Vulnerability Assessment of Six Endemic Tibetan-Himalayan Plants Under Climate Change and Human Activities
Abstract
1. Introduction
2. Results
2.1. Climate Change Vulnerability of Different Species
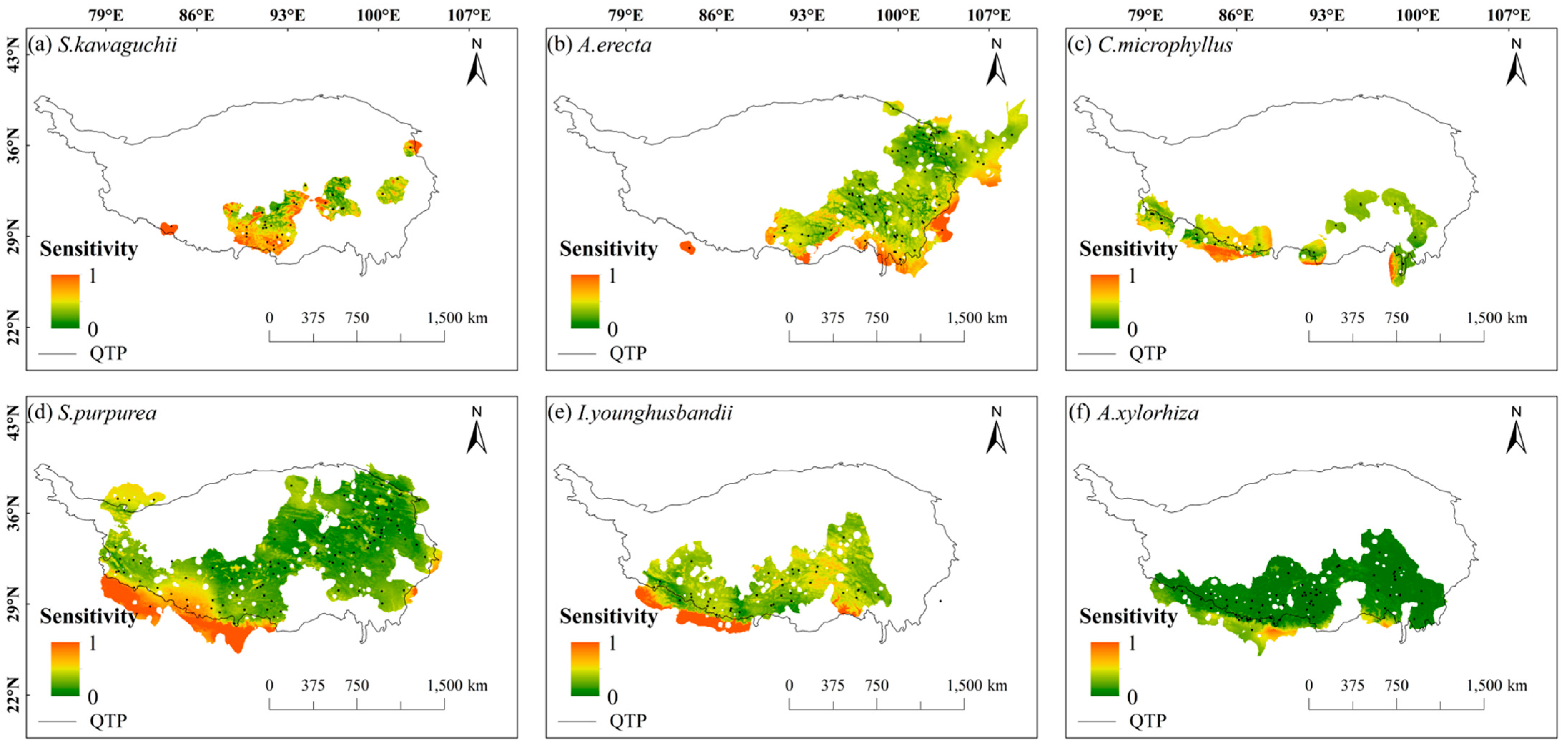
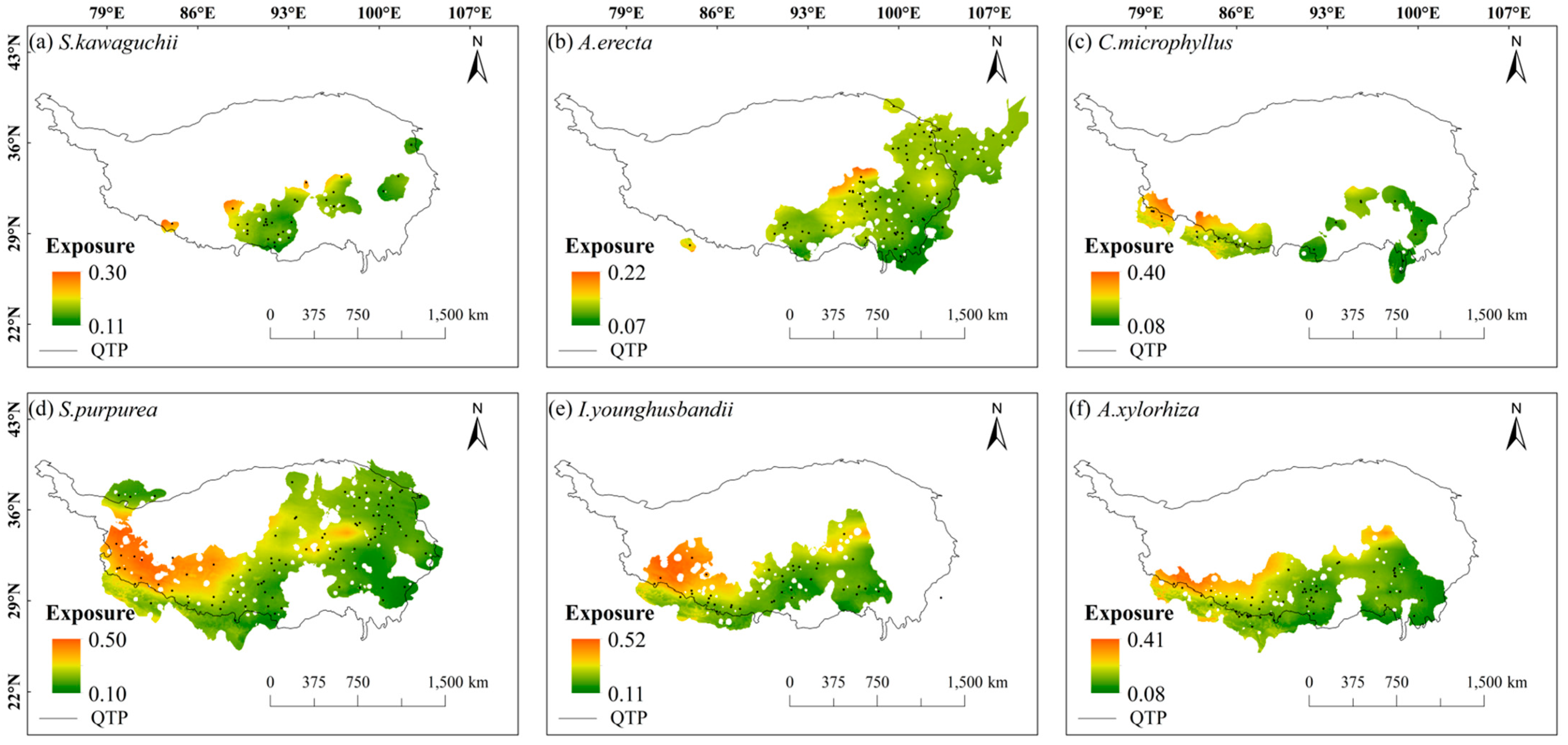
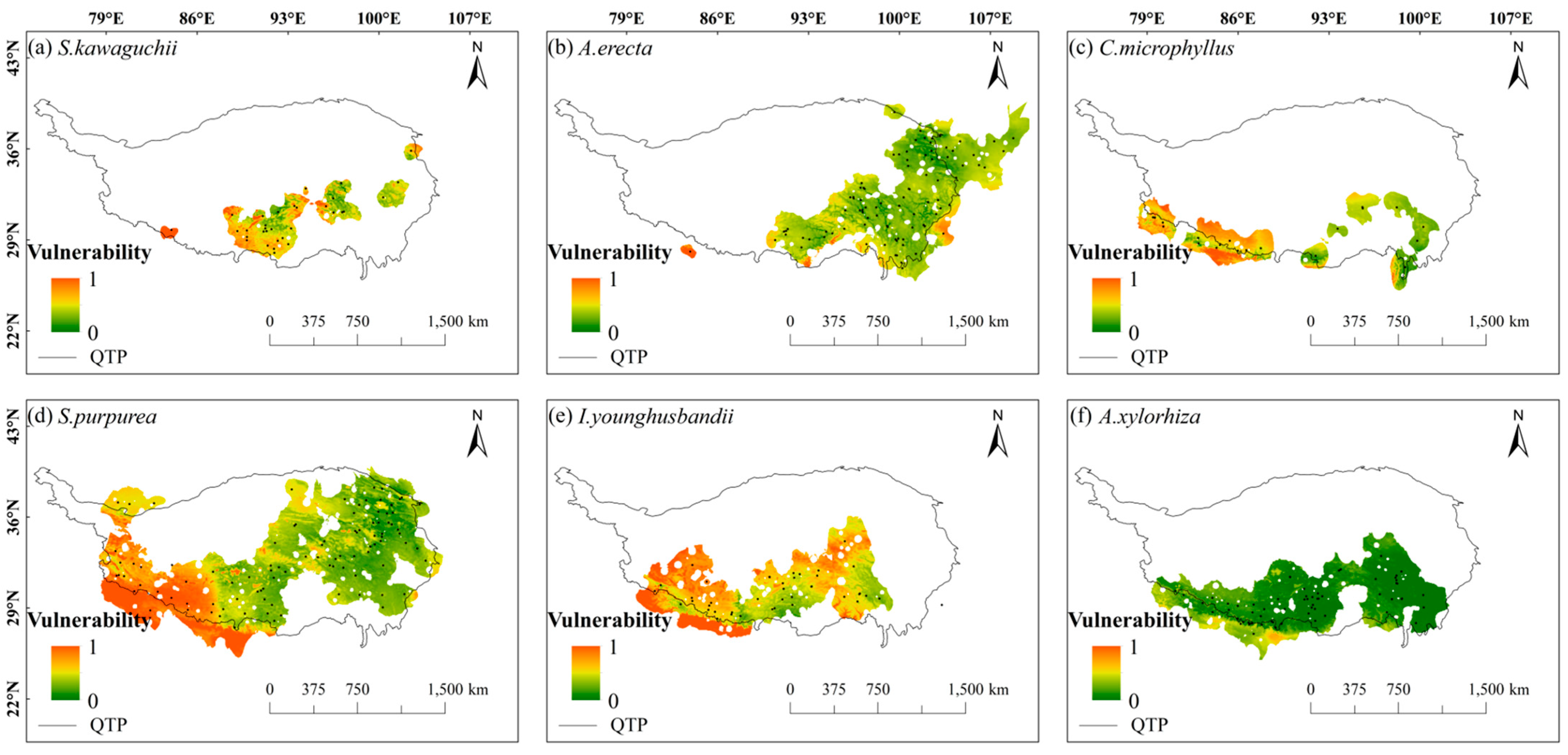
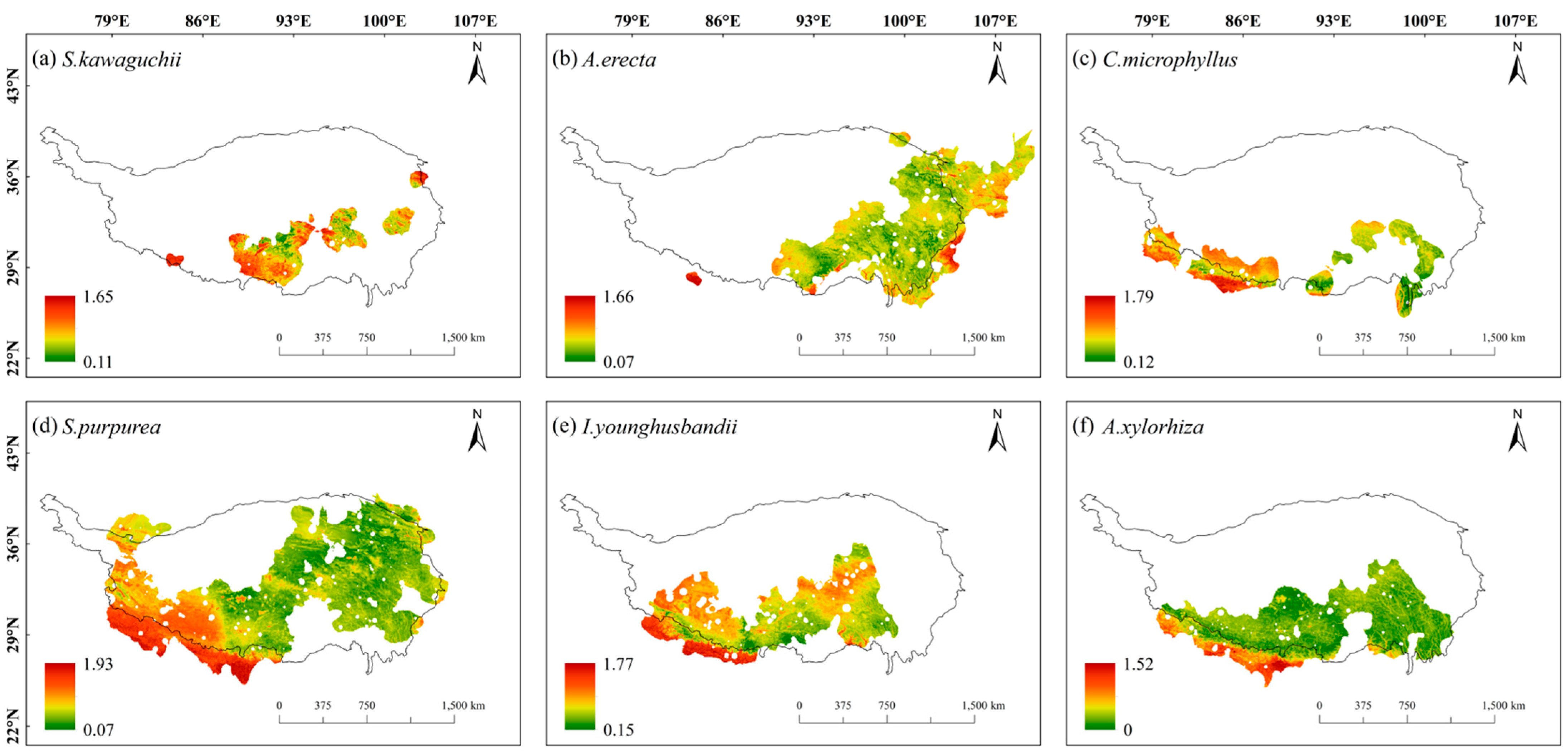
2.2. Spatial Patterns of Sensitivity, Exposure, and Vulnerability to Climate Change
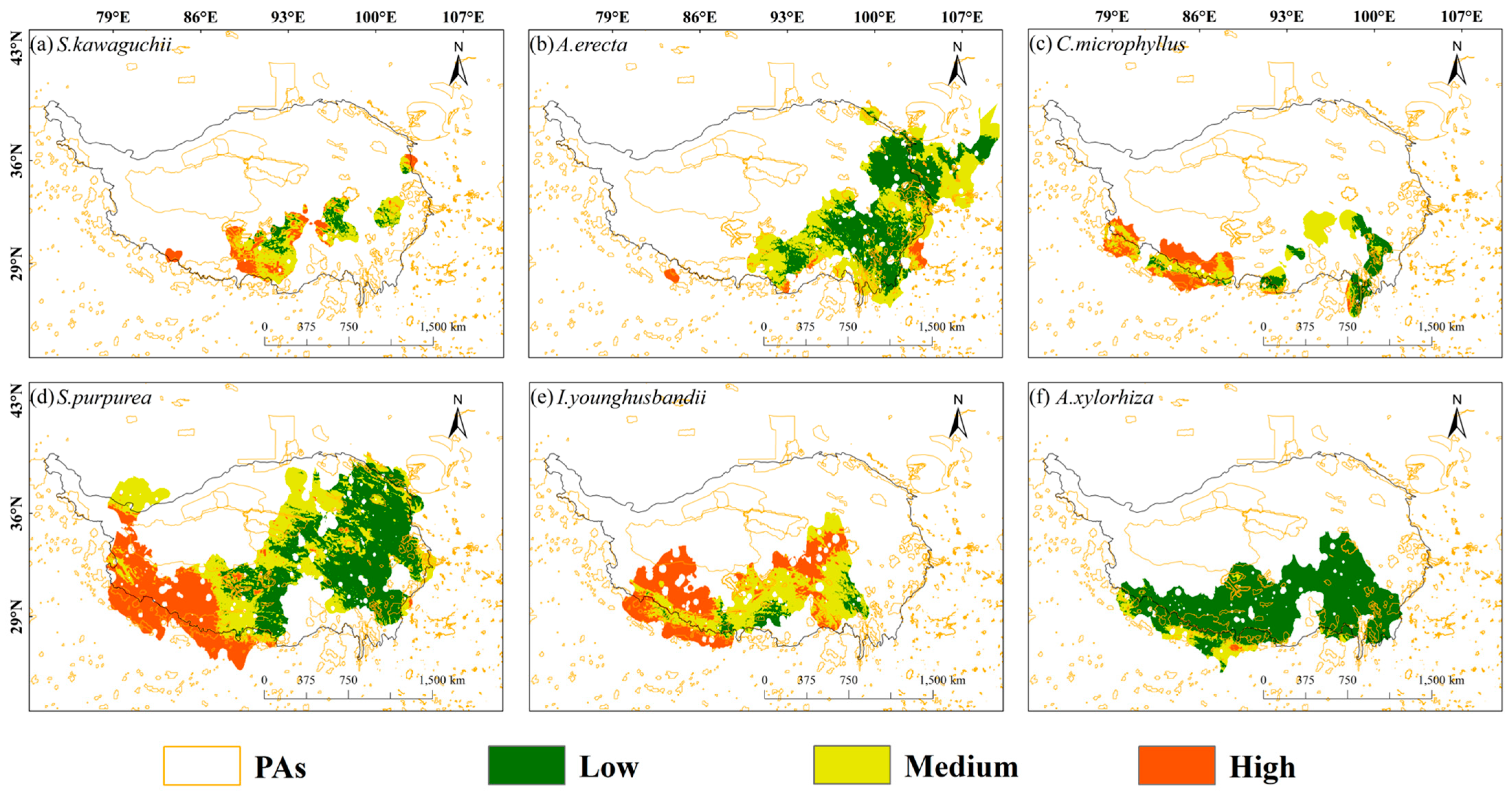
2.3. Integrated Vulnerability of Coupled Human Footprints and Protected Area Distribution
3. Discussion
4. Materials and Methods
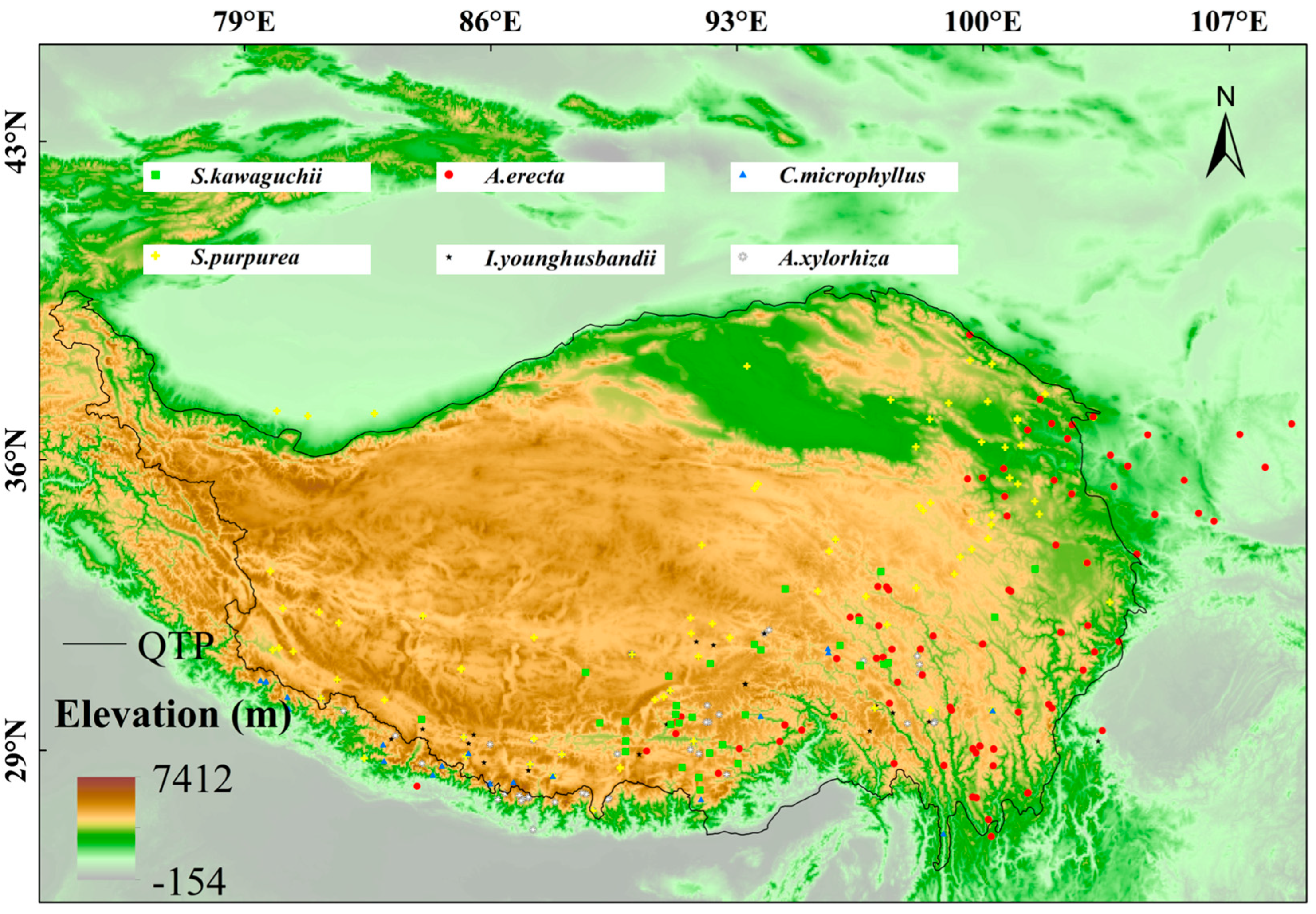
4.1. Occurrence Data and Species Ranges
4.2. Bioclimatic Variables
4.3. Protected Areas and Human Footprint Data
4.4. Climate Change Vulnerability Assessments
4.4.1. Sensitivity
4.4.2. Exposure
4.4.3. Vulnerability
4.5. Spatial Vulnerability Analysis
4.6. Impacts of Protected Areas and Human Footprints on Species Vulnerability
5. Conclusions
- The species patterns of climate change vulnerability revealed a high in the west and low in the east distribution, with the central Tibetan-Himalayan region and HDMs potentially acting as important future climate refugia.
- Species exposure and vulnerability were significantly higher under high carbon emission scenarios (SSP5-8.5) compared with moderate carbon emission scenarios (SSP2-4.5), emphasizing the critical role of climate policy in mitigating biodiversity loss.
- The existing PA coverage is alarmingly inadequate, with less than 25% of our studied species’ habitats currently under protection and coverage dropping below 7% in highly vulnerable zones. Species vulnerability is further exacerbated in areas with dense human activity, such as the areas surrounding Lhasa and Chengdu.
- Limitations in current conservation assessments were observed, as the IUCN Red List does not sufficiently account for future climate risks. For example, S. purpurea (classified as LC) may be more vulnerable to climate change than C. microphylla (classified as NT), highlighting discrepancies in current threat classifications.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, S.; Xu, Y.; You, Q.; Flügel, W.-A.; Pepin, N.; Yao, T. Review of Climate and Cryospheric Change in the Tibetan Plateau. Environ. Res. Lett. 2010, 5, 015101. [Google Scholar] [CrossRef]
- Kuang, X.; Jiao, J.J. Review on Climate Change on the Tibetan Plateau during the Last Half Century. J. Geophys. Res. Atmos. 2016, 121, 3979–4007. [Google Scholar] [CrossRef]
- Hofmann, S.; Stöck, M.; Zheng, Y.; Ficetola, F.G.; Li, J.-T.; Scheidt, U.; Schmidt, J. Molecular Phylogenies Indicate a Paleo-Tibetan Origin of Himalayan Lazy Toads (Scutiger). Sci. Rep. 2017, 7, 3308. [Google Scholar] [CrossRef]
- Lan, T.; Gill, S.; Bellemain, E.; Bischof, R.; Nawaz, M.A.; Lindqvist, C. Evolutionary History of Enigmatic Bears in the Tibetan Plateau–Himalaya Region and the Identity of the Yeti. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171804. [Google Scholar] [CrossRef] [PubMed]
- Muellner-Riehl, A.N. Mountains as Evolutionary Arenas: Patterns, Emerging Approaches, Paradigm Shifts, and Their Implications for Plant Phylogeographic Research in the Tibeto-Himalayan Region. Front. Plant Sci. 2019, 10, 195. [Google Scholar] [CrossRef]
- Xing, Y.; Ree, R.H. Uplift-Driven Diversification in the Hengduan Mountains, a Temperate Biodiversity Hotspot. Proc. Natl. Acad. Sci. USA 2017, 114, E3444–E3451. [Google Scholar] [CrossRef]
- Hughes, C.E.; Atchison, G.W. The Ubiquity of Alpine Plant Radiations: From the Andes to the Hengduan Moun tains. New Phytol. 2015, 207, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Li, R. Protecting Rare and Endangered Species under Climate Change on the Qinghai Plateau, China. Ecol. Evol. 2019, 9, 427–436. [Google Scholar] [CrossRef]
- Li, B.; Liang, C.; Song, P.; Liu, D.; Qin, W.; Jiang, F.; Gu, H.; Gao, H.; Zhang, T. Threatened Birds Face New Distri bution under Future Climate Change on the Qinghai-Tibet Plateau (QTP). Ecol. Indic. 2023, 150, 110217. [Google Scholar] [CrossRef]
- Dimri, A.P.; Allen, S.; Huggel, C.; Mal, S.; Ballesteros-Canovas, J.A.; Rohrer, M.; Shukla, A.; Tiwari, P.; Maharana, P.; Bolch, T.; et al. Climate Change, Cryosphere and Impacts in the Indian Himalayan Region. Curr. Sci. 2021, 120, 774–790. [Google Scholar] [CrossRef]
- Xia, M.; Jia, K.; Zhao, W.; Liu, S.; Wei, X.; Wang, B. Spatio-Temporal Changes of Ecological Vulnerability across the Qinghai-Tibetan Plateau. Ecol. Indic. 2021, 123, 107274. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, R.; Singh, V.P.; Xu, C.-Y.; Fan, K.; Shen, Z.; Wang, G.; Zhao, J. Dynamic Vulnerability of Ecolog ical Systems to Climate Changes across the Qinghai-Tibet Plateau, China. Ecol. Indic. 2022, 134, 108483. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Pacifici, M.; Foden, W.B.; Visconti, P.; Watson, J.E.M.; Butchart, S.H.M.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Akçakaya, H.R.; et al. Assessing Species Vulnerability to Climate Change. Nat. Clim. Change 2015, 5, 215–224. [Google Scholar] [CrossRef]
- Jamwal, P.S.; Di Febbraro, M.; Carranza, M.L.; Savage, M.; Loy, A. Global Change on the Roof of the World: Vul nerability of Himalayan Otter Species to Land Use and Climate Alterations. Divers. Distrib. 2022, 28, 1635–1649. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of Climate Change on the Future of Biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Vanneste, T.; Michelsen, O.; Graae, B.J.; Kyrkjeeide, M.O.; Holien, H.; Hassel, K.; Lindmo, S.; Kapás, R.E.; De Frenne, P. Impact of Climate Change on Alpine Vegetation of Mountain Summits in Norway. Ecol. Res. 2017, 32, 579–593. [Google Scholar] [CrossRef]
- Grabherr, G.; Gottfried, M.; Pauli, H. Climate Change Impacts in Alpine Environments. Geogr. Compass 2010, 4, 1133–1153. [Google Scholar] [CrossRef]
- Pellissier, L.; Bråthen, K.A.; Vittoz, P.; Yoccoz, N.G.; Dubuis, A.; Meier, E.S.; Zimmermann, N.E.; Randin, C.F.; Thuiller, W.; Garraud, L.; et al. Thermal Niches Are More Conserved at Cold than Warm Limits in Arctic-Alpine Plant Species. Glob. Ecol. Biogeogr. 2013, 22, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, J.; Guan, X.; Chen, B.; Zhang, L. Long-Term Trends of Precipitable Water and Precipitation over the Tibetan Plateau Derived from Satellite and Surface Measurements. J. Quant. Spectrosc. Radiat. Transf. 2013, 122, 64–71. [Google Scholar] [CrossRef]
- Duan, A.; Xiao, Z. Does the Climate Warming Hiatus Exist over the Tibetan Plateau? Sci. Rep. 2015, 5, 13711. [Google Scholar] [CrossRef]
- Wiens, J.J. Climate-Related Local Extinctions Are Already Widespread among Plant and Animal Species. PLoS Biol. 2016, 14, e2001104. [Google Scholar] [CrossRef]
- Sinervo, B.; Méndez-de-la-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagrán-Santa Cruz, M.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of Lizard Diversity by Climate Change and Altered Thermal Niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Tittensor, D.P.; Walpole, M.; Hill, S.L.L.; Boyce, D.G.; Britten, G.L.; Burgess, N.D.; Butchart, S.H.M.; Leadley, P.W.; Regan, E.C.; Alkemade, R.; et al. A Mid-Term Analysis of Progress toward International Biodiversity Targets. Science 2014, 346, 241–244. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global Effects of Land Use on Local Terrestrial Biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Banks-Leite, C.; Ewers, R.M.; Folkard-Tapp, H.; Fraser, A. Countering the Effects of Habitat Loss, Fragmentation, and Degradation through Habitat Restoration. One Earth 2020, 3, 672–676. [Google Scholar] [CrossRef]
- Kuipers, K.J.J.; May, R.; Verones, F. Considering Habitat Conversion and Fragmentation in Characterisation Fac tors for Land-Use Impacts on Vertebrate Species Richness. Sci. Total Environ. 2021, 801, 149737. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat Fragmentation and Its Lasting Impact on Earth’s Ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Wang, W.; Yang, T.; Jin, L.; Jiang, J. School of Mathematics and Computer Science, Northwest Minzu University, Lanzhou 730030 Vulnerability of two rhodiola species under climate change in the future. Biodivers. Sci. 2021, 29, 1620–1628. [Google Scholar] [CrossRef]
- Wang, W.-T.; Guo, W.-Y.; Jarvie, S.; Svenning, J.-C. The Fate of Meconopsis Species in the Tibeto-Himalayan Re gion under Future Climate Change. Ecol. Evol. 2021, 11, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Pastor, S.; Coissac, E.; Lavergne, S.; Schwörer, C.; Theurillat, J.-P.; Heintzman, P.D.; Wangensteen, O.S.; Tinner, W.; Rey, F.; Heer, M.; et al. High Resolution Ancient Sedimentary DNA Shows That Alpine Plant Diversity Is Associated with Human Land Use and Climate Change. Nat. Commun. 2022, 13, 6559. [Google Scholar] [CrossRef]
- Dziomber, L.; Gobet, E.; Leunda, M.; Gurtner, L.; Vogel, H.; Tournier, N.; Damanik, A.; Szidat, S.; Tinner, W.; Schwörer, C. Palaeoecological Multiproxy Reconstruction Captures Long-Term Climatic and Anthropogenic Impacts on Vegetation Dynamics in the Rhaetian Alps. Rev. Palaeobot. Palynol. 2024, 321, 105020. [Google Scholar] [CrossRef]
- Ren, Z.; He, J.; Cheng, Q.; Ding, S.; Liu, W.; Duan, P.; Jiao, L. Climate Change Prior to Human Activity Reduces the Immobility of Phosphorus in Eutrophic Alpine Lake. J. Clean. Prod. 2022, 335, 130364. [Google Scholar] [CrossRef]
- Keith, D.A.; Rodríguez, J.P.; Brooks, T.M.; Burgman, M.A.; Barrow, E.G.; Bland, L.; Comer, P.J.; Franklin, J.; Link, J.; McCarthy, M.A.; et al. The IUCN Red List of Ecosystems: Motivations, Challenges, and Applications. Conserv. Lett. 2015, 8, 214–226. [Google Scholar] [CrossRef]
- Cardoso, P.; Borges, P.A.V.; Triantis, K.A.; Ferrández, M.A.; Martín, J.L. Adapting the IUCN Red List Criteria for Invertebrates. Biol. Conserv. 2011, 144, 2432–2440. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Keith, D.A.; Rodríguez-Clark, K.M.; Murray, N.J.; Nicholson, E.; Regan, T.J.; Miller, R.M.; Barrow, E.G.; Bland, L.M.; Boe, K.; et al. A Practical Guide to the Application of the IUCN Red List of Ecosystems Criteria. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140003. [Google Scholar] [CrossRef] [PubMed]
- Akçakaya, H.R.; Butchart, S.H.M.; Watson, J.E.M.; Pearson, R.G. Preventing Species Extinctions Resulting from Climate Change. Nat. Clim. Change 2014, 4, 1048–1049. [Google Scholar] [CrossRef]
- Wheatley, C.J.; Beale, C.M.; Bradbury, R.B.; Pearce-Higgins, J.W.; Critchlow, R.; Thomas, C.D. Climate Change Vulnerability for Species—Assessing the Assessments. Glob. Change Biol. 2017, 23, 3704–3715. [Google Scholar] [CrossRef]
- Foden, W.B.; Young, B.E.; Akçakaya, H.R.; Garcia, R.A.; Hoffmann, A.A.; Stein, B.A.; Thomas, C.D.; Wheatley, C.J.; Bickford, D.; Carr, J.A.; et al. Climate Change Vulnerability Assessment of Species. WIREs Clim. Change 2019, 10, e551. [Google Scholar] [CrossRef]
- Rinnan, D.S.; Lawler, J. Climate-niche Factor Analysis: A Spatial Approach to Quantifying Species Vulnerability to Climate Change. Ecography 2019, 42, 1494–1503. [Google Scholar] [CrossRef]
- Khosravi, R.; Hemami, M.-R.; Malakoutikhah, S.; Ashrafzadeh, M.R.; Cushman, S.A. Prey Availability Modulates Predicted Range Contraction of Two Large Felids in Response to Changing Climate. Biol. Conserv. 2021, 255, 109018. [Google Scholar] [CrossRef]
- Wang, W.-T.; Guo, W.-Y.; Jarvie, S.; Serra-Diaz, J.M.; Svenning, J.-C. Anthropogenic Climate Change Increases Vulnerability of Magnolia Species More in Asia than in the Americas. Biol. Conserv. 2022, 265, 109425. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, J.; Zhao, X.; Wei, W.; Hong, M.; Zhou, H.; Zhang, J.; Zhang, Z. The Fate of Giant Panda and Its Sympatric Mammals under Future Climate Change. Biol. Conserv. 2022, 274, 109715. [Google Scholar] [CrossRef]
- Watson, J.E.M.; Dudley, N.; Segan, D.B.; Hockings, M. The Performance and Potential of Protected Areas. Nature 2014, 515, 67–73. [Google Scholar] [CrossRef]
- Gray, C.L.; Hill, S.L.L.; Newbold, T.; Hudson, L.N.; Börger, L.; Contu, S.; Hoskins, A.J.; Ferrier, S.; Purvis, A.; Scharlemann, J.P.W. Local Biodiversity Is Higher inside than Outside Terrestrial Protected Areas Worldwide. Nat. Commun. 2016, 7, 12306. [Google Scholar] [CrossRef]
- Xu, W.; Xiao, Y.; Zhang, J.; Yang, W.; Zhang, L.; Hull, V.; Wang, Z.; Zheng, H.; Liu, J.; Polasky, S.; et al. Strength ening Protected Areas for Biodiversity and Ecosystem Services in China. Proc. Natl. Acad. Sci. USA 2017, 114, 1601–1606. [Google Scholar] [CrossRef]
- Asamoah, E.F.; Beaumont, L.J.; Maina, J.M. Climate and Land-Use Changes Reduce the Benefits of Terrestrial Protected Areas. Nat. Clim. Change 2021, 11, 1105–1110. [Google Scholar] [CrossRef]
- Naidoo, R.; Gerkey, D.; Hole, D.; Pfaff, A.; Ellis, A.M.; Golden, C.D.; Herrera, D.; Johnson, K.; Mulligan, M.; Ricketts, T.H.; et al. Evaluating the Impacts of Protected Areas on Human Well-Being across the Developing World. Sci. Adv. 2019, 5, eaav3006. [Google Scholar] [CrossRef]
- Thapa Karki, S. Do Protected Areas and Conservation Incentives Contribute to Sustainable Livelihoods? A Case Study of Bardia National Park, Nepal. J. Environ. Manag. 2013, 128, 988–999. [Google Scholar] [CrossRef]
- Abukari, H.; Mwalyosi, R.B. Local Communities’ Perceptions about the Impact of Protected Areas on Livelihoods and Community Development. Glob. Ecol. Conserv. 2020, 22, e00909. [Google Scholar] [CrossRef]
- Guo, W.-Y.; Serra-Diaz, J.M.; Schrodt, F.; Eiserhardt, W.L.; Maitner, B.S.; Merow, C.; Violle, C.; Anand, M.; Belluau, M.; Bruun, H.H.; et al. Half of the World’s Tree Biodiversity Is Unprotected and Is Increasingly Threatened by Human Activities. bioRxiv 2020. [Google Scholar] [CrossRef]
- Williams, D.R.; Rondinini, C.; Tilman, D. Global Protected Areas Seem Insufficient to Safeguard Half of the World’s Mammals from Human-Induced Extinction. Proc. Natl. Acad. Sci. USA 2022, 119, e2200118119. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fang, C.; Watson, J.E.M.; Sun, S.; Qi, W.; Wang, Z.; Liu, J. Mixed Effectiveness of Global Protected Areas in Resisting Habitat Loss. Nat. Commun. 2024, 15, 8389. [Google Scholar] [CrossRef]
- Geldmann, J.; Coad, L.; Barnes, M.D.; Craigie, I.D.; Woodley, S.; Balmford, A.; Brooks, T.M.; Hockings, M.; Knights, K.; Mascia, M.B.; et al. A Global Analysis of Management Capacity and Ecological Outcomes in Terrestrial Protected Areas. Conserv. Lett. 2018, 11, e12434. [Google Scholar] [CrossRef]
- Schleicher, J.; Peres, C.A.; Leader-Williams, N. Conservation Performance of Tropical Protected Areas: How Im portant Is Management? Conserv. Lett. 2019, 12, e12650. [Google Scholar] [CrossRef]
- Mouillot, D.; Velez, L.; Albouy, C.; Casajus, N.; Claudet, J.; Delbar, V.; Devillers, R.; Letessier, T.B.; Loiseau, N.; Manel, S.; et al. The Socioeconomic and Environmental Niche of Protected Areas Reveals Global Conservation Gaps and Opportunities. Nat. Commun. 2024, 15, 9007. [Google Scholar] [CrossRef]
- Soroye, P.; Newbold, T.; Kerr, J. Climate Change Contributes to Widespread Declines among Bumble Bees across Continents. Science 2020, 367, 685–688. [Google Scholar] [CrossRef]
- Ben Abdallah, S.; Lasserre, P. A Real Option Approach to the Protection of a Habitat Dependent Endangered Species. Resour. Energy Econ. 2012, 34, 295–318. [Google Scholar] [CrossRef][Green Version]
- Gibbs, K.E.; Currie, D.J. Protecting Endangered Species: Do the Main Legislative Tools Work? PLoS ONE 2012, 7, e35730. [Google Scholar] [CrossRef]
- Sax, D.F.; Smith, K.F.; Thompson, A.R. Managed Relocation: A Nuanced Evaluation Is Needed. Trends Ecol. Evol. 2009, 24, 472–473. [Google Scholar] [CrossRef]
- Olden, J.D.; Kennard, M.J.; Lawler, J.J.; Poff, N.L. Challenges and Opportunities in Implementing Managed Relo cation for Conservation of Freshwater Species: Climate-Change Effects and Species Translocation. Conserv. Biol. 2011, 25, 40–47. [Google Scholar] [CrossRef]
- Liu, H.; Ren, H.; Liu, Q.; Wen, X.; Maunder, M.; Gao, J. Translocation of threatened plants as a conservation meas ure in China. Conserv. Biol. 2015, 29, 1537–1551. [Google Scholar] [CrossRef] [PubMed]
- Špulerová, J.; Izakovičová, Z.; Vlachovičová, M.; Černecký, J. Natural or Semi-Natural Landscape Features as In dicator of Biocultural Value: Observations from Slovakia. Hum. Ecol. 2022, 50, 531–543. [Google Scholar] [CrossRef]
- Duflot, R.; Aviron, S.; Ernoult, A.; Fahrig, L.; Burel, F. Reconsidering the Role of ‘Semi-natural Habitat’ in Agri cultural Landscape Biodiversity: A Case Study. Ecol. Res. 2015, 30, 75–83. [Google Scholar] [CrossRef]
- Ludovicy, S.; Noroozi, J.; Semenchuk, P.; Moser, D.; Wessely, J.; Talebi, A.; Dullinger, S. Protected Area Network Insufficiently Represents Climatic Niches of Endemic Plants in a Global Biodiversity Hotspot. Biol. Conserv. 2022, 275, 109768. [Google Scholar] [CrossRef]
- Yuan, J.; Kang, J.; Yu, C.; Hu, Z. Energy Conservation and Emissions Reduction in China—Progress and Prospective. Renew. Sustain. Energy Rev. 2011, 15, 4334–4347. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Chang, R.-D.; Zillante, G. Challenges for China’s Energy Conservation and Emission Reduction. Energy Policy 2014, 74, 709–713. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, Z.; Sun, H. Cytological Study on the Genus Syncalathium (Asteraceae-lactuceae), an Endemic Taxon to Alpine Scree of the Sino-himalayas. J. Syst. Evol. 2009, 47, 226–230. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, H.; Yang, Y.-P.; Nie, Z.-L. Polyploidy and New Chromosome Counts in Anaphalis (Asteraceae: Gnaphalieae) from the Qinghai-Tibet Plateau of China. J. Syst. Evol. 2010, 48, 58–64. [Google Scholar] [CrossRef]
- Chen, G.; Sun, W.; Hong, D.; Zhou, Z.; Niu, Y.; Nie, Z.; Sun, H.; Zhang, J.; Li, Z. Systematic Significance of Cytology in Cyananthus (Campanulaceae) Endemic to the Sino-himalayan Region. J. Syst. Evol. 2014, 52, 260–270. [Google Scholar] [CrossRef]
- Chirici, G.; Mura, M.; McInerney, D.; Py, N.; Tomppo, E.O.; Waser, L.T.; Travaglini, D.; McRoberts, R.E. A Meta-Analysis and Review of the Literature on the k-Nearest Neighbors Technique for Forestry Applications That Use Remotely Sensed Data. Remote Sens. Environ. 2016, 176, 282–294. [Google Scholar] [CrossRef]
- Zarco-Perello, S.; Simões, N. Ordinary Kriging vs Inverse Distance Weighting: Spatial Interpolation of the Sessile Community of Madagascar Reef, Gulf of Mexico. PeerJ 2017, 5, e4078. [Google Scholar] [CrossRef]
- Colwell, R.K.; Brehm, G.; Cardelús, C.L.; Gilman, A.C.; Longino, J.T. Global Warming, Elevational Range Shifts, and Lowland Biotic Attrition in the Wet Tropics. Science 2008, 322, 258–261. [Google Scholar] [CrossRef]
- Usta, D.F.B.; Teymouri, M.; Chatterjee, U. Assessment of Temperature Changes over Iran during the Twenty-First Century Using CMIP6 Models under SSP1-26, SSP2-4.5, and SSP5-8.5 Scenarios. Arab. J. Geosci. 2022, 15, 416. [Google Scholar] [CrossRef]
- Pu, J.-Y.; Guo, W.-W.; Zhang, H.-T.; Wang, W.-T. Shifting Distribution Patterns of an Endemic Conifer Species in the Himalayan Region under Climate Change: Past, Present, and Future. Glob. Ecol. Conserv. 2024, 55, e03250. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Hausser, J.; Chessel, D.; Perrin, N. Ecological-Niche Factor Analysis: How to Compute Habitat-Suit ability Maps without Absence Data? Ecology 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Hu, B.; Wu, H.; Han, H.; Cheng, X.; Kang, F. Dramatic Shift in the Drivers of Ecosystem Service Trade-Offs across an Aridity Gradient: Evidence from China’s Loess Plateau. Sci. Total Environ. 2023, 858, 159836. [Google Scholar] [CrossRef]

| Species | Bioclimate | Sensitivity | Exposure | Vulnerability | ||
|---|---|---|---|---|---|---|
| SSP2-4.5 | SSP5-8.5 | SSP2-4.5 | SSP5-8.5 | |||
| S. kawaguchii | MDR | 13.58 | 0.22 | 0.31 | 4.07 | 4.22 |
| ISO | 11.19 | 0.14 | 0.17 | 3.57 | 3.62 | |
| MTWQ | 6.08 | 0.30 | 0.44 | 2.81 | 2.96 | |
| PS | 4.44 | 0.05 | 0.08 | 2.16 | 2.19 | |
| PWQ | 30.62 | 0.09 | 0.16 | 5.78 | 5.96 | |
| PCQ | 7.13 | 0.01 | 0.01 | 2.68 | 2.69 | |
| A. erecta | MDR | 4.35 | 0.13 | 0.19 | 2.22 | 2.28 |
| ISO | 3.06 | 0.09 | 0.14 | 1.83 | 1.87 | |
| MTWQ | 11.89 | 0.30 | 0.44 | 3.93 | 4.14 | |
| PS | 8.88 | 0.06 | 0.09 | 3.07 | 3.11 | |
| PWQ | 14.50 | 0.07 | 0.10 | 3.93 | 3.99 | |
| PCQ | 7.23 | 0.02 | 0.03 | 2.71 | 2.72 | |
| C. microphyllus | MDR | 4.13 | 0.20 | 0.28 | 2.23 | 2.30 |
| ISO | 3.50 | 0.19 | 0.27 | 2.04 | 2.11 | |
| MTWQ | 4.24 | 0.28 | 0.42 | 2.33 | 2.45 | |
| PS | 6.54 | 0.14 | 0.21 | 2.74 | 2.81 | |
| PWQ | 9.22 | 0.13 | 0.19 | 3.23 | 3.32 | |
| PCQ | 2.66 | 0.04 | 0.04 | 1.67 | 1.66 | |
| S. purpurea | MDR | 5.41 | 0.23 | 0.33 | 2.58 | 2.68 |
| ISO | 4.82 | 0.24 | 0.33 | 2.44 | 2.53 | |
| MTWQ | 5.15 | 0.33 | 0.48 | 2.62 | 2.76 | |
| PS | 3.97 | 0.12 | 0.17 | 2.11 | 2.16 | |
| PWQ | 8.70 | 0.09 | 0.14 | 3.09 | 3.15 | |
| PCQ | 6.55 | 0.01 | 0.02 | 2.58 | 2.58 | |
| I. younghusbandii | MDR | 3.17 | 0.26 | 0.37 | 2.00 | 2.09 |
| ISO | 5.32 | 0.26 | 0.34 | 2.59 | 2.67 | |
| MTWQ | 6.15 | 0.32 | 0.47 | 2.85 | 3.00 | |
| PS | 4.94 | 0.14 | 0.21 | 2.37 | 2.45 | |
| PWQ | 14.75 | 0.12 | 0.18 | 4.06 | 4.17 | |
| PCQ | 2.30 | 0.02 | 0.02 | 1.53 | 1.53 | |
| A. xylorhiza | MDR | 3.30 | 0.21 | 0.30 | 2.00 | 2.07 |
| ISO | 2.74 | 0.19 | 0.25 | 1.81 | 1.85 | |
| MTWQ | 3.78 | 0.29 | 0.43 | 2.21 | 2.32 | |
| PS | 2.28 | 0.10 | 0.15 | 1.58 | 1.62 | |
| PWQ | 3.91 | 0.12 | 0.18 | 2.09 | 2.15 | |
| PCQ | 1.93 | 0.02 | 0.03 | 1.41 | 1.41 | |
| Species | Marginality | Sensitivity | Exposure | Vulnerability | ||
|---|---|---|---|---|---|---|
| SSP2-4.5 | SSP5-8.5 | SSP2-4.5 | SSP5-8.5 | |||
| S. kawaguchii | 1.241 | 3.489 | 0.409 | 0.593 | 0.787 | 0.806 |
| A. erecta | 0.667 | 2.884 | 0.350 | 0.520 | 0.700 | 0.716 |
| C. microphyllus | 3.148 | 2.247 | 0.441 | 0.638 | 0.598 | 0.614 |
| S. purpurea | 1.033 | 2.402 | 0.492 | 0.707 | 0.642 | 0.659 |
| I. younghusbandii | 1.810 | 2.470 | 0.523 | 0.741 | 0.632 | 0.651 |
| A. xylorhiza | 1.919 | 1.730 | 0.437 | 0.626 | 0.446 | 0.459 |
| Area (%) | Low | Medium | High | PAs | |
|---|---|---|---|---|---|
| S. kawaguchii | SSP2-4.5 | 17.6 (0.89) | 54.3 (4.41) | 28.1 (3.25) | 8.55 |
| SSP5-8.5 | 14.8 (0.81) | 56.9 (4.54) | 28.3 (3.20) | 8.55 | |
| A. erecta | SSP2-4.5 | 48.7 (4.70) | 48.2 (7.11) | 3.1 (0.67) | 12.48 |
| SSP5-8.5 | 38.6 (3.57) | 57.8 (8.16) | 3.6 (0.75) | 12.48 | |
| C. microphyllus | SSP2-4.5 | 26.6 (8.59) | 43.5 (10.71) | 29.9 (5.72) | 25.02 |
| SSP5-8.5 | 22.1 (8.11) | 48.1 (11.26) | 29.8 (5.65) | 25.02 | |
| S. purpurea | SSP2-4.5 | 40.9 (4.42) | 33.8 (6.08) | 25.3 (5.02) | 15.52 |
| SSP5-8.5 | 38.7 (4.28) | 36.1 (6.11) | 25.2 (5.13) | 15.52 | |
| I. younghusbandii | SSP2-4.5 | 7.5 (1.10) | 48.9 (8.24) | 43.6 (6.95) | 16.29 |
| SSP5-8.5 | 8.3 (0.90) | 48.9 (8.82) | 42.8 (6.57) | 16.29 | |
| A. xylorhiza | SSP2-4.5 | 91.5 (17.80) | 8.1 (1.42) | 0.4 (0.01) | 19.23 |
| SSP5-8.5 | 91.5 (17.75) | 8.4 (1.48) | 0.1 (0.00) | 19.23 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.-D.; Wang, W.-T. Vulnerability Assessment of Six Endemic Tibetan-Himalayan Plants Under Climate Change and Human Activities. Plants 2025, 14, 2424. https://doi.org/10.3390/plants14152424
Wei J-D, Wang W-T. Vulnerability Assessment of Six Endemic Tibetan-Himalayan Plants Under Climate Change and Human Activities. Plants. 2025; 14(15):2424. https://doi.org/10.3390/plants14152424
Chicago/Turabian StyleWei, Jin-Dong, and Wen-Ting Wang. 2025. "Vulnerability Assessment of Six Endemic Tibetan-Himalayan Plants Under Climate Change and Human Activities" Plants 14, no. 15: 2424. https://doi.org/10.3390/plants14152424
APA StyleWei, J.-D., & Wang, W.-T. (2025). Vulnerability Assessment of Six Endemic Tibetan-Himalayan Plants Under Climate Change and Human Activities. Plants, 14(15), 2424. https://doi.org/10.3390/plants14152424




