Preliminary Analysis of Bilberry NaDES Extracts as Versatile Active Ingredients of Natural Dermocosmetic Products: In Vitro Evaluation of Anti-Tyrosinase, Anti-Hyaluronidase, Anti-Collagenase, and UV Protective Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC Analysis
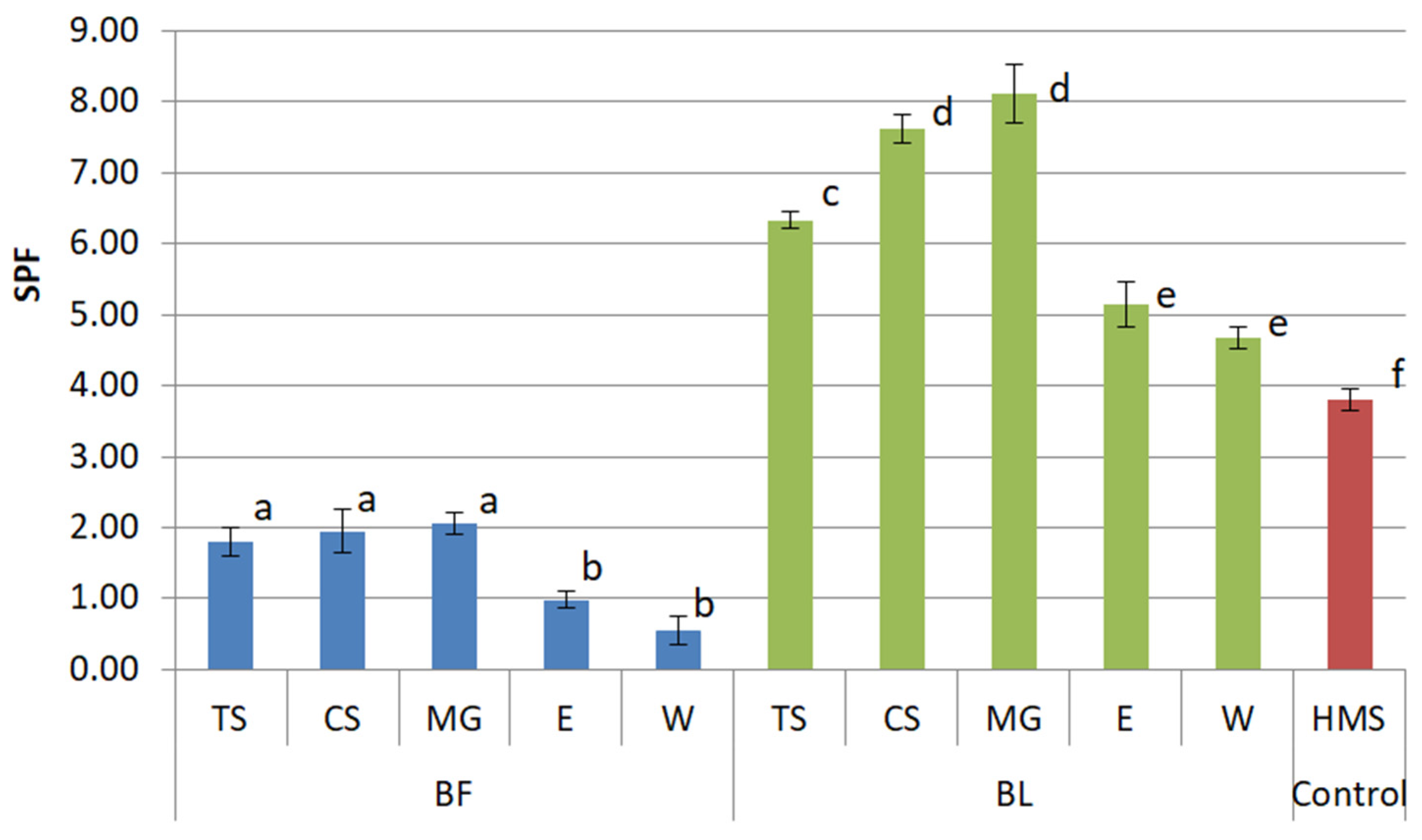
2.2. UV Protective Activity
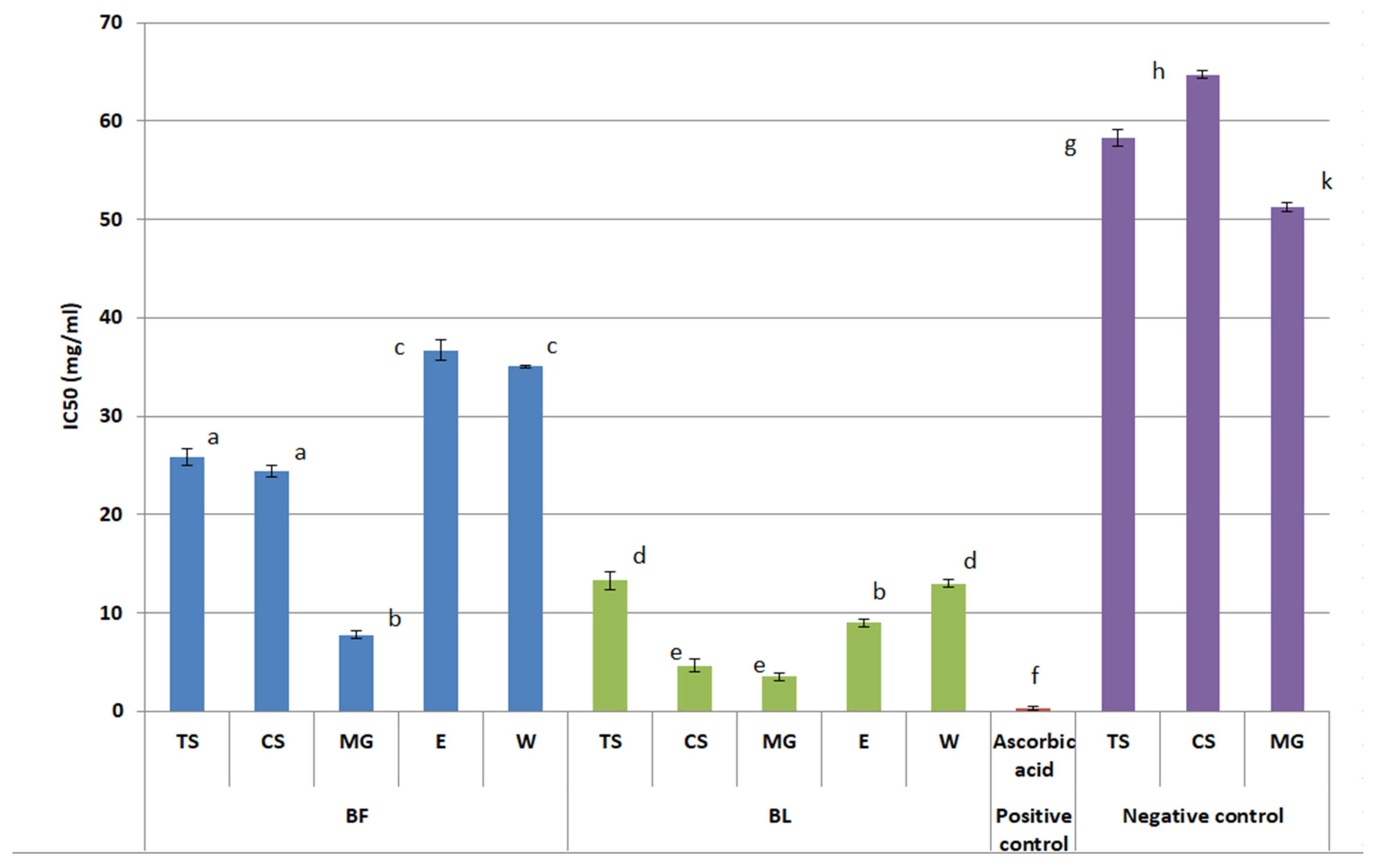
2.3. Anti-Tyrosinase Activity
2.4. Anti-Hyaluronidase Activity
2.5. Anti-Collagenase Activity
2.6. Correlation Study
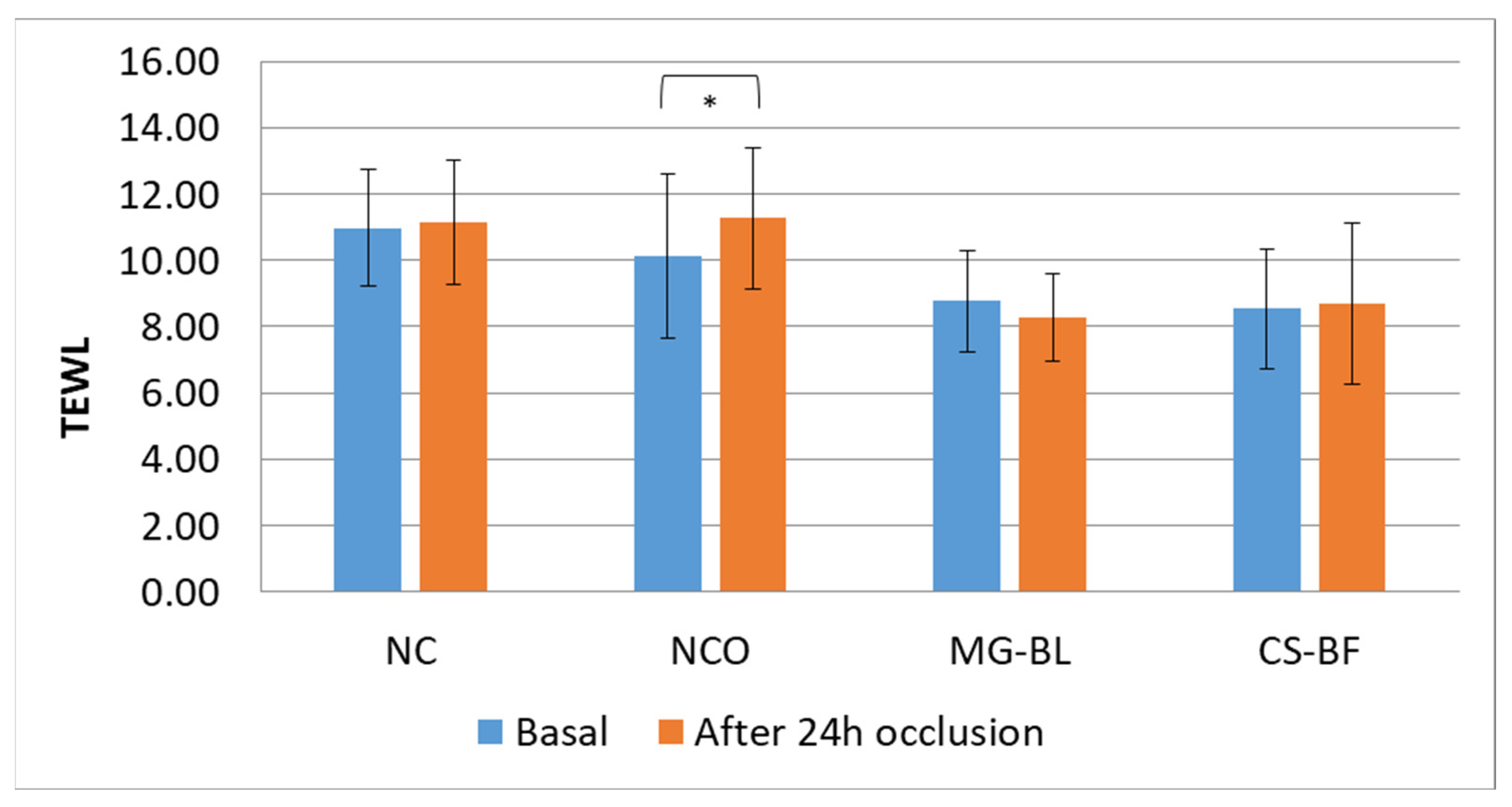
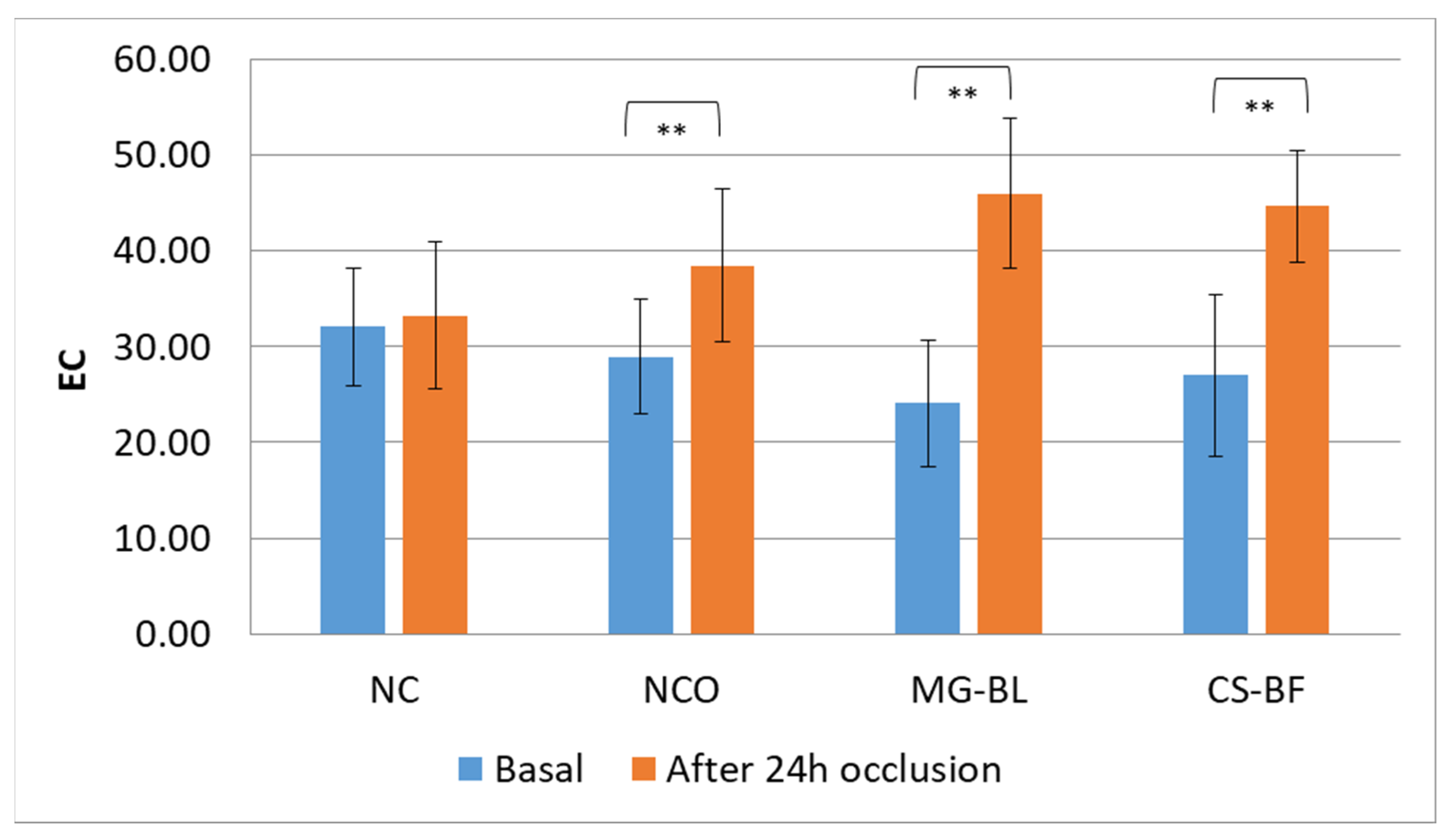
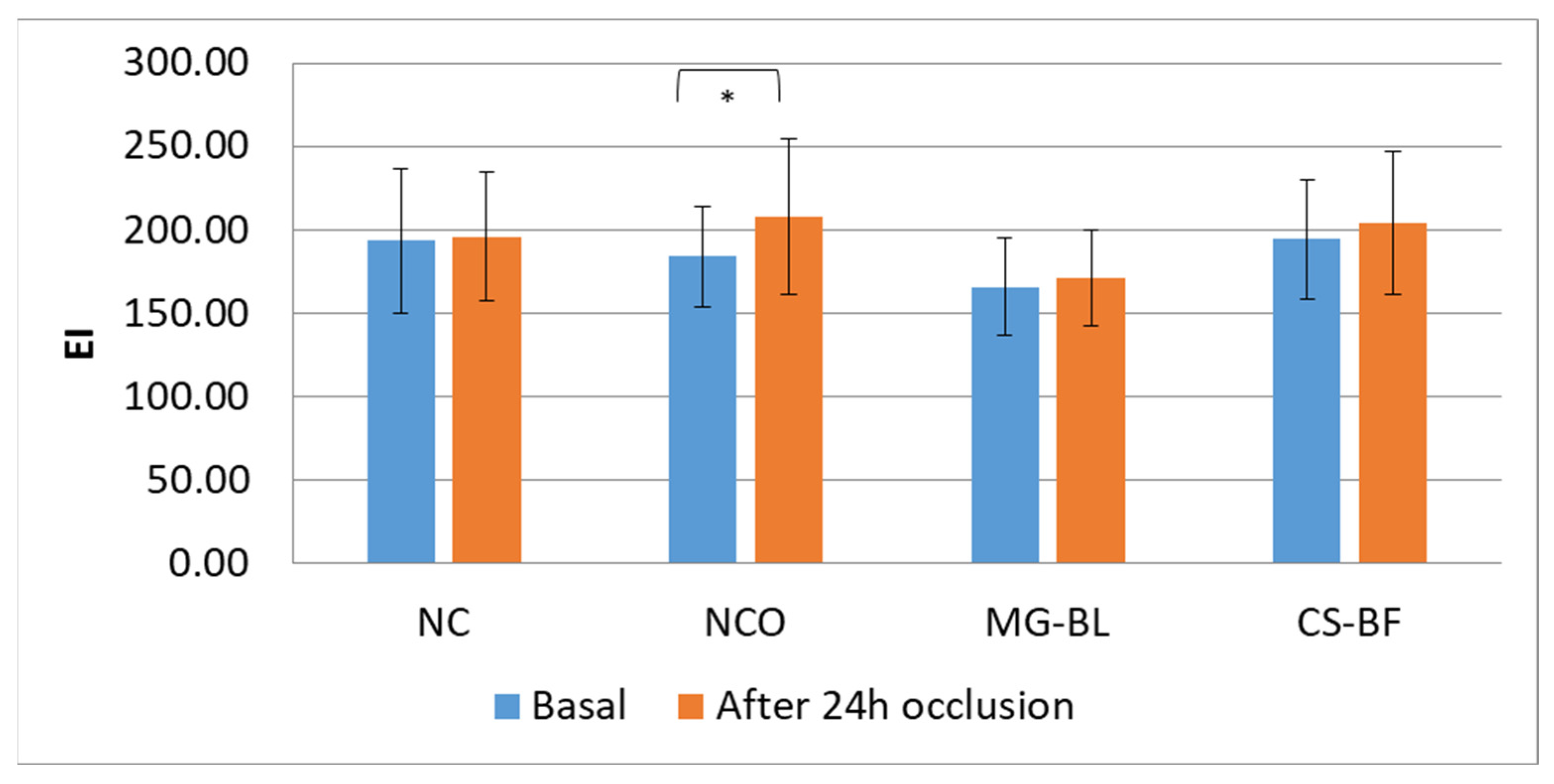
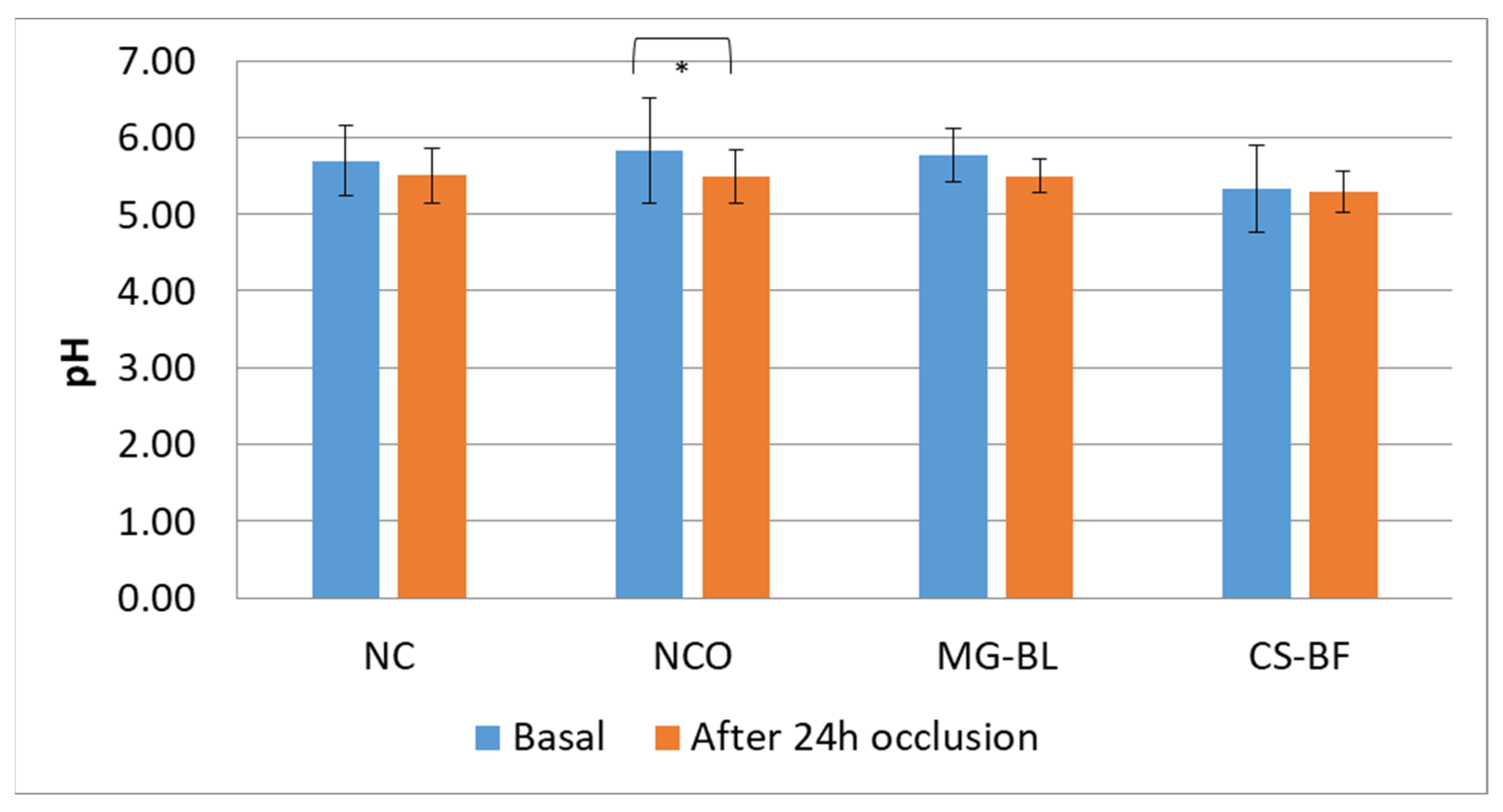
2.7. In Vivo Safety Assessment
3. Materials and Methods
3.1. Materials
3.2. NaDES Extracts Preparation
3.3. SPF Calculation Using Mansur Equation
3.4. HPLC Analysis
- Chlorogenic acid: 0.45 mg/mL;
- Protocatechuic acid: 0.52 mg/mL;
- Delphinidin-3-O-glucoside: 0.39 mg/mL;
- Hyperoside: 0.40 mg/mL;
- Cyanidin-3-O-galactoside: 0.42 mg/mL;
- Cyanidin-3-O-glucoside: 0.42 mg/mL;
- Procyanidin B2: 0.36 mg/mL;
- Quercetin-3-O-glucoside: 0.39 mg/mL;
- Rutin: 0.48 mg/mL;
- Quercitrin: 0.52 mg/mL;
- Epicatechin: 0.40 mg/mL.
3.5. Determination of Anti-Tyrosinase Activity
3.6. Determination of Anti-Hyaluronidase Activity
3.7. Determination of Anti-Collagenase Activity
3.8. In Vivo Safety Assessment
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Franca, C.C.V.; Ueno, H.M. Green Cosmetics: Perspectives and Challenges in the Context of Green Chemistry. Desenvolv. Meio Ambiente 2020, 53, 133–150. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Elferjane, M.R.; Milutinović, V.; Jovanović Krivokuća, M.; Taherzadeh, M.J.; Pietrzak, W.; Marinković, A.; Jovanović, A.A. Vaccinium myrtillus L. Leaf Waste as a Source of Biologically Potent Compounds: Optimization of Polyphenol Extractions, Chemical Profile, and Biological Properties of the Extracts. Pharmaceutics 2024, 16, 740. [Google Scholar] [CrossRef]
- Tadić, V.M.; Nešić, I.; Martinović, M.; Rój, E.; Brašanac-Vukanović, S.; Maksimović, S.; Žugić, A. Old Plant, New Possibilities: Wild Bilberry (Vaccinium myrtillus L., Ericaceae) in Topical Skin Preparation. Antioxidants 2021, 10, 465. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Dycha, N.; Lechwar, P.; Lasota, M.; Okoń, E.; Szczeblewski, P.; Wawruszak, A.; Tarabasz, D.; Hubert, J.; Wilkołek, P.; et al. Vaccinium Species—Unexplored Sources of Active Constituents for Cosmeceuticals. Biomolecules 2024, 14, 1110. [Google Scholar] [CrossRef] [PubMed]
- CosIng-Cosmetics-GROWTH—European Commission. Available online: https://ec.europa.eu/growth/tools-databases/cosing (accessed on 19 March 2025).
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Li, H.; Yang, K.; Yang, Y.; Ding, L.; Li, X. Natural Deep Eutectic Solvents (NADES) in Drug Delivery Systems: Characteristics, Applications, and Future Perspectives. Int. J. Pharm. 2025, 675, 125509. [Google Scholar] [CrossRef]
- Martinović, M.; Krgović, N.; Nešić, I.; Žugić, A.; Tadić, V.M. Conventional vs. Green Extraction Using Natural Deep Eutectic Solvents—Differences in the Composition of Soluble Unbound Phenolic Compounds and Antioxidant Activity. Antioxidants 2022, 11, 2295. [Google Scholar] [CrossRef]
- Cruz, A.M.; Gonçalves, M.C.; Marques, M.S.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. In Vitro Models for Anti-Aging Efficacy Assessment: A Critical Update in Dermocosmetic Research. Cosmetics 2023, 10, 66. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV Radiation-Induced Inflammation and Immunosuppression Accelerate the Aging Process in the Skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Ayad, R.; Lefahal, M.; Makhloufi, E.H.; Akkal, S. Photoprotection Strategies with Antioxidant Extracts: A New Vision. Phys. Sci. Rev. 2024, 9, 2273–2286. [Google Scholar] [CrossRef]
- Palos-Hernández, A.; González-Paramás, A.M.; Santos-Buelga, C. Latest Advances in Green Extraction of Polyphenols from Plants, Foods and Food By-Products. Molecules 2025, 30, 55. [Google Scholar] [CrossRef]
- Jovanović, M.S.; Krgović, N.; Živković, J.; Stević, T.; Zdunić, G.; Bigović, D.; Šavikin, K. Ultrasound-Assisted Natural Deep Eutectic Solvents Extraction of Bilberry Anthocyanins: Optimization, Bioactivities, and Storage Stability. Plants 2022, 11, 2680. [Google Scholar] [CrossRef]
- da Silva, D.T.; Pauletto, R.; da Silva Cavalheiro, S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; de Bona da Silva, C.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural Deep Eutectic Solvents as a Biocompatible Tool for the Extraction of Blueberry Anthocyanins. J. Food Compos. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Grillo, G.; Gunjević, V.; Radošević, K.; Redovniković, I.R.; Cravotto, G. Deep Eutectic Solvents and Nonconventional Technologies for Blueberry-Peel Extraction: Kinetics, Anthocyanin Stability, and Antiproliferative Activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Off. J. Eur. Union L 2009, 342, 59–209. [Google Scholar]
- Santos-Martín, M.; Cubero-Cardoso, J.; González-Domínguez, R.; Cortés-Triviño, E.; Sayago, A.; Urbano, J.; Fernández-Recamales, Á. Ultrasound-Assisted Extraction of Phenolic Compounds from Blueberry Leaves Using Natural Deep Eutectic Solvents (NADES) for the Valorization of Agrifood Wastes. Biomass Bioenergy 2023, 175, 106882. [Google Scholar] [CrossRef]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The Skin Aging Exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- Dimitrovska Cvetkovska, A.; Manfredini, S.; Ziosi, P.; Molesini, S.; Dissette, V.; Magri, I.; Scapoli, C.; Carrieri, A.; Durini, E.; Vertuani, S. Factors affecting SPF in vitro measurement and correlation with in vivo results. Int. J. Cosmet. Sci. 2017, 39, 310–319. [Google Scholar] [CrossRef]
- Skarupova, D.; Vostalova, J.; Rajnochova Svobodova, A. Ultraviolet A Protective Potential of Plant Extracts and Phytochemicals. Biomed. Pap. 2020, 164, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Calò, R.; Marabini, L. Protective Effect of Vaccinium myrtillus Extract against UVA- and UVB-Induced Damage in a Human Keratinocyte Cell Line (HaCaT Cells). J. Photochem. Photobiol. B 2014, 132, 27–35. [Google Scholar] [CrossRef]
- Svobodová, A.; Rambousková, J.; Walterová, D.; Vostalová, J. Bilberry Extract Reduces UVA-induced Oxidative Stress in HaCaT Keratinocytes: A Pilot Study. BioFactors 2008, 33, 249–266. [Google Scholar] [CrossRef]
- Klavins, L.; Mezulis, M.; Nikolajeva, V.; Klavins, M. Composition, Sun Protective and Antimicrobial Activity of Lipophilic Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium Vitis-Idaea L.) Extract Fractions. LWT 2021, 138, 110784. [Google Scholar] [CrossRef]
- Franco, J.G.; Cefali, L.C.; Ataide, J.A.; Santini, A.; Souto, E.B.; Mazzola, P.G. Effect of Nanoencapsulation of Blueberry (Vaccinium myrtillus): A Green Source of Flavonoids with Antioxidant and Photoprotective Properties. Sustain. Chem. Pharm. 2021, 23, 100515. [Google Scholar] [CrossRef]
- Jaakola, L.; Määttä-Riihinen, K.; Kärenlampi, S.; Hohtola, A. Activation of Flavonoid Biosynthesis by Solar Radiation in Bilberry (Vaccinium myrtillus L.) Leaves. Planta 2004, 218, 721–728. [Google Scholar] [CrossRef]
- Li, H.-R.; Habasi, M.; Xie, L.-Z.; Aisa, H.A. Effect of Chlorogenic Acid on Melanogenesis of B16 Melanoma Cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Shi, S.; Yang, G.; Liu, Z.; Wang, J.; Kang, W. Spectrum-Effect Relationship of Antioxidant and Tyrosinase Activity with Malus Pumila Flowers by UPLC-MS/MS and Component Knock-out Method. Food Chem. Toxicol. 2019, 133, 110754. [Google Scholar] [CrossRef]
- Jung, H.G.; Kim, H.H.; Paul, S.; Jang, J.Y.; Cho, Y.H.; Kim, H.J.; Yu, J.M.; Lee, E.S.; An, B.J.; Kang, S.C.; et al. Quercetin-3-O-β-d-Glucopyranosyl-(1 → 6)-β-d-Glucopyranoside Suppresses Melanin Synthesis by Augmenting P38 MAPK and CREB Signaling Pathways and Subsequent cAMP down-Regulation in Murine Melanoma Cells. Saudi J. Biol. Sci. 2015, 22, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.-X.; Yin, S.-J.; Oh, S.; Wang, Z.-J.; Ye, S.; Yan, L.; Yang, J.-M.; Park, Y.-D.; Lee, J.; Qian, G.-Y. An Integrated Study of Tyrosinase Inhibition by Rutin: Progress Using a Computational Simulation. J. Biomol. Struct. Dyn. 2012, 29, 999–1012. [Google Scholar] [CrossRef]
- Pohntadavit, K.; Duangmano, S.; Osiriphan, M.; Leksawasdi, N.; Techapun, C.; Sumonsiri, N.; Sommano, S.R.; Rachtanapun, P.; Nunta, R.; Khemacheewakul, J. Tyrosinase Inhibitory Activity of Crude Procyanidin Extract from Green Soybean Seed and the Stability of Bioactive Compounds in an Anti-Aging Skin Care Formulation. Cosmetics 2024, 11, 178. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Li, M.; Liu, Z.; Wu, J.; Chen, F.; Zhang, S. Inhibition of Tyrosinase and Melanogenesis by Carboxylic Acids: Mechanistic Insights and Safety Evaluation. Molecules 2025, 30, 1642. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; He, Q.; Xu, M.; Chen, F.-F.; Li, F.; Chen, Y.-P. Unveiling Acetobacter Syzygii from Tibetan Kefir Grain: Fermentation-Enhanced Anti-Tyrosinase, and Anti-Melanin. Fermentation 2024, 10, 459. [Google Scholar] [CrossRef]
- Lai, J.; Lin, C.; Chiang, T. Tyrosinase Inhibitory Activity and Thermostability of the Flavonoid Complex from Sophora Japonica L. (Fabaceae). Trop. J. Pharm. Res. 2014, 13, 243–247. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; Gómez-Serranillos, M.P.; Smith, C.; López, V. Anthocyanin Profile, Antioxidant Activity and Enzyme Inhibiting Properties of Blueberry and Cranberry Juices: A Comparative Study. Food Funct. 2017, 8, 4187–4193. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Paczkowska-Walendowska, M.; Erdem, C.; Paluszczak, J.; Kleszcz, R.; Hoszman-Kulisz, M.; Cielecka-Piontek, J. Anti-Aging Properties of Chitosan-Based Hydrogels Rich in Bilberry Fruit Extract. Antioxidants 2024, 13, 105. [Google Scholar] [CrossRef]
- Jung, H. Hyaluronidase: An Overview of Its Properties, Applications, and Side Effects. Arch. Plast. Surg. 2022, 47, 297–300. [Google Scholar] [CrossRef]
- Bravo, B.; Correia, P.; Gonçalves Junior, J.E.; Sant’Anna, B.; Kerob, D. Benefits of Topical Hyaluronic Acid for Skin Quality and Signs of Skin Aging: From Literature Review to Clinical Evidence. Dermatol. Ther. 2022, 35, e15903. [Google Scholar] [CrossRef]
- Luo, H.; Wang, J.; Zhou, Y.; Zou, K. Molecular Docking Study of Chlorogenic Acid as a Hyaluronidase Inhibitor. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Sholihah, I.A.; Raif, M.A.; Kunardi, S.; Million, H.; Widowati, W. Antioxidant and Antiaging Activity of Rutin and Caffeic Acid. Pharmaciana 2020, 10, 147–156. [Google Scholar] [CrossRef]
- M. Mohamed, E.; H. Hetta, M.; Rateb, M.E.; A. Selim, M.; M. AboulMagd, A.; A. Badria, F.; Abdelmohsen, U.R.; Alhadrami, H.A.; M. Hassan, H. Bioassay-Guided Isolation, Metabolic Profiling, and Docking Studies of Hyaluronidase Inhibitors from Ravenala Madagascariensis. Molecules 2020, 25, 1714. [Google Scholar] [CrossRef]
- Tomohara, K.; Ito, T.; Onikata, S.; Kato, A.; Adachi, I. Discovery of Hyaluronidase Inhibitors from Natural Products and Their Mechanistic Characterization under DMSO-Perturbed Assay Conditions. Bioorg. Med. Chem. Lett. 2017, 27, 1620–1623. [Google Scholar] [CrossRef] [PubMed]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Bethasari, M.; Amalia, A.; Widowati, W. Comparison of Antiaging and Antioxidant Activities of Protocatechuic and Ferulic Acids. Mol. Cell. Biomed. Sci. 2020, 4, 68–75. [Google Scholar] [CrossRef]
- Finke, A.; Bétemps, J.B.; Molliard, S.G. In Vitro Study of the Gel Cohesivity and Persistence to Hyaluronidase Degradation of a Novel Stabilized Composition of 26 Mg/mL of High Molecular Weight HA. Plast. Aesthet. Res. 2024, 11, 51. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Y.; Zhou, S.; Kang, L.; Li, C. A Straightforward Ninhydrin-Based Method for Collagenase Activity and Inhibitor Screening of Collagenase Using Spectrophotometry. Anal. Biochem. 2013, 437, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Aziz, J.; Shezali, H.; Radzi, Z.; Yahya, N.A.; Abu Kassim, N.H.; Czernuszka, J.; Rahman, M.T. Molecular Mechanisms of Stress-Responsive Changes in Collagen and Elastin Networks in Skin. Ski. Pharmacol. Physiol. 2016, 29, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Ciganović, P.; Jakupović, L.; Momchev, P.; Nižić Nodilo, L.; Hafner, A.; Zovko Končić, M. Extraction Optimization, Antioxidant, Cosmeceutical and Wound Healing Potential of Echinacea Purpurea Glycerolic Extracts. Molecules 2023, 28, 1177. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Süntar, I.; Ilhan, M.; Aras, E. In Vitro Enzyme Inhibitory Effects of Rubus Sanctus Schreber and Its Active Metabolite as a Function of Wound Healing Activity. J. Herb. Med. 2015, 5, 207–210. [Google Scholar] [CrossRef]
- Mandrone, M.; Lorenzi, B.; Venditti, A.; Guarcini, L.; Bianco, A.; Sanna, C.; Ballero, M.; Poli, F.; Antognoni, F. Antioxidant and Anti-Collagenase Activity of Hypericum hircinum L. Ind. Crops Prod. 2015, 76, 402–408. [Google Scholar] [CrossRef]
- Sin, B.Y.; Kim, H.P. Inhibition of Collagenase by Naturally-Occurring Flavonoids. Arch. Pharm. Res. 2005, 28, 1152–1155. [Google Scholar] [CrossRef]
- Tang, S.-C.; Yang, J.-H. Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules 2018, 23, 863. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Uede, K.; Yonei, N.; Kishioka, A.; Ohtani, T.; Furukawa, F. Effects of Alpha-Hydroxy Acids on the Human Skin of Japanese Subjects: The Rationale for Chemical Peeling. J. Dermatol. 2006, 33, 16–22. [Google Scholar] [CrossRef]
- Penkova, R.; Goshev, I.; Gorinstein, S.; Nedkov, P. Stability of Collagen During Denaturation. J. Protein Chem. 1999, 18, 397–401. [Google Scholar] [CrossRef]
- Buarque, F.S.; Monteiro e Silva, S.A.; Ribeiro, B.D. Choline Chloride-Based Deep Eutectic Solvent as an Inhibitor of Metalloproteases (Collagenase and Elastase) in Cosmetic Formulation. 3 Biotech 2023, 13, 219. [Google Scholar] [CrossRef]
- Juszczak, A.M.; Marijan, M.; Jakupović, L.; Tomczykowa, M.; Tomczyk, M.; Zovko Končić, M. Glycerol and Natural Deep Eutectic Solvents Extraction for Preparation of Luteolin-Rich Jasione Montana Extracts with Cosmeceutical Activity. Metabolites 2023, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Damjanović, A.; Radošević, K.; Cvjetko Bubalo, M.; Dujmić, F.; Škegro, M.; Radojčić Redovniković, I.; Brnčić, M. Enhanced Preparative-Scale Extraction from Graševina Grape Pomace Using Ultrasound-Assisted Extraction and Natural Deep Eutectic Solvents. Appl. Sci. 2024, 14, 6185. [Google Scholar] [CrossRef]
- Tasic-Kostov, M.; Vesic, S.; Savic, S. Objective Skin Performance Evaluation: How Mild Are APGs to the Skin? In Alkyl Polyglucosides; Elsevier: Amsterdam, The Netherlands, 2014; pp. 135–161. ISBN 978-1-907568-65-7. [Google Scholar]
- du Plessis, J.; Stefaniak, A.; Eloff, F.; John, S.; Agner, T.; Chou, T.-C.; Nixon, R.; Steiner, M.; Franken, A.; Kudla, I.; et al. International Guidelines for the in Vivo Assessment of Skin Properties in Non-Clinical Settings: Part 2. Transepidermal Water Loss and Skin Hydration. Ski. Res. Technol. 2013, 19, 265–278. [Google Scholar] [CrossRef]
- Ariffin, N.H.M.; Hasham, R. Assessment of Non-Invasive Techniques and Herbal-Based Products on Dermatological Physiology and Intercellular Lipid Properties. Heliyon 2020, 6, e03955. [Google Scholar] [CrossRef]
- Milošević, S.; Bebek Markovinović, A.; Teslić, N.; Mišan, A.; Pojić, M.; Brčić Karačonji, I.; Jurica, K.; Lasić, D.; Putnik, P.; Bursać Kovačević, D.; et al. Use of Natural Deep Eutectic Solvent (NADES) as a Green Extraction of Antioxidant Polyphenols from Strawberry Tree Fruit (Arbutus Unedo L.): An Optimization Study. Microchem. J. 2024, 200, 110284. [Google Scholar] [CrossRef]
- Wils, L.; Leman-Loubière, C.; Bellin, N.; Clément-Larosière, B.; Pinault, M.; Chevalier, S.; Enguehard-Gueiffier, C.; Bodet, C.; Boudesocque-Delaye, L. Natural Deep Eutectic Solvent Formulations for Spirulina: Preparation, Intensification, and Skin Impact. Algal Res. 2021, 56, 102317. [Google Scholar] [CrossRef]
- Vieira, C.; Rebocho, S.; Craveiro, R.; Paiva, A.; Duarte, A.R.C. Selective Extraction and Stabilization of Bioactive Compounds from Rosemary Leaves Using a Biphasic NADES. Front. Chem. 2022, 10, 954835. [Google Scholar] [CrossRef]
- Mansur, J.d.S.; Breder, M.N.R.; Mansur, M.C.d.; Azulay, R.D. Correlaçäo entre a determinaçäo do fator de proteçäo solar em seres humanos e por espectrofotometria. An. Bras. Dermatol. 1986, 61, 167–172. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A Comparison of in Vivo and in Vitro Testing of Sunscreening Formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Enzymatic Assay of Hyaluronidase (3.2.1.35). Available online: https://www.sigmaaldrich.com/RS/en/technical-documents/protocol/protein-biology/enzyme-activity-assays/enzymatic-assay-of-hyaluronidase?srsltid=AfmBOoo6_9A0TFYXupq7TLPc3_CUqDsZpV360qVM09_od1ych2ooIiaR (accessed on 3 March 2025).
- Phupaisan, N.; Ampasavate, C.; Natakankitkul, S.; Kiattisin, K. Optimizing Antioxidant and Anti-Hyaluronidase Activities of Mixed Coffea Arabica, Centella Asiatica, and Curcuma Longa Extracts for Cosmetic Application. Cosmetics 2024, 11, 201. [Google Scholar] [CrossRef]


| Phenolic Compounds (mg/g) | TS-BF | CS-BF | MG-BF | E-BF | W-BF |
|---|---|---|---|---|---|
| Chlorogenic acid | 1.53 ± 0.11 a | 1.51 ± 0.01 a | 1.47 ± 0.37 a | 0.83 ± 0.03 b | 0.86 ± 0.06 b |
| Protocatechuic acid | 1.48 ± 0.39 ab | 1.30 ± 0.28 ab | 1.35 ± 0.15 ab | 1.03 ± 0.02 ab | 0.94 ± 0.04 b |
| Delphinidin-3-O-glucoside | 1.07 ± 0.02 a | 0.99 ± 0.05 a | 1.01 ± 0.05 a | tr | tr |
| Hyperoside | 0.49 ± 0.01 a | 0.50 ± 0.07 a | 0.57 ± 0.06 a | 0.53 ± 0.03 a | 0.15 ± 0.03 b |
| Cyanidin-3-O-glucoside | 0.19 ± 0.01 a | 0.18 ± 0.01 a | 0.19 ± 0.02 a | tr | tr |
| Cyanidin-3-O-galactoside | 0.10 ± 0.04 a | 0.11 ± 0.03 a | 0.10 ± 0.03 a | tr | tr |
| Phenolic Compounds (mg/g) | TS-BL | CS-BL | MG-BL | E-BL | W-BL |
| Chlorogenic acid | 19.48 ± 0.92 ab | 22.48 ± 1.57 ab | 21.17 ± 1.75 ab | 22.51 ± 0.50 a | 8.37 ± 0.06 c |
| Procyanidin B2 | 7.57 ± 1.16 a | 15.77 ± 0.68 b | 18.59 ± 1.23 c | 14.41 ± 0.80 b | nd |
| Quercetin-3-O-glucoside | 6.76 ± 0.16 a | 8.29 ± 0.12 b | 8.02 ± 0.08 b | 9.58 ± 0.22 c | 4.88 ± 0.20 d |
| Rutin | 3.16 ± 0.05 a | 3.60 ± 0.26 bc | 3.24 ± 0.10 ab | 3.79 ± 0.12 c | 1.93 ± 0.15 |
| Hyperoside | 2.21 ± 0.07 a | 2.85 ± 0.09 b | 2.57 ± 0.11 c | 3.31 ± 0.10 d | 1.32 ± 0.11 e |
| Quercitrin | 0.69 ± 0.04 ab | 0.90 ± 0.06 cd | 0.76 ± 0.05 ad | 0.99 ± 0.10 c | 0.56 ± 0.07 be |
| Epicatechin | 0.51 ± 0.09 a | 1.74 ± 0.08 b | 1.61 ± 0.27 b | 1.92 ± 0.13 b | nd |
| IC50 (mg/mL) | BF | BL | Solvents (Negative Control) |
|---|---|---|---|
| TS | 3.20 ± 0.03 ab | 2.05 ± 0.40 d | n.d. |
| CS | 3.67 ± 0.25 bc | 2.63 ± 0.14 ad | n.d. |
| MG | 3.02 ± 0.36 c | 1.84 ± 0.50 e | n.d. |
| W | >10 f | >10 f | n.d. |
| E | >10 f | >10 f | n.d. |
| Rutin | 0.35 ± 0.13 g (positive control) | ||
| SPF | 1.000 | |||||
|---|---|---|---|---|---|---|
| Tyr | −0.879 ** | 1.000 | ||||
| Hyal | −0.321 | 0.322 | 1.000 | |||
| Col | −0.733 * | 0.512 | 0.817 ** | 1.000 | ||
| Chlorogenic acid | 0.894 ** | −0.759 * | 0.066 | −0.201 | 1.000 | |
| Hyperoside | 0.935 ** | −0.752 * | −0.044 | −0.289 | 0.983 ** | 1 |
| SPF | Tyr | Hyal | Col | Chlorogenic acid | Hyperoside |
| Chromatographic Conditions | Anthocyanins Analysis | Flavonoids and Phenolcarboxylic Acids Analysis |
|---|---|---|
| Column | Lichrospher 100 RP-18e column (250 × 4.6 mm, 5.0 μm particle size) | |
| Mobile phase | 0.1 M phosphoric acid (phase A) pure acetonitrile (phase B). | |
| Gradient program | 0–11% B (5 min), 11–15% B (25 min), 15–18% B (8 min), isocratic 18% B (8 min), 18–30% B (4 min), 30–100% B (3 min), 100% B (7 min) | 11–25% B (35 min), 25–40% B (20 min), 40–65% B (5 min), 65–100% B (10 min) |
| Total run time | 60 min | 70 min |
| Flow rate | 0.8 mL/min | 1.0 mL/min |
| Injection volume | 4 μL | 10 μL |
| Column temperature | 25 °C | 25 °C |
| λ for PDA detector | 520 nm | 260 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinović, M.; Nešić, I.; Žugić, A.; Tadić, V.M. Preliminary Analysis of Bilberry NaDES Extracts as Versatile Active Ingredients of Natural Dermocosmetic Products: In Vitro Evaluation of Anti-Tyrosinase, Anti-Hyaluronidase, Anti-Collagenase, and UV Protective Properties. Plants 2025, 14, 2374. https://doi.org/10.3390/plants14152374
Martinović M, Nešić I, Žugić A, Tadić VM. Preliminary Analysis of Bilberry NaDES Extracts as Versatile Active Ingredients of Natural Dermocosmetic Products: In Vitro Evaluation of Anti-Tyrosinase, Anti-Hyaluronidase, Anti-Collagenase, and UV Protective Properties. Plants. 2025; 14(15):2374. https://doi.org/10.3390/plants14152374
Chicago/Turabian StyleMartinović, Milica, Ivana Nešić, Ana Žugić, and Vanja M. Tadić. 2025. "Preliminary Analysis of Bilberry NaDES Extracts as Versatile Active Ingredients of Natural Dermocosmetic Products: In Vitro Evaluation of Anti-Tyrosinase, Anti-Hyaluronidase, Anti-Collagenase, and UV Protective Properties" Plants 14, no. 15: 2374. https://doi.org/10.3390/plants14152374
APA StyleMartinović, M., Nešić, I., Žugić, A., & Tadić, V. M. (2025). Preliminary Analysis of Bilberry NaDES Extracts as Versatile Active Ingredients of Natural Dermocosmetic Products: In Vitro Evaluation of Anti-Tyrosinase, Anti-Hyaluronidase, Anti-Collagenase, and UV Protective Properties. Plants, 14(15), 2374. https://doi.org/10.3390/plants14152374






