Morphological, Pathogenic and Molecular Characterization of Sclerotinia sclerotiorum, the Causal Agent of White Rot of Cabbage (Brassica oleracea var. capitata), in Serbia
Abstract
1. Introduction
2. Results
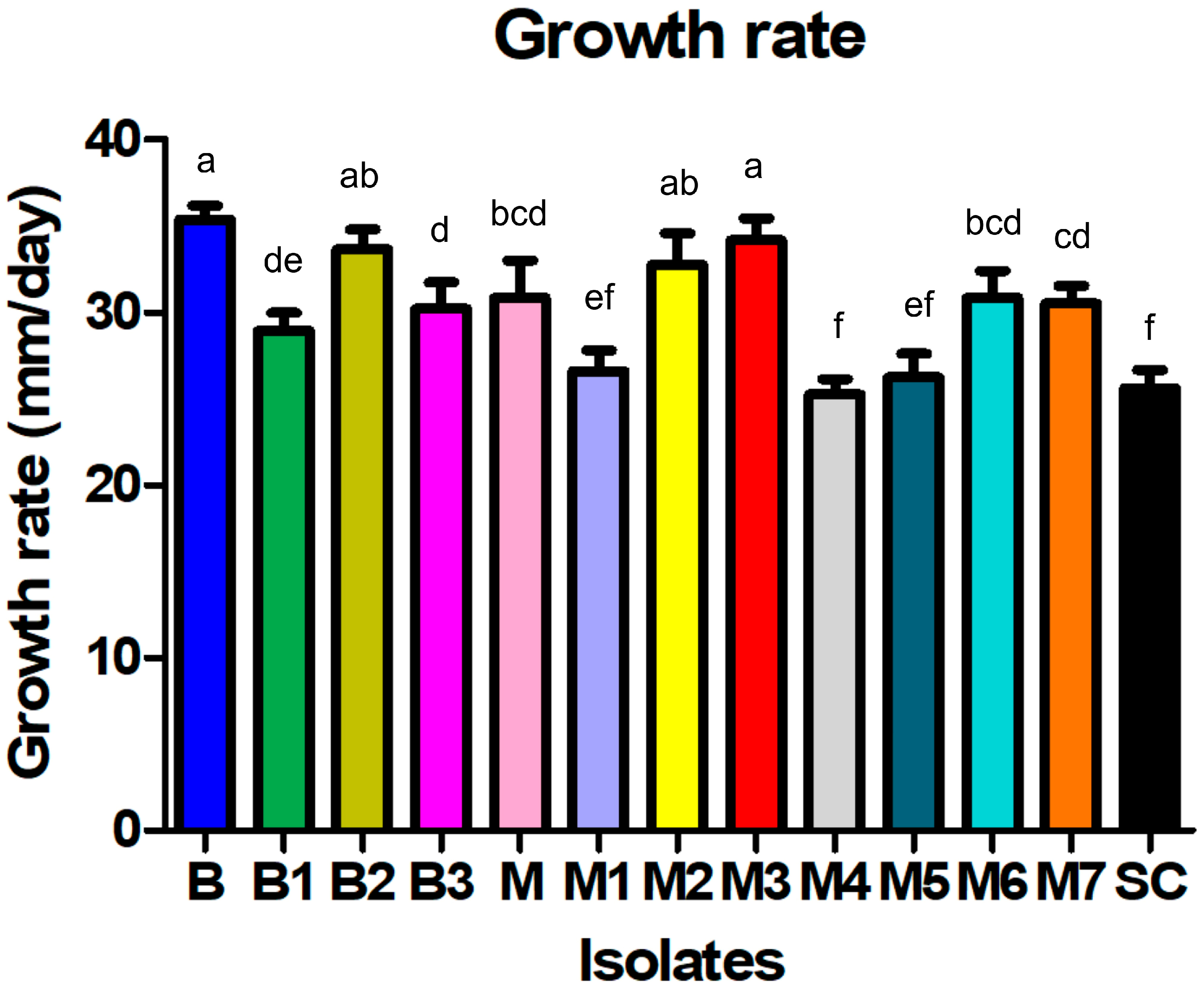
2.1. Disease Symptoms and Morphological Characterization
2.2. Pathogenicity
2.3. Sequence Analysis and Phylogeny
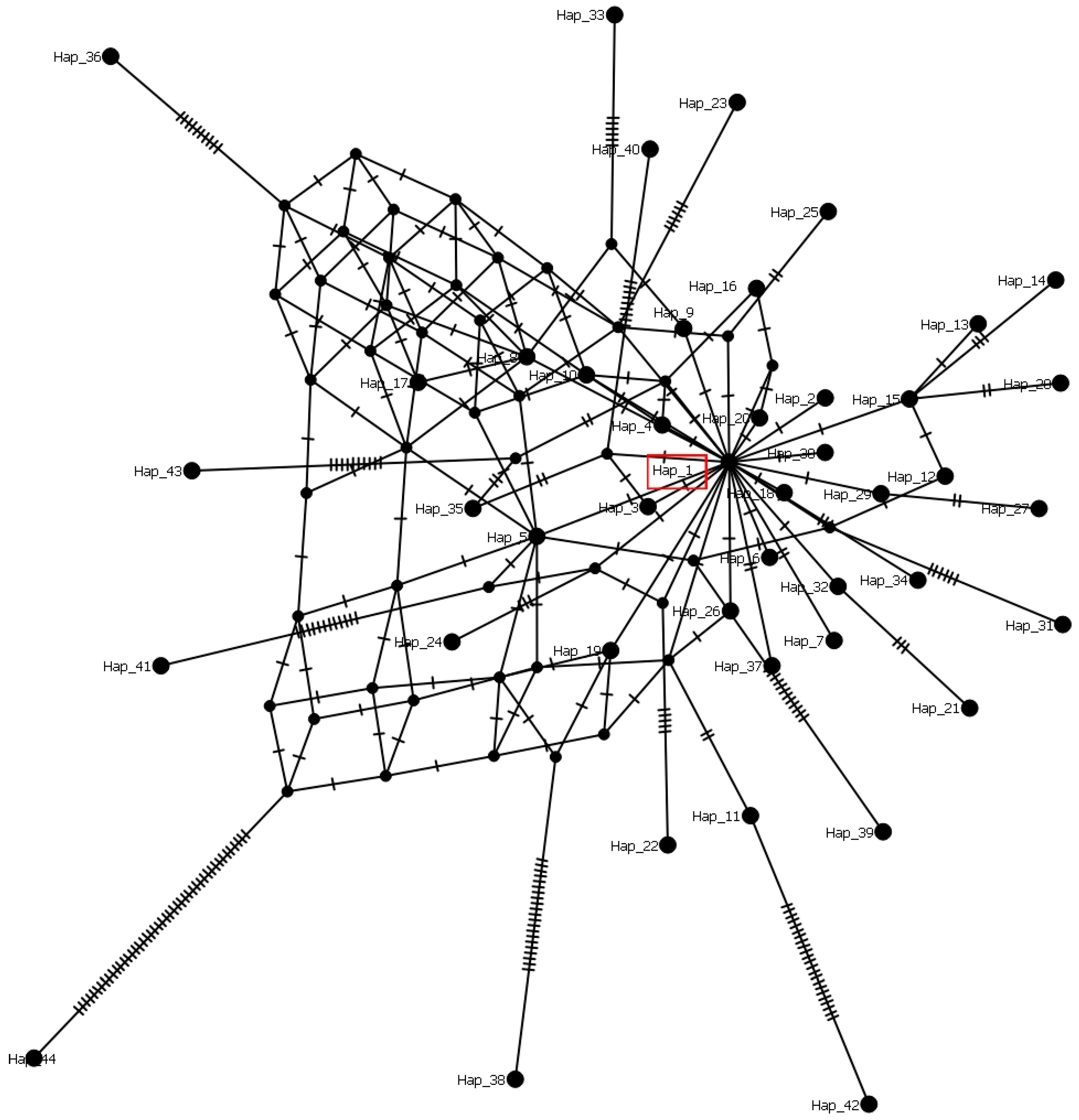
2.4. Haplotype Structure and Genetic Diversity of Sclerotinia sclerotiorum Sequences
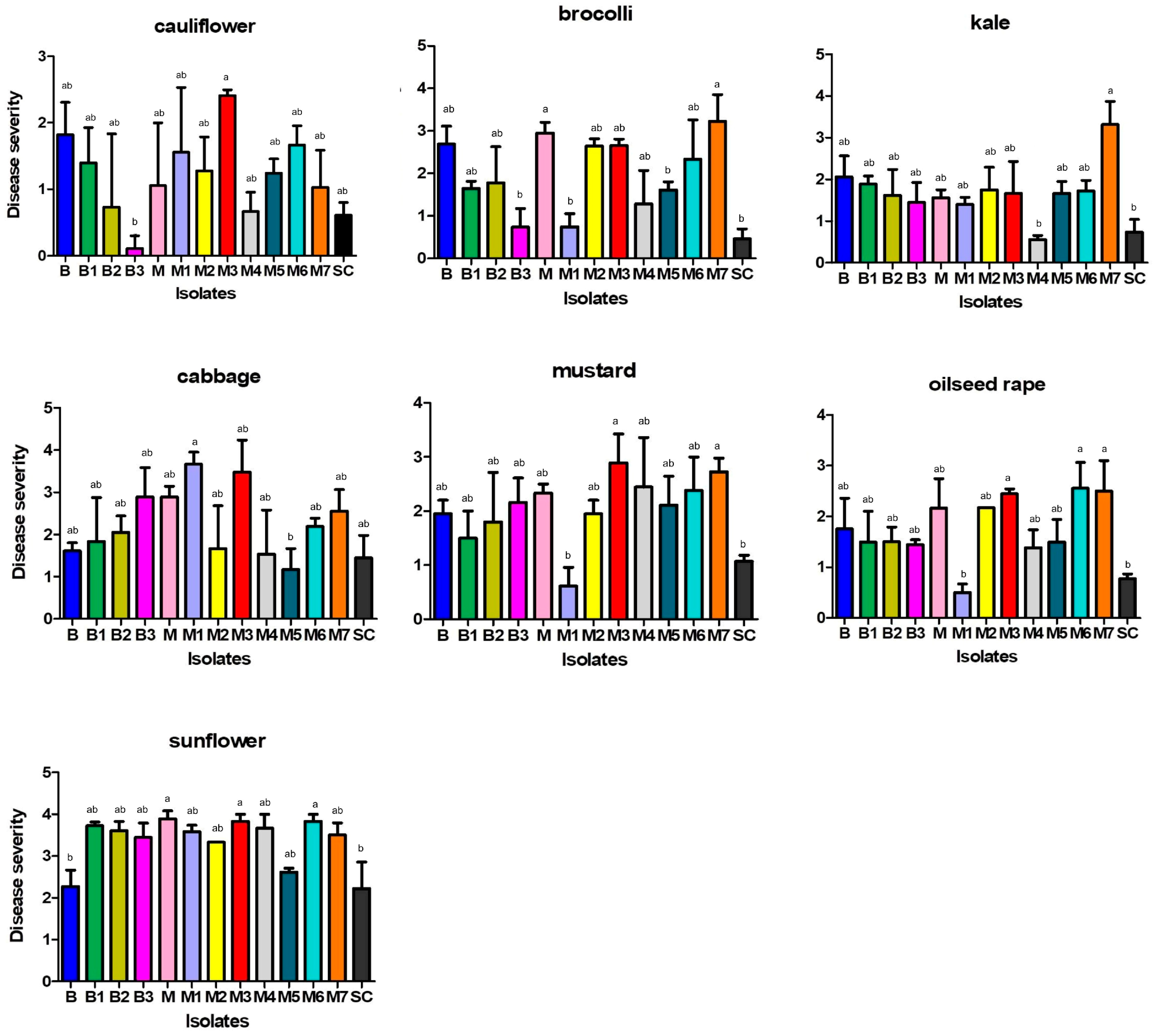
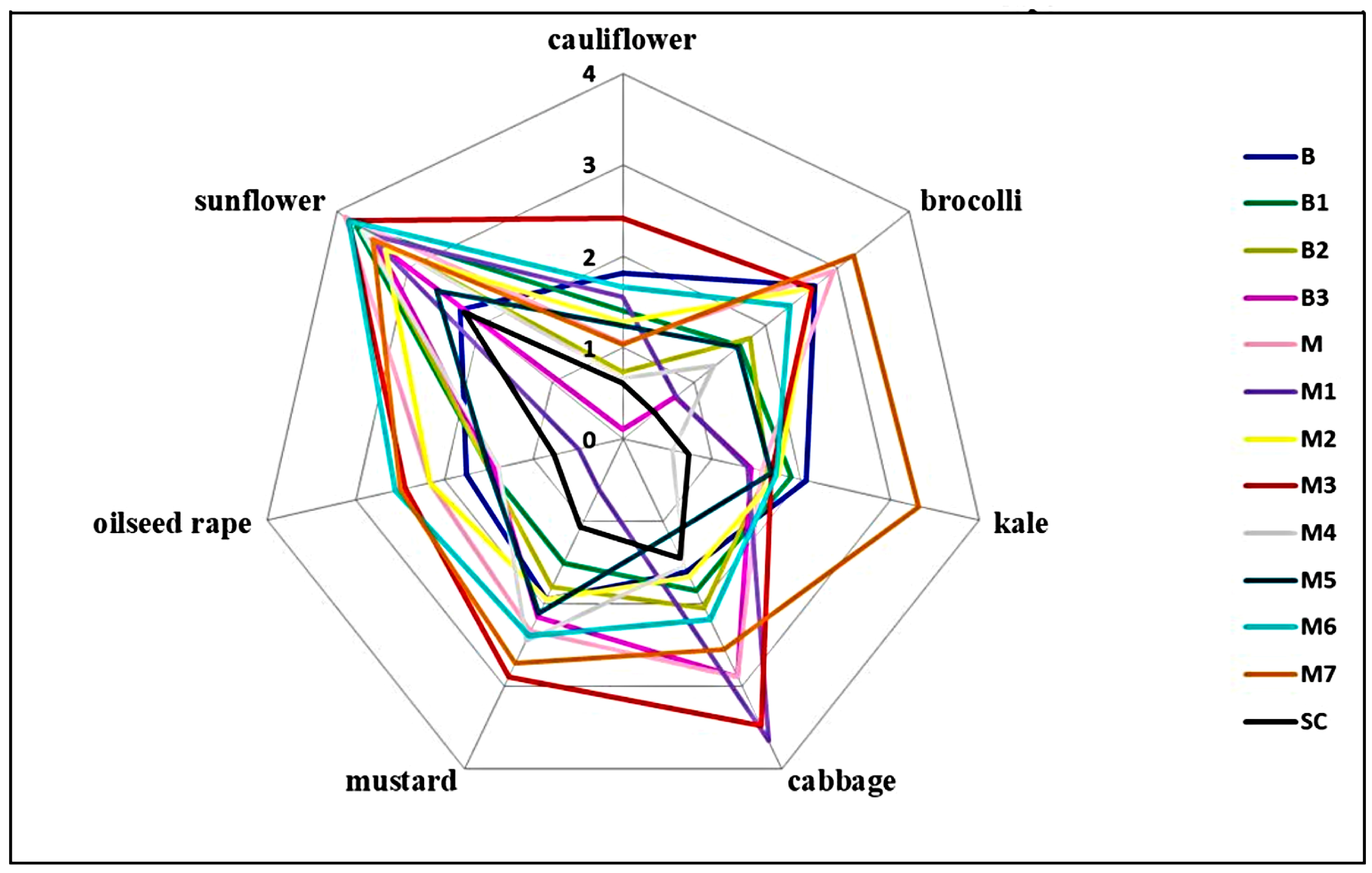
2.5. Aggressiveness Towards Different Plant Species
3. Discussion
4. Materials and Methods
4.1. Sampling and Pathogen Isolation
4.2. Morphological Identification
4.3. Amplification and Sequencing of Isolates’ DNA
4.4. Phylogenetic Analyses
4.5. Haplotype Analysis of Sclerotinia sclerotiourum Sequences
4.6. Pathogenicity and Aggressiveness Testing
4.7. Statistical Analyses
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| bp | Base pair |
| DNA | Deoxyribonucleic acid |
| DSI | Disease severity index |
| Hap | Haplotype |
| ITS | Internal transcribed spacer |
| PCR | Polymerase chain reaction |
| PDA | Potato dextrose agar |
| nt | Nucleotide |
| SD | Standard deviation |
References
- Červenski, J.; Medić-Pap, S. Proizvodnja kupusa [Cabbage Production]; Institute of Field and Vegetable Crops: Novi Sad, Serbia, 2018. [Google Scholar]
- Boland, G.J.; Hall, R. Index of plant hosts of Sclerotinia sclerotiorum. Can. J. Plant Pathol. 1994, 16, 93–108. [Google Scholar] [CrossRef]
- Melzer, M.S.; Smith, E.A.; Boland, G.J. Index of plant hosts of Sclerotinia minor. Can. J. Plant Pathol. 1997, 19, 272–280. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, W.; Lv, Z.; Shi, L.; Ge, B. Evaluation of the inhibitory effects of Wuyiencin, a secondary metabolite of Streptomyces albulus CK-15, against Sclerotinia sclerotiorum in vitro. Plant Dis. 2022, 106, 156–164. [Google Scholar] [CrossRef]
- Li, C.X.; Li, H.; Sivasithamparam, K.; Fu, T.D.; Li, Y.C.; Liu, S.Y.; Barbetti, M.J. Expression of field resistance under Western Australian conditions to Sclerotinia sclerotiorum in Chinese and Australian Brassica napus and Brassica juncea germplasm and its relation with stem diameter. Aust. J. Agric. Res. 2006, 57, 1131–1135. [Google Scholar] [CrossRef]
- Khan, M.A.; Cowling, W.; Banga, S.S.; You, M.P.; Tyagi, V.; Bharti, B.; Barbetti, M.J. Quantitative inheritance of Sclerotinia stem rot resistance in Brassica napus and relationship to cotyledon and leaf resistances. Plant Dis. 2022, 106, 127–139. [Google Scholar] [CrossRef]
- Purdy, L.H. Sclerotinia sclerotiorum: History, diseases and symptomatology, host range, geographic distribution, and impact. Phytopathology 1979, 69, 875–880. [Google Scholar] [CrossRef]
- Dillard, H.; Hunter, J. Association of common ragweed with Sclerotinia rot of cabbage in New York State. Plant Dis. 1986, 70, 26–28. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.K.; Sankaralingam, A.; Nakkeeran, S. Management of head rot of cabbage caused by Sclerotinia sclerotiorum through combined application of fungicides and biocontrol Bacillus amyloliquefaciens. Int. J. Chem. Stud. 2017, 5, 401–404. [Google Scholar]
- Elahi, F.E.; Islam, M.M.; Rahman, M.M. Sclerotinia sclerotiorum infects cabbage in Bangladesh. Australas. Plant Dis. Notes 2023, 18, 4. [Google Scholar] [CrossRef]
- Terrones Salgado, J.; Ortega Acosta, C.; Sánchez Ruiz, F.J.; Ortega Acosta, S.A.; Palemón Alberto, F.; García Sánchez, G.; Rodríguez Márquez, A.; Zárate Aguilar, A. First report of white mold caused by Sclerotinia sclerotiorum on cabbage in Mexico. Plant Dis. 2024, 108, 523. [Google Scholar] [CrossRef]
- Ziqin, L.; Zhang, M.; Wang, Y.; Li, R. Mycelial compatibility group and pathogenicity variation of Sclerotinia sclerotiorum population in sunflower from China, Canada and England. Plant Pathol. J. 2008, 7, 131–139. [Google Scholar] [CrossRef]
- Sharma, P.; Meena, P.D.; Verma, P.R.; Saharan, G.S.; Mehta, N.; Singh, D.; Kumar, A. Sclerotinia sclerotiorum (Lib.) de Bary causing Sclerotinia rot in oilseed Brassicas: A review. J. Oilseed Brass. 2015, 6, 1–44. [Google Scholar]
- Tančić, S.; Dedić, B.; Jocić, S.; Balalić, I.; Lačok, N.; Miladinović, D.; Miklič, V. Sclerotinia wilt occurrence on sunflower in Vojvodina, Serbia. Field Veg. Crop Res. 2011, 48, 353–358. [Google Scholar] [CrossRef]
- Radujkov, D.; Maširević, S.; Vujičić, J.; Tarlanović, J.; Vlajić, S. The appearance of Sclerotinia sclerotiorum on green beans and the examination of antifungal effect of Extrasol®. Res. J. Agric. Sci. 2015, 47, 183–187. [Google Scholar]
- Vasić, T.; Živković, S.; Marković, J.; Stanojević, I.; Filipović, S.; Terzić, D. Phytopathogenic fungi causers fungal diseases of the faba bean (Vicia faba L.) in Serbia. Biol. Nyssana 2019, 10, 17–21. [Google Scholar] [CrossRef]
- Vlajić, S.; Maširević, S.; Barać, R.; Iličić, R.; Gvozdanović–Varga, J.; Božić, V. Diseases of cabbage during 2016. In Proceedings of the XXII Conference on Biotechnology, Čačak, Serbia, 10–11 March 2017; Faculty of Agronomy Čačak, University of Kragujevac: Čačak, Serbia, 2017; pp. 309–314. (In Serbian). [Google Scholar]
- Abawi, G.S.; Grogan, R.G. Epidemiology of diseases caused by Sclerotinia species. Phytopathology 1979, 69, 899–904. [Google Scholar] [CrossRef]
- Choi, I.Y.; Kim, J.; Lee, W.H.; Cho, S.E.; Shin, H.D. First report of Sclerotinia stem rot caused by Sclerotinia sclerotiorum on Chinese chives in Korea. Plant Dis. 2017, 101, 1953. [Google Scholar] [CrossRef]
- Mourde, E.M.; Holliday, P. Sclerotinia sclerotiorum (Sclerotial state). In CMI Descriptions of Pathogenic Fungi and Bacteria; CMI: London, UK, 1976; No. 513. [Google Scholar]
- Borah, T.R.; Dutta, S.; Barman, A.R.; Helim, R.; Sen, K. Variability and host range of Sclerotinia sclerotiorum in Eastern and North Eastern India. J. Plant Pathol. 2021, 103, 809–822. [Google Scholar] [CrossRef]
- Garg, H.; Kohn, L.M.; Andrew, M.; Li, H.; Sivasithamparam, K.; Barbetti, M.J. Pathogenicity of morphologically different isolates of Sclerotinia sclerotiorum with Brassica napus and B. juncea genotypes. Eur. J. Plant Pathol. 2010, 126, 305–315. [Google Scholar] [CrossRef]
- Abreu, M.J.; Souza, E.A. Investigation of Sclerotinia sclerotiorum strains variability in Brazil. Genet. Mol. Res. 2015, 14, 6879–6896. [Google Scholar] [CrossRef]
- Upadhyay, P.; Tiwari, A.K.; Bisht, K.S. Cultural, morphological, pathogenic variability and mycelial compatibility among the isolates of Sclerotinia sclerotiorum (Lib.) de Bary cause of Sclerotinia rot. Bioscan 2015, 10, 1813–1817. [Google Scholar]
- Zanatta, T.P.; Kulczynski, S.M.; Guterres, C.W.; Fontana, D.C.; Meira, D.; Ceolin, E.L.; Balem, E.; Trevisan, M.; Paraginski, J.A.; Buffon, P.A. Morphological and pathogenic characterization of Sclerotinia sclerotiorum. J. Agric. Sci. 2019, 11, 302–313. [Google Scholar] [CrossRef]
- Faruk, M.I.; Rahman, M.M.E. Collection, isolation and characterization of Sclerotinia sclerotiorum, an emerging fungal pathogen causing white mold disease. J. Plant Sci. Phytopathol. 2022, 6, 43–51. [Google Scholar] [CrossRef]
- Willetts, H.J.; Wong, J.A.-L.; Kirst, G.D. The biology of Sclerotinia sclerotiorum, S. trifoliorum, and S. minor with emphasis on specific nomenclature. Bot. Rev. 1980, 46, 101–165. [Google Scholar] [CrossRef]
- Mondal, B.; Khatua, D.C.; Hansda, S.; Sharma, R. Addition to the host range of Sclerotinia sclerotiorum in West Bengal. Sch. Acad. J. Biosci. 2015, 3, 361–364. [Google Scholar] [CrossRef]
- Rather, R.A.; Ahanger, F.A.; Ahanger, S.A.; Basu, U.; Wani, M.A.; Rashid, Z.; Sofi, P.A.; Singh, V.; Javeed, K.; Baazeem, A.; et al. Morpho-cultural and pathogenic variability of Sclerotinia sclerotiorum causing white mold of common beans in temperate climate. J. Fungi 2022, 8, 755. [Google Scholar] [CrossRef]
- Jan, N.; Bhat, M.Y.; Wani, A.H.; Malik, M.A.; Jan, M. Incidence of white mould of bean and characterization of its causal pathogen, Sclerotinia sclerotiorum in Kashmir valley, India. Arch. Phytopathol. Plant Prot. 2023, 56, 636–646. [Google Scholar] [CrossRef]
- Kim, W.G.; Cho, W.D. Occurrence of Sclerotinia rot on composite vegetable crops and the causal Sclerotinia spp. Mycobiology 2002, 30, 41–46. [Google Scholar] [CrossRef]
- Sharma, P.; Samkumar, A.; Rao, M.; Singh, V.V.; Prasad, L.; Mishra, D.C.; Bhattacharya, R.; Gupta, N.C. Genetic diversity studies based on morphological variability, pathogenicity and molecular phylogeny of the Sclerotinia sclerotiorum population from Indian mustard (Brassica juncea). Front. Microbiol. 2018, 9, 1169. [Google Scholar] [CrossRef]
- Prova, A.; Akanda, A.M.; Islam, S.; Hossain, M.M. Characterization of Sclerotinia sclerotiorum, an emerging fungal pathogen causing blight in hyacinth bean (Lablab purpureus). Plant Pathol. J. 2018, 34, 367–380. [Google Scholar] [CrossRef]
- Derbyshire, M.C.; Newman, T.E.; Khentry, Y.; Owolabi Taiwo, A. The evolutionary and molecular features of the broad-host-range plant pathogen Sclerotinia sclerotiorum. Mol. Plant Pathol. 2022, 23, 1075–1090. [Google Scholar] [CrossRef]
- Hu, M.-J.; Cox, K.D.; Schnabel, G.; Luo, C.-X. Monilinia species causing brown rot of peach in China. PLoS ONE 2011, 6, e24990. [Google Scholar] [CrossRef]
- Baltazar, E.; Rodrigues, S.; Ares, A.; Camelo, A.; Brandão, I.; Espirito Santo, C.; Trovão, J.; Garcia, E.; Costa, J. Morphological, Molecular and Genomic Identification and Characterisation of Monilinia fructicola in Prunus persica from Portugal. Agronomy 2023, 13, 1493. [Google Scholar] [CrossRef]
- Faraghati, M.; Abrinbana, M.; Ghosta, Y. Genetic structure of Sclerotinia sclerotiorum populations from sunflower and cabbage in West Azarbaijan province of Iran. Sci. Rep. 2022, 12, 9263. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.G.; Cho, W.D. Occurrence of Sclerotinia rot in cruciferous crops caused by Sclerotinia spp. Plant Pathol. J. 2003, 19, 69–74. [Google Scholar] [CrossRef]
- Kim, W.G.; Cho, W.D.; Jee, H.J. Occurrence of Sclerotinia rot on cucurbitaceous vegetable crops in greenhouses. Korean J. Mycol. 1999, 27, 198–205. [Google Scholar]
- Yu, Y.; Cai, J.; Ma, L.; Huang, Z.; Wang, Y.; Fang, A.; Yang, Y.; Qing, L.; Bi, C. Population structure and aggressiveness of Sclerotinia sclerotiorum from rapeseed (Brassica napus) in Chongqing City. Plant Dis. 2020, 104, 1201–1210. [Google Scholar] [CrossRef]
- Chaudhary, S.; Lal, M.; Sagar, S.; Tyagi, H.; Kumar, M.; Sharma, S.; Chakrabarti, S.K. Genetic diversity studies based on morpho-pathological and molecular variability of the Sclerotinia sclerotiorum population infecting potato (Solanum tuberosum L). World J. Microbiol. Biotechnol. 2020, 36, 177. [Google Scholar] [CrossRef]
- Dhingra, O.; Sinclair, J. Basic Plant Pathology Methods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Mert-Türk, F.; Ipek, M.; Mermer, D.; Nicholson, P. Microsatellite and morphological markers reveal genetic variation within a population of Sclerotinia sclerotiorum from oilseed rape in the Çanakkale Province of Turkey. J. Phytopathol. 2007, 155, 182–187. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; White, T.J., Sninsky, J.J., Gelfand, D.H., Innin, M.A., Eds.; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Baturo-Ciesniewska, A.; Groves, C.; Albrecht, K.; Smith, D.; Grau, C.; Willis, D. Molecular Identification of Sclerotinia trifoliorum and Sclerotinia sclerotiorum Isolates from the United States and Poland. Plant Dis. 2017, 101, 192–199. [Google Scholar] [CrossRef]
- Gargouri, S.; Berraies, S.; Gharbi, M.S.; Paulitz, T.; Murray, T.; Burges, L. Occurrence of Sclerotinia stem rot of fenugreek caused by Sclerotinia trifoliorum and S. sclerotiorum in Tunisia. Eur. J. Plant Pathol. 2017, 149, 587–597. [Google Scholar] [CrossRef]
- Pavani, P.; Singh, L.N.; Sinha, B.; Bathula, P. In-vitro sensitivity test of native Trichoderma spp. against growth of Rhizoctonia solani f.sp. sasakii causing banded leaf and sheath blight of maize in Manipur. Int. J. Environ. Clim. Chang. 2022, 12, 998–1002. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Bandelt, H.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]


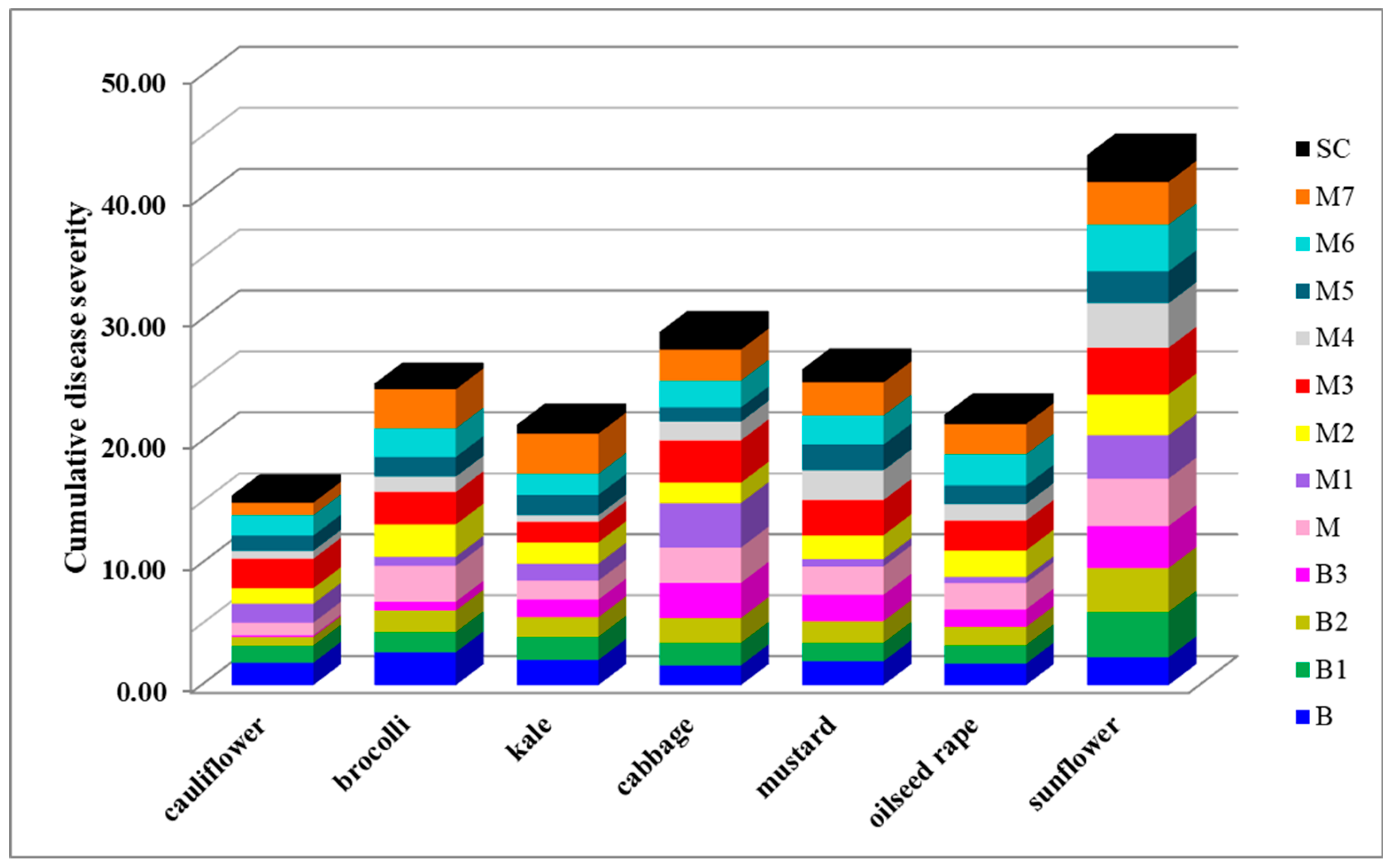
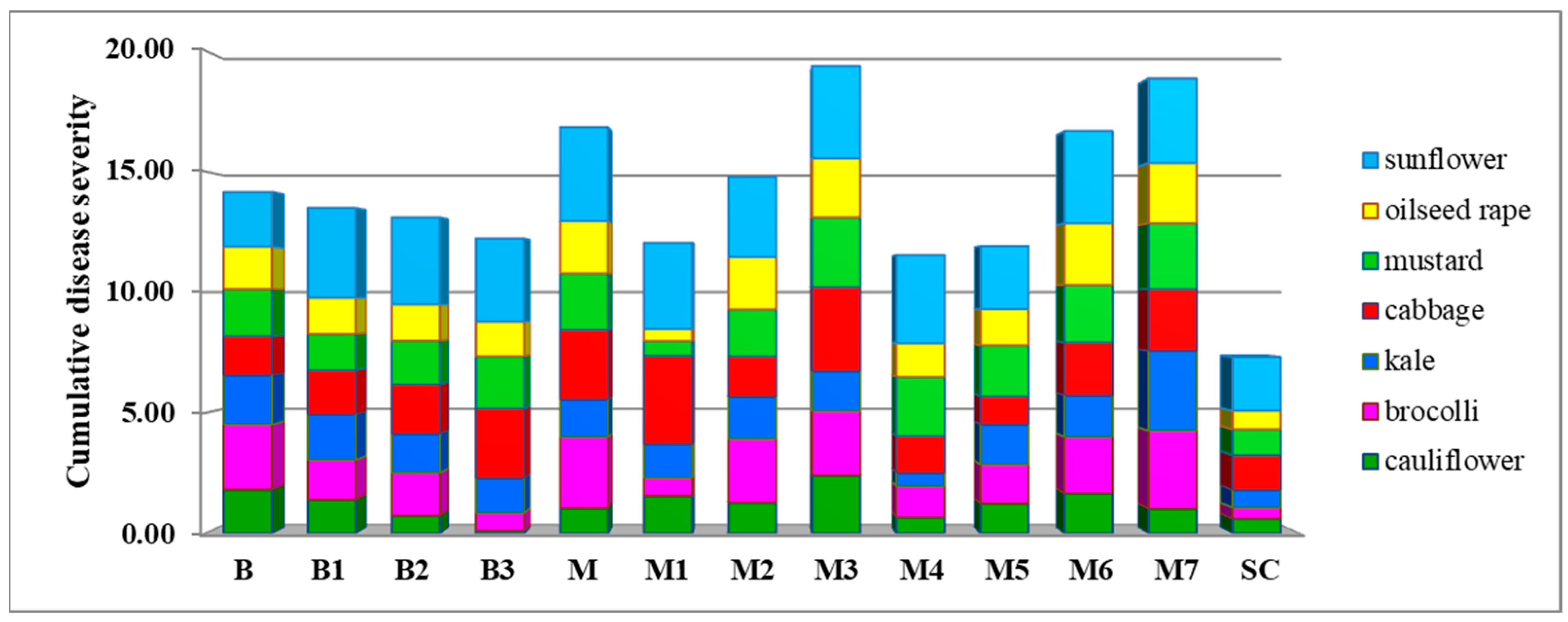
| Isolate | Colony Appearance | Sclerotia | ||||
|---|---|---|---|---|---|---|
| Color | Relative Density and Appearance of Areal Mycelium | Timing of Formation (day) | Average No. ± SD/Plate | Average Size (mm) | Arrangement | |
| B | White | Sparse–very dense, Wooly–floccose | 8 | 15.7 ± 1.5 cd * | 5 × 3 (2–7 × 2–4) | Edge ring |
| B1 | White | Sparse–low dense. Wooly | 8 | 10.7 ± 0.6 de | 4 × 3 (2–5 × 2–4) | Edge ring |
| B2 | White | Sparse–low dense, Floccose | 8 | 10.3 ± 0.6 de | 6 × 3 (1–10 × 1–5) | Edge + middle rings |
| B3 | White | Sparse–very dense, Wooly–floccose | 6 | 15.7 ± 2.5 c | 3 × 2 (1–5 × 1–3) | Edge ring |
| M | White | Dense, Wooly | 8 | 10.7 ± 1.5 cde | 5 × 3 (1–8 × 1–5) | Edge + middle rings |
| M1 | White | Sparse, Floccose | 8 | 10.7 ± 0.6 cde | 4 × 3 (1–7 × 1–4) | Edge + middle rings |
| M2 | White | Sparse, Floccose | 9 | 9.7 ± 0.6 e | 10 × 4 (2–7 × 2–6) | Middle ring |
| M3 | White | Moderately dense, Floccose | 6 | 12.7 ± 0.6 cde | 5 × 4 (2–7 × 2–5) | Edge ring |
| M4 | White | Sparse–dense, Floccose | 8 | 16.7 ± 0.6 bc | 6 × 3 (1–11 × 1–4) | Edge + middle rings |
| M5 | White | Sparse, Floccose | 6 | 23.7 ± 7.0 b | 3 × 3 (1–5 × 1–4) | Edge ring |
| M6 | White | Sparse–very dense, Wooly–floccose | 8 | 16.7 ± 1.5 bc | 5 × 4 (3–6 × 3–4) | Edge + middle rings |
| M7 | White | Sparse–dense, Wooly–floccose | 7 | 33.7 ± 1.5 a | 6 × 3 (2–9 × 2–3) | Edge + middle rings |
| SC | White | Very dense, Wooly | 13 | 2.7 ± 0.6 f | 2 × 2 (1–2 × 1–2) | Edge + middle rings |
| Species | Isolate | Acc No. | Host | Country | Literature |
|---|---|---|---|---|---|
| Sclerotinia sclerotiorum | 2 | KX184720 | Cabbage (Brassica oleracea var. capitata) | Sri Lanka | [33] |
| SSC2JHU | MG249967 | Cotton (Gossypium hirsutum) | USA | [33] | |
| MAFF 306676 | AB233346 | Blueberry (Vaccinium corymbosum) | Japan | [33] | |
| 16-042 | KY073613 | Shepherd’s purse (Capsella bursa-pastoris) | Korea | [33] | |
| SS-BO-SC | KP340898 | Cabbage (Brassica oleracea var. capitata) | New Mexico | [33] | |
| -No data | JN013184 | Fan Columbine (Aquilegia flabellata) | Italy | [33] | |
| DAOM: 241671 | KF859932 | No data | Canada | [33] | |
| B23 | DQ329537 | No data | Alaska | [33] | |
| ms85 | HQ833450 | Mulberry (Morus alba) | China | [33] | |
| SQC-000 | KY750530 | Chinese celery (Oenanthe javanica) | China | [33] | |
| 16-119 | KY073614 | Cucumber (Cucumis sativus) | Korea | [33] | |
| ATCC MYA-4521 | FJ810516 | No data | USA | [33] | |
| Ss1212HA | KT224645 | Caucasian clover (Trifolium ambiguum) | Poland | [33] | |
| JBARES2014A | KJ614564 | Chinese chives (Allium tuberosum) | Korea | [33] | |
| MuRa-103 | AB937095 | Napa cabbage (Brassica napa) | Japan | [33] | |
| B | PP179050 | Cabbage (Brassica oleracea var. capitata) | Serbia | This study | |
| B1 | PP179051 | Cabbage (Brassica oleracea var. capitata) | Serbia | This study | |
| B2 | PP179052 | Cabbage (Brassica oleracea var. capitata) | Serbia | This study | |
| B3 | PP179053 | Cabbage (Brassica oleracea var. capitata) | Serbia | This study | |
| M | PP179054 | Cabbage (Brassica oleracea var. capitata) | Serbia | This study | |
| M1 | PP179057 | Cabbage (Brassica oleracea var. capitata) | Serbia | This study | |
| M2 | PP179055 | Cabbage (Brassica oleracea var. capitata) | Serbia | This study | |
| M3 | PP179056 | Cabbage (Brassica oleracea var. capitata) | Serbia | This study | |
| M4 | PP179058 | Cabbage (Brassica oleracea var. capitata) | Serbia | This study | |
| M5 | PP179059 | Cabbage (Brassica oleracea var. capitata) | Serbia | This study | |
| M6 | PP179060 | Cabbage (Brassica oleracea var. capitata) | Serbia | This study | |
| M7 | PP179061 | Cabbage (Brassica oleracea var. capitata) | Serbia | This study | |
| SC | PP177498 | Cabbage (Brassica oleracea var. capitata) | Serbia | This study | |
| Sclerotinia trifoliorum | St03TP | KT224652 | Caucasian clover (Trifolium ambiguum) | Poland | [33] |
| CBS122377 | KT970794 | Caucasian clover (Trifolium ambiguum) | Poland | [33] | |
| TN Sc10101 | KT819299 | Fenugreek (Trigonella foenum-graecum) | Tunisia | [49] | |
| St2413TA | KT986230 | Caucasian clover (Trifolium ambiguum) | Poland | [48] | |
| St1412TA | KT224642 | Caucasian clover (Trifolium ambiguum) | Poland | [48] | |
| Sclerotinia minor | MAFF 238173 | AB516661 | No data | Japan | [33] |
| 45903A | JF279879 | No data | Australia | [33] | |
| 45802A | JF279877 | No data | Australia | [33] | |
| 62907 | JF279880 | No data | Australia | [33] | |
| Trichoderma lixi | NCIPM-78 | KU904458 | Rhizosphere soil | India | [50] |
| Common Name | Latin Name | Cultivar | Cultivar’s Origin |
|---|---|---|---|
| Cabbage | Brassica oleracea var. capitata | Futoški | Futog, Novi Sad, Serbia |
| Cauliflower | Brassica oleracea var. botrytis | Incline | Sakata Seed Southern Africa (Pty) Ltd., Kempton Park, South Africa |
| Broccoli | Brassica oleracea var. silvestris | Merathon | Sakata Seed Southern Africa (Pty) Ltd., Kempton Park, South Africa |
| Kale | Brassica oleracea | Estoril | Sakata Seed Southern Africa (Pty) Ltd., Kempton Park, South Africa |
| Mustard | Sinapis alba | NS Bela | Institute of field and vegetable crops, Novi Sad, Serbia |
| Oilseed rape | Brassica napus | NS Svetlana | Institute of field and vegetable crops, Novi Sad, Serbia |
| Sunflower | Helianthus annuus | Labud | Institute of field and vegetable crops, Novi Sad, Serbia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pešić, B.; Mitrović, P.; Marjanović Jeromela, A.; Zanetti, F.; Mihajlović, M.; Hrustić, J.; Vojvodić, M.; Grkinić, M.; Bulajić, A. Morphological, Pathogenic and Molecular Characterization of Sclerotinia sclerotiorum, the Causal Agent of White Rot of Cabbage (Brassica oleracea var. capitata), in Serbia. Plants 2025, 14, 2478. https://doi.org/10.3390/plants14162478
Pešić B, Mitrović P, Marjanović Jeromela A, Zanetti F, Mihajlović M, Hrustić J, Vojvodić M, Grkinić M, Bulajić A. Morphological, Pathogenic and Molecular Characterization of Sclerotinia sclerotiorum, the Causal Agent of White Rot of Cabbage (Brassica oleracea var. capitata), in Serbia. Plants. 2025; 14(16):2478. https://doi.org/10.3390/plants14162478
Chicago/Turabian StylePešić, Brankica, Petar Mitrović, Ana Marjanović Jeromela, Federica Zanetti, Milica Mihajlović, Jovana Hrustić, Mira Vojvodić, Miljan Grkinić, and Aleksandra Bulajić. 2025. "Morphological, Pathogenic and Molecular Characterization of Sclerotinia sclerotiorum, the Causal Agent of White Rot of Cabbage (Brassica oleracea var. capitata), in Serbia" Plants 14, no. 16: 2478. https://doi.org/10.3390/plants14162478
APA StylePešić, B., Mitrović, P., Marjanović Jeromela, A., Zanetti, F., Mihajlović, M., Hrustić, J., Vojvodić, M., Grkinić, M., & Bulajić, A. (2025). Morphological, Pathogenic and Molecular Characterization of Sclerotinia sclerotiorum, the Causal Agent of White Rot of Cabbage (Brassica oleracea var. capitata), in Serbia. Plants, 14(16), 2478. https://doi.org/10.3390/plants14162478








