Abstract
Green spaces are essential for urban environments, but urban expansion often results in fragmented patches and narrow pavements unsuitable for tree growth. Consequently, there is a pressing need for alternative vegetation in urban landscapes where tree planting is impractical. Urban spontaneous vegetation (USV)—plants that establish naturally without cultivation—shows promise for urban landscaping, and yet has been underexplored in urban ecology. This study was the first systematic survey to examine the composition of USV in Chiang Mai, Thailand, across seven urban locations. The survey was conducted along 13 sidewalk routes (totaling 33.24 km), documenting all non-tree vascular plant species. A total of 63 USV species from 24 families were recorded, predominantly colonizing pavement gaps, cracks, and curbside cracks. The most diverse family was Poaceae, with 15 species. Among the 61 identified species, 32 species (52%) were non-native. Seven species were found in all surveyed locations, highlighting their adaptability to challenging urban conditions. Fifty USV species are medicinal plants. Many species exhibit characteristics that are ideal for sustainable landscaping, such as drought tolerance, low maintenance requirements, and ornamental value. This study highlights USV as a key component of green infrastructure and provides new insights for urban sustainable landscaping.
1. Introduction
Preserving biodiversity in cities enhances resilience against climate impacts and supports long-term sustainability goals [1,2]. Increasing urban green spaces is essential for sustainable urban systems, as vegetation provides ecosystem services such as air purification, cooling, stormwater regulation, and recreational opportunities [3,4,5,6]. Strategies such as planting trees and creating public parks are widely recognized as effective nature-based solutions for expanding urban green space [7,8]. However, as urban areas develop, the growing number of roads and buildings leave fragmented patches and/or narrow pavements unsuitable for growing trees [9]. Thus, the identification of alternative vegetation for urban landscaping is necessary to sustain urban biodiversity, particularly in areas where trees cannot be planted.
Vegetation in urban environments falls into three broad categories: (1) planted vegetation in managed areas, including parks and street trees; (2) remnant natural habitats; and (3) spontaneous vegetation [10]. Urban spontaneous vegetation (USV) refers to plants that establish naturally without cultivation [11] and are not remnants of natural habitats [10]. USV plays a pivotal role in ecological regeneration within urban areas and provides a variety of ecosystem services [11]. Some USV impose minimal costs on managers [12,13,14]. Urban spontaneous plants can thrive in harsh substrates, such as hard surfaces that lack rooting space, and have limited substrate areas, low moisture availability, and experience disturbances from trampling or urban pollution [14,15,16,17]. Despite these benefits, unmanaged USV may also pose challenges in pedestrian areas. For example, the growth of certain species can disrupt hard surfaces, create uneven pavements, and potentially hinder pedestrian accessibility or clog storm drains [14,15,16,18,19].
USV studies were started in European countries in the 1970s to find alternatives for low-maintenance green spaces in urban areas. In Europe, USV (e.g., perennials, herbaceous dicotyledonous species, non-graminoid monocotyledonous plants, and geophytes) was used in urban meadows because it provides ecosystem services similar to lawns but requires less maintenance [13]. For other countries, such as China, Japan, Iran, and India, the study of USV has only recently received more attention from ecologists. Their studies focus on the composition, diversity, and response mechanisms of USV to urban conditions. It was found that most of the USV in each study area were herbaceous plants, and most of the families were Poaceae and Asteraceae [10,20,21,22]. In Thailand, most studies on spontaneous vegetation have focused on weed control in agricultural areas [23,24,25,26,27]. There has been no study conducted in urban areas.
This study is the first to provide information about USV in the highly developed city of Chiang Mai, Thailand. It aims to determine the species composition and morphological characteristics of USV on urban sidewalks. By identifying species that naturally thrive in these challenging urban conditions, this study contributes to a broader understanding of low-maintenance, biodiversity-friendly landscaping strategies for tropical cities. The findings provide the list of species that can inform future urban greening initiatives, particularly in drought-prone, space-limited areas unsuitable for tree planting.
2. Results
2.1. Pavement Type and Species Found
The roadside pavement consists of two main types of surfaces: (1) solid pavement, such as monolithic concrete pavement or stamped concrete; and (2) tile pavement, which comprises various sizes and types of tiles. USV emerges on curbside cracks of the road, gaps between tile pavements, and cracked pavements. Additionally, in solid pavement, USV is commonly found in regions exhibiting pavement deterioration (Figure 1).
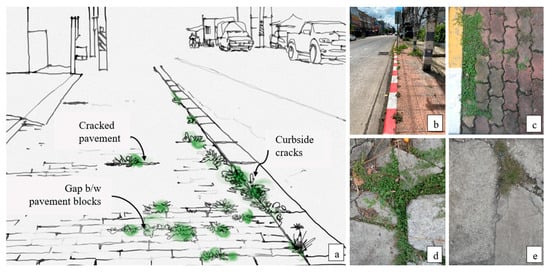
Figure 1.
USVs on the Chiang Mai roadside (a). USVs predominantly thrived in curbside cracks (b), tile pavement gaps (c), cracked tile (d), and solid (e) pavement, along urban roads.
In total, there were 61 identified species and 2 unknown species of USV (Table 1). Two species remained unknown due to unsuitable phenological phases for identification in the survey. Moreover, the permanent elimination of USVs by the city’s staff significantly hinders subsequent efforts to identify these two species. The USV found in this study belonged to 24 families and 49 genera. The most represented USV families were Poaceae (15 species) and Asteraceae (8 species). Poaceae and Asteraceae families accounted for 23 species, representing ~37% of the total recorded USV species. There were seven species—Eleusine indica, Eragrostis tenella, Euphorbia hirta, Euphorbia thymifolia, Evolvulus nummularius, Odenlandia corymbosa, and Phyllanthus amarus—found across all surveyed locations (Figure 2).

Table 1.
USV species recorded in this survey, their native status, their growth habit and life cycle, and some of their pharmacological activities (NAs in the pharmacological activities and references column indicate no pharmacological activities have been reported).

Figure 2.
The seven species commonly found in all seven surveyed locations were Eleusine indica (a), Eragrostis tenella (b), Euphorbia hirta (c), Euphorbia thymifolia (d), Evolvulus nummularius (e), Odenlandia corymbosa (f), and Phyllanthus amarus (g).
The surveyed USV comprised annual plants (57% of the total species) and perennial plants (43% of the total species) (Figure 3). In terms of plant life cycle, upright annual species had the highest proportion of 38%, followed by lying flat perennial species (25%), lying flat annual species (24%), and upright perennial species (13%) (Figure 3). Among lying flat species, four distinct habits were observed: prostrate (11 species), stoloniferous (7 species), procumbent (4 species), and decumbent (4 species). Upright species encompassed four habits: erect (20 species), ascending (5 species), tussock (3 species), and scandent (1 species). Additionally, geniculate growth habits represented five species.
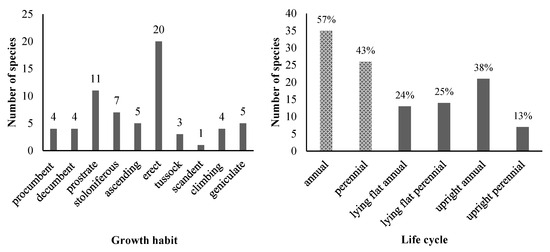
Figure 3.
The number of USV species by growth habit (left) and life cycle (right). In the right-hand side graph, the values on top of the bars show the percentage out of the total of 63 species.
2.2. Native Species of Thailand
Of the 61 species recorded, 29 (48%) were native to Thailand. The most represented family among native species was Poaceae, with 9 species, Eleusine indica and Eragrostis tenella among them, both of which were recorded at all surveyed locations (Figure 2).
Most native species (16 species) exhibited an annual life cycle. In terms of growth habit, many species were lying flat, such as Grona triflora and Indigofera hendecaphylla, which are both members of the Fabaceae family (Table 1). Oldenlandia corymbosa (Figure 2) was found at all surveyed locations and exhibited two growth habits: erect and procumbent.
2.3. Alien and Invasive Alien Species
A total of 32 alien species accounted for 52% of the USV recorded. Most of these alien species (19 species) were annual plants, and the majority exhibited an upright growth habit. Some species displayed two distinct growth habits within the same species; for example, Euphorbia hirta appeared in both erect and prostrate forms, while Portulaca pilosa showed prostrate and ascending habits. Overall, 53% of the alien species belonged to the family Asteraceae, with Bidens pilosa and Tridax procumbens being notable members of this group (Table 1).
Among the alien species, 19 (31% of all USV) were identified as invasive alien species in Thailand. Of the seven species recorded at all surveyed locations, four were alien species—including Euphorbia hirta, Evolvulus nummularius, and Phyllanthus amarus—all of which are recognized as invasive (Figure 2).
3. Discussion
3.1. Urban Roadside Habitats Provide Living Space for USVs of Different Growth Habits and Life Cycles
Urban roadside microhabitats, particularly in pavement gaps and curbside cracks, predominantly provide habitats for USVs. It is likely that wider gaps or cracked pavements facilitate the accumulation of rooting substrates, which provide essential resources for plant growth, such as water and nutrients. Nutrients on urban pavements can originate from multiple sources, including decaying organic matter (e.g., leaves and plant debris), wind-blown dust and soil particles, animal waste, rainwater runoff carrying dissolved nutrients, pollution deposits, and human activities such as littering. These varied nutrient sources contribute to the formation of microhabitats that support both plant growth and the presence of USV [82,83].
Despite spatial limitations, environmental disturbances, and high trampling pressure, roadside habitats can support a diverse range of USVs, with 63 species found across 24 families. These results underscore the ecological potential of constrained urban environments to sustain biodiversity despite harsh conditions. The results align with previous studies that identified Poaceae and Asteraceae as the most common spontaneous plant families in urban areas in China [10,84,85,86] and Japan [20,21,22]. Species from these two families are more prevalent than others among both native and alien species globally [87], and they are widespread, possessing a broad ecological niche that allows them to adapt to diverse habitats [85].
Morphological and physiological adaptations enable Poaceae species to thrive in arid and disturbed urban environments. In this study, grasses such as Cynodon dactylon, Eleusine indica, and Dactyloctenium aegyptium were found growing on compacted soils and roadside pavements. These species are known for drought-tolerance traits. For instance, C. dactylon has a deep root system, high water-use efficiency, and the ability to activate antioxidant enzymes and accumulate osmolyte under water stress [88,89]. E. indica has thickened epidermal layers and expanded cortical cells, which reduce water loss and give aridity tolerance. D. aegyptium adapts by reducing leaf area and closing stomata under water deficit conditions, thereby maintaining growth and water-use efficiency [90]. Other species recorded in the study, such as Eragrostis tenella and Chloris barbata, have structural traits—enlarged vascular bundles, sclerenchyma reinforcement, and Kranz anatomy—that enhance survival and photosynthetic efficiency in a semi-arid environment [91,92].
In addition to morphological and physiological traits, species reproductive strategies enhance the ability to colonize harsh urban habitats. E. indica can produce over 120,000 seeds per plant and establish within 38 days [93]. C. dactylon spreads vegetatively through stolons and rhizomes, facilitating regeneration even in challenging conditions [94]. Asteraceae species such as Tridax procumbens and Cyanthillium cinereum exhibit seed and fruit traits that promote survival and dispersal in disturbed urban microhabitats. Both produce dry, single-seeded achenes topped with pappus structures, enabling effective wind dispersal across compacted and fragmented substrates [95,96]. These reproductive adaptations support their rapid colonization of drought-prone, space-limited, and low-maintenance urban environments.
In our study, almost 60% of USVs were annual plants. When comparing the proportions of growth habit and life cycle, upright annual USVs had the highest proportion. This is consistent with a study conducted in Xi’an, China, which found that most herbaceous species along urban roadsides were annuals with an upright growth habit [10]. Annual plants tend to dominate urban areas due to their adaptability to disturbed environments. Their rapid life cycles allow them to complete growth and reproduction before further disruptions occur. These traits, along with their ability to colonize open spaces and outcompete perennials for limited resources, make annual plants more prevalent in urban settings, particularly in poor, compacted soils [97,98,99,100] and in areas with high disturbance by human management [84].
3.2. Functional Roles and Greening Potential of USVs
USVs exhibit a range of functional traits that position them as strong candidates for low-maintenance urban greening. Traits such as drought tolerance, compact growth habits, and rapid reproduction enable USVs to thrive in degraded soils and overlooked microhabitats. These characteristics support their persistence in challenging urban environments and contribute to important ecological functions, including enhancing vegetative cover and providing habitats for various organisms.
Although this study did not formally assess habitat functions, repeated field observations of insects visiting Evolvulus nummularius and Tridax procumbens suggest that these USVs contribute to pollinator activity in urban areas (Figure 4a–c). Occasional sightings of other arthropods on various USVs (Figure 4d–f) further indicate potential faunal interactions. Similar findings from urban studies have emphasized the role of spontaneous vegetation in boosting pollinator richness within parks and abandoned lots [101,102,103,104,105]. These findings underscore the ecological potential of USVs beyond just vegetative cover.

Figure 4.
USV supports pollinators and insects: (a) A honeybee on the flower of Evolvulus nummularius; (b) A honeybee landing on the flower of Tridax procumbens; (c) A ladybird beetle on the leaf of T. procumbens; (d) A stingless bee and a caterpillar on the flower of Cyanthillium cinereum; (e) A Meranoplus bicolor ant on Grona triflora; and (f) A lynx spider on the petal of Ruellia tuberosa.
Many species can be alternatives to conventional turfgrass in urban settings. Low-growing species such as Grona triflora, Evolvulus nummularius, and Ruellia prostrata commonly form dense, mat-like vegetation that spreads horizontally across bare ground (Figure 5a–c). This growth habit aids soil stabilization and visual integration in the landscape. In addition, their minimal maintenance requirements—requiring little to no irrigation or mowing—further enhance their value for sustainable landscape management. Landscape designers in Thailand have recently adopted E. nummularius as a turfgrass substitute (Figure 5d) and as understory vegetation in coconut orchards to reduce mowing and herbicide use (Figure 5e). Meanwhile, flowering groundcovers such as R. prostrata contribute to ecological aesthetics—a principle that emphasizes the emotional, cultural, and visual appeal of green spaces [106,107]—potentially encouraging public acceptance and appreciation of spontaneous urban flora.

Figure 5.
(a) Grona triflora, (b) Evolvulus nummularius, and (c) Ruellia prostrata exhibit a creeping, prostrate growth habit, making them effective ground covers that soften hard edges in the landscape and fill gaps between larger plants or hardscape elements such as pathways and rocks. (d) E. nummularius used as ground cover in a garden setting, and (e) as understory vegetation in a coconut orchard in Thailand.
Cultural ecosystem services further enrich the value of USVs. For example, Cynodon dactylon (Ya Praek) plays a key role in Thailand’s teacher appreciation ceremony (Pitee Wai Kru) [108], while Oxalis corniculata serves traditional purposes such as cleaning silverware [109]. Additionally, over 80% (50 species) of the USVs recorded in this study have been reported in the scholarly literature to exhibit pharmacological properties, including wound healing, anti-inflammatory, antioxidant, and antimicrobial activities (Table 1), highlighting their relevance to local knowledge and practices.
3.3. Alien Species in Urban Environments
The ecological roles of alien plants in urban environments are still not well understood. While some alien species may contribute to urban ecosystem services, such as enhancing vegetative cover or supporting pollinators, others may outcompete native species and become invasive, posing ecological risks [110,111]. This study adopts a neutral stance on the presence of alien species in cities. The primary objective of this study was not to assess the status of non-native species or propose pavement management strategies. The study provides information on what USV species are alien and invasive alien species (Table 1).
More than half (52%) of the identified USVs were alien species, aligning with previous research suggesting that urban areas act as hotspots for alien species [112]. Previous studies have shown that alien plant species often outperform native species in terms of growth, reproduction, and resistance to natural enemies [113]. Moreover, as urbanization intensifies, native species numbers tend to decline [114,115,116]. In Asia, the number of recognized alien plant species is already substantial, and their spread is expected to increase further due to ineffective management, changes in land use and climate, and the expansion of international trade, travel, and transportation [117].
Being easy to disperse and escaping natural enemies allows alien species to thrive in urban environments. For example, Bidens pilosa and Tridax procumbens are globally recognized invasive species, particularly in tropical and subtropical regions [118]. Seeds of B. pilosa and T. procumbens are wind-dispersed, allowing them to effectively colonize disturbed environments [20,21]. In addition, small seeds of Euphorbia thymifolia and Oldenlandia corymbosa may be dispersed easily through trampling by pedestrians or by attaching to the wheels of vehicles. Additionally, these species likely benefit from escaping pathogens and herbivores, as well as possessing broad habitat adaptability, allowing them to thrive in urban environments [87]. It is important to recognize the invasive alien species and prevent their spread into natural and/or protected ecosystems, where risks to biodiversity and ecological integrity are significantly higher.
3.4. Integrating USVs into Urban Design and Management: Implications and Research Needs
Rather than viewing USVs as nuisances, urban planners and designers can harness their ecological resilience and cultural significance to reduce management costs, promote biodiversity, and reinforce local identity. Future research should aim to identify trait combinations that optimize ecosystem service delivery across diverse urban contexts. However, integrating USVs into urban design must be approached with care. Some species, such as Cenchrus echinatus, produce sharp burs that may pose risks to pedestrians and animals. Such examples highlight the need for trait-based evaluations to distinguish beneficial from potentially harmful species.
In addition, urban pavement maintenance should consider both the function of urban spaces and the composition of USV species present. In densely built-up grey spaces, such as metropolitan areas, the spread of USVs is typically limited due to the restrictive nature of existing pavement materials. However, where invasive alien species are detected, more intensive removal efforts are warranted to prevent their further spread. Conversely, in areas where USVs are native or consist of non-invasive alien species, reduced pavement maintenance may be sufficient to support their persistence. Future research should examine how adaptive maintenance regimes—those that permit USVs to persist in stable microhabitats while actively managing invasive species—can help balance biodiversity conservation with urban infrastructure management.
This study has certain limitations that also present opportunities for future research. First, the number of USV species may be underestimated because the survey was conducted during a single season. Conducting surveys across multiple seasons could help detect species that emerge or establish at different times of the year. Second, the discussion on the potential roles of USV, such as pollinator support (Figure 4) and soil stabilization (Figure 5), is based on field observations made during the plant surveys. Future studies employing systematic assessments are needed to better understand these roles and to explore additional ecosystem functions provided by USVs. Third, this study provides qualitative data in the form of a species list without information on species abundance. Future studies focusing on species abundance and the environmental factors influencing the abundance and distribution of USVs would give deeper insights into effective management in urban ecosystems.
4. Materials and Methods
4.1. Study Area
The study focused on the rapidly developing city of Chiang Mai. Chiang Mai is in northern Thailand along the Ping River (18.78° N latitude and 98.98° E longitude). The city has an average elevation of 313 m above sea level [119]. In 2023, Chiang Mai received approximately 1188 mm of rainfall [120], with temperatures ranging from 22 °C to 34 °C [121]. As one of the fastest-growing cities, Chiang Mai has seen significant expansion in urban communities, industrial sectors, and the tourism industry, driving both economic and social development in the region [122,123,124].
The studied areas were busy roads in urban high-density residential and commercial zones (red colored zones, Figure 6). The surveyed areas were classified as high-density residential and commercial zones by the Ministry of Interior B.E. 2564 (2021). There were seven sampling locations: (1) Mueang Chiang Mai District (23.12 km), (2) Mae Rim District (2 km), (3) Maejo municipality (1.56 km), (4) San Sai District (0.76 km), (5) San Kamphaeng District (2.56 km), (6) Saraphi District (0.92 km), and (7) Hang Dong District (2.32 km) (Figure 6). The total linear distance was 33.24 km.
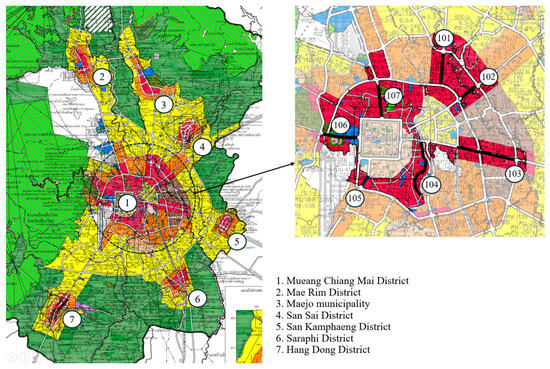
Figure 6.
Seven survey locations in urban high-density residential and commercial zones (left) and seven surveyed sidewalk routes (bold black lines, numbered 101–107) within Location (1) Mueang Chiang Mai District (right).
4.2. Data Collection and Analysis
To determine species composition and characteristics of USV on urban sidewalks, a sampling zone (a sidewalk) was defined as a linear space bounded on one side by a road. The survey covered 13 sidewalk routes. Each location consisted of a single survey route, which was a main road with heavy traffic, except for the location in Mueang Chiang Mai District, which had seven surveyed routes (Figure 6). The plant survey was conducted from June to July 2023, a rainy season in Thailand.
We walked along the sidewalk, and the species of plants growing within the sidewalk area were recorded. For all species found in the sampling zones, plant parts were collected for voucher specimens and identified using the taxonomic literature (i.e., Flora of Thailand, Flora of China, Flora of Java, and Thai Forest Bulletin). The growth habits of USVs were divided into lying flat, upright, and climbing. Other growth habits not included in this division were recorded separately [125,126,127,128] (Table 2). The life cycles were divided into annual and perennial. The growth habits and life cycles of USVs were counted and compared. In addition, we identified non-native and native species based on Plants of the World Online, facilitated by the Royal Botanic Gardens, Kew [129]. Alien invasive species were identified using the list of alien invasive plants in Thailand [130].

Table 2.
The category and characteristics of plant growth habits.
5. Conclusions
This study provides the first comprehensive survey of urban spontaneous vegetation (USV) in Chiang Mai, Thailand, focusing on their species composition and potential use in urban greening. A total of 63 USV species from 24 families were identified, with Poaceae and Asteraceae as the dominant families. The widespread occurrence of certain species across all surveyed sites demonstrates their adaptability to harsh urban conditions, while the predominance of annual species highlights their capacity to colonize disturbed and compacted soils rapidly.
Although this study did not directly assess ecological functions or ecosystem services, field observations indicate that some USV species contribute to urban biodiversity and provide habitats for animals. Many species are drought-tolerant and have low-maintenance traits, suggesting their suitability for enhancing urban green spaces, particularly in fragmented areas where trees cannot establish, such as small urban gardens, roadside verges, and median strips.
More than half of the USV species observed were alien species, some of which may threaten native biodiversity by outcompeting local species and disrupting ecological interactions such as pollination and seed dispersal. These insights highlight the need for targeted management to minimize the ecological risks posed by invasive alien species while harnessing the functional and aesthetic benefits of USV. Future research should focus on developing long-term strategies for integrating USV into urban landscapes, including the identification of resilient and beneficial species, the assessment of pavement maintenance practices, and the implementation of adaptive regimes across varied urban contexts.
Author Contributions
Conceptualization, N.C. and P.T.; data curation, N.C.; formal analysis, N.C.; funding acquisition, N.C. and P.T.; investigation, N.C. and B.B.; methodology, N.C., A.I. and P.T.; supervision, A.I., D.P.S. and P.T.; validation, A.I. and P.T.; visualization, N.C.; writing—original draft, N.C. and P.T.; writing—review and editing, N.C., A.I., D.P.S., B.B. and P.T. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to thank Chiang Mai University for financial support (TA/RA scholarship awarded to NC and the article processing charge).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank Harmless Harvest (Thailand) Ltd., Samut Sakhon, Thailand for a picture of using Evolvulus nummularius in a coconut orchard. We also thank Pongsakorn Suppakittpaisarn (Chiang Mai University) for providing valuable information on urban landscape planning.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Aronson, M.F.; Lepczyk, C.A.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S.; Nilon, C.H.; Vargo, T. Biodiversity in the city: Key challenges for urban green space management. Front. Ecol. Environ. 2017, 15, 189–196. [Google Scholar] [CrossRef]
- Lehmann, S. Growing biodiverse urban futures: Renaturalization and rewilding as strategies to strengthen urban resilience. Sustainability 2021, 13, 2932. [Google Scholar] [CrossRef]
- Semeraro, T.; Scarano, A.; Buccolieri, R.; Santino, A.; Aarrevaara, E. Planning of urban green spaces: An ecological perspective on human benefits. Land 2021, 10, 105. [Google Scholar] [CrossRef]
- Edeigba, B.A.; Ashinze, U.K.; Umoh, A.A.; Biu, P.W.; Daraojimba, A.I.; Edeigba, B.; Ashinze, U.; Umoh, A.; Biu, P.; Daraojimba, A. Urban green spaces and their impact on environmental health: A Global Review. World J. Adv. Res. Rev. 2024, 21, 917–927. [Google Scholar] [CrossRef]
- Grunewald, K.; Bastian, O. Maintaining ecosystem services to support urban needs. Sustainability 2017, 9, 1647. [Google Scholar] [CrossRef]
- Bolund, P.; Hunhammar, S. Ecosystem services in urban areas. Ecol. Econ. 1999, 29, 293–301. [Google Scholar] [CrossRef]
- Bank, W. A Catalogue of Nature-Based Solutions for Urban Resilience; World Bank: Washington, WA, USA, 2021. [Google Scholar]
- Cortinovis, C.; Olsson, P.; Hedlund, K. Scaling up nature-based solutions for climate-change adaptation: Potential and benefits in three European cities. Urban For. Urban Green. 2022, 67, 127450. [Google Scholar] [CrossRef]
- O’Sullivan, O.S.; Holt, A.R.; Warren, P.H.; Evans, K.L. Optimising UK urban road verge contributions to biodiversity and ecosystem services with cost-effective management. J. Environ. Manag. 2017, 191, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, E.; Lundholm, J.; Du, X. Spontaneous urban vegetation and habitat heterogeneity in Xi’an, China. Landsc. Urban Plan. 2013, 120, 25–33. [Google Scholar] [CrossRef]
- Mathey, J.; Arndt, T.; Banse, J.; Rink, D. Public perception of spontaneous vegetation on brownfields in urban areas—Results from surveys in Dresden and Leipzig (Germany). Urban For. Urban Green. 2018, 29, 384–392. [Google Scholar] [CrossRef]
- Kühn, N. Intentions for the unintentional: Spontaneous vegetation as the basis for innovative planting design in urban areas. J. Landsc. Archit. 2006, 1, 46–53. [Google Scholar] [CrossRef]
- Bretzel, F.; Vannucchi, F.; Romano, D.; Malorgio, F.; Benvenuti, S.; Pezzarossa, B. Wildflowers: From conserving biodiversity to urban greening—A review. Urban For. Urban Green. 2016, 20, 428–436. [Google Scholar] [CrossRef]
- Del Tredici, P. Spontaneous urban vegetation: Reflections of change in a globalized world. Nat. Cult. 2010, 5, 299–315. [Google Scholar] [CrossRef]
- Colwill, S. The Root of the Problem: Addressing the Conflicts Between Spontaneous Vegetation and Built Landscape; Technische Universität Berlin: Berlin, Germany, 2019. [Google Scholar]
- Lundholm, J. Vegetation of urban hard surfaces. In Urban Ecology: Patterns, Processes, and Applications; Cambridge University Press: Cambridge, UK, 2011; pp. 93–102. [Google Scholar]
- Yalcinalp, E.; Meral, A. Wall vegetation characteristics of urban and sub-urban areas. Sustainability 2017, 9, 1691. [Google Scholar] [CrossRef]
- Benvenuti, S. Weed dynamics in the Mediterranean urban ecosystem: Ecology, biodiversity and management. Weed Res. 2004, 44, 341–354. [Google Scholar] [CrossRef]
- Rask, A.M. Non-Chemical Weed Control on Hard Surfaces: An Investigation of Long-Term Effects of Thermal Weed Control Methods; Forest & Landscape: Aalborg, Denmark, 2012. [Google Scholar]
- Hayasaka, D.; Akasaka, M.; Miyauchi, D.; Box, E.O.; Uchida, T. Qualitative variation in roadside weed vegetation along an urban–rural road gradient. Flora-Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 126–132. [Google Scholar] [CrossRef]
- Hayasaka, D.; Akasaka, M.; Miyauchi, D.; Uchida, T. Classification of roadside weeds along two highways in different climatic zones according to ecomorphological traits. Weed Technol. 2011, 25, 411–421. [Google Scholar] [CrossRef]
- Uchida, T.; Xue, J.; Hayasaka, D.; Arase, T.; Haller, W.T.; Gettys, L.A. The relation between road crack vegetation and plant biodiversity in urban landscape. GEOMATE J. 2014, 6, 885–891. [Google Scholar] [CrossRef]
- Pinsupa, J.; Chindakul, A.; Intanon, S. Distribution and resistance of barnyardgrass to quinclorac in rice fields in Thailand. Adv. Weed Sci. 2022, 40, e020220004. [Google Scholar] [CrossRef]
- Chouychai, W.; Somtrakoon, K. Weed selection for phytoremediation of fluoranthene. J. Agric. Res. Ext. 2019, 36, 1–10. [Google Scholar]
- Bundit, A.; Ostlie, M.; Prom-U-Thai, C. Sunn hemp (Crotalaria juncea) weed suppression and allelopathy at different timings. Biocontrol Sci. Technol. 2021, 31, 694–704. [Google Scholar] [CrossRef]
- Aekrathok, P.; Songsri, P.; Jongrungklang, N.; Gonkhamdee, S. Efficacy of post-emergence herbicides against important weeds of sugarcane in North-East Thailand. Agronomy 2021, 11, 429. [Google Scholar] [CrossRef]
- Srithi, K.; Balslev, H.; Tanming, W.; Trisonthi, C. Weed diversity and uses: A case study from tea plantations in northern Thailand. Econ. Bot. 2017, 71, 147–159. [Google Scholar] [CrossRef]
- Zahidin, N.S.; Saidin, S.; Zulkifli, R.M.; Muhamad, I.I.; Ya’akob, H.; Nur, H. A review of Acalypha indica L.(Euphorbiaceae) as traditional medicinal plant and its therapeutic potential. J. Ethnopharmacol. 2017, 207, 146–173. [Google Scholar] [CrossRef]
- Mourya, P.; Sharma, N.K.; Gupta, M. Antioxidant activity of ethanolic and aqueous extracts ofAlternanthera pungens Kunth. Asian J. Pharm. Pharmacol. 2019, 5, 1091–1096. [Google Scholar] [CrossRef]
- Saqib, F.; Janbaz, K.H. Rationalizing ethnopharmacological uses of Alternanthera sessilis: A folk medicinal plant of Pakistan to manage diarrhea, asthma and hypertension. J. Ethnopharmacol. 2016, 182, 110–121. [Google Scholar] [CrossRef]
- Reyad-ul-Ferdous, M.; Shahjahan, D.S.; Tanvir, S.; Mukti, M. Present biological status of potential medicinal plant of Amaranthus viridis: A comprehensive review. Am. J. Clin. Exp. Med. 2015, 3, 12. [Google Scholar] [CrossRef]
- Rahman, F.M.; Kabir, S.F.; Nurnabi, M.; Chowdhury, A.S.; Sikder, M.A.A. Chemical and Biological Investigations of Axonopus compressus (Sw.) P. Beauv. Bangladesh Pharm. J. 2014, 17, 113–115. [Google Scholar] [CrossRef]
- Xuan, T.D.; Khanh, T.D. Chemistry and pharmacology of Bidens pilosa: An overview. J. Pharm. Investig. 2016, 46, 91–132. [Google Scholar] [CrossRef]
- Mishra, S.; Aeri, V.; Gaur, P.K.; Jachak, S.M. Phytochemical, therapeutic, and ethnopharmacological overview for a traditionally important herb: Boerhavia diffusa Linn. BioMed Res. Int. 2014, 2014, 808302. [Google Scholar] [CrossRef]
- Dey, A.; Abu, A.; Shakar, S.; Rahman, A.; Amin, M. Evaluation of the anti-inflammatory and antipyretic activities of the plant Boerhavia repens.(Family: Nyctaginaceae). J. Nat. Prod. Plant Resour. 2012, 2, 471–474. [Google Scholar]
- Silva, A.A.; Haraguchi, S.K.; Cellet, T.S.P.; Schuquel, I.T.A.; Sarragiotto, M.H.; Vidotti, G.J.; de Melo, J.O.; Bersani-Amado, C.A.; Zanoli, K.; Nakamura, C.V. Resveratrol-derived stilbenoids and biological activity evaluation of seed extracts of Cenchrus echinatus L. Nat. Prod. Res. 2012, 26, 865–868. [Google Scholar] [CrossRef]
- Natrajan, P.; Elumalai, A.; Soundarajan, C.; Iyyampalayam, T. Study of Antibacterial activity of Chloris barbata (SW) Leaves. Int. Res. J. Pharm. Appl. Sci. 2012, 2, 37–40. [Google Scholar]
- Omokhua, A.G.; McGaw, L.J.; Finnie, J.F.; Van Staden, J. Chromolaena odorata (L.) RM King & H. Rob.(Asteraceae) in sub-Saharan Africa: A synthesis and review of its medicinal potential. J. Ethnopharmacol. 2016, 183, 112–122. [Google Scholar]
- Ghosh, P.; Chatterjee, S.; Das, P.; Karmakar, S.; Mahapatra, S. Natural habitat, phytochemistry and pharmacological properties of a medicinal weed—Cleome rutidosperma DC. (Cleomaceae): A comprehensive review. Int. J. Pharm. Sci. Res. 2019, 10, 1605–1612. [Google Scholar]
- Pekamwar, S.; Kalyankar, T.; Kokate, S. Pharmacological activities of Coccinia grandis. J. Appl. Pharm. Sci. 2013, 3, 114–119. [Google Scholar]
- Ghosh, P.; Dutta, A.; Biswas, M.; Biswas, S.; Hazra, L.; Nag, S.K.; Sil, S.; Chatterjee, S. Phytomorphological, chemical and pharmacological discussions about Commelina benghalensis Linn. (Commelinaceae): A review. Pharma Innov. J. 2019, 8, 12–18. [Google Scholar]
- Thongkhao, K.; Pongkittiphan, V.; Phadungcharoen, T.; Tungphatthong, C.; Urumarudappa, S.K.J.; Pengsuparp, T.; Sutanthavibul, N.; Wiwatcharakornkul, W.; Kengtong, S.; Sukrong, S. Differentiation of Cyanthillium cinereum, a smoking cessation herb, from its adulterant Emilia sonchifolia using macroscopic and microscopic examination, HPTLC profiles and DNA barcodes. Sci. Rep. 2020, 10, 14753. [Google Scholar] [CrossRef]
- Khatun, P.; Das, S.K.; Khulna, B. Medicinal and versatile uses of an amazing, obtainable and valuable grass: Cynodon dactylon. Int. J. Pharm. Med. Res. 2020, 8, 1–11. [Google Scholar]
- Kabir, I.; Biswas, S.; Asaduzzaman, M.; Molla, M.; Rafe, M. Neurobehavioral activity study of methanolic whole plants extract of Cyperus rotundus Linn. J. Pharm. Negat. Results 2019, 10, 36–40. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. A review on Cyperus rotundus A potential medicinal plant. IOSR J. Pharm. 2016, 6, 32–48. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Xu, X.; Sulieman, A.A.; Mahdi, A.A.; Na, Y. Effect of extraction conditions on phenolic compounds and antioxidant properties of koreeb (Dactyloctenium aegyptium) seeds flour. J. Food Meas. Charact. 2020, 14, 799–808. [Google Scholar] [CrossRef]
- Karthikumar, S.; Vigneswari, K.; Jegatheesan, K. Screening of antibacterial and antioxidant activities of leaves of Eclipta prostrata (L). Sci. Res. Essays 2007, 2, 101–104. [Google Scholar]
- Adoho, A.C.C.; Zinsou, F.; Olounlade, P.; Azando, E.; Hounzangbe-Adote, M.S.; Gbangboche, A.B. Review of the literature of Eleusine indica: Phytochemical, toxicity, pharmacological and zootechnical studies. J. Pharmacogn. Phytochem. 2021, 10, 29–33. [Google Scholar] [CrossRef]
- Uche, M.E.; Chinyerea, C.G.; Ekweogu, C.N.; Nwankpa, P.; Ugbogu, E.A. Phytochemical analysis, toxicity assessment, and wound healing properties of Emilia sonchifolia L. leaf extract in rats. S. Afr. J. Bot. 2024, 172, 736–746. [Google Scholar] [CrossRef]
- Petrelli, R.; Orsomando, G.; Sorci, L.; Maggi, F.; Ranjbarian, F.; Biapa Nya, P.C.; Petrelli, D.; Vitali, L.A.; Lupidi, G.; Quassinti, L. Biological activities of the essential oil from Erigeron floribundus. Molecules 2016, 21, 1065. [Google Scholar] [CrossRef]
- Kausar, J.; Muthumani, D.; Hedina, A.; Anand, V. Review of the phytochemical and pharmacological activities of Euphorbia hirta Linn. Pharmacogn. J. 2016, 8, 310–313. [Google Scholar] [CrossRef]
- Kumar, S.; Malhotra, R.; Kumar, D. Euphorbia hirta: Its chemistry, traditional and medicinal uses, and pharmacological activities. Pharmacogn. Rev. 2010, 4, 58. [Google Scholar] [CrossRef]
- Mali, P.Y.; Panchal, S.S. A review on phyto-pharmacological potentials of Euphorbia thymifolia L. Anc. Sci. Life 2013, 32, 165–172. [Google Scholar]
- Pavithra, P.; Sreevidya, N.; Verma, R.S. Antibacterial and antioxidant activity of methanol extract of Evolvulus nummularius. Indian J. Pharmacol. 2009, 41, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Macorini, L.F.B.; Radai, J.A.S.; Maris, R.S.; Silva-Filho, S.E.; Leitao, M.M.; Andrade, S.F.d.; Gelves, D.I.A.; Salvador, M.J.; Arena, A.C.; Kassuya, C.A.L. Antiarthritic and antihyperalgesic properties of ethanolic extract from Gomphrena celosioides Mart. (Amaranthaceae) aerial parts. Evid. Based Complement. Altern. Med. 2020, 2020, 4170589. [Google Scholar] [CrossRef]
- Lai, S.-C.; Ho, Y.-L.; Huang, S.-C.; Huang, T.-H.; Lai, Z.-R.; Wu, C.-R.; Lian, K.-Y.; Chang, Y.-S. Antioxidant and antiproliferative activities of Desmodium triflorum (L.) DC. Am. J. Chin. Med. 2010, 38, 329–342. [Google Scholar] [CrossRef]
- Choudhury, S.; Rahaman, C.H.; Mandal, S. Studies on Ipomoea cairica (L.) sweet-A promising ethnomedicinally important plant. J. Innov. Pharm. Biol. Sci. 2015, 2, 378–395. [Google Scholar]
- Mungole, A.J.; Awati, R.; Chaturvedi, A.; Zanwar, P. Preliminary Phytochemical screening of Ipomoea obscura (L)-A hepatoprotective medicinal plant. Int. J. PharmTech Res. 2010, 2, 2307–2312. [Google Scholar]
- Hamsa, T.; Kuttan, G. Evaluation of the anti-inflammatory and anti-tumor effect of Ipomoea obscura (L) and its mode of action through the inhibition of pro inflammatory cytokines, nitric oxide and COX-2. Inflammation 2011, 34, 171–183. [Google Scholar] [CrossRef]
- Sanghai, D.B.; Kumar, S.V.; Srinivasan, K.; Aswatharam, H.; Shreedhara, C. Pharmacognostic and phytochemical investigation of the leaves of Malvastrum coromandelianum (L.) Garcke. Anc. Sci. Life 2013, 33, 39–44. [Google Scholar] [CrossRef]
- Mamatha, B.; Palaksha, M.; Gnanasekaran, D.; Senthilkumar, G.; Tamizmani, T. Melochia corchorifolia L: A review. World J. Pharm. Res. 2018, 7, 482–491. [Google Scholar]
- Das, S.; Mondal, N.; Mondal, S.; Ghosh, P.; Ghosh, C.; Das, C.; Chatterjee, S.; Sirshendu Chatterjee, C. Botanical features, phytochemical and pharmacological overviews of Oldenlandia corymbosa Linn.: A brief review. Pharma Innov. J. 2019, 8, 464–468. [Google Scholar]
- Srikanth, M.; Swetha, T.; Veeresh, B. Phytochemistry and pharmacology of Oxalis corniculata Linn.: A review. Int. J. Pharm. Sci. Res. 2012, 3, 4077. [Google Scholar]
- Mohanasundari, C.; Natarajan, D.; Srinivasan, K.; Umamaheswari, S.; Ramachandran, A. Antibacterial properties of Passiflora foetida L.–a common exotic medicinal plant. Afr. J. Biotechnol. 2007, 6, 2650–2653. [Google Scholar]
- Patel, J.R.; Tripathi, P.; Sharma, V.; Chauhan, N.S.; Dixit, V.K. Phyllanthus amarus: Ethnomedicinal uses, phytochemistry and pharmacology: A review. J. Ethnopharmacol. 2011, 138, 286–313. [Google Scholar] [CrossRef]
- Novitasari, A.; Rohmawaty, E.; Rosdianto, A.M. Physalis angulata Linn. as a medicinal plant. Biomed. Rep. 2024, 20, 1–16. [Google Scholar] [CrossRef]
- Bansal, P.; Paul, P.; Mudgal, J.; Nayak, P.G.; Pannakal, S.T.; Priyadarsini, K.; Unnikrishnan, M. Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice. Exp. Toxicol. Pathol. 2012, 64, 651–658. [Google Scholar] [CrossRef]
- Modarresi Chahardehi, A.; Ibrahim, D.; Fariza Sulaiman, S. Antioxidant, antimicrobial activity and toxicity test of Pilea microphylla. Int. J. Microbiol. 2010, 2010, 826830. [Google Scholar] [CrossRef]
- Iranshahy, M.; Javadi, B.; Iranshahi, M.; Jahanbakhsh, S.P.; Mahyari, S.; Hassani, F.V.; Karimi, G. A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. J. Ethnopharmacol. 2017, 205, 158–172. [Google Scholar] [CrossRef]
- Mekap, S.K.; Panda, P.K.; Mishra, S.K. Phytochemical and pharmacological profile of Portulaca pilosa Linn.: A review. J. Environ. Life Sci. 2017, 2, 46–51. [Google Scholar]
- Ajaib, M.; Zikrea, A.; Khan, K.M.; Perveen, S.; Shah, S.; Karim, A. Rivina humilis L.: A potential antimicrobial and antioxidant source. J. Chem. Soc. Pak. 2013, 35, 1384–1398. [Google Scholar]
- Lande, S.; Bhogaonkar, P. Physical characterisation of Dipteracanthus prostatus (Poir.) Nees a medicinal herb. Int. J. Biol. Res. 2018, 3, 60–63. [Google Scholar]
- Sharma, A.; Kumar, A.; Singh, A.K.; Kumar, K.J.; Narasimhan, B.; Kumar, P. Ethnomedicinal Uses, Phytochemistry, Pharmacology, and Toxicology of Ruellia tuberosa L.: A Review. Chem. Biodivers. 2024, 21, e202400292. [Google Scholar] [CrossRef]
- Nischitha, R.; Shivanna, M. Phyto-and myco-chemical profiling, bioactivity, and in silico docking study of endophytic fungi and host—Setaria flavida. Int. Microbiol. 2025, 28, 1–14. [Google Scholar] [CrossRef]
- Adjibode, A.; Tougan, U.; Youssao, A.; Mensah, G.; Hanzen, C.; Koutinhouin, G. Synedrella nodiflora (L.) Gaertn: A review on its phytochemical screening and uses in animal husbandry and medicine. Int. J. Adv. Sci. Tech. Res. 2015, 3, 436–443. [Google Scholar]
- Khaing, Y.Y.; Moe, M.M. Morphological, Microscopical Characters and Antioxidant activity of leaves of Talinum fruticosum (L.) Juss. In Proceedings of the 2nd Myanmar Korea Conference Research Journal, Yangon, Myanmar, 1 August 2019; pp. 540–550. [Google Scholar]
- Zhu, W.; Du, Y.; Meng, H.; Dong, Y.; Li, L. A review of traditional pharmacological uses, phytochemistry, and pharmacological activities of Tribulus terrestris. Chem. Cent. J. 2017, 11, 60. [Google Scholar] [CrossRef]
- Naqash, S.Y.; Nazeer, R. Anticoagulant, antiherpetic and antibacterial activities of sulphated polysaccharide from Indian medicinal plant Tridax procumbens L. (Asteraceae). Appl. Biochem. Biotechnol. 2011, 165, 902–912. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Prabakaran, R.; Preetha, V. Antimicrobial and antioxidant activities of ethanolic crude extracts of Turnera ulmifolia L. J. Pharm. Sci. Drug Res. Dec. 2014, 6, 329–333. [Google Scholar] [CrossRef]
- Nascimento, M.; Silva, A.; França, L.; Quignard, E.; López, J.; Almeida, M. Turnera ulmifolia L. (Turneraceae): Preliminary study of its antioxidant activity. Bioresour. Technol. 2006, 97, 1387–1391. [Google Scholar] [CrossRef]
- Dash, S.; Bohidar, J.; Das, C.; Mohanty, A.; Meher, A.; Hota, R. Evaluation of Anthelmintic Activity and GC-MS Characterization of Urochloa distachya (L.). Int. J. Pharm. Investig. 2023, 13, 248–254. [Google Scholar] [CrossRef]
- McDonnell, M.J.; Pickett, S.T. Ecosystem structure and function along urban-rural gradients: An unexploited opportunity for ecology. Ecology 1990, 71, 1232–1237. [Google Scholar] [CrossRef]
- Gilbert, O.; Gilbert, O. Urban Commons. In The Ecology of Urban Habitats; Springer: Dordrecht, The Netherlands, 1989; pp. 68–109. [Google Scholar]
- Hou, S.; Tian, C.; Meng, J.; Liu, C.; Yao, Z. The Impact of Urbanization on the Distribution of Spontaneous Herbaceous Plants in an Ancient City: A Pilot Case Study in Jingzhou, China. Plants 2023, 12, 3353. [Google Scholar] [CrossRef]
- Hu, S.; Jin, C.; Huang, L.; Huang, J.; Luo, M.; Qian, S.; Jim, C.Y.; Song, K.; Chen, S.; Lin, D. Characterizing composition profile and diversity patterns of spontaneous urban plants across China’s major cities. J. Environ. Manag. 2022, 317, 115445. [Google Scholar] [CrossRef]
- Xu, W.; Dai, W.; Ding, Y.; Song, S.; Liu, Q.; Yang, W. Drivers of Spontaneous Plant Communities in Urban Parks: A Case from Nanjing, China. Sustainability 2024, 16, 3841. [Google Scholar] [CrossRef]
- Stohlgren, T.J.; Pyšek, P.; Kartesz, J.; Nishino, M.; Pauchard, A.; Winter, M.; Pino, J.; Richardson, D.M.; Wilson, J.R.; Murray, B.R. Widespread plant species: Natives versus aliens in our changing world. Biol. Invasions 2011, 13, 1931–1944. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Cheng, Z.; Ye, T.; Chan, Z. Analysis of natural variation in bermudagrass (Cynodon dactylon) reveals physiological responses underlying drought tolerance. PLoS ONE 2012, 7, e53422. [Google Scholar] [CrossRef]
- Noor, M.; Fan, J.; Kaleem, M.; Akhtar, M.T.; Jin, S.; Nazir, U.; Zhang, C.-J.; Yan, X. Assessment of the changes in growth, photosynthetic traits and gene expression in Cynodon dactylon against drought stress. BMC Plant Biol. 2024, 24, 235. [Google Scholar] [CrossRef]
- Maroco, J.P.; Pereira, J.S.; Chaves, M.M. Growth, photosynthesis and water-use efficiency of two C4Sahelian grasses subjected to water deficits. J. Arid. Environ. 2000, 45, 119–137. [Google Scholar] [CrossRef]
- Rafique, T.; Hameed, M.; Naseer, M.; Rafique, R.; Sadiq, R.; Zikrea, A.; Tehseen, S.; Sajjad, M.R. Comparative leaf anatomy of grasses (Poaceae) in Faisalabad region of Pakistan. Pol. J. Environ. Stud. 2021, 30, 5701–5709. [Google Scholar] [CrossRef]
- Banan, S.A.; Al-Watban, A.A.; Doaigey, A.R.; Alsahli, A.A.; El-Zaidy, M. Anatomical adaptations in species of Poaceae growing in Al-Hair region of Riyadh, Saudi Arabia. Afr. J. Plant Sci. 2019, 13, 201–208. [Google Scholar] [CrossRef]
- Takano, H.; Oliveira Jr, R.; Constantin, J.; Braz, G.; Padovese, J. Growth, Development and Seed Production of Goosegrass1. Planta Daninha 2016, 34, 249–258. [Google Scholar] [CrossRef]
- Fernandez, O. Establishment of Cynodon dactylon from stolon and rhizome fragments. Weed Res. 2003, 43, 130–138. [Google Scholar] [CrossRef]
- Shaukat, S.; Siddiqui, I.; Zarina, A. Seed dispersal pattern of a composite weed Tridax procumbens L. Int. J. Biol. Biotechnol. 2005, 2, 321–327. [Google Scholar]
- Wijesinghe, S.; Yakandawala, K.; Karunarathne, W. Seed Germination of Selected Wild Flowering Species for Low Maintenance Planting Designs. J. Environ. Prof. Sri Lanka 2016, 5, 15. [Google Scholar]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Kowarik, I. Time lags in biological invasions with regard to the success and failure of alien species. In Plant Invasions: General Aspects and Special Problems; Pyšek, P., Prach, K., Rejmánek, M., Wade, M., Eds.; SPB Academic Publishing: Amsterdam, The Netherlands, 1995; pp. 15–38. [Google Scholar]
- Pouyat, R.V.; Yesilonis, I.D.; Nowak, D.J. Carbon storage by urban soils in the United States. J. Environ. Qual. 2006, 35, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, O.L. The Ecology of Urban Habitats; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Geslin, B.; Le Féon, V.; Kuhlmann, M.; Vaissière, B.E.; Dajoz, I. The bee fauna of large parks in downtown Paris, France. Ann. Soc. Entomol. Fr. (N.S.) 2015, 51, 487–493. [Google Scholar] [CrossRef]
- Matteson, K.C.; Ascher, J.S.; Langellotto, G.A. Bee richness and abundance in New York City urban gardens. Ann. Entomol. Soc. Am. 2008, 101, 140–150. [Google Scholar] [CrossRef]
- Baldock, K.C.; Goddard, M.A.; Hicks, D.M.; Kunin, W.E.; Mitschunas, N.; Osgathorpe, L.M.; Potts, S.G.; Robertson, K.M.; Scott, A.V.; Stone, G.N. Where is the UK’s pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142849. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, J.; Guan, M.; Lu, Y.; Liu, H.; Zhao, D. Effects of vegetation structure and environmental characteristics on pollinator diversity in urban green spaces. Urban For. Urban Green. 2023, 84, 127928. [Google Scholar] [CrossRef]
- Berger, J.L.; Daum, S.N.; Hartlieb, M. Simply the green: Urban refuges. Basic Appl. Ecol. 2024, 80, 108–119. [Google Scholar] [CrossRef]
- Wartmann, F.M.; Lorimer, J. Messy natures: The political aesthetics of nature recovery. People Nat. 2024, 6, 2564–2576. [Google Scholar] [CrossRef]
- Nassauer, J.I. Messy ecosystems, orderly frames. Landsc. J. 1995, 14, 161–170. [Google Scholar] [CrossRef]
- Thailand Foundation. Pitee Wai Khru: The Thai Teacher Appreciation Ceremony. Available online: https://thailandfoundation.or.th/pitee-wai-khru-the-thai-teacher-appreciation-ceremony/ (accessed on 7 August 2025).
- Wisetkomolmat, J.; Suppakittpaisarn, P.; Sommano, S.R. Detergent plants of Northern Thailand: Potential sources of natural saponins. Resources 2019, 8, 10. [Google Scholar] [CrossRef]
- Robinson, S.L.; Lundholm, J.T. Ecosystem services provided by urban spontaneous vegetation. Urban Ecosyst. 2012, 15, 545–557. [Google Scholar] [CrossRef]
- van Wilgen, B.W.; Reyers, B.; Le Maitre, D.; Richardson, D.; Schonegevel, L. A biome-scale assessment of the impact of invasive alien plants on ecosystem services in South Africa. J. Environ. Manag. 2008, 89, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, M.; Wilson, J.R.; Cadotte, M.W.; MacIvor, J.S.; Zenni, R.D.; Richardson, D.M. Non-native species in urban environments: Patterns, processes, impacts and challenges. Biol. Invasions 2017, 19, 3461–3469. [Google Scholar] [CrossRef]
- Sheppard, C.S.; Lüpke, N. Are alien plant species superior to natives, and is this determined by performance measure and study design? A meta-analysis. Basic Appl. Ecol. 2024, 77, 16–25. [Google Scholar] [CrossRef]
- Alue, B.A.; Salleh Hudin, N.; Mohamed, F.; Mat Said, Z.; Ismail, K. Plant diversity along an urbanization gradient of a tropical city. Diversity 2022, 14, 1024. [Google Scholar] [CrossRef]
- Ilie, D.; Cosmulescu, S. Spontaneous plant diversity in urban contexts: A review of its impact and importance. Diversity 2023, 15, 277. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Delgado, J.D.; Otto, R.; Naranjo, A.; Salas, M.; Fernández-Palacios, J.M. Distribution of alien vs. native plant species in roadside communities along an altitudinal gradient in Tenerife and Gran Canaria (Canary Islands). Perspect. Plant Ecol. Evol. Syst. 2005, 7, 185–202. [Google Scholar] [CrossRef]
- Shrestha, B.B.; Witt, A.B.; Shen, S.; Khuroo, A.A.; Shrestha, U.B.; Naqinezhad, A. Plant invasions in Asia. In Global Plant Invasions; Springer: Berlin/Heidelberg, Germany, 2022; pp. 89–127. [Google Scholar]
- Jayasundera, M.; Florentine, S.; Tennakoon, K.U.; Chauhan, B.S. Medicinal value of three agricultural weed species of the asteraceae family: A review. Pharmacogn. J. 2021, 13. [Google Scholar] [CrossRef]
- Meteostat. Chiang Mai Weather Station (Station 48327)—Daily Weather Data. Available online: https://meteostat.net/en/station/48327?t=2025-07-16/2025-07-23 (accessed on 30 July 2025).
- Thai Meteorological Department. Monthly Weather Summary in Thailand (December 2023); Meteorological Development Division, Climate Center, Thai Meteorological Department: Bangkok, Thailand, 4 January 2024; 16p. Available online: https://www.tmd.go.th/media/climate/climate-monthly/weather_summary_dec_2023_rev1-0.pdf (accessed on 14 August 2025).
- Tutiempo. Climate Data for Chiang Mai Airport (Station 483270). 2023. Available online: https://en.tutiempo.net/climate/2023/ws-483270.html (accessed on 30 July 2025).
- Pongruengkiat, W.; Tippayawong, K.Y.; Aggarangsi, P.; Pichayapan, P.; Katongtung, T.; Tippayawong, N. Assessing sustainability of Chiang Mai urban development. Discov. Sustain. 2023, 4, 54. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Z.; Crabbe, M.J.C.; Suntichaikul, P. Effects of urban land-use planning on housing prices in Chiang Mai, Thailand. Land 2024, 13, 1136. [Google Scholar] [CrossRef]
- Wanitchakorn, T.; Muangasame, K. The identity change of rural–urban transformational tourism development in Chiang Mai heritage city: Local residents’ perspectives. Int. J. Tour. Cities 2021, 7, 1008–1028. [Google Scholar] [CrossRef]
- Ibrahim, K.M.; Peterson, P.M. Grasses of Washington, DC.; Smithsonian Institution Scholarly Press: Washington, WA, USA, 2019. [Google Scholar]
- Nelson, L.S.; Shih, R.D.; Balick, M.J. Glossary of botanical terms. In Handbook of Poisonous and Injurious Plants; Springer: Berlin/Heidelberg, Germany, 2007; pp. 9–17. [Google Scholar]
- Crozier, A.A. A Dictionary of Botanical Terms; H. Holt: New York, NY, USA, 1892. [Google Scholar]
- Beentje, H. Plant Glossary; Kew, Royal Botanical Gardens: London, UK, 2010. [Google Scholar]
- POWO. Plants of the World Online; Facilitated by the Royal Botanic Gardens, Kew: London, UK, 2024. [Google Scholar]
- Research Center of Forest and Plant Species Conservation. Alien Invasive Plants “พืชต่างถิ่นรุกราน” [in TH]; Department of National Park, Wildlife, and Plant Conservation: Bangkok, Thailand, 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).