of rice (Oryza sativa L.). Euphytica 2016, 209, 715–723. [Google Scholar] [CrossRef]
- Hirabayashi, H.; Sasaki, K.; Kambe, T.; Gannaban, R.B.; Miras, M.A.; Mendioro, M.S.; Simon, E.V.; Lumanglas, P.D.; Fujita, D.; Takemoto-Kuno, Y.; et al. qEMF3, a novel QTL for the early-morning flowering trait from wild rice, Oryza officinalis, to mitigate heat stress damage at flowering in rice, O. sativa. J. Exp. Bot. 2015, 66, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Jiang, J.; Li, Y.; Song, S.; Zou, Y.; Jing, C.; Zhang, Y.; Wang, D.; He, Q.; Dang, X. QTL mapping and identification of candidate genes using a genome-wide association study for heat tolerance at anthesis in rice (Oryza sativa L.). Front. Genet. 2022, 13, 983525. [Google Scholar] [CrossRef]
- Murata, K.; Iyama, Y.; Yamaguchi, T.; Ozaki, H.; Kidani, Y.; Ebitani, T. Identification of a novel gene (Apq1) from the indica rice cultivar ‘Habataki’ that improves the quality of grains produced under high temperature stress. Breed. Sci. 2014, 64, 273–281. [Google Scholar] [CrossRef]
- Kobayashi, A.; Sonoda, J.; Sugimoto, K.; Kondo, M.; Iwasawa, N.; Hayashi, T.; Tomita, K.; Yano, M.; Shimizu, T. Detection and verification of QTLs associated with heat-induced quality decline of rice (Oryza sativa L.) using recombinant inbred lines and near-isogenic lines. Breed. Sci. 2013, 63, 339–346. [Google Scholar] [CrossRef]
- El-Kereamy, A.; Bi, Y.M.; Ranathunge, K.; Beatty, P.H.; Good, A.G.; Rothstein, S.J. The rice R2R3-MYB transcription factor OsMYB55 is involved in the tolerance to high temperature and modulates amino acid metabolism. PLoS ONE 2012, 7, e52030. [Google Scholar] [CrossRef]
- Moon, J.-C.; Ham, D.J.; Hwang, S.-G.; Park, Y.C.; Lee, C. Molecular characterization of a heat inducible rice gene, OsHSP1, and implications for rice heat tolerance. Genes Genom. 2013, 36, 151–161. [Google Scholar] [CrossRef]
- Fang, Y.; Liao, K.; Du, H.; Xu, Y.; Song, H.; Li, X.; Xiong, L. A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J. Exp. Bot. 2015, 66, 6803–6817. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Zhang, Q.; Liu, D.; Wang, H.; Yin, J.; Wang, R.; He, M.; Cui, M.; Shang, Z.; Wang, D.; et al. A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J. Exp. Bot. 2015, 66, 5853–5866. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Wei, C.; Liu, X.; Wang, M.; Yu, F.; Xie, Q.; Tu, J. The RING Finger Ubiquitin E3 Ligase OsHTAS Enhances Heat Tolerance by Promoting H2O2-Induced Stomatal Closure in Rice. Plant Physiol. 2016, 170, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Yarra, R.; Xue, Y. Ectopic expression of nucleolar DEAD-Box RNA helicase OsTOGR1 confers improved heat stress tolerance in transgenic Chinese cabbage. Plant Cell Rep. 2020, 39, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of Rice Rab7 Gene Improves Drought and Heat Tolerance and Increases Grain Yield in Rice (Oryza sativa L.). Genes 2019, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lim, S.D.; Jang, C.S. Oryza sativa heat-induced RING finger protein 1 (OsHIRP1) positively regulates plant response to heat stress. Plant Mol. Biol. 2019, 99, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Lu, S.; Li, Z.; Cheng, J.; Hu, P.; Zhu, T.; Wang, X.; Jin, M.; Wang, X.; Li, L.; et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 Promote Tolerance to Heat and Chilling in Rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, C.C.; Gao, Y.; Yang, Y.; Shi, B.; Yu, J.L.; Lyu, C.; Sun, B.F.; Wang, H.L.; Xu, Y.; et al. OsNSUN2-Mediated 5-Methylcytosine mRNA Modification Enhances Rice Adaptation to High Temperature. Dev. Cell 2020, 53, 272–286.e277. [Google Scholar] [CrossRef]
- Chen, F.; Dong, G.; Wang, F.; Shi, Y.; Zhu, J.; Zhang, Y.; Ruan, B.; Wu, Y.; Feng, X.; Zhao, C.; et al. A β-ketoacyl carrier protein reductase confers heat tolerance via the regulation of fatty acid biosynthesis and stress signaling in rice. New Phytol. 2021, 232, 655–672. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, J.X. TT3.1: A journey to protect chloroplasts upon heat stress. Stress Biol. 2022, 2, 27. [Google Scholar] [CrossRef]
- Gu, X.; Si, F.; Feng, Z.; Li, S.; Liang, D.; Yang, P.; Yang, C.; Yan, B.; Tang, J.; Yang, Y.; et al. The OsSGS3-tasiRNA-OsARF3 module orchestrates abiotic-biotic stress response trade-off in rice. Nat. Commun. 2023, 14, 4441. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, S.; Ma, X.; Dong, G.; Liu, C.; Ding, Y.; Hou, B. A high temperature responsive UDP-glucosyltransferase gene OsUGT72F1 enhances heat tolerance in rice and Arabidopsis. Plant Cell Rep. 2025, 44, 48. [Google Scholar] [CrossRef]
- Li, G.; Wang, H.; Li, H.; Feng, B.; Fu, W.; Ma, J.; Li, J.; Wu, Z.; Islam, M.R.; Chen, T.; et al. GRAIN SIZE ON CHROMOSOME 2 orchestrates phytohormone, sugar signaling and energy metabolism to confer thermal resistance in rice. Physiol. Plant. 2025, 177, e70113. [Google Scholar] [CrossRef]
- Qiu, R.; Yao, P.; Yang, J.; Hou, J.; Xiao, H.; Wu, Y.; Tu, D.; Ma, X.; Zhao, Y.; Li, L. OsIAA7 enhances heat stress tolerance by inhibiting the activity of OsARF6 in rice. Int. J. Biol. Macromol. 2025, 288, 138746. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhao, X.; Wang, F.; Wang, H.; Zhang, Y.; Ruan, B.; Dong, G.; Yu, Y.; Wu, L.; Chen, F. Rice Cytochrome P450 Protein CYP71P1 Is Required for Heat Stress Tolerance by Regulating Serotonin Biosynthesis and ROS Homeostasis. Plants 2025, 14, 1072. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Lu, C.A. Characterization of OsCAF1 Protein Function in Rice Response to Thermal Stress. Plants 2025, 14, 1036. [Google Scholar] [CrossRef]
- Dong, G.R.; Zhao, S.M.; Ding, Y.; Ma, Y.Q.; Ma, X.M.; Liu, C.L.; Hou, B.K. Rice glycosyltransferase OsDUGT1 is involved in heat stress tolerance by glycosylating flavonoids and regulating flavonoid metabolism. Front. Plant Sci. 2024, 15, 1516990. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Wang, G.; Peng, Z.; Wang, T.; Meng, Y.; Huang, J.; Huo, J.; Li, X.; Zhu, X.; et al. The OsEBF1-OsEIL5-OsPP91 module regulates rice heat tolerance via ubiquitination and transcriptional activation. Cell Rep. 2025, 44, 115271. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Islam, M.N.; Sarker, S.; Tuteja, N.; Seraj, Z.I. Overexpression of heterotrimeric G protein beta subunit gene (OsRGB1) confers both heat and salinity stress tolerance in rice. Plant Physiol. Biochem. PPB 2019, 144, 334–344. [Google Scholar] [CrossRef]
- Yang, C.; Luo, A.; Lu, H.P.; Davis, S.J.; Liu, J.X. Diurnal regulation of alternative splicing associated with heat tolerance in rice by two glycine-rich RNA-binding proteins. Sci. Bull. 2024, 69, 59–71. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Wang, K.; Wang, T.; Meng, Y.; Peng, Z.; Huang, J.; Huo, J.; Zhu, X.; Yang, J.; et al. The splicing auxiliary factor OsU2AF35a enhances heat tolerance via protein separation and promoting proper splicing of OsHSA32 pre-mRNA in rice. Plant Biotechnol. J. 2025, 23, 1308–1328. [Google Scholar] [CrossRef]
- Yu, H.X.; Cao, Y.J.; Yang, Y.B.; Shan, J.X.; Ye, W.W.; Dong, N.Q.; Kan, Y.; Zhao, H.Y.; Lu, Z.Q.; Guo, S.Q.; et al. A TT1-SCE1 module integrates ubiquitination and SUMOylation to regulate heat tolerance in rice. Mol. Plant 2024, 17, 1899–1918. [Google Scholar] [CrossRef]
- Shen, H.; Zhong, X.; Zhao, F.; Wang, Y.; Yan, B.; Li, Q.; Chen, G.; Mao, B.; Wang, J.; Li, Y.; et al. Overexpression of receptor-like kinase ERECTA improves heat tolerance in rice and tomato. Nat. Biotechnol. 2015, 33, 996–1003. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Ou, S.; Wang, R.; Wang, Y.; Chu, C.; Yao, S. Natural variations of SLG1 confer high-temperature tolerance in indica rice. Nat. Commun. 2020, 11, 5441. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; Zhang, Y.; Xiao, Y.; Liu, X.; Deng, H.; Lu, X.; Tang, W.; Zhang, G. Comparative Analysis of Heat-Tolerant and Heat-Susceptible Rice Highlights the Role of OsNCED1 Gene in Heat Stress Tolerance. Plants 2022, 11, 1062. [Google Scholar] [CrossRef]
- Takehara, K.; Murata, K.; Yamaguchi, T.; Yamaguchi, K.; Chaya, G.; Kido, S.; Iwasaki, Y.; Ogiwara, H.; Ebitani, T.; Miura, K. Thermo-responsive allele of sucrose synthase 3 (Sus3) provides high-temperature tolerance during the ripening stage in rice (Oryza sativa L.). Breed. Sci. 2018, 68, 336–342. [Google Scholar] [CrossRef]
- Xu, W.; Dou, Y.; Geng, H.; Fu, J.; Dan, Z.; Liang, T.; Cheng, M.; Zhao, W.; Zeng, Y.; Hu, Z.; et al. OsGRP3 Enhances Drought Resistance by Altering Phenylpropanoid Biosynthesis Pathway in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2022, 23, 7045. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Lin, Q.; Zhang, X.; Zhang, S.; Ma, W.; Sheng, P.; Zhang, L.; Wu, M.; Zhu, X.; Li, Z.; et al. OsPRMT6b balances plant growth and high temperature stress by feedback inhibition of abscisic acid signaling. Nat. Commun. 2025, 16, 5173. [Google Scholar] [CrossRef]
- Liu, X.H.; Lyu, Y.S.; Yang, W.; Yang, Z.T.; Lu, S.J.; Liu, J.X. A membrane-associated NAC transcription factor OsNTL3 is involved in heat tolerance in rice. Plant Biotechnol. J. 2020, 18, 1317–1329. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Zhu, B.; Xie, G. Overexpressing OsFBN1 enhances plastoglobule formation, reduces grain-filling percent and jasmonate levels under heat stress in rice. Plant Sci. Int. J. Exp. Plant Biol. 2019, 285, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, X.; Xu, F.; Zhang, Y.; Zhang, Q.; Miao, R.; Zhang, J.; Liang, J.; Xu, W. Suppression of OsMDHAR4 enhances heat tolerance by mediating H2O2-induced stomatal closure in rice plants. Rice 2018, 11, 38. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Huo, C.; Wang, H.; An, Z.; Sun, D.; Liu, J.; Tang, W.; Zhang, B. A Quantitative Proteomics Study of Early Heat-Regulated Proteins by Two-Dimensional Difference Gel Electrophoresis Identified OsUBP21 as a Negative Regulator of Heat Stress Responses in Rice. Proteomics 2019, 19, e1900153. [Google Scholar] [CrossRef]
- Lu, H.P.; Liu, X.H.; Wang, M.J.; Zhu, Q.Y.; Lyu, Y.S.; Xu, J.H.; Liu, J.X. The NAT1-bHLH110-CER1/CER1L module regulates heat stress tolerance in rice. Nat. Genet. 2025, 57, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Fu, Y.; Cui, Y.; Wu, N.; Li, Y.; Yang, Z.; Zhang, C.; Song, H.; He, G.; et al. HTT1, a Stearoyl-Acyl Carrier Protein Desaturase Involved Unsaturated Fatty Acid Biosynthesis, Affects Rice Heat Tolerance. Plant Cell Environ. 2025, 48, 3391–3405. [Google Scholar] [CrossRef]
- Liu, X.; Ji, P.; Liao, J.; Duan, X.; Luo, Z.; Yu, X.; Jiang, C.J.; Xu, C.; Yang, H.; Peng, B.; et al. CRISPR/Cas knockout of the NADPH oxidase gene OsRbohB reduces ROS overaccumulation and enhances heat stress tolerance in rice. Plant Biotechnol. J. 2025, 23, 336–351. [Google Scholar] [CrossRef]
- Kan, Y.; Mu, X.R.; Zhang, H.; Gao, J.; Shan, J.X.; Ye, W.W.; Lin, H.X. TT2 controls rice heat tolerance through SCT1-dependent alteration of wax biosynthesis. Nat. Plants 2022, 8, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, K.; Hu, C.; Abbas, W.; Zhang, J.; Xu, P.; Cheng, B.; Zhang, J.; Yin, W.; Shalmani, A.; et al. A natural gene on-off system confers field heat tolerance for grain quality and yield in rice. Cell 2025, 188, 3661–3678. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; AlMutairi, K.A.; Siddiqui, Z.H. Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. PPB 2017, 110, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. PPB 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Ma, Y.; Ding, Y.; Dong, G.; Liu, C.; Ma, X.; Hou, B. Rice glycosyltransferase UGT706F1 functions in heat tolerance through glycosylating flavonoids under the regulation of transcription factor MYB61. Plant J. Cell Mol. Biol. 2025, 121, e17252. [Google Scholar] [CrossRef]
- Wu, K.; Wang, S.; Song, W.; Zhang, J.; Wang, Y.; Liu, Q.; Yu, J.; Ye, Y.; Li, S.; Chen, J.; et al. Enhanced sustainable green revolution yield via nitrogen-responsive chromatin modulation in rice. Science 2020, 367, eaaz2046. [Google Scholar] [CrossRef]
- Li, X.M.; Chao, D.Y.; Wu, Y.; Huang, X.; Chen, K.; Cui, L.G.; Su, L.; Ye, W.W.; Chen, H.; Chen, H.C.; et al. Natural alleles of a proteasome α2 subunit gene contribute to heat tolerance and adaptation of African rice. Nat. Genet. 2015, 47, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xie, Y.; Liu, Y. A novel NF-Ys-QT12-IRE1 module controlling grain quality and yield heat tolerance in rice. Mol. Plant 2025, 18, 1106–1108. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Wang, X.; Gu, C.; Lu, Z.; Ma, R.; Wang, X.; Lu, Y.; Cai, K.; Tang, Z.; Zhou, Z.; et al. Rice Heat Stress Response: Physiological Changes and Molecular Regulatory Network Research Progress. Plants 2025, 14, 2573. https://doi.org/10.3390/plants14162573
Ma W, Wang X, Gu C, Lu Z, Ma R, Wang X, Lu Y, Cai K, Tang Z, Zhou Z, et al. Rice Heat Stress Response: Physiological Changes and Molecular Regulatory Network Research Progress. Plants. 2025; 14(16):2573. https://doi.org/10.3390/plants14162573
Chicago/Turabian StyleMa, Weiwei, Xiaole Wang, Chuanwei Gu, Zhengfei Lu, Rongrong Ma, Xiaoyan Wang, Yongfa Lu, Kefeng Cai, Zhiming Tang, Zhuoqi Zhou, and et al. 2025. "Rice Heat Stress Response: Physiological Changes and Molecular Regulatory Network Research Progress" Plants 14, no. 16: 2573. https://doi.org/10.3390/plants14162573
APA StyleMa, W., Wang, X., Gu, C., Lu, Z., Ma, R., Wang, X., Lu, Y., Cai, K., Tang, Z., Zhou, Z., Chen, Z., Zhou, H., & Bao, X. (2025). Rice Heat Stress Response: Physiological Changes and Molecular Regulatory Network Research Progress. Plants, 14(16), 2573. https://doi.org/10.3390/plants14162573






