Carbon Dot Nanoparticles Synthesized from Horticultural Extracts for Postharvest Shelf-Life Extension of Fruits and Vegetables
Abstract
1. Introduction
2. Overview of Physicochemical Properties and Various Approaches to Synthesizing Carbon Dots from Agricultural Extracts
2.1. Biogenic Synthesis of CDs Using the Hydrothermal Method
2.2. Biogenic Synthesis of CDs Using the Microwave-Assisted Method
2.3. Biogenic Synthesis of CDs Using Chemical Oxidation Approach
2.4. Biogenic Synthesis of CDs Using the Ultrasonic Method
2.5. Biogenic Synthesis of CDs: Pyrolysis and Carbonization Method
3. The Major Factor Affecting the Properties of CDs
3.1. The Impact of Biological Carbon Precursor
3.2. The Effect of Reaction Time
3.3. The Effect of Synthesis Temperature
4. Characterization of Carbon Dots
5. Recent Applications of Carbon Dots from Agricultural Extracts in Food Preservation
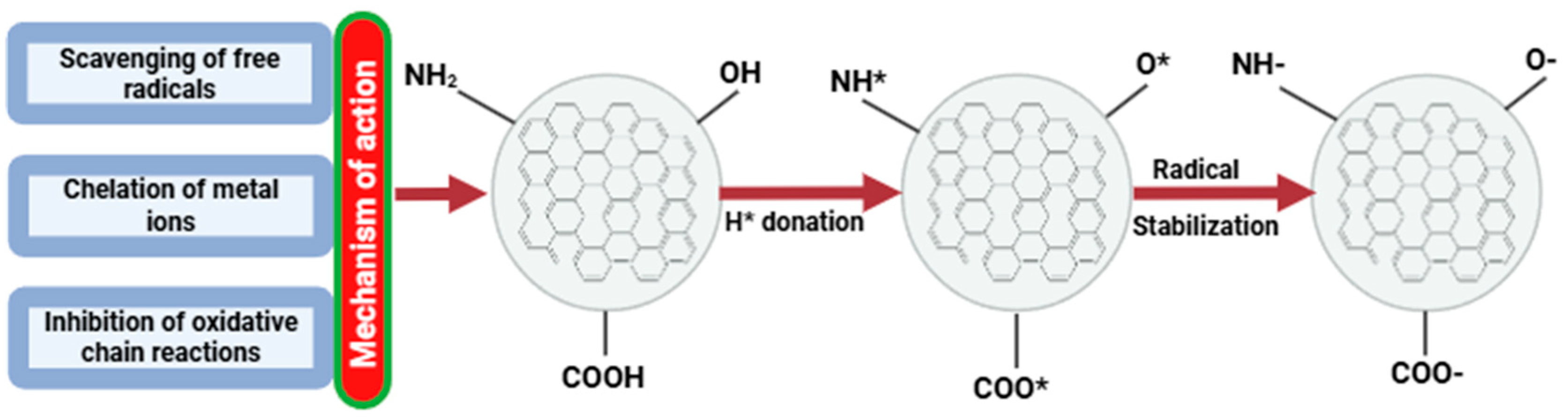
5.1. Carbon Dot Nanoparticles Applied as Antioxidant Agents
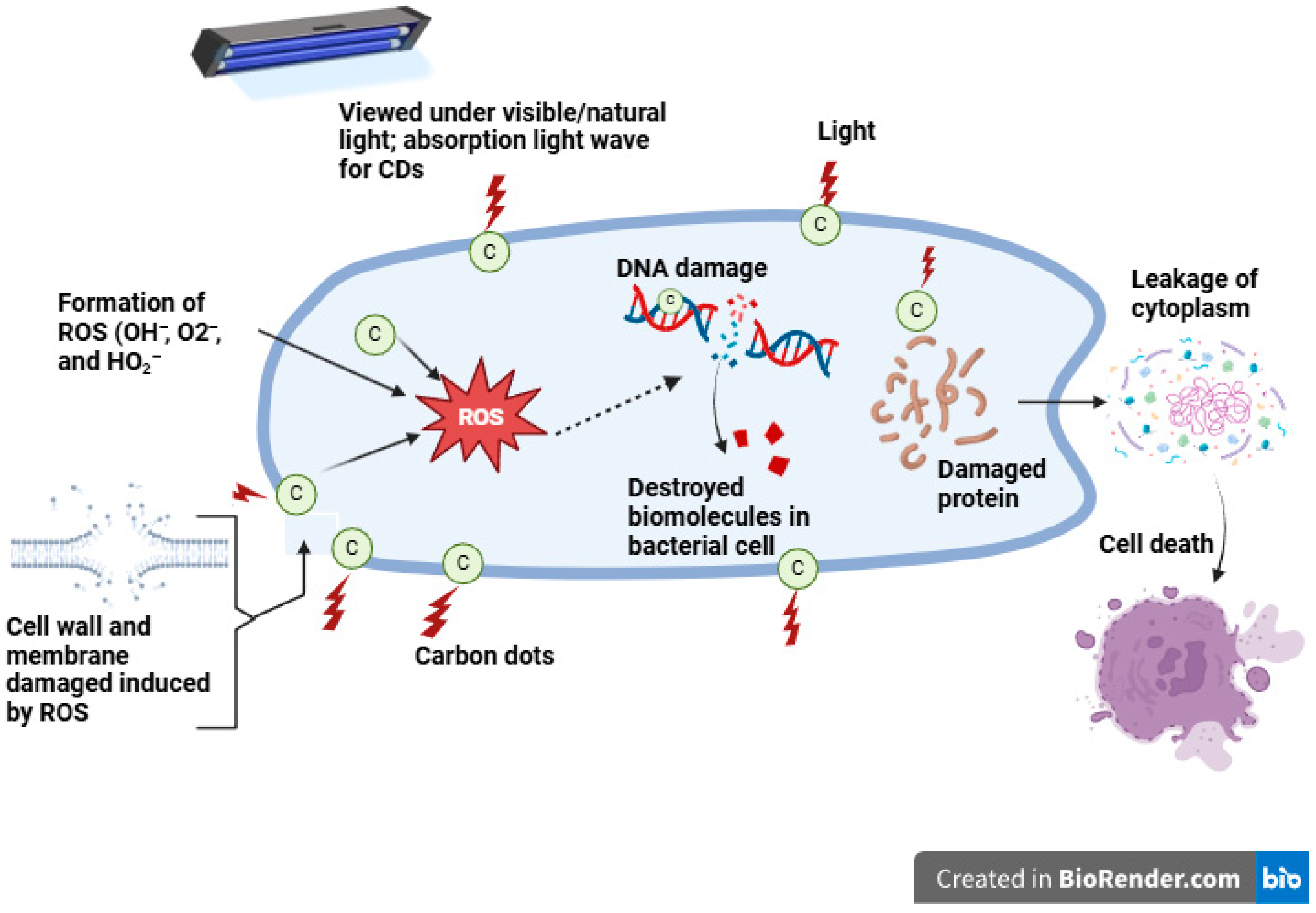
5.2. Microbial Properties of Carbon Dot Nanoparticles
5.3. Cytotoxicity of Carbon Dots
6. Application of Carbon Dots in Food Packaging
6.1. UV Barrier Properties of CDs on Polymers
6.2. The Effect of CDs on the Mechanical Properties of Nanocomposite Films
6.3. The Effect of CDs on Water Vapor Permeability of Nanocomposite Films
6.4. The Effect of CDs on the Antioxidant Properties of Nanocomposite Films
6.5. Antimicrobial Properties of CDs on Polymers
7. Application of Edible Coatings Enriched with CDs for Food Preservation: Enhancing Shelf Life and Food Safety
7.1. UV Barrier Capabilities of CDs Enhance the Shelf Life
7.2. The Effects of CDs on Microbial Count
7.3. The Effects of CDs on the Physiological Properties of Food Products
8. Challenges and Future Directions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDs | Carbon dots |
| CNT | Carbon nanotubes |
| ABTS | 2,2′ -azino-bis (3-ethyl-benzothiazoline-6-sulfonic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| IC50 | 50% inhibition concentration |
| AMR | Antimicrobial resistance |
| CAGR | Compound Annual Growth Rate |
| CNF | Cellulose nanofibers |
| CMC | Carboxymethyl cellulose |
| GO | Graphene oxide |
| HDPE | High-density polyethylene |
| FTIR | Fourier Transform Infrared spectroscopy |
| LDPE | Low-density polyethylene |
| PCL | Polycaprolactone |
| PVC | Polyvinyl chloride |
| PET | Polyethylene terephthalate |
| PLA | Poly lactic acid |
| PBS | Polybutylene succinate |
| QY | Quantum yield |
| UV | Ultraviolet |
| AFM | Atomic force microscopy |
| SEM | Scanning Electron Microscopy |
| ROS | Reactive oxygen species |
| SET | Single electron transfer |
| TS | Tensile strength |
| EVOH | Thylene vinyl alcohol copolymer |
| TEM | Transmission Electron Microscopy |
| TGA | Thermogravimetric analysis |
| PL | Photoluminescence |
| WVP | Water vapor permeability |
| WHO | World Health Organization |
| XRD | X-ray Diffraction |
| XPS | X-ray Photoelectron Spectroscopy |
| ZnO | Zinc oxide |
References
- Chowmasundaram, Y.A.P.; Tan, T.L.; Nulit, R.; Jusoh, M.; Rashid, S.A. Recent Developments, Applications and Challenges for Carbon Quantum Dots as a Photosynthesis Enhancer in Agriculture. RSC Adv. 2023, 13, 25093–25117. [Google Scholar] [CrossRef]
- Vollset, S.E.; Goren, E.; Yuan, C.W.; Cao, J.; Smith, A.E.; Hsiao, T.; Bisignano, C.; Azhar, G.S.; Castro, E.; Chalek, J.; et al. Fertility, Mortality, Migration, and Population Scenarios for 195 Countries and Territories from 2017 to 2100: A Forecasting Analysis for the Global Burden of Disease Study. Lancet 2020, 396, 1285–1306. [Google Scholar] [CrossRef]
- Sardella, D.; Gatt, R.; Valdramidis, V.P. Physiological Effects and Mode of Action of ZnO Nanoparticles against Postharvest Fungal Contaminants. Food Res. Int. 2017, 101, 274–279. [Google Scholar] [CrossRef]
- Alegbeleye, O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial Spoilage of Vegetables, Fruits and Cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
- UN Environment Programme. UNEP Food Waste Index Report 2024; UN Environment Programme: Nairobi, Kenya, 2024.
- Mmereki, D.; David, V.E.; Wreh Brownell, A.H. The Management and Prevention of Food Losses and Waste in Low- and Middle-Income Countries: A Mini-Review in the Africa Region. Waste Manag. Res. 2024, 42, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.; Bhattacharjee, T.; Nag, P.; Ritika; Ghati, A.; Kuila, A. Valorization of Agro-Waste into Value Added Products for Sustainable Development. Bioresour. Technol. Rep. 2021, 16, 100834. [Google Scholar] [CrossRef]
- Dhanker, R.; Rawat, S.; Chandna, V.; Deepa; Kumar, R.; Das, S.; Sharma, A.; Kumar, V. Recovery of Silver Nanoparticles and Management of Food Wastes: Obstacles and Opportunities. Environ. Adv. 2022, 9, 100303. [Google Scholar] [CrossRef]
- Leta, T.B.; Adeyemi, J.O.; Fawole, O.A. Utilizing Fruit Waste-Mediated Nanoparticles for Sustainable Food Packaging Materials to Combat Food Loss and Waste. Food Biosci. 2024, 59, 104151. [Google Scholar] [CrossRef]
- Paul, A.; Kurian, M. Facile Synthesis of Nitrogen Doped Carbon Dots from Waste Biomass: Potential Optical and Biomedical Applications. Clean. Eng. Technol. 2021, 3, 100103. [Google Scholar] [CrossRef]
- Manzoor, S.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Srivastava, S.; Bashir, I.; Khan, S.A. Carbon Dots Applications for Development of Sustainable Technologies for Food Safety: A Comprehensive Review. Appl. Food Res. 2023, 3, 100263. [Google Scholar] [CrossRef]
- Panniello, A.; Di Mauro, A.E.; Fanizza, E.; Depalo, N.; Agostiano, A.; Curri, M.L.; Striccoli, M. Luminescent Oil-Soluble Carbon Dots toward White Light Emission: A Spectroscopic Study. J. Phys. Chem. C 2018, 122, 839–849. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Safardoust-Hojaghan, H.; Salavati-Niasari, M.; Amiri, O.; Rashki, S.; Ashrafi, M. Green Synthesis, Characterization and Antimicrobial Activity of Carbon Quantum Dots-Decorated ZnO Nanoparticles. Ceram. Int. 2021, 47, 5187–5197. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, S.H. Carbon Dots: Large-Scale Synthesis, Sensing and Bioimaging. Mater. Today 2016, 19, 382–393. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.J. Carbon Quantum Dots and Their Biomedical and Therapeutic Applications: A Review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Manikandan, V.; Lee, N.Y. Green Synthesis of Carbon Quantum Dots and Their Environmental Applications. Environ. Res. 2022, 212, 113283. [Google Scholar] [CrossRef]
- Saikia, M.; Hower, J.C.; Das, T.; Dutta, T.; Saikia, B.K. Feasibility Study of Preparation of Carbon Quantum Dots from Pennsylvania Anthracite and Kentucky Bituminous Coals. Fuel 2019, 243, 433–440. [Google Scholar] [CrossRef]
- Soni, H.; Pamidimukkala, P.S. Green Synthesis of N, S Co-Doped Carbon Quantum Dots from Triflic Acid Treated Palm Shell Waste and Their Application in Nitrophenol Sensing. Mater. Res. Bull. 2018, 108, 250–254. [Google Scholar] [CrossRef]
- Dash, K.K.; Ali, N.A.; Das, D.; Mohanta, D. Thorough Evaluation of Sweet Potato Starch and Lemon-Waste Pectin Based-Edible Films with Nano-Titania Inclusions for Food Packaging Applications. Int. J. Biol. Macromol. 2019, 139, 449–458. [Google Scholar] [CrossRef]
- Malavika, J.P.; Shobana, C.; Ragupathi, M.; Kumar, P.; Lee, Y.S.; Govarthanan, M.; Selvan, R.K. A Sustainable Green Synthesis of Functionalized Biocompatible Carbon Quantum Dots from Aloe Barbadensis Miller and Its Multifunctional Applications. Environ. Res. 2021, 200, 111414. [Google Scholar] [CrossRef]
- Riahi, Z.; Rhim, J.W.; Bagheri, R.; Pircheraghi, G.; Lotfali, E. Carboxymethyl Cellulose-Based Functional Film Integrated with Chitosan-Based Carbon Quantum Dots for Active Food Packaging Applications. Prog. Org. Coat. 2022, 166, 106794. [Google Scholar] [CrossRef]
- Konwar, A.; Gogoi, N.; Majumdar, G.; Chowdhury, D. Green Chitosan-Carbon Dots Nanocomposite Hydrogel Film with Superior Properties. Carbohydr. Polym. 2015, 115, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Rhim, J.W.; Molaei, R.; Priyadarshi, R.; Roy, S.; Min, S.; Kim, Y.H.; Lee, S.G.; Han, S. Preparation and Characterization of B, S, and N-Doped Glucose Carbon Dots: Antibacterial, Antifungal, and Antioxidant Activity. Sustain. Mater. Technol. 2022, 32, e00397. [Google Scholar] [CrossRef]
- Kwon, E.E.; Lee, T.; Ok, Y.S.; Tsang, D.C.W.; Park, C.; Lee, J. Effects of Calcium Carbonate on Pyrolysis of Sewage Sludge. Energy 2018, 153, 726–731. [Google Scholar] [CrossRef]
- Gunjal, D.B.; Naik, V.M.; Waghmare, R.D.; Patil, C.S.; Shejwal, R.V.; Gore, A.H.; Kolekar, G.B. Sustainable Carbon Nanodots Synthesised from Kitchen Derived Waste Tea Residue for Highly Selective Fluorimetric Recognition of Free Chlorine in Acidic Water: A Waste Utilization Approach. J. Taiwan Inst. Chem. Eng. 2019, 95, 147–154. [Google Scholar] [CrossRef]
- Yu, H.H.; Chin, Y.W.; Paik, H.D. Application of Natural Preservatives for Meat and Meat Products against Food-borne Pathogens and Spoilage Bacteria: A Review. Foods 2021, 10, 2418. [Google Scholar] [CrossRef] [PubMed]
- Imran, A.; Ahmed, F.; Ali, Y.A.; Naseer, M.S.; Sharma, K.; Bisht, Y.S.; Alawadi, A.H.; Shehzadi, U.; Islam, F.; Shah, M.A. A Comprehensive Review on Carbon Dot Synthesis and Food Applications. J. Agric. Food Res. 2025, 21, 101847. [Google Scholar] [CrossRef]
- Kalaš, B.; Radovanov, B.; Milenković, N.; Horvat, A.M. Macroeconomic Determinants of Circular Economy Investments: An ECM Approach. Sustainability 2024, 16, 6666. [Google Scholar] [CrossRef]
- Khalufi, N.A.M.; Sheikh, R.A.; Khan, S.M.F.A.; Onn, C.W. Evaluating the Impact of Sustainability Practices on Customer Relationship Quality: An SEM-PLS Approach to Align with SDG. Sustainability 2025, 17, 798. [Google Scholar] [CrossRef]
- Choirunnisa, N.I.; Ravenska, N. The Role of Green Marketing Through Brand Image to Increase Interest to Buy Gen Y in Love Beauty and Planet Products. In Proceedings of the Fourth International Conference on Administrative Science (ICAS 2022); Atlantis Press: Dordrecht, The Netherlands, 2024; pp. 200–211. [Google Scholar]
- de Oliveira, B.P.; da Silva Abreu, F.O.M. Carbon Quantum Dots Synthesis from Waste and By-Products: Perspectives and Challenges. Mater. Lett. 2021, 282, 128764. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Shim, J.J.; Lee, Y.R. Microwave Assisted Green Synthesis of Fluorescent N-Doped Carbon Dots: Cytotoxicity and Bio-Imaging Applications. J. Photochem. Photobiol. B Biol. 2016, 161, 154–161. [Google Scholar] [CrossRef]
- Sahu, V.; Sahoo, S.K. Biogenic Synthesis of Carbon Dots with Inbuilt Biological Activity. Next Nanotechnol. 2024, 5, 100034. [Google Scholar] [CrossRef]
- Rajapandi, S.; Pandeeswaran, M.; Jesuraj, D.; Kousalya, G.N. Synthesis of Green Fluorescent Nitrogen Doped Vitis Vinifera Derived Carbon Dots and Their In-Vitro Antimicrobial Studies. J. Mol. Struct. 2023, 1275, 134660. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, M.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Natural-Product-Derived Carbon Dots: From Natural Products to Functional Materials. ChemSusChem 2018, 11, 11–24. [Google Scholar] [CrossRef]
- Diao, H.; Li, T.; Zhang, R.; Kang, Y.; Liu, W.; Cui, Y.; Wei, S.; Wang, N.; Li, L.; Wang, H.; et al. Facile and Green Synthesis of Fluorescent Carbon Dots with Tunable Emission for Sensors and Cells Imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Bai, L.F.; Geng, Z.R.; Chen, H.; Xu, L.T.; Xie, Y.C.; Wang, D.J.; Gu, H.W.; Wang, X.M. Carbon Quantum Dots: Preparation, Optical Properties, and Biomedical Applications. Mater. Today Adv. 2023, 18, 100376. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Banger, A.; Gautam, S.; Jadoun, S.; Jangid, N.K.; Srivastava, A.; Pulidindi, I.N.; Dwivedi, J.; Srivastava, M. Synthetic Methods and Applications of Carbon Nanodots. Catalysts 2023, 13, 858. [Google Scholar] [CrossRef]
- Gupta, D.A.; Desai, M.L.; Malek, N.I.; Kailasa, S.K. Fluorescence Detection of Fe3+ Ion Using Ultra-Small Fluorescent Carbon Dots Derived from Pineapple (Ananas Comosus): Development of Miniaturized Analytical Method. J. Mol. Struct. 2020, 1216, 128343. [Google Scholar] [CrossRef]
- Sabet, M.; Mahdavi, K. Green Synthesis of High Photoluminescence Nitrogen-Doped Carbon Quantum Dots from Grass via a Simple Hydrothermal Method for Removing Organic and Inorganic Water Pollutions. Appl. Surf. Sci. 2019, 463, 283–291. [Google Scholar] [CrossRef]
- Lin, X.; Xiong, M.; Zhang, J.; He, C.; Ma, X.; Zhang, H.; Kuang, Y.; Yang, M.; Huang, Q. Carbon Dots Based on Natural Resources: Synthesis and Applications in Sensors. Microchem. J. 2021, 160, 105604. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Yang, M.; Yan, Y.; Liu, E.; Ji, Z.; Fan, J. Facile Synthesis of Novel Carbon Quantum Dots from Biomass Waste for Highly Sensitive Detection of Iron Ions. Mater. Res. Bull. 2020, 124, 110730. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Y.; Niu, N.; Chen, L. Synthesis of Molecularly Imprinted Fluorescent Probe Based on Biomass-Derived Carbon Quantum Dots for Detection of Mesotrione. Anal. Bioanal. Chem. 2019, 411, 5519–5530. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Troganis, A.; Stalikas, C. Two of a Kind but Different: Luminescent Carbon Quantum Dots from Citrus Peels for Iron and Tartrazine Sensing and Cell Imaging. Talanta 2017, 175, 305–312. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Jha, S.; Park, T.J.; Kailasa, S.K. Green Synthesis of Multi-Color Emissive Carbon Dots from Manilkara Zapota Fruits for Bioimaging of Bacterial and Fungal Cells. J. Photochem. Photobiol. B Biol. 2019, 191, 150–155. [Google Scholar] [CrossRef]
- Kishore, S.C.; Perumal, S.; Atchudan, R.; Edison, T.N.J.I.; Sundramoorthy, A.K.; Alagan, M.; Sangaraju, S.; Lee, Y.R. Eco-Friendly Synthesis of Functionalized Carbon Nanodots from Cashew Nut Skin Waste for Bioimaging. Catalysts 2023, 13, 547. [Google Scholar] [CrossRef]
- Ng, H.K.M.; Lim, G.K.; Leo, C.P. Comparison between Hydrothermal and Microwave-Assisted Synthesis of Carbon Dots from Biowaste and Chemical for Heavy Metal Detection: A Review. Microchem. J. 2021, 165, 106116. [Google Scholar] [CrossRef]
- Xiao, P.; Ke, Y.; Lu, J.; Huang, Z.; Zhu, X.; Wei, B.; Huang, L. Photoluminescence Immunoassay Based on Grapefruit Peel-Extracted Carbon Quantum Dots Encapsulated into Silica Nanospheres for P53 Protein. Biochem. Eng. J. 2018, 139, 109–116. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Muthuchamy, N.; Lee, Y.R. Hydrophilic Nitrogen-Doped Carbon Dots from Biowaste Using Dwarf Banana Peel for Environmental and Biological Applications. Fuel 2020, 275, 117821. [Google Scholar] [CrossRef]
- Shi, X.; Wei, W.; Fu, Z.; Gao, W.; Zhang, C.; Zhao, Q.; Deng, F.; Lu, X. Review on Carbon Dots in Food Safety Applications. Talanta 2019, 194, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Ma, C.; Niu, N.; Chen, Z.; Liu, S.; Li, J.; Li, S. Seeking Value from Biomass Materials: Preparation of Coffee Bean Shell-Derived Fluorescent Carbon Dots via Molecular Aggregation for Antioxidation and Bioimaging Applications. Mater. Chem. Front. 2018, 2, 1269–1275. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Zhu, J.J.; Tong, Q.X. Pd-Au@carbon Dots Nanocomposite: Facile Synthesis and Application as an Ultrasensitive Electrochemical Biosensor for Determination of Colitoxin DNA in Human Serum. Biosens. Bioelectron. 2017, 94, 507–512. [Google Scholar] [CrossRef]
- De, B.; Karak, N. A Green and Facile Approach for the Synthesis of Water Soluble Fluorescent Carbon Dots from Banana Juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Aslandaş, A.M.; Balci, N.; Arik, M.; Şakiroğlu, H.; Onganer, Y.; Meral, K. Liquid Nitrogen-Assisted Synthesis of Fluorescent Carbon Dots from Blueberry and Their Performance in Fe3+ Detection. Appl. Surf. Sci. 2015, 356, 747–752. [Google Scholar] [CrossRef]
- Jin, H.; Gui, R.; Wang, Y.; Sun, J. Carrot-Derived Carbon Dots Modified with Polyethyleneimine and Nile Blue for Ratiometric Two-Photon Fluorescence Turn-on Sensing of Sulfide Anion in Biological Fluids. Talanta 2017, 169, 141–148. [Google Scholar] [CrossRef]
- Arul, V.; Edison, T.N.J.I.; Lee, Y.R.; Sethuraman, M.G. Biological and Catalytic Applications of Green Synthesized Fluorescent N-Doped Carbon Dots Using Hylocereus Undatus. J. Photochem. Photobiol. B Biol. 2017, 168, 142–148. [Google Scholar] [CrossRef]
- Sun, X.; He, J.; Yang, S.; Zheng, M.; Wang, Y.; Ma, S.; Zheng, H. Green Synthesis of Carbon Dots Originated from Lycii Fructus for Effective Fluorescent Sensing of Ferric Ion and Multicolor Cell Imaging. J. Photochem. Photobiol. B Biol. 2017, 175, 219–225. [Google Scholar] [CrossRef]
- Chandra, S.; Bano, D.; Sahoo, K.; Kumar, D.; Kumar, V.; Kumar Yadav, P.; Hadi Hasan, S. Synthesis of Fluorescent Carbon Quantum Dots from Jatropha Fruits and Their Application in Fluorometric Sensor for the Detection of Chlorpyrifos. Microchem. J. 2022, 172, 106953. [Google Scholar] [CrossRef]
- Arul, V.; Sethuraman, M.G. Facile Green Synthesis of Fluorescent N-Doped Carbon Dots from Actinidia Deliciosa and Their Catalytic Activity and Cytotoxicity Applications. Opt. Mater. 2018, 78, 181–190. [Google Scholar] [CrossRef]
- So, R.C.; Sanggo, J.E.; Jin, L.; Diaz, J.M.A.; Guerrero, R.A.; He, J. Gram-Scale Synthesis and Kinetic Study of Bright Carbon Dots from Citric Acid and Citrus Japonica via a Microwave-Assisted Method. ACS Omega 2017, 2, 5196–5208. [Google Scholar] [CrossRef] [PubMed]
- Monte-Filho, S.S.; Andrade, S.I.E.; Lima, M.B.; Araujo, M.C.U. Synthesis of Highly Fluorescent Carbon Dots from Lemon and Onion Juices for Determination of Riboflavin in Multivitamin/Mineral Supplements. J. Pharm. Anal. 2019, 9, 209–216. [Google Scholar] [CrossRef]
- Su, A.; Wang, D.; Shu, X.; Zhong, Q.; Chen, Y.; Liu, J.; Wang, Y. Synthesis of Fluorescent Carbon Quantum Dots from Dried Lemon Peel for Determination of Carmine in Drinks. Chem. Res. Chin. Univ. 2018, 34, 164–168. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green Synthesis of Carbon Quantum Dots from Lemon Peel Waste: Applications in Sensing and Photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Jiao, X.Y.; Li, L.S.; Qin, S.; Zhang, Y.; Huang, K.; Xu, L. The Synthesis of Fluorescent Carbon Dots from Mango Peel and Their Multiple Applications. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 306–314. [Google Scholar] [CrossRef]
- Desai, M.L.; Jha, S.; Basu, H.; Singhal, R.K.; Park, T.J.; Kailasa, S.K. Acid Oxidation of Muskmelon Fruit for the Fabrication of Carbon Dots with Specific Emission Colors for Recognition of Hg2+ Ions and Cell Imaging. ACS Omega 2019, 4, 19332–19340. [Google Scholar] [CrossRef]
- Fatimah, S.; Isnaeni; Abdullah, B.; Tahir, D. Strong Luminescence Carbon Nanodots by Green Synthesis Based Microwave Assisted from Fruit Peel. J. Phys. Conf. Ser. 2019, 1242, 012038. [Google Scholar] [CrossRef]
- Prasannan, A.; Imae, T. One-Pot Synthesis of Fluorescent Carbon Dots from Orange Waste Peels. Ind. Eng. Chem. Res. 2013, 52, 15673–15678. [Google Scholar] [CrossRef]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Das, B.; Mitra, A.; Dhara, S. Onion Derived Carbon Nanodots for Live Cell Imaging and Accelerated Skin Wound Healing. J. Mater. Chem. B 2017, 5, 6579–6592. [Google Scholar] [CrossRef]
- Ma, X.; Dong, Y.; Sun, H.; Chen, N. Highly Fluorescent Carbon Dots from Peanut Shells as Potential Probes for Copper Ion: The Optimization and Analysis of the Synthetic Process. Mater. Today Chem. 2017, 5, 1–10. [Google Scholar] [CrossRef]
- Pooja, D.; Singh, L.; Thakur, A.; Kumar, P. Green Synthesis of Glowing Carbon Dots from Carica Papaya Waste Pulp and Their Application as a Label-Freechemo Probe for Chromium Detection in Water. Sens. Actuators B Chem. 2019, 283, 363–372. [Google Scholar] [CrossRef]
- Ramezani, Z.; Qorbanpour, M.; Rahbar, N. Green Synthesis of Carbon Quantum Dots Using Quince Fruit (Cydonia oblonga) Powder as Carbon Precursor: Application in Cell Imaging and As3+ Determination. Colloids Surf. A Physicochem. Eng. Asp. 2018, 549, 58–66. [Google Scholar] [CrossRef]
- Başoğlu, A.; Ocak, Ü.; Gümrükçüoğlu, A. Synthesis of Microwave-Assisted Fluorescence Carbon Quantum Dots Using Roasted–Chickpeas and Its Applications for Sensitive and Selective Detection of Fe3+ Ions. J. Fluoresc. 2020, 30, 515–526. [Google Scholar] [CrossRef]
- Tan, X.W.; Romainor, A.N.B.; Chin, S.F.; Ng, S.M. Carbon Dots Production via Pyrolysis of Sago Waste as Potential Probe for Metal Ions Sensing. J. Anal. Appl. Pyrolysis 2014, 105, 157–165. [Google Scholar] [CrossRef]
- Boruah, A.; Saikia, M.; Das, T.; Goswamee, R.L.; Saikia, B.K. Blue-Emitting Fluorescent Carbon Quantum Dots from Waste Biomass Sources and Their Application in Fluoride Ion Detection in Water. J. Photochem. Photobiol. B Biol. 2020, 209, 111940. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, L.; Chen, L.; Chen, Y.; Zhou, T.; Li, H.; Xu, Y.; Zhao, L.; Huang, N. A Facile, Green Synthesis of Biomass Carbon Dots Coupled with Molecularly Imprinted Polymers for Highly Selective Detection of Oxytetracycline. J. Ind. Eng. Chem. 2019, 69, 455–463. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Ha, S.; Baek, S.H.; Phan, L.M.T.; Kim, S.; Kwak, K.; Park, T.J. Tuning of Carbon Dots Emission Color for Sensing of Fe3+ Ion and Bioimaging Applications. Mater. Sci. Eng. C 2019, 98, 834–842. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Lee, Y.R. Nitrogen-Doped Carbon Dots Originating from Unripe Peach for Fluorescent Bioimaging and Electrocatalytic Oxygen Reduction Reaction. J. Colloid. Interface Sci. 2016, 482, 8–18. [Google Scholar] [CrossRef]
- Kim, M.I.; Park, S.Y.; Park, K.S.; Kim, S.-r.; Kim, J.P.; Lee, Y.C.; Lee, H.U.; Park, H.G. Label-Free Fluorescent Detection of Alkaline Phosphatase with Vegetable Waste-Derived Green Carbon Probes. Sens. Actuators B Chem. 2018, 262, 469–476. [Google Scholar] [CrossRef]
- Zhou, J.; Sheng, Z.; Han, H.; Zou, M.; Li, C. Facile Synthesis of Fluorescent Carbon Dots Using Watermelon Peel as a Carbon Source. Mater. Lett. 2012, 66, 222–224. [Google Scholar] [CrossRef]
- Schwenke, A.M.; Hoeppener, S.; Schubert, U.S. Synthesis and Modification of Carbon Nanomaterials Utilizing Microwave Heating. Adv. Mater. 2015, 27, 4113–4141. [Google Scholar] [CrossRef]
- Vázquez, E.; Prato, M. Carbon Nanotubes and Microwaves: Interactions, Responses, and Applications. ACS Nano 2009, 3, 3819–3824. [Google Scholar] [CrossRef]
- Rosana, M.R.; Hunt, J.; Ferrari, A.; Southworth, T.A.; Tao, Y.; Stiegman, A.E.; Dudley, G.B. Microwave-Specific Acceleration of a Friedel-Crafts Reaction: Evidence for Selective Heating in Homogeneous Solution. J. Org. Chem. 2014, 79, 7437–7450. [Google Scholar] [CrossRef]
- Jusuf, B.N.; Sambudi, N.S.; Isnaeni, I.; Samsuri, S. Microwave-Assisted Synthesis of Carbon Dots from Eggshell Membrane Ashes by Using Sodium Hydroxide and Their Usage for Degradation of Methylene Blue. J. Environ. Chem. Eng. 2018, 6, 7426–7433. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Chen, M.; Zhou, X.; Chen, W. Microwave-Assisted Synthesis of Polyamine-Functionalized Carbon Dots from Xylan and Their Use for the Detection of Tannic Acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 301–308. [Google Scholar] [CrossRef]
- He, G.; Shu, M.; Yang, Z.; Ma, Y.; Huang, D.; Xu, S.; Wang, Y.; Hu, N.; Zhang, Y.; Xu, L. Microwave Formation and Photoluminescence Mechanisms of Multi-States Nitrogen Doped Carbon Dots. Appl. Surf. Sci. 2017, 422, 257–265. [Google Scholar] [CrossRef]
- Magesh, V.; Sundramoorthy, A.K.; Ganapathy, D. Recent Advances on Synthesis and Potential Applications of Carbon Quantum Dots. Front. Mater. 2022, 9, 906838. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small Molecules Derived Carbon Dots: Synthesis and Applications in Sensing, Catalysis, Imaging, and Biomedicine. J. Nanobiotechnology 2019, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- González-González, R.B.; González, L.T.; Madou, M.; Leyva-Porras, C.; Martinez-Chapa, S.O.; Mendoza, A. Synthesis, Purification, and Characterization of Carbon Dots from Non-Activated and Activated Pyrolytic Carbon Black. Nanomaterials 2022, 12, 298. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Zhang, C.; Feng, Y.; Shao, H.; Chen, J.; Hu, J.; Zhang, L. Fabricating Carbon Quantum Dots of Graphitic Carbon Nitride Vis Ultrasonic Exfoliation for Highly Efficient H2O2 Production. Ultrason. Sonochemistry 2023, 99, 106582. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Y.; Wang, C.F.; Wu, G.; Chen, S. The Rapid and Large-Scale Production of Carbon Quantum Dots and Their Integration with Polymers. Angew. Chem. Int. Ed. 2021, 60, 8585–8595. [Google Scholar] [CrossRef] [PubMed]
- Chelladurai, D.; Alaguthevar, R.; Murugesan, B.; Subburamu, K.; Khan, A.; Rhim, J.W. Carbon Quantum Dots: Progress toward Food Safety and Sustainability. Food Biosci. 2024, 61, 105016. [Google Scholar] [CrossRef]
- Shen, X.H.; Zhang, J.H.; Li, H.; Wang, J.J.; Wang, X.C. Ultrasonic Vibration-Assisted Milling of Aluminum Alloy. Int. J. Adv. Manuf. Technol. 2012, 63, 41–49. [Google Scholar] [CrossRef]
- Houtmeyers, S.; Degrève, J.; Willems, K.; Dewil, R.; Appels, L. Comparing the Influence of Low Power Ultrasonic and Microwave Pre-Treatments on the Solubilisation and Semi-Continuous Anaerobic Digestion of Waste Activated Sludge. Bioresour. Technol. 2014, 171, 44–49. [Google Scholar] [CrossRef]
- Salimi Shahraki, H.; Ahmad, A.; Bushra, R. Green Carbon Dots with Multifaceted Applications– Waste to Wealth Strategy. FlatChem 2022, 31, 100310. [Google Scholar] [CrossRef]
- Wang, H.; Di, J.; Sun, Y.; Fu, J.; Wei, Z.; Matsui, H.; Alonso, A.d.C.; Zhou, S. Biocompatible PEG-Chitosan@Carbon Dots Hybrid Nanogels for Two-Photon Fluorescence Imaging, Near-Infrared Light/PH Dual-Responsive Drug Carrier, and Synergistic Therapy. Adv. Funct. Mater. 2015, 25, 5537–5547. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, A.; Sahu, S.K.; Kumar, S. Synthesis of Green Fluorescent Carbon Quantum Dots Using Waste Polyolefins Residue for Cu2+ Ion Sensing and Live Cell Imaging. Sens. Actuators B Chem. 2018, 254, 197–205. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Wang, J.; Zhao, X.; Zhao, Y.; Qian, J.; Wang, T. Pyrolysis and Hydrothermal Carbonization of Biowaste: A Comparative Review on the Conversion Pathways and Potential Applications of Char Product. Sustain. Chem. Pharm. 2023, 33, 101106. [Google Scholar] [CrossRef]
- Aji, M.P.; Susanto; Wiguna, P.A.; Sulhadi. Facile Synthesis of Luminescent Carbon Dots from Mangosteen Peel by Pyrolysis Method. J. Theor. Appl. Phys. 2017, 11, 119–126. [Google Scholar] [CrossRef]
- Dehvari, K.; Liu, K.Y.; Tseng, P.J.; Gedda, G.; Girma, W.M.; Chang, J.Y. Sonochemical-Assisted Green Synthesis of Nitrogen-Doped Carbon Dots from Crab Shell as Targeted Nanoprobes for Cell Imaging. J. Taiwan Inst. Chem. Eng. 2019, 95, 495–503. [Google Scholar] [CrossRef]
- Ranjan, P.; Khan, R.; Yadav, S.; Sadique, M.A.; Murali, S.; Ban, M.K. Physical and Chemical Properties of Carbon Dots. In Carbon Dots in Agricultural Systems Strategies to Enhance Plant Productivity; Academic Press: Cambridge, MA, USA, 2022; pp. 117–133. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The Photoluminescence Mechanism in Carbon Dots (Graphene Quantum Dots, Carbon Nanodots, and Polymer Dots): Current State and Future Perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The Fluorescence Mechanism of Carbon Dots, and Methods for Tuning Their Emission Color: A Review. Microchim. Acta 2019, 186, 583. [Google Scholar] [CrossRef] [PubMed]
- Pramudita, R.; Marpongahtun; Gea, S.; Daulay, A.; Harahap, M.; Tan, Y.Z.; Goei, R.; Tok, A.I.Y. Synthesis of Fluorescent Citric Acid Carbon Dots Composites Derived from Empty Fruit Bunches of Palm Oil Tree and Its Anti-Bacterial Property. Case Stud. Chem. Environ. Eng. 2022, 6, 100277. [Google Scholar] [CrossRef]
- Hola, K.; Bourlinos, A.B.; Kozak, O.; Berka, K.; Siskova, K.M.; Havrdova, M.; Tucek, J.; Safarova, K.; Otyepka, M.; Giannelis, E.P.; et al. Photoluminescence Effects of Graphitic Core Size and Surface Functional Groups in Carbon Dots: COO- Induced Red-Shift Emission. Carbon 2014, 70, 279–286. [Google Scholar] [CrossRef]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A Review of Carbon Dots Produced from Biomass Wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Ozyurt, D.; Kobaisi, M.A.; Hocking, R.K.; Fox, B. Properties, Synthesis, and Applications of Carbon Dots: A Review. Carbon Trends 2023, 12, 100276. [Google Scholar] [CrossRef]
- Hussen, N.H.; Hasan, A.H.; FaqiKhedr, Y.M.; Bogoyavlenskiy, A.; Bhat, A.R.; Jamalis, J. Carbon Dot Based Carbon Nanoparticles as Potent Antimicrobial, Antiviral, and Anticancer Agents. ACS Omega 2024, 9, 9849–9864. [Google Scholar] [CrossRef]
- Himaja, A.L.; Karthik, P.S.; Sreedhar, B.; Singh, S.P. Synthesis of Carbon Dots from Kitchen Waste: Conversion of Waste to Value Added Product. J. Fluoresc. 2014, 24, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, T.; Ang, W.L.; Mahmoudi, E.; Mohammad, A.W.; Sambudi, N.S. Formation Mechanism and Application Potential of Carbon Dots Synthesized from Palm Kernel Shell via Microwave Assisted Method. Carbon Resour. Convers. 2022, 5, 150–166. [Google Scholar] [CrossRef]
- Bandi, R.; Gangapuram, B.R.; Dadigala, R.; Eslavath, R.; Singh, S.S.; Guttena, V. Facile and Green Synthesis of Fluorescent Carbon Dots from Onion Waste and Their Potential Applications as Sensor and Multicolour Imaging Agents. RSC Adv. 2016, 6, 28633–28639. [Google Scholar] [CrossRef]
- Ngu, P.Z.Z.; Chia, S.P.P.; Fong, J.F.Y.; Ng, S.M. Synthesis of Carbon Nanoparticles from Waste Rice Husk Used for the Optical Sensing of Metal Ions. New Carbon Mater. 2016, 31, 135–143. [Google Scholar] [CrossRef]
- Yi, H.; Liu, J.; Yao, J.; Wang, R.; Shi, W.; Lu, C. Photoluminescence Mechanism of Carbon Dots: Triggering Multiple Color Emissions through Controlling the Degree of Protonation. Molecules 2022, 27, 6517. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Li, M.; Li, Q.; Liang, S.; Tan, Y.; Sheng, L.; Shi, W.; Zhang, S.X.A. Carbon Dots with Continuously Tunable Full-Color Emission and Their Application in Ratiometric PH Sensing. Chem. Mater. 2014, 26, 3104–3112. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Cai, J.; Huang, X.; Lin, L.; Lin, Y.; Yang, H.; Li, S. Nitrogen and Sulfur Co-Doped Carbon Dots Synthesis via One Step Hydrothermal Carbonization of Green Alga and Their Multifunctional Applications. Microchem. J. 2019, 147, 1038–1047. [Google Scholar] [CrossRef]
- Ezati, P.; Priyadarshi, R.; Rhim, J.W. Prospects of Sustainable and Renewable Source-Based Carbon Quantum Dots for Food Packaging Applications. Sustain. Mater. Technol. 2022, 33, e00494. [Google Scholar] [CrossRef]
- Rajapandi, S.; Pandeeswaran, M.; Kousalya, G.N. Novel Green Synthesis of N-Doped Carbon Dots from Fruits of Opuntia Ficus Indica as an Effective Catalyst for the Photocatalytic Degradation of Methyl Orange Dye and Antibacterial Studies. Inorg. Chem. Commun. 2022, 146, 110041. [Google Scholar] [CrossRef]
- Riahi, Z.; Khan, A.; Rhim, J.W.; Shin, G.H.; Kim, J.T. Carrageenan-Based Active and Intelligent Packaging Films Integrated with Anthocyanin and TiO2-Doped Carbon Dots Derived from Sweet Potato Peels. Int. J. Biol. Macromol. 2024, 259, 129371. [Google Scholar] [CrossRef]
- Bao, J.; Hu, Y.; Farag, M.A.; Huan, W.; Wu, J.; Yang, D.; Song, L. Carbon Dots, Cellulose Nanofiber, and Essential Oil Nanoemulsion from Torreya Grandis Aril Added to Fish Scale Gelatin Film for Tomato Preservation. Int. J. Biol. Macromol. 2023, 245, 125482. [Google Scholar] [CrossRef]
- Khan, A.; Ezati, P.; Kim, J.T.; Rhim, J.W. Biocompatible Carbon Quantum Dots for Intelligent Sensing in Food Safety Applications: Opportunities and Sustainability. Mater. Today Sustain. 2023, 21, 100306. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, Q.; Tan, X.; Ye, M.; Zhang, Y.; Zou, L.; Liu, S.; Yang, Y.; Liu, A.; He, L.; et al. Photodynamic Antibacterial Chitosan/Nitrogen-Doped Carbon Dots Composite Packaging Film for Food Preservation Applications. Carbohydr. Polym. 2023, 314, 120938. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Rhim, J.W.; Molaei, R.; Priyadarshi, R.; Han, S. Cellulose Nanofiber-Based Coating Film Integrated with Nitrogen-Functionalized Carbon Dots for Active Packaging Applications of Fresh Fruit. Postharvest Biol. Technol. 2022, 186, 111845. [Google Scholar] [CrossRef]
- Slewa, L.H. Antifungal Films for Strawberry Packaging Using Carbon Quantum Dots Derived from Lemon and Onion Juice via Green Hydrothermal Method. Food Biosci. 2024, 61, 104653. [Google Scholar] [CrossRef]
- Surendran, P.; Lakshmanan, A.; Priya, S.S.; Geetha, P.; Rameshkumar, P.; Kannan, K.; Hegde, T.A.; Vinitha, G. Fluorescent Carbon Quantum Dots from Ananas Comosus Waste Peels: A Promising Material for NLO Behaviour, Antibacterial, and Antioxidant Activities. Inorg. Chem. Commun. 2021, 124, 108397. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J. Antioxidants: Classification, Natural Sources, Activity/Capacity. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Frankel, E.N. Antioxidants. In Lipid Oxidation; Woodhead Publishing: Sawston, UK, 2012; pp. 209–258. [Google Scholar] [CrossRef]
- Rao, V.R. Antioxidant Agents. In Advances in Structure and Activity Relationship of Coumarin Derivatives; Academic Press: Cambridge, MA, USA, 2016; pp. 137–150. [Google Scholar] [CrossRef]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Sapanidou, V.; Tsantarliotou, M.P.; Lavrentiadou, S.N. A Review of the Use of Antioxidants in Bovine Sperm Preparation Protocols. Anim. Reprod. Sci. 2023, 251, 107215. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive Oxygen Species—Sources, Functions, Oxidative Damage. Pol. Merkur. Lek. 2020, 48, 124–127. [Google Scholar]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Mukherjee, A.; Ghosh, K.K.; Chakrabortty, S.; Gulyás, B.; Padmanabhan, P.; Ball, W.B. Mitochondrial Reactive Oxygen Species in Infection and Immunity. Biomolecules 2024, 14, 670. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in Antioxidant Active Food Packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Lai, W.F. Design of Polymeric Films for Antioxidant Active Food Packaging. Int. J. Mol. Sci. 2022, 23, 12. [Google Scholar] [CrossRef]
- Chang, B.P.; Trinh, B.M.; Tadele, D.T.; Bandara, N.; Mekonnen, T.H. Natural Antioxidant and Antimicrobial Agents and Processing Technologies for the Design of Active Food Packaging Polymers. Polym. Rev. 2023, 63, 961–1013. [Google Scholar] [CrossRef]
- Kuai, L.; Liu, F.; Chiou, B.S.; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled Release of Antioxidants from Active Food Packaging: A Review. Food Hydrocoll. 2021, 120, 106992. [Google Scholar] [CrossRef]
- Ezati, P.; Roy, S.; Rhim, J.W. Pectin/Gelatin-Based Bioactive Composite Films Reinforced with Sulfur Functionalized Carbon Dots. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128123. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Chen, H. Effect of Ultrasound Treatment Combined with Carbon Dots Coating on the Microbial and Physicochemical Quality of Fresh-Cut Cucumber. Food Bioprocess Technol. 2020, 13, 648–660. [Google Scholar] [CrossRef]
- Murru, C.; Badía-Laíño, R.; Díaz-García, M.E. Synthesis and Characterization of Green Carbon Dots for Scavenging Radical Oxygen Species in Aqueous and Oil Samples. Antioxidants 2020, 9, 1147. [Google Scholar] [CrossRef] [PubMed]
- Neffe-Skocińska, K.; Karbowiak, M.; Kruk, M.; Kołożyn-Krajewska, D.; Zielińska, D. Polyphenol and Antioxidant Properties of Food Obtained by the Activity of Acetic Acid Bacteria (AAB)—A Systematic Review. J. Funct. Foods 2023, 107, 105691. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Chen, K.; Qing, W.; Hu, W.; Lu, M.; Wang, Y.; Liu, X. On-off-on Fluorescent Carbon Dots from Waste Tea: Their Properties, Antioxidant and Selective Detection of CrO42−, Fe3+, ascorbic Acid and L-Cysteine in Real Samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 228–234. [Google Scholar] [CrossRef]
- Min, S.; Ezati, P.; Rhim, J.W. Gelatin-Based Packaging Material Incorporated with Potato Skins Carbon Dots as Functional Filler. Ind. Crops Prod. 2022, 181, 114820. [Google Scholar] [CrossRef]
- Smrithi, S.P.; Kottam, N.; Muktha, H.; Mahule, A.M.; Chamarti, K.; Vismaya, V.; Sharath, R. Carbon Dots Derived from Beta Vulgaris: Evaluation of Its Potential as Antioxidant and Anticancer Agent. Nanotechnology 2022, 33, 045403. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Lin, B.; Gao, Y.; Jiao, Y.; Li, L.; Dong, C.; Shuang, S. Highly Luminescent N-Doped Carbon Dots from Black Soya Beans for Free Radical Scavenging, Fe3+ Sensing and Cellular Imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 211, 363–372. [Google Scholar] [CrossRef]
- Roshni, V.; Gujar, V.; Pathan, H.; Islam, S.; Tawre, M.; Pardesi, K.; Santra, M.K.; Ottoor, D. Bioimaging Applications of Carbon Dots (C. Dots) and Its Cystamine Functionalization for the Sensitive Detection of Cr(VI) in Aqueous Samples. J. Fluoresc. 2019, 29, 1381–1392. [Google Scholar] [CrossRef]
- Guo, Y.; Li, T.; Xie, L.; Tong, X.; Tang, C.; Shi, S. Red Pitaya Peels-Based Carbon Dots for Real-Time Fluorometric and Colorimetric Assay of Au3+, cellular Imaging, and Antioxidant Activity. Anal. Bioanal. Chem. 2021, 413, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fan, Y.; Zhang, L.; Wang, Q.; Fu, H.; She, Y. A Novel Enhanced Fluorescence Method Based on Multifunctional Carbon Dots for Specific Detection of Hg2+ in Complex Samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 220, 117109. [Google Scholar] [CrossRef]
- Gudimella, K.K.; Appidi, T.; Wu, H.F.; Battula, V.; Jogdand, A.; Rengan, A.K.; Gedda, G. Sand Bath Assisted Green Synthesis of Carbon Dots from Citrus Fruit Peels for Free Radical Scavenging and Cell Imaging. Colloids Surf. B Biointerfaces 2021, 197, 111362. [Google Scholar] [CrossRef]
- Rajamanikandan, S.; Biruntha, M.; Ramalingam, G. Blue Emissive Carbon Quantum Dots (CQDs) from Bio-Waste Peels and Its Antioxidant Activity. J. Clust. Sci. 2022, 33, 1045–1053. [Google Scholar] [CrossRef]
- Sharma, N.; Das, G.S.; Yun, K. Green Synthesis of Multipurpose Carbon Quantum Dots from Red Cabbage and Estimation of Their Antioxidant Potential and Bio-Labeling Activity. Appl. Microbiol. Biotechnol. 2020, 104, 7187–7200. [Google Scholar] [CrossRef]
- Rodríguez-Varillas, S.; Fontanil, T.; Obaya, Á.J.; Fernández-González, A.; Murru, C.; Badía-Laíño, R. Biocompatibility and Antioxidant Capabilities of Carbon Dots Obtained from Tomato (Solanum lycopersicum). Appl. Sci. 2022, 12, 773. [Google Scholar] [CrossRef]
- Roy, S.; Ezati, P.; Rhim, J.W.; Molaei, R. Preparation of Turmeric-Derived Sulfur-Functionalized Carbon Dots: Antibacterial and Antioxidant Activity. J. Mater. Sci. 2022, 57, 2941–2952. [Google Scholar] [CrossRef]
- Cruz-Luna, A.R.; Vásquez-López, A.; Rojas-Chávez, H.; Valdés-Madrigal, M.A.; Cruz-Martínez, H.; Medina, D.I. Engineered Metal Oxide Nanoparticles as Fungicides for Plant Disease Control. Plants 2023, 12, 2461. [Google Scholar] [CrossRef]
- Sundin, G.W.; Castiblanco, L.F.; Yuan, X.; Zeng, Q.; Yang, C.H. Bacterial Disease Management: Challenges, Experience, Innovation and Future Prospects: Challenges in Bacterial Molecular Plant Pathology. Mol. Plant Pathol. 2016, 17, 1506–1518. [Google Scholar] [CrossRef]
- Phani, V.; Khan, M.R.; Dutta, T.K. Plant-Parasitic Nematodes as a Potential Threat to Protected Agriculture: Current Status and Management Options. Crop Prot. 2021, 144, 105573. [Google Scholar] [CrossRef]
- Pujari, J.D.; Yakkundimath, R.; Byadgi, A.S. Image Processing Based Detection of Fungal Diseases in Plants. Procedia Comput. Sci. 2015, 46, 1802–1808. [Google Scholar] [CrossRef]
- Islam, M.S.; Haque, M.S.; Islam, M.M.; Emdad, E.M.; Halim, A.; Hossen, Q.M.M.; Hossain, M.Z.; Ahmed, B.; Rahim, S.; Rahman, M.S.; et al. Tools to Kill: Genome of One of the Most Destructive Plant Pathogenic Fungi Macrophomina phaseolina. BMC Genom. 2012, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Charles, H.; Godfray, J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; et al. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Huang, C.C.; Hung, Y.S.; Weng, Y.M.; Chen, W.; Lai, Y.S. Sustainable Development of Carbon Nanodots Technology: Natural Products as a Carbon Source and Applications to Food Safety. Trends Food Sci. Technol. 2019, 86, 144–152. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Wu, L.; Wu, W.; Zheng, Y.; Lin, L.; Weng, S.; Lin, X. Nitrogen-Doped Carbon Quantum Dots as an Antimicrobial Agent against Staphylococcus for the Treatment of Infected Wounds. Colloids Surf. B Biointerfaces 2019, 179, 17–27. [Google Scholar] [CrossRef]
- Yan, Y.; Kuang, W.; Shi, L.; Ye, X.; Yang, Y.; Xie, X.; Shi, Q.; Tan, S. Carbon Quantum Dot-Decorated TiO2 for Fast and Sustainable Antibacterial Properties under Visible-Light. J. Alloys Compd. 2019, 777, 234–243. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Mujumdar, A.S.; Wang, H. Application of Carbon Dots in Food Preservation: A Critical Review for Packaging Enhancers and Food Preservatives. Crit. Rev. Food Sci. Nutr. 2023, 63, 6738–6756. [Google Scholar] [CrossRef]
- Ye, S.L.; Huang, J.J.; Luo, L.; Fu, H.J.; Sun, Y.M.; Shen, Y.D.; Lei, H.T.; Xu, Z.L. Preparation of Carbon Dots and Their Application in Food Analysis as Signal Probe. Chin. J. Anal. Chem. 2017, 45, 1571–1581. [Google Scholar] [CrossRef]
- Moradi, M.; Molaei, R.; Kousheh, S.A.; Guimarães, J.T.; McClements, D.J. Carbon Dots Synthesized from Microorganisms and Food By-Products: Active and Smart Food Packaging Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 1943–1959. [Google Scholar] [CrossRef]
- Kasibabu, B.S.B.; D’Souza, S.L.; Jha, S.; Kailasa, S.K. Imaging of Bacterial and Fungal Cells Using Fluorescent Carbon Dots Prepared from Carica Papaya Juice. J. Fluoresc. 2015, 25, 803–810. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Jha, S.; Singhal, R.K.; Park, T.J.; Kailasa, S.K. Facile Green Synthesis of Carbon Dots from Pyrus pyrifolia Fruit for Assaying of Al3+ Ion via Chelation Enhanced Fluorescence Mechanism. J. Mol. Liq. 2018, 264, 9–16. [Google Scholar] [CrossRef]
- Mehta, V.N.; Jha, S.; Basu, H.; Singhal, R.K.; Kailasa, S.K. One-Step Hydrothermal Approach to Fabricate Carbon Dots from Apple Juice for Imaging of Mycobacterium and Fungal Cells. Sens. Actuators B Chem. 2015, 213, 434–443. [Google Scholar] [CrossRef]
- Bukasov, R.; Filchakova, O.; Gudun, K.; Bouhrara, M. Strong Surface Enhanced Florescence of Carbon Dot Labeled Bacteria Cells Observed with High Contrast on Gold Film. J. Fluoresc. 2018, 28, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Unnikrishnan, B.; Wei, S.C.; Chou, C.P.; Zhang, L.Z.; Huang, C.C. Graphene Oxide and Carbon Dots as Broad-Spectrum Antimicrobial Agents-a Minireview. Nanoscale Horiz. 2019, 4, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ezati, P.; Rhim, J.W. Chitosan/Starch-Based Active Packaging Film with N, P-Doped Carbon Dots for Meat Packaging. ACS Appl. Bio Mater. 2023, 6, 1294–1305. [Google Scholar] [CrossRef]
- Li, Y.J.; Harroun, S.G.; Su, Y.C.; Huang, C.F.; Unnikrishnan, B.; Lin, H.J.; Lin, C.H.; Huang, C.C. Synthesis of Self-Assembled Spermidine-Carbon Quantum Dots Effective against Multidrug-Resistant Bacteria. Adv. Healthc. Mater. 2016, 5, 2545–2554. [Google Scholar] [CrossRef]
- Verma, A.; Arshad, F.; Ahmad, K.; Goswami, U.; Samanta, S.K.; Sahoo, A.K.; Sk, M.P. Role of Surface Charge in Enhancing Antibacterial Activity of Fluorescent Carbon Dots. Nanotechnology 2020, 31, 095101. [Google Scholar] [CrossRef]
- Bing, W.; Sun, H.; Yan, Z.; Ren, J.; Qu, X. Programmed Bacteria Death Induced by Carbon Dots with Different Surface Charge. Small 2016, 12, 4713–4718. [Google Scholar] [CrossRef]
- Dou, Q.; Fang, X.; Jiang, S.; Chee, P.L.; Lee, T.C.; Loh, X.J. Multi-Functional Fluorescent Carbon Dots with Antibacterial and Gene Delivery Properties. RSC Adv. 2015, 5, 46817–46822. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial Carbon-Based Nanomaterials. Adv. Mater. 2019, 31, e1804838. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Ma, Y.H.; Gao, G.; Chen, X.; Jia, H.R.; Li, Y.H.; Chen, Z.; Wu, F.G. Carbon Dot-Based Platform for Simultaneous Bacterial Distinguishment and Antibacterial Applications. ACS Appl. Mater. Interfaces 2016, 8, 32170–32181. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Camacho, D.M.; Kohanski, M.A.; Callura, J.M.; Collins, J.J. Antibiotic-Induced Bacterial Cell Death Exhibits Physiological and Biochemical Hallmarks of Apoptosis. Mol. Cell 2012, 46, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Ye, Y.; Ping, J.; Sun, X. Carbon Dots: Current Advances in Pathogenic Bacteria Monitoring and Prospect Applications. Biosens. Bioelectron. 2020, 156, 112085. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Rhim, J.W.; Molaei, R.; Rezaei, Z. Carbon Quantum Dots-Based Antifungal Coating Film for Active Packaging Application of Avocado. Food Packag. Shelf Life 2022, 33, 100878. [Google Scholar] [CrossRef]
- Adnan, S.; Ahmed, N.; Yousaf, A. Green Synthesis of Carbon Dots Employing Beetroot for The Evaluation of Antibacterial Activity. Adv. Food Sci. 2024, 46, 38–47. [Google Scholar]
- Wang, N.; Wang, Y.; Guo, T.; Yang, T.; Chen, M.; Wang, J. Green Preparation of Carbon Dots with Papaya as Carbon Source for Effective Fluorescent Sensing of Iron (III) and Escherichia coli. Biosens. Bioelectron. 2016, 85, 68–75. [Google Scholar] [CrossRef]
- Yan, H.; Li, P.; Wen, F.; Xu, Q.; Guo, Q.; Su, W. Green Synthesis of Carbon Quantum Dots from Plant Turmeric Holds Promise as Novel Photosensitizer for in Vitro Photodynamic Antimicrobial Activity. J. Mater. Res. Technol. 2023, 22, 17–34. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Salavati-Niasari, M.; Ghanbari, D. Hydrothermal Green Synthesis of Magnetic Fe3O4-Carbon Dots by Lemon and Grape Fruit Extracts and as a Photoluminescence Sensor for Detecting of E. coli Bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 203, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Chen, P.C.; Periasamy, A.P.; Chen, Y.N.; Chang, H.T. Photoluminescent Carbon Nanodots: Synthesis, Physicochemical Properties and Analytical Applications. Mater. Today 2015, 18, 447–458. [Google Scholar] [CrossRef]
- Aksu, M.; Güzdemir, Ö. Food Waste-Derived Carbon Quantum Dots and Their Applications in Food Technology: A Critical Review. Food Bioprocess Technol. 2025, 18, 6753–6778. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, X.; Sun, Z.; Fu, C.; Liu, T.; Meng, X.; Wang, Z. Toxicity and Bio-Distribution of Carbon Dots after Single Inhalation Exposure in Vivo. Chin. Chem. Lett. 2018, 29, 895–898. [Google Scholar] [CrossRef]
- Myrzagali, S.; Omarova, Z.; Zeitkaziyeva, D.; Madet, A.; Xie, Y. Carbon Nanoparticle-Induced Cell Death. Carbon Trends 2024, 15, 100352. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C. Cytotoxicity of Carbon Nanotubes, Graphene, Fullerenes, and Dots. Nanomaterials 2023, 13, 1458. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yu, N.Y.; Fang, W.D.; Tan, Q.G.; Ji, R.; Yang, L.Y.; Wei, S.; Zhang, X.W.; Miao, A.J. Photodegradation of Carbon Dots Cause Cytotoxicity. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef]
- Dias, C.; Vasimalai, N.; Sárria, M.P.; Pinheiro, I.; Vilas-Boas, V.; Peixoto, J.; Espiña, B. Biocompatibility and Bioimaging Potential of Fruit-Based Carbon Dots. Nanomaterials 2019, 9, 199. [Google Scholar] [CrossRef]
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Shanmugam, M.; Perumal, S.; Somanathan, T.; Lee, Y.R. Sustainable Synthesis of Carbon Quantum Dots from Banana Peel Waste Using Hydrothermal Process for in Vivo Bioimaging. Phys. E Low. Dimens. Syst. Nanostruct 2021, 126, 114417. [Google Scholar] [CrossRef]
- Nguyen, D.H.H.; El-Ramady, H.; Prokisch, J. Food Safety Aspects of Carbon Dots: A Review. Environ. Chem. Lett. 2025, 23, 337–360. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.M.; Williams, L. Antimicrobial-Loaded Nanocarriers for Food Packaging Applications. Adv. Colloid Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef]
- Hoelzer, K.; Moreno Switt, A.I.; Wiedmann, M.; Boor, K.J. Emerging Needs and Opportunities in Foodborne Disease Detection and Prevention: From Tools to People. Food Microbiol. 2018, 75, 65–71. [Google Scholar] [CrossRef]
- Todd, E.C.D. Foodborne Diseases: Overview of Biological Hazards and Foodborne Diseases. In Encyclopedia of Food Safety; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 221–242. ISBN 9780123786128. [Google Scholar]
- Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Rhim, J.W. Carrageenan/Alginate-Based Functional Films Incorporated with Allium Sativum Carbon Dots for UV-Barrier Food Packaging. Food Bioproc Technol. 2023, 16, 2001–2015. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial Agents and Packaging Systems in Antimicrobial Active Food Packaging: An Overview of Approaches and Interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Rhim, J.W. Cellulose Nanofiber-Based PH Indicator Integrated with Resazurin-Modified Carbon Dots for Real-Time Monitoring of Food Freshness. Food Biosci. 2023, 53, 102679. [Google Scholar] [CrossRef]
- Abotbina, W.; Sapuan, S.M.; Ilyas, R.A.; Sultan, M.T.H.; Alkbir, M.F.M.; Sulaiman, S.; Harussani, M.M.; Bayraktar, E. Recent Developments in Cassava (Manihot esculenta) Based Biocomposites and Their Potential Industrial Applications: A Comprehensive Review. Materials 2022, 15, 6992. [Google Scholar] [CrossRef] [PubMed]
- Tammina, S.K.; Rhim, J.W. Carboxymethylcellulose/Agar-Based Functional Film Incorporated with Nitrogen-Doped Polyethylene Glycol-Derived Carbon Dots for Active Packaging Applications. Chemosphere 2023, 313, 137627. [Google Scholar] [CrossRef] [PubMed]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current Status of Biobased and Biodegradable Food Packaging Materials: Impact on Food Quality and Effect of Innovative Processing Technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Li, P.; Yan, H.; Su, W. Turmeric Carbon Quantum Dots Enhanced Chitosan Nanocomposite Films Based on Photodynamic Inactivation Technology for Antibacterial Food Packaging. Carbohydr. Polym. 2023, 311, 120784. [Google Scholar] [CrossRef] [PubMed]
- Sul, Y.; Ezati, P.; Rhim, J.W. Preparation of Chitosan/Gelatin-Based Functional Films Integrated with Carbon Dots from Banana Peel for Active Packaging Application. Int. J. Biol. Macromol. 2023, 246, 125600. [Google Scholar] [CrossRef] [PubMed]
- Deepika; Kumar, L.; Gaikwad, K.K. Carbon Dots for Food Packaging Applications. Sustain. Food Technol. 2023, 1, 185–199. [Google Scholar] [CrossRef]
- Khoshkalampour, A.; Ghorbani, M.; Ghasempour, Z. Cross-Linked Gelatin Film Enriched with Green Carbon Quantum Dots for Bioactive Food Packaging. Food Chem. 2023, 404, 134742. [Google Scholar] [CrossRef]
- Kousheh, S.A.; Moradi, M.; Tajik, H.; Molaei, R. Preparation of Antimicrobial/Ultraviolet Protective Bacterial Nanocellulose Film with Carbon Dots Synthesized from Lactic Acid Bacteria. Int. J. Biol. Macromol. 2020, 155, 216–225. [Google Scholar] [CrossRef]
- Aigaje, E.; Riofrio, A.; Baykara, H. Processing, Properties, Modifications, and Environmental Impact of Nanocellulose/Biopolymer Composites: A Review. Polymers 2023, 15, 1219. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y.; Hui, D. Influences of Nanoparticles Aggregation/Agglomeration on the Interfacial/Interphase and Tensile Properties of Nanocomposites. Compos. Part B Eng. 2017, 122, 41–46. [Google Scholar] [CrossRef]
- Kar, D.K.; Praveenkumar, V.; Si, S.; Panigrahi, H.; Mishra, S. Carbon Dots and Their Polymeric Nanocomposites: Insight into Their Synthesis, Photoluminescence Mechanisms, and Recent Trends in Sensing Applications. ACS Omega 2024, 9, 11050–11080. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Effect of Chitosan Modified Halloysite on the Physical and Functional Properties of Pullulan/Chitosan Biofilm Integrated with Rutin. Appl. Clay Sci. 2021, 211, 106205. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Gelatin/Agar-Based Functional Film Integrated with Pickering Emulsion of Clove Essential Oil Stabilized with Nanocellulose for Active Packaging Applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127220. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Advances in Controlled Release Devices for Food Packaging Applications. Trends Food Sci. Technol. 2010, 21, 591–598. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Ban, Z.; Zhang, J.; Li, L.; Luo, Z.; Wang, Y.; Yuan, Q.; Zhou, B.; Liu, H. Ginger Essential Oil-Based Microencapsulation as an Efficient Delivery System for the Improvement of Jujube (Ziziphus jujuba Mill.) Fruit Quality. Food Chem. 2020, 306, 125628. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Liu, Q.; Liu, M.; Chen, X.; Lin, H.; Zheng, Z.; Zhu, J.; Dai, C.; Dong, X.; Yang, D.P. Carbon Dots Enhanced Gelatin/Chitosan Bio-Nanocomposite Packaging Film for Perishable Foods. Chin. Chem. Lett. 2022, 33, 4577–4582. [Google Scholar] [CrossRef]
- Koshy, R.R.; Reghunadhan, A.; Mary, S.K.; Sadanandan, S.; Jose, S.; Thomas, S.; Pothen, L.A. AgNP Anchored Carbon Dots and Chitin Nanowhisker Embedded Soy Protein Isolate Films with Freshness Preservation for Active Packaging. Food Packag. Shelf Life 2022, 33, 100876. [Google Scholar] [CrossRef]
- Koshy, R.R.; Koshy, J.T.; Mary, S.K.; Sadanandan, S.; Jisha, S.; Pothan, L.A. Preparation of PH Sensitive Film Based on Starch/Carbon Nano Dots Incorporating Anthocyanin for Monitoring Spoilage of Pork. Food Control 2021, 126, 108039. [Google Scholar] [CrossRef]
- Roy, S.; Ezati, P.; Rhim, J.W. Gelatin/Carrageenan-Based Functional Films with Carbon Dots from Enoki Mushroom for Active Food Packaging Applications. ACS Appl. Polym. Mater. 2021, 3, 6437–6445. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, Y.; Jiang, Y.; Miao, M.; Cao, S.; Fang, J. Use of Carbon Dots to Enhance UV-Blocking of Transparent Nanocellulose Films. Carbohydr. Polym. 2017, 161, 253–260. [Google Scholar] [CrossRef]
- Salimi, F.; Moradi, M.; Tajik, H.; Molaei, R. Optimization and Characterization of Eco-Friendly Antimicrobial Nanocellulose Sheet Prepared Using Carbon Dots of White Mulberry (Morus alba L.). J. Sci. Food Agric. 2021, 101, 3439–3447. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Pan, H.; Xu, N.; Mei, C.; Mao, H.; Zhang, W.; Cai, J.; Xu, C. Preparation and Performance of Radiata-Pine-Derived Polyvinyl Alcohol/Carbon Quantum Dots Fluorescent Films. Materials 2020, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Gao, S.; Niu, Y.; Liu, H.; Mei, C.; Cai, J.; Xu, C. Preparation and Properties of Cyanobacteria-Based Carbon Quantum Dots/Polyvinyl Alcohol/Nanocellulose Composite. Polymers 2020, 12, 1143. [Google Scholar] [CrossRef]
- Patil, A.S.; Waghmare, R.D.; Pawar, S.P.; Salunkhe, S.T.; Kolekar, G.B.; Sohn, D.; Gore, A.H. Photophysical Insights of Highly Transparent, Flexible and Re-Emissive PVA @ WTR-CDs Composite Thin Films: A next Generation Food Packaging Material for UV Blocking Applications. J. Photochem. Photobiol. A Chem. 2020, 400, 112647. [Google Scholar] [CrossRef]
- Xu, N.; Gao, S.; Xu, C.; Fang, Y.; Xu, L.; Zhang, W. Carbon Quantum Dots Derived from Waste Acorn Cups and Its Application as an Ultraviolet Absorbent for Polyvinyl Alcohol Film. Appl. Surf. Sci. 2021, 556, 149774. [Google Scholar] [CrossRef]
- Kalanidhi, K.; Nagaraaj, P. N-Doped Carbon Dots Incorporated Chitosan/Polyvinylpyrrolidone Based Polymer Film for Advanced Packaging Applications. Chem. Phys. Lett. 2022, 805, 139960. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Qi, P.; Sun, J.; Jiang, S.; Li, H.; Gu, X.; Zhang, S. Enhancing the Thermostability, UV Shielding and Antimicrobial Activity of Transparent Chitosan Film by Carbon Quantum Dots Containing N/P. Carbohydr. Polym. 2022, 278, 118957. [Google Scholar] [CrossRef]
- Ananthi, P.; Hemkumar, K.; Subasini, S.; Pius, A. Development of Biodegradable Films Reinforced with Silver Functionalized Cow Milk Carbon Dots for Active Food Packaging Applications. Mater. Today Sustain. 2023, 24, 100609. [Google Scholar] [CrossRef]
- You, Y.; Zhang, H.; Liu, Y.; Lei, B. Transparent Sunlight Conversion Film Based on Carboxymethyl Cellulose and Carbon Dots. Carbohydr. Polym. 2016, 151, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Zinc Nanoparticles: Mode of Action and Efficacy against Boscalid-Resistant Alternaria Alternata Isolates. Sci. Total Environ. 2022, 829, 54638. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liu, G.; Ye, W.; Xu, Z.; Li, W.; Zhuang, J.; Zhang, X.; Wang, L.; Lei, B.; Hu, C.; et al. Multifunctional Carbon Dots Reinforced Gelatin-Based Coating Film for Strawberry Preservation. Food Hydrocoll. 2024, 147, 109327. [Google Scholar] [CrossRef]
- Davoodi, S.; Davachi, S.M.; Ghorbani Golkhajeh, A.; Shekarabi, A.S.; Abbaspourrad, A. Development and Characterization of Salvia Macrosiphon/Chitosan Edible Films. ACS Sustain. Chem. Eng. 2020, 8, 1487–1496. [Google Scholar] [CrossRef]
- Soto-Muñoz, L.; Palou, L.; Argente-Sanchis, M.; Ramos-López, M.A.; Pérez-Gago, M.B. Optimization of Antifungal Edible Pregelatinized Potato Starch-Based Coating Formulations by Response Surface Methodology to Extend Postharvest Life of ‘Orri’ Mandarins. Sci. Hortic. 2021, 288, 110394. [Google Scholar] [CrossRef]
- Mustapha, F.A.; Jai, J.; Nik Raikhan, N.H.; Sharif, Z.I.M.; Yusof, N.M. Response Surface Methodology Analysis towards Biodegradability and Antimicrobial Activity of Biopolymer Film Containing Turmeric Oil against Aspergillus Niger. Food Control 2019, 99, 106–113. [Google Scholar] [CrossRef]
- Mafe, A.N.; Edo, G.I.; Makia, R.S.; Joshua, O.A.; Akpoghelie, P.O.; Gaaz, T.S.; Jikah, A.N.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; et al. A Review on Food Spoilage Mechanisms, Food Borne Diseases and Commercial Aspects of Food Preservation and Processing. Food Chem. Adv. 2024, 5, 100852. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, M.; Deng, Y.; Zheng, L.; Wang, T.; Sun, B.; Chen, G. Multifunctional Carbon Dots with Full-Band UV-to-HEBL Shielding and Antibacterial Properties in Polyvinyl Alcohol Film for Enhancing Food Preservation. Food Packag. Shelf Life 2025, 48, 101468. [Google Scholar] [CrossRef]
- Ananda, B.; Radha Krushna, B.R.; Gagana, M.; Sharma, S.C.; Ray, S.; Subha, V.J.; Kumari, B.N.; Manjunatha, K.; Wu, S.Y.; Nagabhushana, H. Biodegradable Chitosan-Based Carbon Dot-Infused Intelligent Films with UV-Blocking and Shape Memory Properties for Shrimp Preservation and Milk Freshness Monitoring. J. Ind. Eng. Chem. 2025, in press. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Guo, C.; Dan, W.; Devahastin, S. Laser-Induced Microporous Modified Atmosphere Packaging and Chitosan Carbon-Dot Coating as a Novel Combined Preservation Method for Fresh-Cut Cucumber. Food Bioprocess Technol. 2021, 14, 968–983. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Fan, D.; Jiang, F. Effect of Carbon Dots with Chitosan Coating on Microorganisms and Storage Quality of Modified-Atmosphere-Packaged Fresh-Cut Cucumber. J. Sci. Food Agric. 2019, 99, 6032–6041. [Google Scholar] [CrossRef] [PubMed]
- Cosme Silva, G.M.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The Chitosan Affects Severely the Carbon Metabolism in Mango (Mangifera indica L. Cv. Palmer) Fruit during Storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, W.Y.; Fang, X.Q.; Li, W.; Sun, Y. Carbon Dots-Based Reinforced Hydrogen-Rich Water Nanocomposite Coating for Storage Quality of Fresh-Cut Pear. Food Biosci. 2023, 53, 102837. [Google Scholar] [CrossRef]
- Chen, H.; Hu, X.; Chen, E.; Wu, S.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. Preparation, Characterization, and Properties of Chitosan Films with Cinnamaldehyde Nanoemulsions. Food Hydrocoll. 2016, 61, 662–671. [Google Scholar] [CrossRef]
- Gao, Y.; Kan, C.; Wan, C.; Chen, C.; Chen, M.; Chen, J. Quality and Biochemical Changes of Navel Orange Fruits during Storage as Affected by Cinnamaldehyde -Chitosan Coating. Sci. Hortic. 2018, 239, 80–86. [Google Scholar] [CrossRef]
- Mei, S.; Fu, B.; Su, X.; Chen, H.; Lin, H.; Zheng, Z.; Dai, C.; Yang, D.P. Developing Silk Sericin-Based and Carbon Dots Reinforced Bio-Nanocomposite Films and Potential Application to Litchi Fruit. LWT 2022, 164, 113630. [Google Scholar] [CrossRef]
- Su, X.; Lin, H.; Fu, B.; Mei, S.; Lin, M.; Chen, H.; Zheng, Z.; Bo, H.; Yang, D.P.; Lin, Y. Egg-Yolk-Derived Carbon Dots@albumin Bio-Nanocomposite as Multifunctional Coating and Its Application in Quality Maintenance of Fresh Litchi Fruit during Storage. Food Chem. 2023, 405, 134813. [Google Scholar] [CrossRef]
- Tarhan, Ö. Safety and Regulatory Issues of Nanomaterials in Foods. In Handbook of Food Nanotechnology: Applications and Approaches; Elsevier: Amsterdam, The Netherlands, 2020; pp. 655–703. ISBN 9780128158661. [Google Scholar]
- Jafari, S.M.; Katouzian, I.; Akhavan, S. Nanoencapsulation Technologies for the Food and Nutraceutical Industries Safety and Regulatory Issues of Nanocapsules; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Amenta, V.; Aschberger, K.; Arena, M.; Bouwmeester, H.; Botelho Moniz, F.; Brandhoff, P.; Gottardo, S.; Marvin, H.J.P.; Mech, A.; Quiros Pesudo, L.; et al. Regulatory Aspects of Nanotechnology in the Agri/Feed/Food Sector in EU and Non-EU Countries. Regul. Toxicol. Pharmacol. 2015, 73, 463–476. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon Quantum Dots from Natural Resource: A Review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Huo, X.; He, Y.; Ma, S.; Jia, Y.; Yu, J.; Li, Y.; Cheng, Q. Green Synthesis of Carbon Dots from Grapefruit and Its Fluorescence Enhancement. J. Nanomater. 2020, 2020, 8601307. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Rhim, J.W.; Kim, J.T.; Molaei, R. PH-Responsive Strips Integrated with Resazurin and Carbon Dots for Monitoring Shrimp Freshness. Colloids Surf. B Biointerfaces 2023, 221, 113013. [Google Scholar] [CrossRef]
- Gogoi, S.; Sarmah, J.K.; Khan, R.; Murali, S. Postharvest Applications of Carbon Dots in Agriculture: Food Safety. In Carbon Dots in Agricultural Systems: Strategies to Enhance Plant Productivity; Elsevier: Amsterdam, The Netherlands, 2022; pp. 241–261. ISBN 9780323902601. [Google Scholar]
- Havrdova, M.; Hola, K.; Skopalik, J.; Tomankova, K.; Petr, M.; Cepe, K.; Polakova, K.; Tucek, J.; Bourlinos, A.B.; Zboril, R. Toxicity of Carbon Dots-Effect of Surface Functionalization on the Cell Viability, Reactive Oxygen Species Generation and Cell Cycle. Carbon 2016, 99, 238–248. [Google Scholar] [CrossRef]
- Wang, H.; Su, W.; Tan, M. Endogenous Fluorescence Carbon Dots Derived from Food Items. Innovation 2020, 1, 100009. [Google Scholar] [CrossRef]
- Wang, J.; Liang, X.; Hou, H.; Deng, J.; Lin, Q.; Li, W.; Bfai, J. A Review on Migration Behavior and Safety Regulation of Inorganic Nanoparticles in Food Packaging: Multi-Scale Mechanisms, Risk Assessment, and Innovative Strategies. Food Control 2025, 178, 111490. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.D.; Trivedi, D.H.; Jadhav, N.L.; Pinjari, D.V. Sustainable and Green Synthesis of Carbon Nanomaterials: A Review. J. Environ. Chem. Eng. 2021, 9, 106118. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Liang, L.; Gao, Y.; Cheng, H.; Li, X.; Zou, M.; Cao, A.; Ma, R.; Yuan, Q.; et al. Large-Area Graphene-Nanomesh/Carbon-Nanotube Hybrid Membranes for Ionic and Molecular Nanofiltration. Science 2019, 364, 1057–1062. [Google Scholar] [CrossRef]
- Mohammadzadeh kakhki, R.; Mohammadpoor, M. Machine Learning-Driven Approaches for Synthesizing Carbon Dots and Their Applications in Photoelectrochemical Sensors. Inorg. Chem. Commun. 2024, 159, 111859. [Google Scholar] [CrossRef]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and Characterization of Mono-Disperse Carbon Quantum Dots from Fennel Seeds: Photoluminescence Analysis Using Machine Learning. Sci. Rep. 2019, 9, 14004. [Google Scholar] [CrossRef]
- Senanayake, R.D.; Yao, X.; Froehlich, C.E.; Cahill, M.S.; Sheldon, T.R.; McIntire, M.; Haynes, C.L.; Hernandez, R. Machine Learning-Assisted Carbon Dot Synthesis: Prediction of Emission Color and Wavelength. J. Chem. Inf. Model. 2022, 62, 5918–5928. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, Y.; Shi, C.; Pei, Y.T. Large-Scale Synthesis of Defect-Selective Graphene Quantum Dots by Ultrasonic-Assisted Liquid-Phase Exfoliation. Carbon 2016, 109, 373–383. [Google Scholar] [CrossRef]
- Singh, A.K.; Itkor, P.; Lee, M.; Saenjaiban, A.; Lee, Y.S. Synergistic Integration of Carbon Quantum Dots in Biopolymer Matrices: An Overview of Current Advancements in Antioxidant and Antimicrobial Active Packaging. Molecules 2024, 29, 5138. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]






| Carbon Source | Type of Carbon-Based NPs | Production Techniques | Size | Key Findings QY (%) | Reference |
|---|---|---|---|---|---|
| Banana (Musa spp.) peels | CDs | Microwave treatment 500 W for 20 min | 2.2 nm | NR | [56] |
| Banana (Musa acuminata.) Juice | CDs | 150 °C for 4 h. | 3 nm | 8.95%. | [57] |
| Blueberry (Vaccinium spp.) | CDs | Liquid nitrogen-assisted, for 30 min | NR | NR | [58] |
| Carrot (Daucus carota) | CDs | Hydrothermal carbonization for 5 h at 180 °C | 2–7 nm | 11.5%. | [59] |
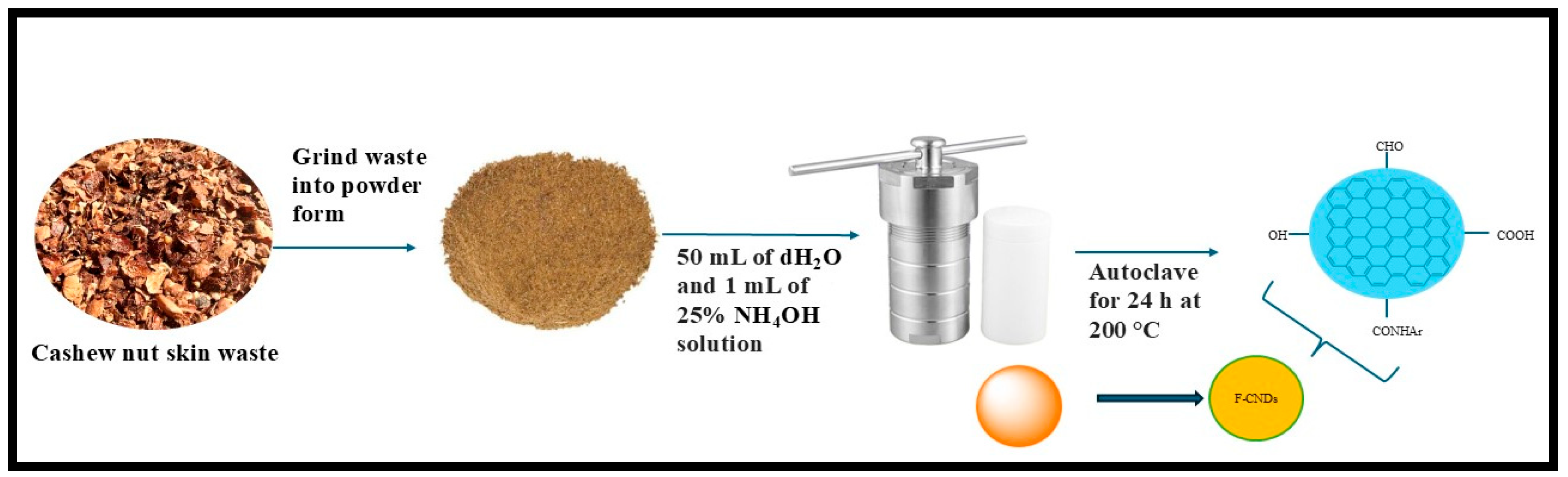
| Cashew Nut Skin (Anacardium occidentale) Waste | F-CNDs | Hydrothermal, at 200 °C for 24 h. | 2.5 nm | 15.5% | [49] |
| Dragon (Hylocereus undatus) fruits | N-CDs | Hydrothermal carbonization, 180 °C for 12 h | 2.5 nm | NR | [60] |
| Dwarf banana (Musa spp.) peel | N-CDs | 200 °C for 24 h. | 4.0 nm | 23%. | [53] |
| Grapefruit (Citrus × paradisi) peel | CDs | Hydrothermal, 190 °C for 12 h | 4.2 nm | NR | [52] |
| Goji Berry (Lycii Fructus) | CDs | Hydrothermal, 200 °C for 5 h | 3.3 nm | 17.2%. | [61] |
| Jackfruit (Artocarpus heterophyllus) peel and tamarind (Tamarindus indica) peel | N-CDs | Hydrothermal, 180 °C for 12 h | 6.4 nm and 5.3 nm | QY of Jackfruit peel was 13.04% while that of tamarind peel was 6.13%. | [10] |
| Jatropha (Jatropha curcas) fruits | CDs | Carbonization, 5 h at 180 °C | 3.2 nm | 13.7% | [62] |
| Kiwifruit (Actinidia deliciosa) fruit | N-CDs | 180 °C for 12 h | 3.59 nm | NR | [63] |
| Kumquat (Citrus Japonica) | CDs | Microwave-assisted, at 630 W for 2 min | NR | 7% | [64] |
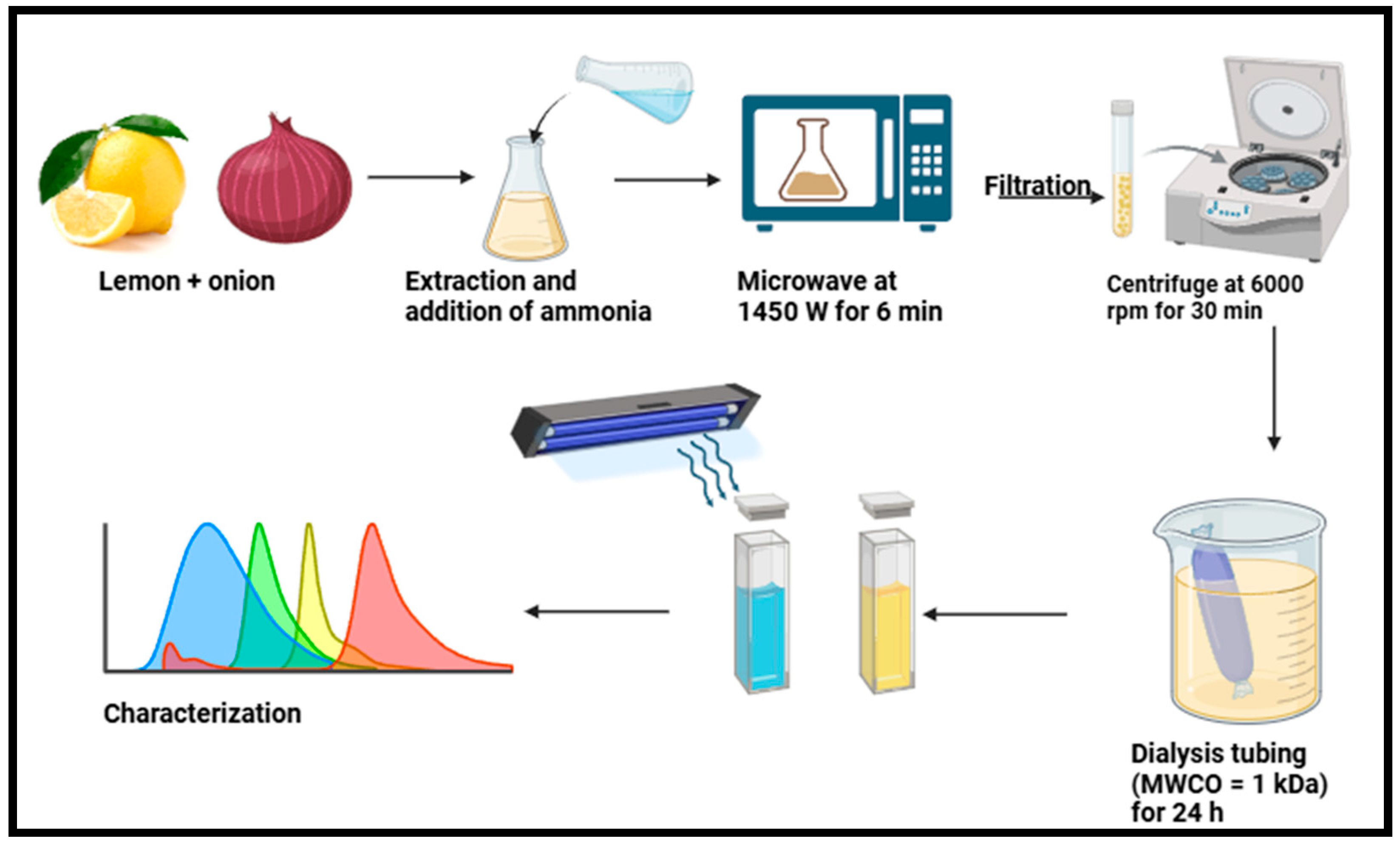
| Lemon (Citrus limon) juice and onion (Allium cepa) juice | CDs | Microwave-assisted, power= 1450 W) for 6 min | 6.15 nm | 23.6% | [65] |
| Lemon (Citrus limon) peel | CDs | 200 °C for 6 h | 9.5 nm | 11%. | [66] |
| Lemon (Citrus limon) peel waste | CDs | Hydrothermal 200 °C for 12 h. | 1–3 nm | 14%. | [67] |
| Mango (Mangifera indica) peels | CDs | Carbonization and oxygenolysis at 300 °C for 2 h to 6 h | 2–6 nm | 8.5% | [68] |
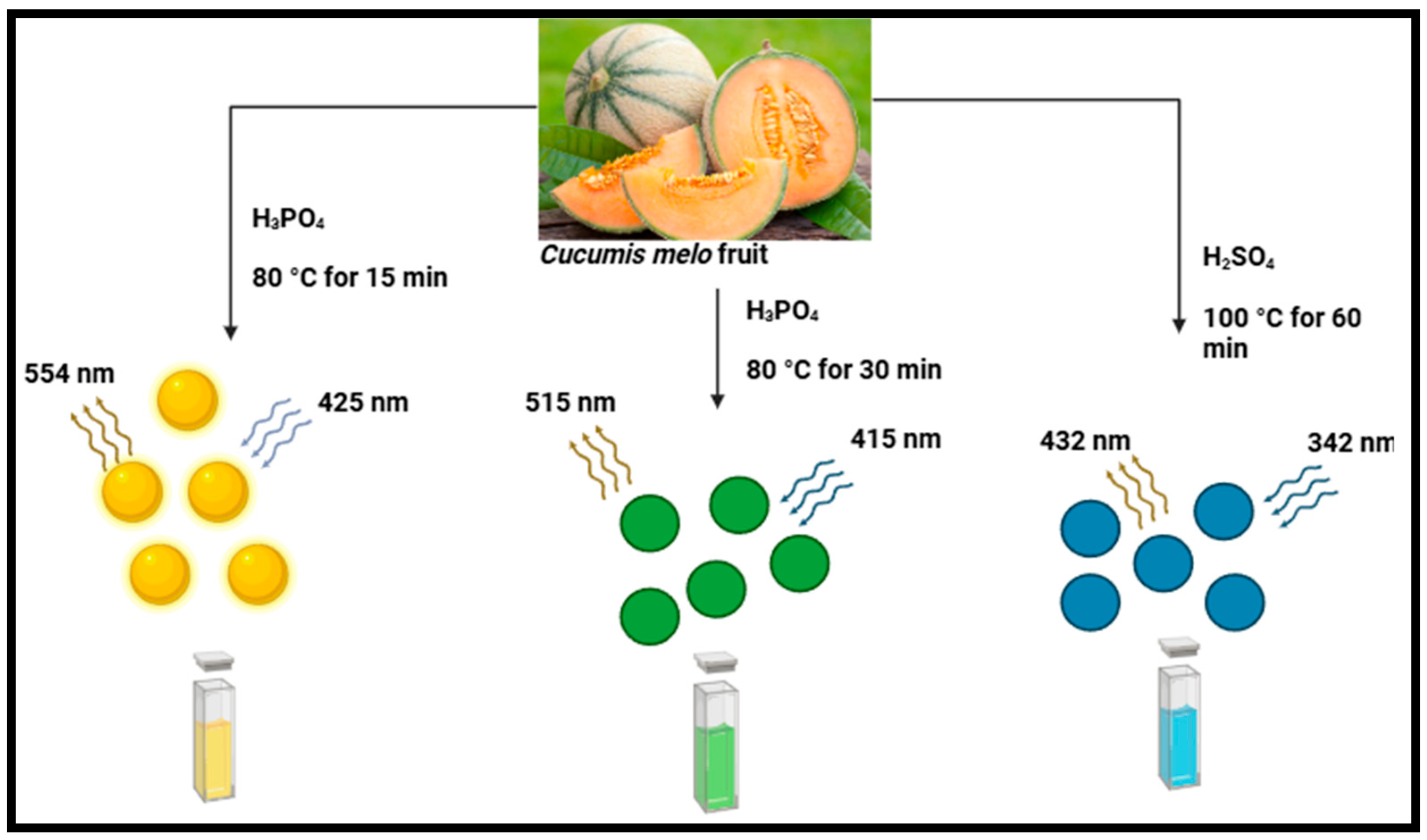
| Muskmelon (Cucumis melo) fruit | CMCDs | Acid oxidation at 80 °C for 15–30 min. | The B-, G-, and Y-CMCD were approximately 3.5, 4.3, and 5.8 nm, respectively. | B-, G-, and Y-CMCD were 14.3%, 26.9%, and 7.07% | [69] |
| Orange (Citrus sinensis) and limon (Citrus limon) peels | CDs | Heated at 180 °C for 2 h | 0.35 and 0.37 nm | Orange and limon CDs were found to have QYs of 16.8% and 15.5%, respectively. | [48] |
| Orange (Citrus sinensis) peel and banana (Musa spp.) peel | CDs | Microwave for 2 × 5 min | NR | NR | [70] |
| Orange (Citrus sinensis) peels | CDs | Hydrothermal carbonization at 180 °C for 12 h | 2–7 nm | 36%. | [71] |
| Onion (Allium cepa) peels | CDs | Microwave treatment 1000 W at specific time intervals | NR | NR | [72] |
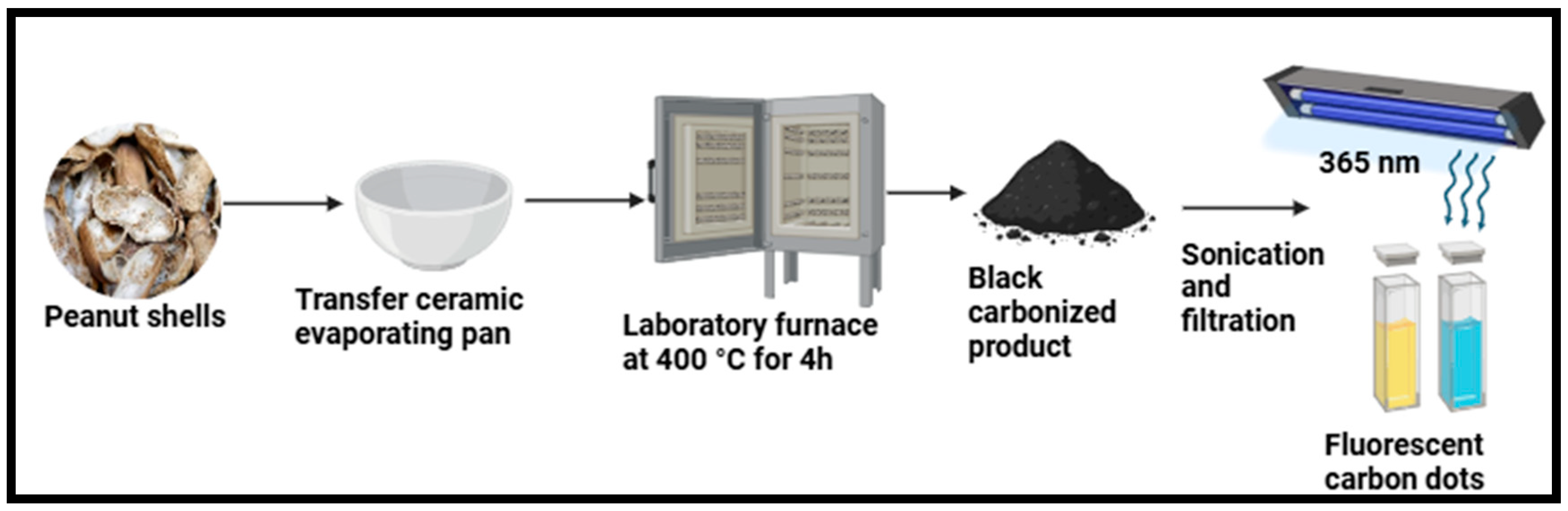
| Peanut (Arachis hypogaea) shells | CDs | Pyrolysis at 400 °C for 4 h in a laboratory furnace. | 3.3 nm | 10.58% | [73] |
| Pear (Pyrus communis) fruit | CDs | Hydrothermal 180 °C for 6 h | 2.0 nm | NR | [48] |
| Papaya (Carica papaya) pulp waste | CDs | Pyrolysis | 7 nm | 23.7% | [74] |
| Pineapple (Ananas comosus) fruit | CDs | Acid oxidation, 80–100 °C, 15–60 min | B-, G-, and Y- CDs were 2.08, 2.8, and 4.0 nm, respectively. | B-, G-, and Y- CDs QY were 18.0%, 37.6%, and 44.7%, respectively. | [42] |
| Quince (Cydonia oblonga) fruit powder | CDs | Microwave irradiation, 220 °C in 1 min using 850 W. Hydrothermal, 200 °C for 4 h in furnace | 4.85 nm | 8.55% | [75] |
| Roasted chickpeas (Cicer arietinum). | CDs | Microwave-Assisted, 350 watts for 2 min | 4.5–10.3 nm | 1.8%. | [76] |
| Sago (Metroxylon sagu) waste | CDs | Pyrolysis temperature ranging from 250 °C to 450 °C for 1 h | 6–17 nm | NR | [77] |
| Sapodilla (Manilkara zapota) fruits | CDs | Sonicated and heated at 100 °C for 60 min, 80 °C for 30 min, and 80 °C for 15 min | Blue, green, and yellow C-dots were 1.9, 2.9 and 4.5 nm, respectively | The QYs for the C-dots in blue, green, and yellow were 5.2%, 7.9%, and 5.7%, respectively. | [49] |
| Sugarcane bagasse (Saccharum officinarum), garlic (Allium sativum) peels, and taro (Colocasia esculenta) peels | CDs | Ultrasonic-assisted wet-chemical-oxidation method (~40 kHz, output power ~ 700 W) | 8–12 nm | QY ranging from 4 to 27%. | [78] |
| Sweet Potato (Ipomoea batatas) peels | CDs | Hydrothermal, 200 C for 3 h. | 2.0 nm | 8.9% | [79] |
| Tomato (Solanum lycopersicum) fruits | CDs | Chemical oxidation method, 40 N H3PO4, and heated at 80 °C for 25 min. | 5.0 to 10.0 nm | QY of blue, green, and yellow CDs were found to be 12.70%, 4.21%, and 2.76%, respectively. | [80] |
| Unripe Peach (Prunus persica) | N-CDs | Hydrothermal 180 °C for 5 h | 8 nm | 15%. | [81] |
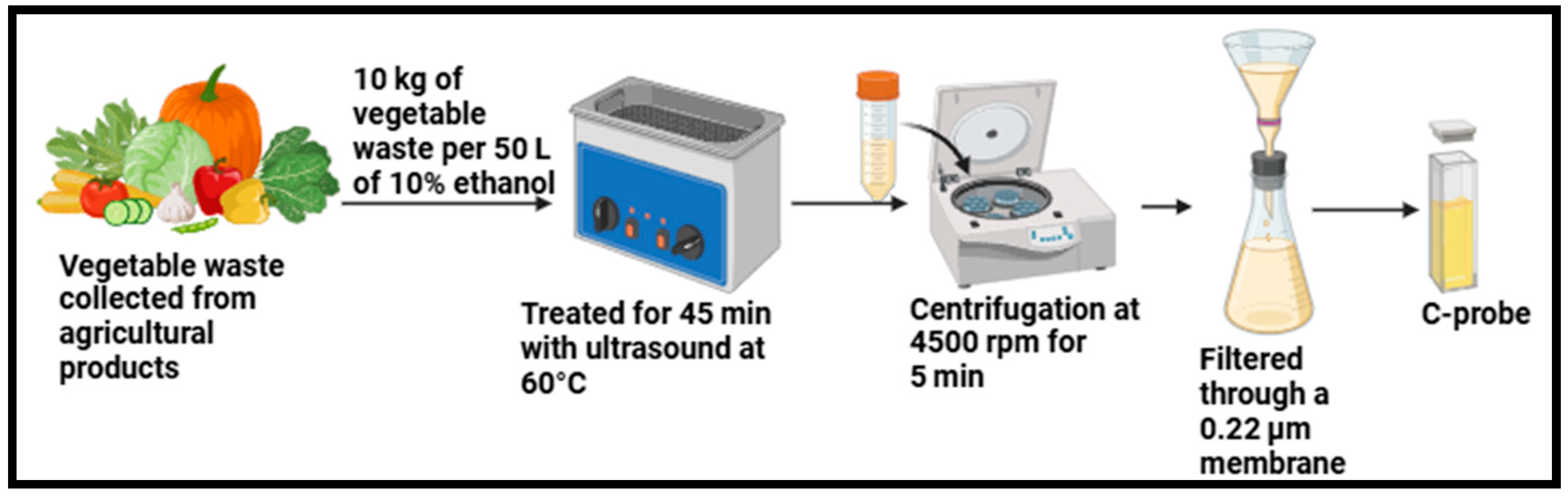
| Vegetable waste | CDs | Treated for 45 min with ultrasound irradiation at 60 °C | 6.03 nm | NR | [82] |
| Watermelon (Citrullus lanatus) peel | CDs | Carbonization, 220 °C for 2 h | 2.0 nm | NR | [83] |
| Plant Extract | Carbon Dots | Production Techniques | Key Findings | Reference |
|---|---|---|---|---|
| Beetroot (Beta vulgaris) | b-CDs | 160 °C for 8 h, hydrothermal | The DPPH assay is used to assess the antioxidant properties of b-CDs. It yields a maximum scavenging activity of 94.5% at a dose of 1000 μg mL−1. | [148] |
| Black soya (Glycine max) beans | N-CDs | Pyrolyzed at 200 °C for 4 h | According to estimates, N-CDs had a final scavenging activity of 62.8% against DPPH. The maximum amount of superoxide anion that N-CDs could scavenge was 81.3%. DPPH and superoxide anion radicals were scavenged with 93.8% and 99.3% efficiency, respectively, using 1 μg·mL−1 ascorbic acid as a positive control. | [149] |
| Cumin (Cuminum cyminum) seeds | CDs | 6 h at a temperature of 250 °C, hydrothermal | The concentration of CDs increased from 220 to 1540 μg/mL. The antioxidant capability of CDs increased by up to 80%, and the EC50 value was 1.2 mg/mL. | [150] |
| Dragon (Hylocereus undatus) fruit peels | CDs | Solvothermal treatment (acetic acid). | The antioxidant capacity of ACDs was very high; their DPPH radical scavenging IC50 value (0.70 μgmL−1) was significantly lower than that of the known antioxidant agent, ascorbic acid (4.34 μgmL−1). | [151] |
| Green (Xinyang Maojian) tea | CDs | 200 °C for 3 h, hydrothermal | The linear regression result showed a good linear association between the inhibition value and the concentration of carbon dots when the concentration was between 1.40 and 11.20 μg·mL−1. | [152] |
| Lemons (Citrus limon) and onions (Allium cepa) | CDs | Hydrothermal, 200 °C for 3 h | At 100 μg/mL, LCDs, and OCDs displayed 80 and 90% radical scavenging activity at 100 μgmL−1. | [126] |
| Orange (Citrus sinensis) fruit peel | CDs | Sand bath at 180 °C under magnetic stirring for 12 h | Ascorbic acid and CDs were found to have estimated EC50 μg mL−1 values of 0.80 ug mL−1 and 4.73829, respectively. | [153] |
| Pineapple (Ananas comosus) waste | CDs | 6 h at 200 °C, hydrothermal | The scavenging potential of CDs was 23.3% at a concentration of 5 mg/mL, whereas ascorbic acid exhibited the highest radical scavenging activity at the same dose, around 33.9%. At a dosage of 5 mg/mL, CDs scavenged the superoxide radical in a dose-dependent manner, reaching up to 42.9%; however, standard ascorbic acid demonstrated higher scavenging capacity (73.4% at 5 mg/mL). At a concentration of 5 mg/mL, CDs have hydrogen peroxide and hydroxyl scavenging activity of up to 93.4% and 50.2%, respectively. | [154] |
| Potato (Solanum tuberosum) Peel | CDs | 200 °C for 6 h, hydrothermal | ABTS and DPPH approaches demonstrated significant antioxidant activity from the CDs, contingent upon the CD concentration. | [147] |
| Red cabbage (Brassica oleracea) | rcCDs | 220 °C for 36 h, hydrothermal | Strong antioxidant properties were demonstrated by the rcCDs, which scavenged 61, 56, and 91% of the DPPH, hydroxyl, and potassium permanganate radicals, respectively. | [155] |
| Tea (Camellia sinensis) waste Grape (Vitis vinifera) pomace | TCDs GCDs | Carbonization method, 200 °C for 6 h in an oven. Hydrothermal-assisted process, 180 °C for 4 h in an oven | The DPPH radical scavenging activity of TCDs and GCDs was 75% and 56%, respectively, at a concentration of 375 µg·mL−1 CDs. For TCDs and GCDs, the EC50 values were 50 μg·mL−1 and 175 μg·mL−1, respectively. | [143] |
| Tomato (Solanum lycopersicum) | TCDs GCDs | 160 °C for 3 h, hydrothermal | Carbon dots from tomatoes (TCDs) exhibit robust inhibition even at the lowest concentration, while carbon dots based on glutathione (GCDs) require a concentration at least four times higher to get equivalent antioxidant strength. The concentration required to achieve 50% of DPPH inhibition, or EC50, is estimated to be less than 4 ppm for TCD (0.16 ppm·nmol−1) and approximately 14 ppm for GCD (0.56 ppm·nmol−1). | [156] |
| Tumeric (Curcuma longa) | CD S-CDs | 200 °C for 6 h, hydrothermal | At 200 lg/mL of CD, both CD and S-CD demonstrated significant free radical scavenging activity, around 90% and 80% observed in the ABTS method, and roughly 70% and 60% in the DPPH radical scavenging activity assay. | [157] |
| Waste (Camellia sinensis) tea | CDs | 150 °C for 6 h, hydrothermal | The hydroxyl and superoxide radicals had IC50 values of 80 and 24.2 μg/mL, respectively. | [146] |
| Plant Extract | Carbon Dots | Production Techniques | Key Findings | Reference |
|---|---|---|---|---|
| Apple (Malus spp.) juice | CDs | Hydrothermal at 150 °C for 12 h | For bioimaging of Mycobacterium tuberculosis and Pseudomonas aeruginosa cells. | [172] |
| Beetroot (Beta vulgaris) | CDs | (100, 150, 200, 250 and 300 C) for 10 h/hydrothermal | The synthesized CDs exhibited noteworthy antibacterial activity against Bacillus subtilis and Escherichia coli bacteria, with a higher inhibition zone. | [185] |
| Dried papaya (Carica papaya) flesh | CDs | Thermal at 200 ° for 5 h | The CDs have also been demonstrated to be an excellent probe for Escherichia coli O157: H7 fluorescence sensing, with a 9.5 × 104 cfu mL−1 detection limit. | [186] |
| Lemons (Citrus limon) and onions (Allium cepa) | CDs | Hydrothermal 200 °C for 6 h | Agar-well diffusion method was used to evaluate he antifungal activity of both CDs screened against Aspergillus sp., Candida albicans, Rhizopus sp., Botrytis cinerea, and Penicillium sp. Both LCDs (lemon) and OCDs (onion) displayed inhibition zones ranging from 12.6 to 44.5 mm, respectively. | [126] |
| Papaya (Carica papaya) juice | CDs | Single-step hydrothermal at 170 °C for 12 h | When activated at 488 (green) and 561 (red) nm, CD-labeled Bacillus subtilis cells produced a strong green and red fluorescence, demonstrating that the cells efficiently absorbed the manufactured CDs. Similarly, when excited at 488 and 561 nm, CD-labeled Aspergillus aculeatus produced green and red fluorescence images. | [170] |
| Pear (Pyrus pyrifolia) fruit | CDs | Hydrothermal 180 °C for 6 h | Demonstrate that CDs can bioimage Bacillus subtilis bacterial cells, indicating the possibility of using the nanoprobe for cell imaging applications. | [49] |
| Pineapple (Ananas comosus) waste peels | CDs | 200 °C for 6 h. | Antimicrobial against Pseudomonas aeruginosa, Bacillus cereus (28 mm), Staphylococcus aureus (25 mm), Escherichia coli (30 mm), and Vibrio cholerae (14 mm). | [127] |
| Potato (Solanum tuberosum) peel | CDs | 200 °C for 6 h | According to the findings of the disk-diffusion and well-diffusion tests, the CDs demonstrated considerable effectiveness against Listeria monocytogenes but no inhibitory zone against Escherichia coli. The growth inhibition zones in the well-diffusion and disk-diffusion approaches were 7 mm and approximately 6 mm, respectively. | [147] |
| Sapodilla (Manilkara zapota) | CDs | Hydrothermal 80–100° for 15–60 min | The blue, green, and yellow C-Dots demonstrated promise as bioimaging agents for imaging the cells of Aspergillus aculeatus, Escherichia coli, and Fomitopsis sp. | [49] |
| Soya (Glycine max) chunks | CDs | Hydrothermal, 180 °C for 12 h | The investigated pathogens were not inhibited by ZnO/CDs nanocomposite, while ZnO NPs demonstrated a minimum inhibitory concentration screened against Staphylococcus aureus (19.53 μg/mL). The inhibitory impact of ZnO NPs is significantly diminished when CDs are present in ZnO/CDs nanocomposite. | [14] |
| Turmeric (Curcuma longa) extract | CDs | 180 C for 10 h, solvent method | The antibacterial properties of CDs against Escherichia coli and Staphylococcus aureus under blue light irradiation were found to be dependent on carbonization levels, concentration, and light duration in vitro. | [187] |
| Turmeric (Curcuma longa) | CDs | 200 °C for 6 h | The CDs demonstrated no antibacterial efficacy against Escherichia coli but showed strong antibacterial activity against Listeria monocytogenes. | [157] |
| Turmeric (Curcuma longa), lemon (Citrus limon), citric acid and grapefruit (Citrus × paradisi) | CDs | 180 °C for 6 h | Escherichia coli pathogens were detected using CDs, a non-toxic photoluminescent sensor. The photoluminescence of the CDs nanocomposite was inhibited by increasing the number of Escherichia coli bacteria. | [188] |
| Polymer Matrix | Type of Carbon-Based NPs | Production Techniques and Condition | Key Findings | Reference |
|---|---|---|---|---|
| Carboxymethyl cellulose | CDs | Hydrothermally at 180 °C for 12 h | The polymer matrix contained uniformly distributed CDs, producing a highly translucent UV-blocking film. Increased the tensile strength by up to 27.6% and elastic modulus by up to 61.5%. Displayed excellent antioxidant and strong antimicrobial activity. | [23]. |
| Gelatin/chitosan | CDs | Hydrothermal, 180 °C for 800 min | When compared to the gelatin/chitosan film, the gelatin/chitosan/CDs film exhibited improved UV shielding, antioxidant, and antibacterial properties with an optimal addition of 20% CDs. | [220] |
| Chitosan | N-CDs | Hydrothermal, 180 °C for 8 h | The CS/N-CDs composite film exhibits superior UV light barrier performance and strength when compared to the CS film. Improved mechanical properties and displayed high photodynamic antibacterial rates of 91.2% and 99.9% for Escherichia coli and Staphylococcus aureus, respectively, were demonstrated by the produced CS/7% N-CDs composites. | [124] |
| Chitosan | CDs | Hydrothermal, 180 °C for 12 h | Chitosan films incorporated with CDs demonstrated better mechanical, UV, and hydrophobic qualities. Reduction in populations of Staphylococcus aureus and Escherichia coli of about 3.19 and 2.05 Log10 CFU/mL, respectively, within 40 min. | [207] |
| Chitin nanowhisker (CNW) embedded soy protein | AgNP anchored carbon dots | Hydrothermal autoclave at 180 °C for 6 h | When CNW and AgNP were added, the mechanical strength and thermal stability of the SPI film were greatly enhanced, and the moisture content decreased. Superior antioxidant, antibacterial, and antifungal activities were conferred by the synergistic effect of AgNP and CNW. | [221] |
| Starch + anthocyanin | CDs | Hydrothermal, 180 °C for 5 h | CDs and clitoria ternatea flower (CTE) were distributed uniformly in the starch matrix, according to SEM, FTIR, and XRD analyses. Because of the complementary effects of CD and CTE films have the best mechanical, barrier, thermal, and antioxidant qualities. | [222] |
| Carboxymethylcellulose and agar-based | Nitrogen-doped polyethylene glycol-derived CD (NPCD) | Hydrothermal, 180 °C for 6 h | The NPCD-loaded film demonstrated strong antibacterial activity and high antioxidant levels (DPPH 12.7% and ABTS 67%). CMC/agar films incorporated with 8% NPCD prevented the proliferation of Listeria monocytogenes and Escherichia coli. | [205] |
| Gelatin/Carrageenan-Based | mushroom-CDs (mCDs) | Hydrothermal, 200 °C for 6 h | The addition of mCDs in the film significantly improved the mechanical characteristics, while its water vapor permeability and hydrophobicity remained mostly unchanged, and the films were highly transparent. Gelatin/carrageenan films with mCDs added showed significant antioxidant activity as assessed by DPPH and ABTS assays. | [223] |
| Cellulose nanofiber-based | Modified carbon dots with resazurin (R-CD) | Hydrothermal, 160 °C for 6 h | The CNF/R-CD indicator film exhibited enhanced thermal stability and a marginally reduced water contact angle in comparison to the clean CNF film. UV-barrier qualities of the CNF/R-CD film were good, as evidenced by 98.3% and 87.7% of UV-B and UV-A light barriers, respectively. | [203] |
| Nanocellulose oxidized by applying TEMPO oxidation | CDs and ZnO | One-step microwave reaction at 600 W and heated at 200 °C for 2 min | Films demonstrated better UV-blocking capabilities, superior thermal stability, and excellent transparency to visible light. When the same amounts of ZnO were used in CDs-ONC-ZnO films, the UV-blocking ratio (UVR) was significantly higher than in previously proposed NC-ZnO films. Furthermore, CDs-ONC-s-ZnO film with 4 wt% sheet-like ZnO (s-ZnO) at 300 and 225 nm has a higher UVR (92.74% and 98.99%) than CDs-ONC-b-ZnO film supplemented with belt-like ZnO (b-ZnO) and CDs-ONC-p-ZnO film under the same conditions. | [224] |
| Chitosan nanocomposite hydrogel films | CH-CDs | Hydrothermal, 200 °C for 8 h | Chitosan + CDs hydrogel films were found to have better UV-visible blocking. Transmittance for CH-CD4 was up to 20% lower than that of CH hydrogel film in the 300–600 nm wavelength range. Compared to CH hydrogel film (5.1 MPa), the tensile strength (TS) of the CH-CD1 nanocomposite film increased significantly to 18.6 MPa. The hydrophobicity of the hydrogel nanocomposite films was indicated by an increase in contact angle values, which went from 64.95° for CH films to 88.75° for CH-CD3 films. | [24] |
| Nanocellulose | CDs | Hydrothermal, 160 °C for 6 h | Listeria monocytogenes was used to test antibacterial activity. C-dots considerably enhanced the tensile strength and reduced strain in relation to breaking BNC when added to BNC. C-dots were used to create a BNC sheet with highly effective UV-blocking properties. | [225] |
| Polyvinyl Alcohol | CD | Hydrothermal, 200 °C for 8 h | The composite’s tensile strength and modulus increase dramatically when CNF and CDs are added to the PVA matrix. PVA-based films become more water-resistant when CNF and CDs are doped. When the produced films contain more CDs, the water contact angle reduces, and their wettability improves. | [226] |
| Chitosan/gelatin-based | CDs | Hydrothermal, 200 °C for 6 h | The composite film exhibited a notable improvement in UV protection qualities but a slight drop in transparency. The produced film demonstrated a high level of antioxidant efficacy, with >74% DPPH and 100% ABTS radical scavenging capacity. Additionally, the Chitosan/gelatin-based films demonstrated strong antibacterial efficacy against Listeria monocytogenes. | [208] |
| Pectin/gelatin-based | CD | Hydrothermal, 200 °C for 6 h | The CD-added film demonstrated excellent UV protection qualities without significantly affecting the transparency of the pectin/gelatin film. Hydrophobicity, vapor permeability, and mechanical properties of the film were impacted by the addition of CD. Furthermore, the DPPH and ABTS tests revealed that the pectin/gelatin-based films with CD added had strong antioxidant activity. Also, the sulfur-functionalized CD film exhibited potent antibacterial activity against Listeria monocytogenes and E. coli. | [141] |
| Polyvinyl Alcohol | CDs | Hydrothermal, 200 °C for 8 h | Emission intensity of the PVA/CNF/CDs films decreased gradually. Increased water absorption rate. The light barrier of the composite improved. | [227] |
| Nanocellulose film | CDs | Hydrothermal, 200 °C for 2 h | Nanocellulose with CDs improved flexibility in comparison to neat nanocellulose. Improved ultraviolet barrier property and prevented the growth of Gram-positive bacteria more than Gram-negative bacteria. | [141] |
| Polyvinyl Alcohol | CDs | Hydrothermal, 200 °C for 8 h | Emission intensity of the PVA/CNF/CDs films decreased gradually. Increased water absorption rate. The light barrier of the composite improved. | [227] |
| Polyvinyl alcohol (PVA) | CDs | Hydrothermal, 200 °C for 5 h | Improved UV barrier properties of the composite PVA films. PVA@WTR-CDs-3 films were able to completely block (100%) the UV-C (230–280 nm) and UV-B (280–315 nm) regions, and only 20–60% of the UV-A (315–400 nm) region. PVA@WTR-CDs-5 composite films also achieved a maximum UV barrier. No significant changes in the intrinsic mechanical and tensile strength of PVA films. No changes were observed in the thermal analysis study when WTR-CDs were incorporated in PVA films. | [228] |
| Chitosan and PVA | CDs | Hydrothermal, 200 °C for 8 h | The PVA/CS/1-CDs film demonstrated a UV-A barrier capacity of 83.58% and a transparency of 72.34%, respectively. While PVA/CS/2-CDs film achieved a UV-A barrier capacity of 94.53%. | [229] |
| Chitosan-polyvinylpyrrolidone | N-doped carbon dots (NCDs) | Hydrothermal, 200 °C for 12 h | The film with NCDs integrated displayed a smooth surface with evenly dispersed NCDs in the chitosan-PVP film. while NCDs with chitosan-PVP-orange peel film had a uniformly smooth surface. Chitosan-PVP films with NCDs displayed better tensile strength, elongation at the break, reduced moisture content, a contact angle value of 89.6°, and showed degradation exceeding 40% over a 50-day period. | [230] |
| Cellulose nanofiber-based | Glucose (GCD) and N-functionalized CDs (NGCD) | Hydrothermal, 200 °C for 6 h | GCD and NGCD reduced T280 by 91–28% and T660 by 12–10% while maintaining high UV blocking qualities for the CNF film without affecting its transparency. No changes in mechanical properties were observed when GCD and NGCD were added, but the films’ water vapor permeability (WVP) and water contact angle (WCA) increased. The composite films incorporated with GCD and NGCD exhibited a high level of antioxidant activity, scavenging 80–85% of DPPH radicals and 99% of ABTS. The CNF/NGCD film demonstrated better antibacterial and antifungal activity than the CNF/GCD film. | [125] |
| Chitosan | Nitrogen and phosphorus (NP-CDs) | Hydrothermal, 180 °C for 8 h | The NP-CDs increased the density of the film, water contact angle from 79.2° to 105.8°, UV-A and UV-B transmittance, and antibacterial activity to both E. coli and S. aureus compared to the chitosan film. However, NP-CDs reduced water vapor permeation. | [231] |
| Agar-based film | Ag-CMCDs | Hydrothermal, 180 °C for 12 h | Agar-based films had high UV barrier properties as well as good biodegradability, with roughly 86% degradation in 60 days. Improved tensile strength value to 41.85 MPa. In addition, the agar-based films demonstrate excellent antibacterial activity against Staphylococcus aureus and Escherichia coli. | [232] |
| Gelatin-based | CDs | Hydrothermal, 200 °C for 6 h | When CDs were added, the water vapor permeability (by 28%) and hydrophobicity (by 9% and 13%) of the very transparent film significantly improved without affecting the mechanical properties. The gelatin films with CD added showed strong antioxidant activity and UV barrier properties. Moreover, gelatin-based films with CDs significantly improved antibacterial efficacy. | [147] |
| Carboxymethyl cellulose | CDs | Hydrothermal, 200 °C for 5 h | Improved structural homogeneity, optical properties, and tensile strength. | [233] |
| Food Product | Polymer Matrix | (%) Weight of CDs | Storage Condition | Impact of Coating | Reference |
|---|---|---|---|---|---|
| Avocado (Persea americana) | Chitosan + gelatin | CDs 1% and 2% wt | 25 °C for 21 days | On day 14, mold growth was noted. The antifungal activity of CDs films was dependent on CDs concentration. | [184] |
| Banana (Musa spp.) | Polyvinyl alcohol (PVA) | CDs at 0.5% | 23 °C | The appearance of bananas coated with CS-CDs/PVA showed less decay compared to those coated with PVA and the control (no coating). The bananas gradually deteriorated during storage, as seen by black spots. | [55] |
| Lemon (Citrus limon) | Carboxymethyl cellulose | CDs 3.0 wt% | Room humidity at 25 °C for 21 days | Retained their original flavor and color, and the surface of the lemons displayed no signs of mold growth. | [23] |
| Sliced tomatoes (Solanum lycopersicum) | Agar based | CD and AgNO3 1, 2, and 3% | Ambient temperature with 50% RH for 5 days | Reduced microbial growth, minimal water loss, and delayed the decay of tomatoes and prolonged shelf life. | [232] |
| Fresh-cut cucumber (Cucumis sativus) | Chitosan | CDs at 0%, 1.5%, 3%, and 4.5% | 4 ∘C for 15 days. | During storage, the total number of colonies, mold, and yeast growth in fresh-cut cucumbers packaged in a regulated environment was suppressed by the CDs/CH coating. Moreover, the 4.5% CDs/CH coating successfully inhibited peroxidase activity, lowered water mobility, and prevented weight, firmness, and total soluble solids losses in fresh-cut cucumbers during storage. It also prevented the ascorbic acid content and flavor from degrading. | [243] |
| Fresh-Cut Cucumber (Cucumis sativus) | Chitosan and ultrasound | CDs at 4.5% | 4 °C for 15 days | The findings showed that after 15 days of storage, US treatment coupled with CDs coating significantly reduced the overall bacterial count to 5.18 log CFU g−1, mold, and yeast to 3.45 log CFU g−1. The treatment also obtained a lower weight loss of 8.54%, respiration rate of 4.67 mg kg−1 h−1 CO2 and malondialdehyde content of 2.24 μmol kg−1. Furthermore, the US treatment inhibited polyphenol oxidase activity to 137.17 U kg−1 s −1 and peroxidase activity to 139.83 U kg−1 s−1. They also maintained higher firmness of 6.78 N, ascorbic acid content of 0.0243 g kg−1 and total soluble solids of 2.29 °Brix, and after 15 days of storage, there was less of a change in the water content. | [142] |
| Fresh-cut pears (Pyrus communis) | Carboxymethyl chitosan | CDs at 1, 2 and 3% | 4 °C for 5 days | The CS/2%PER-CDs coating solution effectively reduced respiration (51.67 mgCO2/Kg⋅h) and ethylene production rate (0.75 μg/kg⋅h). | [245] |
| Litchi fruit (Litchi chinensis) | Silk sericin and chitosan | CDs at 20% | 25 °C and 85% relative humidity for 6 days | Effectively reduced water loss, nutritional components, such as ascorbic acid and soluble solids | [248] |
| Litchi fruit (Litchi chinensis) | Chitosan (CS) | CDs | 25 °C and 85–90% RH | Litchi fruit treated with EA/CS/CDs displayed higher levels (L*, a*, and b*) compared to the control treatment. Maintain higher levels of vitamin C, sucrose, and total sugar to ensure optimal nutritional quality and minimize weight loss. Effectively postponed the pericarp from browning. | [249] |
| Strawberries (Fragaria × ananassa) | Gelatin-based | R-CDs | Room temperature for 8 days | Most of the strawberries in the control group were shriveled or bacterially contaminated by the end of the eighth day of storage, with a rotting rate of almost 75%. In contrast, most of the strawberries coated with Gelatin + 1.5% R-CDs had a rotting rate of roughly 4.17%. On the fourth day, the control group showed a significant weight reduction, yet the coated group that contained 1.5% R-CDs experienced comparatively less weight loss. This pattern persisted until the last day of storage, when the 1.5% R-CDs group experienced a weight loss rate of 29.63%, and the control treatment was 45.47%. | [235] |
| Strawberries (Fragaria × ananassa) | Packaging material | CDs | 25–27 °C and 68% RH | Weight loss on the fourth day of observation was 5.21% for strawberries packed with OCDs and 8.14% for those packaged with LCDs, compared to 18.71% for the control. The rate of decay for OCDs was 11%, LCDs was 40%, and control was 96.65% on the same day, as shown in Figure 12. | [126] |
| Tangerine (Citrus reticulata) and strawberry (Fragaria × ananassa) fruit | Cellulose nanofiber-based coating | GCD and NGCD at 1 wt % based on polymer | NR | Mold developed on the surface of the uncoated and coated fruit with the neat CNF and CNF/GCD films; the mold became worse after 15 and 4 days of storage. CNF/NGCD film maintained better quality without exhibiting any signs of mold growth. | [125] |
| Tomato (Solanum lycopersicum) | Pure gelatin cellulose nanofiber (CNF) | CDs | 28 °C with 40% humidity | Compared to CK, the hardness reduction in the G/10CD/3CNF/EON treatment condition was slower, demonstrating that the intricate layer that formed a barrier around the tomato helped preserve the fruit firmness by preventing water loss. Moreover, G/10CD/3CNF/EON treatment maintained soluble solids content and TA better than EON and the control group. | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leta, T.B.; Adeyemi, J.O.; Fawole, O.A. Carbon Dot Nanoparticles Synthesized from Horticultural Extracts for Postharvest Shelf-Life Extension of Fruits and Vegetables. Plants 2025, 14, 2523. https://doi.org/10.3390/plants14162523
Leta TB, Adeyemi JO, Fawole OA. Carbon Dot Nanoparticles Synthesized from Horticultural Extracts for Postharvest Shelf-Life Extension of Fruits and Vegetables. Plants. 2025; 14(16):2523. https://doi.org/10.3390/plants14162523
Chicago/Turabian StyleLeta, Tshiamo B., Jerry O. Adeyemi, and Olaniyi A. Fawole. 2025. "Carbon Dot Nanoparticles Synthesized from Horticultural Extracts for Postharvest Shelf-Life Extension of Fruits and Vegetables" Plants 14, no. 16: 2523. https://doi.org/10.3390/plants14162523
APA StyleLeta, T. B., Adeyemi, J. O., & Fawole, O. A. (2025). Carbon Dot Nanoparticles Synthesized from Horticultural Extracts for Postharvest Shelf-Life Extension of Fruits and Vegetables. Plants, 14(16), 2523. https://doi.org/10.3390/plants14162523









