Advances and Application of Polyphenol Oxidase Immobilization Technology in Plants
Abstract
1. Introduction
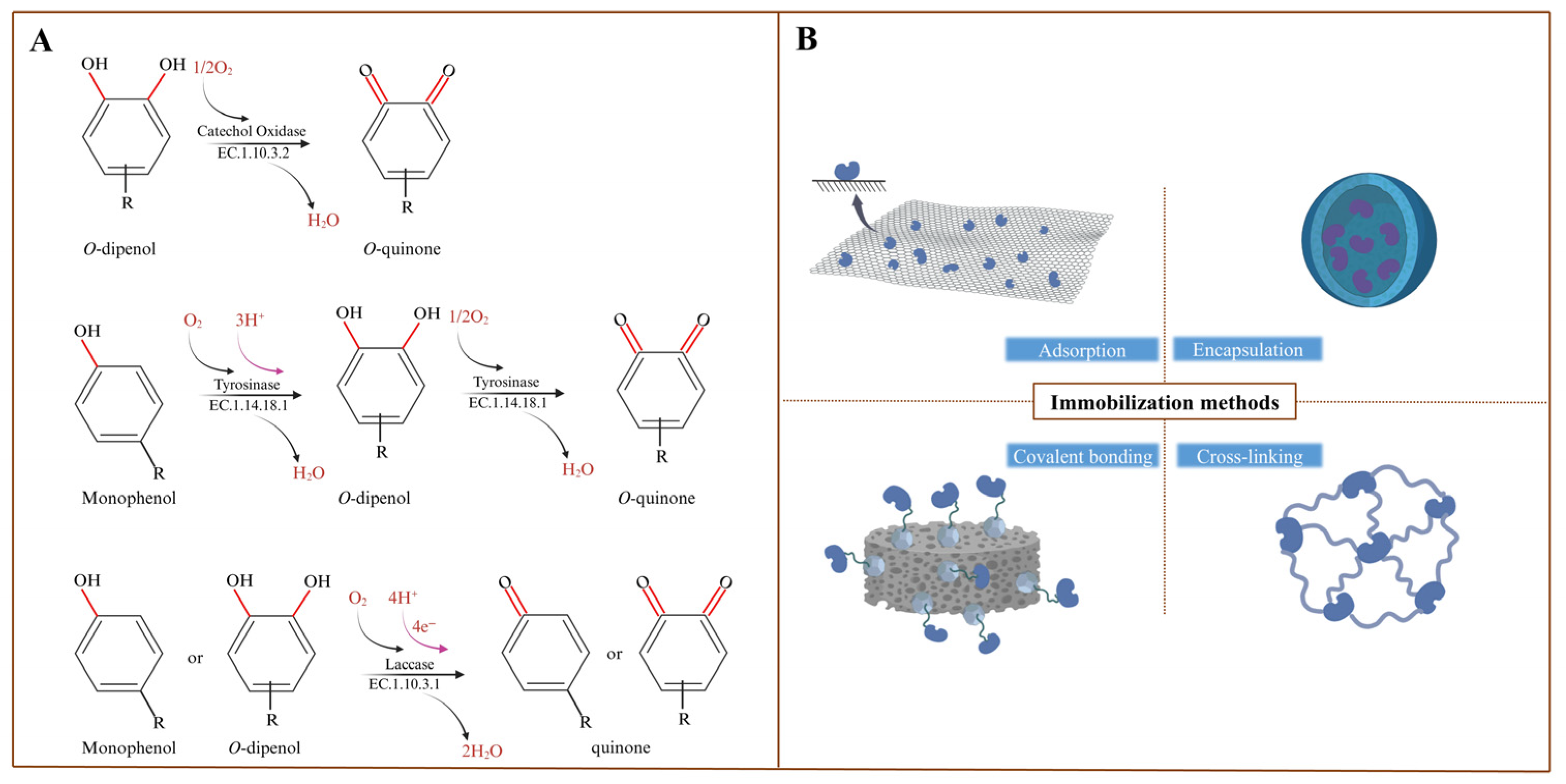
2. PPO in Plants
2.1. Sources and Functions of PPO in Plants
2.2. Classification of PPO in Plants
3. Plant PPO Immobilization
3.1. Physical Methods
3.1.1. Adsorption
3.1.2. Encapsulation
| Physical Methods | PPO Source | Materials | Payload Capacity | Retention Rate of Enzyme Activity | pH Stability | Temperature Stability | Storage Stability | Cyclic Stability | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Adsorption | Mushroom | Transition metal carbides/Yttrium oxide | - | 100% | - | - | Retained 84.1% of initial activity after 2 weeks storage at 4 °C | After 10 cycles of measurement, the RSD was 2.34% | [81] |
| Mushroom | SBA-15 silica | 7.20 μmol·g−1 silica | 91% | At pH = 3, the free enzyme was inactivated and the immobilized enzyme maintained 25% of the initial enzyme activity | At 70 °C, the free enzyme was inactivated and the immobilized enzyme maintained 22% of the initial enzyme activity | Retained 66% of initial activity after 6 months storage at 4 °C | After 15 cycles, 63% of initial enzyme activity was retained | [69] | |
| Kombucha tea | Carbon paste/Mineral oil | - | - | - | - | - | - | [82] | |
| Solanum lycocarpum | Carbon paste/Mineral oil | - | - | - | - | Retained 68.4% of initial activity after 15 days storage at 4 °C | After 5 cycles, 58.7% of the initial enzyme activity was retained | [83] | |
| Mushroom | Chitosan/Organic rectorite | 17.30 mg/g | - | - | - | - | After 10 cycles, 60.3% of the initial enzyme activity was retained | [22] | |
| Mushroom | Chitosan/Montmorillonite | 13.17 mg/g | - | At pH 7, free PPO and immobilized enzyme retained 88.4% and 96.6% of their initial activity at 8 h, respectively. | Ed is 56.77 kJ/mol and 65.57 kJ/mol for free and immobilized enzymes, respectively (Ed: energy required for enzyme inactivation) | - | After 10 cycles, 52.1% of the initial enzyme activity was retained | [72] | |
| Chitosan gold nanoparticles/Montmorillonite | 18.93 mg/g | - | At pH 7, free PPO and immobilized enzyme retained 88.4% and 98.4% of their initial activity at 8 h, respectively | Ed is 56.77 kJ/mol and 72.24 kJ/mol for free and immobilized enzymes, respectively | - | After 10 cycles, 59.1% of the initial enzyme activity was retained | |||
| Potato | Poly(glycine)/Reduced graphene oxide | 24 µg/cm2 | 12% | - | - | Retained 85% of initial activity after a week storage at 4 °C | After 10 cycles, optimal catalytic activity is maintained | [11] | |
| Ipomoea batatas | Strontium copper oxide/Polypyrrole nanotubes | - | - | - | - | Retained 81% of initial activity after 18 days storage at 4 °C | After 6 cycles of measurement, the RSD was 0.76% | [84] | |
| Potato | Filter paper | - | - | - | - | Retained 98.7% of initial activity after 4 weeks storage at 4 °C | After 3 cycles of measurement, the RSD was 1.6% | [85] | |
| Grape | Graphene oxide | -- | - | - | - | - | - | [74] | |
| Banana | Graphite powder/Paraffin | - | - | - | - | Retained 75% of initial activity after 40 days storage at 4 °C | After 6 cycles of measurement, the RSD was 3.2% | [86] | |
| Sapota | Graphite/Polypyrrole/Silver nanoparticles | - | - | - | The optimum temperature is increased to 40 °C | Retained 82% of initial activity after 2 weeks storage at 4 °C | - | [87] | |
| Jenipapo | Carbon paste/Mineral oil | - | - | - | - | Retained 88.22% of initial activity after 15 days storage at 4 °C | - | [88] | |
| Jurubeba | Carbon paste/Mineral oil | - | - | - | - | Retained 87.79% of initial activity after 6 weeks storage at 4 °C | - | [89] | |
| Sapota | Graphite/Graphene nanoribbons/Silver nanoparticles | Retained 80.82% of initial activity after 25 days storage at 4 °C | After 15 cycles of measurement, the RSD was 3.2% | [90] | |||||
| Sapota | Graphite/Graphene nanoribbons/Silver nanoparticles | - | - | - | - | Retained 90% of initial activity after 6 days storage at 4 °C | - | [91] | |
| Mushroom | SBA-15 silica, SBA-3 silica, MCM-48 silica | 300 mg/g, 100 mg/g, 140 mg/g | - | - | - | - | - | [25] | |
| Banana | Graphite powder/Hydrogel | - | - | - | - | -- | - | [92] | |
| Pear | Tyrosinase | Retained 70% of initial activity after 3 weeks storage at 4 °C | After 21 cycles, 70% of the initial enzyme activity was retained | [93] | |||||
| Potato | Diethylaminoethyl/Cellulose fibers | - | - | pH activity distribution, the immobilized enzyme has a wider distribution than the free enzyme | At 0–90 °C, the free enzyme is more sensitive than the immobilized enzyme | - | After 21 cycles, 55% to 60% of the initial enzyme activity was retained | [94] | |
| Entrapment | Mushroom | Polyacrylamide gel | - | - | - | - | Retained 100% of initial activity after 4 weeks storage at 4 °C | - | [79] |
| Apple | Thin layer chromatography | - | - | - | - | - | - | [80] | |
| Mushroom | Sodium alginate/Polyvinyl alcohol | - | - | At pH 6–10, the free enzyme is more sensitive to pH than the immobilized enzyme | At 30–50 °C, the free enzyme is more sensitive than the immobilized enzyme | - | After 8 cycles, 100% of the initial enzyme activity was retained | [77] | |
| Mushroom | Sodium alginate/Polyvinyl alcohol/Silver nanoparticles | - | - | At pH 6–10, the free enzyme is more sensitive to pH than the immobilized enzyme | At 30–50 °C, the free enzyme is more sensitive than the immobilized enzyme | - | After 12 cycles, 100% of the initial enzyme activity was retained | [77] | |
| Quince | Calcium alginate | - | - | pH activity distribution, the immobilized enzyme has a wider distribution than the free enzyme | The optimum temperature for free enzyme is 30 °C, and in the case of immobilized enzyme, it is 35 °C | - | - | [95] |
3.2. Chemical Methods
3.2.1. Cross-Linking
3.2.2. Covalent Binding
3.3. Mixed Methods
| Physical Methods | PPO Source | Materials | Payload Capacity | Retention Rate of Enzyme Activity | pH Stability | Temperature Stability | Storage Stability | Cyclic Stability | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Cross-linking | Tea tree | Polyethylene glycol | - | - | - | - | - | After 3 cycles, 80% of the initial enzyme activity was retained | [103] |
| Mushroom | 3,4-ethylenedioxythiophene/Graphene oxide | - | - | - | The optimum temperature is increased to 45 °C | Retained 74% of initial activity after 25 days storage at 4 °C | After 30 cycles, 50% of the initial enzyme activity was retained | [23] | |
| Green tea | Alginate/Magnetic Fe3O4/Glutaraldehyde | - | - | At pH 4–11, the free enzyme is more sensitive to pH than the immobilized enzyme | At 40–80 °C, the free enzyme is more sensitive than the immobilized enzyme | Retained 87% of initial activity after 12 days storage at 4 °C | After 7 cycles, 41% of the initial enzyme activity was retained | [10] | |
| Mushroom | Porous graphene/Polypyrrole | Retained 65% of initial activity after 60 days storage at 4 °C | After 21 cycles, 80% of the initial enzyme activity was retained | [110] | |||||
| Mushroom | NH2-SBA-15 silica/L-tyrosine/Gold nano-particles/Glass carbon | - | - | - | - | Retained 92.6% of initial activity after 12 days storage at 4 °C | - | [111] | |
| Mushroom | Thiophene-3-boronic acid/Gold nanopartilces/Glutaraldehyde | - | - | - | - | - | - | [112] | |
| Ipomoea batatas | Babassu mesocarp nanoparticles/Glutaraldehyde | - | - | - | - | - | After 30 days of cycling, the total current loss was 7.5% | [113] | |
| Mushroom | Pt/CoOx/Glassy carbon | - | - | - | - | - | - | [114] | |
| Pear | Fe3O4/Chitosan nanoparticles | 145 μg/mg | 67.1% | - | - | Retained 90% of initial activity after 4 weeks storage at 4 °C | After 8 cycles, 85% of the initial enzyme activity was retained | [104] | |
| Mushroom | Polypyrrole nanotubes/GA | - | - | - | - | Retained 88% of initial activity after 30 days storage at 4 °C | - | [100] | |
| Banana | Acrylamide/N, N′-methylenebisacrylamided | - | - | - | - | Retained 100% of initial activity after 20 days storage at 4 °C | After 5 cycles of measurement, the RSD was 3.2% | [92] | |
| Mushroom | Polyaniline/Glutaraldehyde | - | - | - | - | Retained 80% of initial activity after 20 weeks storage at 4 °C | After 25 cycles of measurement, the RSD was 2.8% | [115] | |
| Mushroom | Silica/Acrylamide/Diacrylamide | - | - | - | - | Retained 100% of initial activity after 20 days storage at 4 °C | - | [116] | |
| Covalent binding | Mushroom | Chitosan/Organic rectorite/Glutaraldehyde | 26.5 mg/g | After 10 cycles, 73.2% of the initial enzyme activity was retained | [22] | ||||
| Adsorption/Cross-linking | Plant tissue | Polyaniline/Porous polyacrylonitrile/Nanostructured graphene | - | - | - | - | - | After 10 cycles of measurement, the RSD was 3.9% | [86] |
| Adsorption/Covalent binding | Potato | Propylamine functionalized silica nanoparticles | - | - | - | - | Retained 40% of initial activity after 80 days storage at 4 °C | After 10 cycles of measurement, the RSD was 4.1% to 5.2% | [24] |
3.4. Nanomaterials for Plant PPO Immobilization
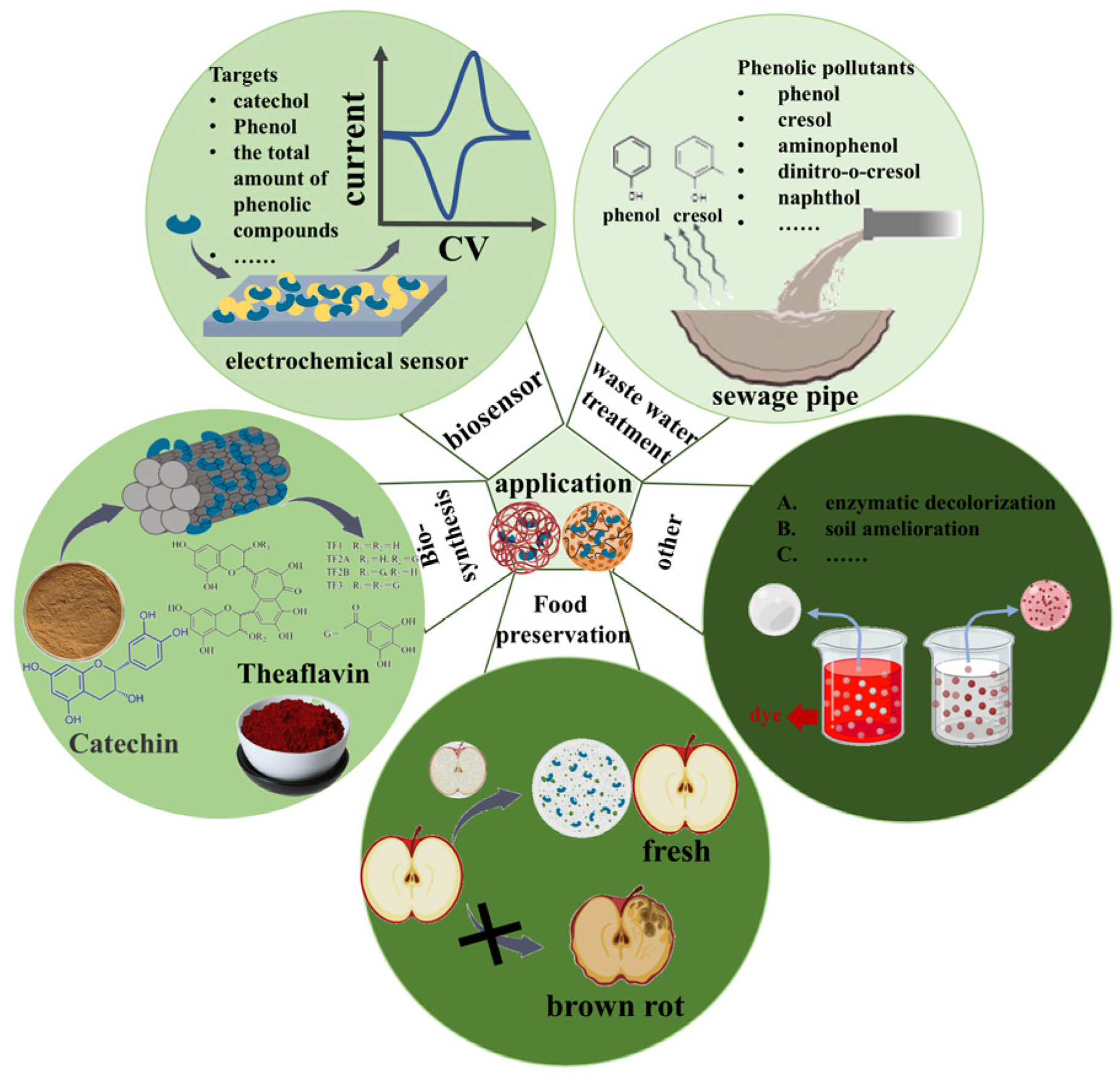
4. Multi-Field Applications of Plant PPO Immobilization
4.1. Wastewater Treatment
4.2. Biosensors
4.3. Food Preservation
4.4. Theaflavin Synthesis
4.5. Other Applications
5. Future Prospects
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef]
- Zhang, S. Recent Advances of Polyphenol Oxidases in Plants. Molecules 2023, 28, 2158. [Google Scholar] [CrossRef]
- Wei, S.; Xiang, Y.; Zhang, Y.; Fu, R. The unexpected flavone synthase-like activity of polyphenol oxidase in tomato. Food Chem. 2022, 377, 131958. [Google Scholar] [CrossRef]
- Fujieda, N.; Umakoshi, K.; Ochi, Y.; Nishikawa, Y.; Yanagisawa, S.; Kubo, M.; Kurisu, G.; Itoh, S. Copper–Oxygen Dynamics in the Tyrosinase Mechanism. Angew. Chem. Int. Ed. 2020, 59, 13385–13390. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, D.; Li, J.; Zhang, Z.; Wang, Y.; Qiu, C.; Sun, Y.; Pan, C. Research progress on the functions and biosynthesis of theaflavins. Food Chem. 2024, 450, 139285. [Google Scholar] [CrossRef] [PubMed]
- Gooding, P.S.; Bird, C.; Robinson, S.P. Molecular cloning and characterisation of banana fruit polyphenol oxidase. Planta 2001, 213, 748–757. [Google Scholar] [CrossRef]
- Huang, S.; Li, R.; Zhang, Z.; Li, L.; Gu, X.; Fan, W.; Lucas, W.J.; Wang, X.; Xie, B.; Ni, P.; et al. The genome of the cucumber, Cucumis sativus L. Nat. Genet. 2009, 41, 1275–1281. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Silva, T.A.; Caetano, F.R.; Ribovski, L.; Zapp, E.; Brondani, D.; Bergamini, M.F.; Marcolino, L.H.; Banks, C.E.; Oliveira, O.N.; et al. Polyphenol oxidase-based electrochemical biosensors: A review. Anal. Chim. Acta 2020, 1139, 198–221. [Google Scholar] [CrossRef]
- Li, S.; Zhong, L.; Wang, H.; Li, J.; Cheng, H.; Ma, Q. Process optimization of polyphenol oxidase immobilization: Isotherm, kinetic, thermodynamic and removal of phenolic compounds. Int. J. Biol. Macromol. 2021, 185, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Marjani, A.; Zare, M.H.; Sadeghi, M.H.; Shirazian, S.; Ghadiri, M. Synthesis of alginate-coated magnetic nanocatalyst containing high-performance integrated enzyme for phenol removal. J. Environ. Chem. Eng. 2021, 9, 104884. [Google Scholar] [CrossRef]
- Kıranşan, K.D.; Aksoy, M. A novel polyphenol oxidase immobilized polyglycine/reduced graphene oxide composite electrode for sensitive determination of catechol. J. Appl. Electrochem. 2020, 50, 863–873. [Google Scholar] [CrossRef]
- Mohammadi, M.; As’habi, M.A.; Salehi, P.; Yousefi, M.; Nazari, M.; Brask, J. Immobilization of laccase on epoxy-functionalized silica and its application in biodegradation of phenolic compounds. Int. J. Biol. Macromol. 2018, 109, 443–447. [Google Scholar] [CrossRef]
- Datta, S.; Veena, R.; Samuel, M.S.; Selvarajan, E. Immobilization of laccases and applications for the detection and remediation of pollutants: A review. Environ. Chem. Lett. 2021, 19, 521–538. [Google Scholar] [CrossRef]
- Pei, X.; Luo, Z.; Qiao, L.; Xiao, Q.; Zhang, P.; Wang, A.; Sheldon, R.A. Putting precision and elegance in enzyme immobilisation with bio-orthogonal chemistry. Chem. Soc. Rev. 2022, 51, 7281–7304. [Google Scholar] [CrossRef]
- Dong, C.-D.; Tiwari, A.; Anisha, G.S.; Chen, C.-W.; Singh, A.; Haldar, D.; Patel, A.K.; Singhania, R.R. Laccase: A potential biocatalyst for pollutant degradation. Environ. Pollut. 2023, 319, 120999. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, S.A.; Almulaiky, Y.Q. Cu-trimesic acid-based metal organic frameworks for improved ß-galactosidase immobilization: Enhanced stability, reusability and lactose hydrolysis. J. Mol. Liq. 2023, 392, 123456. [Google Scholar] [CrossRef]
- Bao, C.; Wang, Y.; Xu, X.; Li, D.; Chen, J.; Guan, Z.; Wang, B.; Hong, M.; Zhang, J.; Wang, T.; et al. Reversible immobilization of laccase onto glycopolymer microspheres via protein-carbohydrate interaction for biodegradation of phenolic compounds. Bioresour. Technol. 2021, 342, 126026. [Google Scholar] [CrossRef] [PubMed]
- Ulu, A.; Birhanli, E.; Boran, F.; Köytepe, S.; Yesilada, O.; Ateş, B. Laccase-conjugated thiolated chitosan-Fe3O4 hybrid composite for biocatalytic degradation of organic dyes. Int. J. Biol. Macromol. 2020, 150, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Oh, W.-D.; Yap, P.-S. Recent advances in the utilization of immobilized laccase for the degradation of phenolic compounds in aqueous solutions: A review. Chemosphere 2022, 307, 135824. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Du, G.; Shao, X.; Feng, K.-N.; Zeng, Y. Recombinant polyphenol oxidases for production of theaflavins from tea polyphenols. Int. J. Biol. Macromol. 2019, 134, 139–145. [Google Scholar] [CrossRef]
- Zhong, L.; Li, J.; Tian, D.; Cai, J.; Wang, H.; Ma, Q. Immobilization of polyphenol oxidase on chitosan/organic rectorite composites for phenolic compounds removal. Water Sci. Technol. 2021, 83, 906–921. [Google Scholar] [CrossRef]
- Ramu, P.; Vimal, S.P.; Suresh, P.; Sanmugam, A.; Saravanakumar, U.; Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Vikraman, D. Investigation of the one-step electrochemical deposition of graphene oxide-doped poly(3,4-ethylenedioxythiophene)–polyphenol oxidase as a dopamine sensor. RSC Adv. 2022, 12, 15575–15583. [Google Scholar] [CrossRef]
- Rahimi-Mohseni, M.; Raoof, J.B.; Aghajanzadeh, T.A.; Ojani, R. Rapid Determination of Phenolic Compounds in Water Samples: Development of a Paper-based Nanobiosensor Modified with Functionalized Silica Nanoparticles and Potato Tissue. Electroanalysis 2019, 31, 2311–2318. [Google Scholar] [CrossRef]
- Corell Escuin, P.; García-Bennett, A.; Ros-Lis, J.V.; Argüelles Foix, A.; Andrés, A. Application of mesoporous silica materials for the immobilization of polyphenol oxidase. Food Chem. 2017, 217, 360–363. [Google Scholar] [CrossRef]
- Uzunoğlu, S.B.; Uzunoğlu, T.; Koçsuz, S.; Evyapan, M.; Arslan, O. Metal ion effects on Polyphenol Oxidase Covalently immobilized on a Bio-Composite. Cell. Mol. Biol. 2021, 67, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Derardja, A.e.; Pretzler, M.; Barkat, M.; Rompel, A. Extraction, Purification, and Characterization of Olive (Olea europaea L., cv. Chemlal) Polyphenol Oxidase. J. Agric. Food Chem. 2024, 72, 3099–3112. [Google Scholar] [CrossRef]
- Ji, D.; Wang, Q.; Lu, T.; Ma, H.; Chen, X. The effects of ultrasonication on the phytochemicals, antioxidant, and polyphenol oxidase and peroxidase activities in coffee leaves. Food Chem. 2022, 373, 131480. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.G.; Zhang, S.; Xiong, L.G.; Zhou, J.H.; Huang, J.A.; Zhao, A.Q.; Liu, Z.H.; Liu, A.L. A comprehensive review of polyphenol oxidase in tea (Camellia sinensis): Physiological characteristics, oxidation manufacturing, and biosynthesis of functional constituents. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2267–2291. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, J.-H.; Gan, Z.-L.; Ni, Y.-Y. Comparison of membrane-bound and soluble polyphenol oxidase in Fuji apple (Malus domestica Borkh. cv. Red Fuji). Food Chem. 2015, 173, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Murtaza, A.; Liu, Y.; Hu, W.; Xu, X.; Pan, S. Catalytic and Structural Characterization of a Browning-Related Protein in Oriental Sweet Melon (Cucumis melo var. Makuwa Makino). Front. Chem. 2018, 6, 354. [Google Scholar] [CrossRef]
- Han, Q.; Liu, F.; Hao, Y.; Ni, Y. Characterization of membrane-bound polyphenol oxidase from Granny Smith apple (Malus × domestica Borkh.). Int. J. Biol. Macromol. 2020, 158, 977–984. [Google Scholar] [CrossRef]
- Fan, X. Chemical inhibition of polyphenol oxidase and cut surface browning of fresh-cut apples. Crit. Rev. Food Sci. Nutr. 2023, 63, 8737–8751. [Google Scholar] [CrossRef]
- Xu, X.; Pan, S.; Cheng, S.; Zhang, B.; Mu, D.; Ni, P.; Zhang, G.; Yang, S.; Li, R.; Wang, J.; et al. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Bøjer Rasmussen, C.; Enghild, J.J.; Scavenius, C. Identification of polyphenol oxidases in potato tuber (Solanum tuberosum) and purification and characterization of the major polyphenol oxidases. Food Chem. 2021, 365, 130454. [Google Scholar] [CrossRef]
- Kuijpers, T.F.M.; van Herk, T.; Vincken, J.-P.; Janssen, R.H.; Narh, D.L.; van Berkel, W.J.H.; Gruppen, H. Potato and Mushroom Polyphenol Oxidase Activities Are Differently Modulated by Natural Plant Extracts. J. Agric. Food Chem. 2014, 62, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-Y.; Sharma, V.K.; Sharma, N. Mushroom Tyrosinase: Recent Prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.-Y.; Liu, P.; Meng, A.-l.; Deng, L.; Xue, W.; Chen, F.; Che, Z.-m. Comparative study of the biochemical properties of membrane-bound and soluble polyphenol oxidase from Prunus mume. LWT 2022, 171, 114156. [Google Scholar] [CrossRef]
- Liu, Y.; Han, X.; Zhao, M.; Liu, L.; Deng, Z.; Zhao, Q.; Yu, Y. Functional characterization of polyphenol oxidase OfPPO2 supports its involvement in parallel biosynthetic pathways of acteoside. Plant J. 2024, 119, 927–941. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X. Recent advances in polyphenol oxidase-mediated plant stress responses. Phytochemistry 2021, 181, 112588. [Google Scholar] [CrossRef]
- Khodadadi, F.; Tohidfar, M.; Vahdati, K.; Dandekar, A.M.; Leslie, C.A. Functional analysis of walnut polyphenol oxidase gene (JrPPO1) in transgenic tobacco plants and PPO induction in response to walnut bacterial blight. Plant Pathol. 2020, 69, 756–764. [Google Scholar] [CrossRef]
- George, A.S.; Simko, I.; Brandl, M.T. Identification and characterization of lettuce cultivars with high inhibitory activity against the human pathogen Escherichia coli O157:H7: Toward a plant-intrinsic hurdle approach to microbial safety. Postharvest Biol. Technol. 2024, 211, 112816. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, J.; Liu, Y.; Yan, D.; Wu, H.; Li, R.; Jiang, Z.; Yang, Y.; Ren, X. Polyphenol oxidase plays a critical role in melanin formation in the fruit skin of persimmon (Diospyros kaki cv. ‘Heishi’). Food Chem. 2020, 330, 127253. [Google Scholar] [CrossRef]
- Sui, X.; Meng, Z.; Dong, T.; Fan, X.; Wang, Q. Enzymatic browning and polyphenol oxidase control strategies. Curr. Opin. Biotechnol. 2023, 81, 102921. [Google Scholar] [CrossRef]
- Pretzler, M.; Rompel, A. What causes the different functionality in type-III-copper enzymes? A state of the art perspective. Inorg. Chim. Acta 2018, 481, 25–31. [Google Scholar] [CrossRef]
- Decker, H.; Schweikardt, T.; Tuczek, F. The First Crystal Structure of Tyrosinase: All Questions Answered? Angew. Chem. Int. Ed. 2006, 45, 4546–4550. [Google Scholar] [CrossRef] [PubMed]
- Goldfeder, M.; Kanteev, M.; Isaschar-Ovdat, S.; Adir, N.; Fishman, A. Determination of tyrosinase substrate-binding modes reveals mechanistic differences between type-3 copper proteins. Nat. Commun. 2014, 5, 4505. [Google Scholar] [CrossRef] [PubMed]
- Penttinen, L.; Rutanen, C.; Jänis, J.; Rouvinen, J.; Hakulinen, N. Unraveling Substrate Specificity and Catalytic Promiscuity of Aspergillus oryzae Catechol Oxidase. ChemBioChem 2018, 19, 2348–2352. [Google Scholar] [CrossRef] [PubMed]
- Claus, H.; Decker, H. Bacterial tyrosinases. Syst. Appl. Microbiol. 2006, 29, 3–14. [Google Scholar] [CrossRef]
- Yoruk, R.; Marshall, M.R. Physicochemical properties and function of plant polyphenol oxidase: A review. J. Food Biochem. 2003, 27, 361–422. [Google Scholar] [CrossRef]
- Lin, H.; Yu, Z.; Wang, Q.; Liu, Y.; Jiang, L.; Xu, C.; Xian, M. Application of Laccase Catalysis in Bond Formation and Breakage: A Review. Catalysts 2023, 13, 750. [Google Scholar] [CrossRef]
- Jones, S.M.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef]
- Bassanini, I.; Ferrandi, E.E.; Riva, S.; Monti, D. Biocatalysis with Laccases: An Updated Overview. Catalysts 2021, 11, 26. [Google Scholar] [CrossRef]
- Yuan, Y.; Jin, W.; Nazir, Y.; Fercher, C.; Blaskovich, M.A.T.; Cooper, M.A.; Barnard, R.T.; Ziora, Z.M. Tyrosinase inhibitors as potential antibacterial agents. Eur. J. Med. Chem. 2020, 187, 111892. [Google Scholar] [CrossRef]
- Kakkar, P.; Wadhwa, N. In silico and in vitro analysis of polyphenol oxidase: Study in bioremediation of phenol in wastewater. Environ. Dev. Sustain. 2023, 27, 9955–9976. [Google Scholar] [CrossRef]
- Soozanipour, A.; Taheri-Kafrani, A. Chapter Fourteen—Enzyme Immobilization on Functionalized Graphene Oxide Nanosheets: Efficient and Robust Biocatalysts. In Methods in Enzymology; Kumar, C.V., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 609, pp. 371–403. [Google Scholar]
- Cui, J.; Jia, S. Organic–inorganic hybrid nanoflowers: A novel host platform for immobilizing biomolecules. Coord. Chem. Rev. 2017, 352, 249–263. [Google Scholar] [CrossRef]
- Filippovich, S.Y.; Isakova, E.P.; Gessler, N.N.; Deryabina, Y.I. Advances in immobilization of phytases and their application. Bioresour. Technol. 2023, 379, 129030. [Google Scholar] [CrossRef]
- Wang, H.; Tang, L.-X.; Ye, Y.-F.; Ma, J.-X.; Li, X.; Si, J.; Cui, B.-K. Laccase immobilization and its degradation of emerging pollutants: A comprehensive review. J. Environ. Manag. 2024, 359, 120984. [Google Scholar] [CrossRef] [PubMed]
- Zofair, S.F.F.; Ahmad, S.; Hashmi, M.A.; Khan, S.H.; Khan, M.A.; Younus, H. Catalytic roles, immobilization and management of recalcitrant environmental pollutants by laccases: Significance in sustainable green chemistry. J. Environ. Manag. 2022, 309, 114676. [Google Scholar] [CrossRef] [PubMed]
- Bounegru, A.V.; Apetrei, C. Tyrosinase Immobilization Strategies for the Development of Electrochemical Biosensors—A Review. Nanomaterials 2023, 13, 760. [Google Scholar] [CrossRef]
- Hussain, A.; Rafeeq, H.; Qasim, M.; Jabeen, Z.; Bilal, M.; Franco, M.; Iqbal, H.M.N. Engineered tyrosinases with broadened bio-catalysis scope: Immobilization using nanocarriers and applications. 3 Biotech. 2021, 11, 365. [Google Scholar] [CrossRef]
- Han, Z.; Fan, X.; Yu, S.; Li, X.; Wang, S.; Lu, L. Metal-organic frameworks (MOFs): A novel platform for laccase immobilization and application. J. Environ. Chem. Eng. 2022, 10, 108795. [Google Scholar] [CrossRef]
- Li, W.; Chen, S.; Lu, Y.; Yu, J.; Zhao, S. Co-immobilization of laccase-mediator system to catalyze the synthesis of theaflavins from tea polyphenols. Results Eng. 2024, 22, 102062. [Google Scholar] [CrossRef]
- Carballares, D.; Rocha-Martin, J.; Fernandez-Lafuente, R. Chemical amination of immobilized enzymes for enzyme coimmobilization: Reuse of the most stable immobilized and modified enzyme. Int. J. Biol. Macromol. 2022, 208, 688–697. [Google Scholar] [CrossRef]
- Aghaee, M.; Salehipour, M.; Rezaei, S.; Mogharabi-Manzari, M. Bioremediation of organic pollutants by laccase-metal–organic framework composites: A review of current knowledge and future perspective. Bioresour. Technol. 2024, 406, 131072. [Google Scholar] [CrossRef] [PubMed]
- Fried, D.I.; Brieler, F.J.; Fröba, M. Designing Inorganic Porous Materials for Enzyme Adsorption and Applications in Biocatalysis. ChemCatChem 2013, 5, 862–884. [Google Scholar] [CrossRef]
- Guillet-Nicolas, R.; Wainer, M.; Marcoux, L.; Thommes, M.; Kleitz, F. Exploring the confinement of polymer nanolayers into ordered mesoporous silica using advanced gas physisorption. J. Colloid. Interface Sci. 2020, 579, 489–507. [Google Scholar] [CrossRef]
- Iriarte-Mesa, C.; Pretzler, M.; von Baeckmann, C.; Kählig, H.; Krachler, R.; Rompel, A.; Kleitz, F. Immobilization of Agaricus bisporus Polyphenol Oxidase 4 on mesoporous silica: Towards mimicking key enzymatic processes in peat soils. J. Colloid. Interface Sci. 2023, 646, 413–425. [Google Scholar] [CrossRef]
- Arsalan, A.; Younus, H. Enzymes and nanoparticles: Modulation of enzymatic activity via nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1833–1847. [Google Scholar] [CrossRef]
- Li, J.; Cai, J.; Zhong, L.; Cheng, H.; Wang, H.; Ma, Q. Adsorption of reactive red 136 onto chitosan/montmorillonite intercalated composite from aqueous solution. Appl. Clay Sci. 2019, 167, 9–22. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Li, J.; Zhong, L.; Cheng, H.; Ma, Q. Immobilized polyphenol oxidase: Preparation, optimization and oxidation of phenolic compounds. Int. J. Biol. Macromol. 2020, 160, 233–244. [Google Scholar] [CrossRef]
- Moura, D.; Rohringer, S.; Ferreira, H.P.; Pereira, A.T.; Barrias, C.C.; Magalhães, F.D.; Bergmeister, H.; Gonçalves, I.C. Long-term in vivo degradation and biocompatibility of degradable pHEMA hydrogels containing graphene oxide. Acta Biomater. 2024, 173, 351–364. [Google Scholar] [CrossRef]
- Gür, B.; Ayhan, M.E.; Türkhan, A.; Gür, F.; Kaya, E.D. A facile immobilization of polyphenol oxidase enzyme on graphene oxide and reduced graphene oxide thin films: An insight into in-vitro activity measurements and characterization. Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 179–185. [Google Scholar] [CrossRef]
- Su, X.; Yang, X.; Long, H.; Li, Y.; Sun, W.; Mo, T.; Lyu, H.; Cavaco-Paulo, A.; Wang, H.; Su, J. Construction of immobilized laccase hydrogels via sodium alginate-dopamine/polyethylene glycol and its efficient degradation of dyeing wastewater. Int. J. Biol. Macromol. 2024, 279, 134929. [Google Scholar] [CrossRef]
- Zhao, Z.; Ren, D.; Zhuang, M.; Wang, Z.; Zhang, X.; Zhang, S.; Chen, W. Degradation of 2,4-DCP by the immobilized laccase on the carrier of sodium alginate-sodium carboxymethyl cellulose. Bioprocess. Biosyst. Eng. 2022, 45, 1739–1751. [Google Scholar] [CrossRef] [PubMed]
- Edalli, V.A.; Mulla, S.I.; Eqani, S.A.M.A.S.; Mahadevan, G.D.; Sharma, R.; Shouche, Y.; Kamanavalli, C.M. Evaluation of p-cresol degradation with polyphenol oxidase (PPO) immobilized in various matrices. 3 Biotech. 2016, 6, 229. [Google Scholar] [CrossRef]
- Almulaiky, Y.Q.; Almaghrabi, O. Polyphenol Oxidase from Coleus forskohlii: Purification, Characterization, and Immobilization Onto Alginate/ZnO Nanocomposite Materials. Catal. Lett. 2022, 152, 3089–3099. [Google Scholar] [CrossRef]
- Mishra, S.K.; Chiang, K.S. Phenolic-compounds sensor based on immobilization of tyrosinase in polyacrylamide gel on long-period fiber grating. Opt. Laser Technol. 2020, 131, 106464. [Google Scholar] [CrossRef]
- Micheloni, O.B.; Farroni, A.E.; García, P.; Furlan, R.L.E. Rapid autographic method for detection of enzymatic browning inhibitors based on enzyme immobilization. Food Chem. 2018, 269, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xia, G.; Chen, J.; Zheng, H. Biosensor based on MXene-Y2O3 composite for ultrasensitive detection of catechol in water samples. Int. J. Electrochem. Sci. 2024, 19, 100823. [Google Scholar] [CrossRef]
- Batista, E.A.; Pereira, M.O.A.; Macêdo, I.Y.L.; Machado, F.B.; Moreno, E.K.G.; Diniz, E.P.; Frazzão, I.G.V.; Bernardes, L.S.C.; Oliveira, S.C.B.; Gil, E.S. Electroanalytical Enzyme Biosensor Based on Cordia superba Enzyme Extract for the Detection of Phytomarkers in Kombucha. Biosensors 2022, 12, 1112. [Google Scholar] [CrossRef]
- Antunes, R.S.; Thomaz, D.V.; Garcia, L.F.; Gil, E.S.; Lopes, F.M. Development and Optimization of Solanum Lycocarpum Polyphenol Oxidase-Based Biosensor and Application towards Paracetamol Detection. Adv. Pharm. Bull. 2021, 11, 469–476. [Google Scholar] [CrossRef]
- Yashas, S.R.; Sandeep, S.; Shivakumar, B.P.; Swamy, N.K. Potentiometric polyphenol oxidase biosensor for sensitive determination of phenolic micropollutant in environmental samples. Environ. Sci. Pollut. Res. 2020, 27, 27234–27243. [Google Scholar] [CrossRef]
- Noori, R.; Perwez, M.; Mazumder, J.A.; Sardar, M. Development of low-cost paper-based biosensor of polyphenol oxidase for detection of phenolic contaminants in water and clinical samples. Environ. Sci. Pollut. Res. 2020, 27, 30081–30092. [Google Scholar] [CrossRef]
- Broli, N.; Vallja, L.; Shehu, A.; Vasjari, M. Determination of catechol in extract of tea using carbon paste electrode modified with banana tissue. J. Food Process. Preserv. 2019, 43, e13838. [Google Scholar] [CrossRef]
- Sandeep, S.; Santhosh, A.S.; Swamy, N.K.; Suresh, G.S.; Melo, J.S. Detection of Catechol Using a Biosensor Based on Biosynthesized Silver Nanoparticles and Polyphenol Oxidase Enzymes. Port. Electrochim. Acta 2019, 37, 257–270. [Google Scholar] [CrossRef]
- Antunes, R.S.; Ferraz, D.; Garcia, L.F.; Thomaz, D.V.; Luque, R.; Lobón, G.S.; Gil, E.D.S.; Lopes, F.M. Development of a Polyphenol Oxidase Biosensor from Jenipapo Fruit Extract (Genipa americana L.) and Determination of Phenolic Compounds in Textile Industrial Effluents. Biosensors 2018, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Antunes, R.S.; Garcia, L.F.; Somerset, V.S.; Gil, E.D.S.; Lopes, F.M. The Use of a Polyphenoloxidase Biosensor Obtained from the Fruit of Jurubeba (Solanum paniculatum L.) in the Determination of Paracetamol and Other Phenolic Drugs. Biosensors 2018, 8, 36. [Google Scholar] [CrossRef]
- Sandeep, S.; Santhosh, A.S.; Swamy, N.K.; Suresh, G.S.; Melo, J.S.; Chamaraja, N.A. A biosensor based on a graphene nanoribbon/silver nanoparticle/polyphenol oxidase composite matrix on a graphite electrode: Application in the analysis of catechol in green tea samples. New J. Chem. 2018, 42, 16620–16629. [Google Scholar] [CrossRef]
- Sandeep, S.; Santhosh, A.S.; Swamy, N.K.; Suresh, G.S.; Melo, J.S.; Nithin, K.S. Electrochemical detection of L-dopa using crude Polyphenol oxidase enzyme immobilized on electrochemically reduced RGO-Ag nanocomposite modified graphite electrode. Mater. Sci. Eng. B 2018, 232–235, 15–21. [Google Scholar] [CrossRef]
- Aliabadi, A.; Rounaghi, G.H.; Arbab Zavar, M.H. A new droplet-based polymeric banana electrochemical biosensor for analysis of one microliter solution of paracetamol. Sens. Actuators B Chem. 2017, 241, 182–189. [Google Scholar] [CrossRef]
- Brisolari, A.; Gonçalves, D. Immobilization of Tyrosinase from Avocado Crude Extract in Polypyrrole Films for Inhibitive Detection of Benzoic Acid. Chemosensors 2014, 2, 182–192. [Google Scholar] [CrossRef]
- Lončar, N.; Vujčić, Z. Tentacle carrier for immobilization of potato phenoloxidase and its application for halogenophenols removal from aqueous solutions. J. Hazard. Mater. 2011, 196, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Arabaci, G.; Usluoglu, A. The Enzymatic Decolorization of Textile Dyes by the Immobilized Polyphenol Oxidase from Quince Leaves. Sci. World J. 2014, 2014, 685975. [Google Scholar] [CrossRef]
- Guo, S.; Wang, S.; Meng, J.; Gu, D.; Yang, Y. Immobilized enzyme for screening and identification of anti-diabetic components from natural products by ligand fishing. Crit. Rev. Biotechnol. 2023, 43, 242–257. [Google Scholar] [CrossRef]
- Ashkan, Z.; Hemmati, R.; Homaei, A.; Dinari, A.; Jamlidoost, M.; Tashakor, A. Immobilization of enzymes on nanoinorganic support materials: An update. Int. J. Biol. Macromol. 2021, 168, 708–721. [Google Scholar] [CrossRef]
- Ahrari, F.; Yousefi, M.; Habibi, Z.; Mohammadi, M. Cross-linked lipase particles with improved activity; application of a non-toxic linker for cross-linking. LWT 2023, 173, 114371. [Google Scholar] [CrossRef]
- Naseer, S.; Ouyang, J.; Chen, X.; Pu, S.; Guo, Y.; Zhang, X.; Li, D.; Yang, C. Immobilization of β-glucosidase by self-catalysis and compared to crosslinking with glutaraldehyde. Int. J. Biol. Macromol. 2020, 154, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, X.; Zhu, H.; Zang, Y.; Xue, H. Amperometric Phenol Biosensor Based on a New Immobilization Matrix: Polypyrrole Nanotubes Derived from Methyl Orange as Dopant. Int. J. Electrochem. Sci. 2017, 12, 6714–6728. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef]
- Alam, S.; Nagpal, T.; Singhal, R.; Kumar Khare, S. Immobilization of L-asparaginase on magnetic nanoparticles: Kinetics and functional characterization and applications. Bioresour. Technol. 2021, 339, 125599. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, J.; Huang, J.; Xu, W.; Liu, Z. Study on the Synthesis of Theaflavin-3,3′-Digallate Catalyzed by Escherichia coli Expressing Tea Tree Polyphenol Oxidase Isozymes and Its Enzymatic Solution. Fermentation 2023, 9, 770. [Google Scholar] [CrossRef]
- Lei, S.; Xie, M.; Hu, B.; Zhou, L.; Sun, Y.; Saeeduddin, M.; Zhang, H.; Zeng, X. Effective synthesis of theaflavin-3,3′-digallate with epigallocatechin-3-O-gallate and epicatechin gallate as substrates by using immobilized pear polyphenol oxidase. Int. J. Biol. Macromol. 2017, 94, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.S.; Thirlway, J.; Micklefield, J. Direct Site-Selective Covalent Protein Immobilization Catalyzed by a Phosphopantetheinyl Transferase. J. Am. Chem. Soc. 2008, 130, 12456–12464. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. New electrochemiluminescent biosensors combining polyluminol and an enzymatic matrix. Anal. Bioanal. Chem. 2009, 394, 971–980. [Google Scholar] [CrossRef]
- Zheng, H.; Yan, Z.; Wang, M.; Chen, J.; Zhang, X. Biosensor based on polyaniline-polyacrylonitrile-graphene hybrid assemblies for the determination of phenolic compounds in water samples. J. Hazard. Mater. 2019, 378, 120714. [Google Scholar] [CrossRef]
- Mondal, M.K.; Mukherjee, S.; Saha, S.K.; Chowdhury, P.; Sinha Babu, S.P. Design and synthesis of reduced graphene oxide based supramolecular scaffold: A benign microbial resistant network for enzyme immobilization and cell growth. Mater. Sci. Eng. C 2017, 75, 1168–1177. [Google Scholar] [CrossRef]
- Ramu, P.; Vimal, S.P.; Suresh, P.; Saravanakumar, U.; Sethuraman, V.; Anandhavelu, S. Electrochemically Deposited Porous Graphene−Polypyrrole−Polyphenol Oxidase for Dopamine Biosensor. Electroanalysis 2021, 33, 774–780. [Google Scholar] [CrossRef]
- Zhong, T.; Guo, Q.; Zhu, X.; Liu, R.; Huang, S. Based on Gold Nanoparticles-l-Tyr-Amino Functionalized Mesoporous Materials-Polyphenol Oxidase Modified Biosensor for the Detection of Resorcinol. Anal. Sci. 2021, 37, 817–823. [Google Scholar] [CrossRef]
- Sethuraman, V.; Sridhar, T.M.; Sasikumar, R. Development of an electrochemical biosensor for determination of dopamine by gold modified poly(thiophene-3-boronic acid)-polyphenol oxidase modified electrode. Mater. Lett. 2021, 302, 130387. [Google Scholar] [CrossRef]
- do Nascimento Marreiro Teixeira, A.S.S.; Teixeira, P.R.S.; de Oliveira Farias, E.A.; Ferraz e Sousa, B.; Moura Sérvulo, K.B.d.L.; da Silva, D.A.; Eiras, C. Babassu mesocarp (Orbignya phalerata Mart) nanoparticle-based biosensors for indirect sulfite detection in industrial juices. J. Solid. State Electrochem. 2020, 24, 1143–1155. [Google Scholar] [CrossRef]
- Kuşcu, C.; Özdokur, K.V.; Koçak, S.; Ertaş, F.N. Development of cobalt oxide film modified electrode decorated with platinum nanoparticles as a biosensing platform for phenol. Int. J. Environ. Anal. Chem. 2020, 100, 873–881. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Wang, S.; Tan, Y.; Kan, J. A New Catechol Biosensor Immobilized Polyphenol Oxidase by Combining Electropolymerization and Cross-Linking Process. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 620–626. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, S.K.; Gupta, B.D. SPR based fibre optic biosensor for phenolic compounds using immobilization of tyrosinase in polyacrylamide gel. Sens. Actuators B Chem. 2013, 186, 388–395. [Google Scholar] [CrossRef]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar] [CrossRef]
- Deng, J.; Wang, H.; Zhan, H.; Wu, C.; Huang, Y.; Yang, B.; Mosa, A.; Ling, W. Catalyzed degradation of polycyclic aromatic hydrocarbons by recoverable magnetic chitosan immobilized laccase from Trametes versicolor. Chemosphere 2022, 301, 134753. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Shen, Y.; Wu, D.; Wu, R.; Sheng, J.; Feng, X.; Tang, X. Biodegradable films of chitosan and tea polyphenols catalyzed by laccase and their physical and antioxidant activities. Food Biosci. 2022, 46, 101513. [Google Scholar] [CrossRef]
- Prakash, O.; Verma, D.; Singh, P.C. Exploring enzyme-immobilized MOFs and their application potential: Biosensing, biocatalysis, targeted drug delivery and cancer therapy. J. Mater. Chem. B 2024, 12, 10198–10214. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Lv, Y.-Z.; Yao, L.; Wang, L.; Liu, W.-R.; Zhao, J.-L.; He, L.-Y.; Ying, G.-G. Bioaccumulation, metabolism, and risk assessment of phenolic endocrine disrupting chemicals in specific tissues of wild fish. Chemosphere 2019, 226, 607–615. [Google Scholar] [CrossRef]
- Gill, S.; Kumara, V. Comparative Neurodevelopment Effects of Bisphenol A and Bisphenol F on Rat Fetal Neural Stem Cell Models. Cells 2021, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Lara-Guzmán, O.J.; Álvarez-Quintero, R.; Osorio, E.; Naranjo-Cano, M.; Muñoz-Durango, K. GC/MS method to quantify bioavailable phenolic compounds and antioxidant capacity determination of plasma after acute coffee consumption in human volunteers. Food Res. Int. 2016, 89, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K.; Sentkowska, A. Recent Developments in the HPLC Separation of Phenolic Food Compounds. Crit. Rev. Anal. Chem. 2015, 45, 41–51. [Google Scholar] [CrossRef]
- Wu, L.; Deng, D.; Jin, J.; Lu, X.; Chen, J. Nanographene-based tyrosinase biosensor for rapid detection of bisphenol A. Biosens. Bioelectron. 2012, 35, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Shu, J.; Tang, D. Bioresponsive Release System for Visual Fluorescence Detection of Carcinoembryonic Antigen from Mesoporous Silica Nanocontainers Mediated Optical Color on Quantum Dot-Enzyme-Impregnated Paper. Anal. Chem. 2017, 89, 5152–5160. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.S.-P.; Leroux, F.; Mousty, C. Amperometric biosensors based on LDH-ALGINATE hybrid nanocomposite for aqueous and non-aqueous phenolic compounds detection. Sens. Actuators B Chem. 2010, 150, 36–42. [Google Scholar] [CrossRef]
- Wang, P.; Liu, M.; Kan, J. Amperometric phenol biosensor based on polyaniline. Sens. Actuators B Chem. 2009, 140, 577–584. [Google Scholar] [CrossRef]
- Wang, B.; Zheng, J.; He, Y.; Sheng, Q. A sandwich-type phenolic biosensor based on tyrosinase embedding into single-wall carbon nanotubes and polyaniline nanocomposites. Sens. Actuators B Chem. 2013, 186, 417–422. [Google Scholar] [CrossRef]
- Xue, H.; Shen, Z. A highly stable biosensor for phenols prepared by immobilizing polyphenol oxidase into polyaniline–polyacrylonitrile composite matrix. Talanta 2002, 57, 289–295. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Wu, W.; Farag, M.A.; Wang, L.; Xiao, S.; Gao, H.; Jiang, W. Critical assessment of the delivery methods of chemical and natural postharvest preservatives for fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2023, 65, 1070–1092. [Google Scholar] [CrossRef]
- Jukanti, A. Polyphenol Oxidase(s): Importance in Food Industry. In Polyphenol Oxidases (PPOs) in Plants; Jukanti, A., Ed.; Springer Singapore: Singapore, 2017; pp. 93–106. [Google Scholar]
- Stohs, S.J.; Miller, M.J.S. A case study involving allergic reactions to sulfur-containing compounds including, sulfite, taurine, acesulfame potassium and sulfonamides. Food Chem. Toxicol. 2014, 63, 240–243. [Google Scholar] [CrossRef]
- Lynn, L.; Scholes, R.C.; Kim, J.H.; Wilson-Welder, J.H.; Orts, W.J.; Hart-Cooper, W.M. Antimicrobial, Preservative, and Hazard Assessments from Eight Chemical Classes. ACS Omega 2024, 9, 17869–17877. [Google Scholar] [CrossRef]
- Liu, J.; Liang, C.; Jiang, H.; Yu, Z.; Zou, L.; Zhou, L.; Liu, W. Identification of Polyphenol Oxidase Inhibitors from the Root of Pueraria Lobata: Inhibitor Profiles, Kinetics Analysis and Molecular Interaction. Food Biophys. 2024, 19, 730–744. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, T.; Liu, W.; Zou, L.; Liu, C.; Terefe, N.S. Inhibitory effects of organic acids on polyphenol oxidase: From model systems to food systems. Crit. Rev. Food Sci. Nutr. 2020, 60, 3594–3621. [Google Scholar] [CrossRef] [PubMed]
- Tinello, F.; Lante, A. Recent advances in controlling polyphenol oxidase activity of fruit and vegetable products. Innov. Food Sci. Emerg. Technol. 2018, 50, 73–83. [Google Scholar] [CrossRef]
- Narai-Kanayama, A.; Chiku, K.; Ono, H.; Momoi, T.; Hiwatashi-Kanno, M.; Kobayashi, A.; Matsuda, H.; Yoshida, M.; Nakayama, T. Inhibitory effects of thiol compounds on theaflavin browning and structural analysis of the causative substances. Food Chem. 2022, 384, 132488. [Google Scholar] [CrossRef]
- Zhong, S.; Luo, L.; Pittia, P.; Xie, J.; Wen, H.; Luo, W.; Zeng, L. Studies on the effects of preheated β-lactoglobulin on the physicochemical properties of theaflavin-3,3′-digallate and the interaction mechanism. Food Hydrocoll. 2024, 154, 110087. [Google Scholar] [CrossRef]
- Shi, M.; Lu, Y.; Wu, J.; Zheng, Z.; Lv, C.; Ye, J.; Qin, S.; Zeng, C. Beneficial Effects of Theaflavins on Metabolic Syndrome: From Molecular Evidence to Gut Microbiome. Int. J. Mol. Sci. 2022, 23, 7595. [Google Scholar] [CrossRef]
- Xiao, Y.-Z.; Yang, M.; Xiao, Y.; Guo, Q.; Huang, Y.; Li, C.-J.; Cai, D.; Luo, X.-H. Reducing Hypothalamic Stem Cell Senescence Protects against Aging-Associated Physiological Decline. Cell Metab. 2020, 31, 534–548.e535. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Su, L.; Zhang, F.; Zhu, X.; Zhu, Y.; Wei, L.; Jiao, X.; Hou, Y.; Chen, X.; Wang, W.; et al. Thevebioside, the active ingredient of traditional Chinese medicine, promotes ubiquitin-mediated SRC-3 degradation to induce NSCLC cells apoptosis. Cancer Lett. 2020, 493, 167–177. [Google Scholar] [CrossRef]
- Ye, J.-H.; Ye, Y.; Yin, J.-F.; Jin, J.; Liang, Y.-R.; Liu, R.-Y.; Tang, P.; Xu, Y.-Q. Bitterness and astringency of tea leaves and products: Formation mechanism and reducing strategies. Trends Food Sci. Technol. 2022, 123, 130–143. [Google Scholar] [CrossRef]
- Cai, H.; Zhong, Z.; Chen, Y.; Zhang, S.; Ling, H.; Fu, H.; Zhang, L. Genes cloning, sequencing and function identification of recombinant polyphenol oxidase isozymes for production of monomeric theaflavins from Camellia sinensis. Int. J. Biol. Macromol. 2023, 240, 124353. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, W.; Dong, Y.; Zhan, C.; Tao, T.; Kang, M.; Zhang, C.; Liu, Z. Advances in metabolism pathways of theaflavins: Digestion, absorption, distribution and degradation. Crit. Rev. Food Sci. Nutr. 2024, 65, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Liu, Y.; Zeng, W.; Zhou, M.; Liu, Y.; Huang, Y.; Chen, Q. In vitro enzymatic synthesis of a monomeric theaflavin using a polyphenol oxidase isozyme from tea (Camellia sinensis) leaf. Int. J. Food Sci. Technol. 2022, 57, 5621–5631. [Google Scholar] [CrossRef]
- Jeyabalan, J.; Veluchamy, A.; Priyan, V.V.; Kumar, A.; Chandrasekar, R.; Narayanasamy, S. A review on the laccase assisted decolourization of dyes: Recent trends and research progress. J. Taiwan Inst. Chem. Eng. 2023, 151, 105081. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, B.; Song, Z.; Wang, H.; He, F.; Han, X. Wheat straw biochar amendments on the removal of polycyclic aromatic hydrocarbons (PAHs) in contaminated soil. Ecotoxicol. Environ. Saf. 2016, 130, 248–255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Lin, H.; Luo, Y.; Liu, C. Advances and Application of Polyphenol Oxidase Immobilization Technology in Plants. Plants 2025, 14, 2335. https://doi.org/10.3390/plants14152335
Zhou F, Lin H, Luo Y, Liu C. Advances and Application of Polyphenol Oxidase Immobilization Technology in Plants. Plants. 2025; 14(15):2335. https://doi.org/10.3390/plants14152335
Chicago/Turabian StyleZhou, Fang, Haiyan Lin, Yong Luo, and Changwei Liu. 2025. "Advances and Application of Polyphenol Oxidase Immobilization Technology in Plants" Plants 14, no. 15: 2335. https://doi.org/10.3390/plants14152335
APA StyleZhou, F., Lin, H., Luo, Y., & Liu, C. (2025). Advances and Application of Polyphenol Oxidase Immobilization Technology in Plants. Plants, 14(15), 2335. https://doi.org/10.3390/plants14152335





