Genome-Wide Linkage Mapping of QTL for Adult-Plant Resistance to Stripe Rust in a Chinese Wheat Population Lantian 25 × Huixianhong
Abstract
1. Introduction
2. Results
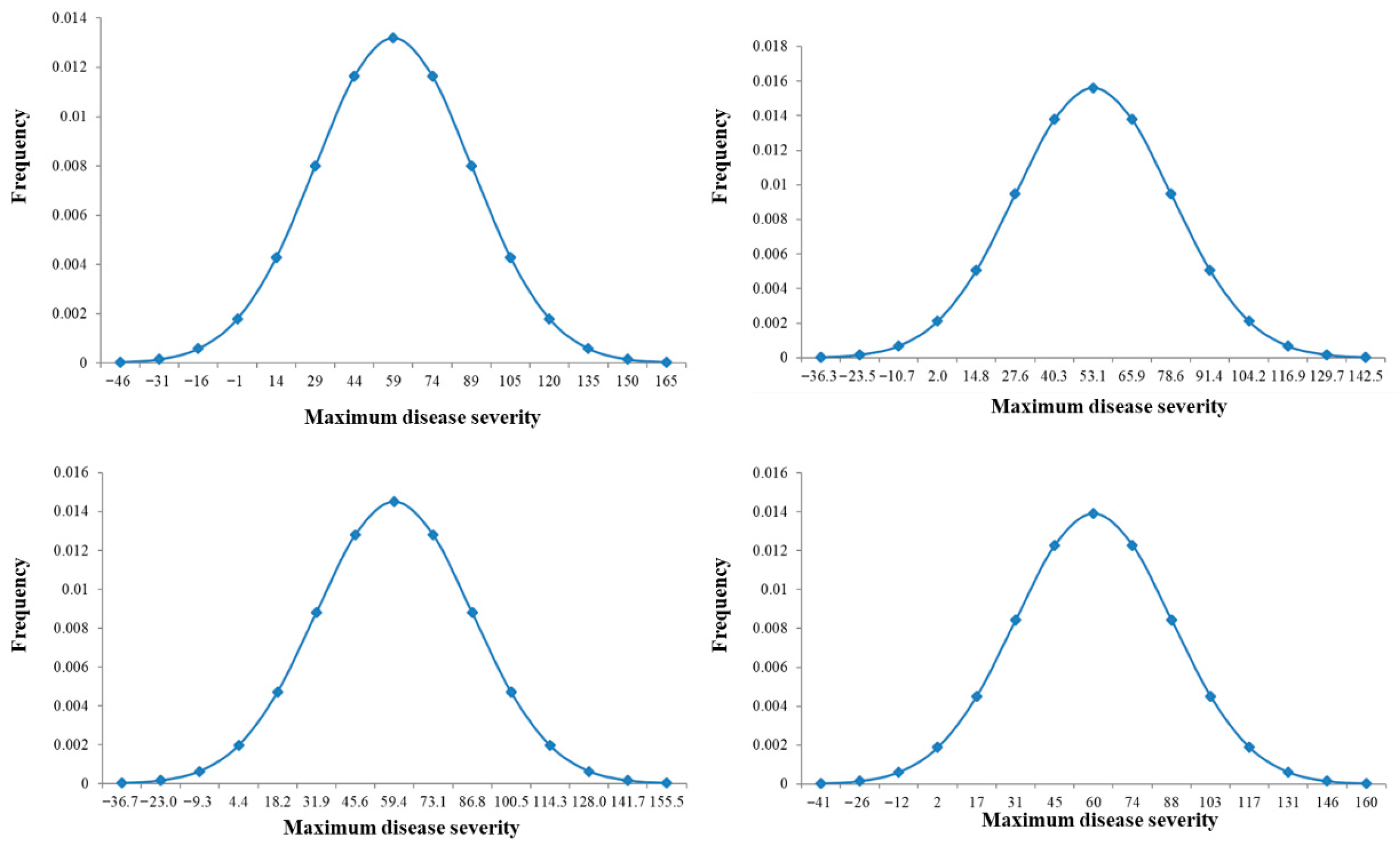
2.1. Phenotypic Evaluation
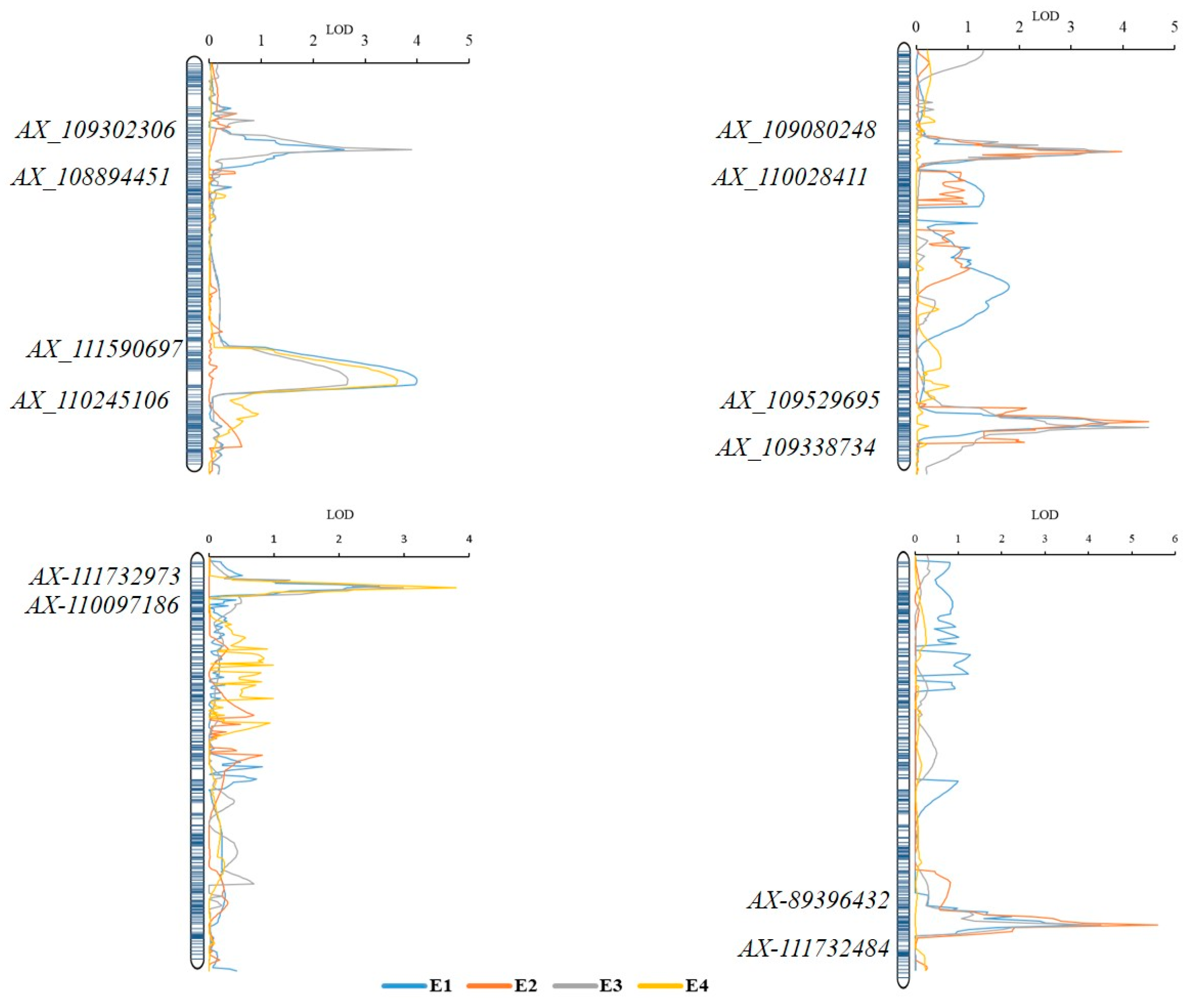
2.2. QTL for APR to Stripe Rust
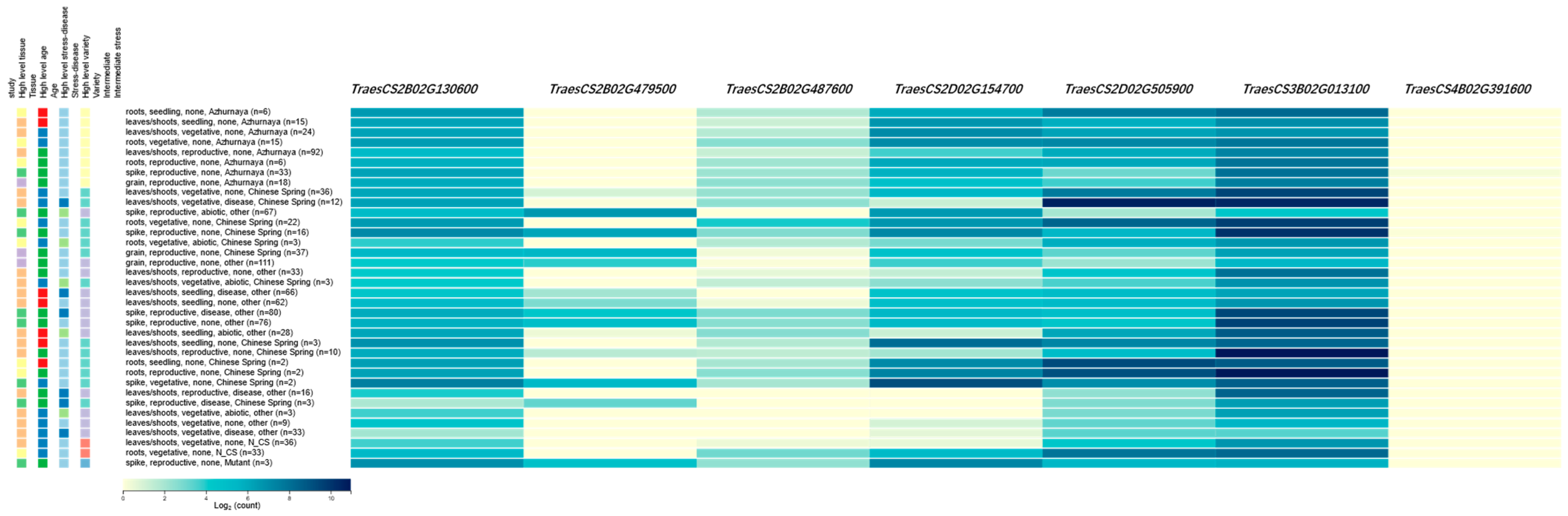
2.3. Candidate Gene Identification
2.4. Development and Validation of Kompetitive Allele-Specific PCR (KASP) Markers
3. Discussion
4. Materials and Methods
4.1. Germplasm Development and Characterization
4.2. Field Experimentation
4.3. Genotyping and Genetic Map Construction and QTL Analysis
4.4. KASP Marker Development and Candidate Gene Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| APR | Adult-plant resistance |
| RILs | Recombinant inbred lines |
| SNP | Single-nucleotide polymorphism |
| SR | Stripe rust |
| QTL | Quantitative trait loci |
| KASP | Kompetitive allele-specific PCR |
| MAS | Marker-assisted selection |
| PVE | Phenotypic variance explained |
| YR | Yellow rust |
Appendix A
| Source of Variance | Df | Mean Square | F Value |
|---|---|---|---|
| Replicate (environment) | 2 | 1571 | 8.8 * |
| Environment | 4 | 38,028 | 212.0 * |
| Line | 218 | 3125 | 17.4 * |
| Line × Environment | 1088 | 311 | 1.7 * |
| Error | 1654 | 179 |
| Kasp Marker | Prime | Sequence |
|---|---|---|
| Kasp_2BS_Y | FAX | GAAGGTGACCAAGTTCATGCTtgCtagtgaaatcaagtgtatgatA |
| HEM | GAAGGTCGGAGTCAACGGATTtgCtagtgaaatcaagtgtatgatG | |
| Common | ||
| Kasp_2BL_YR | FAX | GAAGGTGACCAAGTTCATGCTGGGTAGCATTGATCTAAACTCCTG |
| HEM | GAAGGTCGGAGTCAACGGATTGGGTAGCATTGATCTAAACTCCTC | |
| Common | ctgagcgactgtttcttattgtT | |
| Kasp_2DS_YR | FAX | GAAGGTGACCAAGTTCATGCTCTACAAACATGGTTCACATTGAC |
| HEM | GAAGGTCGGAGTCAACGGATTACTACAAACATGGTTCACATTGAT | |
| Common | TGCATCATCTCCACCGGAAC | |
| Kasp_2DL_Y | FAX | GAAGGTGACCAAGTTCATGCTgttctcgatctgtatgctgaagtA |
| HEM | GAAGGTCGGAGTCAACGGATTgttctcgatctgtatgctgaagtG | |
| Common | agatgactttccagatgtagggA |
| Name | Origin | MDS (%) | Kasp_2BS_YR | Kasp_2BL_YR | Kasp_2DS_YR | Kasp_2DL_YR |
|---|---|---|---|---|---|---|
| Wheatear | USA | 33.2 | CC | CC | GG | CC |
| 11CA40 | Beijing | 21.8 | CC | CC | AA | CC |
| 12CA29 | Beijing | 64.3 | CC | CC | GG | CC |
| CA0548 | Beijing | 83.3 | CC | CC | AA | TT |
| CA0816 | Beijing | 22.3 | CC | CC | GG | CC |
| CA0958 | Beijing | 59.7 | CC | GG | AA | TT |
| CA1055 | Beijing | 60.3 | TT | GG | GG | CC |
| CA1090 | Beijing | 69.5 | TT | GG | AA | TT |
| CA1119 | Beijing | 30.4 | TT | GG | GG | CC |
| CA1133 | Beijing | 30.7 | CC | CC | GG | CC |
| CA9722 | Beijing | 61.4 | CC | CC | AA | TT |
| Jing411 | Beijing | 27.8 | CC | CC | GG | CC |
| Jing9428 | Beijing | 68.3 | CC | CC | AA | TT |
| Zhongmai175 | Beijing | 22.3 | CC | GG | AA | TT |
| Zhongmai415 | Beijing | 74.6 | CC | CC | GG | CC |
| Zhongyou9507 | Beijing | 50.5 | CC | CC | AA | TT |
| Holdfast | Britain | 18.5 | CC | CC | GG | CC |
| 00127-2-2 | Gansu | 7.9 | CC | CC | GG | CC |
| 863-13 | Gansu | 19.4 | CC | CC | GG | CC |
| Baigetiao | Gansu | 20.1 | CC | CC | GG | TT |
| Baimangmai1 | Gansu | 24 | TT | GG | GG | CC |
| CP07-9-1-1-2F6 | Gansu | 41.3 | CC | CC | GG | CC |
| Dabaijiankou | Gansu | 21.6 | CC | GG | AA | TT |
| Damai1 | Gansu | 18.9 | CC | CC | GG | CC |
| Hangxuan2 | Gansu | 25.8 | CC | CC | GG | CC |
| Honghuomai | Gansu | 33 | CC | CC | GG | CC |
| Honglaomangmai | Gansu | 43.6 | TT | CC | GG | TT |
| Huomaizi | Gansu | 31.5 | CC | CC | AA | TT |
| Jinhuangmai | Gansu | 24.4 | TT | GG | GG | CC |
| Lanhangxuan122 | Gansu | 34.2 | CC | CC | AA | TT |
| Lantian 25 | Gansu | 36.4 | TT | GG | AA | TT |
| Lantian26 | Gansu | 10.3 | CC | CC | GG | CC |
| Longdonghong | Gansu | 23.8 | CC | CC | GG | CC |
| Longjian103 | Gansu | 32.3 | CC | CC | AA | TT |
| Longjian196 | Gansu | 26 | CC | CC | GG | CC |
| Longjian301 | Gansu | 59.9 | CC | CC | GG | CC |
| Pingliang45 | Gansu | 37.5 | CG | CG | GG | CC |
| Tutoumai | Gansu | 29.5 | CC | CC | AA | TT |
| Xiaobeijiekou | Gansu | 23.7 | CC | CC | GG | CC |
| Xifeng20 | Gansu | 56.5 | CC | CC | AA | CC |
| Youmangmazhamai | Gansu | 47.3 | CC | CC | GG | CC |
| Hengguan33 | Hebei | 45 | CC | CC | AA | TT |
| Shijiazhuang8 | Hebei | 57.1 | CC | GG | GG | CC |
| Ag303-29 | Henan | 39.8 | TT | GG | AA | TT |
| Ag303-8 | Henan | 45 | GC | GC | GG | CC |
| Ag311-8 | Henan | 47.1 | CC | CC | AA | TT |
| Aikang58 | Henan | 58.2 | CC | CC | GG | CC |
| Bainong64 | Henan | 37.5 | CC | CC | AA | TT |
| C40468 | Henan | 46.7 | TT | CC | GG | CC |
| C40469 | Henan | 51.9 | TT | CC | AA | CC |
| C40470 | Henan | 54 | TT | GG | GG | CC |
| C40472 | Henan | 55.3 | CC | CC | AA | TT |
| C40473 | Henan | 48.4 | CC | GG | GG | CC |
| C40477 | Henan | 38.2 | CC | CC | GG | CC |
| C40481 | Henan | 46.1 | TT | CC | AA | TT |
| C40485 | Henan | 24.1 | CC | CC | GG | CC |
| C50835 | Henan | 42.1 | TT | GG | AA | TT |
| C50837 | Henan | 26.6 | TT | GG | AA | TT |
| C50843 | Henan | 39.2 | CC | CC | GG | CC |
| C50845 | Henan | 25.2 | TT | GG | GG | CC |
| C50850 | Henan | 52.5 | CC | CC | AA | TT |
| C50851 | Henan | 51.3 | CC | CC | AA | TT |
| C50855 | Henan | 53.6 | CC | CC | GG | CC |
| C50865 | Henan | 39.3 | GC | GC | AG | CC |
| He106 | Henan | 31 | CC | CC | GG | CC |
| He109 | Henan | 45.6 | TT | CC | AA | TT |
| He12 | Henan | 18.8 | CC | CC | GG | CC |
| He120 | Henan | 25.8 | CC | CC | AA | TT |
| He18 | Henan | 31.5 | CC | CC | AA | TT |
| He193 | Henan | 19 | CC | CC | GG | CC |
| He2 | Henan | 47.3 | TT | CC | AA | TT |
| He222 | Henan | 26.6 | CC | CC | AA | TT |
| He231 | Henan | 33.9 | TT | GG | AA | TT |
| He244 | Henan | 19.4 | CC | CC | GG | CC |
| He256 | Henan | 48.7 | CC | CC | GG | CC |
| He3 | Henan | 30.3 | TT | GG | AA | TT |
| He48 | Henan | 30.9 | TT | GG | GG | CC |
| He53 | Henan | 38.5 | TT | GG | AA | TT |
| He7 | Henan | 28.6 | CC | CC | GG | CC |
| He87 | Henan | 27.8 | CC | CC | AA | TT |
| Pingyuan50 | Henan | 11.1 | CC | CC | GG | CC |
| Zheng9023 | Henan | 49.3 | CC | CC | AA | TT |
| Zhengmai366 | Henan | 29.4 | CC | CC | GG | CC |
| Zhongmai871 | Henan | 36.6 | CC | CC | AA | TT |
| Zhongmai875 | Henan | 35.5 | CC | GG | AA | TT |
| Zhongmai895 | Henan | 34.7 | CC | CC | AA | TT |
| Zhoumai13 | Henan | 45.8 | TT | CC | AA | TT |
| Zhoumai16 | Henan | 41.2 | CC | CC | GG | CC |
| Zhoumai18 | Henan | 22.8 | CC | CC | GG | CC |
| Libellula | Italy | 19.3 | TT | GG | AA | TT |
| Pascal | Italy | 7.1 | CC | CC | GG | CC |
| Abbondanza | Italy | 64.2 | TT | GG | GG | CC |
| Funo | Italy | 8.6 | CC | CC | AA | TT |
| Ningdong10 | Ningxia | 25 | CC | CC | GG | CC |
| Ningdong10 | Ningxia | 25 | CC | CC | GG | CC |
| Ningdong11 | Ningxia | 83.2 | CC | CC | AA | TT |
| Lovrin10 | Romania | 69 | TT | GG | GG | CC |
| Changwu131 | Shaanxi | 52.9 | CC | CC | GG | CC |
| Xiaoyan6 | Shaanxi | 75.9 | CC | CC | AA | TT |
| Jimai19 | Shandong | 46 | TT | GG | GG | CC |
| Jimai22 | Shandong | 36.6 | TT | GG | AA | TT |
| Jinan13 | Shandong | 74.2 | CC | CC | GG | CC |
| Jinan17 | Shandong | 59.8 | CC | CC | GG | CC |
| Liangxing66 | Shandong | 52.3 | TT | GG | AA | TT |
| Linmai4 | Shandong | 51.8 | TT | GG | AA | TT |
| Lumai21 | Shandong | 72.5 | TT | CC | GG | CC |
| Weimai8 | Shandong | 60.4 | CC | CC | GG | CC |
| Yannong19 | Shandong | 90.9 | TT | GG | AA | TT |
| ChineseSpring | Sichuan | 34.4 | CC | CC | GG | CC |
| Chuanmai107 | Sichuan | 62.1 | CG | CG | AA | TT |
| Fan6 | 75.9 | TT | GG | GG | CC |
References
- Schwessinger, B. Fundamental wheat stripe rust research in the 21st century. New Phytol. 2017, 213, 1625–1631. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, T.; Li, K.; Huang, K.; Ma, C.; Zhang, M.; Zhao, X.; Zhang, Z.; Liu, X. Yield losses associated with different levels of stripe rust resistance of commercial wheat cultivars in China. Phytopathology 2022, 112, 1244–1254. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, Q.; Han, D.; Wu, J.; Wu, L.; Tong, J.; Zhang, Z.; Liu, X. Molecular characterization and validation of adult-plant stripe rust resistance gene Yr86 in Chinese wheat cultivar Zhongmai 895. Theor. Appl. Genet. 2023, 136, 142. [Google Scholar] [CrossRef]
- Yang, F.; Liu, J.; Guo, Y.; He, Z.; Rasheed, A.; Wu, L.; Zhang, Z.; Zhang, H. Genome-wide association mapping of adult-plant resistance to stripe rust in common wheat (Triticum aestivum). Plant Dis. 2020, 104, 2174–2180. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, D.; Ni, Z.; Zou, X.; Xu, X.; Sun, M.; Liu, H. Molecular identification and validation of four stable QTL for adult-plant resistance to powdery mildew in Chinese wheat cultivar Bainong 64. Theor. Appl. Genet. 2023, 136, 232. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, X.; Gebrewahid, T.W.; Zhou, Y.; Yang, E.; Xia, X.; Zhao, Y.; Zhang, Z. Genome-wide association mapping of leaf rust and stripe rust resistance in wheat accessions using the 90K SNP array. Theor. Appl. Genet. 2021, 134, 1233–1251. [Google Scholar] [CrossRef]
- Wu, J.; Xu, D.; Fu, L.; Wu, L.; Hao, W.; Li, J.; Zhang, Z.; Liu, X. Fine mapping of a stripe rust resistance gene YrZM175 in bread wheat. Theor. Appl. Genet. 2022, 135, 3485–3496. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Singh, R.P.; Yuan, C.; Liu, D.; Randhawa, M.S.; Huerta-Espino, J.; Wang, X. Three co-located resistance genes confer resistance to leaf rust and stripe rust in wheat variety Borlaug 100. Crop J. 2022, 10, 490–497. [Google Scholar] [CrossRef]
- Zhao, J.; Kang, Z. Fighting wheat rusts in China: A look back and into the future. Phytopathol. Res. 2023, 5, 6. [Google Scholar] [CrossRef]
- Bariana, H.S.; Parry, N.; Barclay, I.R.; Brown, G.N.; McLean, R.J.; Shankar, M.; Wilson, R.E.; Willey, N.J.; Francki, M. Identification and characterization of stripe rust resistance gene Yr34 in common wheat. Theor. Appl. Genet. 2006, 112, 1143–1148. [Google Scholar] [CrossRef]
- Bariana, H.; Forrest, K.; Qureshi, N.; Miah, H.; Hayden, M.; Bansal, U. Adult plant stripe rust resistance gene Yr71 maps close to Lr24 in chromosome 3D of common wheat. Mol. Breed. 2016, 36, 98. [Google Scholar] [CrossRef]
- Basnet, B.R.; Singh, R.P.; Ibrahim, A.M.H.; Herrera-Foessel, S.A.; Huerta-Espino, J.; Lan, C.; Rudd, J.C. Characterization of Yr54 and other genes associated with adult plant resistance to yellow rust and leaf rust in common wheat Quaiu 3. Mol. Breed. 2014, 33, 385–399. [Google Scholar] [CrossRef]
- Chhetri, M.; Bariana, H.; Kandiah, P.; Bansal, U. Yr58: A new stripe rust resistance gene and its interaction with Yr46 for enhanced resistance. Phytopathology 2016, 106, 1530–1534. [Google Scholar] [CrossRef]
- Chugunkova, T.V.; Pastukhova, N.L.; Pirko, Y.V.; Blume, Y.B. Genetic Basis of Resistance to Wheat Yellow Rust. Cytol. Genet. 2025, 59, 186–196. [Google Scholar] [CrossRef]
- Coram, T.E.; Settles, M.L.; Chen, X. Transcriptome analysis of high-temperature adult-plant resistance conditioned by Yr39 during the wheat–Puccinia striiformis f. sp. tritici interaction. Mol. Plant Pathol. 2008, 9, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Zhang, J.; Dubcovsky, J. High-resolution mapping of Yr78, an adult plant resistance gene to wheat stripe rust. Plant Genome 2022, 15, e20212. [Google Scholar] [CrossRef]
- Herrera-Foessel, S.A.; Singh, R.P.; Lan, C.X.; Huerta-Espino, J.; Calvo-Salazar, V.; Bansal, U.K.; Bariana, H.S.; Lagudah, E.S. Yr60, a gene conferring moderate resistance to stripe rust in wheat. Plant Dis. 2015, 99, 508–511. [Google Scholar] [CrossRef]
- Kanwal, M.; Qureshi, N.; Gessese, M.; Forrest, K.; Babu, P.; Bariana, H.; Bansal, U. An adult plant stripe rust resistance gene maps on chromosome 7A of Australian wheat cultivar Axe. Theor. Appl. Genet. 2021, 134, 2213–2220. [Google Scholar] [CrossRef]
- Krattinger, S.G.; Lagudah, E.S.; Spielmeyer, W.; Singh, R.P.; Huerta-Espino, J.; McFadden, H.; Bossolini, E.; Selter, L.L.; Keller, B. A Putative ABC Transporter Confers Durable Resistance to Multiple Fungal Pathogens in Wheat. Science 2009, 323, 1360–1363. [Google Scholar] [CrossRef]
- Li, J.; Dundas, I.; Dong, C.; Li, G.; Trethowan, R.; Yang, Z.; Hoxha, S.; Zhang, P. Identification and characterization of a new stripe rust resistance gene Yr83 on rye chromosome 6R in wheat. Theor. Appl. Genet. 2020, 133, 1095–1107. [Google Scholar] [CrossRef]
- Lowe, I.; Jankuloski, L.; Chao, S.; Chen, X.; See, D.; Dubcovsky, J. Mapping and validation of QTL which confer partial resistance to broadly virulent post-2000 North American races of stripe rust in hexaploid wheat. Theor. Appl. Genet. 2011, 123, 143–157. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, M.; Chen, X.; See, D.; Chao, S.; Jing, J. Mapping of Yr62 and a small-effect QTL for high-temperature adult-plant resistance to stripe rust in spring wheat PI 192252. Theor. Appl. Genet. 2014, 127, 1449–1459. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Morris, C.; Appels, R.; Xia, X.C. Catalogue of gene symbols. KOMUGI Integr. Wheat Sci. Database 2012. [Google Scholar]
- McIntosh, R.A.; Dubcovsky, J.; Rogers, J. Catalogue of Gene Symbols for Wheat: 2013–2014 Supplement; National Institute of Genetics (NIG): Mishima, Japan, 2014. [Google Scholar]
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Morris, C.; Appels, R.; Xia, X.C. Catalogue of Gene Symbols for Wheat: 2015–2016 Supplement; National Institute of Genetics (NIG): Mishima, Japan, 2016. [Google Scholar]
- Moore, J.W.; Herrera-Foessel, S.; Lan, C.; Schnippenkoetter, W.; Ayliffe, M.; Huerta-Espino, J.; Lillemo, M.; Viccars, L.; Milne, R.; Periyannan, S.; et al. A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 2015, 47, 1494–1498. [Google Scholar] [CrossRef]
- Nsabiyera, V.; Bariana, H.S.; Qureshi, N.; Wong, D.; Hayden, M.J.; Bansal, U.K. Characterization and mapping of adult plant stripe rust resistance in wheat accession Aus27284. Theor. Appl. Genet. 2018, 131, 1459–1467. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Bai, B.; Li, J.; Huang, L.; Xu, Z.; Chen, Y.; Liu, X.; Cao, T.; Li, M.; et al. Current Status and Strategies for Utilization of Stripe Rust Resistance Genes in Wheat Breeding Program of China. Sci. Agric. Sin. 2024, 57, 34–51. [Google Scholar]
- Kumar, S.; Saini, D.K.; Jan, F.; Jan, S.; Tahir, M.; Djalovic, I.; Latkovic, D.; Khan, M.A.; Kumar, S.; Vikas, V.K.; et al. Comprehensive meta-QTL analysis for dissecting the genetic architecture of stripe rust resistance in bread wheat. BMC Genom. 2023, 24, 259. [Google Scholar] [CrossRef]
- Jan, I.; Saripalli, G.; Kumar, K.; Kumar, A.; Singh, R.; Batra, R.; Sharma, P.K.; Balyan, H.S.; Gupta, P.K. Meta-QTLs and candidate genes for stripe rust resistance in wheat. Sci. Rep. 2021, 11, 22923. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Zhang, M.; Ma, C.; Zheng, X.; Tan, W.; Tian, R.; Yan, Y.; Zhou, X.; Li, X.; Yang, S.; et al. Application of Yr52 gene in wheat improvement for stripe rust resistance. Sci. Agric. Sin. 2022, 55, 2077–2091. [Google Scholar]
- Uauy, C.; Brevis, J.C.; Chen, X.; Khan, I.; Jackson, L.; Chicaiza, O.; Distelfeld, A.; Fahima, T.; Dubcovsky, J. High-temperature adult-plant (HTAP) stripe rust resistance gene Yr36 from Triticum turgidum ssp. dicoccoides is closely linked to the grain protein content locus Gpc-B1. Theor. Appl. Genet. 2005, 112, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Spielmeyer, W.; Lagudah, E.S.; Appels, R. Characterization of genetic loci conferring adult plant resistance to leaf rust and stripe rust in spring wheat. Genome 2006, 49, 977–990. [Google Scholar] [CrossRef]
- Wang, X.; Xiang, M.; Li, H.; Li, X.; Mu, K.; Huang, S.; Zhang, Y.; Cheng, X.; Yang, S.; Yuan, X.; et al. High-density mapping of durable and broad-spectrum stripe rust resistance gene Yr30 in wheat. Theor. Appl. Genet. 2024, 137, 152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Wang, M.N.; Chen, X.M.; Lu, Y.; Kang, Z.S.; Jing, J.X. Identification of Yr59 conferring high-temperature adult-plant resistance to stripe rust in wheat germplasm PI 178759. Theor. Appl. Genet. 2014, 127, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, M.; See, D.R.; Chao, S.; Zheng, Y.; Chen, X. Characterization of novel gene Yr79 and four additional QTL for all-stage and high-temperature adult-plant resistance to stripe rust in spring wheat PI 182103. Phytopathology 2018, 108, 737–747. [Google Scholar] [CrossRef]
- Marchal, C.; Zhang, J.; Zhang, P.; Fenwick, P.; Steuernagel, B.; Adamski, N.M.; Boyd, L.; McIntosh, R.; Wulff, B.B.H.; Berry, S.; et al. BED-domain-containing immune receptors confer diverse resistance spectra to yellow rust. Nat. Plants 2018, 4, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Frick, M.; Huel, R.; Nykiforuk, C.L.; Wang, X.; Gaudet, D.A.; Eudes, F.; Conner, R.L.; Kuzyk, A.; Chen, Q.; et al. The stripe rust resistance gene Yr10 encodes an evolutionary-conserved and unique CC–NBS–LRR sequence in wheat. Mol. Plant 2014, 7, 1740–1755. [Google Scholar] [CrossRef]
- Klymiuk, V.; Yaniv, E.; Huang, L.; Raats, D.; Fatiukha, A.; Chen, S.; Feng, L.; Frenkel, Z.; Krugman, T.; Lidzbarsky, G.; et al. Cloning of the wheat Yr15 resistance gene sheds light on the plant tandem kinase-pseudokinase family. Nat. Commun. 2018, 9, 3735. [Google Scholar] [CrossRef]
- Wang, H.; Zou, S.; Li, Y.; Lin, F.; Tang, D. An ankyrin-repeat and WRKY-domain-containing immune receptor confers stripe rust resistance in wheat. Nat. Commun. 2020, 11, 1353. [Google Scholar] [CrossRef]
- Fu, D.; Uauy, C.; Distelfeld, A.; Blechl, A.; Epstein, L.; Chen, X.; Sela, H.; Fahima, T.; Dubcovsky, J. A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 2009, 323, 1357–1360. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, L.; Zhang, H.; Hao, Q.; Lyu, B.; Wang, M.; Epstein, L.; Liu, M.; Kou, C.; Qi, J.; et al. An ancestral NB-LRR with duplicated 3′ UTRs confers stripe rust resistance in wheat and barley. Nat. Commun. 2019, 10, 4023. [Google Scholar] [CrossRef]
- Ni, F.; Zheng, Y.; Liu, X.; Yu, Y.; Zhang, G.; Epstein, L.; Mao, X.; Wu, J.; Yuan, C.; Lv, B.; et al. Sequencing trait-associated mutations to clone wheat rust-resistance gene YrNAM. Nat. Commun. 2023, 14, 4353. [Google Scholar] [CrossRef] [PubMed]
- Athiyannan, N.; Abrouk, M.; Boshoff, W.H.; Cauet, S.; Rodde, N.; Kudrna, D.; Mohammed, N.; Bettgenhaeuser, J.; Botha, K.S.; Derman, S.S.; et al. Long-read genome sequencing of bread wheat facilitates disease resistance gene cloning. Nat. Genet. 2022, 54, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Mallard, S.; Gaudet, D.; Aldeia, A.; Abelard, C.; Besnard, A.L.; Sourdille, P.; Dedryver, F. Genetic analysis of durable resistance to yellow rust in bread wheat. Theor. Appl. Genet. 2005, 110, 1401–1409. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, Z.J.; Xu, Y.B.; Li, G.H.; Feng, J.; Zhou, Y. Quantitative trait loci for high-temperature adult-plant and slow-rusting resistance to Puccinia striiformis f. sp. tritici in wheat cultivars. Phytopathology 2008, 98, 803–809. [Google Scholar] [CrossRef]
- Ren, Y.; He, Z.; Li, J.; Lillemo, M.; Wu, L.; Bai, B.; Zhang, Z.; Zhao, X.; Liu, X.; Zhang, H. QTL mapping of adult-plant resistance to stripe rust in a population derived from common wheat cultivars Naxos and Shanghai 3/Catbird. Theor. Appl. Genet. 2012, 125, 1211–1221. [Google Scholar] [CrossRef]
- Singh, A.; Knox, R.E.; DePauw, R.M.; Singh, A.K.; Cuthbert, R.D.; Campbell, H.L.; Li, W.; Bhavani, S. Stripe rust and leaf rust resistance QTL mapping, epistatic interactions, and co-localization with stem rust resistance loci in spring wheat evaluated over three continents. Theor. Appl. Genet. 2014, 127, 2465–2477. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, J.; Yao, F.; Long, L.; Wang, Y.; Wu, Y.; Chen, G. Dissection of loci conferring resistance to stripe rust in Chinese wheat landraces from the middle and lower reaches of the Yangtze River via genome-wide association study. Plant Sci. 2019, 287, 110204. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Chen, N.; Yu, R.; Yu, S.; Wang, Q.; Li, X.; Zhang, H.; Zhang, Z.; Liu, Y. Association analysis identifies new loci for resistance to Chinese Yr26-virulent races of the stripe rust pathogen in a diverse panel of wheat germplasm. Plant Dis. 2020, 104, 1751–1762. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhao, Y.Y.; Kang, Z.S.; Zhao, J. First report of a Puccinia striiformis f. sp. tritici race virulent to wheat stripe rust resistance gene Yr5 in China. Plant Dis. 2020, 104, 284. [Google Scholar] [CrossRef]
- Dibley, K.; Jost, M.; McIntosh, R.; Lagudah, E.; Zhang, P. The wheat stripe rust resistance gene YrNAM is Yr10. Nat. Commun. 2024, 15, 3291. [Google Scholar] [CrossRef]
- Suenaga, K.; Singh, R.P.; Huerta-Espino, J.; William, H.M. Microsatellite markers for genes Lr34/Yr18 and other quantitative trait loci for leaf rust and stripe rust resistance in bread wheat. Phytopathology 2003, 93, 881–890. [Google Scholar] [CrossRef]
- Lu, Y.; Lan, C.; Liang, S.; Zhou, X.; Liu, D.; Zhou, G.; Zhang, Z. QTL mapping for adult-plant resistance to stripe rust in Italian common wheat cultivars Libellula and Strampelli. Theor. Appl. Genet. 2009, 119, 1349–1359. [Google Scholar] [CrossRef]
- Coram, T.E.; Huang, X.; Zhan, G.; Settles, M.L.; Chen, X. Meta-analysis of transcripts associated with race-specific resistance to stripe rust in wheat demonstrates common induction of blue copper-binding protein, heat-stress transcription factor, pathogen-induced WIR1A protein, and ent-kaurene synthase transcripts. Funct. Integr. Genom. 2010, 10, 383–392. [Google Scholar]
- Agenbag, G.M.; Pretorius, Z.A.; Boyd, L.A.; Bender, C.M.; Prins, R. Identification of adult plant resistance to stripe rust in the wheat cultivar Cappelle-Desprez. Theor. Appl. Genet. 2012, 125, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.M.; Royo, C. Dissecting the genetic architecture of leaf rust resistance in wheat by QTL meta-analysis. Phytopathology 2015, 105, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Amo, A.; Soriano, J.M. Unravelling consensus genomic regions conferring leaf rust resistance in wheat via meta-QTL analysis. Plant Genome 2022, 15, e20185. [Google Scholar] [CrossRef]
- Saini, D.K.; Chahal, A.; Pal, N.; Srivastava, P.; Gupta, P.K. Meta-analysis reveals consensus genomic regions associated with multiple disease resistance in wheat (Triticum aestivum L.). Mol. Breed. 2022, 42, 11. [Google Scholar] [CrossRef]
- Rosewarne, G.M.; Singh, R.P.; Huerta-Espino, J.; Jankuloski, L.C. Analysis of leaf and stripe rust severities reveals pathotype changes and multiple minor QTLs associated with resistance in an Avocet × Pastor wheat population. Theor. Appl. Genet. 2012, 124, 1283–1294. [Google Scholar] [CrossRef]
- Jagger, L.J.; Newell, C.; Berry, S.T.; MacCormack, R.; Boyd, L.A. The genetic characterization of stripe rust resistance in the German wheat cultivar Alcedo. Theor. Appl. Genet. 2011, 122, 723–733. [Google Scholar] [CrossRef]
- Naruoka, Y.; Garland-Campbell, K.A.; Carter, A.H. Genome-wide association mapping for stripe rust (Puccinia striiformis f. sp. tritici) in US Pacific Northwest winter wheat (Triticum aestivum L.). Theor. Appl. Genet. 2015, 128, 1083–1101. [Google Scholar] [CrossRef]
- Wu, J.; Huang, S.; Zeng, Q.; Liu, S.; Wang, Q.; Mu, J.; Zhao, X.; Zhang, Z.; Zhang, H.; Liu, Y. Comparative genome-wide mapping versus extreme pool-genotyping and development of diagnostic SNP markers linked to QTL for adult plant resistance to stripe rust in common wheat. Theor. Appl. Genet. 2018, 131, 1777–1792. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.P.; Zeng, Q.D.; Wu, J.H.; Wang, Q.L.; Yang, Z.J.; Liang, B.P.; Zhang, H.; Liu, X. QTL mapping and validation of adult plant resistance to stripe rust in Chinese wheat landrace Humai 15. Front. Plant Sci. 2018, 9, 968. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, H.; Gu, T.; Xing, L.; Han, G.; Ma, P.; Zhang, S.; Zhao, X.; Zhang, Z.; Zhang, G.; et al. PM2b, a CC-NBS-LRR protein, interacts with TaWRKY76-D to regulate powdery mildew resistance in common wheat. Front. Plant Sci. 2022, 13, 973065. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, F.; Su, C.; Zhang, Y.; Li, J.; Wang, J.; Hu, X. NBS-LRR gene TaRPS2 is positively associated with the high-temperature seedling plant resistance of wheat against Puccinia striiformis f. sp. tritici. Phytopathology 2021, 111, 1449–1458. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Zhang, L.; Huang, J.; Dang, C.; Xie, C.; Wang, Z. TaRPP13-3, a CC-NBS-LRR-like gene located on chr 7D, promotes disease resistance to wheat powdery mildew in Brock. J. Phytopathol. 2020, 168, 688–699. [Google Scholar] [CrossRef]
- Dinh, H.X.; Singh, D.; Periyannan, S.; Park, R.F.; Pourkheirandish, M. Molecular genetics of leaf rust resistance in wheat and barley. Theor. Appl. Genet. 2020, 133, 2035–2050. [Google Scholar] [CrossRef]
- Kumar Kushwaha, S.; Vetukuri, R.R.; Odilbekov, F.; Pareek, N.; Henriksson, T.; Chawade, A. Differential gene expression analysis of wheat breeding lines reveal molecular insights in yellow rust resistance under field conditions. Agronomy 2020, 10, 1888. [Google Scholar] [CrossRef]
- Long, L.; Li, J.; Huang, L.; Jin, H.; Guan, F.; Zhang, H.; Zhao, Y.; Zhang, Z.; Liu, X.; Wang, Y. Fine mapping and characterization of stripe rust resistance gene YrAYH in near-isogenic lines derived from a cross involving wheat landrace Anyuehong. Crop J. 2024, 12, 826–835. [Google Scholar] [CrossRef]
- Wu, J.; Yu, R.; Wang, H.; Zhou, C.E.; Huang, S.; Jiao, H.; Zhang, Z.; Liu, X. A large-scale genomic association analysis identifies the candidate causal genes conferring stripe rust resistance under multiple field environments. Plant Biotechnol. J. 2021, 19, 177–191. [Google Scholar] [CrossRef]
- Li, H.; Wei, C.; Meng, Y.; Fan, R.; Zhao, W.; Wang, X.; Zhang, J.; Wu, J.; Li, X.; Liu, X.; et al. Identification and expression analysis of some wheat F-box subfamilies during plant development and infection by Puccinia triticina. Plant Physiol. Biochem. 2020, 155, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Gidhi, A.; Mohapatra, A.; Fatima, M.; Jha, S.K.; Kumar, M.; Mukhopadhyay, K. Insights of auxin signaling f-box genes in wheat (Triticum aestivum L.) and their dynamic expression during the leaf rust infection. Protoplasma 2023, 260, 723–739. [Google Scholar] [CrossRef]
- Lu, M.; Zhao, Y.; Feng, Y.; Tang, X.; Zhao, W.; Yu, K.; Li, J.; Zhang, J.; Song, C. 2,4-Dihydroxybenzoic Acid, a Novel SA Derivative, Controls Plant Immunity via UGT95B17-Mediated Glucosylation: A Case Study in Camellia sinensis. Adv. Sci. 2024, 11, 2307051. [Google Scholar] [CrossRef]
- Rasheed, A.; Hao, Y.F.; Xia, X.C.; Khan, A.; Xu, Y.B.; Varshney, R.K.; He, Z.H. Crop breeding chips and genotyping platforms: Progress, challenges, and perspectives. Mol. Plant 2017, 10, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; He, Z.; Gao, F.; Liu, J.; Jin, H.; Zhai, S.; Qu, Y.; Xia, X. A high-density consensus map of common wheat integrating four mapping populations scanned by the 90K SNP array. Front. Plant Sci. 2017, 8, 1389. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- Liu, J.; He, Z.; Wu, L.; Bai, B.; Wen, W.; Xie, C.; Xia, X. Genome-wide linkage mapping of QTL for black point reaction in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2016, 129, 2179–2190. [Google Scholar] [CrossRef]
| QTL | Environment | Genetic Interval | Start (Mb) | End (Mb) | LOD | PVE (%) | Add |
|---|---|---|---|---|---|---|---|
| QYr.gaas-2BS | E1, E3 | AX_109302306- A_108894451 | 96.0 | 102.8 | 2.6–3.9 | 5.0–8.4 | 6.1–8.0 |
| QYr.gaas-2BL | E1, E3, E4 | AX_111590697- AX_110245106 | 672.3 | 697.7 | 2.5–4.0 | 4.8–8.5 | 6.3–8.1 |
| QYr.gaas-2DS | E1, E2, E3 | AX_109080248- AX_110028411 | 96.1 | 102.9 | 3.6–3.9 | 7.5–8.6 | 6.5–8.3 |
| QYr.gaas-2DL | E1, E2, E3 | AX_109529695- AX_109338734 | 596.0 | 608.6 | 2.7–4.5 | 5.2–9.6 | 6.3–8.3 |
| QYr.gaas-3BS | E1, E3, E4 | AX-111732973- AX-110097186 | 2.49 | 8.04 | 2.6–3.8 | 4.9–8.5 | 6.2–7.9 |
| QYr.gaas-4BL | E1, E2, E3 | AX-89396432- AX-111732484 | 665.4 | 667.9 | 3.9–5.6 | 8.6–12.0 | 7.5–10.2 |
| QTL | Candidate Gene | Start (Mb) | Annotation |
|---|---|---|---|
| QYr.gaas-2BS | TraesCS2B02G130600 | 97.9 | F-box family proteins |
| QYr.gaas-2BL | TraesCS2B02G479500 | 676.8 | Disease resistance protein family |
| QYr.gaas-2BL | TraesCS2B02G487600 | 683.7 | Disease resistance protein RGA2 |
| QYr.gaas-2DS | TraesCS2D02G154700 | 98.3 | Serine/threonine-protein kinase |
| QYr.gaas-2DL | TraesCS2D02G505900 | 599.9 | Serine/threonine-protein kinase |
| QYr.gaas-3BS | TraesCS3B02G013100 | 6.06 | Glycosyltransferase |
| QYr.gaas-4BL | TraesCS4B02G391600 | 667.4 | F-box family protein |
| QTL | Genotype | Number of Lines | MDS (%) | p-Value |
|---|---|---|---|---|
| Kasp_2BS_YR | TT | 32 | 45.9 | 0.030 * |
| CC | 75 | 38.0 | ||
| Kasp_2BL_YR | GG | 31 | 43.9 | 0.039 * |
| CC | 77 | 39.1 | ||
| Kasp_2DS_YR | GG | 61 | 37.8 | 0.043 * |
| AA | 49 | 44.2 | ||
| Kasp_2DL_YR | TT | 48 | 43.7 | 0.022 * |
| CC | 63 | 38.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Wang, Y.; Wu, L.; Guo, Y.; Liu, X.; Wang, H.; Zhang, X.; Ren, K.; Bai, B.; Zhan, Z.; et al. Genome-Wide Linkage Mapping of QTL for Adult-Plant Resistance to Stripe Rust in a Chinese Wheat Population Lantian 25 × Huixianhong. Plants 2025, 14, 2571. https://doi.org/10.3390/plants14162571
Yang F, Wang Y, Wu L, Guo Y, Liu X, Wang H, Zhang X, Ren K, Bai B, Zhan Z, et al. Genome-Wide Linkage Mapping of QTL for Adult-Plant Resistance to Stripe Rust in a Chinese Wheat Population Lantian 25 × Huixianhong. Plants. 2025; 14(16):2571. https://doi.org/10.3390/plants14162571
Chicago/Turabian StyleYang, Fangping, Yamei Wang, Ling Wu, Ying Guo, Xiuyan Liu, Hongmei Wang, Xueting Zhang, Kaili Ren, Bin Bai, Zongbing Zhan, and et al. 2025. "Genome-Wide Linkage Mapping of QTL for Adult-Plant Resistance to Stripe Rust in a Chinese Wheat Population Lantian 25 × Huixianhong" Plants 14, no. 16: 2571. https://doi.org/10.3390/plants14162571
APA StyleYang, F., Wang, Y., Wu, L., Guo, Y., Liu, X., Wang, H., Zhang, X., Ren, K., Bai, B., Zhan, Z., & Liu, J. (2025). Genome-Wide Linkage Mapping of QTL for Adult-Plant Resistance to Stripe Rust in a Chinese Wheat Population Lantian 25 × Huixianhong. Plants, 14(16), 2571. https://doi.org/10.3390/plants14162571






