Sustainable Agronomical Practices Affect Essential Oil Composition of Tanacetum balsamita L.
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Experimental Set Up
4.2. Tanacetum balsamita L. Harvest, Essential Oil Extraction, and GC-MS Analysis
- -
- Column: Agilent DB-Wax UI polar capillary column (160 m length, i.d. 0.250 mm, thickness 0.5 μm);
- -
- Carrier gas: Helium at 1.2 mL min−1;
- -
- Oven temperature program: initial = 40 °C; ramp = 5 °C min−1 to 200 °C, then 10 °C min−1 to 240 °C;
- -
- Injection: splitless, 1 µL volume, 1 min splitless time;
- -
- Injection temperature: 250 °C;
- -
- Mass spectrometer settings: ionization energy = 70 eV, scan range = 29–330 amu, scan rate = 4.5 scans sec−1.
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Hart, M.; Liu, H. Paving the way from the lab to the field: Using synthetic microbial consortia to produce high-quality crops. Front. Plant Sci. 2018, 9, 1467. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Pandey, R. Bioinoculant with Vermicompost Augments Essential Oil Constituents and Antioxidants in Mentha arvensis L. J. Plant Growth Regul. 2021, 40, 1281–1297. [Google Scholar] [CrossRef]
- Sen, N.K.; Duran, A. A new approach on essential oil production of Origanum onites L.: Microbial fertilization and microwave extraction. Helyion 2023, 9, e20211. [Google Scholar] [CrossRef]
- Lobo, C.B.; Juárez Tomás, M.S.; Viruel, E.; Ferrero, M.A.; Lucca, M.E. Development of low-cost formulations of plant growth-promoting bacteria to be used as inoculants in beneficial agricultural technologies. Microbiol. Res. 2019, 219, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Lanfranco, L.; Fiorilli, V.; Gutjahr, C. Partner communication and role of nutrients in the arbuscular mycorrhizal symbiosis. New Phytol. 2018, 220, 1031–1046. [Google Scholar] [CrossRef]
- Bianciotto, V.; Victorino, I.; Scariot, V.; Berruti, A. Arbuscular mycorrhizal fungi as natural biofertilizers: Current role and potential for the horticulture industry. Acta Hortic. 2018, 1191, 207–216. [Google Scholar] [CrossRef]
- Kour, R.; Ambardar, S.; Vakhlu, J. Plant growth promoting bacteria associated with corm of Crocus sativus during three growth stages. Lett. Appl. Microbiol. 2018, 67, 458–464. [Google Scholar] [CrossRef]
- Kumar, S.; Arora, N.; Upadhyay, H. Arbuscular mycorrhizal fungi: Source of secondary metabolite production in medicinal plants. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Hoboken, NJ, USA, 2021; pp. 155–164. [Google Scholar]
- Pandey, D.K.; Kaur, P.; Dey, A. Arbuscular Mycorrhizal Fungi: Effects on Secondary Metabolite Production in Medicinal Plants. In Fungi and Their Role in Sustainable Development: Current Perspectives; Gehlot, P., Singh, J., Eds.; Springer: Singapore, 2018; pp. 507–538. [Google Scholar]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; Pascale, S.D.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Evanylo, G.; Sherony, C.; Spargo, J.; Starner, D.; Brosius, M.; Haering, K. Soil and water environmental effects of fertilizer-, manure-, and compost-based fertility practices in an organic vegetable cropping system. Agric. Ecosyst. Environ. 2008, 127, 50–58. [Google Scholar] [CrossRef]
- Lakhdar, A.; Hafsi, C.; Debez, A.; Montemurro, F.; Jedidi, N.; Abdelly, C. Assessing solid waste compost application as a practical approach for salt-affected soil reclamation. Acta Agric. Scand. Sect. B Soil Plant Sci. 2011, 61, 284–288. [Google Scholar] [CrossRef]
- Yasmin, N.; Jamuda, M.; Panda, A.K.; Samal, K.; Nayak, J.K. Emission of greenhouse gases (GHGs) during composting and vermicomposting: Measurement, mitigation, and perspectives. Energy Nexus 2002, 7, 100092. [Google Scholar] [CrossRef]
- Hargreaves, J.; Adl, M.; Warman, P. A review of the use of composted municipal solid waste in agriculture. Agric. Ecosyst. Environ. 2008, 123, 1–14. [Google Scholar] [CrossRef]
- Guerrero, L.A.; Maas, G.; Hogland, W. Solid waste management challenges for cities in developing countries. Waste Manag. 2013, 33, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.D.; Yadav, R.K.; Narjary, B.; Yadav, G.; Jat, H.S.; Sheoran, P.; Meena, M.K.; Antil, R.S.; Meena, B.L.; Singh, H.V.; et al. Municipal solid waste (MSW): Strategies to improve salt affected soil sustainability: A review. Waste Manag. 2019, 84, 38–53. [Google Scholar] [CrossRef]
- Mbarki, S.; Cerdà, A.; Zivcak, M.; Brestic, M.; Rabhi, M.; Mezni, M.; Jedidi, N.; Abdelly, C.; Pascual, J.A. Alfalfa crops amended with MSW compost can compensate the effect of salty water irrigation depending on the soil texture. Process Saf. Environ. Prot. 2018, 115, 8–16. [Google Scholar] [CrossRef]
- Emmerling, C.; Udelhoven, T.; Schneider, R. Long-lasting impact of biowaste-compost application in agriculture on soil-quality parameters in three different crop-rotation systems. J. Plant Nutr. Soil Sci. 2010, 173, 391–398. [Google Scholar] [CrossRef]
- Weber, J.; Kocowicz, A.; Bekier, J.; Jamroz, E.; Tyszka, R.; Debicka, M.; Parylak, D.; Kordas, L. The effect of a sandy soil amendment with municipal solid waste (MSW) compost on nitrogen uptake efficiency by plants. Eur. J. Agron. 2014, 54, 54–60. [Google Scholar] [CrossRef]
- Scotti, R.; Pane, C.; Spaccini, R.; Palese, A.M.; Piccolo, A.; Celano, G.; Zaccardelli, M. On-farm compost: A useful tool to improve soil quality under intensive farming systems. Appl. Soil Ecol. 2016, 107, 13–23. [Google Scholar] [CrossRef]
- Mbarki, S.; Labidi, N.; Mahmoudi, H.; Jedidi, N.; Abdelly, C. Contrasting effects of municipal compost on alfalfa growth in clay and in sandy soils: N, P, K, content and heavy metal toxicity. Bioresour. Technol. 2008, 99, 6745–6750. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, O. Influence of municipal solid waste compost application on heavy metal content in soil. Environ. Monit. Assess. 2015, 187, 313. [Google Scholar] [CrossRef]
- Khatib, S.; Faraloni, C.; Bouissane, L. Tanacetum balsamita L.: Botany, Traditional Uses, Phytochemical Profiling, and Biological Activities. Drugs Drug Candidates 2025, 4, 10. [Google Scholar] [CrossRef]
- Kubo, A.; Kubo, I. Antimicrobial Agents from Tanacetum balsamita. J. Nat. Prod. 1995, 58, 1565–1569. [Google Scholar] [CrossRef]
- Hanganu, D.; Marculescu, A.; Oprean, R.; Tamas, M.; Popescu, H. Identification of some compounds of the essential oil from Chrysanthemum balsamita L. (Asteraceae). Clujul. Med. 1995, 68, 244–247. [Google Scholar]
- Todorova, M.N.; Ognyanov, I.V. Sesquiterpene lactones in a population of Balsamita major cultivated in Bulgaria. Phytochemistry 1989, 28, 1115–1117. [Google Scholar] [CrossRef]
- Bylaitė, E.; Venskutonis, R.; Roozen, J.P.; Posthumus, M.A. Composition of Essential Oil of Costmary Balsamita major (L.) Desf. at Different Growth Phases. J. Agric. Food Chem. 2000, 48, 2409–2414. [Google Scholar] [CrossRef]
- Bestmann, H.J.; Claßen, B.; Kobold, U.; Vostrowsky, O.; Klingauf, F.; Strobel, H.; Knobloch, K. Pflanzliche Insektizide II [1]. Das ätherische Öl aus Blättern des Balsamkrautes, Chrysanthemum balsamita L. Insektizide Wirkung und Zusammensetzung/Herbal Insecticides II [1]. The Essential Oil from Leaves of Chrysanthemum balsamita L. Insecticidal Activity and Composition. Z. Naturforschung C 1984, 39, 543–547. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.B.; Tabatabaie, S.J.; Nazemiyeh, H.; Vojodi, L.; Aazami, M.A.; Shoja, A.M. Chrysanthemum balsamita (L.) Baill.: A forgotten medicinal plant. Facta Univ. Ser. Med. Biol. 2008, 15, 119–124. [Google Scholar]
- Bagci, E.; Kursat, M.; Kocak, A.; Gur, S. Composition and Antimicrobial Activity of the Essential Oils of Tanacetum balsamita L. subsp. balsamita and T. chiliophyllum (Fisch. et Mey.) Schultz Bip. var. chiliophyllum (Asteraceae) from Turkey. J. Essent. Oil Bear. Plants 2008, 11, 476–484. [Google Scholar] [CrossRef]
- Ivashchenko, I. Antimicrobial properties of Tanacetum balsamita L. (Asteraceae) introduced in Ukrainian Polissya. Ukr. J. Ecol. 2017, 7, 52–57. [Google Scholar] [CrossRef]
- Sharif, M.; Najafizadeh, P.; Asgarpanah, J.; Mousavi, Z. In vivo analgesic and anti-inflammatory effects of the essential oil from Tanacetum balsamita L. Braz. J. Pharm. Sci. 2020, 56, e18357. [Google Scholar] [CrossRef]
- Venskutonis, P.R. Costmary (Chrysanthemum balsamita) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 365–375. [Google Scholar]
- Bonetti, A.; Grattacaso, M.; Di Lonardo, S.; D’Acqui, L.P. Impact of Sustainable Soil Cropping Management on the Production and Stability of Bioactive Compounds in Tanacetum balsamita L. by Cold Pressure Extraction. Plants 2025, 14, 948. [Google Scholar] [CrossRef]
- Bączek, K.B.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Costa, R.; Mondello, L.; Gniewosz, M.; Synowiec, A.; Węglarz, Z. Antibacterial and antioxidant activity of essential oils and extracts from costmary (Tanacetum balsamita L.) and tansy (Tanacetum vulgare L.). Ind. Crops Prod. 2017, 102, 154–163. [Google Scholar] [CrossRef]
- Gharib, A.F.; Moussa, L.A.; Massoud, O.N. Effect of compost and bio-fertilizers on growth, yield and essential oil of sweet marjoram (Majorana hortensis) plant. Int. J. Agric. Biol. 2008, 10, 381–387. [Google Scholar]
- Boutahiri, S.; Benr’Kia, R.; Tembeni, B.; Idowu, O.E.; Olatunji, O.J. Effect of biostimulants on the chemical profile of food crops under normal and abiotic stress conditions. Curr. Plant Biol. 2024, 40, 100410. [Google Scholar] [CrossRef]
- Vukic, M.D.; Vukovic, N.L.; Obradovic, A.D.; Galovičová, L.; Čmiková, N.; Kačániová, M.; Matic, M.M. Chemical Composition and Biological Activity of Tanacetum balsamita Essential Oils Obtained from Different Plant Organs. Plants 2022, 11, 3474. [Google Scholar] [CrossRef]
- Gallori, S.; Flamini, G.; Bilia, A.R.; Morelli, I.; Landini, A.; Vincieri, F.F. Chemical Composition of Some Traditional Herbal Drug Preparations: Essential Oil and Aromatic Water of Costmary (Balsamita suaveolens Pers.). J. Agric. Food Chem. 2001, 49, 5907–5910. [Google Scholar] [CrossRef]
- Rosselli, S.; Bruno, M.; Raimondo, F.M.; Spadaro, V.; Varol, M.; Koparal, A.T.; Maggio, A. Cytotoxic Effect of Eudesmanolides Isolated from Flowers of Tanacetum Vulgare ssp. Siculum. Mol. 2012, 17, 8186–8195. [Google Scholar] [CrossRef]
- Prerna, J.C.; Khullar, L.; Mudgil, U.; Harjai, K. A comprehensive review on the pharmacological prospects of Terpinen-4-ol: From nature to medicine and beyond. Fitoterapia 2024, 176, 106051. [Google Scholar] [CrossRef] [PubMed]
- Vuković, N.L.; Vukić, M.; Branković, J.; Petrović, V.; Galovičova, L.; Čmikova, N.; Kačaniova, M. The antimicrobial and antibiofilm potential of the Piper nigrum L. essential oil: In vitro, in situ, and in silico study. Ind. Crops Prod. 2024, 209, 118075. [Google Scholar] [CrossRef]
- Formisano, C.; Senatore, F.; Bruno, M.; Rosselli, S.; Bellone, G.; Spadaro, V. Essential Oil Composition of Tanacetum vulgare Subsp. siculum (Guss.) Raimondo et Spadaro (Asteraceae) from Sicily. Nat. Prod. Commun. 2009, 4, 567–570. [Google Scholar]
- EMA (European Medicines Agency). Public Statement on the Use of Herbal Medicinal Products Containing Thujone EMA/HMPC/732886/2010. Available online: https://www.ema.europa.eu/en/documents/public-statement/draft-public-statement-use-herbal-medicinal-products-containing-thujone_en.pdf (accessed on 22 May 2025).
- EC (European Commission). Opinion of the Scientific Committee on Food on Thujone. Available online: https://ec.europa.eu/food/fs/sc/scf/out162_en.pdf (accessed on 22 May 2025).
- Lachenmeier, D.W.; Emmert, J.; Kuballa, T.; Sartor, G. Thujone—Cause of absinthism? Forensic Sci. Int. 2006, 158, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Walch, S.G.; Padosch, S.A.; Kröner, L.U. Absinthe—A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 365–377. [Google Scholar] [CrossRef]
- Kumar, V.; Traji, D. Chemical composition and biological activity of essential oil of genus Tanacetum—A review. J. Pharmacogn. Phytochem. 2013, 2, 155–159. [Google Scholar]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals; Elsevier Health Science: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Selvaraj, A.; Valliammai, A.; Sivasankar, C.; Suba, M.; Sakthivel, G.; Pandian, S.K. Antibiofilm and antivirulence efficacy of myrtenol enhances the antibiotic susceptibility of Acinetobacter baumannii. Sci. Rep. 2020, 10, 21975. [Google Scholar] [CrossRef]
- Barbhuiya, P.A.; Pathak, M.P. Myrtenol: A promising terpene with potent pharmacological properties. Pharmacol. Res. Nat. Prod. 2024, 4, 100067. [Google Scholar] [CrossRef]
- Silva, R.O.; Salvadori, M.S.; Sousa, F.B.M.; Santos, M.S.; Carvalho, N.S.; Sousa, D.P.; Gomes, B.S.; Oliveira, F.A.; Barbosa, A.L.R.; Freitas, R.M.; et al. Evaluation of the anti-inflammatory and antinociceptive effects of myrtenol, a plant-derived monoterpene alcohol, in mice. Flavour Fragr. J. 2014, 29, 184–192. [Google Scholar] [CrossRef]
- Touhtouh, J.; Laghmari, M.; Benali, T.; Aanniz, T.; Lemhadri, A.; Akhazzane, M.; Habbadi, K.; Bouyahya, A.; Zengin, G.; Hammani, K. Determination of the antioxidant and enzyme-inhibiting activities and evaluation of selected terpenes’ ADMET properties: In vitro and in silico approaches. Biochem. Syst. Ecol. 2023, 111, 104733. [Google Scholar] [CrossRef]
- Moreira, M.R.C.; Salvadori, M.G.D.S.S.; De Almeida, A.A.C.; De Sousa, D.P.; Jordán, J.; Satyal, P.; De Freitas, R.M.; De Almeida, R.N. Anxiolytic-like effects and mechanism of (−)-myrtenol: A monoterpene alcohol. Neurosci. Lett. 2014, 579, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.; La Pietra, A.; De Alteriis, E.; Mileo, A.; De Falco, M.; Guida, M.; Galdiero, E. Effect of Myrtenol and Its Synergistic Interactions with Antimicrobial Drugs in the Inhibition of Single and Mixed Biofilms of Candida auris and Klebsiella pneumoniae. Microorganisms 2022, 10, 1773. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Abreu, F.F.; Bispo, J.M.M.; Cerqueira, A.R.A.; Santos, J.R.D.; Correa, C.B.; Costa, S.K.P.; Camargo, E.A. Myrtenol Reduces Orofacial Nociception and Inflammation in Mice Through p38-MAPK and Cytokine Inhibition. Front. Pharmacol. 2022, 13, 910219. [Google Scholar] [CrossRef] [PubMed]
- Viana, A.F.S.C.; Lopes, M.T.P.; Oliveira, F.T.B.; Nunes, P.I.G.; Santos, V.G.; Braga, A.D.; Silva, A.C.A.; Sousa, D.P.; Viana, D.A.; Rao, V.S.; et al. (−)-Myrtenol accelerates healing of acetic acid-induced gastric ulcers in rats and in human gastric adenocarcinoma cells. Eur. J. Pharmacol. 2019, 854, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Prakash, B. Effect of Environmental Factors on Essential Oil Biosynthesis, Chemical Stability, and Yields. In Plant Essential Oils; Prakash, B., Dubey, N.K., de São José, J.F., Eds.; Springer Nature: Singapore, 2024; pp. 225–247. [Google Scholar]

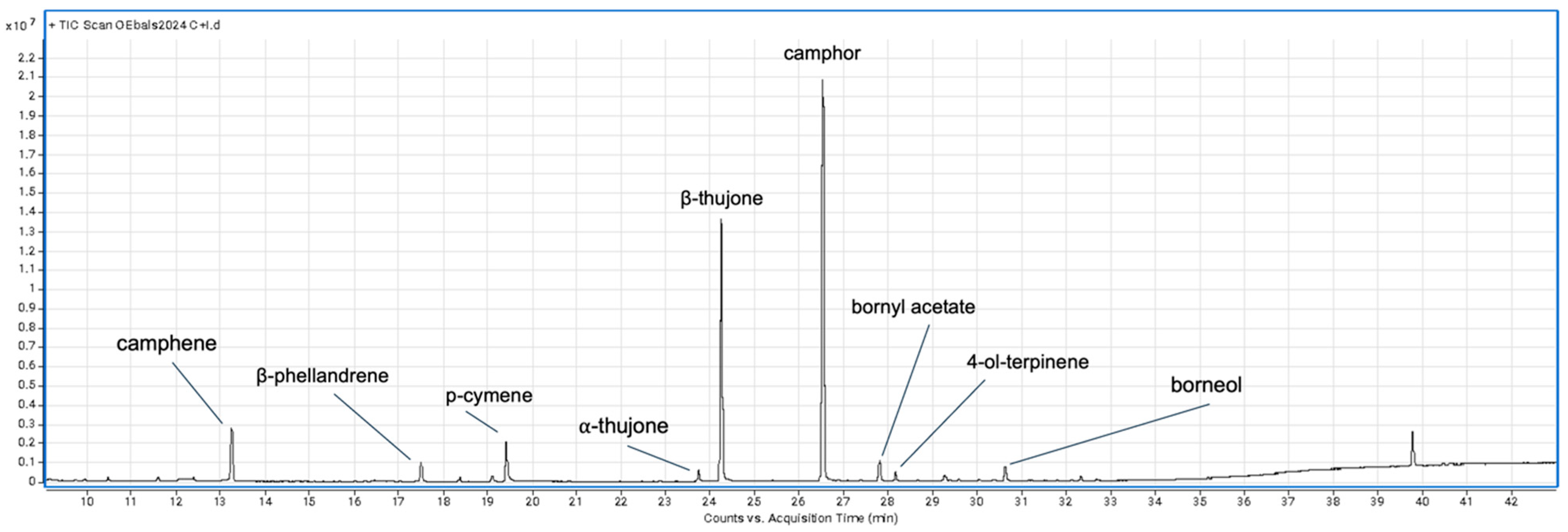
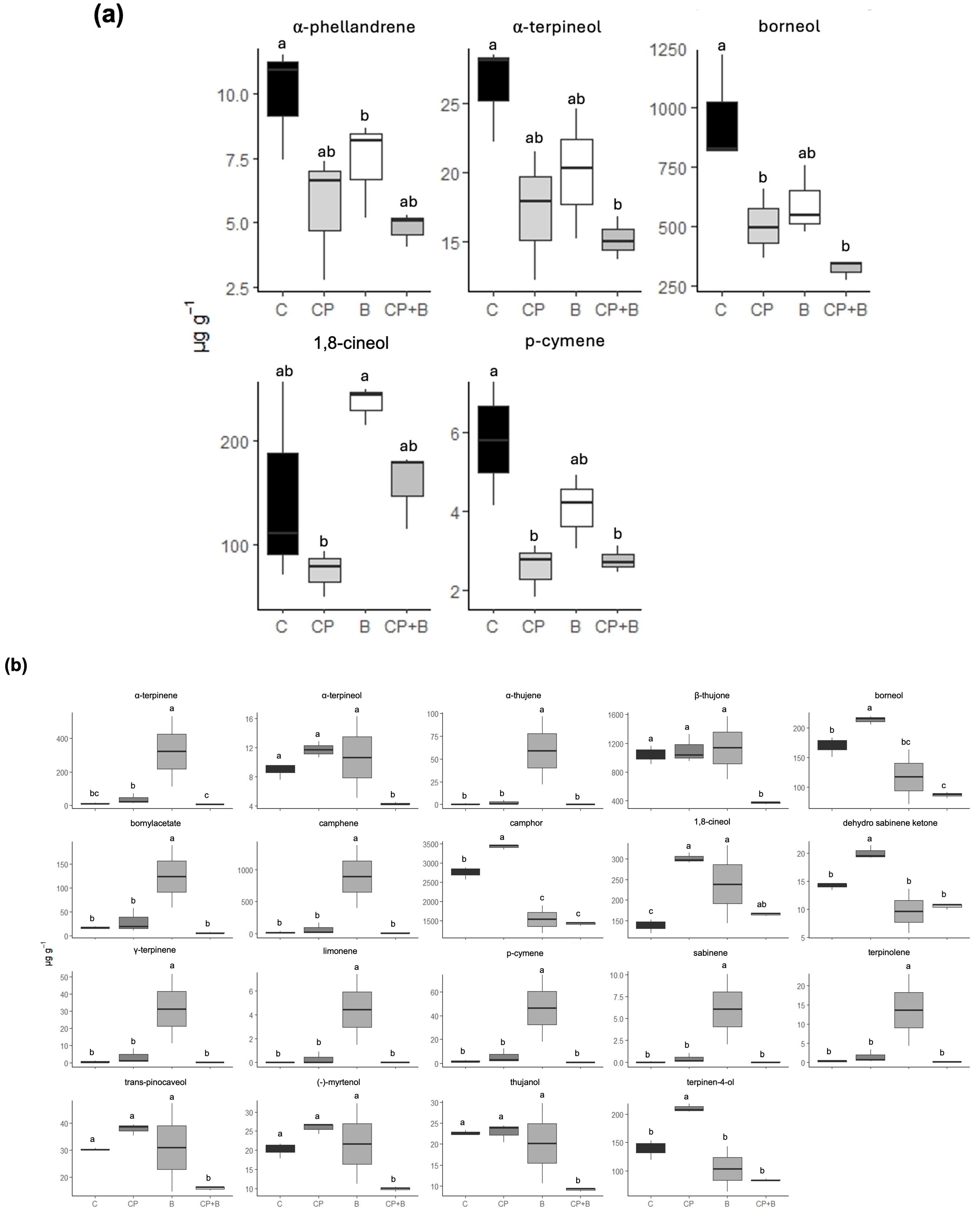

| 2023 | 2024 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | B | CP | C + I | C | B | CP | C + I | ||||||||||
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | ||
| (µg g−1 FW) | (µg g−1 FW) | (µg g−1 FW) | (µg g−1 FW) | (µg g−1 FW) | (µg g−1 FW) | (µg g−1 FW) | (µg g−1 FW) | ||||||||||
| 1 | α-thujene | 3.3 | 8.2 | 3.4 | 7.2 | 2.3 | 4.3 | 2.5 | 5.9 | 0.1 | 1.1 | 0.1 | 97.0 | 0.2 | 4.9 | 0.2 | 0.4 |
| 2 | camphene | 1.2 | 15.9 | 1.9 | 3.0 | 0.3 | 0.4 | 0.5 | 1.0 | 4.9 | 36.3 | 2.5 | 1380.1 | 9.8 | 172.1 | 9.1 | 13.3 |
| 3 | Sabinene | 6.0 | 14.2 | 6.4 | 13.3 | 3.9 | 7.5 | 4.3 | 10.2 | n.d. | 0.1 | n.d. | 10.0 | 0.1 | 1.1 | n.d. | 0.1 |
| 4 | α-phellandrene | 7.4 | 11.5 | 5.2 | 8.7 | 2.8 | 7.4 | 4.0 | 5.3 | 60.4 | 67.0 | 14.0 | 206.7 | 83.4 | 94.3 | 30.5 | 33.7 |
| 5 | α-terpinene | 8.6 | 17.3 | 7.5 | 14.6 | 5.3 | 9.8 | 5.8 | 10.8 | 8.1 | 17.1 | 2.1 | 532.0 | 13.9 | 70.3 | 6.3 | 8.4 |
| 6 | limonene | 1.3 | 2.9 | 1.1 | 2.1 | 1.0 | 1.5 | 0.9 | 1.9 | n.d. | n.d. | n.d. | n.d. | ||||
| 7 | β-phellandrene | 12.0 | 19.6 | 9.3 | 14.6 | 4.9 | 12.2 | 7.1 | 9.8 | 94.0 | 109.0 | 21.0 | 333.0 | 141.0 | 157.0 | 51.0 | 55.0 |
| 8 | 1.8-cineol | 70.7 | 266 | 214.1 | 248.1 | 49.5 | 93.9 | 114.0 | 181.0 | 119.0 | 153.0 | 43.0 | 334.0 | 292.0 | 316.0 | 161.0 | 169.0 |
| 9 | γ-terpinene | 5.0 | 11.9 | 5.1 | 11.4 | 3.3 | 6.5 | 3.5 | 8.7 | 0.2 | 1.2 | 0.1 | 51.5 | 0.6 | 8.2 | 0.3 | 0.5 |
| 10 | p-cymene | 4.1 | 7.5 | 3.0 | 4.9 | 1.8 | 3.1 | 2.5 | 3.1 | 1.0 | 2.8 | 0.3 | 74.7 | 1.8 | 12.2 | 0.8 | 1.1 |
| 11 | terpinolene | 3.0 | 6.6 | 2.7 | 6.1 | 1.8 | 3.7 | 1.9 | 4.6 | n.d. | 0.6 | 0.1 | 23.0 | 0.4 | 3.3 | 0.2 | 0.3 |
| 12 | α-thujone | 40.2 | 165 | 59.8 | 162.7 | 9.8 | 47.3 | n.d. | 107.0 | 61.1 | 81.2 | 15.4 | 188.7 | 62.2 | 87.3 | 21.4 | 24.1 |
| 13 | β-thujone | n.d. | n.d. | n.d. | n.d. | 911.4 | 1160.0 | 233.5 | 1575.0 | 952.3 | 1324.0 | 352.6 | 383.8 | ||||
| 14 | Camphor | 3191.0 | 5828.0 | 3110.0 | 4563.0 | 3592.0 | 4163.0 | 2470.0 | 4259.0 | 2567.0 | 2882.0 | 806.0 | 1897.0 | 3349.0 | 3461.0 | 1363.0 | 1449.0 |
| 15 | bornylacetate | 67.2 | 202.0 | 36.8 | 86.0 | 31.7 | 52.9 | 32.0 | 60.1 | 14.9 | 20.3 | 4.9 | 189.0 | 11.3 | 58.1 | 5.7 | 7.8 |
| 16 | terpinen 4-ol | 283.0 | 388.0 | 183.0 | 342.0 | 0.0 | 257.0 | 41.2 | 190.0 | 119.5 | 153.2 | 31.9 | 143.4 | 204 | 218.5 | 83.2 | 86.2 |
| 17 | dehydro sabinene ketone | n.d. | n.d. | n.d. | n.d. | 0.27 | 0.34 | 0.18 | 0.30 | 13.5 | 14.6 | 3.7 | 13.6 | ||||
| 18 | 3-thujanol | n.d. | n.d. | n.d. | n.d. | 0.44 | 0.52 | 0.35 | 0.40 | 22.4 | 23.3 | 4.8 | 29.7 | ||||
| 19 | trans-Pinocarveol | n.d. | n.d. | n.d. | n.d. | 0.60 | 0.70 | 0.48 | 0.65 | 29.9 | 30.9 | 8.2 | 47.3 | ||||
| 20 | trans-Verbenol | n.d. | n.d. | n.d. | n.d. | 0.26 | 0.28 | 0.28 | 0.60 | 12.1 | 13.1 | 3.5 | 44.7 | ||||
| 21 | α-terpinenol | 22.2 | 28.5 | 15.2 | 24.6 | 12.2 | 21.5 | 13.8 | 16.9 | 7.5 | 9.6 | 1.9 | 16.3 | 10.6 | 12.9 | 4.2 | 4.5 |
| 22 | Borneol | 813.0 | 1222.0 | 478.0 | 753.0 | 365.0 | 654.0 | 272.0 | 349.0 | 150.8 | 182.8 | 38.3 | 163.2 | 205.1 | 219.2 | 81.4 | 90.8 |
| 23 | verbenone | 6.6 | 12.1 | 8.7 | 3.5 | 6.0 | 11.9 | 7.5 | 10.5 | n.d. | n.d. | n.d. | n.d. | ||||
| 24 | Carvone | 17.0 | 30.8 | 13.7 | 16.8 | 13.9 | 25.5 | 12.6 | 17.1 | n.d. | n.d. | n.d. | n.d. | ||||
| 25 | (−)-myrtenol | 44.5 | 72.7 | 34.1 | 59.5 | 28.7 | 48.6 | 24.1 | 50.4 | 17.8 | 21.7 | 5.7 | 32.2 | 24.2 | 26.7 | 9.2 | 10.3 |
| Compound | Factor | |||
|---|---|---|---|---|
| Treatment | Year | Treatment × Year | ||
| 1 | α-thujene | n.s. | n.s. | n.s. |
| 2 | camphene | n.s. | n.s. | n.s. |
| 3 | sabinene | n.s. | * | n.s. |
| 4 | α-phellandrene | n.s. | ** | n.s. |
| 5 | α-terpinene | n.s. | n.s. | n.s. |
| 6 | limonene | n.s. | n.s. | n.s. |
| 7 | β-phellandrene | n.s. | ** | n.s. |
| 8 | 1,8-cineol | n.s. | n.s. | ** |
| 9 | γ-terpinene | n.s. | n.s. | n.s. |
| 10 | p-cymene | n.s. | n.s. | n.s. |
| 11 | Terpinolene | n.s. | n.s. | n.s. |
| 12 | α-thujone | n.s. | n.s. | n.s. |
| 13 | β-thujone | n.s. | ** | n.s. |
| 14 | camphor | ** | *** | n.s. |
| 15 | bornylacetate | n.s. | n.s. | n.s. |
| 16 | terpinen 4-ol | * | * | * |
| 17 | dehydro sabinene ketone | *** | *** | *** |
| 18 | 3-thujanol | * | *** | * |
| 19 | trans-pinocarveol | n.s. | *** | n.s. |
| 20 | trans-verbenol | n.s. | *** | n.s. |
| 21 | α-terpineol | * | *** | n.s. |
| 22 | borneol | *** | *** | ** |
| 23 | Verbenone | n.s. | *** | n.s. |
| 24 | Carvone | n.s. | *** | n.s. |
| 25 | (−)-myrtenol | n.s. | *** | n.s. |
| Mean Temperature (°C) | T Min (°C) | T Max (°C) | Mean Humidity (%) | Mean Wind Speed (km h−1) | Rain (Days) | Hail (Days) | Summer Storms (Days) | Fog (Days) | |
|---|---|---|---|---|---|---|---|---|---|
| 2023 | |||||||||
| May | 18.7 | 12.9 | 23.7 | 67.1 | 9.3 | 15 | 0 | 5 | 2 |
| June | 23.3 | 17.1 | 29.6 | 65.8 | 7.1 | 14 | 0 | 12 | 0 |
| 2024 | |||||||||
| May | 18.7 | 13.1 | 24.2 | 69.0 | 3.7 | 11 | 0 | 2 | 1 |
| June | 22.4 | 16.2 | 28.3 | 65.2 | 0.0 | 7 | 0 | 4 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grattacaso, M.; Bonetti, A.; Di Lonardo, S.; D’Acqui, L.P. Sustainable Agronomical Practices Affect Essential Oil Composition of Tanacetum balsamita L. Plants 2025, 14, 2406. https://doi.org/10.3390/plants14152406
Grattacaso M, Bonetti A, Di Lonardo S, D’Acqui LP. Sustainable Agronomical Practices Affect Essential Oil Composition of Tanacetum balsamita L. Plants. 2025; 14(15):2406. https://doi.org/10.3390/plants14152406
Chicago/Turabian StyleGrattacaso, Martina, Alessandra Bonetti, Sara Di Lonardo, and Luigi Paolo D’Acqui. 2025. "Sustainable Agronomical Practices Affect Essential Oil Composition of Tanacetum balsamita L." Plants 14, no. 15: 2406. https://doi.org/10.3390/plants14152406
APA StyleGrattacaso, M., Bonetti, A., Di Lonardo, S., & D’Acqui, L. P. (2025). Sustainable Agronomical Practices Affect Essential Oil Composition of Tanacetum balsamita L. Plants, 14(15), 2406. https://doi.org/10.3390/plants14152406








