Chemical Constituents, In Silico Studies and In Vitro Antioxidant, Enzyme Inhibitory and Antibacterial Activities of the Algerian Tamarix boveana Essential Oil and Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. T. boveana Essential Oil Chemical Composition
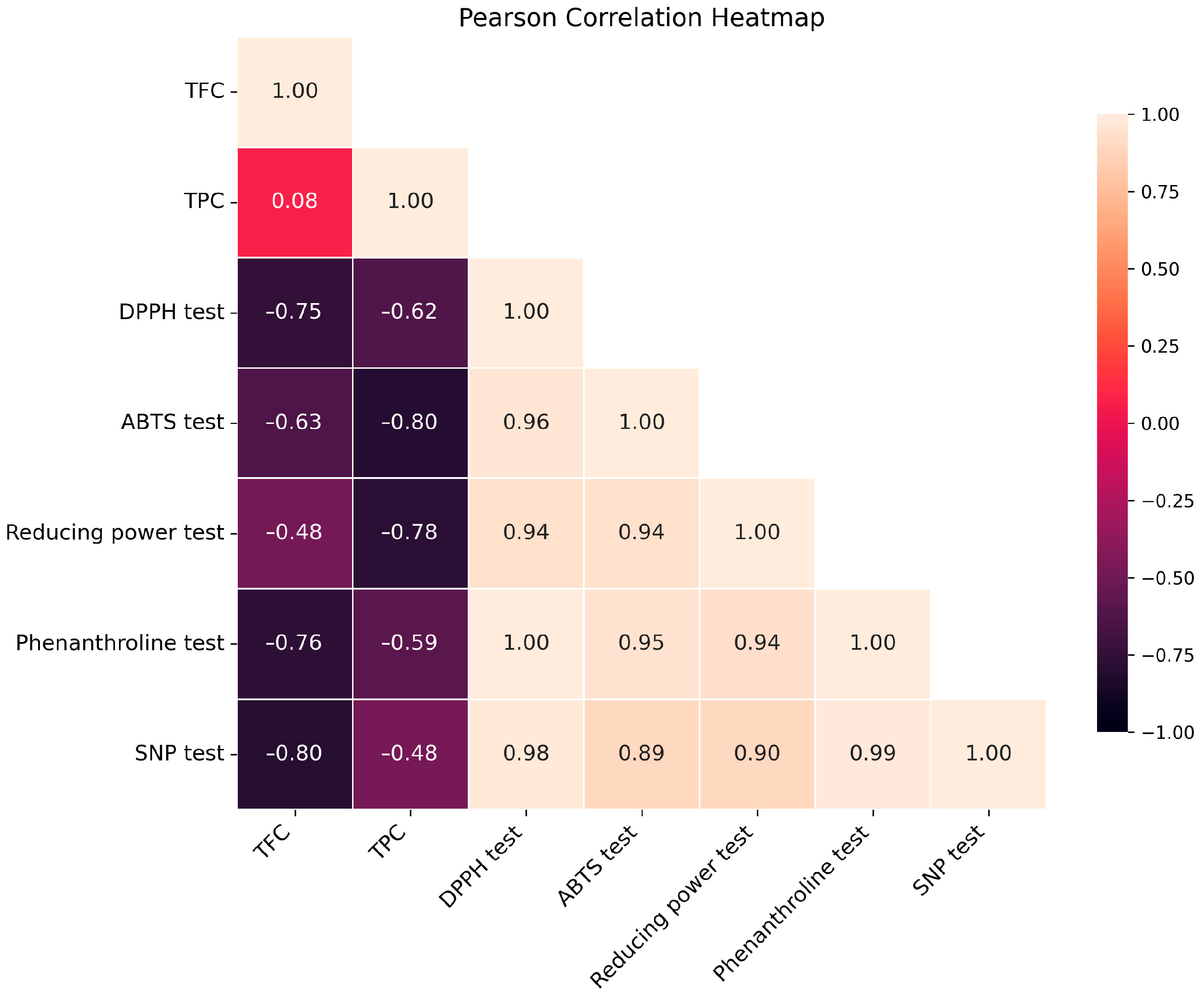
2.2. In Vitro Antioxidant Ability
2.3. Enzyme Inhibition Effects
2.4. Photoprotective Activity
2.5. Antibacterial Activity
2.6. Density Functional Theory Calculation
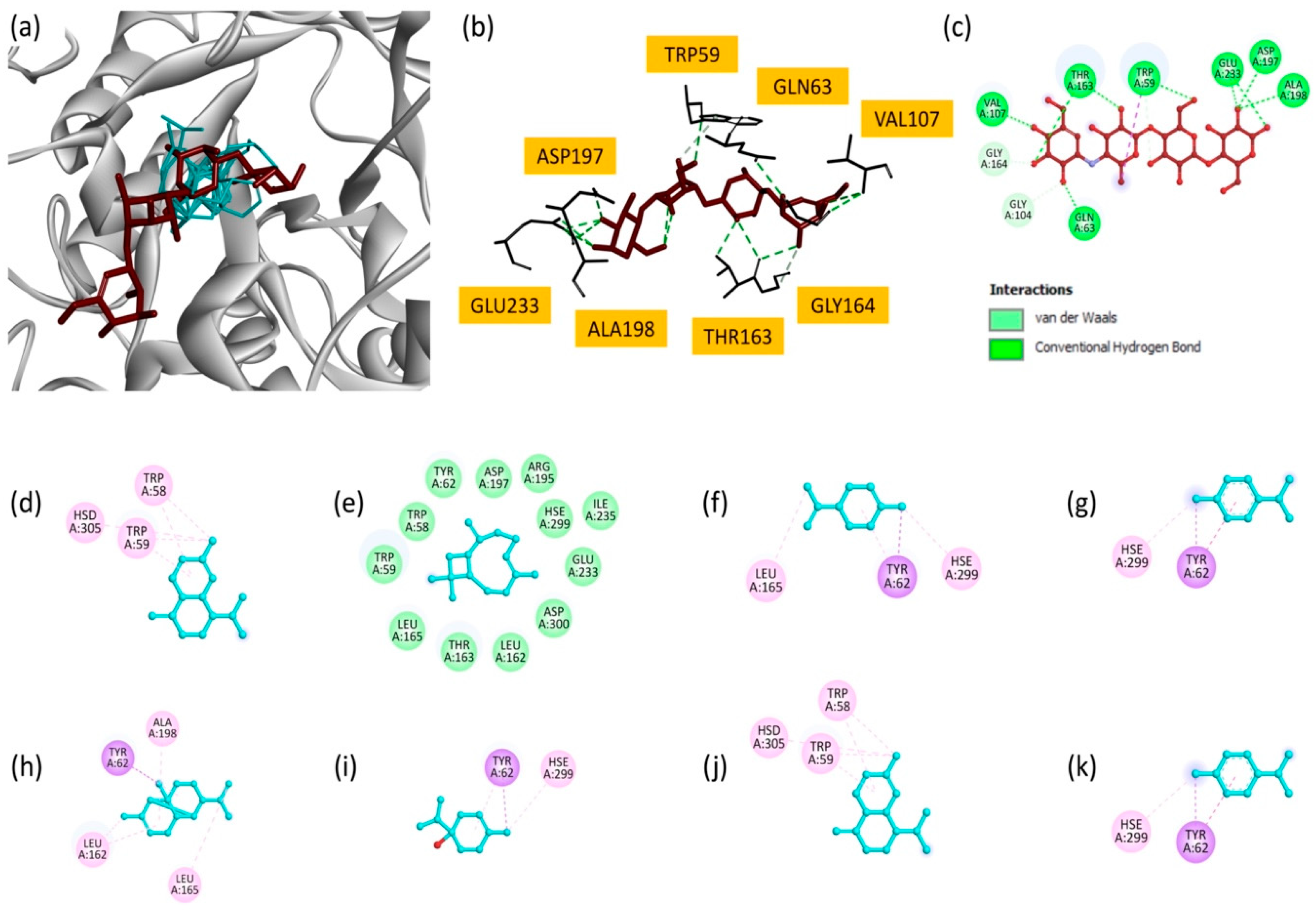
2.7. Docking Studies
3. Conclusions
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Extraction and Organic Extracts Preparation
4.3. GC-FID Analysis
4.4. GC/MS Analysis
4.5. Antioxidant Activity
4.5.1. Total Flavonoid Content
4.5.2. Total Polyphenol Content
4.5.3. Scavenging Activity on DPPH Radical
4.5.4. ABTS Test
4.5.5. Ferric Reducing Antioxidant Power
4.5.6. Phenanthroline Test
4.5.7. Silver Nanoparticles SNP Activity
4.6. Photoprotective Activity (SPF)
4.7. Enzyme Inhibitory Effect
4.7.1. α-Amylase Inhibition
4.7.2. Cholinesterase Inhibition
4.8. Antimicrobial Activity
MBC and MIC Determination
4.9. DFT Calculations
4.10. Molecular Docking
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EO | Essential Oil |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| GC-FID | Gas Chromatography–Flame Ionization Detector |
| CHCl3 | Chloroform |
| AcOEt | Ethyl Acetate |
| n-BuOH | n-Butanol |
| MIC | Minimum Inhibitory Concentration |
| MIB | Minimum Bactericidal Concentration |
| DFT | Density Functional Theory |
| BuChE | Butyryl Cholinesterase Enzyme |
| AD | Alzheimer’s Disease |
| DM | Diabetes Mellitus |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FRAP | Ferric Reducing Antioxidant Power |
| IC50 | Half Maximal Inhibitory Concentration |
| RT | Retention Time |
| RI | Retention Index |
| Relat. | Conc. Relative Concentration Expressed as a Percentage |
| TPC | Total Phenolic Content |
| AGE | Gallic Acid Equivalents |
| QE | Quercetin Equivalents |
| TFC | Total Flavonoids Content |
| ROS | Reactive Oxygen Species |
| SNP | Silver Nanoparticles |
| ABTS | 2,2′-Azino-Bis(3-ethylbenzoThiazoline-6-Sulfonic Acid) |
| BHA | Butylated HydroxyAnisole |
| BHT | Butylated HydroxyToluene |
| SPF | Sun Protection Factor |
| HOMO | Highest-Occupied Molecular Orbital |
| LUMO | Lowest-Occupied Molecular Orbital |
| ESP | Electrostatic Potential Maps |
References
- Butler, M.S. The Role of Natural Product Chemistry in Drug Discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Mici, D.; Ðurovi, S.; Riabov, P.; Tomi, A.; Šovljanski, O.; Filip, S.; Tosti, T.; Dojčinovi, B.; Božovi, R.; Jovanovi, D.; et al. Rosemary Essential Oils as a Promising Source of Bioactive Compounds: Chemical Composition, Thermal Properties, Biological Activity, and Gastronomical Perspectives. Foods 2021, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Salanță, L.C.; Cropotova, J. An Update on Effectiveness and Practicability of Plant Essential Oils in the Food Industry. Plants 2022, 11, 2488. [Google Scholar] [CrossRef]
- Achagar, R.; Ait-Touchente, Z.; El Ati, R.; Boujdi, K.; Thoume, A.; Abdou, A.; Touzani, R. A Comprehensive Review of Essential Oil–Nanotechnology Synergy for Advanced Dermocosmetic Delivery. Cosmetics 2024, 11, 48. [Google Scholar] [CrossRef]
- Rodilla, J.M.; Rosado, T.; Gallardo, E. Essential Oils: Chemistry and Food Applications. Foods 2024, 13, 1074. [Google Scholar] [CrossRef]
- Visan, A.I.; Negut, I. Coatings Based on Essential Oils for Combating Antibiotic Resistance. Antibiotics 2024, 13, 625. [Google Scholar] [CrossRef]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial Effects of Polyphenols on Cardiovascular Disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef]
- Del Rio, D.; Costa, L.G.; Lean, M.E.J.; Crozier, A. Polyphenols and Health: What Compounds Are Involved? Nutr. Metab. Cardiovasc. Dis. 2010, 20, 1–6. [Google Scholar] [CrossRef]
- Ajila, C.M.; Jaganmohan Rao, L.; Prasada Rao, U.J.S. Characterization of Bioactive Compounds from Raw and Ripe Mangifera Indica L. Peel Extracts. Food Chem. Toxicol. 2010, 48, 3406–3411. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Howes, M.J.R.; Houghton, P.J. Plants Used in Chinese and Indian Traditional Medicine for Improvement of Memory and Cognitive Function. Pharmacol. Biochem. Behav. 2003, 75, 513–527. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Etxeberria, U.; De La Garza, A.L.; Campin, J.; Martnez, J.A.; Milagro, F.I. Antidiabetic Effects of Natural Plant Extracts via Inhibition of Carbohydrate Hydrolysis Enzymes with Emphasis on Pancreatic Alpha Amylase. Expert. Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A Review of Alpha-Glucosidase Inhibitors from Plants as Potential Candidates for the Treatment of Type-2 Diabetes; Springer: Dordrecht, The Netherlands, 2022; Volume 21, ISBN 0123456789. [Google Scholar]
- Thompson, S.; Lanctôt, K.L.; Hermann, N. The Benefits and Risks Associated with Cholinesterase Inhibitor Therapy in Alzheimer’s Disease. Expert Opin. Drug Saf. 2004, 3, 425–440. [Google Scholar] [CrossRef]
- Li, F.; Xie, W.; Ding, X.; Xu, K.; Fu, X. Phytochemical and Pharmacological Properties of the Genus Tamarix: A Comprehensive Review. Arch. Pharm. Res. 2024, 47, 410–441. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Xu, X.; Shi, X.; Ji, X.; Wang, Y. Revealing the Salt Tolerance Mechanism of Tamarix Hispida by Large-Scale Identification of Genes Conferring Salt Tolerance. Tree Physiol. 2021, 41, 2153–2170. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, S.; Du, S.; Wang, G.; Zhang, J.; Jiang, J. Effects of Exogenous (K+) Potassium Application on Plant Hormones in the Roots of Tamarix Ramosissima under NaCl Stress. Genes 2022, 13, 1803. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Farzaei, M.H.; Sajadimajd, S.; Iranpanah, A.; Khazaei, M.; Pourjabar, Z.; Hajimahmoodi, M.; Rahimi, R. In Vitro and in Vivo Antidiabetic Activity of Tamarix Stricta Boiss.: Role of Autophagy. J. Ethnopharmacol. 2021, 269, 113692. [Google Scholar] [CrossRef] [PubMed]
- Alnuqaydan, A.M.; Almutary, A.G.; Alsahli, M.A.; Alnasser, S.; Rah, B. Tamarix Articulata Induced Prevention of Hepatotoxicity Effects of In Vivo Carbon Tetrachloride by Modulating Pro-Inflammatory Serum and Antioxidant Enzymes to Reverse the Liver Fibrosis. Antioxidants 2022, 11, 1824. [Google Scholar] [CrossRef]
- Alnuqaydan, A.M.; Rah, B. Tamarix Articulata (T. Articulata)—An Important Halophytic Medicinal Plant with Potential. Pharmacological Properties. Curr. Pharm. Biotechnol. 2019, 20, 285–292. [Google Scholar] [CrossRef]
- Alnuqaydan, A.M.; Ali Zainy, F.M.; Almutary, A.G.; Sadier, N.S.; Rah, B. Tamarix Articulata Extract Offers Protection against Toxicity Induced by Beauty Products in Hs27 Human Skin Fibroblasts. PLoS ONE 2023, 18, e0287071. [Google Scholar] [CrossRef]
- Alshehri, S.A.; Wahab, S.; Abullais, S.S.; Das, G.; Hani, U.; Ahmad, W.; Amir, M.; Ahmad, A.; Kandasamy, G.; Vasudevan, R. Pharmacological Efficacy of Tamarix Aphylla: A Comprehensive Review. Plants 2022, 11, 118. [Google Scholar] [CrossRef]
- Fayez, N.; Khalil, W.; Abdel-Sattar, E.; Abdel-Fattah, A.F.M. Involvement of TNFα, IL-1β, COX-2 and NO in the Anti-Inflammatory Activity of Tamarix Aphylla in Wistar Albino Rats: An in-Vivo and in-Vitro Study. BMC Complement. Med. Ther. 2024, 24, 57. [Google Scholar] [CrossRef] [PubMed]
- Saïdana, D.; Mahjoub, M.A.; Boussaada, O.; Chriaa, J.; Chéraif, I.; Daami, M.; Mighri, Z.; Helal, A.N. Chemical Composition and Antimicrobial Activity of Volatile Compounds of Tamarix Boveana (Tamaricaceae). Microbiol. Res. 2008, 163, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Saïdana, D.; Ben Halima-Kamel, M.; Mahjoub, M.A.; Haouas, D.; Mighri, Z.; Helal, A.N. Insecticidal Activities of Tunisian Halophytic Plant Extracts against Larvae and Adults of Tribolium Confusum. Tropicultura 2007, 25, 193–199. [Google Scholar]
- Mennai, I.; Lamera, E.; Slougui, N.; Benaicha, B.; Gasmi, S.; Samai, Z.; Rahmouni, N.; Bensouici, C.; Pinto, D.C.G.A. Chemical Composition and Antioxidant, Antiparasitic, Cytotoxicity and Antimicrobial Potential of the Algerian Limonium Oleifolium Mill. Essential Oil and Organic Extracts. Chem. Biodivers. 2021, 18, e2100278. [Google Scholar] [CrossRef]
- Mennai, I.; Hanfer, M.; Esseid, C.; Benayache, S.; Ameddah, S.; Menad, A.; Benayache, F. Chemical Composition, in Vitro Antiparasitic, Antimicrobial and Antioxidant Activities of Frankenia Thymifolia Desf. Nat. Prod. Res. 2020, 34, 3363–3368. [Google Scholar] [CrossRef]
- Rahmouni, N.; Pinto, D.C.G.A.; Santos, S.A.O.; Beghidja, N.; Silva, A.M.S. Lipophilic Composition of Scabiosa Stellata L.: An Underexploited Plant from Batna (Algeria). Chem. Pap. 2018, 72, 753–762. [Google Scholar] [CrossRef]
- Alhourani, N.; Kasabri, V.; Bustanji, Y.; Abbassi, R.; Hudaib, M. Potential Antiproliferative Activity and Evaluation of Essential Oil Composition of the Aerial Parts of Tamarix Aphylla (L.) H.Karst.: A Wild Grown Medicinal Plant in Jordan. Evid. Based Complement. Alternat. Med. 2018, 2018, 9363868. [Google Scholar] [CrossRef]
- Ghaffari, Z.; Rahimmalek, M.; Sabzalian, M.R. Variations in Essential Oil Composition and Antioxidant Activity in Perovskia Abrotanoides Kar. Collected from Different Regions in Iran. Chem. Biodivers. 2018, 15, e1700565. [Google Scholar] [CrossRef]
- Sözmen, F.; Uysal, B.; Köse, E.O.; Aktaş, Ö.; Cinbilgel, I.; Oksal, B.S. Extraction of the Essential Oil from Endemic Origanum Bilgeri P.H. Davis with Two Different Methods: Comparison of the Oil Composition and Antibacterial Activity. Chem. Biodivers. 2012, 9, 1356–1363. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour. Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria Crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Deen, J.I.; Zawad, A.N.M.S.; Uddin, M.; Chowdhury, M.A.H.; Al Araby, S.Q.; Rahman, M.A. Terpinen-4-Ol, A Volatile Terpene Molecule, Extensively Electrifies the Biological Systems against the Oxidative Stress-Linked Pathogenesis. Adv. Redox Res. 2023, 9, 100082. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, Y.W.; Hou, C.Y. Antioxidant and Antibacterial Activity of Seven Predominant Terpenoids. Int. J. Food Prop. 2019, 22, 230–238. [Google Scholar] [CrossRef]
- Santos, E.L.; Freitas, P.R.; Araújo, A.C.J.; Almeida, R.S.; Tintino, S.R.; Paulo, C.L.R.; Silva, A.C.A.; Silva, L.E.; do Amaral, W.; Deschamps, C.; et al. Enhanced Antibacterial Effect of Antibiotics by the Essential Oil of Aloysia Gratissima (Gillies & Hook.) Tronc. and Its Major Constituent Beta-Caryophyllene. Phytomedicine Plus 2021, 1, 100100. [Google Scholar] [CrossRef]
- Prerna, P.; Chadha, J.; Khullar, L.; Mudgil, U.; Harjai, K. A Comprehensive Review on the Pharmacological Prospects of Terpinen-4-Ol: From Nature to Medicine and Beyond. Fitoterapia 2024, 176, 106051. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Saha, S.; Walia, S.; Ahluwalia, V.; Kaur, C. Antioxidant Potential of Essential Oil and Cadinene Sesquiterpenes of Eupatorium Adenophorum. Toxicol. Environ. Chem. 2013, 95, 127–137. [Google Scholar] [CrossRef]
- Nakamura, M.; Ra, J.H.; Jee, Y.; Kim, J.S. Impact of Different Partitioned Solvents on Chemical Composition and Bioavailability of Sasa Quelpaertensis Nakai Leaf Extract. J. Food Drug Anal. 2017, 25, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Jayakody, J.T.M.; Kim, J.I.; Jeong, J.W.; Choi, K.M.; Kim, T.S.; Seo, C.; Azimi, I.; Hyun, J.M.; Ryu, B.M. The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review. Foods 2024, 13, 3151. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between Phenolic Content and Antioxidant Properties in Twenty-Four Plant Species of Traditional Ethnoveterinary Use in the Mediterranean Area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Naija, D.S.; Helal, A.N. The Antioxidant and Free-Radical Scavenging Activities of Tamarix Boveana and Suaeda Fruticosa Fractions and Related Active Compound. Eur. Sci. J. 2014, 10, 201–219. [Google Scholar]
- Spinelli, R.; Sanchis, I.; Aimaretti, F.M.; Attademo, A.M.; Portela, M.; Humpola, M.V.; Tonarelli, G.G.; Siano, A.S. Natural Multi-Target Inhibitors of Cholinesterases and Monoamine Oxidase Enzymes with Antioxidant Potential from Skin Extracts of Hypsiboas Cordobae and Pseudis Minuta (Anura: Hylidae). Chem. Biodivers. 2019, 16, e1800472. [Google Scholar] [CrossRef]
- Srief, M.; Bani, M.; Mokrani, E.H.; Mennai, I.; Hamdi, M.; Boumechhour, A.; Mustapha, M.A.; Derdour, M.; Kerkatou, M.; El-Shazly, M.; et al. Evaluation of In Vitro and In Silico Anti-Alzheimer Potential of Nonpolar Extracts and Essential Oil from Mentha Piperita. Foods 2023, 12, 190. [Google Scholar] [CrossRef]
- Arya, A.; Chahal, R.; Rao, R.; Rahman, M.H.; Kaushik, D.; Akhtar, M.F.; Saleem, A.; Khalifa, S.M.A.; El-Seedi, H.R.; Kamel, M.; et al. Acetylcholinesterase Inhibitory Potential of Various Sesquiterpene Analogues for Alzheimer’s Disease Therapy. Biomolecules 2021, 11, 350. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Alqahtani, Y.S.; Alyami, B.A.; Alqarni, A.O.; Ayaz, M.; Ghufran, M.; Ullah, F.; Sadiq, A.; Ullah, I.; Haq, I.U.; et al. Phytochemical Analysis, α-Glucosidase and Amylase Inhibitory, and Molecular Docking Studies on Persicaria Hydropiper L. Leaves Essential Oils. Evid. Based Complement. Alternat. Med. 2022, 2022, 7924171. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Nazzaro, F. Anti-Cholinesterase and Anti-α-Amylase Activities and Neuroprotective Effects of Carvacrol and p-Cymene and Their Effects on Hydrogen Peroxide Induced Stress in SH-SY5Y Cells. Int. J. Mol. Sci. 2023, 24, 6073. [Google Scholar] [CrossRef]
- Mahfoudhi, A.; Grosso, C.; Gonçalves, R.F.; Khelifi, E.; Hammami, S.; Achour, S.; Trabelsi-Ayadi, M.; Valentão, P.; Andrade, P.B.; Mighri, Z. Evaluation of Antioxidant, Anticholinesterase, and Antidiabetic Potential of Dry Leaves and Stems in Tamarix Aphylla Growing Wild in Tunisia. Chem. Biodivers. 2016, 13, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- El Aanachi, S.; Gali, L.; Nacer, S.N.; Bensouici, C.; Dari, K.; Aassila, H. Phenolic Contents and in Vitro Investigation of the Antioxidant, Enzyme Inhibitory, Photoprotective, and Antimicrobial Effects of the Organic Extracts of Pelargonium Graveolens Growing in Morocco. Biocatal. Agric. Biotechnol. 2020, 29, 101819. [Google Scholar] [CrossRef]
- Li, L.; Chong, L.; Huang, T.; Ma, Y.; Li, Y.; Ding, H. Natural Products and Extracts from Plants as Natural UV Filters for Sunscreens: A Review. Animal. Model. Exp. Med. 2023, 6, 183–195. [Google Scholar] [CrossRef]
- Boulebd, H. Comparative Study of the Radical Scavenging Behavior of Ascorbic Acid, BHT, BHA and Trolox: Experimental and Theoretical Study. J. Mol. Struct. 2020, 1201, 127210. [Google Scholar] [CrossRef]
- Bajda, M.; Więckowska, A.; Hebda, M.; Guzior, N.; Sotriffer, C.A.; Malawska, B. Structure-Based Search for New Inhibitors of Cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608–5632. [Google Scholar] [CrossRef]
- Evaristus, N.A.; Abdullah, W.N.W.; Gan, C.-Y. Extraction and Identification of α-Amylase Inhibitor Peptides from Nephelium Lappacheum and Nephelium Mutabile Seed Protein Using Gastro-Digestive Enzymes. Peptides 2018, 102, 61–67. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oils by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Topçu, G.; Ay, M.; Bilici, A.; Sarikürkcü, C.; Öztürk, M.; Ulubelen, A. A New Flavone from Antioxidant Extracts of Pistacia Terebinthus. Food Chem. 2007, 103, 816–822. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on Products of Browning Reaction Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szłyk, E. Determination of Antioxidant Capacities of Vegetable Oils by Ferric-Ion Spectrophotometric Methods. Talanta 2008, 76, 899–905. [Google Scholar] [CrossRef]
- Özyürek, M.; Güngör, N.; Baki, S.; Güçlü, K.; Apak, R. Development of a Silver Nanoparticle-Based Method for the Antioxidant Capacity Measurement of Polyphenols. Anal. Chem. 2012, 84, 8052–8059. [Google Scholar] [CrossRef]
- Mansur, J.d.S.; Breder, M.N.; Mansur, M.C.; Azulay, R.D. Determination of Sun Protection Factor by Spectrophotometry. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Dutra, E.A.; Oliveira, D.A.G.D.C.E.; Kedor-Hackmann, E.R.M.; Santoro, M.I.R.M. Determination of Sun Protection Factor (SPF) of Sunscreens by Ultraviolet Spectrophotometry. Rev. Bras. Cienc. Farm. 2004, 40, 381–385. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R. Sideritis Galatica Bornm.: A Source of Multifunctional Agents for the Management of Oxidative Damage, Alzheimer’s’s and Diabetes Mellitus. J. Funct. Foods 2014, 11, 538–547. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y. Chemical Composition and Antibacterial Activity of Essential Oils from Different Parts of Litsea Cubeba. Chem. Biodivers. 2010, 7, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.; López, S.; Luna, L.; Aguero, M.B.; Aragón, L.; Tapia, A.; Zacchino, S.; López, M.L.; Zygadlo, J.; Feresin, G.E. Essential Oils of Medicinal Plants from the Central Andes of Argentina: Chemical Composition, and Antifungal, Antibacterial, and Insect-Repellent Activities. Chem. Biodivers. 2011, 8, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Syu, W.; Shen, C.; Lu, J.; Lee, G.; Sun, C. Antimicrobial and Cytotoxic Activities of Neolignans from Magnolia Officinalis. Chem. Biodivers. 2004, 1, 530–537. [Google Scholar] [CrossRef]
- Frisch, M.J. Gaussian 09, Revision D. 01/Gaussian 2009; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, T. Efficient Evaluation of Electrostatic Potential with Computerized Optimized Code. Phys. Chem. Chem. Phys. 2021, 23, 20323–20328. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G. Incorporation of by-products of rosemary and thyme in the diet of ewes: Effect on the fatty acid profile of lamb. Eur. Food Res. Technol. 2013, 236, 379–389. [Google Scholar] [CrossRef]
- Nieto, G.; Banón, S.; Garrido, M. Administration of distillate thyme leaves into the diet of Segureña ewes: Effect on lamb meat quality. Animal 2012, 6, 2048–2056. [Google Scholar] [CrossRef]
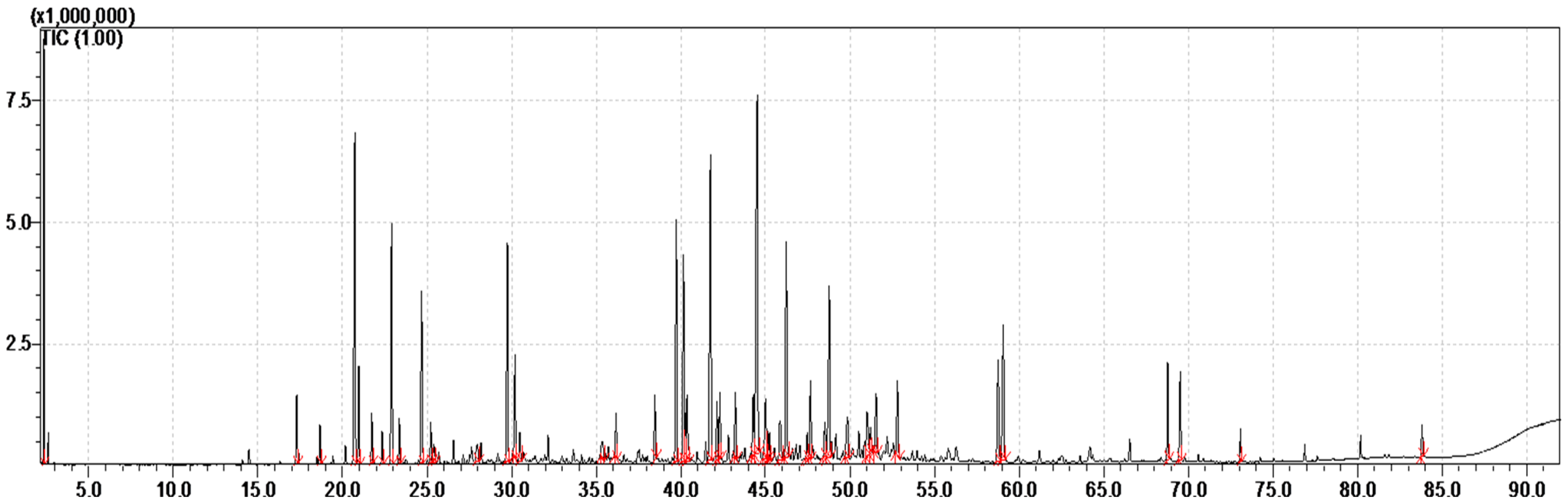



| No. | RT [a] | RIcalc [b] | RIlit [c] | Compunds [d] | Relat. Conc [e] |
|---|---|---|---|---|---|
| 01 | 2.021 | 923 | 927 | Tricyclene | 1.38 ± 0.04 |
| 02 | 14.475 | 933 | 939 | α-Pinene | 2.59 ± 0.18 |
| 03 | 14.480 | 942 | - | NI [f] | 0.42 ± 0.02 |
| 04 | 17.301 | 964 | 1029 | β-Phellandrene | 1.12 ± 0.08 |
| 05 | 18.677 | 1018 | 1019 | α-Terpinene | 0.56 ± 0.02 |
| 06 | 20.131 | 1022 | - | NI | 0.82 ± 0.04 |
| 07 | 20.738 | 1025 | 1027 | p-Cymene | 6.16 ± 0.44 |
| 08 | 21.967 | 1030 | 1032 | Limonene | 6.50 ± 0.86 |
| 09 | 22.354 | 1046 | 1040 | (Z)-β-Ocimene | 0.46 ± 0.02 |
| 10 | 22.918 | 1058 | 1062 | γ-Terpinene | 4.11 ± 0.93 |
| 11 | 24.685 | 1087 | 1089 | Terpinolene | 3.13 ± 0.95 |
| 12 | 25.217 | 1102 | 1102 | Linalool | 0.62 ± 0.05 |
| 13 | 25.392 | 1122 | 1124 | (Z)-p-Mentha-2,8-dien-1-ol | 0.42 ± 0.09 |
| 14 | 26.612 | 1129 | - | NI | 1.50 ± 0.07 |
| 15 | 27.984 | 1136 | 1143 | Trans-verbenol | 0.47 ± 0.03 |
| 16 | 29.753 | 1137 | 1175 | Terpinen-4-ol | 4.23 ± 0.28 |
| 17 | 30.204 | 1197 | 1185 | Cymen-8-ol | 1.72 ± 0.05 |
| 18 | 30.476 | 1198 | 1192 | α-terpineol | 0.53 ± 0.07 |
| 19 | 32.034 | 1120 | - | NI | 1.60 ± 0.10 |
| 20 | 35.380 | 1250 | 1256 | Carvotanacetone | 0.51 ± 0.01 |
| 21 | 36.173 | 1262 | 1290 | Thymol | 0.65 ± 0.02 |
| 22 | 38.469 | 1344 | 1348 | α-Cubebene | 1.00 ± 0.04 |
| 23 | 39.736 | 1375 | 1378 | Copaene | 4.37 ± 0.32 |
| 24 | 40.160 | 1382 | 1387 | β-bourbonene | 3.74 ± 0.45 |
| 25 | 40.371 | 1435 | 1474 | γ-Muurolene | 1.36 ± 0.07 |
| 26 | 41.765 | 1414 | 1417 | β-Caryophyllene | 6.71 ± 0.83 |
| 27 | 42.142 | 1454 | 1456 | α-Humulene | 0.96 ± 0.02 |
| 28 | 42.315 | 1464 | 1467 | cis-Muurola-4 (14), 5-diene | 1.05 ± 0.03 |
| 29 | 43.227 | 1485 | 1486 | Germacrene D | 1.05 ± 0.06 |
| 30 | 44.286 | 1502 | 1510 | β-Bisabolene | 1.16 ± 0.09 |
| 31 | 45.525 | 1512 | 1515 | γ-Cadinene | 9.41 ± 1.02 |
| 32 | 46.867 | 1515 | 1518 | α-Alaskene | 1.13 ± 0.32 |
| 33 | 46.238 | 1518 | 1520 | δ-Cadinene | 4.21 ± 0.62 |
| 34 | 47.483 | 1537 | 1538 | α-Cadinene | 0.44 ± 0.03 |
| 35 | 47.672 | 1557 | 1560 | Germacrene –B- | 1.31 ± 0.04 |
| 36 | 48.522 | 1576 | 1578 | Spathulenol | 0.66 ± 0.08 |
| 37 | 48.771 | 1587 | 1583 | Caryophyllene oxide | 3.54 ± 0.92 |
| 38 | 49.852 | 1595 | 1635 | σ-Cadinol | 1.08 ± 0.08 |
| 39 | 50.737 | 1615 | - | NI | 1.26 ± 0.15 |
| 40 | 51.003 | 1631 | 1642 | trans-Muurulol | 1.15 ± 0.06 |
| 41 | 51.217 | 1662 | 1660 | α-Cadinol | 0.45 ± 0.05 |
| 42 | 51.540 | 1681 | 1692 | Acorenone | 1.24 ± 0.12 |
| 43 | 52.800 | 1699 | 1685 | Edusma-4 (15),7-diene-1-β-ol | 1.35 ± 0.22 |
| 44 | 55.813 | 1720 | - | NI | 0.06 ± 0.02 |
| 45 | 56.232 | 1728 | - | NI | 0.16± 0.01 |
| 46 | 58.752 | 1774 | 1827 | Neophytadiene | 2 ± 0.31 |
| 47 | 59.060 | 1841 | 1845 | Phytone | 3 ± 0.73 |
| 48 | 59.914 | 1853 | - | NI | 0.06 ± 0.01 |
| 49 | 61.102 | 1886 | - | NI | 0.10 ± 0.02 |
| 50 | 62.561 | 1903 | - | NI | 0.04 ± 0.002 |
| 51 | 63.765 | 1934 | - | NI | 0.02 ± 0.001 |
| 52 | 64.131 | 1961 | - | NI | 0.06 ± 0.01 |
| 53 | 66.425 | 2005 | - | NI | 1.20 ± 0.24 |
| 54 | 68.153 | 2035 | - | NI | 0.02 ± 0.003 |
| 55 | 68.781 | 2045 | 2110 | Phytol | 1.3 ± 0.08 |
| 56 | 69.532 | 2059 | 2140 | Osthole | 1.46 ± 0.12 |
| 57 | 70.821 | 2130 | - | NI | 0.02 ± 0.005 |
| 58 | 73.075 | 2407 | 2400 | Tetracosane | 0.40 ± 0.02 |
| 59 | 76.931 | 2841 | - | NI | 0.08 ± 0.01 |
| 60 | 80.123 | 3230 | - | NI | 1.40 ± 0.11 |
| 61 | 83.807 | 3600 | 3600 | Hexatriacontane | 0.49 ± 0.03 |
| Hydrocarbon monoterpenes | 26.01 | ||||
| Oxygenated monoterpenes | 9.15 | ||||
| Hydrocarbon sesquiterpenes | 37.9 | ||||
| Oxygenated sesquiterpenes | 9.47 | ||||
| Other | 8.65 | ||||
| Unidentified compounds (%) | 8.82 | ||||
| Number identified | 44 | ||||
| Total identified (%) | 91.18 |
| DPPH Test IC50 [a] | ABTS Test IC50 [a] | Reducing Power Test A0.5 [a] | Phenanthroline Test A0.5 [a] | SNP Test A0.5 [a] | |
|---|---|---|---|---|---|
| Essential oil | 223.59 ± 1.01 | 593.33 ± 15.65 | 442.67 ± 14.85 | 746.25 ± 12.37 | 205.67 ± 0.88 |
| Hydroalcoholic extract | 64.16 ± 1.77 | 167.093 ± 3.53 | 33.02 ± 0.81 | 11.64 ± 2.88 | 44.31 ± 0.06 |
| CHCl3 extract | 131.68 ± 0.01 | 410.07 ± 8.07 | 174.41 ± 8.35 | 30.08 ± 1.83 | 176.5 ± 0.50 |
| AcOEt extract | 91.34 ± 0.65 | 315.777 ± 17.18 | 145.33 ± 6.75 | 18.67 ± 0.60 | 84.92 ± 3.79 |
| n-BuOH extract | 47.45 ± 4.82 | 9.17 ± 0.8 | 23.42 ± 0.5 | 7.40 ± 0.82 | 32.21 ± 0.73 |
| BHA [b] | 6.14 ± 0.41 | 1.81 ± 0.10 | 7.99 ± 0.87 | 0.93 ± 0.07 | 73.47 ± 0.88 |
| BHT [c] | 12.99 ± 0.41 | 1.29 ± 0.30 | 152.24 ± 2.43 | 2.24 ± 0.17 | >200 |
| α-Tocopherol | 13.02 ± 5.17 | 7.59 ± 0.53 | 34.93 ± 2.38 | 5.78 ± 0.30 | 63.41 ± 4.39 |
| Tannic acid | 7.74 ± 0.19 | 1.01 ± 0.16 | 41.07 ± 2.36 | - | - |
| Ascorbic acid | 13.94 ± 2.81 | 1.74 ± 0.10 | 6.37 ± 0.42 | 8.30 ± 0.76 | >200 |
| DPPH Test | ABTS Test | Reducing Power Test | Phenanthroline Test | SNP Test | |
|---|---|---|---|---|---|
| DPPH test | 1 | ||||
| ABTS test | 0.986915566 | 1 | |||
| Reducing power test | 0.929473080 | 0.960317460 | 1 | ||
| Phenanthroline test | 0.790179245 | 0.861792735 | 0.842666085 | 1 | |
| SNP test | 0.830664209 | 0.907226194 | 0.915478964 | 0.948088307 | 1 |
| Extract | Total Phenolic Content (μg GAE/mg) | Total Flavonoid Content (μg QE/mg) |
|---|---|---|
| Hydroalcoholic extract | 391.26 ± 2.8 | 120.31 ± 0.28 |
| CHCl3 extract | 303.85 ± 2.8 | 114.79 ± 0.22 |
| AcOEt extract | 119.44 ± 1.02 | 79.48 ± 0.83 |
| n-BuOH extract | 563.70 ± 3.40 | 124.79 ± 0.26 |
| SPF1 | SPF2 | SPF3 | Mean ± SD | |
|---|---|---|---|---|
| Essential oil | 8.25 | 9.85 | 9.92 | 9.08 ± 1.18 |
| Hydroalcoholic extract | 46.10 | 46.07 | 46.13 | 46.10 ± 0.03 |
| CHCl3 extract | 41.28 | 41.58 | 40.98 | 41.28 ± 0.3 |
| AcOEt extract | 37.22 | 38.47 | 36.14 | 37.25 ± 1.25 |
| n-BuOH extract | 46.40 | 46.40 | 46.40 | 46.40 ± 0.00 |
| Vichy sunscreen | 44.11 | 44.33 | 44.22 | 44.22 ± 0.1 |
| Nivea sunscreen | 50.11 | 49.89 | 50.33 | 50.11 ± 0.22 |
| Bacteria Strain | Essential Oil | Hydroalcoholic Extract | CHCl3 Extract | AcOEt Extract | n-BuOH Extract | Control [a] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC [b] μg/mL | MBC [b] μg/mL | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Staphyloccocus aureus (ATCC 25923) | 1.5 ± 0.2 | 8 ± 0.6 | 6 ± 0.6 | 20 ± 0.2 | 3 ± 0.1 | 5 ± 0.3 | 3 ± 0.2 | 7 ± 0.9 | 6 ± 0.4 | 18 ± 2.4 | 2 ± 0.2 | 6 ± 0.4 |
| Micrococcus luteus strain MM DSM 113600 | 1.5 ± 0.1 | 4 ± 0.3 | 3 ± 0.1 | 10 ± 0.2 | 1.5± 0.1 | 8 ± 0.6 | 24 ± 0.2 | 50 ± 5.2 | 3 ± 0.2 | 10 ± 1.2 | 2 ± 0.1 | 6 ± 0.3 |
| Pseudomonas aeruginosa (ATCC 27853) | 3 ± 0.3 | 5 ± 0.4 | 48 ± 2.51 | 80 ± 4.74 | 6 ± 0.9 | 20 ± 3.2 | 12 ± 1.25 | 36 ± 3.4 | 48 ± 4.5 | 90 ± 5.2 | 8 ± 0.3 | 14 ± 1.85 |
| Escherichia coli (ATCC 25922) | 3 ± 0.6 | 8 ± 0.7 | 12 ± 1.2 | 36 ± 4.2 | 6 ± 0.8 | 17 ± 0.2 | 24 ± 2.23 | 72 ± 4.2 | 6 ± 0.4 | 10 ± 0.4 | 15 ± 3.1 | 23 ± 4.15 |
| Name | Docking Score kcal/mol | Interaction Types | Interacting Residues | Interaction Distance/Å |
|---|---|---|---|---|
| γ-Cadinene | −7.6 | Pi–alkyl | HSD438 | 4.90 |
| Pi–sigma, Pi–alkyl | TRP82 | 3.58, 5.14, 4.12 | ||
| β-caryophyllene | −7.5 | Pi–sigma | TRP82 | 3.76, 3.86 |
| Pi–alkyl | TYR332 | 5.30 | ||
| Pi–alkyl | ALA328 | 4.30 | ||
| Limonene | −5.9 | Pi–alkyl, alkyl | TRP82 | 4.21, 4.47 |
| Pi–alkyl, alkyl | HSD438 | 5.17, 5.48 | ||
| Pi–alkyl, alkyl | MET437 | 5.48 | ||
| Pi–alkyl, alkyl | TYR440 | 5.17 | ||
| Pi–alkyl, alkyl | ALA328 | 4.26 | ||
| p-Cymene | −6.1 | Pi–sigma, Pi–alkyl | TRP82 | 4.15, 5.32 |
| Pi–sigma | HSD438 | 5.73 | ||
| Pi–alkyl | ALA328 | 4.37 | ||
| Copaene | −7.3 | Pi–sigma, Pi–alkyl | TRP82 | 3.77, 5.20, 4.77 |
| Pi–alkyl | TYR332 | 5.29 | ||
| Pi–alkyl | HSD438 | 5.27 | ||
| Terpinen-4-ol | −5.9 | Pi–alkyl | TRP82 | 3.88, 5.22 |
| Pi–alkyl | ALA328 | 4.38 | ||
| δ-Cadinene | −7.6 | Pi–alkyl | HSD438 | 4.72 |
| Pi–sigma, Pi–alkyl | TRP82 | 3.74, 4.60, 4.52, 4.16 | ||
| γ-Terpinene | −6.1 | Pi–sigma, Pi–alkyl | TRP82 | 4.16, 5.26 |
| Pi–sigma | HSD438 | 5.74 | ||
| Pi–alkyl | ALA328 | 4.34 |
| Name | Docking Score kcal/mol | Interaction Nature | Interacting Residues | Interaction Distance/Å |
|---|---|---|---|---|
| γ-Cadinene | −7.3 | Pi–alkyl | TRP58 | 5.08, 5.27 |
| Pi–alkyl | TRP59 | 4.67, 4.52 | ||
| Pi–alkyl | HSD305 | 5.13 | ||
| β-caryophyllene | −6.9 | - | - | - |
| Limonene | −5.8 | Pi–sigma, Pi–alkyl | TYR62 | 3.94, 4.61 |
| Pi–alkyl | LEU165 | 4.77 | ||
| Pi–alkyl | HSE299 | 4.84 | ||
| p-Cymene | −5.9 | Pi–sigma, Pi–alkyl | TYR62 | 3.87, 4.47 |
| Pi–alkyl | HSE299 | 4.74 | ||
| Copaene | −7.3 | Pi–sigma, Pi–alkyl | TYR62 | 3.90, 5.25 |
| Pi–alkyl | ALA198 | 4.73 | ||
| Pi–alkyl | LEU165 | 4.98 | ||
| Pi–alkyl | LEU162 | 5.25, 5.27 | ||
| Terpinen-4-ol | −5.1 | Pi–sigma, Pi–alkyl | TYR62 | 3.84, 4.42 |
| Pi–alkyl | HSE299 | 4.90 | ||
| δ-Cadinene | −7.0 | Pi–alkyl | TRP59 | 4.98 |
| Pi–alkyl | TRP58 | 5.20 | ||
| Pi–alkyl | HSE299 | 4.58 | ||
| Pi–sigma, Pi–alkyl | TYR62 | 3.86, 4.97 | ||
| γ-Terpinene | −5.9 | Pi–sigma, Pi–alkyl | TYR62 | 3.90, 4.46 |
| Pi–alkyl | HSE299 | 4.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamera, E.; Mennai, I.; Slougui, N.; Bensouici, C.; Hazmoune, H.; Boulebd, H.; Akkal, S.; Nieto, G. Chemical Constituents, In Silico Studies and In Vitro Antioxidant, Enzyme Inhibitory and Antibacterial Activities of the Algerian Tamarix boveana Essential Oil and Extracts. Plants 2025, 14, 2497. https://doi.org/10.3390/plants14162497
Lamera E, Mennai I, Slougui N, Bensouici C, Hazmoune H, Boulebd H, Akkal S, Nieto G. Chemical Constituents, In Silico Studies and In Vitro Antioxidant, Enzyme Inhibitory and Antibacterial Activities of the Algerian Tamarix boveana Essential Oil and Extracts. Plants. 2025; 14(16):2497. https://doi.org/10.3390/plants14162497
Chicago/Turabian StyleLamera, Esma, Imad Mennai, Nabila Slougui, Chawki Bensouici, Hichem Hazmoune, Houssem Boulebd, Salah Akkal, and Gema Nieto. 2025. "Chemical Constituents, In Silico Studies and In Vitro Antioxidant, Enzyme Inhibitory and Antibacterial Activities of the Algerian Tamarix boveana Essential Oil and Extracts" Plants 14, no. 16: 2497. https://doi.org/10.3390/plants14162497
APA StyleLamera, E., Mennai, I., Slougui, N., Bensouici, C., Hazmoune, H., Boulebd, H., Akkal, S., & Nieto, G. (2025). Chemical Constituents, In Silico Studies and In Vitro Antioxidant, Enzyme Inhibitory and Antibacterial Activities of the Algerian Tamarix boveana Essential Oil and Extracts. Plants, 14(16), 2497. https://doi.org/10.3390/plants14162497








