Exploring Reproductive Timing in Olive Tree: Male Meiosis and Anthesis Events
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
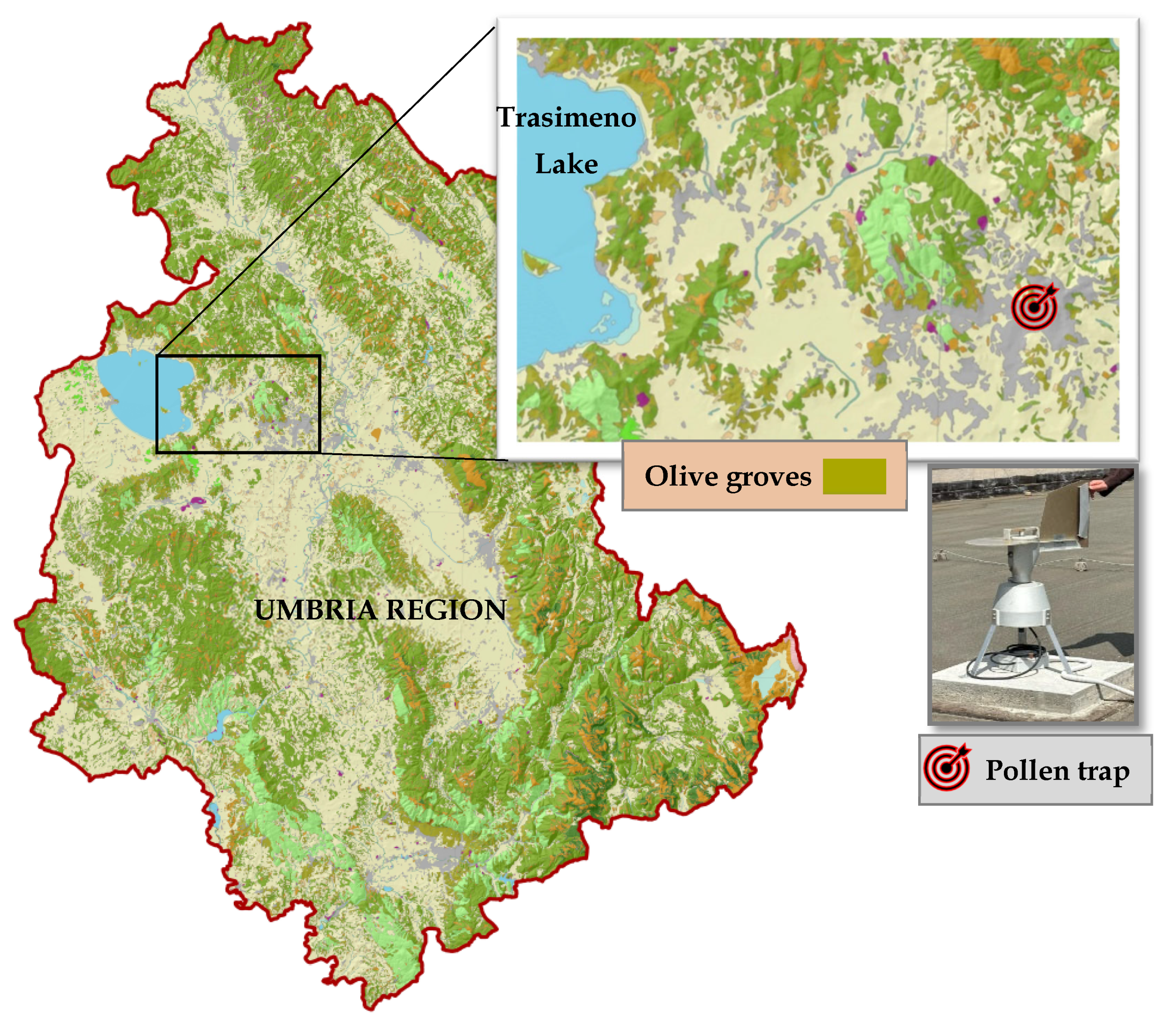
4.1. Study Area
4.2. Aerobiological and Meteorological Data
4.3. GDD Calculations
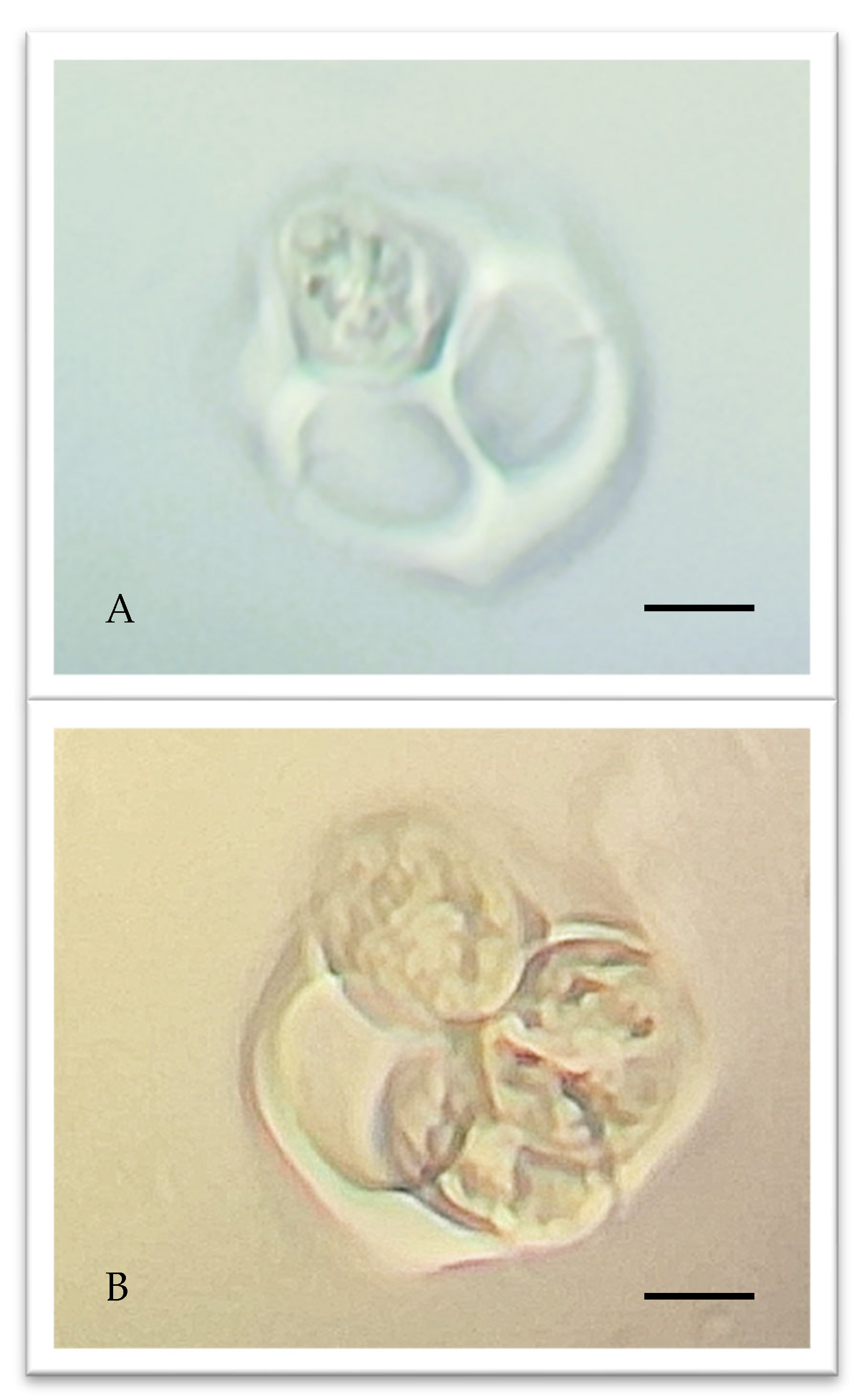
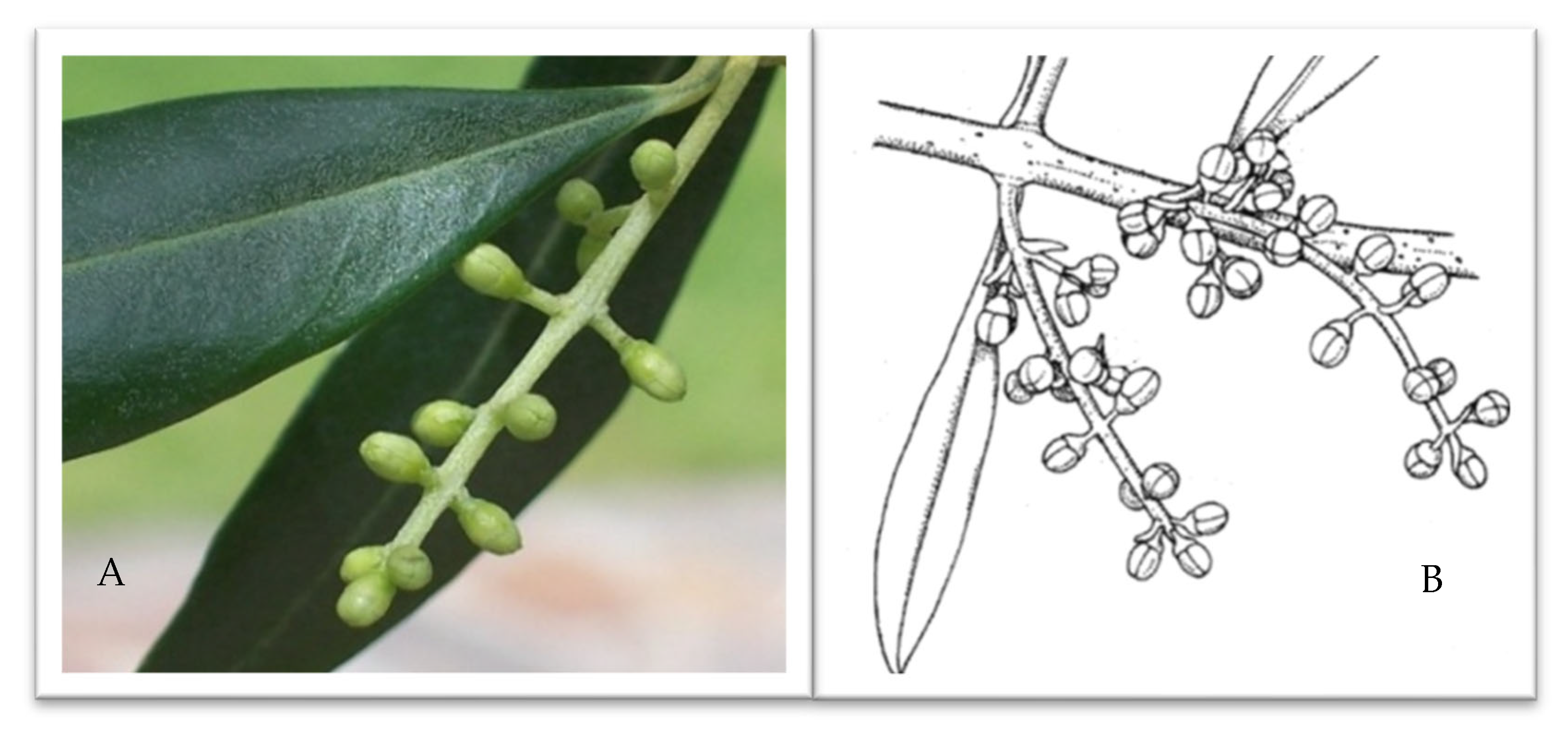
4.4. Male Meiosis Phase Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hafidh, S.; Fíla, J.; Honys, D. Male gametophyte development and function in angiosperms: A general concept. Plant Reprod. 2016, 29, 31–51. [Google Scholar] [CrossRef] [PubMed]
- De Storme, N.; Geelen, D. The impact of environmental stress on male reproductive development in plants: Biological processes and molecular mechanisms. Plant Cell Environ. 2014, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lohani, N.; Singh, M.B.; Bhalla, P.L. High temperature susceptibility of sexual reproduction in crop plants. J. Exp. Bot. 2020, 71, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Dolferus, R.; Ji, X.; Richards, R.A. Abiotic stress and control of grain number in cereal. Plant Sci. 2011, 181, 331–341. [Google Scholar] [CrossRef]
- Bomblies, K.; Higgins, J.D.; Yant, L. Meiosis evolves: Adaptation to external and internal environments. New Phytol. 2015, 208, 306–323. [Google Scholar] [CrossRef]
- Ma, Y.; Schwenke, G.; Sun, L.; Liu, D.L.; Wang, B.; Yang, B. Modeling the impact of crop rotation with legume on nitrous oxide emissions from rain-fed agricultural systems in Australia under alternative future climate scenarios. Sci. Total Environ. 2018, 15, 1544–1552. [Google Scholar] [CrossRef]
- Muller, F.; Rieu, I. Acclimation to high temperature during pollen development. Plant Reprod. 2016, 29, 107–118. [Google Scholar] [CrossRef]
- Begcy, K.; Weigert, A.; Egesa, A.O.; Dresselhaus, T. Compared to Australian cultivars, European summer wheat (Triticum aestivum) overreacts when moderate heat stress is applied at the pollen development stage. Agronomy 2018, 8, 99. [Google Scholar] [CrossRef]
- Tedeschini, E.; RodrÍguez-Rajo, F.J.; Caramiello, R.; Jato, V.G.; Frenguelli, G. The influence of climate changes in Platanus spp. pollination in Spain and Italy. Grana 2006, 45, 222–229. [Google Scholar] [CrossRef]
- Draeger, T.; Moore, G. Short periods of high temperature during meiosis prevent normal meiotic progression and reduce grain number in hexaploid wheat (Triticum aestivum L.). Theor. Appl. Genet. 2017, 130, 1785–1800. [Google Scholar] [CrossRef]
- Endo, M.; Tsuchiya, T.; Hamada, K.; Kawamura, S.; Yano, K.; Ohshima, M.; Higashitani, A.; Watanabe, M.; Kawagishi-Kobayashi, M. High Temperatures Cause Male Sterility in Rice Plants with Transcriptional Alterations During Pollen Development. Plant Cell Physiol. 2009, 50, 1911–1922. [Google Scholar] [CrossRef]
- Masoomi-Aladizgeh, F.; Atwell, B.J.; Bokshi, A.I.; Thistlethwaite, R.J.; Khoddami, A.; Trethowan, R.; Tan, D.K.Y.; Roberts, T.H. Pinpointing the timing of meiosis: A critical factor in evaluating the impact of abiotic stresses on the fertility of cereal crops. New Phytol. 2024, 245, 1341–1354. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, X.; Sami Khanal, S.; Robyn Wilson, R.; Leng, G.; Toman, E.M.; Wang, X.; Li, Y.; Zhao, K. Climate change impacts on crop yields: A review of empirical findings, statistical crop models, and machine learning methods. Environ. Model. Softw. 2024, 179, 106119. [Google Scholar] [CrossRef]
- Tedeschini, E.; Mariani, M.; Frenguelli, G. The effects of cold stress on cypress pollen intine permeability. Aerobiologia 2019, 35, 567–570. [Google Scholar] [CrossRef]
- Begcy, K.; Nosenko, T.; Zhou, L.-Z.; Fragner, L.; Weckwerth, W.; Dresselhaus, T. Male sterility in maize after transient heat stress during the tetrad stage of pollen development. Plant Physiol. 2019, 181, 683–700. [Google Scholar] [CrossRef]
- Frenguelli, G.; Ferranti, F.; Tedeschini, E.; Andreutti, R. Volume changes in the pollen grain of Corylus avellana L. (Corylaceae) during development. Grana 1997, 36, 289–292. [Google Scholar] [CrossRef]
- Tottman, D.R. The Decimal Code for the Growth Stages of Cereals, with Illustrations. Ann. Appl. Biol. 1987, 110, 441–454. [Google Scholar] [CrossRef]
- Wang, E.; Engel, T. Simulation of phenological development of wheat crops. Agric. Syst. 1998, 58, 1–24. [Google Scholar] [CrossRef]
- Aguilera, F.; Ruiz Valenzuela, L.; Fornaciari, M.; Romano, B.; Galán, C.; Oteros, J.; Dhiab, A.B.; Msallem, M.; Orlandi, F. Heat accumulation period in the Mediterranean region: Phenological response of the olive in different climate areas (Spain, Italy and Tunisia) Int. J. Biometeorol. 2014, 58, 867–876. [Google Scholar] [CrossRef]
- Galán, J.M.G.; Passarelli, L.M.; Prada, C.; Roller, C.H. Sporophyte morphology and gametophyte development of the fern Blechnum sprucei (Pteridophyta: Blechnaceae). Rev. Biol. Trop. 2008, 56, 2027–2040. [Google Scholar] [CrossRef][Green Version]
- Fornaciari, M.; Orlandi, F.; Tedeschini, E. Long term analysis on Olive flowering and climatic relationships in central Italy. Eur. J. Agron. 2025, 162, 127435. [Google Scholar] [CrossRef]
- Recio, M.; Picornell, A.; Trigo, M.M.; Gharbi, D.; García-Sánchez, J.; Cabezudo, B. Intensity and temporality of airborne Quercus pollen in the southwest Mediterranean area: Correlation with meteorological and phenoclimatic variables, trends and possible adaptation to climate change. Agric. For. Meteorol. 2018, 250–251, 308–318. [Google Scholar] [CrossRef]
- Marchand, L.J.; Dox, I.; Gričar, J.; Prislan, P.; Leys, S.; Van den Bulcke, J.; Fonti, P.; Lange, H.; Matthysen, J.; Peñuelas, E.; et al. Inter-individual variability in spring phenology of temperate deciduous trees depends on species, tree size and previous year autumn phenology. Agric. For. Meteorol. 2020, 290, 108031. [Google Scholar] [CrossRef] [PubMed]
- Menzel, A.; Sparks, T.; Estrella, N.; Koch, E.; Aasa, A.R.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Moriondo, M.; Trombi, G.; Ferrise, R.; Brandani, G.; Dibari, C.; Ammann, C.M.; Lippi, M.M.; Bindi, M. Olive trees as bio-indicators of climate evolution in the Mediterranean Basin. Glob. Ecol. Biogeogr. 2013, 22, 818–833. [Google Scholar] [CrossRef]
- Chuine, I.; Cour, P.; Rousseau, D.D. Selecting models to predict the timing of flowering of temperate trees: Implications for tree phenology modelling. Plant Cell Environ. 1999, 22, 1–13. [Google Scholar] [CrossRef]
- Aguilera, F.; Ruiz Valenzuela, L. Study of the floral phenology of Olea europaea L. in Jaen province (SE Spain) and its relation with pollen emission. Aerobiologia 2009, 25, 217–225. [Google Scholar] [CrossRef]
- Orlandi, F.; Sgromo, C.; Bonofiglio, T.; Ruga, L.; Romano, B.; Fornaciari, M. Spring Influences on Olive Flowering and Threshold Temperatures Related to Reproductive Structure Formation. HortScience 2010, 45, 1052–1057. [Google Scholar] [CrossRef]
- Orlandi, F.; Garcia-Mozo, H.; Galán, C.; Romano, B.; de la Guardia, C.D.; Ruiz, L.; del Mar Trigo, M.; Dominguez-Vilches, E.; Fornaciari, M. Olive flowering trends in a large Mediterranean area (Italy and Spain). Int. J. Biometeorol. 2010, 54, 151–163. [Google Scholar] [CrossRef]
- Tedeschini, E.; Proietti, P.; Timorato, V.; D’Amato, R.; Nasini, L.; Del Buono, D.; Frenguelli, G. Selenium as stressor and antioxidant affects pollen performance in Olea europaea L. Flora 2015, 215, 16–22. Flora 2015, 215, 16–22. [Google Scholar] [CrossRef]
- Didevarasl, A.; Costa Saura, J.M.; Spano, D.; Deiana, P.; Snyder, R.L.; Mulas, M.; Nieddu, G.; Zelasco, S.; Santona, M.; Trabucco, A. Modeling Phenological Phases across Olive Cultivars in the Mediterranean. Plants 2023, 12, 3181. [Google Scholar] [CrossRef]
- Chmielewski, F.M. Phenology and Agriculture. In Phenology: An Integrative Environmental Science; Schwartz, M.D., Ed.; Kluwer Academic Publishers: Boston, MA, USA; Dordrecht, The Netherlands; London, UK, 2003; Chapter 7; pp. 505–522. [Google Scholar]
- Qian, W.; Hu, Y.; Lin, X.; Yu, D.; Jia, S.; Ye, Y.; Mao, Y.; Yi, L.; Gao, S. Phenological Growth Stages of Abelmoschus manihot: Codification and Description According to the BBCH Scale. Agronomy 2023, 13, 1328. [Google Scholar] [CrossRef]
- Wadgymar, S.M.; Ogilvie, J.E.; Inouye, D.W.; Weis, A.E.; Anderson, J.T. Phenological responses to multiple environmental drivers under climate change: Insights from a long-term observational study and a manipulative field experiment. New Phytol. 2018, 218, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, Y.; Yang, X.; Zhang, H.; Guo, Z.; Hu, G.; Bai, H.; Sun, Y.; Huang, L.; Ma, M. Indirect effects of warming via phenology on reproductive success of alpine plants. J. Ecol. 2025, 113, 180–193. [Google Scholar] [CrossRef]
- Hamze, L.M.; Trentacoste, E.R.; Searles, P.S.; Rousseaux, M.C. Spring Reproductive and Vegetative Phenology of Olive (Olea europaea L.) Cultivars at Different Air Temperatures along a Latitudinal-Altitudinal Gradient in Argentina. Sci. Hortic. 2022, 304, 111327. [Google Scholar] [CrossRef]
- Di Paola, A.; Chiriacò, M.V.; Di Paola, F.; Nieddu, G. A phenological model for olive (Olea europaea L. var europaea) growing in Italy. Plants 2021, 10, 1115. [Google Scholar] [CrossRef]
- Basler, D.; Körner, C. Photoperiod and temperature responses of bud swelling and bud burst in four temperate forest tree species. Tree Physiol. 2014, 34, 377–388. [Google Scholar] [CrossRef]
- CEN/TS 16868:2019; Ambient Air—Sampling and Analysis of Airborne Pollen and Fungal Spores for Aerobiological Monitoring-Volumetric Hirst Method. UNI, Italian National Unification: Milano, Italy, 2019.
- Aguilera, F.; Fornaciari, M.; Ruiz-Valenzuela, L.; Galán, C.; Msallem, M.; Dhiab, A.B.; la Guardia, C.D.-D.; del Mar Trigo, M.; Bonofiglio, T.; Orlandi, F. Phenological models to predict the main flowering phases of olive (Olea europaea L.) along a latitudinal and longitudinal gradient across the Mediterranean region. Int. J. Biometeorol. 2015, 59, 629–641. [Google Scholar] [CrossRef]
- Papadogiannaki, S.; Karatzas, K.; Kontos, S.; Poupkou, A.; Melas, D. A Multi-Model Approach to Pollen Season Estimations: Case Study for Olea and Quercus in Thessaloniki, Greece. Atmosphere 2025, 16, 454. [Google Scholar] [CrossRef]
- Arnold, C.Y. Maximum-minimum temperatures as a basis for computing heat units. Proc. Am. Soc. Hort. Sci. 1960, 76, 682–692. [Google Scholar]
- Meier, U. Growth Stages of Mono and Dicotyledonous Plants; BBCH Monograph; Federal Biological Research Centre for Agriculture and Forestry: Bonn, Germany, 2001. [Google Scholar]


| Meiosis | SP_10% | MP | GDD_Meiosis | |
|---|---|---|---|---|
| Meiosis | 1.00 | |||
| SP_10% | −0.18 | 1.00 | ||
| MP | −0.04 | 0.91 | 1.00 | |
| GDD_Meiosis | 0.65 * | −0.70 ** | −0.55 | 1.00 |
| GDD_SP_10% | 0.24 | 0.83 ** | 0.75 | −0.46 |
| GDD_MP | 0.31 | 0.43 | 0.65 * | −0.25 |
| Δ_GDD_Meiosis_SP_10% | −0.31 | 0.88 ** | 0.74 ** | −0.89 ** |
| Δ_GDD_Meiosis_MP | −0.34 | 0.74 ** | 0.73 ** | −0.88 ** |
| Σ_Rain_Meiosis | −0.62 * | −0.08 | 0.02 | −0.05 |
| Σ_Rain_SP_10% | −0.62 | 0.06 | 0.14 | −0.08 |
| Σ_Rain_MP | −0.54 | −0.01 | 0.12 | 0.02 |
| Δ_Rain_Meiosis_SP_10% | −0.38 | 0.35 | 0.36 | −0.12 |
| Δ_Rain_Meiosis_MP | −0.16 | 0.13 | 0.28 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedeschini, E.; Orlandi, F.; Fornaciari, M. Exploring Reproductive Timing in Olive Tree: Male Meiosis and Anthesis Events. Plants 2025, 14, 2522. https://doi.org/10.3390/plants14162522
Tedeschini E, Orlandi F, Fornaciari M. Exploring Reproductive Timing in Olive Tree: Male Meiosis and Anthesis Events. Plants. 2025; 14(16):2522. https://doi.org/10.3390/plants14162522
Chicago/Turabian StyleTedeschini, Emma, Fabio Orlandi, and Marco Fornaciari. 2025. "Exploring Reproductive Timing in Olive Tree: Male Meiosis and Anthesis Events" Plants 14, no. 16: 2522. https://doi.org/10.3390/plants14162522
APA StyleTedeschini, E., Orlandi, F., & Fornaciari, M. (2025). Exploring Reproductive Timing in Olive Tree: Male Meiosis and Anthesis Events. Plants, 14(16), 2522. https://doi.org/10.3390/plants14162522







