Endophytic Bacterial and Fungal Communities of Spruce Picea jezoensis in the Russian Far East
Abstract
1. Introduction
2. Results
2.1. Illumina MiSeq Sequencing
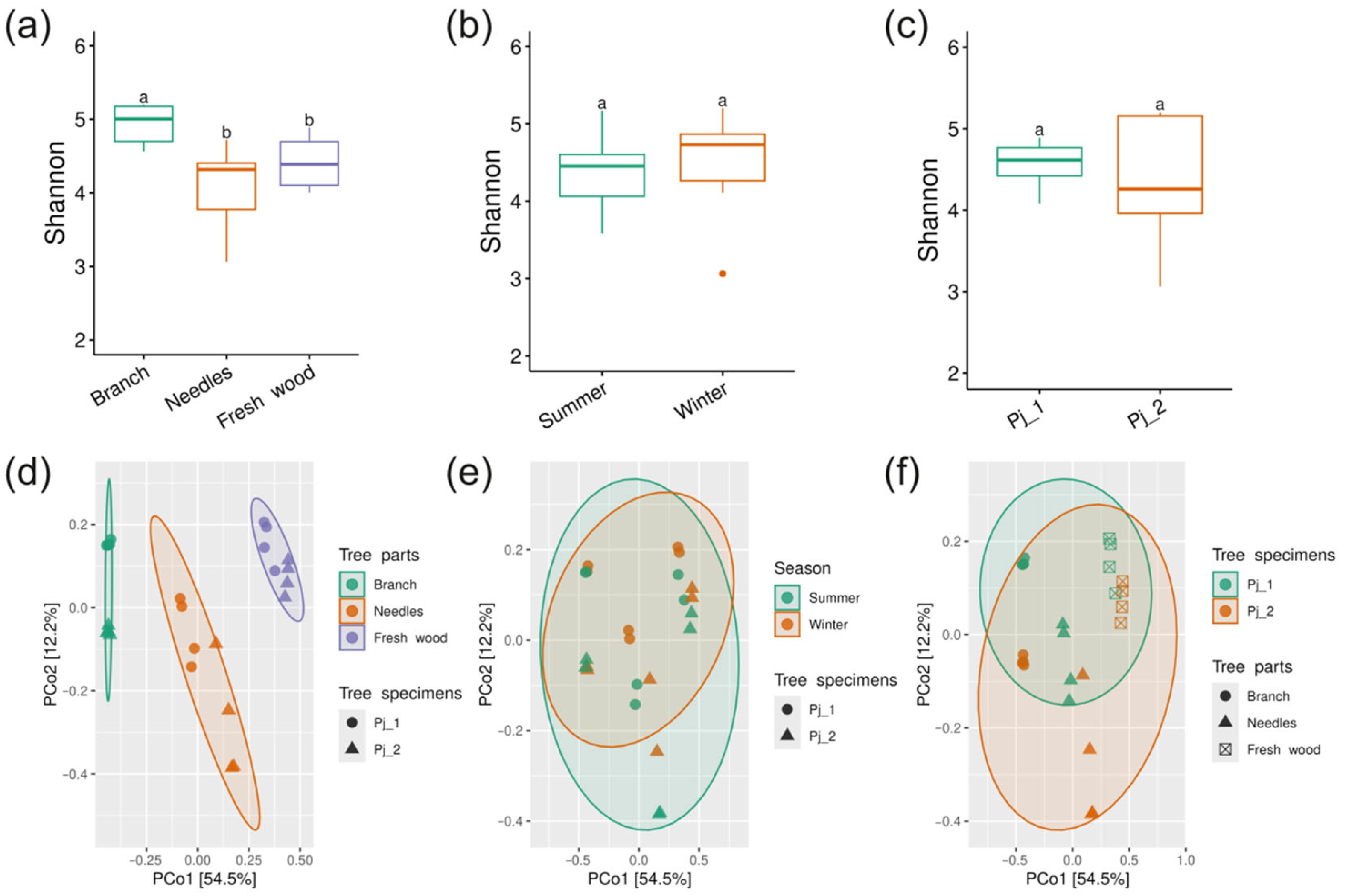
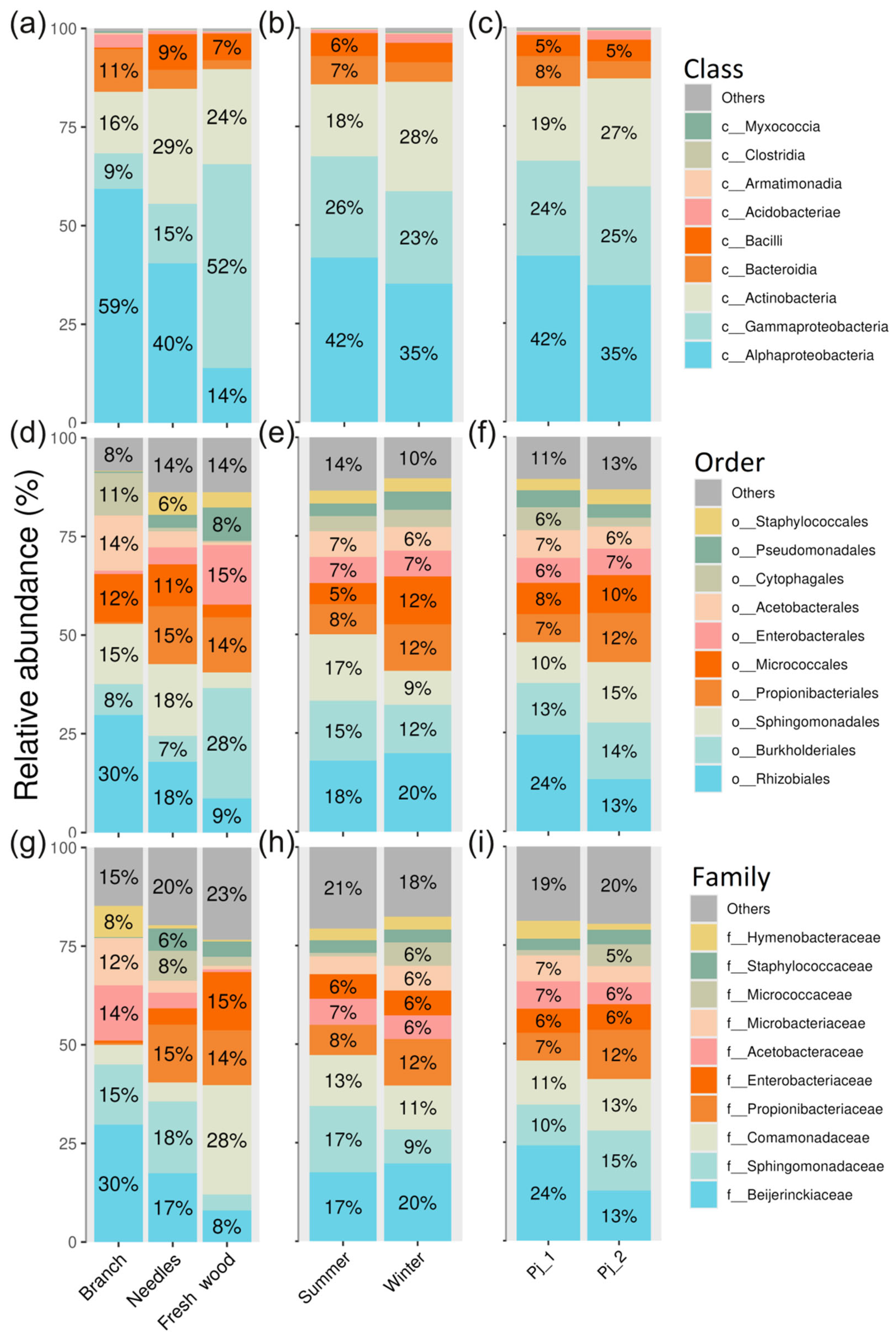
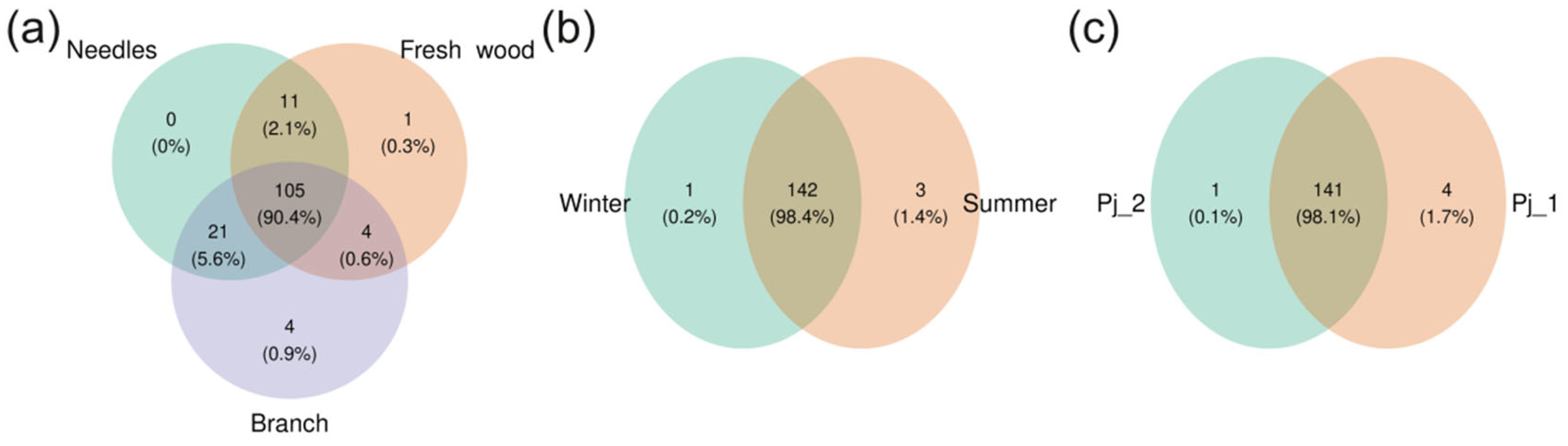
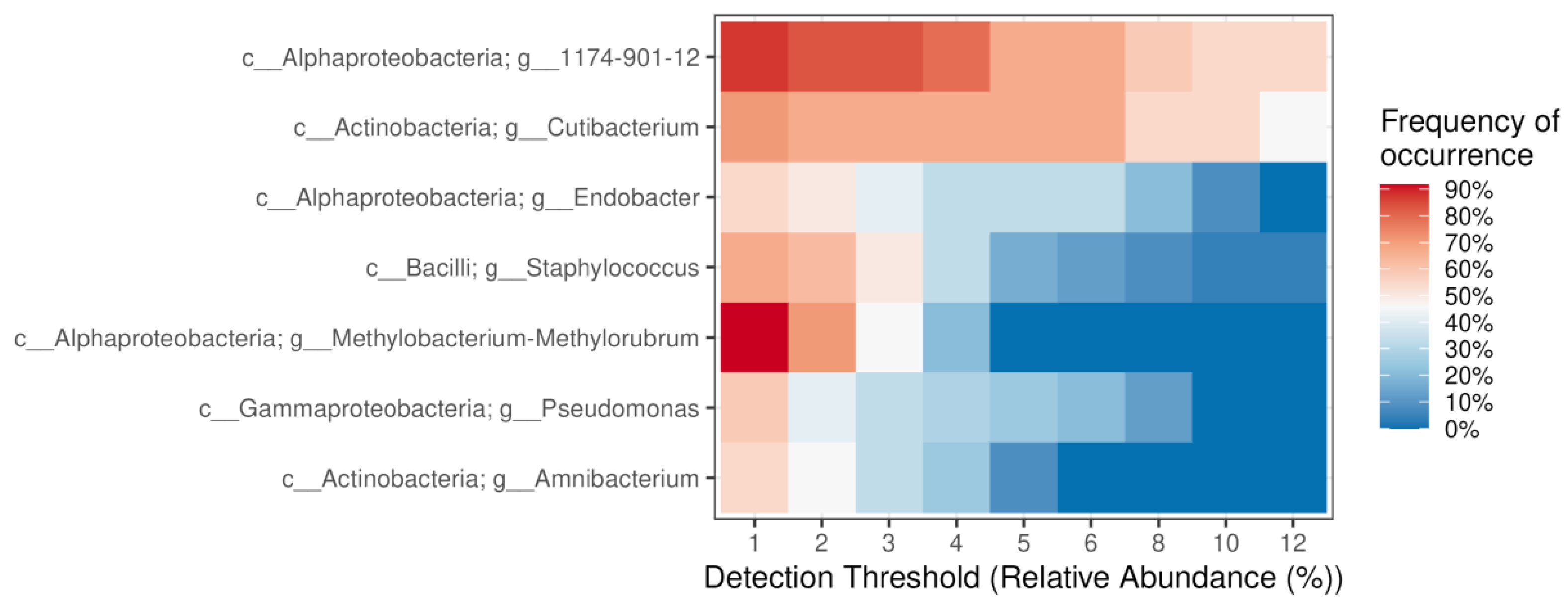
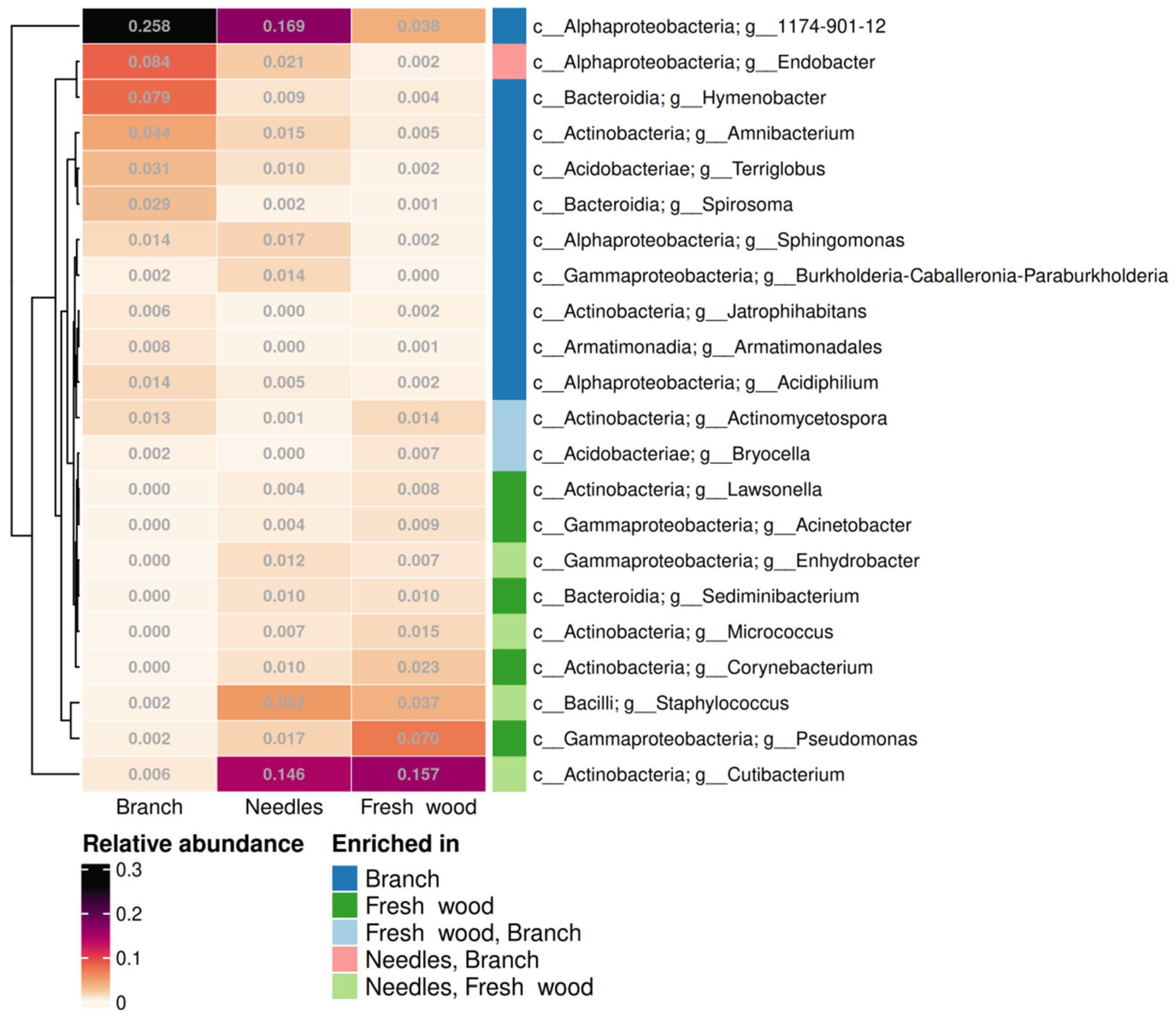
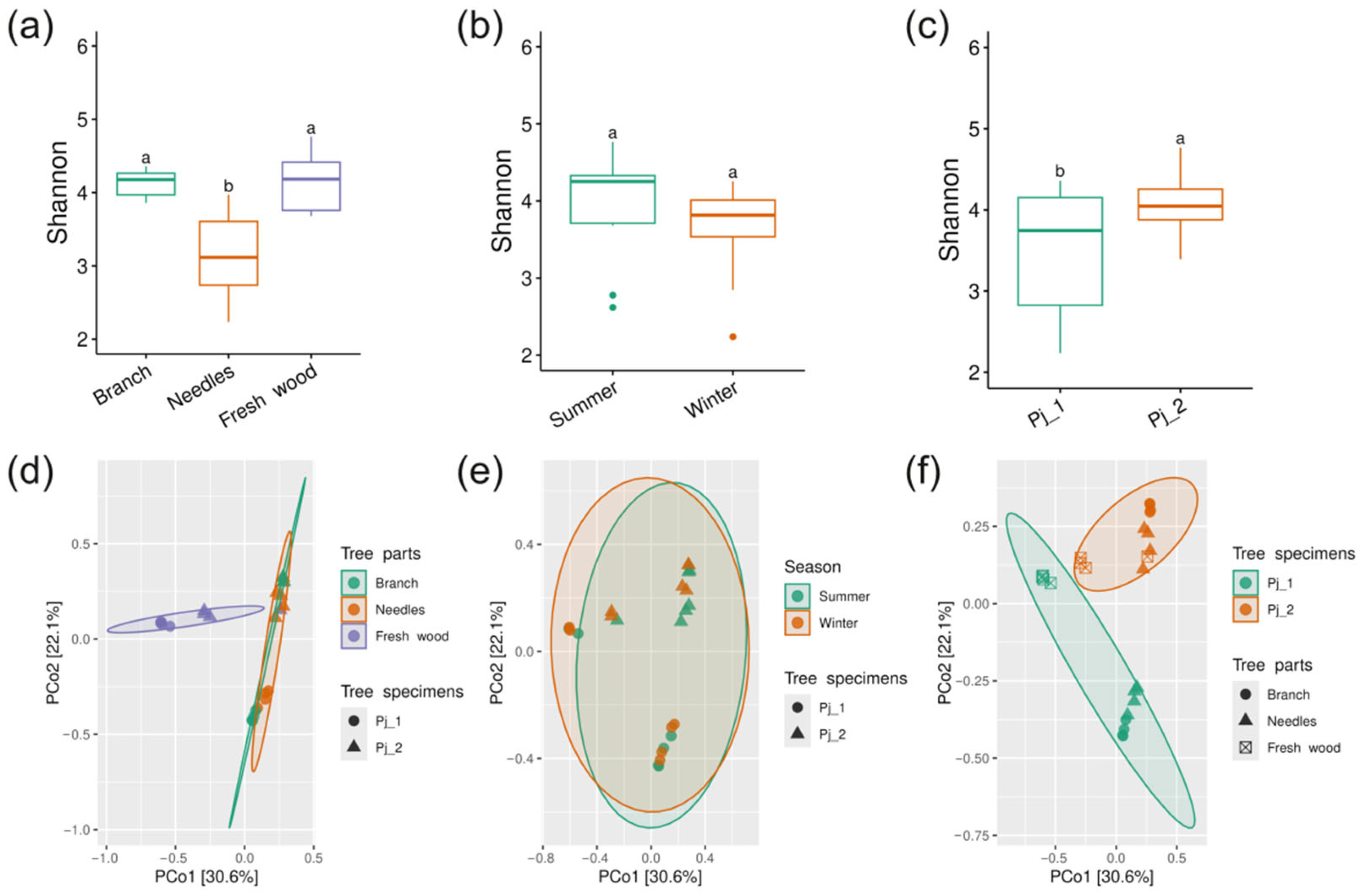
2.2. Diversity and Taxonomical Composition of Endophytic Communities of Bacteria in Various Tree Parts and Specimens of Picea jezoensis in Winter and Summer
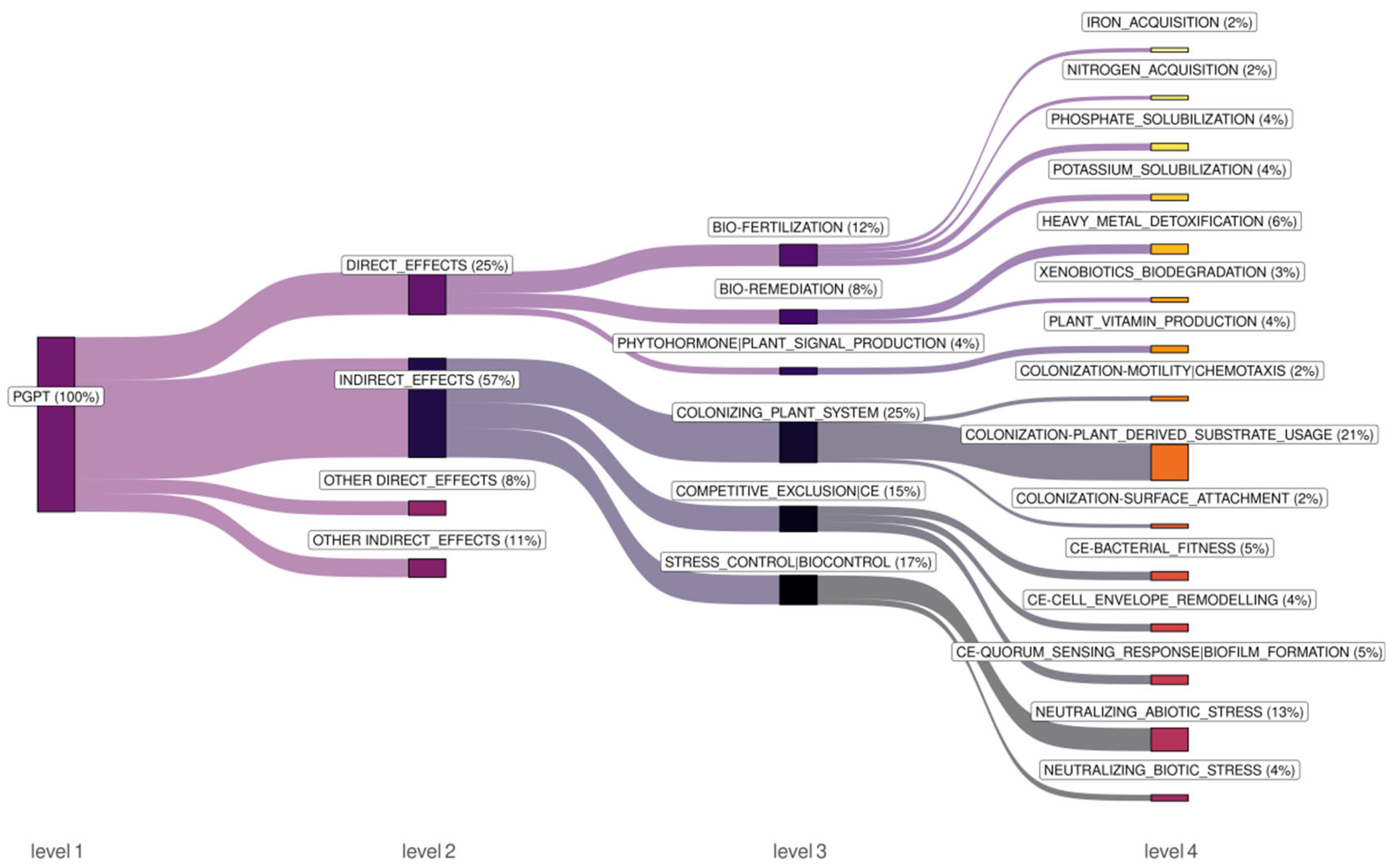
2.3. Functional Analysis of Endophytic Bacterial Communities in Picea jezoensis
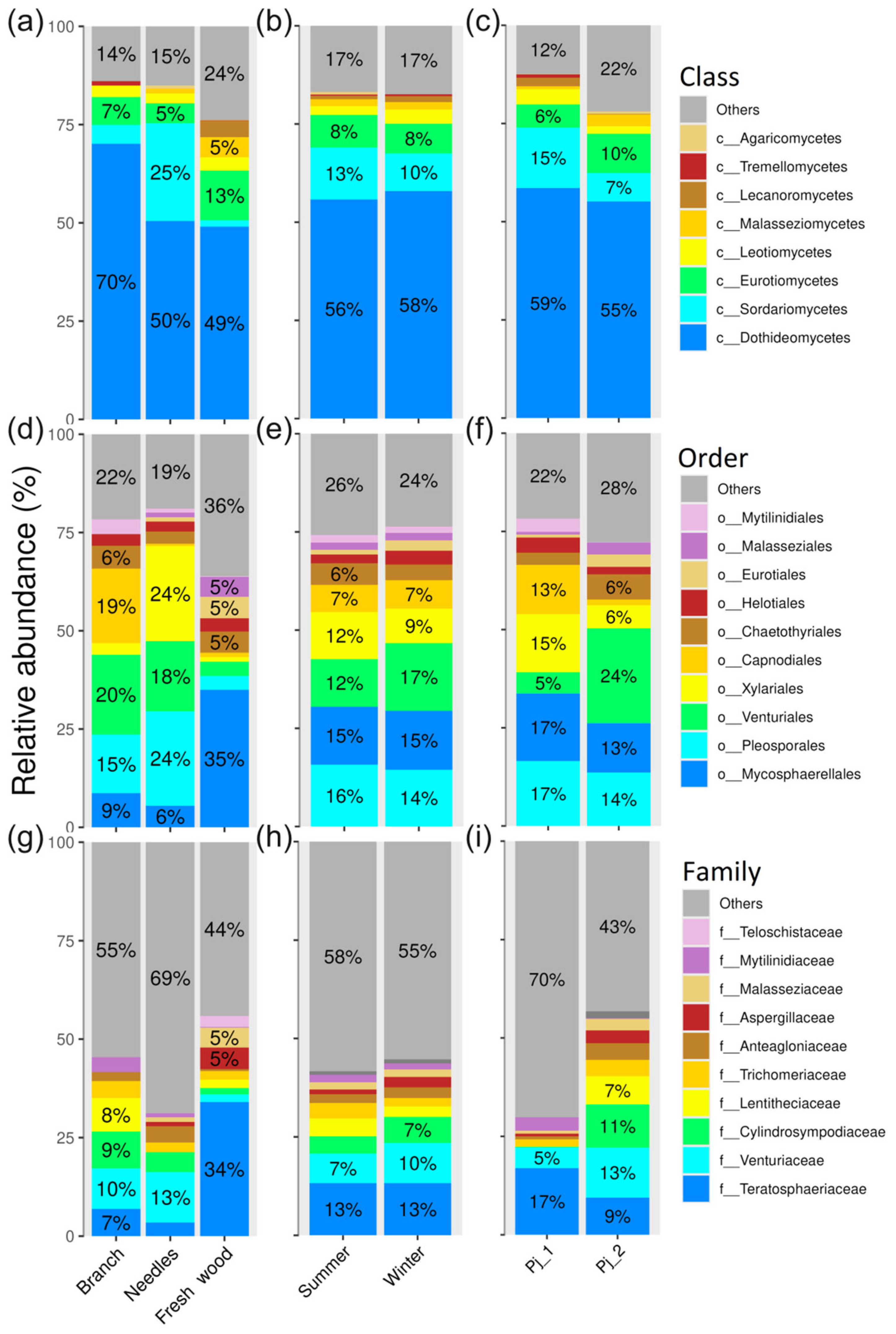
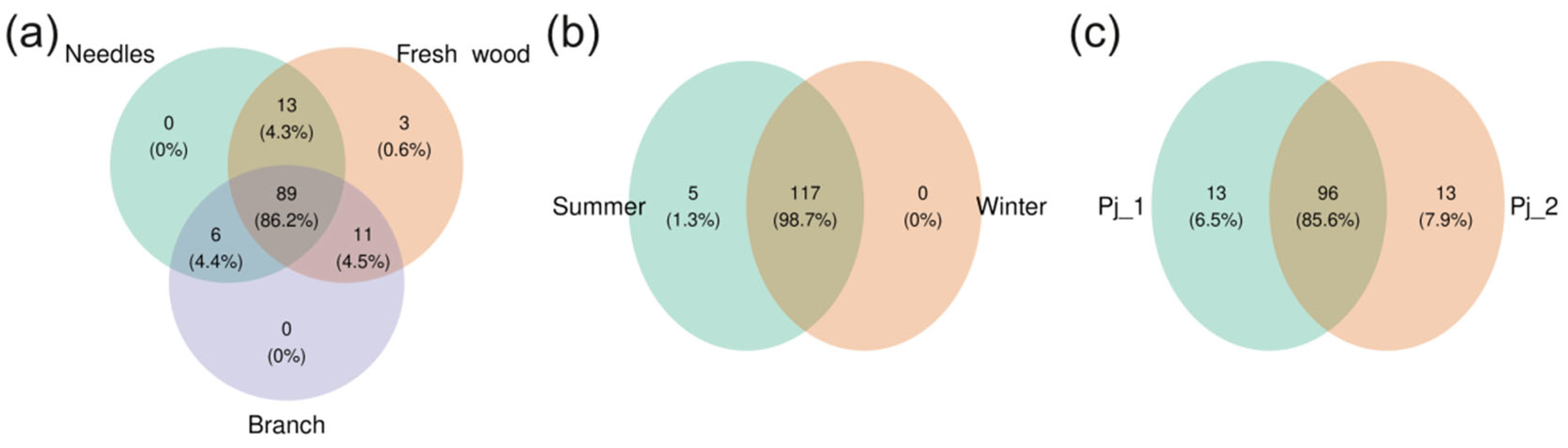
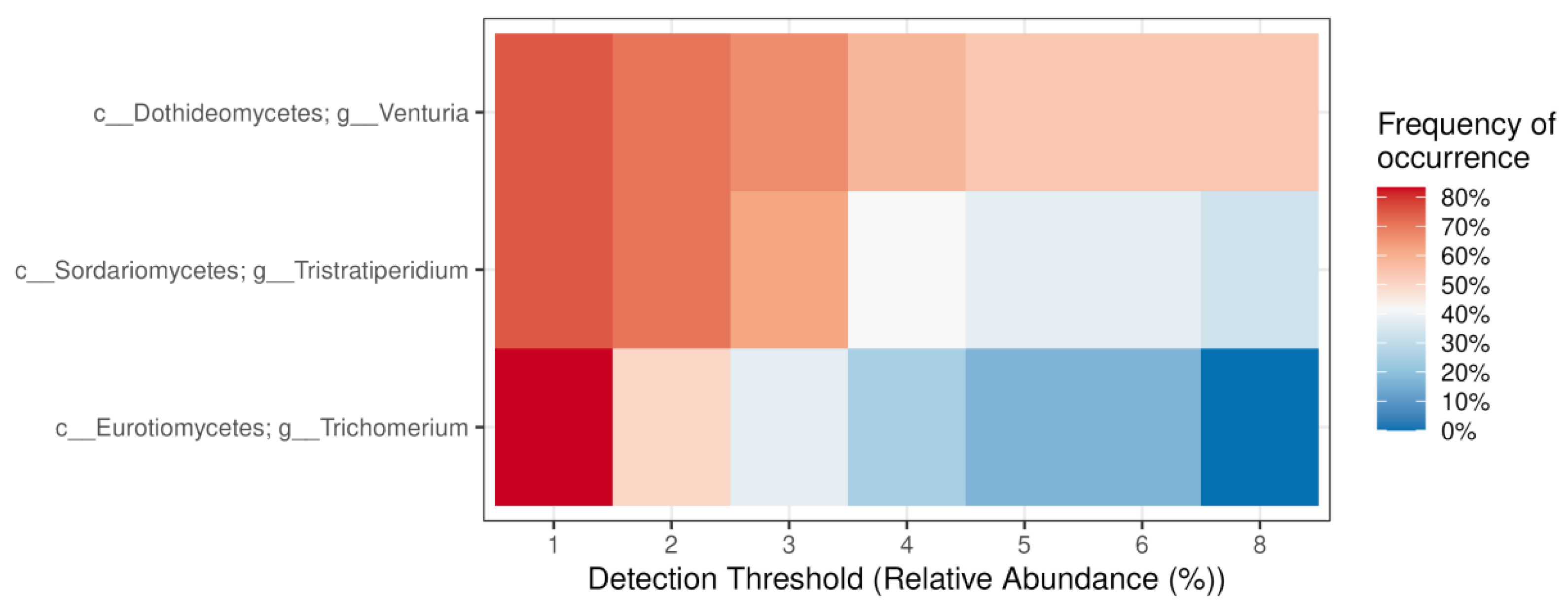
2.4. Diversity and Composition of Endophytic Fungi in Different Tree Parts and Tree Specimens of Picea jezoensis in Winter and Summer
3. Discussion
4. Materials and Methods
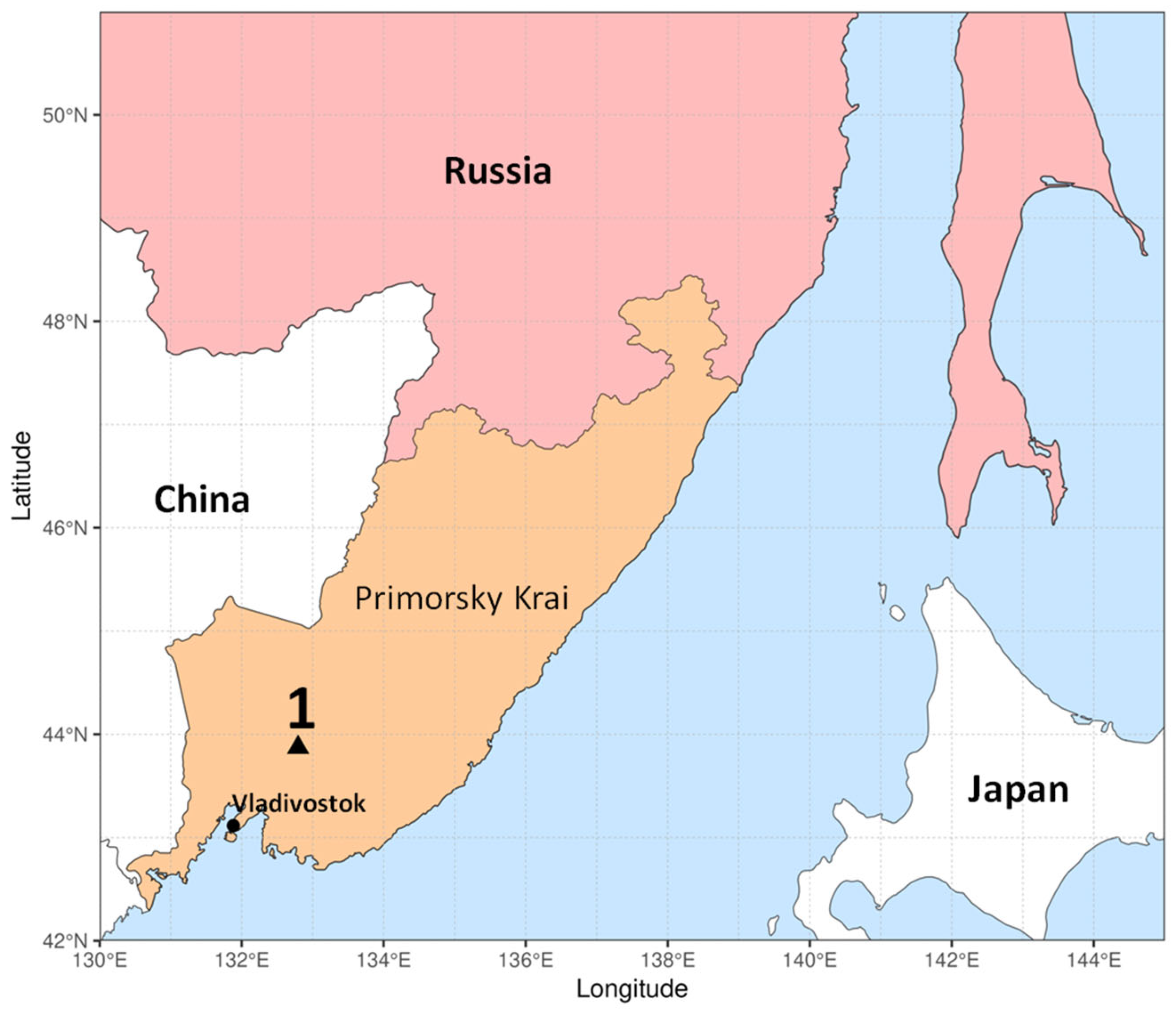
4.1. Site Description
4.2. Sampling
4.3. DNA Extraction, Library Preparation, and Illumina MiSeq Sequencing
4.4. Bioinformatics Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, D. Endophyte: The Evolution of a Term, and Clarification of Its Use and Definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Jia, M.; Chen, L.; Xin, H.-L.; Zheng, C.-J.; Rahman, K.; Han, T.; Qin, L.-P. A Friendly Relationship between Endophytic Fungi and Medicinal Plants: A Systematic Review. Front. Microbiol. 2016, 7, 906. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, J.; Chen, C.; Mo, X.; Tan, Q.; He, Y.; Wang, Z.; Yin, J.; Zhou, G. The Multifunctions and Future Prospects of Endophytes and Their Metabolites in Plant Disease Management. Microorganisms 2022, 10, 1072. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial Effects of Endophytic Fungi Colonization on Plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Malla, M.A.; Kumar, A.; Dayanandan, S.; Khan, M.L. Plants Endophytes: Unveiling Hidden Agenda for Bioprospecting toward Sustainable Agriculture. Crit. Rev. Biotechnol. 2020, 40, 1210–1231. [Google Scholar] [CrossRef] [PubMed]
- Rabiey, M.; Hailey, L.E.; Roy, S.R.; Grenz, K.; Al-Zadjali, M.A.S.; Barrett, G.A.; Jackson, R.W. Endophytes vs Tree Pathogens and Pests: Can They Be Used as Biological Control Agents to Improve Tree Health? Eur. J. Plant Pathol. 2019, 155, 711–729. [Google Scholar] [CrossRef]
- Ganley, R.J.; Newcombe, G. Fungal Endophytes in Seeds and Needles of Pinus monticola. Mycol. Res. 2006, 110, 318–327. [Google Scholar] [CrossRef]
- Müller, M.M.; Hallaksela, A.-M. Diversity of Norway Spruce Needle Endophytes in Various Mixed and Pure Norway Spruce Stands. Mycol. Res. 1998, 102, 1183–1189. [Google Scholar] [CrossRef]
- Rajala, T.; Velmala, S.M.; Tuomivirta, T.; Haapanen, M.; Müller, M.; Pennanen, T. Endophyte Communities Vary in the Needles of Norway Spruce Clones. Fungal Biol. 2013, 117, 182–190. [Google Scholar] [CrossRef]
- Bailey, J.K.; Deckert, R.; Schweitzer, J.A.; Rehill, B.J.; Lindroth, R.L.; Gehring, C.; Whitham, T.G. Host Plant Genetics Affect Hidden Ecological Players: Links among Populus, Condensed Tannins, and Fungal Endophyte Infection. Can. J. Bot. 2005, 83, 356–361. [Google Scholar] [CrossRef]
- Rajala, T.; Velmala, S.M.; Vesala, R.; Smolander, A.; Pennanen, T. The Community of Needle Endophytes Reflects the Current Physiological State of Norway Spruce. Fungal Biol. 2014, 118, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Farjon, A. Pinaceae, Drawings and Descriptions of the Genera: Abies, Cedrus, Pseudolarix, Keteleeria, Nothotsuga, Tsuga, Cathaya, Pseudotsuga, Larix and Picea; Regnum vegetabile; Koeltz Scientific Books; Lubrecht & Cramer Ltd.: New York, NY, USA, 1990; ISBN 978-1-878762-04-7. [Google Scholar]
- Suprun, A.R.; Dubrovina, A.S.; Aleynova, O.A.; Kiselev, K.V. The Bark of the Spruce Picea jezoensis Is a Rich Source of Stilbenes. Metabolites 2021, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Ishikawa, Y.; Minami, T.; Minoura, K.; Tokuda, H.; Matsunaga, S. Two New Anti-Tumor Promoting Serratane-Type Triterpenoids from the Stem Bark of Picea jezoensis var. jezoensis. Planta Medica 2003, 69, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Minami, T.; Tokuda, H. Anti-Initiating Activity of 3beta-Methoxy-13alpha,14alpha-Epoxyserratan-21beta-Ol (PJJ-34) from the Stem Bark of Picea jezoensis Carr. var. jezoensis. Chem. Biodivers. 2006, 3, 818–824. [Google Scholar] [CrossRef]
- Wada, S.; Yasui, Y.; Tokuda, H.; Tanaka, R. Anti-Tumor-Initiating Effects of Phenolic Compounds Isolated from the Bark of Picea jezoensis var. jezoensis. Bioorg. Med. Chem. 2009, 17, 6414–6421. [Google Scholar] [CrossRef]
- Tanney, J.B.; McMullin, D.R.; Green, B.D.; Miller, J.D.; Seifert, K.A. Production of Antifungal and Antiinsectan Metabolites by the Picea Endophyte Diaporthe maritima sp. nov. Fungal Biol. 2016, 120, 1448–1457. [Google Scholar] [CrossRef]
- McMullin, D.R.; Green, B.D.; Prince, N.C.; Tanney, J.B.; Miller, J.D. Natural Products of Picea Endophytes from the Acadian Forest. J. Nat. Prod. 2017, 80, 1475–1483. [Google Scholar] [CrossRef]
- Wang, K.; Wen, Z.; Asiegbu, F.O. The Dark Septate Endophyte Phialocephala sphaeroides Suppresses Conifer Pathogen Transcripts and Promotes Root Growth of Norway Spruce. Tree Physiol. 2022, 42, 2627–2639. [Google Scholar] [CrossRef]
- Puri, A.; Padda, K.P.; Chanway, C.P. Sustaining the Growth of Pinaceae Trees under Nutrient-Limited Edaphic Conditions via Plant-Beneficial Bacteria. PLoS ONE 2020, 15, e0238055. [Google Scholar] [CrossRef]
- Sumarah, M.W.; Puniani, E.; Blackwell, B.A.; Miller, J.D. Characterization of Polyketide Metabolites from Foliar Endophytes of Picea glauca. J. Nat. Prod. 2008, 71, 1393–1398. [Google Scholar] [CrossRef]
- Tanabe, J.; Tamura, A.; Hamanaka, M.; Ishiguri, F.; Takashima, Y.; Ohshima, J.; Iizuka, K.; Yokota, S. Wood Properties and Their Among-Family Variations in 10 Open-Pollinated Families of Picea jezoensis. J. Wood Sci. 2014, 60, 297–304. [Google Scholar] [CrossRef]
- Alberton, O.; Kuyper, T.W.; Summerbell, R.C. Dark Septate Root Endophytic Fungi Increase Growth of Scots Pine Seedlings under Elevated CO2 through Enhanced Nitrogen Use Efficiency. Plant Soil 2010, 328, 459–470. [Google Scholar] [CrossRef]
- Lacava, P.T.; Bogas, A.C.; de Paula Nogueira Cruz, F. Plant Growth Promotion and Biocontrol by Endophytic and Rhizospheric Microorganisms from the Tropics: A Review and Perspectives. Front. Sustain. Food Syst. 2022, 6, 796113. [Google Scholar] [CrossRef]
- Vidholdová, Z.; Kačík, F.; Reinprecht, L.; Kučerová, V.; Luptáková, J. Changes in Chemical Structure of Thermally Modified Spruce Wood Due to Decaying Fungi. J. Fungi 2022, 8, 739. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Escobedo, R.; Briones-Roblero, C.I.; Pineda-Mendoza, R.M.; Rivera-Orduña, F.N.; Zúñiga, G. Bacteriome from Pinus arizonica and P. durangensis: Diversity, Comparison of Assemblages, and Overlapping Degree with the Gut Bacterial Community of a Bark Beetle That Kills Pines. Front. Microbiol. 2018, 9, 77. [Google Scholar] [CrossRef]
- Dendooven, L.; Pérez-Hernández, V.; Navarro-Pérez, G.; Tlalmis-Corona, J.; Navarro-Noya, Y.E. Spatial and Temporal Shifts of Endophytic Bacteria in Conifer Seedlings of Abies religiosa (Kunth) Schltdl. & Cham. Microb. Ecol. 2024, 87, 90. [Google Scholar] [CrossRef]
- Padda, K.P.; Puri, A.; Nguyen, N.K.; Philpott, T.J.; Chanway, C.P. Evaluating the Rhizospheric and Endophytic Bacterial Microbiome of Pioneering Pines in an Aggregate Mining Ecosystem Post-Disturbance. Plant Soil 2022, 474, 213–232. [Google Scholar] [CrossRef]
- Carrell, A.A.; Frank, A.C. Pinus flexilis and Picea engelmannii Share a Simple and Consistent Needle Endophyte Microbiota with a Potential Role in Nitrogen Fixation. Front. Microbiol. 2014, 5, 333. [Google Scholar] [CrossRef]
- Carrell, A.A.; Carper, D.L.; Frank, A.C. Subalpine Conifers in Different Geographical Locations Host Highly Similar Foliar Bacterial Endophyte Communities. FEMS Microbiol. Ecol. 2016, 92, fiw124. [Google Scholar] [CrossRef]
- Proença, D.N.; Francisco, R.; Kublik, S.; Schöler, A.; Vestergaard, G.; Schloter, M.; Morais, P.V. The Microbiome of Endophytic, Wood Colonizing Bacteria from Pine Trees as Affected by Pine Wilt Disease. Sci. Rep. 2017, 7, 4205. [Google Scholar] [CrossRef]
- Cankar, K.; Kraigher, H.; Ravnikar, M.; Rupnik, M. Bacterial Endophytes from Seeds of Norway Spruce (Picea abies L. Karst). FEMS Microbiol. Lett. 2005, 244, 341–345. [Google Scholar] [CrossRef]
- Koskimäki, J.J.; Pirttilä, A.M.; Ihantola, E.-L.; Halonen, O.; Frank, A.C. The Intracellular Scots Pine Shoot Symbiont Methylobacterium extorquens DSM13060 Aggregates around the Host Nucleus and Encodes Eukaryote-Like Proteins. mBio 2015, 6, e00039-15. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Carbohydrate Sources and Sinks in Woody Plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Ata, J.P.; Ibarra Caballero, J.R.; Abdo, Z.; Mondo, S.J.; Stewart, J.E. Transitions of Foliar Mycobiota Community and Transcriptome in Response to Pathogenic Conifer Needle Interactions. Sci. Rep. 2022, 12, 7832. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.N.; Sundh, J.; Sundström, G.; Richau, K.; Delhomme, N.; Grabherr, M.; Hurry, V.; Street, N.R. Comparative Fungal Community Analyses Using Metatranscriptomics and Internal Transcribed Spacer Amplicon Sequencing from Norway Spruce. mSystems 2021, 6, e00884-20. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-L.; Chen, Y.; Vilgalys, R. Metatranscriptomic Study of Common and Host-Specific Patterns of Gene Expression between Pines and Their Symbiotic Ectomycorrhizal Fungi in the Genus Suillus. PLoS Genet. 2016, 12, e1006348. [Google Scholar] [CrossRef]
- Nerva, L.; Garcia, J.F.; Favaretto, F.; Giudice, G.; Moffa, L.; Sandrini, M.; Cantu, D.; Zanzotto, A.; Gardiman, M.; Velasco, R.; et al. The Hidden World within Plants: Metatranscriptomics Unveils the Complexity of Wood Microbiomes. J. Exp. Bot. 2022, 73, 2682–2697. [Google Scholar] [CrossRef]
- Saarenpää, S.; Shalev, O.; Ashkenazy, H.; Carlos, V.; Lundberg, D.S.; Weigel, D.; Giacomello, S. Spatial Metatranscriptomics Resolves Host–Bacteria–Fungi Interactomes. Nat. Biotechnol. 2024, 42, 1384–1393. [Google Scholar] [CrossRef]
- Choi, B.Y.; Lee, S.; Kim, J.; Park, H.; Kim, J.-H.; Kim, M.; Park, S.-J.; Kim, K.-T.; Ryu, H.; Shim, D. Comparison of Endophytic and Epiphytic Microbial Communities in Surviving and Dead Korean Fir (Abies koreana) Using Metagenomic Sequencing. Forests 2022, 13, 1932. [Google Scholar] [CrossRef]
- Eo, J.-K.; Eom, A.-H. Community of Endophytic Fungi from Alpine Conifers on Mt. Seorak. Mycobiology 2022, 50, 317–325. [Google Scholar] [CrossRef]
- Ata, J.P.; Schoettle, A.W.; Sitz, R.A.; Caballero, J.R.I.; Holtz, C.T.; Abdo, Z.; Stewart, J.E. Characterization of Foliar Fungal Endophyte Communities from White Pine Blister Rust Resistant and Susceptible Pinus flexilis in Natural Stands in the Southern Rocky Mountains. Phytobiomes J. 2022, 7, 259–269. [Google Scholar] [CrossRef]
- González-Domínguez, E.; Armengol, J.; Rossi, V. Biology and Epidemiology of Venturia Species Affecting Fruit Crops: A Review. Front. Plant Sci. 2017, 8, 1496. [Google Scholar] [CrossRef]
- Ullah, A.; Shah, A.; Chen, S.; Shah, A.; Rodriguez-Ramos, J.C.; Zaman, R.; Erbilgin, N. Alliance Between Conifer Trees and Endophytic Fungi Against Insect Defoliators. Plant Cell Environ. 2025, 48, 5236–5249. [Google Scholar] [CrossRef] [PubMed]
- Daranagama, D.A.; Camporesi, E.; Liu, X.Z.; Bhat, D.J.; Chamyuang, S.; Bahkali, A.H.; Stadler, M.; Hyde, K.D. Tristratiperidium microsporum gen. et sp. nov. (Xylariales) Dead Leaves Arundo plinii. Mycol. Prog. 2015, 15, 8. [Google Scholar] [CrossRef]
- Elfstrand, M.; Zhou, L.; Baison, J.; Olson, Å.; Lundén, K.; Karlsson, B.; Wu, H.X.; Stenlid, J.; García-Gil, M.R. Genotypic Variation in Norway Spruce Correlates to Fungal Communities in Vegetative Buds. Mol. Ecol. 2020, 29, 199–213. [Google Scholar] [CrossRef]
- Chomnunti, P.; Bhat, D.J.; Jones, E.B.G.; Chukeatirote, E.; Bahkali, A.H.; Hyde, K.D. Trichomeriaceae, a New Sooty Mould Family of Chaetothyriales. Fungal Divers. 2012, 56, 63–76. [Google Scholar] [CrossRef]
- Lazarević, J.; Menkis, A. Exploring Fungal Communities in the Needles of Marginal Conifer Tree Populations. Forests 2025, 16, 968. [Google Scholar] [CrossRef]
- Yamaji, K.; Fukushi, Y.; Hashidoko, Y.; Yoshida, T.; Tahara, S. Penicillium Fungi from Picea glehnii Seeds Protect the Seedlings from Damping-off. New Phytol. 2001, 152, 521–531. [Google Scholar] [CrossRef]
- Mahendran, T.R.; Thottathil, G.P.; Surendran, A.; Nagao, H.; Sudesh, K. Biocontrol Potential of Aspergillus terreus, Endophytic Fungus against Rigidoporus microporus and Corynespora cassiicola, Pathogens of Rubber Tree. Arch. Phytopathol. Plant Prot. 2021, 54, 1014–1032. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, S.; Kaur, R.; Kaur, A. Impact of Plant Symbiotic Endophytic Fungus, Aspergillus terreus on Insect Herbivore Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2023, 52, 932–944. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Nityagovsky, N.N.; Dubrovina, A.S.; Kiselev, K.V. The Biodiversity of Grapevine Bacterial Endophytes of Vitis amurensis Rupr. Plants 2022, 11, 1128. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Nityagovsky, N.N.; Suprun, A.R.; Ananev, A.A.; Dubrovina, A.S.; Kiselev, K.V. The Diversity of Fungal Endophytes from Wild Grape Vitis amurensis Rupr. Plants 2022, 11, 2897. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Nityagovsky, N.N.; Aleynova, O.A. A Method of DNA Extraction from Plants for Metagenomic Analysis Based on the Example of Grape Vitis amurensis Rupr. Appl. Biochem. Microbiol. 2023, 59, 361–367. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Abarenkov, K.; Zirk, A.; Piirmann, T.; Pöhönen, R.; Ivanov, F.; Nilsson, R.H.; Kõljalg, U. UNITE QIIME Release for Eukaryotes; Version 29.11.2022; UNITE Community: London, UK, 2022. [Google Scholar] [CrossRef]
- Bisanz, J. qiime2R: Importing QIIME2 Artifacts and Associated Data into R Sessions. 2018. Available online: https://github.com/jbisanz/qiime2R (accessed on 18 June 2025).
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R Package for Data Mining in Microbial Community Ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- Barnett, D.J.M.; Arts, I.C.W.; Penders, J. microViz: An R Package for Microbiome Data Visualization and Statistics. J. Open Source Softw. 2021, 6, 3201. [Google Scholar] [CrossRef]
- Lahti, L.; Shetty, S. Microbiome R Package. 2019. Available online: https://www.bioconductor.org/packages/release/bioc/html/microbiome.html (accessed on 18 June 2025).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Lin, H.; Peddada, S.D. Multigroup Analysis of Compositions of Microbiomes with Covariate Adjustments and Repeated Measures. Nat. Methods 2024, 21, 83–91. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Patz, S.; Gautam, A.; Becker, M.; Ruppel, S.; Rodríguez-Palenzuela, P.; Huson, D. PLaBAse: A Comprehensive Web Resource for Analyzing the Plant Growth-Promoting Potential of Plant-Associated Bacteria. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological Systems Database as a Model of the Real World. Nucleic Acids Res. 2025, 53, D672–D677. [Google Scholar] [CrossRef]
- Sjoberg, D. ggsankey R package. 2025. Available online: https://github.com/davidsjoberg/ggsankey (accessed on 18 June 2025).

| Month | Average t, °C | Precipitation, mm |
|---|---|---|
| February | −8 | 24 |
| July | 25 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nityagovsky, N.N.; Ananev, A.A.; Suprun, A.R.; Dneprovskaya, A.A.; Kiselev, K.V.; Aleynova, O.A. Endophytic Bacterial and Fungal Communities of Spruce Picea jezoensis in the Russian Far East. Plants 2025, 14, 2534. https://doi.org/10.3390/plants14162534
Nityagovsky NN, Ananev AA, Suprun AR, Dneprovskaya AA, Kiselev KV, Aleynova OA. Endophytic Bacterial and Fungal Communities of Spruce Picea jezoensis in the Russian Far East. Plants. 2025; 14(16):2534. https://doi.org/10.3390/plants14162534
Chicago/Turabian StyleNityagovsky, Nikolay N., Alexey A. Ananev, Andrey R. Suprun, Alina A. Dneprovskaya, Konstantin V. Kiselev, and Olga A. Aleynova. 2025. "Endophytic Bacterial and Fungal Communities of Spruce Picea jezoensis in the Russian Far East" Plants 14, no. 16: 2534. https://doi.org/10.3390/plants14162534
APA StyleNityagovsky, N. N., Ananev, A. A., Suprun, A. R., Dneprovskaya, A. A., Kiselev, K. V., & Aleynova, O. A. (2025). Endophytic Bacterial and Fungal Communities of Spruce Picea jezoensis in the Russian Far East. Plants, 14(16), 2534. https://doi.org/10.3390/plants14162534







