Tree Age-Related Differences in Chilling Resistance and Bark-Bleeding Physiological Responses to Chemical Component and Fiber Morphology Changes in Cell Walls of Hevea brasiliensis Bark
Abstract
1. Introduction
2. Results
2.1. Differences in Physiological Parameters, Chemical Components, Fiber Morphologies, and Tensile Properties in Bark of Hevea brasiliensis at Different Ages
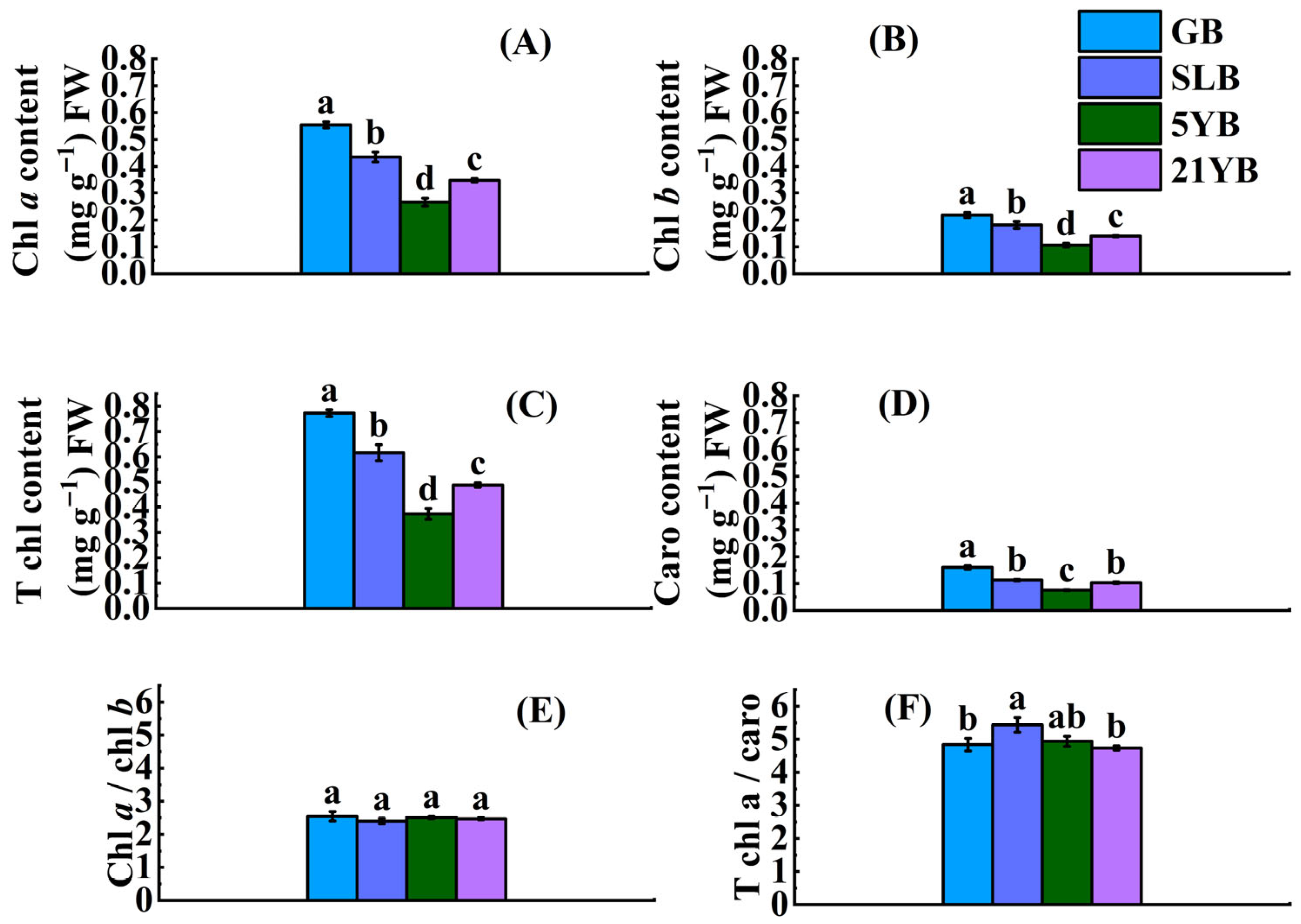
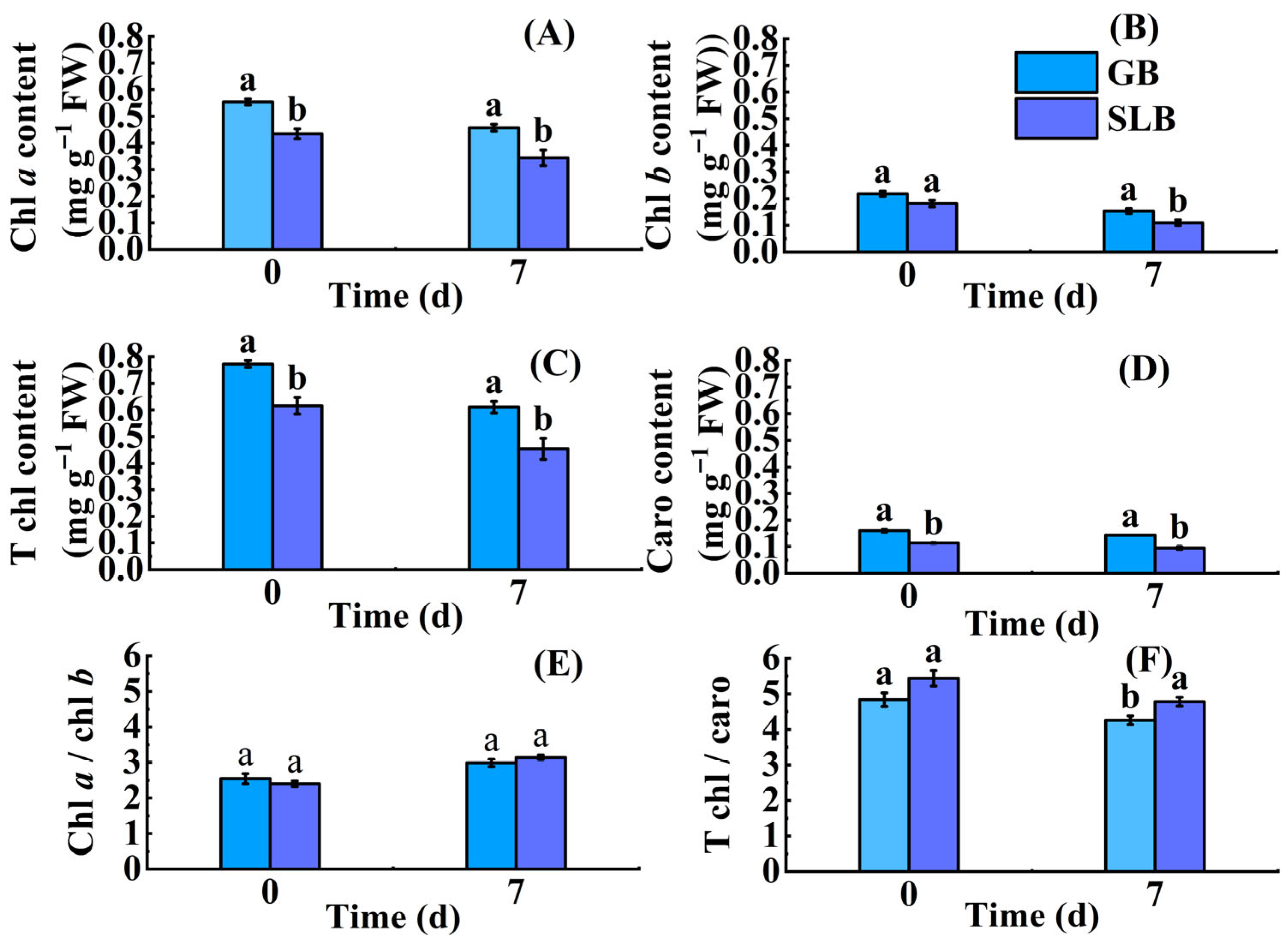
2.1.1. Differences in Chlorophyll Content in Bark of Hevea brasiliensis at Different Ages
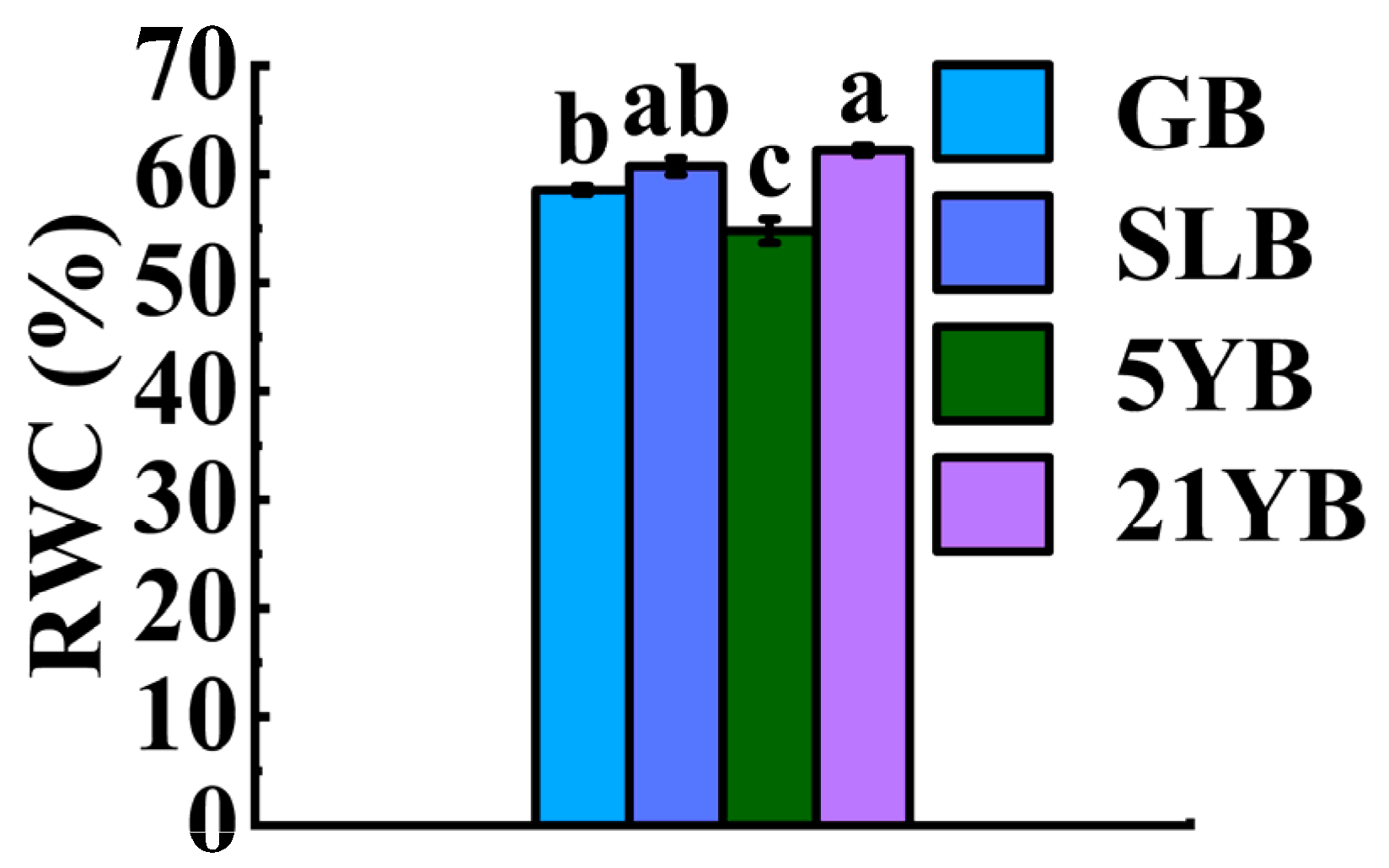
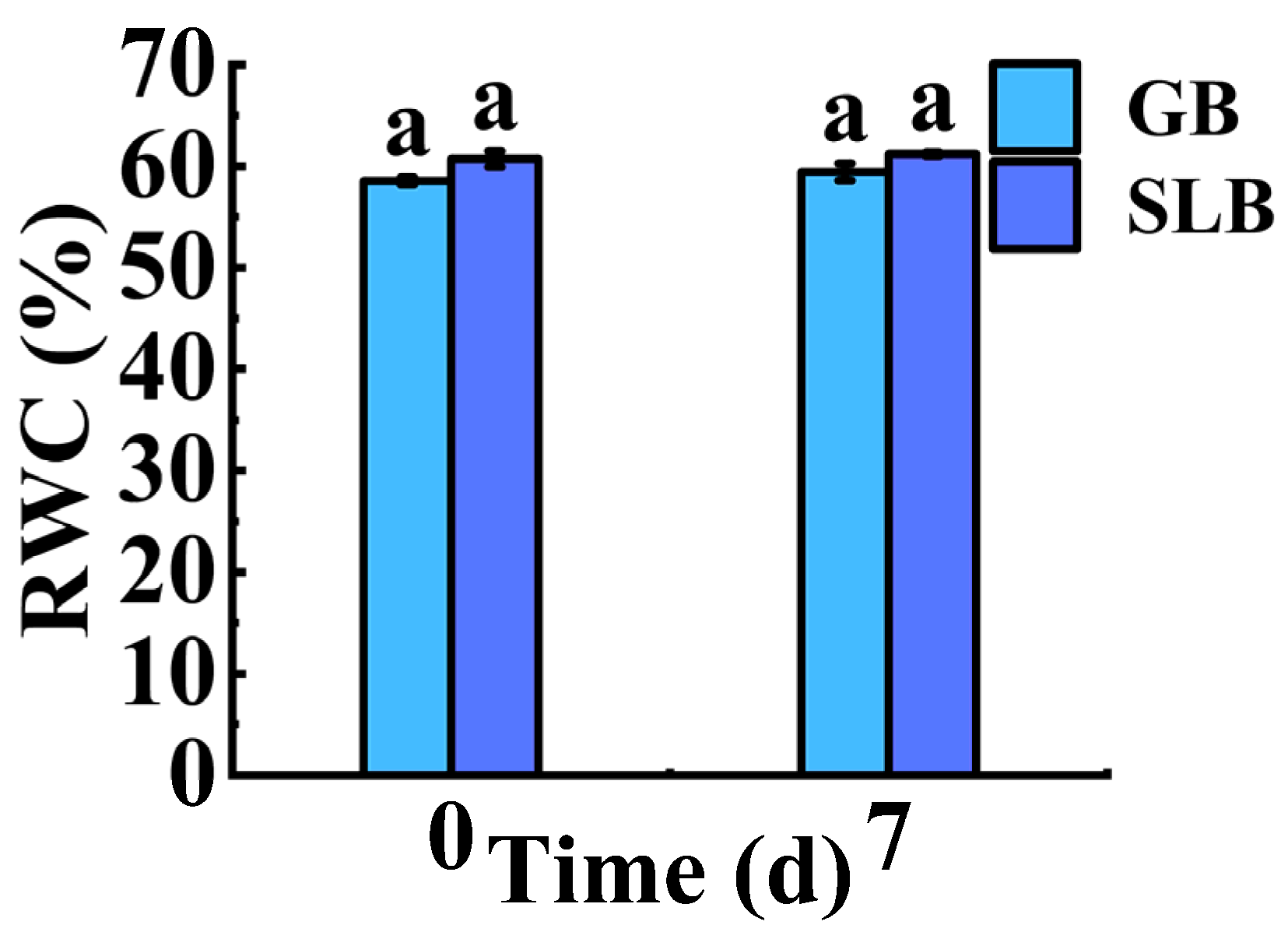
2.1.2. Differences in RWC in Bark from Hevea brasiliensis at Different Ages
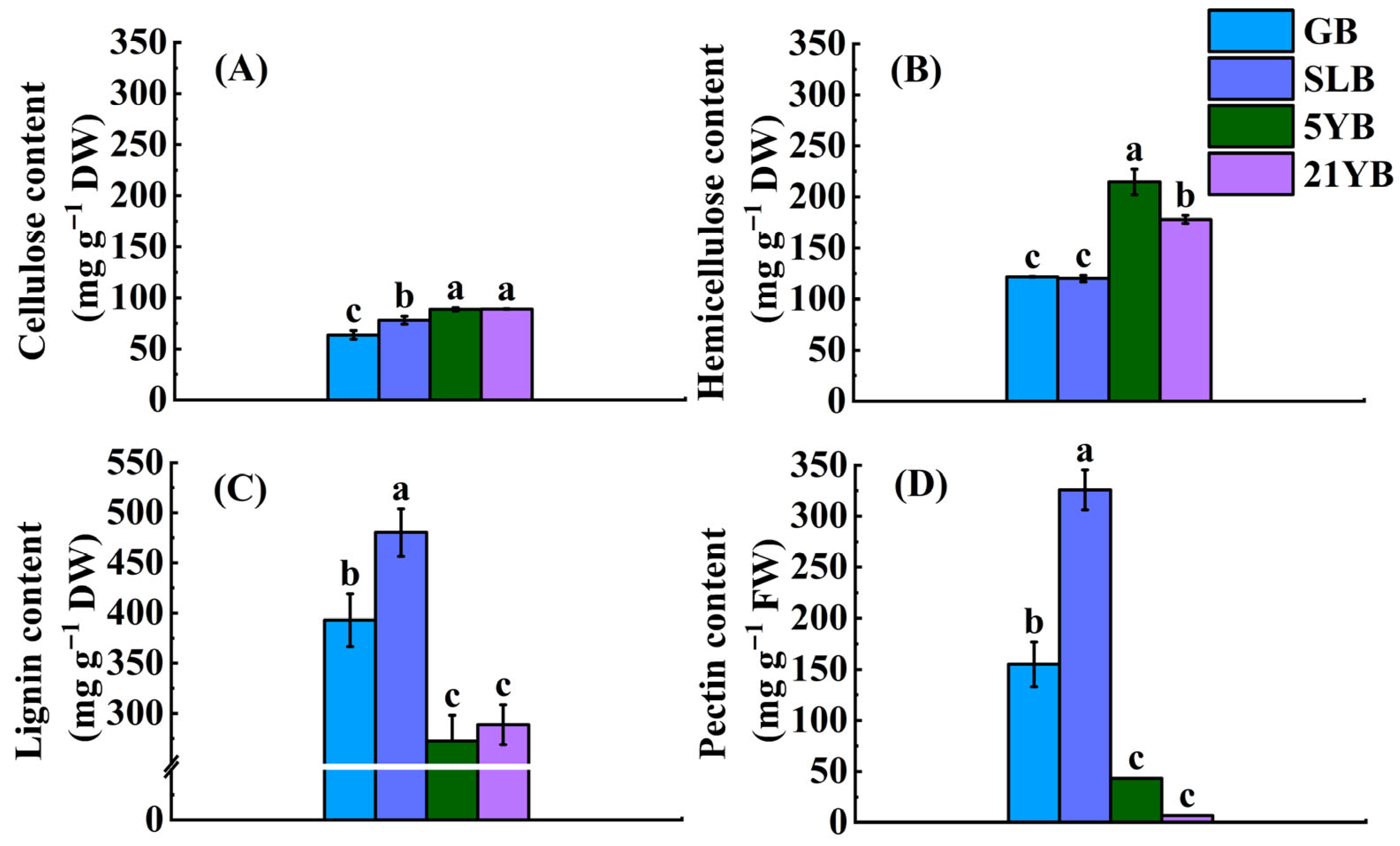
2.1.3. Differences in Chemical Components in Cell Walls of Hevea brasiliensis Bark at Different Ages
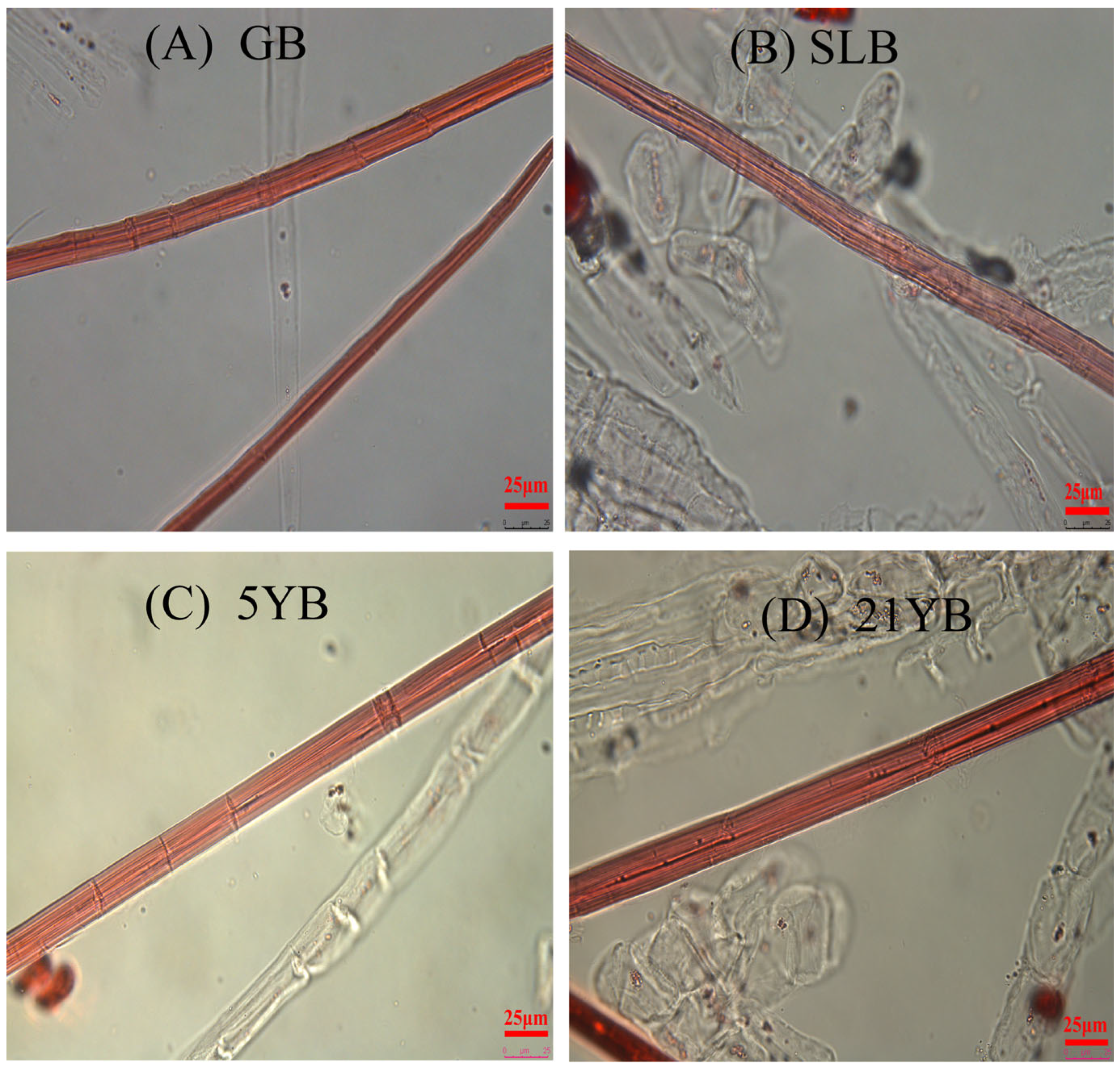
2.1.4. Differences in Fiber Dimensions in Cell Walls of Hevea brasiliensis Bark at Different Ages
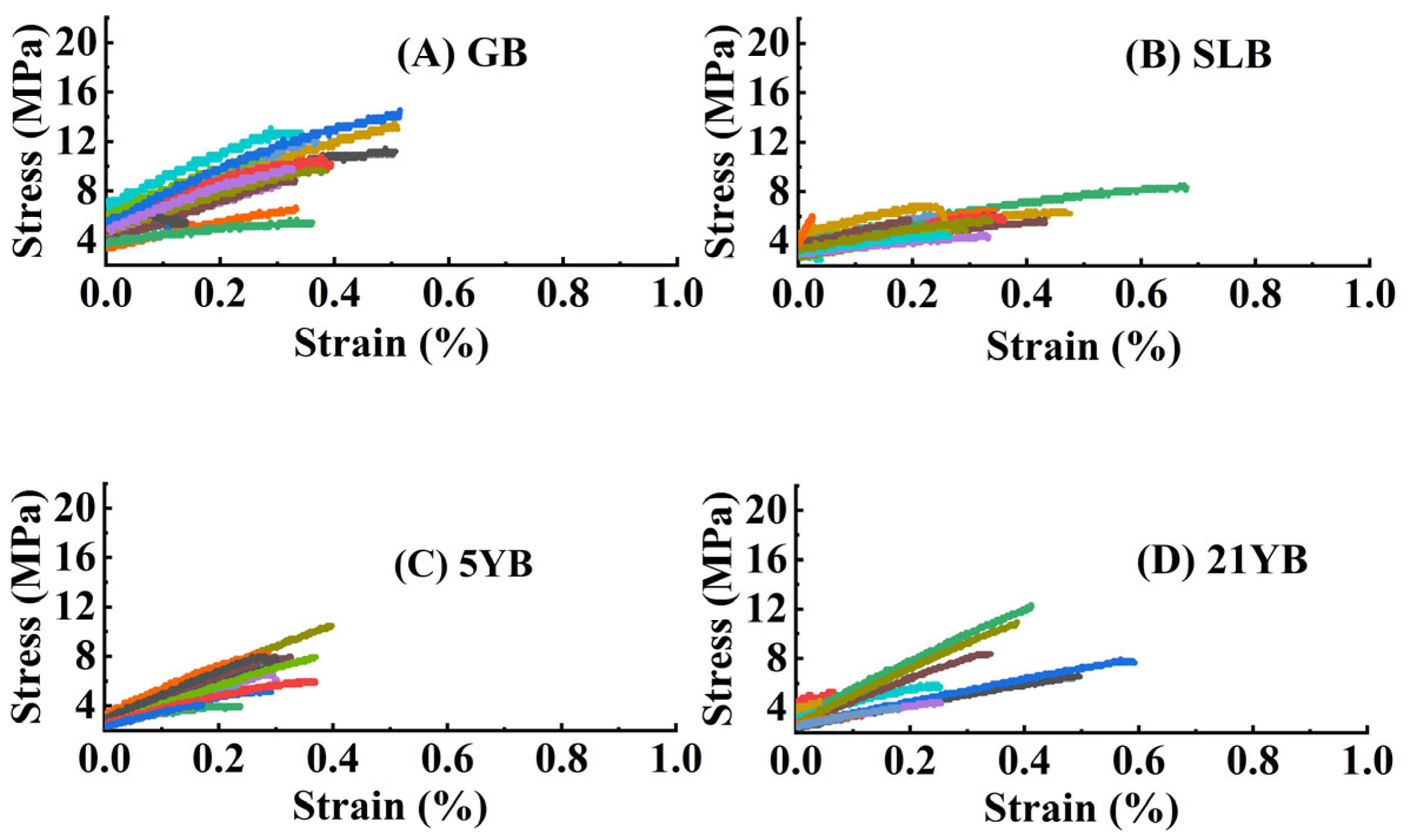
2.1.5. Differences in Tensile Properties in Hevea brasiliensis Bark at Different Ages
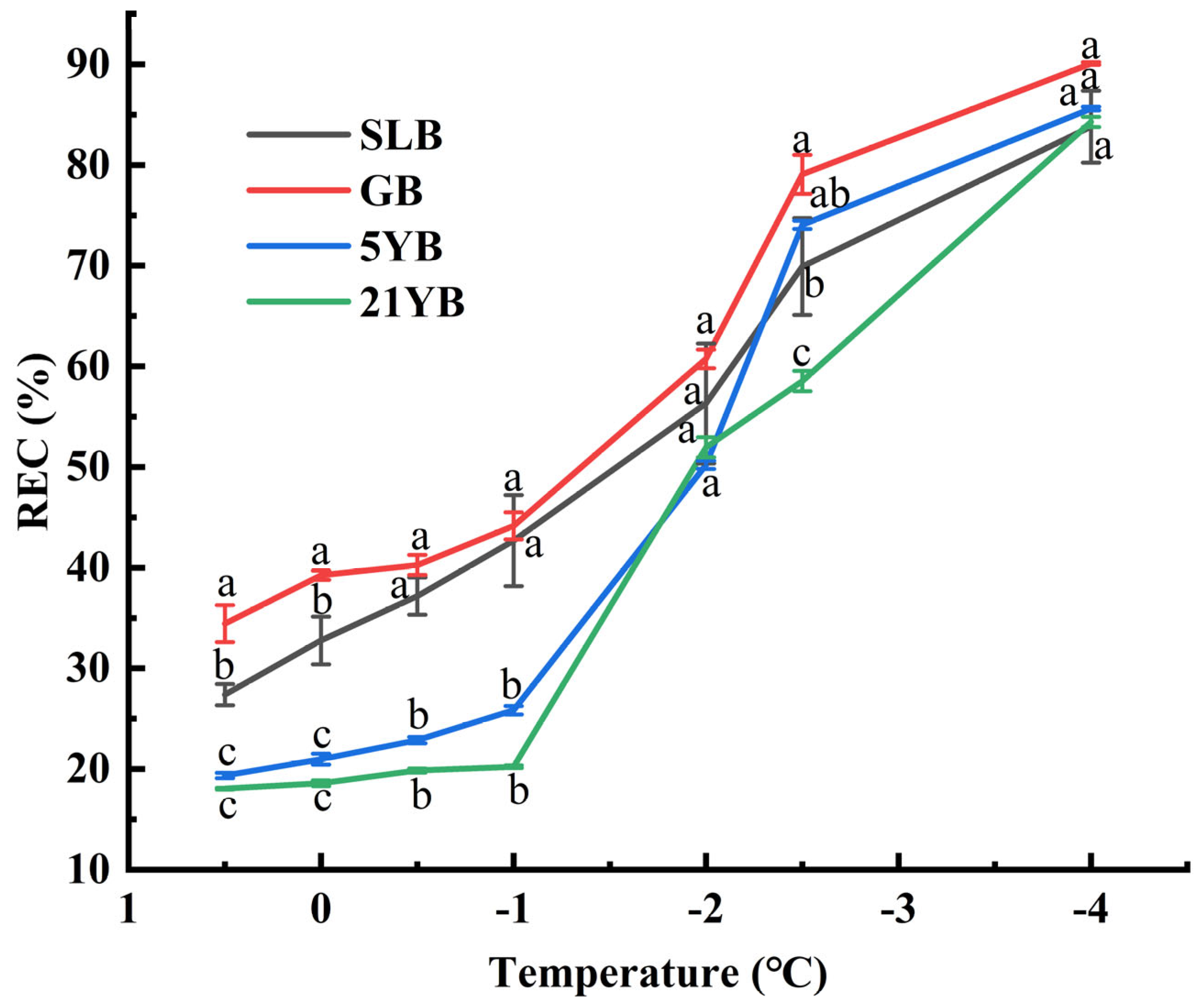
2.2. Changes in Relative Electrical Conductivity (REC) Under Low-Temperature Stresses and Semi-Lethal Temperature (LT50) Estimation
2.3. Stem Age-Mediation of Seedling Responses to Chilling Stress
2.3.1. Changes in Chlorophyll Content in Stem Bark of Hevea brasiliensis Seedlings at Different Stem Ages Under Chilling Stress
2.3.2. Changes in RWC in Stem Bark of Hevea brasiliensis Seedlings at Different Stem Ages Under Chilling Stress
2.3.3. Changes in Chemical Components in Stem Bark of Hevea brasiliensis Seedlings at Different Stem Ages Under Chilling Stress
2.3.4. Changes in Fiber Dimensions in Stem Bark of Hevea brasiliensis Seedlings at Different Stem Ages Under Chilling Stress
2.3.5. Changes in Tensile Properties in Stem Bark of Hevea brasiliensis Seedlings at Different Stem Ages Under Chilling Stress
2.4. Results of Bark Bleeding
2.5. A Comprehensive Analysis of Chilling Resistance and Bark-Bleeding Characteristics in Bark at Different Ages in Rubber Trees
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Chilling-Stress Treatment and Experiment Design
4.3. Electrical Conductivity Measurement
4.4. Photosynthetic Pigment Content Determination
4.5. Relative Water Content Calculation
4.6. Cell-Wall Chemical Component Content Quantitation
4.7. Fiber Dimension Measurement
4.8. Tensile Property Test
4.9. Visual Observation of Bark Bleeding
4.10. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, K.; Huang, Z. Rubber Culture in the Northern Part of Tropical Area, 1st ed.; GuangDong Science & Technology Press: Guangzhou, China, 1987; pp. 23, 110, 114, 128. [Google Scholar]
- Cheng, H.; Cai, H.B.; Fu, H.T.; An, Z.W.; Fang, J.L.; Hu, Y.S.; Guo, D.J.; Huang, H.S. Functional characterization of Hevea brasiliensis CRT/DRE binding factor 1 gene revealed regulation potential in the CBF pathway of rropical perennial tree. PLoS ONE 2015, 10, 137634. [Google Scholar]
- Zhai, D.; Wang, J.; Thaler, P.; Luo, Y.; Xu, J. Contrasted effects of temperature during defoliation vs. refoliation periods on the infection of rubber powdery mildew (Oidium heveae) in Xishuangbanna, China. Int. J. Biometeorol. 2020, 64, 1835–1845. [Google Scholar] [CrossRef]
- Lai, S.C.; Wang, J.G.; Fu, Y.G.; Duan, B.; Hongchang, A.; Zhang, L.; Tarno, H. Infestation of Platypodine Beetles (Coleoptera: Curculionidae) on Rubber Trees in China. Coleopt. Bull. 2020, 74, 626–631. [Google Scholar] [CrossRef]
- Lim, C.; Krebs, S.L.; Arora, R. Cold hardiness increases with age in juvenile Rhododendron populations. Front. Plant Sci. 2014, 5, 542. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A. Comparison of signaling interactions determining annual and perennial plant growth in response to low temperature. Front. Plant Sci. 2015, 5, 794. [Google Scholar] [CrossRef]
- Jaikumar, N.S.; Snapp, S.S.; Sharkey, T.D. Older Thinopyrum intermedium (Poaceae) plants exhibit superior photosynthetic tolerance to cold stress and greater increases in two photosynthetic enzymes under freezing stress compared with young plants. J. Exp. Bot. 2016, 67, 4743–4753. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.R.; Zhou, T.; Chen, B.M.; Peng, S.L. How does tree age influence damage and recovery in forests impacted by freezing rain and snow. Sci. China Life Sci. 2015, 58, 472–479. [Google Scholar] [CrossRef]
- Taylor-Teeples, M.; Lin, L.; de Lucas, M.; Turco, G.; Total, T.W.; Gaudinier, A.; Young, N.F.; Trabucco, G.M.; Veling, M.T.; Lamothe, R.; et al. An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature 2015, 517, 571–575. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Structure and growth of plant cell walls. Nat. Rev. Mol. Cell Biol. 2024, 25, 340–358. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Cavender-Bares. Impacts of freezing on long distance transport in woody plants. In Vascular Transport in Plants; Holbrook, N.M., Zwieniecki, M.A., Eds.; Elsevier Academic Press: London, UK, 2005; pp. 401–424. [Google Scholar]
- Chanliaud, E.; Burrows, K.M.; Jeronimidis, G.; Gidley, M.J. Mechanical properties of primary plant cell wall analogues. Planta 2002, 215, 989–996. [Google Scholar] [CrossRef]
- Ryden, P.; Sugimoto-Shirasu, K.; Smith, A.C.; Findlay, K. Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes1. Plant Physiol. 2003, 132, 1033–1040. [Google Scholar] [CrossRef]
- Goudenhooft, C.; Bourmaud, A.; Baley, C. Flax (Linum usitatissimum L.) Fibers for composite reinforcement: Exploring the link between plant growth, cell walls development, and fiber properties. Front. Plant Sci. 2019, 10, 411. [Google Scholar] [CrossRef]
- Girma, A.; Skidmore, A.K.; de Bie, C.A.J.M.; Bongers, F.; Schlerf, M. Photosynthetic bark: Use of chlorophyll absorption continuum index to estimate Boswellia papyrifera bark chlorophyll content. Int. J. Appl. Earth Obs. 2013, 23, 71–80. [Google Scholar] [CrossRef]
- Aschan, G.; Wittmann, C.; Pfanz, H. Age-dependent bark photosynthesis of aspen twigs. Trees 2001, 15, 431–437. [Google Scholar] [CrossRef]
- Tausz, M.; Warren, C.R.; Adams, M.A. Is the bark of shining gum (Eucalyptus nitens) a sun or a shade leaf? Trees 2005, 19, 415–421. [Google Scholar] [CrossRef]
- Rosell, J.A.; Gleason, S.; Méndez Alonzo, R.; Chang, Y.; Westoby, M. Bark functional ecology: Evidence for tradeoffs, functional coordination, and environment producing bark diversity. New Phytol. 2014, 201, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Charrier, G.; Poirier, M.; Bonhomme, M.; Lacointe, A.; Ameglio, T. Frost hardiness in walnut trees (Juglans regia L.): How to link physiology and modelling? Tree Physiol. 2013, 33, 1229–1241. [Google Scholar] [CrossRef]
- Ilek, A.; Płachta, A.; Siegert, C.; Campos, S.D.; Szostek, M.; Tonello, K.C. Vertical variation in swelling properties of Norway spruce bark depending on tree age and bark moisture content. Eur. J. Forest Res. 2024, 143, 1225–1235. [Google Scholar] [CrossRef]
- Berveiller, D.; Kierzkowski, D.; Damesin, C. Interspecific variability of stem photosynthesis among tree species. Tree Physiol. 2007, 27, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, J. Gradient of photosynthetic pigments in the bark and leaves of lilac (Syringa vulgaris L.). Acta Physiol. Plant 1999, 21, 365–373. [Google Scholar] [CrossRef]
- Levizou, E.; Manetas, Y. Photosynthetic pigment contents in twigs of 24 woody species assessed by in vivo reflectance spectroscopy indicate low chlorophyll levels but high carotenoid/chlorophyll ratios. Environ. Exp. Bot. 2007, 59, 293–298. [Google Scholar] [CrossRef]
- Tran, T.T. The Effect of Light Exposure on the Total Chlorophyll Content, Chl a/b Ratio, and Car/chl Ratio in the Barks of Fraxinus latifolia Seedlings; University Honors Theses: Portland, OR, USA, 2018; p. 575. [Google Scholar]
- Pfanz, H.; Aschan, G.; Langenfeld-Heyser, R.; Wittmann, C.; Loose, M. Ecology and ecophysiology of tree stems: Corticular and wood photosynthesis. Naturwissenschaften 2002, 89, 147–162. [Google Scholar]
- Pástory, Z.; Mohácsiné, I.R.; Gorbacheva, G.; Börcsök, Z. The utilization of tree bark. BioResources 2016, 11, 7859–7888. [Google Scholar] [CrossRef]
- Paine, C.E.T.; Stahl, C.; Courtois, E.A.; Patiño, S.; Sarmiento, C.; Baraloto, C. Functional explanations for variation in bark thickness in tropical rain forest trees. Funct. Ecol. 2010, 24, 1202–1210. [Google Scholar] [CrossRef]
- Vázquez, G.; Antorrena, G.; Parajó, J.C. Studies on the utilization of Pinus pinaster bark. Wood. Sci. Technol. 1987, 21, 65–74. [Google Scholar] [CrossRef]
- Özgenç, Ö.; Durmaz, S.; Kuştaş, S. Chemical analysis of tree barks using ATR-FTIR spectroscopy and conventional techniques. Bioresources 2017, 12, 9143–9151. [Google Scholar] [CrossRef]
- Cabalová, I.; Belik, M.; Kucerova, V.; Jurczykova, T. Chemical and morphological composition of Norway spruce wood (Picea abies, L.) in the dependence of its Storage. Polymers 2021, 13, 1619. [Google Scholar] [CrossRef] [PubMed]
- Kathirselvam, M.; Kumaravel, A.; Arthanarieswaran, V.P.; Saravanakumar, S.S. Assessment of cellulose in bark fibers of Thespesia populnea: Influence of stem maturity on fiber characterization. Carbohyd. Polym. 2019, 212, 439–449. [Google Scholar] [CrossRef]
- Tanguy, M.; Bourmaud, A.; Baley, C. Plant cell walls to reinforce composite materials: Relationship between nanoindentation and tensile modulus. Mater. Lett. 2016, 167, 161–164. [Google Scholar] [CrossRef]
- Petrova, A.; Gorshkova, T.; Liudmila, K. Gradients of cell wall nano-mechanical properties along and across elongating primary roots of maize. J. Exp. Bot. 2021, 72, 1764–1781. [Google Scholar] [CrossRef]
- Cardoso, S.; Ferreira, J.; Miranda, I.; Pereira, H. Age variation of Douglas-fir bark chemical composition. J. Wood. Chem. Technol. 2018, 38, 385–396. [Google Scholar] [CrossRef]
- Kopanina, A.V.; Talskikh, A.I.; Vlasova, I.I.; Kotina, E.L. Age-related pattern in bark formation of Betula ermanii growing in volcanic environments from southern Sakhalin and Kuril Islands (Northeast Asia). Trees 2022, 36, 915–939. [Google Scholar] [CrossRef]
- Bold, G.; Langer, M.; Bornert, L.; Speck, T. The protective role of bark and bark fibers of the giant sequoia (Sequoiadendron giganteum) during high-energy impacts. Int. J. Mol. Sci. 2020, 21, 3355. [Google Scholar] [CrossRef]
- McNamara, S.; Pellett, H. Cold Hardiness of Phellodendron sachalinense Friedr. Schmidt seedlings increases with age. Hortscience 2000, 35, 304–305. [Google Scholar] [CrossRef]
- Ambavaram, M.M.; Basu, S.; Krishnan, A.; Ramegowda, V.; Batlang, U.; Rahman, L.; Baisakh, N.; Pereira, A. Coordinated regulation of photosynthesis in rice increases yield and tolerance to environmental stress. Nat. Commun. 2014, 5, 5302. [Google Scholar] [CrossRef] [PubMed]
- Arco Molina, J.G.; Hadad, M.A.; Patón Domínguez, D.; Roig, F.A. Tree age and bark thickness as traits linked to frost ring probability on Araucaria araucana trees in northern Patagonia. Dendrochronologia 2016, 37, 116–125. [Google Scholar] [CrossRef]
- Ilek, A.; Van Stan, J.T.; Morkisz, K.; Kucza, J. Vertical variability in bark hydrology for two coniferous tree species. Front. For. Glob. Change 2021, 4, 687907. [Google Scholar] [CrossRef]
- Domon, J.; Baldwin, L.; Acket, S.; Caudeville, E.; Arnoult, S.; Zub, H.; Gillet, F.; Lejeune-Hénaut, I.; Brancourt-Hulmel, M.; Pelloux, J.; et al. Cell wall compositional modifications of Miscanthus ecotypes in response to cold acclimation. Phytochemistry 2013, 85, 51–61. [Google Scholar] [CrossRef]
- Hausman, J.F.; Evers, D.; Thiellement, H.; Jouve, L. Compared responses of poplar cuttings and in vitro raised shoots to short-term chilling treatments. Plant Cell. Rep. 2000, 19, 954–960. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Y.H.; Liu, K.; Shu, H.M.; Zhou, Z.G. Protein expression changes during cotton fiber elongation in response to low temperature stress. J. Plant Physiol. 2012, 169, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Galindo, F.G.; Vaughan, D.; Herppich, W.; Smallwood, M.; Sommarin, M.; Gekas, V.; Sjöholm, I. Influence of cold acclimation on the mechanical strength of carrot (Daucus carota L.) tissue. Eur. J. Hortic. Sci. 2004, 69, 229–234. [Google Scholar] [CrossRef]
- Solecka, D.; Zebrowski, J.; Kacperska, A. Are pectins involved in cold acclimation and de-acclimation of winter oil-seed rape plants? Ann. Bot. 2008, 101, 521–530. [Google Scholar] [CrossRef]
- Huang, G.X.; Zhou, X.M.; LIU, X.B.; Cai, J.M.; Li, B.X. First report of rubber tree gummosis disease caused by Fusarium solani in China. Plant. Dis. 2016, 100, 1788. [Google Scholar] [CrossRef]
- Mancero-Castillo, D.; Sarkhosh, A.; Sherman, S.; Olmstead, M.; Harmon, P.; Beckman, T. Fungal gummosis in peach. UF/IFAS Ext. 2018, HS1625, 1–6. [Google Scholar] [CrossRef]
- Cheng, L.; Jiang, H.; Xie, G.; Wang, J.; Peng, W.; Zhou, L.; An, F. Photosynthesis and Latex Burst Characteristics in Different Varieties of Rubber Trees (Hevea brasiliensis) under Chilling Stress, Combing Bark Tensile Property and Chemical Component Analysis. Forests 2024, 15, 1408. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wu, D.; Hui, M.; Wang, Y.; Han, X.; Yao, F.; Cao, X.; Li, Y.H.; Li, H.; Wang, H. Screening of cold hardiness-related indexes and establishment of a comprehensive evaluation method for grapevines (V. vinifera). Front. Plant Sci. 2022, 13, 1014330. [Google Scholar] [CrossRef]
- Gao, J.F. Experimental Gaidance for Plant Physiolgoy; Higher Education Press: Beijing, China, 2006; pp. 74–77. [Google Scholar]
- Hao, Y.Y.; Lu, F.C.; Pyo, S.W.; Kim, M.H.; Ko, J.H.; Yan, X.J.; Ralph, J.; Li, Q.Z. PagMYB128 regulates secondary cell wall formation by direct activation of cell wall biosynthetic genes during wood formation in poplar. J. Integr. Plant Biol. 2024, 66, 1658–1674. [Google Scholar] [CrossRef] [PubMed]
- Housseinpour, R.; Latibari, A.J.; Farnood, R.; Fatehi, P.; Sepiddehdam, S.J. Fiber morphology and chemical compostion of rape-seed (Brassica napus) stems. Iawa J. 2010, 31, 457–464. [Google Scholar] [CrossRef]
- Wang, Y.F.; Tian, S.H.; Shuai, H.G.; Jin, B.C.; Zhang, Y.Y.; Wei, J.P.; Niu, Z.J.; Ma, Y.F.; Zhao, X.C. Effects of fertilization gradient on the production performance and nutritional quality of cultivated grasslands in karst areas. Front. Plant Sci. 2023, 14, 1228621. [Google Scholar] [CrossRef]


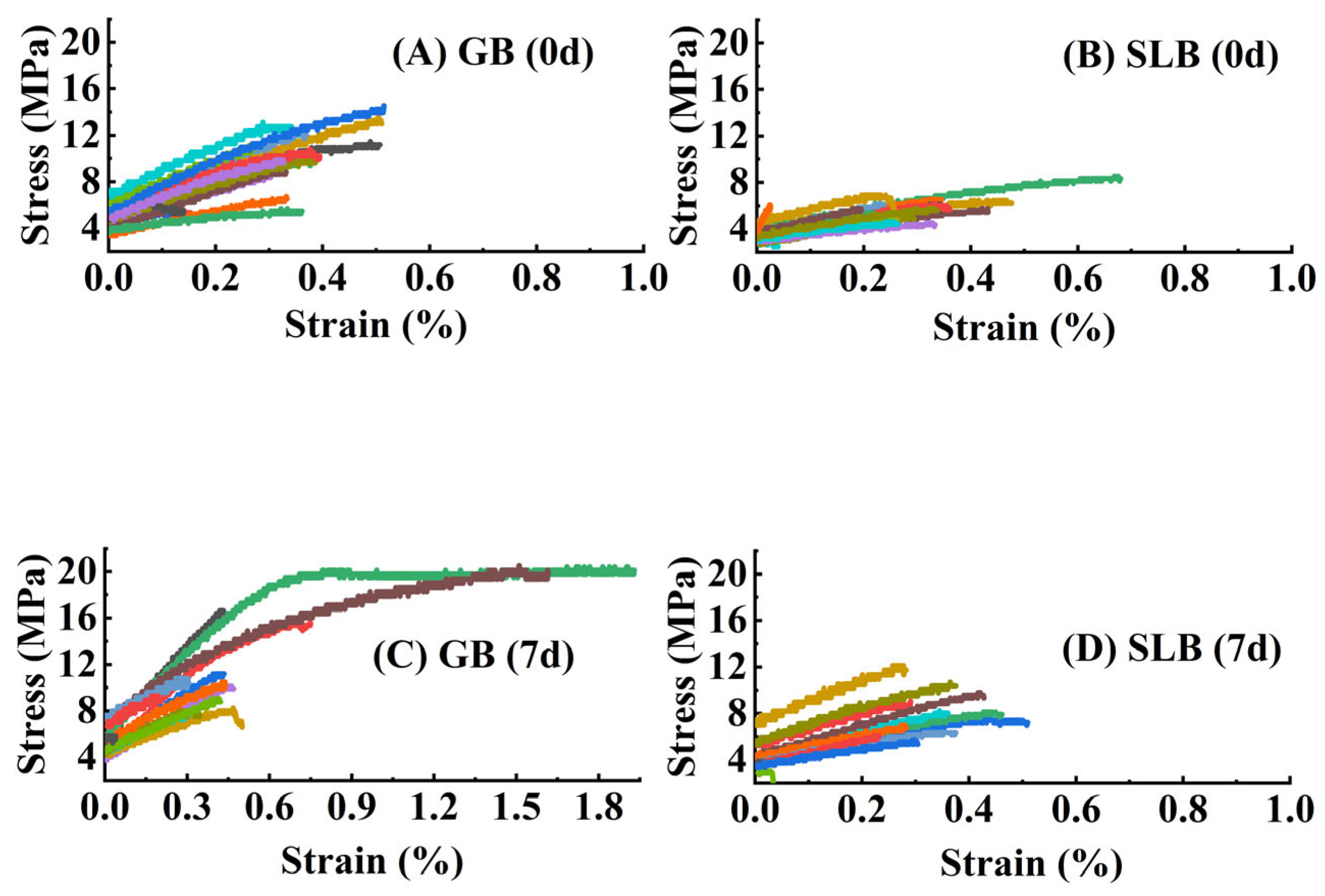

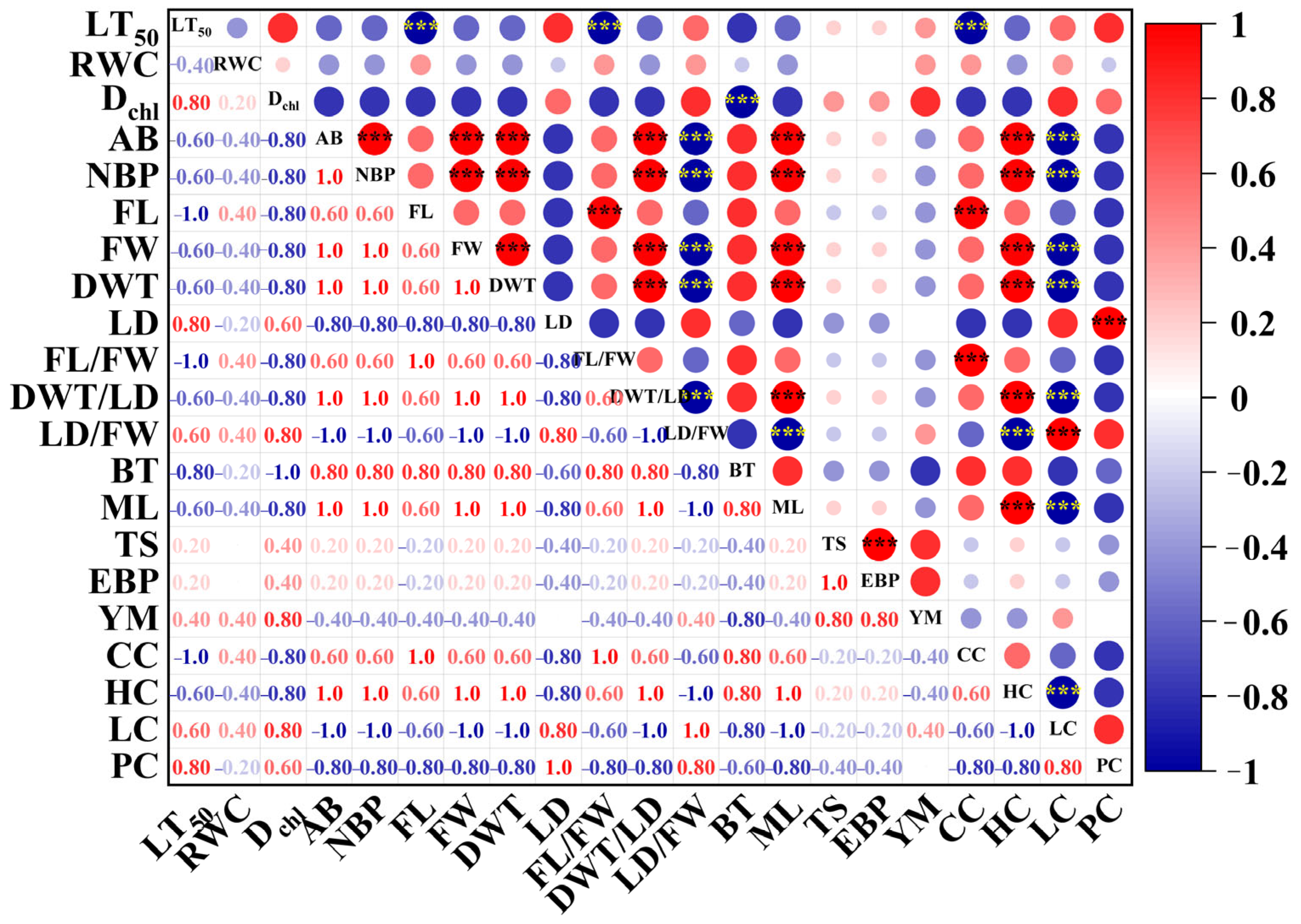
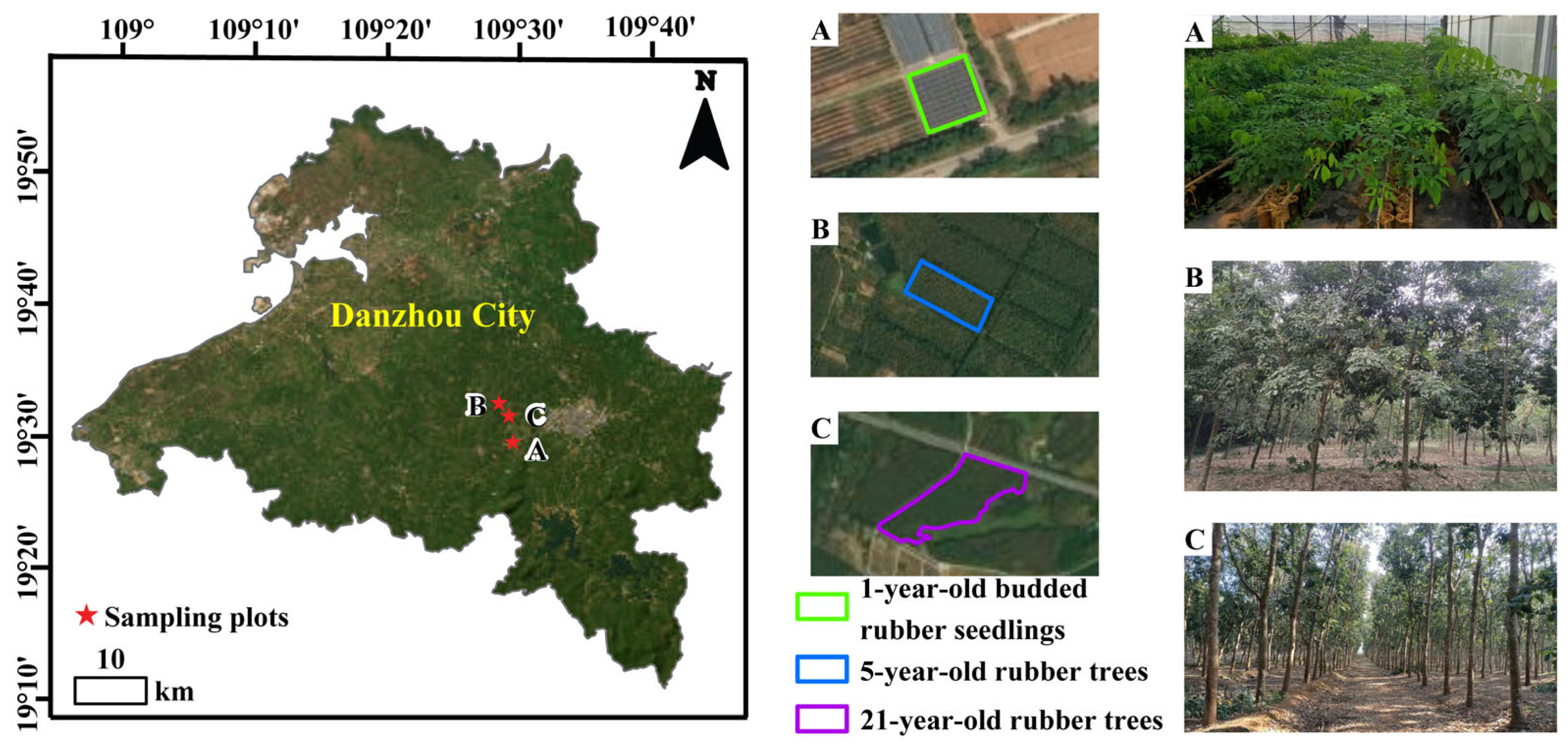
| Fiber Properties | GB | SLB | 5YB | 21YB |
|---|---|---|---|---|
| FL (μm) | 2722.3660 ± 66.4041 c | 2786.0260 ± 110.3086 c | 3381.9610 ± 93.4299 b | 4014.6650 ± 134.4203 a |
| FW (μm) | 19.9108 ± 0.4474 bc | 18.8703 ± 0.41 c | 22.1818 ± 0.5015 a | 20.8010 ± 0.3888 b |
| DWT(μm) | 18.6548 ± 0.4249 bc | 17.5515 ± 0.3385 c | 21.0406 ± 0.5013 a | 19.6665 ± 0.3842 b |
| LD (μm) | 1.2560 ± 0.0571 a | 1.3187 ± 0.1808 a | 1.1411 ± 0.0264 a | 1.1345 ± 0.0274 a |
| FL/FW | 137.7251 ± 4.6514 c | 152.1800 ± 6.4443 bc | 156.5164 ± 5.3717 b | 197.3573 ± 7.8216 a |
| DWT/LD | 16.7325 ± 0.8078 b | 16.5424 ± 0.6522 b | 19.1053 ± 0.6690 a | 17.9940 ± 0.5807 ab |
| LD/FW | 0.0627 ± 0.0029 ab | 0.0665 ± 0.0054 a | 0.0530 ± 0.0017 b | 0.0554 ± 0.0016 b |
| Tensile Properties | GB | SLB | 5YB | 21YB |
|---|---|---|---|---|
| BT (mm) | 0.6271 ± 0.0258 c | 0.8327 ± 0.0243 b | 1.0993 ± 0.0239 a | 1.0209 ± 0.0566 a |
| ML (N) | 5.8500 ± 0.3084 b | 4.6123 ± 0.1744 c | 7.2080 ± 0.4582 a | 7.0164 ± 0.9192 ab |
| TS (MPa) | 9.6647 ± 0.7067 a | 5.6038 ± 0.2386 b | 6.6170 ± 0.4965 b | 6.9109 ± 0.8401 b |
| EBP (%) | 0.3374 ± 0.0291 a | 0.2821 ± 0.0299 a | 0.2916 ± 0.0180 a | 0.3072 ± 0.0477 a |
| YM (MPa) | 3074.6760 ± 212.9404 a | 2677.2310 ± 422.0297 a | 2283.2720 ± 108.3105 a | 2716.7490 ± 472.8373 a |
| Sample | Logistic Equation | LT50 (°C) | R2 | Order of Chilling Resistance |
|---|---|---|---|---|
| GB | y = 1/(1 + 1.7789 × e0.6622x) | −0.8695 | 0.9433 ** | 4 |
| SLB | y = 1/(1 + 2.1641 × e0.5932x) | −1.3021 | 0.9863 ** | 3 |
| 5YB | y = 1/(1 + 4.0082 × e0.7931x) | −1.7504 | 0.9364 ** | 2 |
| 21YB | y = 1/(1 + 4.7676 × e0.7661x) | −2.0387 | 0.9341 ** | 1 |
| Fiber Properties | GB | SLB | ||
|---|---|---|---|---|
| 0 d | 7 d | 0 d | 7 d | |
| FL (μm) | 2722.3660 ± 66.4041 b | 3133.6330 ± 88.4555 a | 2786.0260 ± 110.3086 b | 3140.588 ± 80.059 a |
| FW (μm) | 19.9108 ± 0.4474 a | 18.9018 ± 0.4352 a | 18.8703 ± 0.41 a | 17.6059 ± 0.3794 b |
| DWT (μm) | 18.6548 ± 0.4249 a | 17.1258 ± 0.4673 bc | 17.5515 ± 0.3385 ab | 16.3246 ± 0.3793 c |
| LD (μm) | 1.2560 ± 0.0571 b | 2.1262 ± 0.3068 a | 1.3187 ± 0.1808 b | 1.2813 ± 0.0321 b |
| FL/FW | 137.7251 ± 4.6514 c | 164.9380 ± 4.9622 b | 152.1800 ± 6.4443 bc | 182.3958 ± 5.7221 a |
| DWT/LD | 16.7325 ± 0.8078 a | 12.9035 ± 0.6635 b | 16.5424 ± 0.6522a | 13.3049 ± 0.5021 b |
| LD/FW | 0.0627 ± 0.0029 b | 0.1133 ± 0.0169 a | 0.0665 ± 0.0054 b | 0.0748 ± 0.0025 b |
| Tensile Properties | GB | SLB | ||
|---|---|---|---|---|
| 0 d | 7 d | 0 d | 7 d | |
| BT (mm) | 0.6271 ± 0.0258 c | 0.5771 ± 0.0306 c | 0.8327 ± 0.0243 a | 0.7247 ± 0.0367 b |
| ML (N) | 5.8500 ± 0.3084 ab | 6.6579 ± 0.6656 a | 4.6123 ± 0.1744 c | 4.9207 ± 0.2767 bc |
| TS (MPa) | 9.6647 ± 0.7067 b | 11.7743 ± 1.2474 a | 5.6038 ± 0.2386 c | 7.1953 ± 0.652 c |
| EBP (%) | 0.3374 ± 0.0291 b | 0.5872 ± 0.1417 a | 0.2821 ± 0.0299 b | 0.2827 ± 0.0377 b |
| YM (MPa) | 3074.6760 ± 212.9404 a | 3354.7546 ± 913.5118 a | 2677.2310 ± 422.0297 a | 3469.5580 ± 579.3064 a |
| The Situation of Bark Bleeding | GB | SLB | 5YB | 21YB |
|---|---|---|---|---|
| NBPs | 9 | 0 | 85 | 82 |
| AB (mm2) | 23.1130 | 0 | 789.6621 | 656.5682 |
| PL | Petiole | / | Bundle scar, lenticel | Bundle scar, lenticel |
| RBSs/RBTSs | 16.6670% | 0 | 100% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Jiang, H.; Xie, G.; Wang, J.; Peng, W.; Zhou, L.; Liu, W.; Wu, D.; An, F. Tree Age-Related Differences in Chilling Resistance and Bark-Bleeding Physiological Responses to Chemical Component and Fiber Morphology Changes in Cell Walls of Hevea brasiliensis Bark. Plants 2025, 14, 2531. https://doi.org/10.3390/plants14162531
Cheng L, Jiang H, Xie G, Wang J, Peng W, Zhou L, Liu W, Wu D, An F. Tree Age-Related Differences in Chilling Resistance and Bark-Bleeding Physiological Responses to Chemical Component and Fiber Morphology Changes in Cell Walls of Hevea brasiliensis Bark. Plants. 2025; 14(16):2531. https://doi.org/10.3390/plants14162531
Chicago/Turabian StyleCheng, Linlin, Huichuan Jiang, Guishui Xie, Jikun Wang, Wentao Peng, Lijun Zhou, Wanting Liu, Dingquan Wu, and Feng An. 2025. "Tree Age-Related Differences in Chilling Resistance and Bark-Bleeding Physiological Responses to Chemical Component and Fiber Morphology Changes in Cell Walls of Hevea brasiliensis Bark" Plants 14, no. 16: 2531. https://doi.org/10.3390/plants14162531
APA StyleCheng, L., Jiang, H., Xie, G., Wang, J., Peng, W., Zhou, L., Liu, W., Wu, D., & An, F. (2025). Tree Age-Related Differences in Chilling Resistance and Bark-Bleeding Physiological Responses to Chemical Component and Fiber Morphology Changes in Cell Walls of Hevea brasiliensis Bark. Plants, 14(16), 2531. https://doi.org/10.3390/plants14162531







