Development of a Green-Synthesized WA-CDs@MIL-101 Fluorescent Sensor for Rapid Detection of Panax notoginseng Leaf Pathogen Spores
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
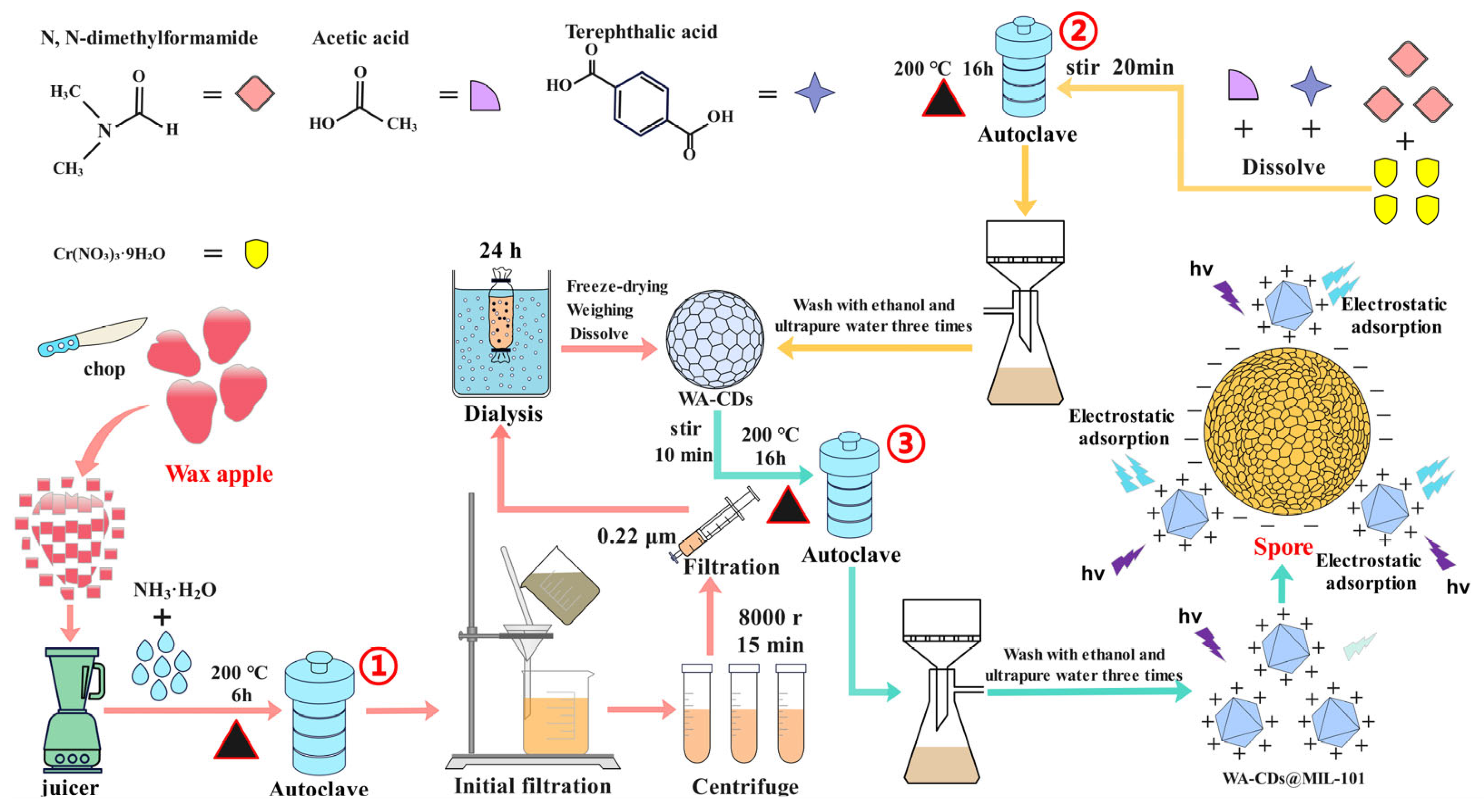
2.2. Synthesis of WA-CDs@MIL-101 Composite
2.3. Characterization and Fluorescence Detection
2.4. Calibration and Real Sample Analysis
3. Results and Discussion
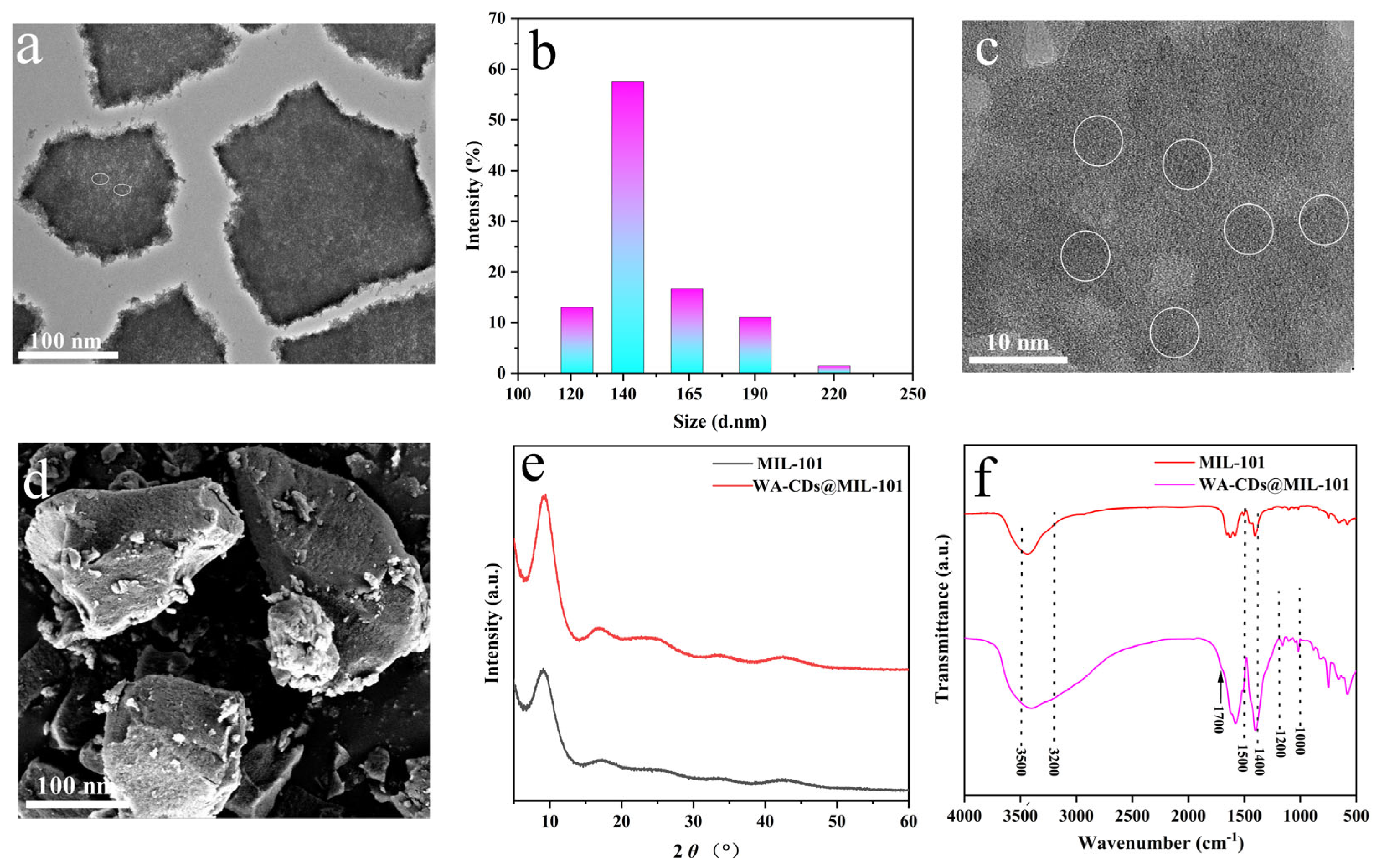
3.1. Characterization
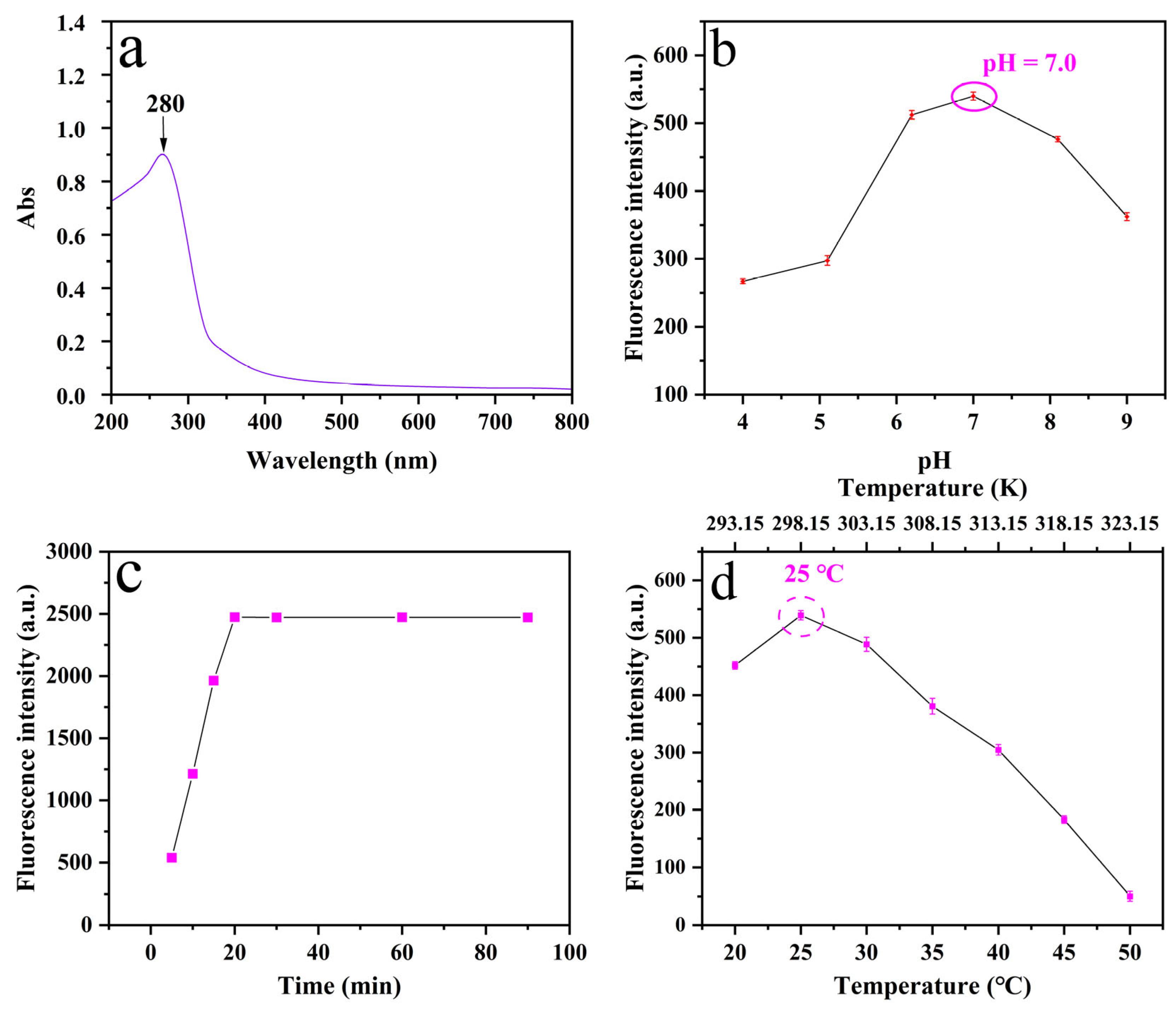
3.2. Optimization of Experimental Conditions
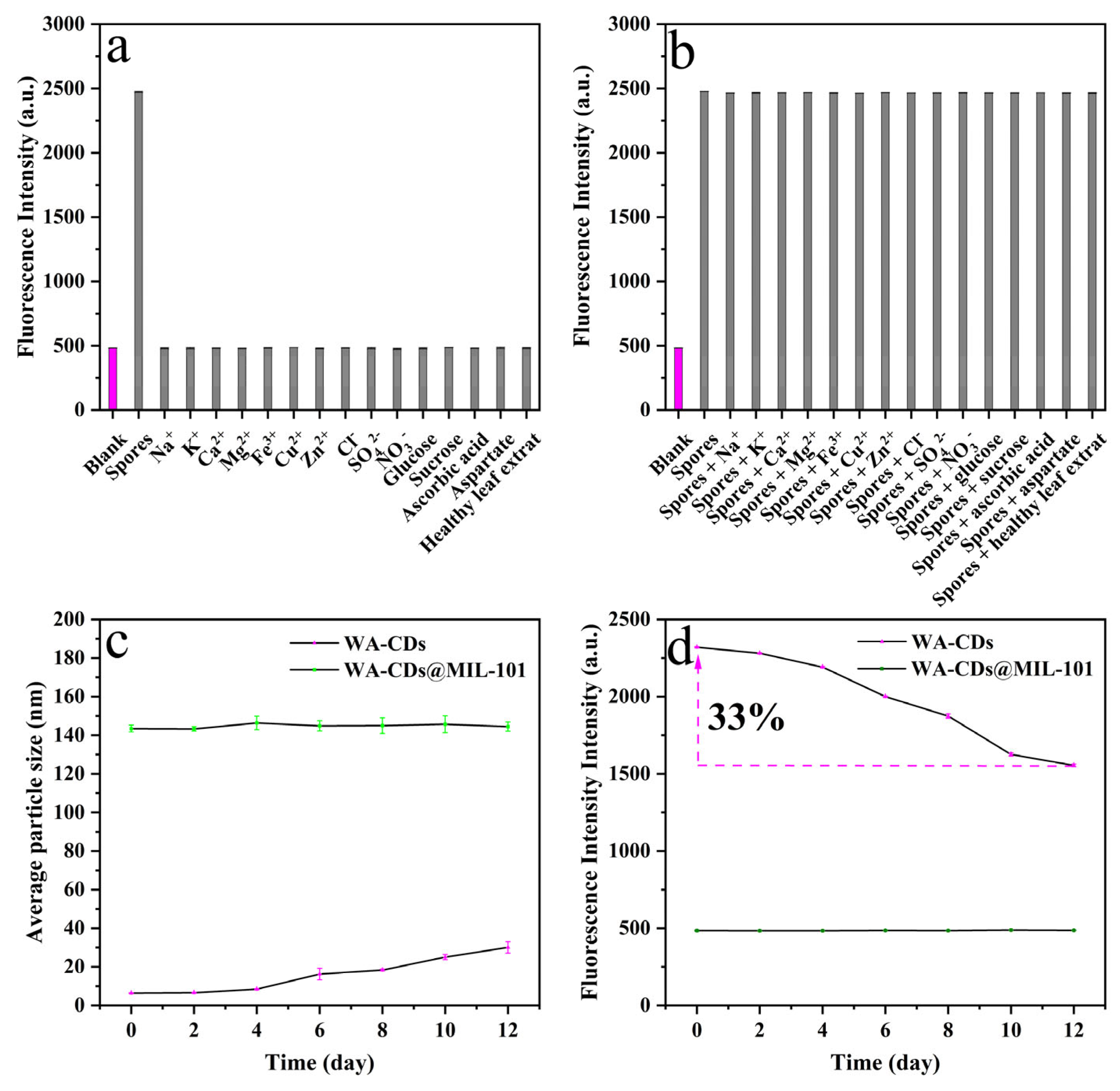
3.3. Selectivity and Anti-Interference Ability
3.4. Verification of the Inhibitory Effect of MIL-101 on the Aggregation Behavior of WA-CDs
3.5. Standard Curve
3.6. Detection Mechanism
3.7. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDs | Carbon Dots |
| WA-CDs | Wax Apple-Derived Carbon Dots |
| MIL-101 | Materials of Institute Lavoisier-101 (a type of metal–organic framework) |
| WA-CDs@MIL-101 | Composite of WA-CDs encapsulated in MIL-101 |
| QY | Quantum Yield |
| MOFs | Metal–Organic Frameworks |
| TEM | Transmission Electron Microscopy |
| SEM | Scanning Electron Microscopy |
| EDX | Energy-Dispersive X-ray Spectroscopy |
| DLS | Dynamic Light Scattering |
| XRD | X-ray Diffraction |
| FTIR | Fourier Transform Infrared Spectroscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| UV–vis | Ultraviolet–Visible Spectroscopy |
| qPCR | Quantitative Polymerase Chain Reaction |
| LOD | Limit of Detection |
| RSD | Relative Standard Deviation |
| SI | Supporting Information |
| FCDMP | Fluorescent Carbon Dots by Flow-Assisted Melt Polymerization |
| BES | Bio-Electrochemical Sensor |
| FEAB | Freeze-Dried Electrochemically Active Bacteria |
| HOSN | Hybrid Graphene Oxide-Immobilized Silver Nanocomposite |
| GCDC | Green Fluorescent Carbon Dots from Chitosan |
| DUECD | Deep Ultraviolet Emissive Carbon Dots |
References
- Zhang, X.; Zhang, B.; Zhang, C.; Sun, G.; Sun, X. Effect of Panax notoginseng saponins and major anti-obesity components on weight loss. Front. Pharmacol. 2020, 11, 601751. [Google Scholar] [CrossRef]
- Yang, K.; Wang, H.L.; Ye, C.; Wang, Z.H.; Ye, K.H.; Zhang, S.; Huang, H.P.; Wei, Z.X.; Zhu, S.S.; Zhu, Y.Y.; et al. Infection characteristics and physical prevention strategy of Panax notoginseng round spot disease caused by mycocentrospora acerina. Plant Dis. 2022, 106, 2607–2617. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Qiu, B.; Zhang, Y.; Cui, X.; Ge, F.; Liu, D. Thaumatin-like protein genes of Panax notoginseng confers resistance to Alternaria panax. Physiol. Mol. Plant Pathol. 2020, 112, 101537. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Li, S.; Yang, Q. The combination of biochar and bacillus subtilis biological agent reduced the relative abundance of pathogenic bacteria in the rhizosphere soil of Panax notoginseng. Microorganisms 2024, 12, 783. [Google Scholar] [CrossRef]
- Chen, C.-J.; Li, Q.-Q.; Zeng, Z.-Y.; Duan, S.-S.; Wang, W.; Xu, F.-R.; Cheng, Y.-X.; Dong, X. Efficacy and mechanism of Mentha haplocalyx and Schizonepeta tenuifolia essential oils on the inhibition of Panax notoginseng pathogens. Ind. Crops Prod. 2020, 145, 112073. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, H.; Lu, Y.; Ao, D.; Liang, Z.; Zhao, M.; Yang, S.; Tang, Q. Microencapsulation of total saponins from stem and leaf of Panax notoginseng by freeze and spray drying: Process optimization, physicochemical properties, structure, antioxidant activity, and stability. J. Food Sci. 2024, 89, 7888–7906. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, X.; Yang, Q.; Lei, L.; Liang, J.; Yang, L. Enhancing Panax notoginseng leaf disease classification with Inception-SSNet and image generation via improved diffusion model. Agronomy 2024, 14, 1982. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Wang, R.; Lei, L.; Ding, H.; Yang, Q. WE-DeepLabV3+: A lightweight segmentation model for Panax notoginseng leaf diseases. Comput. Electron. Agric 2024, 227, 109612. [Google Scholar] [CrossRef]
- Yi, K.; Zhang, X.; Zhang, L. Eu3+@metal–organic frameworks encapsulating carbon dots as ratiometric fluorescent probes for rapid recognition of anthrax spore biomarker. Sci. Total Environ. 2020, 743, 140692. [Google Scholar] [CrossRef]
- Ma, M.; Pan, J.; Wang, J.; Jing, Y.; Fu, Y.; Shen, Y.; Wang, D.; Wang, C.; Li, J. Cu2+-cross-linked tannic acid carbon dot nanoparticles for mold inhibition. ACS Appl. Nano Mater. 2025, 8, 4138–4150. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Liu, L.; Jia, M.; Li, X.; Li, J. Nano-biosensor based on manganese dioxide nanosheets and carbon dots for dual-mode determination of Staphylococcus aureus. Food Chem. 2024, 432, 137144. [Google Scholar] [CrossRef]
- Ren, Y.; Fan, Z. Synthesis of fluorescent probe based on molecularly imprinted polymers on nitrogen-doped carbon dots for determination of tobramycin in milk. Food Chem. 2023, 416, 135792. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, L.; Chen, H.; Hu, S.; Huang, C.; Zhen, S.; Zhan, L. Antibacterial properties of folic acid-based hydrogel loaded with CeCDs and its potential application in food preservation. Food Chem. 2025, 481, 144031. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, L.; Song, Z.; Zhang, J.; Yang, Y.; Liu, X. Revealing the in-situ growth mechanism of carbon dots confined in ZIF-8 as multicolor fluorescent material with high photothermal stability. J. Colloid Interface Sci. 2025, 688, 172–182. [Google Scholar] [CrossRef]
- Tan, P.; Chen, Y.; Chang, H.; Liu, T.; Wang, J.; Lu, Z.; Sun, M.; Su, G.; Wang, Y.; Wang, H.D.; et al. Deep learning assisted logic gates for real-time identification of natural tetracycline antibiotics. Food Chem. 2024, 454, 139705. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Su, Y.; Xu, R.; Song, D.; Ding, L.; Chen, Y. Highly sensitive fluoroprobe for detecting Sudan dyes in paprika utilizing carbon dot-embedded zeolitic imidazolate framework-8. Food Chem. 2024, 438, 137975. [Google Scholar] [CrossRef]
- Li, W.; Yu, X.; Tang, Y.; Li, Z.; Shah, S.J.; Liu, Y.; Zhao, H.; Song, M.; Li, J.; Wang, G.; et al. Confined construction of CDs@MIL-101 as reusable and turn-on fluorescence sensor for highly sensitive water detection based on adsorption-solvent synergistic mechanism. Sens. Actuators B Chem. 2023, 390, 133954. [Google Scholar] [CrossRef]
- He, Q.; Zhuang, S.; Yu, Y.; Li, H.; Liu, Y. Ratiometric dual-emission of Rhodamine-B grafted carbon dots for full-range solvent components detection. Anal. Chim. Acta 2021, 1174, 338743. [Google Scholar] [CrossRef]
- Li, C.; Li, N.; Yang, L.; Liu, L.; Zhang, D. Synthesis of fluorescent carbon dots by B/P doping and application for Co2+ and methylene blue detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 309, 123824. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, K.; Yuan, B.; Zhu, X.; Chen, X.; Huang, K. Gasoline residue-prepared carbon dots embedded metal–organic frameworks for selective and sensitive detection of sulfide ions. Microchem. J. 2023, 193, 109035. [Google Scholar] [CrossRef]
- Li, Y.; Chi, C.; Zhao, Y.; Jiang, G.; Wu, J.; Song, J. Formaldehyde detection based on tannin carbon dots. Chem. Eng. Sci. 2025, 304, 120914. [Google Scholar] [CrossRef]
- Zhao, Z.; Jing, Y.; Shen, Y.; Liu, Y.; Wang, J.; Ma, M.; Pan, J.; Wang, D.; Wang, C.; Li, J. Silicon-doped carbon dots crosslinked carboxymethyl cellulose gel: Detection and adsorption of Fe3+. Gels 2024, 10, 285. [Google Scholar] [CrossRef]
- McEnroe, A.; Brunt, E.; Mosleh, N.; Yu, J.; Hailstone, R.; Sun, X. Bright, green fluorescent carbon dots for sensitive and selective detection of ferrous ions. Talanta Open 2023, 7, 100236. [Google Scholar] [CrossRef]
- Yang, D.; Ma, C.; Chen, G.; Li, L.; Hu, A.; Huang, A.; Zhou, Y.; Cai, Z.; Yang, T.; Gao, H.; et al. Investigation of the application and mechanism of nitrogen and phosphorus co-doped carbon dots for mercury ion detection. J. Fluoresc. 2025, 35, 1181–1189. [Google Scholar] [CrossRef]
- Yang, D.; Lin, J.; Ying, W.; Wen, P.; Zhang, J.; Chen, Z. Xylooligosaccharides, monosaccharides, and pH-sensitive carbon dots production from Toona sinensis branches using organic acid hydrolysis and hydrothermal treatment. Int. J. Biol. Macromol. 2025, 310, 142851. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, K.; Wei, S.; Li, S.; Peng, D.; Nie, J.; Duan, J.; Wu, D.; Chang, X. Converting waste chrysanthemi flos residues into high-value fluorescent carbon dots for rapid and selective detection of mercury (II) ions in aqueous environments. Ind. Crops Prod. 2025, 228, 120914. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, M.; Zheng, N.; Liu, B.; Tang, J.; Tang, J.; Zhang, F.; Chen, G. Green synthesis of carbon dots and their application as fluorescent probes for rutin detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 337, 126084. [Google Scholar] [CrossRef]
- Zhao, C.-Y.; Tseng, W.-B.; Hung, K.-H.; Tseng, W.-L. Ultrasensitive detection of tetracycline using the disruption of crosslink-enhanced emission and inner-filter effect-induced phosphorescence quenching of carbonized polymer dots. Biosens. Bioelectron. 2025, 279, 117409. [Google Scholar] [CrossRef]
- Miao, C.; Shi, X.; Li, Z.; Zhang, X.; Wang, X.; Yang, D.; Wang, Q. Norfloxacin-derived carbon dots with rich electron pyrrolic nitrogen for copper corrosion inhibition and antibacterial functions. Langmuir 2025, 41, 8951–8964. [Google Scholar] [CrossRef]
- Maria, S.A.P.; Lima, G.M.; André, R.; Matiuzzi, C.M.; Leonardo, D.B.; Pequeno, O.H. Antibacterial activity of ciprofloxacin-based carbon dot@silver nanoparticle composites. ACS Omega 2025, 10, 11505–11515. [Google Scholar]
- Atchudan, R.; Karuppasamy, B.D.; Perumal, S.; Gangadaran, P.; Sundramoorthy, A.K.; Manoj, D.; Rajendran, R.L.; Ahn, B.-C.; Ahamed, M.; Lee, S.W.; et al. Sustainable-biomass-derived multifunctional carbon dots as fluorescent probes for multi-purpose advanced imaging, migration and security solutions. Surf. Interfaces 2025, 62, 106238. [Google Scholar] [CrossRef]
- Jalili, R.; Irani-nezhad, M.H.; Khataee, A.; Joo, S.W. A ratiometric fluorescent probe based on carbon dots and gold nanocluster encapsulated metal–organic framework for detection of cephalexin residues in milk. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 262, 120089. [Google Scholar] [CrossRef]
- Zang, Y.; Zhao, T.; Xie, B.; Feng, Y.; Yi, Y.; Liu, H. A bio-electrochemical sensor based on suspended Shewanella oneidensis MR-1 for the sensitive assessment of water biotoxicity. Sens. Actuators B Chem. 2021, 341, 130004. [Google Scholar] [CrossRef]
- Zang, Y.; Cao, B.; Zhao, H.; Xie, B.; Ge, Y.; Yi, Y.; Liu, H. On-site determination of water toxicity based on freeze-dried electrochemically active bacteria. Sci. Total Environ. 2023, 867, 161432. [Google Scholar] [CrossRef]
- Liu, Z.; Ni, L.; Chi, J.; Qin, Y.; Shi, Z.; Wang, Y.; Wei, H.; Feng, L.; Sun, C. Preparation of fluorescent carbon dots by flow-assisted melt polymerization for tetracycline detection in medical wastewater. Chin. J. Chromatogr. 2024, 42, 1068–1076. [Google Scholar] [CrossRef]
- Dat, N.M.; Quan, T.H.; Nguyet, D.M.; Anh, T.N.M.; Thinh, D.B.; Diep, T.C.; Huy, L.A.; Tai, L.T.; Hai, N.D.; Khang, P.T.; et al. Hybrid graphene oxide-immobilized silver nanocomposite with optimal fabrication route and multifunctional application. Appl. Surf. Sci. 2021, 551, 149434. [Google Scholar] [CrossRef]
- Xu, J.; Qi, Q.; Sun, L.; Guo, X.; Zhang, H.; Zhao, X. Green fluorescent carbon dots from chitosan as selective and sensitive “off-on” probes for nitrite and “on-off-on” probes for enrofloxacin detection. J. Alloys Compd. 2022, 908, 164519. [Google Scholar] [CrossRef]
- Yin, W.; Gu, J.; Zhu, T.; Gao, H.; Ma, C.; Zhu, C.; Li, L.; Yang, Z.; Chen, G. Use of deep ultraviolet emissive carbon dots as a novel optical sensor for the detection of tetracyclines in milk. IEEE Sens. J. 2022, 22, 17777–17783. [Google Scholar] [CrossRef]
- Wu, M.; Liu, T.; Yin, C.; Jiang, X.; Sun, Q.; Gao, L.; Niu, N.; Chen, L.; Gang, H. Portable smartphone-assisted RGB-dependent ratiometric sensing platform for the detection of tetrachloro-p-benzoquinone in river samples. Microchem. J. 2023, 190, 108686. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, S.; Zhang, Y. Green fluorescent carbon dots for sensing of quercetin and pH and cell imaging. Luminescence 2023, 39, e4638. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.A.; Afonso, M.M.; Palenzuela, J.A.; Martín, I.R.; Soler-Carracedo, K. Carbon dots as temperature nanosensors in the physiological range. J. Lumin. 2018, 196, 313–315. [Google Scholar] [CrossRef]


| Analytes | Technique | Linear Range (mg/L) | Limit of Detection (mg/L) | Reference |
|---|---|---|---|---|
| Organic pollutants | BES a | 0.2–1 | 0.1 | [33] |
| Dichlorophenol | FEAB b | 0.5–2 | 0.5 | [34] |
| Tetracycline | FCDMP c | 4–20 | 0.2 | [35] |
| Methylene blue | HOSN d | 40–100 | 98.04 | [36] |
| Enrofloxacin | GCDC e | 1–50 | 0.04 | [37] |
| Tetracyclines | DUECD f | 0.5–40 | 0.07061 | [38] |
| Spores | WA-CDs@MIL-101 | 0.0025–5 | 0.00515 | This work |
| Sample | Method | Spiked (mg/L) | Detected (mg/L) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Panax notoginseng leaf | WA-CDs@MIL-101 | 0 | / | / | / |
| 0.50 | 0.47 | 94 | 2.5 | ||
| 2.00 | 1.98 | 99 | 1.3 | ||
| 4.00 | 4.08 | 102 | 3.4 | ||
| qPCR | 0 | / | / | / | |
| 0.50 | 0.48 | 96 | 3.3 | ||
| 2.00 | 1.93 | 97 | 2.2 | ||
| 4.00 | 3.88 | 97 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, C.; Sun, W.; Yang, L.; Yang, Q. Development of a Green-Synthesized WA-CDs@MIL-101 Fluorescent Sensor for Rapid Detection of Panax notoginseng Leaf Pathogen Spores. Plants 2025, 14, 2316. https://doi.org/10.3390/plants14152316
Cao C, Sun W, Yang L, Yang Q. Development of a Green-Synthesized WA-CDs@MIL-101 Fluorescent Sensor for Rapid Detection of Panax notoginseng Leaf Pathogen Spores. Plants. 2025; 14(15):2316. https://doi.org/10.3390/plants14152316
Chicago/Turabian StyleCao, Chunhao, Wei Sun, Ling Yang, and Qiliang Yang. 2025. "Development of a Green-Synthesized WA-CDs@MIL-101 Fluorescent Sensor for Rapid Detection of Panax notoginseng Leaf Pathogen Spores" Plants 14, no. 15: 2316. https://doi.org/10.3390/plants14152316
APA StyleCao, C., Sun, W., Yang, L., & Yang, Q. (2025). Development of a Green-Synthesized WA-CDs@MIL-101 Fluorescent Sensor for Rapid Detection of Panax notoginseng Leaf Pathogen Spores. Plants, 14(15), 2316. https://doi.org/10.3390/plants14152316







