Establishment of Singleplex and Duplex TaqMan RT-qPCR Detection Systems for Strawberry Mottle Virus (SMoV) and Strawberry Vein Banding Virus (SVBV)
Abstract
1. Introduction
2. Results
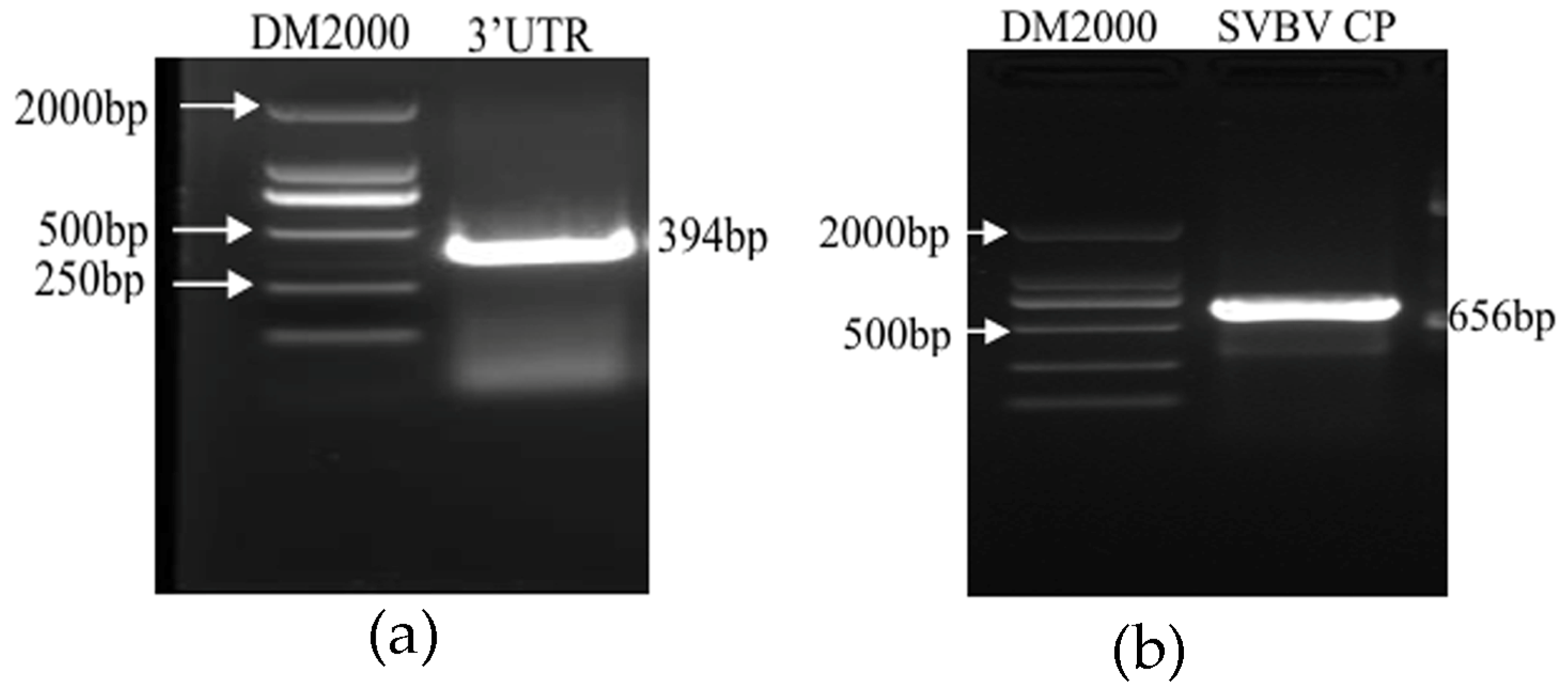
2.1. Amplification of SMoV and SVBV Templates
2.2. Establishment of the Standard Curve for SMoV Singleplex TaqMan RT-qPCR
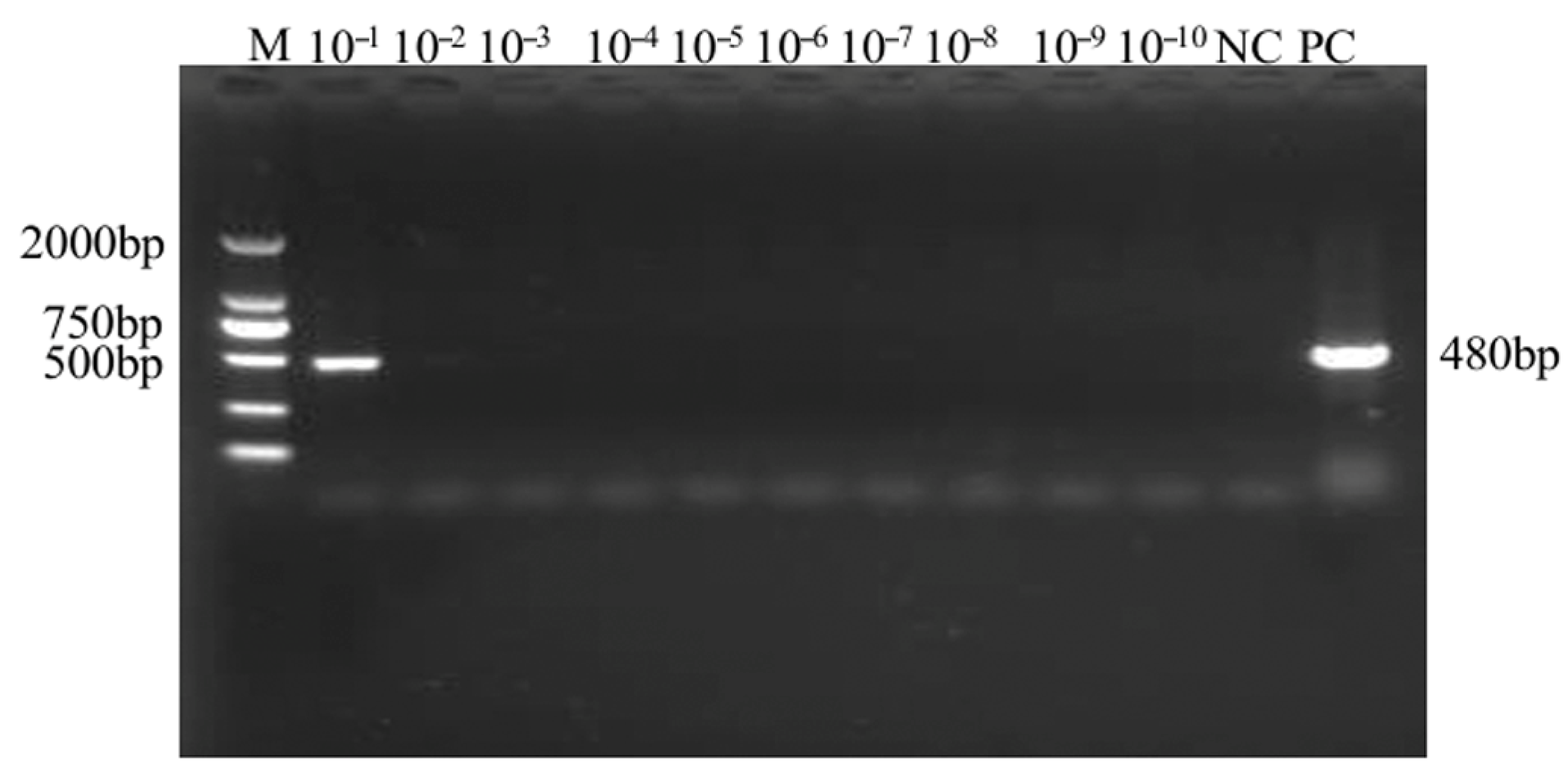
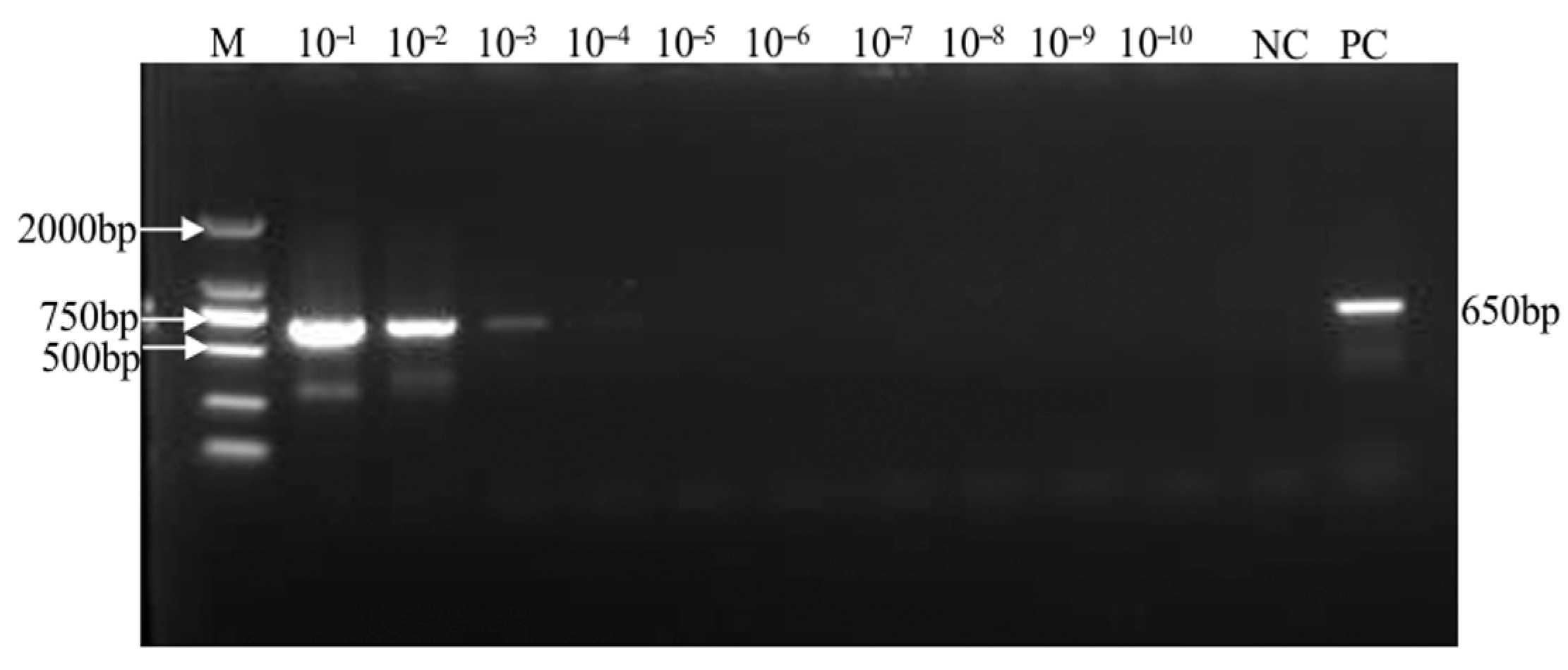
2.3. Sensitivity Analysis of the SMoV Singleplex TaqMan RT-qPCR Assay
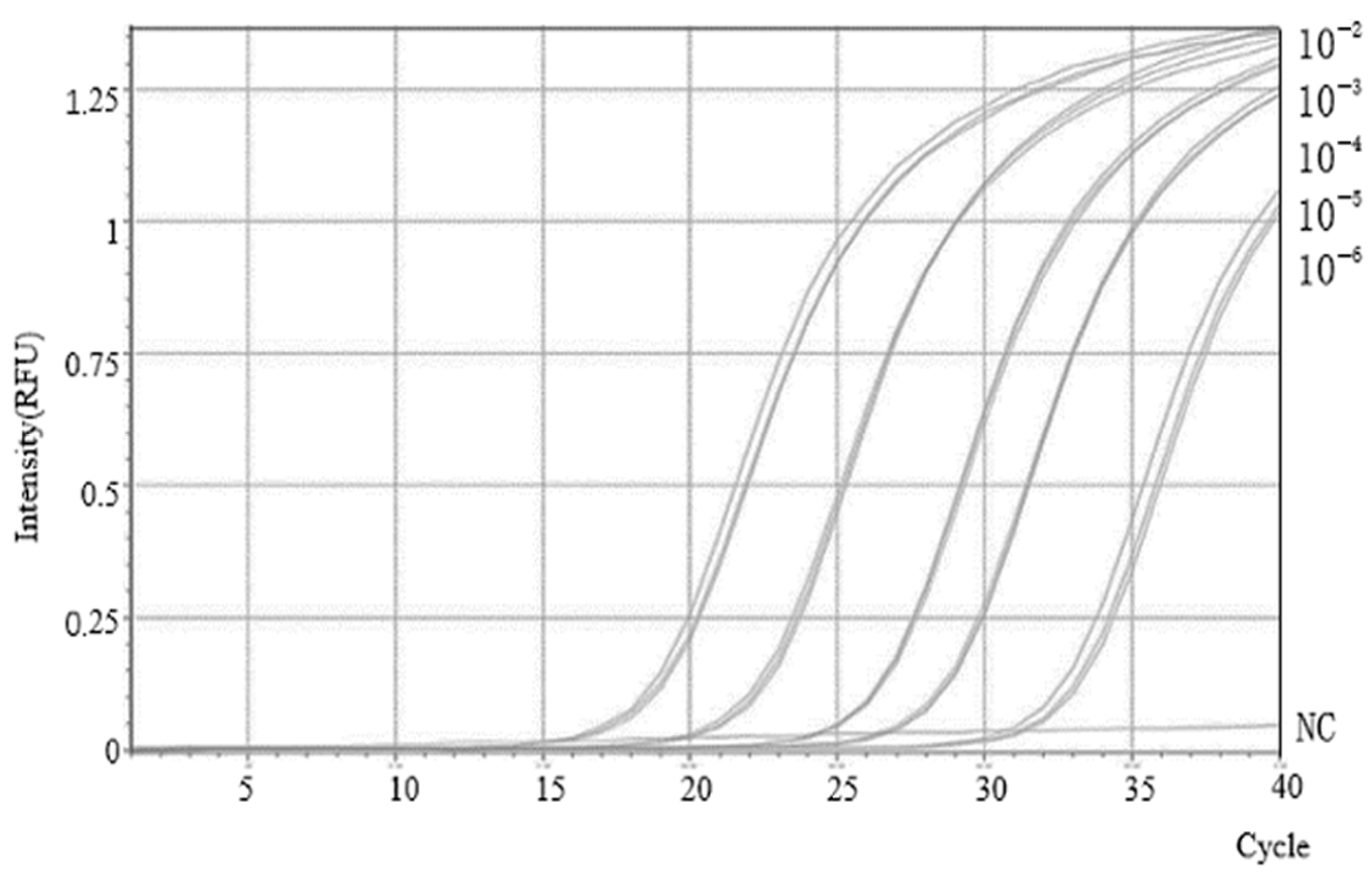
2.4. Establishment of the SVBV Singleplex TaqMan RT-qPCR Standard Curve
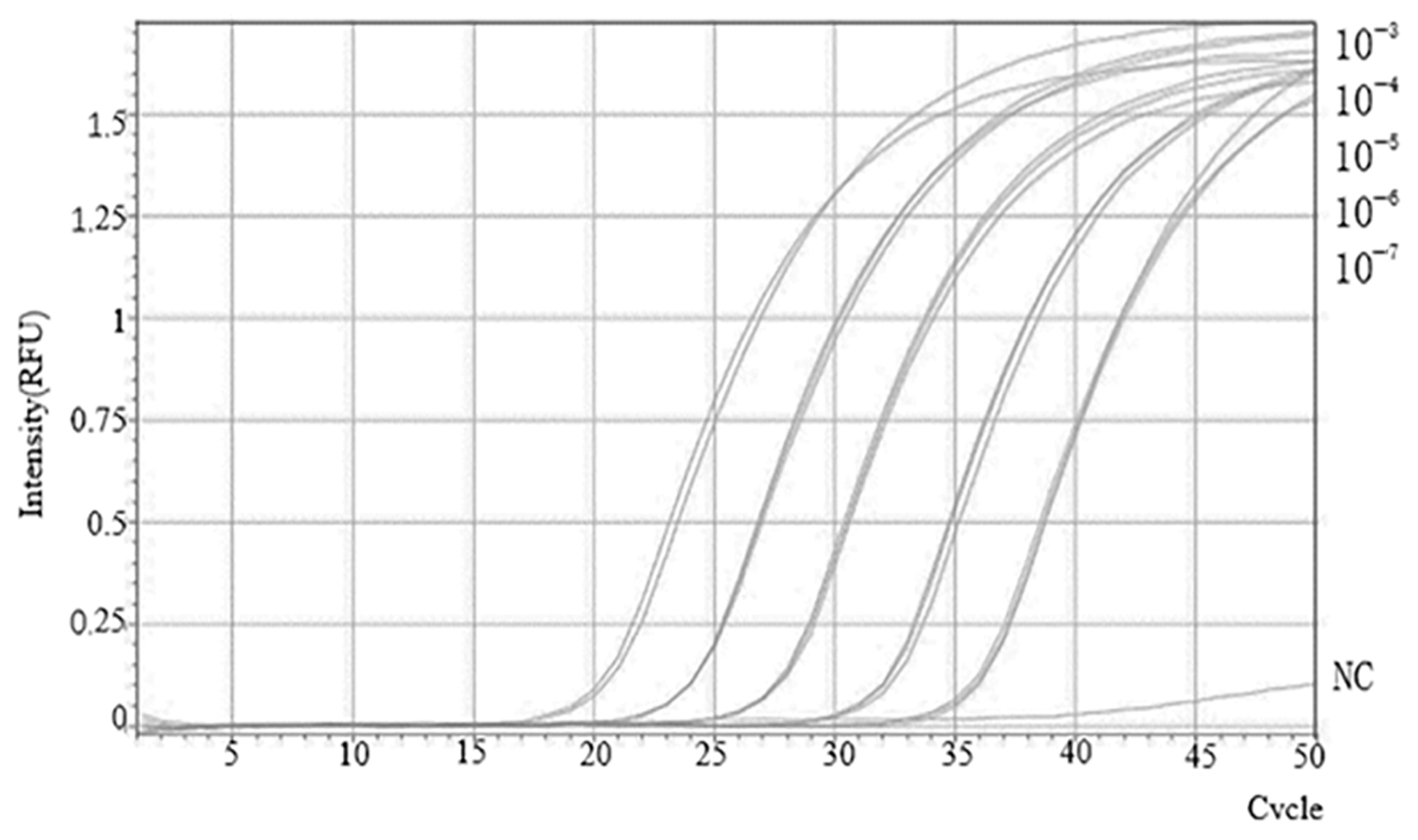
2.5. Sensitivity Analysis of the SVBV Singleplex TaqMan RT-qPCR Assay
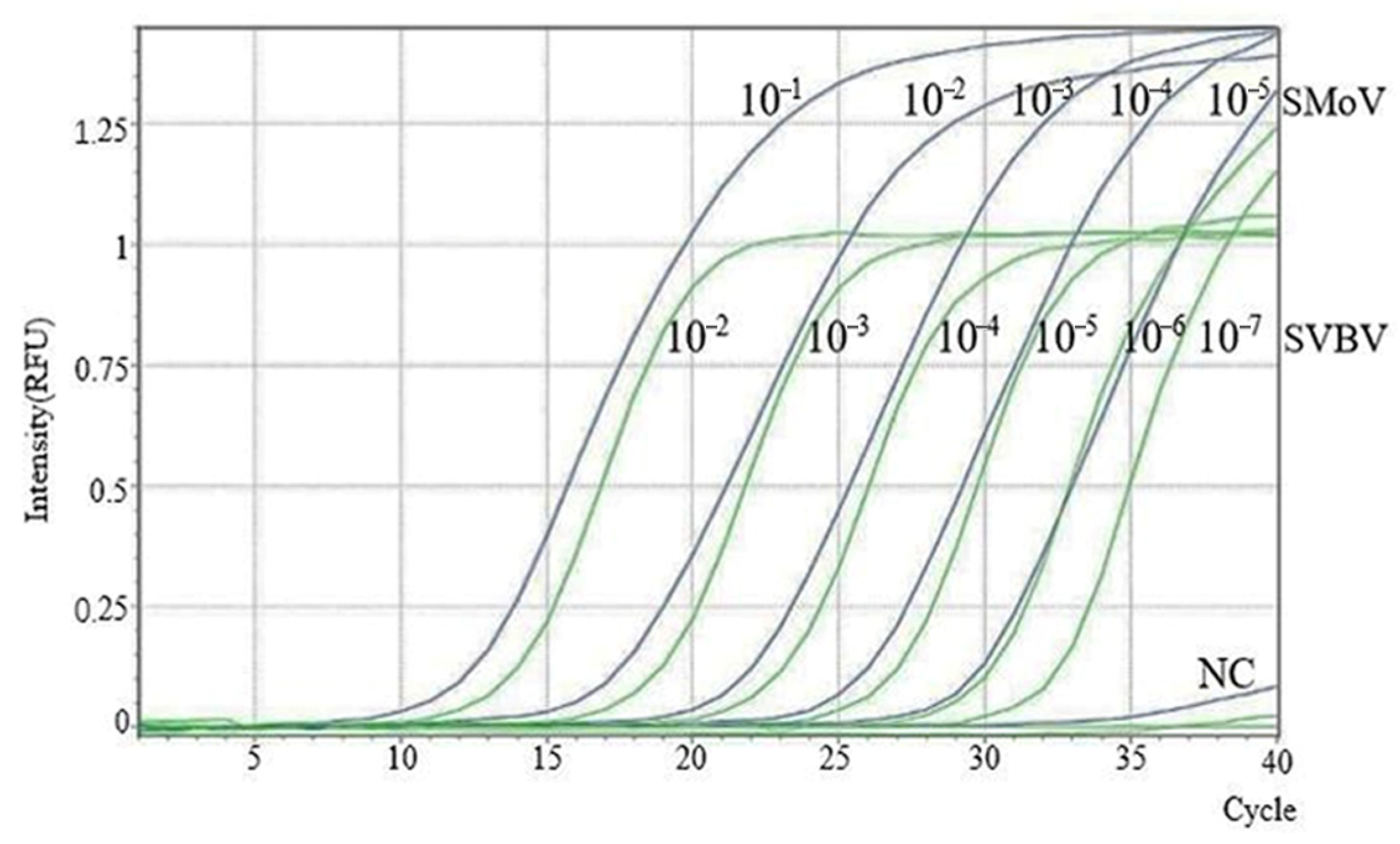
2.6. Establishment of the SMoV and SVBV Duplex TaqMan RT-qPCR Standard Curve
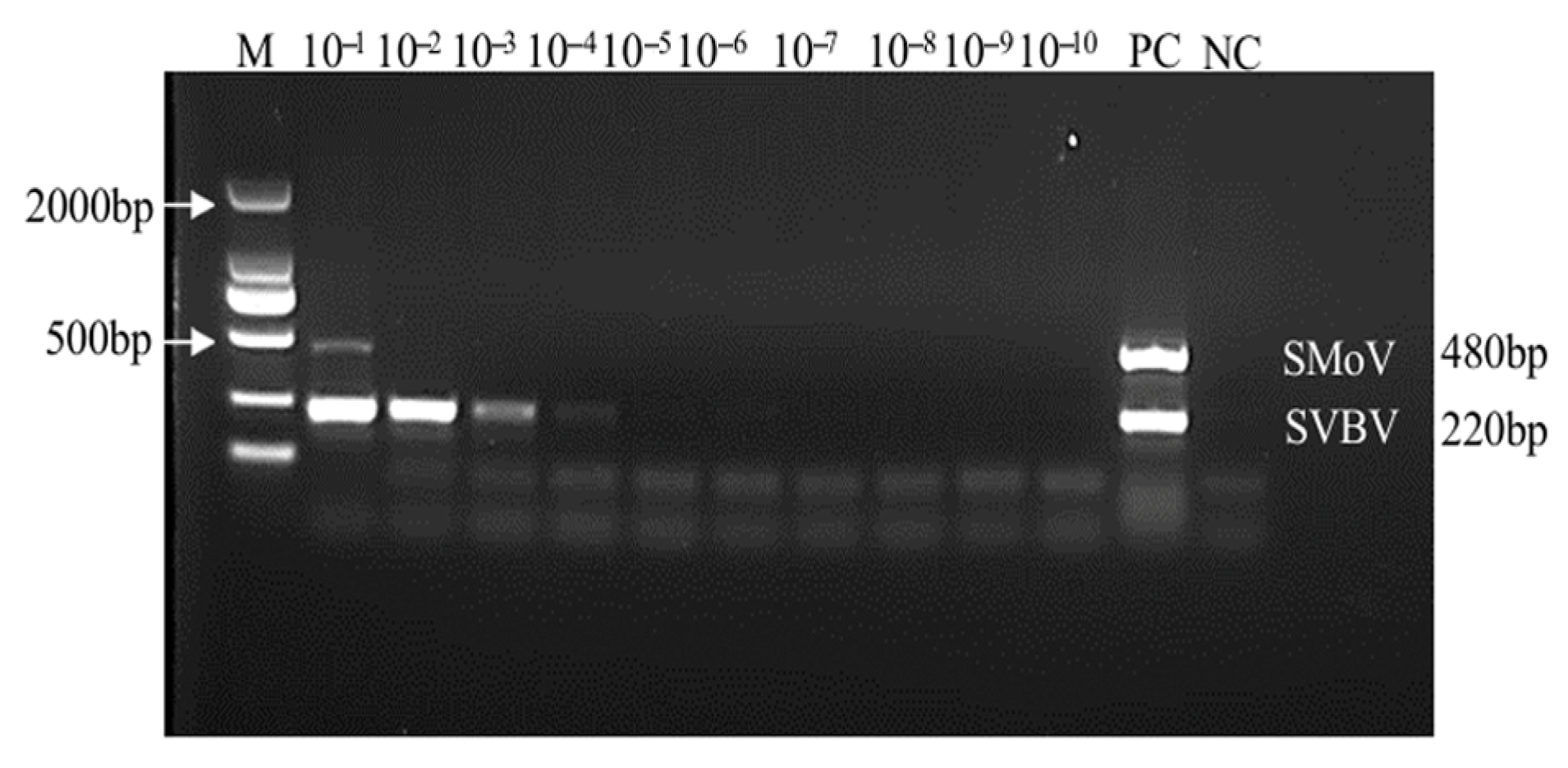
2.7. Sensitivity Analysis of the Duplex TaqMan RT-qPCR Assay for SMoV and SVBV
2.8. Comparison Between RT-PCR and Duplex TaqMan RT-qPCR Techniques on Field Samples
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Total RNA Extraction and Reverse Transcription from Plant Tissues
4.3. Primer and Probe Design and Synthesis
4.4. Preparation of TaqMan RT-qPCR Standard Curve
4.5. Singleplex TaqMan RT-qPCR
4.6. Duplex TaqMan RT-qPCR
4.7. PCR
4.8. Gene Cloning and Plasmid Extraction
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, L.; He, C.; Gao, D.; Xu, T.; Xing, F.; Yan, J.; Zhan, B.; Li, S.; Wang, H. Identification of silencing suppressor protein encoded by strawberry mottle virus. Front. Plant Sci. 2022, 13, 786489. [Google Scholar] [CrossRef]
- Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; González-Paramás, A.M.; Santos-Buelga, C.; Mezzetti, B.; Quiles, J.L.; Battino, M.; Giampieri, F. Strawberry (cv. Romina) methanolic extract and anthocyanin-enriched fraction improve lipid profile and antioxidant status in HepG2 cells. Int. J. Mol. Sci. 2017, 18, 1149. [Google Scholar] [CrossRef]
- Xu, S.; Shi, D.; Chen, H.; Ma, F.; Tao, G.; Meng, L.; Lin, D.; Wu, S.; Fei, Q. Comparison of the flavor characteristics in three kinds of strawberry fruits. Food Res. Int. 2024, 198, 115363. [Google Scholar] [CrossRef]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Kowalska, J.; Kowalska, H.; Marzec, A.; Brzeziński, T.; Samborska, K.; Lenart, A. Dried strawberries as a high nutritional value fruit snack. Food Sci. Biotechnol. 2018, 27, 799–807. [Google Scholar] [CrossRef]
- Martin, R.R.; Tzanetakis, I.E. High risk strawberry viruses by region in the United States and Canada: Implications for certification, nurseries, and fruit production. Plant Dis. 2013, 97, 1358–1362. [Google Scholar] [CrossRef]
- Su, D.-F.; Tong, J.-Y.; Yang, J.-Y.; Chen, S.-Y.; Luo, Z.-W.; Shen, X.-M.; Lai, Y.-H.; Arslan, J.; Wei, S.-J.; Cui, X.-L. Strawberry viruses diseases and its research advances. J. Yunnan Univ. (Nat. Sci. Ed.) 2019, 41, 1221–1237. [Google Scholar]
- Martin, R.R.; Tzanetakis, I.E. Characterization and recent advances in detection of strawberry viruses. Plant Dis. 2006, 90, 384–396. [Google Scholar] [CrossRef]
- Freeman, J.A.; Mellor, F.C. Influences of latent viruses on vigor, yield and quality of British Sovereign strawberries. Can. J. Plant Sci. 1962, 42, 602–610. [Google Scholar] [CrossRef]
- Sharma, A.; Handa, A.; Kapoor, S.; Watpade, S.; Gupta, B.; Verma, P. Viruses of strawberry and production of virus free planting material—A critical review. Int. J. Sci. Environ. Technol. 2018, 7, 521–545. [Google Scholar]
- Xu, T.; Zhan, B.; Zhang, Z.; Fan, L.; Gao, D.; Dong, Z.; Li, S.; Wang, H. FaNDUFB9 Attenuates Strawberry Mottle Virus Infection by Inhibiting the Activity of the Viral Gene Silencing Suppressor, Pro2Glu. Mol. Plant Pathol. 2025, 26, e70061. [Google Scholar] [CrossRef]
- Mann, K.S.; Walker, M.; Sanfaçon, H. Identification of cleavage sites recognized by the 3C-Like cysteine protease within the two polyproteins of strawberry mottle virus. Front. Microbiol. 2017, 8, 745. [Google Scholar]
- Wang, G.; Liu, F.; Guo, J. Identification of strawberry viruses in China. Acta Phytopathol. Sin. 1991, 21, 9–14. [Google Scholar]
- Thompson, J.; Jelkmann, W. The detection and variation of Strawberry mottle virus. Plant Dis. 2003, 87, 385–390. [Google Scholar] [CrossRef]
- Cieślińska, M. Detection of Strawberry mottle virus (SMoV) using RT-PCR–comparison of two RNA extraction methods. J. Fruit Ornam. Plant Res. 2004, 12, 17–22. [Google Scholar]
- Thompson, J.R.; Wetzel, S.; Klerks, M.; Vašková, D.; Schoen, C.; Špak, J.; Jelkmann, W. Multiplex RT-PCR detection of four aphid-borne strawberry viruses in Fragaria spp. in combination with a plant mRNA specific internal control. J. Virol. Methods 2003, 111, 85–93. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, X.; Xiao, G.; Zhang, D.; Wen, X.; Zhou, X.; Wang, Z.; Deng, J.; Han, Y. Development of a Multiplex RT-PCR Detection for Six Viruses Infecting Strawberry. Viruses 2024, 16, 1858. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, H.; Pan, Y.; Hu, Y.; Chen, J.; Zuo, D.; Jiang, T. Complete nucleotide sequence of strawberry vein banding virus Chinese isolate and infectivity of its full-length DNA clone. Virol. J. 2016, 13, 164. [Google Scholar] [CrossRef]
- Chun, S.; Luping, W.; Zhihong, Z. Detection of strawberry vein banding virus in strawberries by PCR. Acta Hortic. Sin. 2003, 30, 82. [Google Scholar]
- Xiang, M.L.; Ning, R.N.; Chao, Z.Y.; Fang, Y.X.; Qin, Z.; Qin, Z.H.; Hua, J.G. Real time fluorescent quantitative PCR for detection of Strawberry vein banding virus. Acta Agric. Zhejiangensis 2016, 28, 1030–1036. [Google Scholar]
- Heid, C.A.; Stevens, J.; Livak, K.J.; Williams, P.M. Real time quantitative PCR. Genome Res. 1996, 6, 986–994. [Google Scholar] [CrossRef]
- Kubista, M.; Andrade, J.M.; Bengtsson, M.; Forootan, A.; Jonák, J.; Lind, K.; Sindelka, R.; Sjöback, R.; Sjögreen, B.; Strömbom, L. The real-time polymerase chain reaction. Mol. Asp. Med. 2006, 27, 95–125. [Google Scholar] [CrossRef]
- Watson, D.E.; Li, B. TaqMan applications in genetic and molecular toxicology. Int. J. Toxicol. 2005, 24, 139–145. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, M.; Li, Y.; Tian, J.; Xian, J.; Chen, Q. A Taq Man probe-based multiplex real-time quantitative pcr for simultaneous detection of kobuvirus, parechovirus B, rosavirus B, and hunnivirus carried by murine rodents and shrews. Virol. J. 2025, 22, 61. [Google Scholar] [CrossRef]
- Bustin, S.A.; Nolan, T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J. Biomol. Tech. JBT 2004, 15, 155. [Google Scholar]
- Kralik, P.; Ricchi, M. A basic guide to real time PCR in microbial diagnostics: Definitions, parameters, and everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Mackay, I.M.; Arden, K.E.; Nitsche, A. Real-time PCR in virology. Nucleic Acids Res. 2002, 30, 1292–1305. [Google Scholar] [CrossRef]
- Fan, L.; Song, D.; Khoo, Y.; Wu, M.; Xu, T.; Zhao, X.; Wang, H. Effect of strawberry vein banding virus and strawberry mottle virus co-infection on the growth and development of strawberry. Biocell 2022, 46, 263. [Google Scholar] [CrossRef]

| Primer Concentration (μmol/L) | Probe Concentration (μmol/L) | ||||
|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
| 0.2 | 11.842 | 11.935 | 12.028 | 11.923 | 11.793 |
| 0.4 | 11.721 | 11.753 | 12.001 | 11.646 | 11.657 |
| 0.6 | 11.732 | 11.746 | 11.745 | 11.732 | 11.638 |
| 0.8 | 11.628 | 11.711 | 12.095 | 11.702 | 11.683 |
| Group | Cq Value | CV | |||
|---|---|---|---|---|---|
| Intra-Group | Inter-Group | ||||
| 1 | 17.659 | 18.047 | 17.937 | 1.11% | 1.54% |
| 2 | 18.934 | 17.564 | 18.332 | 3.75% | |
| 3 | 17.892 | 18.457 | 18.921 | 2.79% | |
| Primer Concentration (μmol/L) | Probe Concentration (μmol/L) | ||||
|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
| 0.2 | 13.159 | 13.445 | 13.403 | 13.421 | 13.381 |
| 0.4 | 13.380 | 13.598 | 13.504 | 13.521 | 13.428 |
| 0.6 | 13.061 | 13.165 | 13.237 | 13.439 | 13.372 |
| 0.8 | 13.363 | 13.461 | 13.470 | 13.377 | 13.600 |
| Group | Cq Value | CV | |||
|---|---|---|---|---|---|
| Intra-Group | Inter-Group | ||||
| 1 | 19.347 | 19.669 | 18.584 | 2.90% | 2.77% |
| 2 | 17.653 | 18.418 | 18.614 | 2.79% | |
| 3 | 18.349 | 17.893 | 19.012 | 3.06% | |
| Primer Concentration (μmol/L) | Probe Concentration (μmol/L) | ||||
|---|---|---|---|---|---|
| 0.1 | 0.15 | 0.2 | 0.25 | 0.3 | |
| 0.4 | 30.056 | 30.302 | 30.542 | 30.925 | 32.490 |
| 0.5 | 29.411 | 28.870 | 29.394 | 31.541 | 31.631 |
| 0.6 | 28.229 | 28.602 | 29.049 | 30.189 | 30.527 |
| 0.7 | 29.587 | 28.199 | 30.387 | 29.463 | 30.051 |
| 0.8 | 27.300 | 28.835 | 28.963 | 37.628 | 30.428 |
| 0.9 | 26.444 | 27.603 | 29.154 | 30.388 | 33.390 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Gao, D.; Wu, M.; Wang, H.; He, C. Establishment of Singleplex and Duplex TaqMan RT-qPCR Detection Systems for Strawberry Mottle Virus (SMoV) and Strawberry Vein Banding Virus (SVBV). Plants 2025, 14, 2330. https://doi.org/10.3390/plants14152330
Xu T, Gao D, Wu M, Wang H, He C. Establishment of Singleplex and Duplex TaqMan RT-qPCR Detection Systems for Strawberry Mottle Virus (SMoV) and Strawberry Vein Banding Virus (SVBV). Plants. 2025; 14(15):2330. https://doi.org/10.3390/plants14152330
Chicago/Turabian StyleXu, Tengfei, Dehang Gao, Mengmeng Wu, Hongqing Wang, and Chengyong He. 2025. "Establishment of Singleplex and Duplex TaqMan RT-qPCR Detection Systems for Strawberry Mottle Virus (SMoV) and Strawberry Vein Banding Virus (SVBV)" Plants 14, no. 15: 2330. https://doi.org/10.3390/plants14152330
APA StyleXu, T., Gao, D., Wu, M., Wang, H., & He, C. (2025). Establishment of Singleplex and Duplex TaqMan RT-qPCR Detection Systems for Strawberry Mottle Virus (SMoV) and Strawberry Vein Banding Virus (SVBV). Plants, 14(15), 2330. https://doi.org/10.3390/plants14152330





