Improved Biomass Production and Secondary Metabolism: A Critical Review of Grafting in Cannabis sativa
Abstract
1. Introduction
2. Literature Search Strategy
3. Recent Advances in Grafting-Mediated Alteration in Biomass and Secondary Metabolism in Cannabis sativa
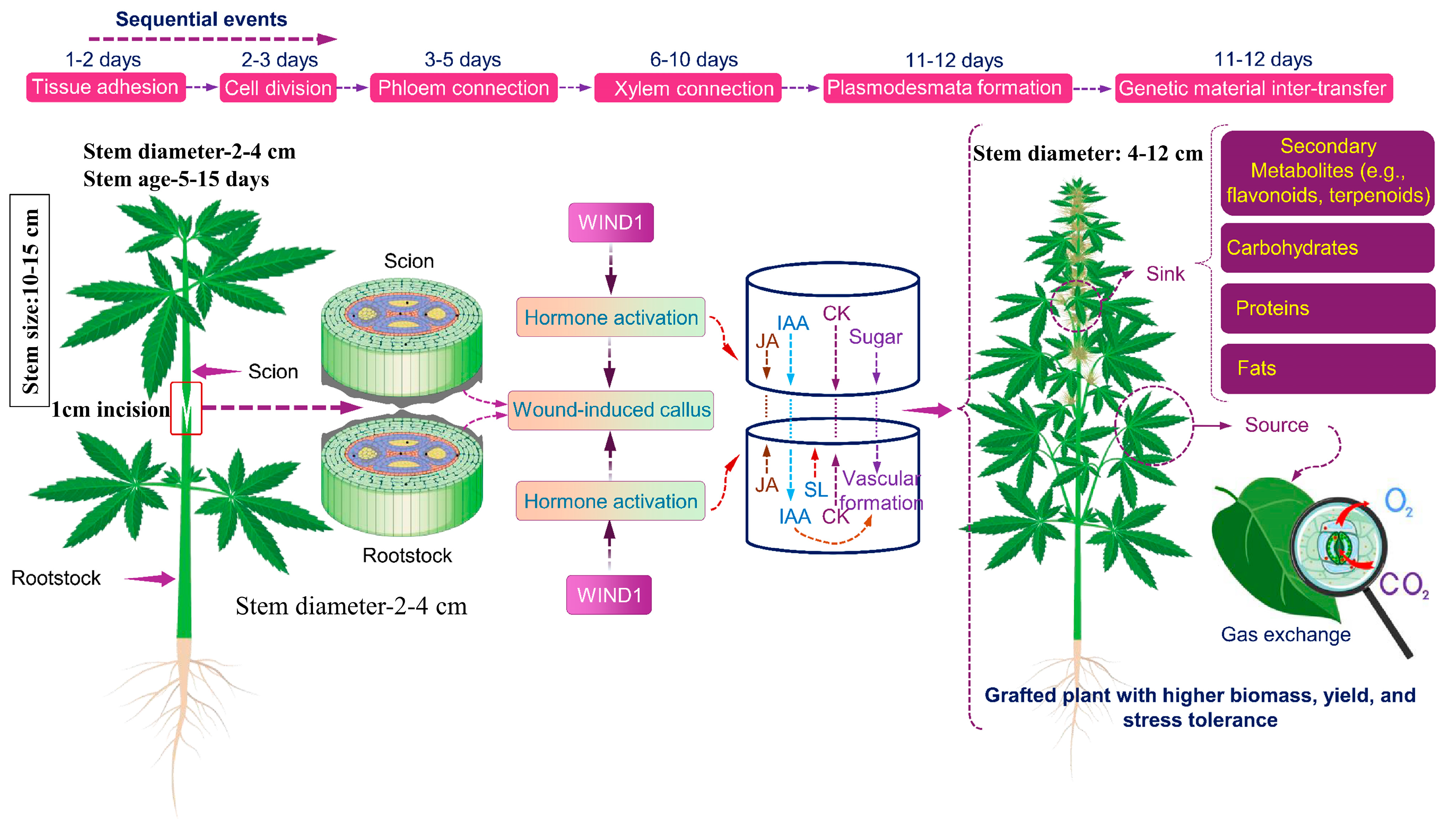
4. Grafting: Molecular Background of Rootstock–Scion Joining
5. Implication of Grafting for Crop Improvement: Prospects for Cannabis sativa: Yield and Secondary Metabolite Production
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bernstein, N.; Gorelick, J.; Koch, S. Interplay between chemistry and morphology in medical cannabis (Cannabis sativa L.). Ind. Crops Prod. 2019, 129, 185–194. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Jones, A.M.P. Current status and future prospects in cannabinoid production through in vitro culture and synthetic biology. Biotechnol. Adv. 2023, 62, 108074. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef] [PubMed]
- Wiles, D.; Shanbhag, B.K.; O’Brien, M.; Doblin, M.S.; Bacic, A.; Beddoe, T. Heterologous production of Cannabis sativa-derived specialised metabolites of medicinal significance—Insights into engineering strategies. Phytochemistry 2022, 203, 113380. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; McKinney, A.E.; Holmes, A.E. Minor Cannabinoids: Biosynthesis, Molecular Pharmacology and Potential Therapeutic Uses. Front. Pharmacol. 2021, 12, 777804. [Google Scholar] [CrossRef] [PubMed]
- Lazare, S.; Golshmid, P.; Krassin, A.; Simhon, E.; Cohen, T.L.; Dag, A. Grafting of Cannabis—The effect of the rootstock on vegetative and reproductive indices of the scion. Plant Sci. 2024, 348, 112210. [Google Scholar] [CrossRef]
- Parker, K.A.; Di Mattia, A.; Shaik, F.; Cerón Ortega, J.C.; Whittle, R. Risk management within the cannabis industry: Building a framework for the cannabis industry. Financ. Mark. Inst. Instrum. 2019, 28, 3–55. [Google Scholar] [CrossRef]
- Sorrentino, G. Introduction to emerging industrial applications of cannabis (Cannabis sativa L.). Rend. Lincei. Sci. Fis. E Nat. 2021, 32, 233–243. [Google Scholar] [CrossRef]
- Trancoso, I.; de Souza, G.A.R.; dos Santos, P.R.; dos Santos, K.D.; de Miranda, R.M.d.S.N.; da Silva, A.L.P.M.; Santos, D.Z.; García-Tejero, I.F.; Campostrini, E. Cannabis sativa L.: Crop Management and Abiotic Factors That Affect Phytocannabinoid Production. Agronomy 2022, 12, 1492. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Shape Matters: Plant Architecture Affects Chemical Uniformity in Large-Size Medical Cannabis Plants. Plants 2021, 10, 1834. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Too Dense or Not Too Dense: Higher Planting Density Reduces Cannabinoid Uniformity but Increases Yield/Area in Drug-Type Medical Cannabis. Front. Plant Sci. 2022, 13, 713481. [Google Scholar] [CrossRef]
- Bernstein, N.; Gorelick, J.; Zerahia, R.; Koch, S. Impact of N, P, K, and Humic Acid Supplementation on the Chemical Profile of Medical Cannabis (Cannabis sativa L). Front. Plant Sci. 2019, 10, 736. [Google Scholar] [CrossRef]
- Baas, R.; Wijnen, D. Salinity effects on yield and nutrient uptake in Cannabis sativa L. In Proceedings of the XXXI International Horticultural Congress (IHC2022): International Symposium on Innovative Technologies and Production 1377, Angers, France, 14–20 August 2023; pp. 785–792. [Google Scholar]
- Morgan, W.; Singh, J.; Kesheimer, K.; Davis, J.; Sanz-Saez, A. Severe drought significantly reduces floral hemp (Cannabis sativa L.) yield and cannabinoid content but moderate drought does not. Environ. Exp. Bot. 2024, 219, 105649. [Google Scholar] [CrossRef]
- Schober, T.; Präger, A.; Hartung, J.; Hensmann, F.; Graeff-Hönninger, S. Growth dynamics and yield formation of Cannabis (Cannabis sativa) cultivated in differing growing media under semi-controlled greenhouse conditions. Ind. Crops Prod. 2023, 203, 117172. [Google Scholar] [CrossRef]
- Magagnini, G.; Grassi, G.; Kotiranta, S. The Effect of Light Spectrum on the Morphology and Cannabinoid Content of Cannabis sativa L. Med. Cannabis Cannabinoids 2018, 1, 19–27. [Google Scholar] [CrossRef]
- Sainz Martinez, A.; Lanaridi, O.; Stagel, K.; Halbwirth, H.; Schnürch, M.; Bica-Schröder, K. Extraction techniques for bioactive compounds of cannabis. Nat. Prod. Rep. 2023, 40, 676–717. [Google Scholar] [CrossRef] [PubMed]
- Bitežnik, L.; Štukelj, R.; Flajšman, M. The Efficiency of CBD Production Using Grafted Cannabis sativa L. Plants Is Highly Dependent on the Type of Rootstock: A Study. Plants 2024, 13, 1117. [Google Scholar] [CrossRef]
- Purdy, S.J.; Hewavitharana, A.K.; Azman Halimi, R.; Magner, N.J.; Peterswald, T.J.; Trebilco, A.; Kretzschmar, T.; Hailstones, D. A One-Step Grafting Methodology Can Adjust Stem Morphology and Increase THCA Yield in Medicinal Cannabis. Agronomy 2022, 12, 852. [Google Scholar] [CrossRef]
- Welling, M.T.; Deseo, M.A.; Bacic, A.; Doblin, M.S. Untargeted Metabolomic Analyses Reveal Chemical Complexity of Dioecious Cannabis Flowers. Aust. J. Chem. 2021, 74, 463–479. [Google Scholar] [CrossRef]
- Sae-Tang, W.; Heuvelink, E.; Kohlen, W.; Argyri, E.; Nicole, C.C.S.; Marcelis, L.F.M. Effect of far-red and blue light on rooting in medicinal cannabis cuttings and related changes in endogenous auxin and carbohydrates. Sci. Hortic. 2024, 325, 112614. [Google Scholar] [CrossRef]
- Lee, J.-M.; Oda, M. Grafting of Herbaceous Vegetable and Ornamental Crops. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 61–124. [Google Scholar]
- Davis, A.R.; Perkins-Veazie, P.; Hassell, R.; Levi, A.; King, S.R.; Zhang, X. Grafting Effects on Vegetable Quality. HortScience 2008, 43, 1670–1672. [Google Scholar] [CrossRef]
- Ulas, F.; Kılıç, F.N.; Ulas, A. Alleviate the Influence of Drought Stress by Using Grafting Technology in Vegetable Crops: A Review. J. Crop Health 2025, 77, 51. [Google Scholar] [CrossRef]
- Morales, C.; Riveros-Burgos, C.; Espinoza Seguel, F.; Maldonado, C.; Mashilo, J.; Pinto, C.; Contreras-Soto, R.I. Rootstocks Comparison in Grafted Watermelon under Water Deficit: Effects on the Fruit Quality and Yield. Plants 2023, 12, 509. [Google Scholar] [CrossRef]
- Hayat, F.; Iqbal, S.; Coulibaly, D.; Razzaq, M.K.; Nawaz, M.A.; Jiang, W.; Shi, T.; Gao, Z. An insight into dwarfing mechanism: Contribution of scion-rootstock interactions toward fruit crop improvement. Fruit Res. 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Rachappanavar, V.; Padiyal, A.; Sharma, J.K.; Gupta, S.K. Plant hormone-mediated stress regulation responses in fruit crops- a review. Sci. Hortic. 2022, 304, 111302. [Google Scholar] [CrossRef]
- Kumari, S.; Nazir, F.; Maheshwari, C.; Kaur, H.; Gupta, R.; Siddique, K.H.M.; Khan, M.I.R. Plant hormones and secondary metabolites under environmental stresses: Enlightening defense molecules. Plant Physiol. Biochem. 2024, 206, 108238. [Google Scholar] [CrossRef]
- Trono, D. Elicitation as a tool to improve the accumulation of secondary metabolites in Cannabis sativa. Phytochem. Rev. 2024, 1–37. [Google Scholar] [CrossRef]
- Kapazoglou, A.; Tani, E.; Avramidou, E.V.; Abraham, E.M.; Gerakari, M.; Megariti, S.; Doupis, G.; Doulis, A.G. Epigenetic Changes and Transcriptional Reprogramming Upon Woody Plant Grafting for Crop Sustainability in a Changing Environment. Front. Plant Sci. 2021, 11, 613004. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Gautier, A.T.; Chambaud, C.; Brocard, L.; Ollat, N.; Gambetta, G.A.; Delrot, S.; Cookson, S.J. Merging genotypes: Graft union formation and scion–rootstock interactions. J. Exp. Bot. 2018, 70, 747–755. [Google Scholar] [CrossRef]
- Feng, M.; Augstein, F.; Kareem, A.; Melnyk, C.W. Plant grafting: Molecular mechanisms and applications. Mol. Plant 2024, 17, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Loupit, G.; Brocard, L.; Ollat, N.; Cookson, S.J. Grafting in plants: Recent discoveries and new applications. J. Exp. Bot. 2023, 74, 2433–2447. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.K.; Melnyk, C.W. The role of plant hormones during grafting. J. Plant Res. 2018, 131, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, L.; Wu, R. Plant grafting: How genetic exchange promotes vascular reconnection. N. Phytol. 2017, 214, 56–65. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Meyerowitz, E.M. Plant grafting. Curr. Biol. 2015, 25, R183–R188. [Google Scholar] [CrossRef]
- Goldschmidt, E.E. Plant grafting: New mechanisms, evolutionary implications. Front. Plant Sci. 2014, 5, 727. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Cheng, F.; Ahmed, W.; Huang, Y.; Bie, Z. Grafting: A Technique to Modify Ion Accumulation in Horticultural Crops. Front. Plant Sci. 2016, 7, 1457. [Google Scholar] [CrossRef]
- Chilukamarri, L.; Ashrafzadeh, S.; Leung, D.W.M. In-vitro grafting—Current applications and future prospects. Sci. Hortic. 2021, 280, 109899. [Google Scholar] [CrossRef]
- Wang, L.; Liao, Y.; Liu, J.; Zhao, T.; Jia, L.; Chen, Z. Advances in understanding the graft healing mechanism: A review of factors and regulatory pathways. Hortic. Res. 2024, 11, uhae175. [Google Scholar] [CrossRef]
- Rasool, A.; Mansoor, S.; Bhat, K.M.; Hassan, G.I.; Baba, T.R.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; Paray, B.A.; Ahmad, P. Mechanisms Underlying Graft Union Formation and Rootstock Scion Interaction in Horticultural Plants. Front. Plant Sci. 2020, 11, 590847. [Google Scholar] [CrossRef]
- Melnyk, C.W. Plant grafting: Insights into tissue regeneration. Regeneration 2017, 4, 3–14. [Google Scholar] [CrossRef]
- Gaion, L.A.; Trevisan, B.L.; and Carvalho, R.F. Grafting in Vegetable Crops: A Great Technique for Agriculture. Int. J. Veg. Sci. 2018, 24, 85–102. [Google Scholar] [CrossRef]
- Tedesco, S.; Fevereiro, P.; Kragler, F.; Pina, A. Plant grafting and graft incompatibility: A review from the grapevine perspective. Sci. Hortic. 2022, 299, 111019. [Google Scholar] [CrossRef]
- Thomas, H.R.; Gevorgyan, A.; Frank, M.H. Anatomical and biophysical basis for graft incompatibility within the Solanaceae. J. Exp. Bot. 2023, 74, 4461–4470. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-w.; Han, F.; Liu, X.-l.; Lv, H.-y.; Li, L.-l.; Tian, H.; Zhong, C.-h. Localized graft incompatibility in kiwifruit: Analysis of homografts and heterografts with different rootstock & scion combinations. Sci. Hortic. 2021, 283, 110080. [Google Scholar] [CrossRef]
- Aubin, E.; El Baidouri, M.; Panaud, O. Horizontal Gene Transfers in Plants. Life 2021, 11, 857. [Google Scholar] [CrossRef]
- Bock, R. The give-and-take of DNA: Horizontal gene transfer in plants. Trends Plant Sci. 2010, 15, 11–22. [Google Scholar] [CrossRef]
- Richardson, A.O.; Palmer, J.D. Horizontal gene transfer in plants. J. Exp. Bot. 2006, 58, 1–9. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, T.; Yuan, L.; Shen, Y.; Liu, K.; Liu, B.; Xu, K.; Elsadek, M.A.; Wang, Y.; Wu, L.; et al. Horizontal transfer of plasmid-like extrachromosomal circular DNAs across graft junctions in Solanaceae. Mol. Hortic. 2024, 4, 41. [Google Scholar] [CrossRef]
- Abdul Aziz, M.; Brini, F.; Rouached, H.; Masmoudi, K. Genetically engineered crops for sustainably enhanced food production systems. Front. Plant Sci. 2022, 13, 1027828. [Google Scholar] [CrossRef]
- Moralez, J.; Szenkiel, K.; Hamilton, K.; Pruden, A.; Lopatkin, A.J. Quantitative analysis of horizontal gene transfer in complex systems. Curr. Opin. Microbiol. 2021, 62, 103–109. [Google Scholar] [CrossRef]
- Filip, E.; Skuza, L. Horizontal Gene Transfer Involving Chloroplasts. Int. J. Mol. Sci. 2021, 22, 4484. [Google Scholar] [CrossRef] [PubMed]
- Philips, J.G.; Martin-Avila, E.; Robold, A.V. Horizontal gene transfer from genetically modified plants—Regulatory considerations. Front. Bioeng. Biotechnol. 2022, 10, 971402. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Singh, S.P.; Iqbal, H.M.N.; Parra-Saldivar, R.; Varjani, S.; Tong, Y.W. Genetic modifications associated with sustainability aspects for sustainable developments. Bioengineered 2022, 13, 9509–9521. [Google Scholar] [CrossRef]
- Halder, K.; Chaudhuri, A.; Abdin, M.Z.; Majee, M.; Datta, A. RNA Interference for Improving Disease Resistance in Plants and Its Relevance in This Clustered Regularly Interspaced Short Palindromic Repeats-Dominated Era in Terms of dsRNA-Based Biopesticides. Front. Plant Sci. 2022, 13, 885128. [Google Scholar] [CrossRef] [PubMed]
- Touzdjian Pinheiro Kohlrausch Távora, F.; de Assis dos Santos Diniz, F.; de Moraes Rêgo-Machado, C.; Chagas Freitas, N.; Barbosa Monteiro Arraes, F.; Chumbinho de Andrade, E.; Furtado, L.L.; Osiro, K.O.; Lima de Sousa, N.; Cardoso, T.B.; et al. CRISPR/Cas- and Topical RNAi-Based Technologies for Crop Management and Improvement: Reviewing the Risk Assessment and Challenges Towards a More Sustainable Agriculture. Front. Bioeng. Biotechnol. 2022, 10, 913728. [Google Scholar] [CrossRef]
- Augstein, F.; Melnyk, C.W. Modern and historical uses of plant grafting to engineer development, stress tolerance, chimeras, and hybrids. Plant J. 2025, 121, e70057. [Google Scholar] [CrossRef]
- Shujat, S.; Robinson, G.I.; Norouzkhani, F.; Kovalchuk, I. Using advanced biotechnological techniques to improve cannabis cultivars. Biocatal. Agric. Biotechnol. 2024, 60, 103250. [Google Scholar] [CrossRef]
- Schambach, A.; Buchholz, C.J.; Torres-Ruiz, R.; Cichutek, K.; Morgan, M.; Trapani, I.; Büning, H. A new age of precision gene therapy. Lancet 2024, 403, 568–582. [Google Scholar] [CrossRef]
- Izawa, T. What is going on with the hormonal control of flowering in plants? Plant J. 2021, 105, 431–445. [Google Scholar] [CrossRef]
- Motoki, K.; Kinoshita, Y.; Nakano, R.; Hosokawa, M.; Nakazaki, T. Quantitative Analysis of Florigen for the Variability of Floral Induction in Cabbage/Radish Inter-generic Grafting. Plant Cell Physiol. 2022, 63, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Rabeh, K.; Hnini, M.; Oubohssaine, M. A comprehensive review of transcription factor-mediated regulation of secondary metabolites in plants under environmental stress. Stress Biol. 2025, 5, 15. [Google Scholar] [CrossRef]
- Burch-Smith, T.M.; Zambryski, P.C. Plasmodesmata Paradigm Shift: Regulation from Without Versus Within. Annu. Rev. Plant Biol. 2012, 63, 239–260. [Google Scholar] [CrossRef]
- Bayer, E.M.; Benitez-Alfonso, Y. Plasmodesmata: Channels Under Pressure. Annu. Rev. Plant Biol. 2024, 75, 291–317. [Google Scholar] [CrossRef]

| SL. | Scion Genotype | Rootstock Type | Effect on Biomass Accumulation | Effect on Metabolic Traits | References |
|---|---|---|---|---|---|
| 1. |
|
|
|
| [7] |
| 2. |
|
|
|
| [20] |
| 3. |
| Three rootstock categories:
|
|
| [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahsan, S.M.; Injamum-Ul-Hoque, M.; Rahman, M.M.; Kang, S.-M.; Lee, I.-J.; Choi, H.W. Improved Biomass Production and Secondary Metabolism: A Critical Review of Grafting in Cannabis sativa. Plants 2025, 14, 2347. https://doi.org/10.3390/plants14152347
Ahsan SM, Injamum-Ul-Hoque M, Rahman MM, Kang S-M, Lee I-J, Choi HW. Improved Biomass Production and Secondary Metabolism: A Critical Review of Grafting in Cannabis sativa. Plants. 2025; 14(15):2347. https://doi.org/10.3390/plants14152347
Chicago/Turabian StyleAhsan, S. M., Md. Injamum-Ul-Hoque, Md. Mezanur Rahman, Sang-Mo Kang, In-Jung Lee, and Hyong Woo Choi. 2025. "Improved Biomass Production and Secondary Metabolism: A Critical Review of Grafting in Cannabis sativa" Plants 14, no. 15: 2347. https://doi.org/10.3390/plants14152347
APA StyleAhsan, S. M., Injamum-Ul-Hoque, M., Rahman, M. M., Kang, S.-M., Lee, I.-J., & Choi, H. W. (2025). Improved Biomass Production and Secondary Metabolism: A Critical Review of Grafting in Cannabis sativa. Plants, 14(15), 2347. https://doi.org/10.3390/plants14152347








