Insights into Biological and Ecological Features of Four Rare and Endemic Plants from the Northern Tian Shan (Kazakhstan)
Abstract
1. Introduction
2. Results
2.1. Ecological, Anatomical, and Genomic Features of Taraxacum kok-saghyz
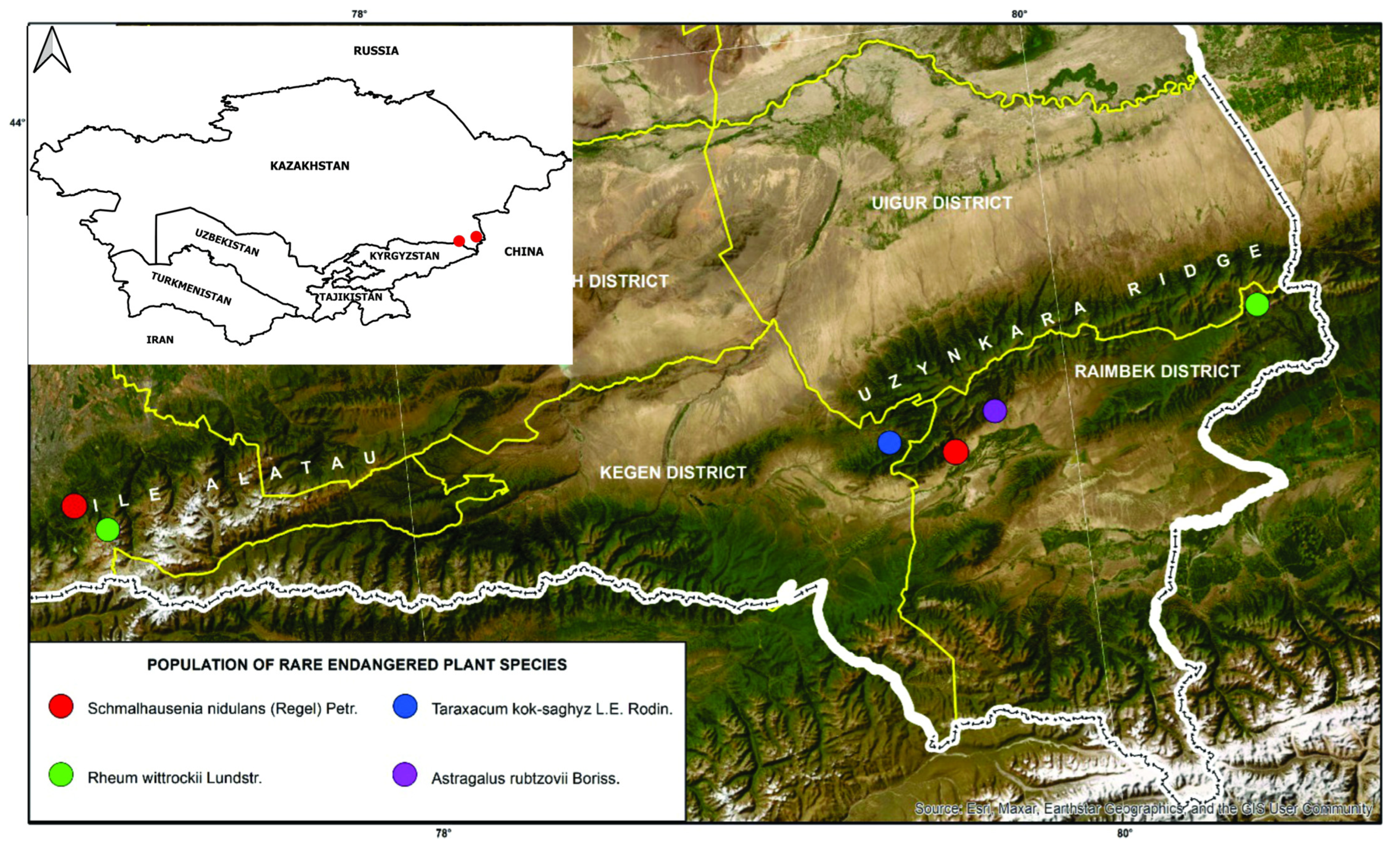
2.1.1. Habitat and Population Structure of T. kok-saghyz
2.1.2. Morphological and Anatomical Characteristics of T. kok-saghyz
2.1.3. Chloroplast Genome Analysis of T. kok-saghyz
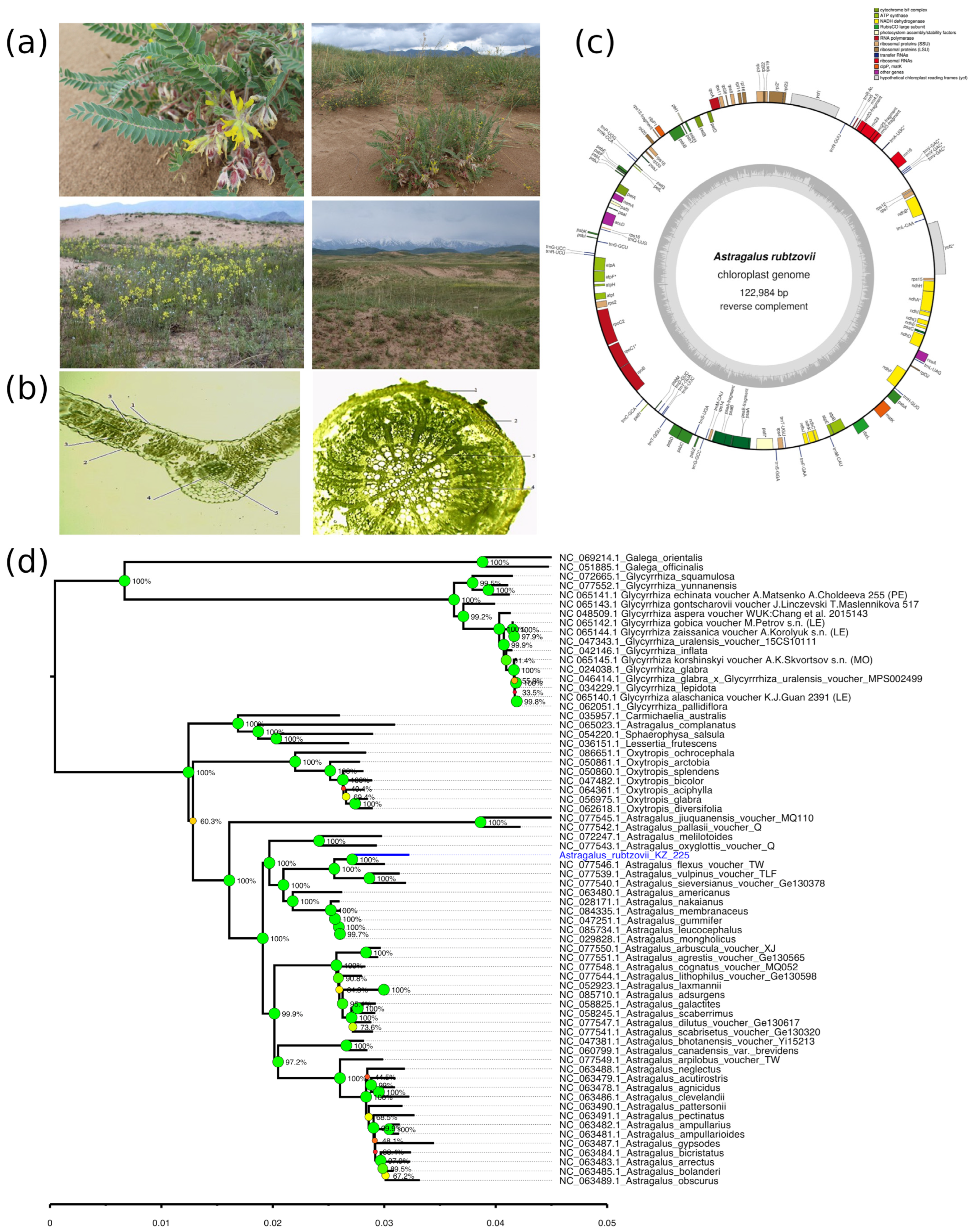
2.2. Ecological, Anatomical, and Genomic Features of Astragalus rubtzovii
2.2.1. Habitat and Population Structure of A. rubtzovii
2.2.2. Morphological and Anatomical Characteristics of A. rubtzovii
2.2.3. Chloroplast Genome Analysis of A. rubtzovii
2.3. Ecological, Anatomical, and Genomic Features of Schmalhausenia nidulans
2.3.1. Habitat and Population Structure of S. nidulans
2.3.2. Morphological and Anatomical Characteristics of S. nidulans
2.3.3. Chloroplast Genome Analysis of S. nidulans
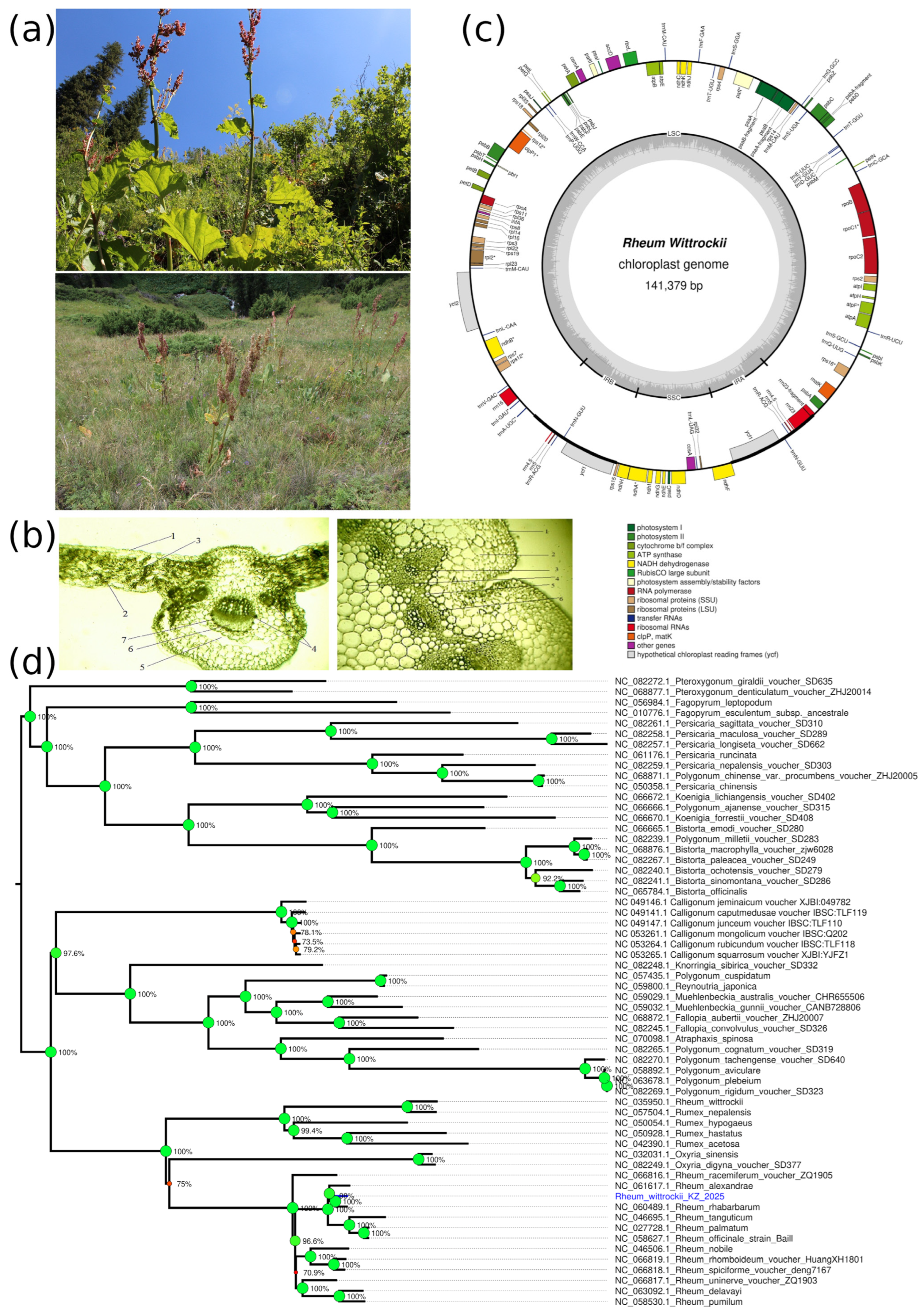
2.4. Ecological, Anatomical, and Genomic Features of Rheum wittrockii
2.4.1. Habitat and Population Structure of R. wittrockii
2.4.2. Morphological and Anatomical Characteristics of R. wittrockii
2.4.3. Chloroplast Genome Analysis of R. wittrockii
3. Discussion
3.1. T. kok-saghyz
3.2. A. rubtzovii
3.3. S. nidulans
3.4. R. wittrockii
4. Materials and Methods
4.1. Plant Material
4.2. Geobotanical Survey and Population Structure
4.3. Morphological and Anatomical Studies
4.4. DNA Extraction and Sequencing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kubentayev, S.A.; Alibekov, D.T.; Perezhogin, Y.V.; Lazkov, G.A.; Kupriyanov, A.N.; Ebel, A.L.; Izbastina, K.S.; Borodulina, O.V.; Kubentayeva, B.B. Revised Checklist of Endemic Vascular Plants of Kazakhstan. PhytoKeys 2024, 238, 241–279. [Google Scholar] [CrossRef] [PubMed]
- Baitulin, I. The Red Book of Kazakhstan (Plants); Art Print XXI Astana: Astana, Kazakhstan, 2014; Volume 2. [Google Scholar]
- Moore, J.W.; Schindler, D.E. Getting Ahead of Climate Change for Ecological Adaptation and Resilience. Science 2022, 376, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Sala, O.E.; Stuart Chapin, F.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.; Chan, K.M. Pervasive Human-Driven Decline of Life on Earth Points to the Need for Transformative Change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef] [PubMed]
- Jaureguiberry, P.; Titeux, N.; Wiemers, M.; Bowler, D.E.; Coscieme, L.; Golden, A.S.; Guerra, C.A.; Jacob, U.; Takahashi, Y.; Settele, J. The Direct Drivers of Recent Global Anthropogenic Biodiversity Loss. Sci. Adv. 2022, 8, eabm9982. [Google Scholar] [CrossRef] [PubMed]
- Navas Sastre, S.; Sánchez De Dios, R.; Domínguez Lozano, F. Contrasting Patterns for Endangered Flora Revealed by 60-Year Land-Use Change Analysis; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Bauer, H. Local Perceptions of Waza National Park, Northern Cameroon. Environ. Conserv. 2003, 30, 175–181. [Google Scholar] [CrossRef]
- Anthony, B. The Dual Nature of Parks: Attitudes of Neighbouring Communities towards Kruger National Park, South Africa. Environ. Conserv. 2007, 34, 236–245. [Google Scholar] [CrossRef]
- Bennett, N.J. Using Perceptions as Evidence to Improve Conservation and Environmental Management. Conserv. Biol. 2016, 30, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, T.D.; Radeloff, V.C.; Keuler, N.S. People’s Perceptions of Protected Areas Across Spatial Scales. Parks 2019, 25, 25–38. [Google Scholar] [CrossRef]
- Abukari, H.; Mwalyosi, R.B. Local Communities’ Perceptions about the Impact of Protected Areas on Livelihoods and Community Development. Glob. Ecol. Conserv. 2020, 22, e00909. [Google Scholar] [CrossRef]
- Mwasaga, R.U.; Mbise, F.P.; Nyahongo, J.W.; Røskaft, E. Awareness of Urban Communities on Biodiversity Conservation in Tanzania’s Protected Areas. Glob. Ecol. Conserv. 2022, 38, e02251. [Google Scholar] [CrossRef]
- Lemenkova, P. Rural Sustainability and Management of Natural Resources in Tian Shan Region, Central Asia. In Proceedings of the Int’l Conference “Celebrating Pastoral Life. Heritage and Economic Development”; Papageorgiou, F., Ed.; CANEPAL: Athens, Greece, 2014; pp. 81–89. [Google Scholar]
- Uteulin, K.; Bari, G.; Zheksenbai, A. Dandelion Kok-Saghyz (Taraxacum kok-saghyz L. Rodin) as a One-Year Culture Developed Under Conditions of South East Kazakhstan. Bull. Natl. Acad. Sci. Repub. Kazakhstan 2020, 3, 20–28. [Google Scholar]
- Sadyrova, G.; Shimshikov, B.; Tynybekov, B.; Nurmakhanova, A.; Orazbekova, K.; Tastybay, M.; Imanaliyeva, M.; Bekbossyn, N. Ecological and Biological Features of Some Rare and Endemic Plant Species of South-East Kazakhstan. Bull. LN Gumilyov Eurasian Natl. Univ. Chem. Geogr. Ecol. Ser. 2024, 149, 166–184. [Google Scholar] [CrossRef]
- Syedina, I.A.; Otradnykh, I.G.; Ualieva, B.B. New Habitats and Population Status of the Rare Species Schmalhausenia nidulans in the Kungey and Terskey Alatau Mountains (Northern Tien Shan) (Novye mesta obitaniya i sostoyanie populyatsiy redkogo vida Schmalhausenia nidulans v gorakh Kungey i Terskey Alatau (Severnyy Tyan‘-Shan’)). Probl. Bot. South. Sib. Mong. 2024, 23, 436–439. [Google Scholar] [CrossRef]
- Sitpayeva, G. Conservation of Biodiversity of Wild Plant of Rheum Wittrockii Lundstr of Kazakhstan. Biosci. Biotechnol. Res. Asia 2017, 14, 93–98. [Google Scholar] [CrossRef]
- Sadyrova, G.; Tynybekov, B.; Nazarbekova, S.; Orazbekova, K.; Ibragimov, T.; Shimshikov, B.; Mamytova, N.; Bekbossyn, N.; Tastybay, M.; Mussina, A. Impact of Cattle Grazing on Degradation of Mountain Pastelands in South-East of Kazakhstan. ES Energy Environ. 2025, 27, 1430. [Google Scholar] [CrossRef]
- Sarasan, V.; Cripps, R.; Ramsay, M.M.; Atherton, C.; McMICHEN, M.; Prendergast, G.; Rowntree, J.K. Conservation in Vitro of Threatened Plants—Progress in the Past Decade. Vitr. Cell. Dev. Biol. Plant 2006, 42, 206–214. [Google Scholar] [CrossRef]
- Georghiou, K.; Delipetrou, P. Patterns and Traits of the Endemic Plants of Greece. Bot. J. Linn. Soc. 2010, 162, 130–153. [Google Scholar] [CrossRef]
- Türe, C.; Böcük, H. Distribution Patterns of Threatened Endemic Plants in Turkey: A Quantitative Approach for Conservation. J. Nat. Conserv. 2010, 18, 296–303. [Google Scholar] [CrossRef]
- El-Keblawy, A. Impact of Climate Change on Biodiversity Loss and Extinction of Endemic Plants of Arid Land Mountains. J. Biodivers. Endanger. Species 2014, 2, 2. [Google Scholar] [CrossRef]
- Kidane, Y.O.; Steinbauer, M.J.; Beierkuhnlein, C. Dead End for Endemic Plant Species? A Biodiversity Hotspot under Pressure. Glob. Ecol. Conserv. 2019, 19, e00670. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic Plant Species Conservation: Biotechnological Approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Liu, J.; Yu, J.; Wang, L.; Zhou, S. Highly Variable Chloroplast Markers for Evaluating Plant Phylogeny at Low Taxonomic Levels and for DNA Barcoding. PLoS ONE 2012, 7, e35071. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zheng, Y. Dynamic Evolution and Phylogenomic Analysis of the Chloroplast Genome in Schisandraceae. Sci. Rep. 2018, 8, 9285. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Parajuli, S.; Nepal, M.P. Reporting Complete Chloroplast Genome of Endangered Red Mulberry, Useful for Understanding Hybridization and Phylogenetic Relationships. Sci. Rep. 2025, 15, 13403. [Google Scholar] [CrossRef] [PubMed]
- Akhmetova, A.; Mukhitdinov, N.; Ydyrys, A.; Ametov, A.; Inelova, Z.; Öztürk, M. Studies on the Root Anatomy of Rubber Producing Endemic of Kazakhstan, Taraxacum kok-saghyz LE Rodin. JAPS J. Anim. Plant Sci. 2018, 28, 1400. [Google Scholar]
- Uteulin, K.; Suleimenov, B.; Pachikin, K. The Soils of Natural (In Situ) Coenopopulations of Taraxacum kok-saghyz LE Rodin in Kazakhstan. Agronomy 2023, 13, 2737. [Google Scholar] [CrossRef]
- Ivashchenko, A.A.; Mukhitdinov, N.; Abidkulova, K.T.; Ametov, A.; Tashev, A.; Ydyrys, A. Floristic Analysis of Plant Communities with the Participation of a Narrow Tien Shan Endemic, Taraxacum kok-saghyz L.E Rodin. For. Ideas 2021, 27, 195–209. [Google Scholar]
- Seilkhan, A. An Overview of Green Applications of Natural Products for Pharmaceutical, Biofuel, and Rubber Industries: Case Study of Kazakh Dandelion (Taraxacum kok-saghyz Rodin.). ES Energy Environ. 2024, 25, 1171. [Google Scholar] [CrossRef]
- Stewart-Wade, S.; Neumann, S.; Collins, L.; Boland, G. The Biology of Canadian Weeds. 117. Taraxacum officinale GH Weber Ex Wiggers. Can. J. Plant Sci. 2002, 82, 825–853. [Google Scholar] [CrossRef]
- Molina-Montenegro, M.A.; Penuelas, J.; Munné-Bosch, S.; Sardans, J. Higher Plasticity in Ecophysiological Traits Enhances the Performance and Invasion Success of Taraxacum officinale (Dandelion) in Alpine Environments. Biol. Invasions 2012, 14, 21–33. [Google Scholar] [CrossRef]
- Pyurko, O.; Khrystova, T.; Pyurko, V.; Tipenko, L.A. Endo-Adaptive Component Taraxacum officinale L. as a Determinant of the Adaptation Syndrome of Plant Organisms in the Background of Ecosystem Stability. Rev. Univ. Zulia 2024, 15, 491–503. [Google Scholar] [CrossRef]
- Zhang, Y.; Iaffaldano, B.J.; Zhuang, X.; Cardina, J.; Cornish, K. Chloroplast Genome Resources and Molecular Markers Differentiate Rubber Dandelion Species from Weedy Relatives. BMC Plant Biol. 2017, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, J.; Štěpánek, J.; Černý, T.; De Heer, P.; van Dijk, P.J. Available Ex Situ Germplasm of the Potential Rubber Crop Taraxacum koksaghyz Belongs to a Poor Rubber Producer, T. brevicorniculatum(Compositae–Crepidinae). Genet. Resour. Crop Evol. 2013, 60, 455–471. [Google Scholar] [CrossRef]
- Verma, D.; Samson, N.P.; Koya, V.; Daniell, H. A Protocol for Expression of Foreign Genes in Chloroplasts. Nat. Protoc. 2008, 3, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Wittzell, H. Chloroplast DNA Variation and Reticulate Evolution in Sexual and Apomictic Sections of Dandelions. Mol. Ecol. 1999, 8, 2023–2035. [Google Scholar] [CrossRef] [PubMed]
- Townsend, C.E. Breeding, Physiology, Culture, and Utilization of Cicer Milkvetch (Astragalus cicer L.). In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: New York, NY, USA, 1993; Volume 49, pp. 253–308. ISBN 0065-2113. [Google Scholar]
- Acharya, S.; Kastelic, J.; Beauchemin, K.; Messenger, D. A Review of Research Progress on Cicer Milkvetch (Astragalus cicer L.). Can. J. Plant Sci. 2006, 86, 49–62. [Google Scholar] [CrossRef]
- Yang, M.; Li, Z.; Liu, L.; Bo, A.; Zhang, C.; Li, M. Ecological Niche Modeling of Astragalus membranaceus Var. Mongholicus Medicinal Plants in Inner Mongolia, China. Sci. Rep. 2020, 10, 12482. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, M.F.; Lavin, M.; Sanderson, M.J. A Phylogeny of Legumes (Leguminosae) Based on Analysis of the Plastid matK Gene Resolves Many Well-supported Subclades within the Family. Am. J. Bot. 2004, 91, 1846–1862. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.; Ohta, A.; Shimizu, M.; Terauchi, R.; Kazempour-Osaloo, S. The Complete Chloroplast Genome of Onobrychis gaubae (Fabaceae-Papilionoideae): Comparative Analysis with Related IR-Lacking Clade Species. BMC Plant Biol. 2022, 22, 75. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Thompson, W.F. Chloroplast DNA Rearrangements Are More Frequent When a Large Inverted Repeat Sequence Is Lost. Cell 1982, 29, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Charboneau, J.L.; Cronn, R.C.; Liston, A.; Wojciechowski, M.F.; Sanderson, M.J. Plastome Structural Evolution and Homoplastic Inversions in Neo-Astragalus (Fabaceae). Genome Biol. Evol. 2021, 13, evab215. [Google Scholar] [CrossRef] [PubMed]
- Rakhimova, N.; Duschanova, G.; Abdinazarov, S.; Temirov, E.; Nosirov, S.; Samadov, I. Adaptive Features of the Leaf of Juno vicaria (Vved.) T. Hall & Seisums in Different Ecological Conditions of Uzbekistan. Am. J. Plant Sci. 2019, 10, 947–957. [Google Scholar] [CrossRef]
- Abidkulova, D.M.; Ivashchenko, A.A.; Sramko, G.; Kurbatova, N.V.; Abidkulova, K.T. Gymnospermium altaicum (PALL.) Spach (BERBERIDACEAE), an Early Spring Element of Wild Fruit Forests of the Trans-Ili Alatau. Exp. Biol. 2021, 86, 14–26. [Google Scholar] [CrossRef]
- Almabek, D.; Abidkulova, K.; Kurbatova, N.; Mukhitdinov, N.; Turalin, B. Comparative Anatomical and Morphological Study of Vegetative Organs of Gymnospermium altaicum (Pall.) Spach from Natural Populations. BIO Web Conf. 2024, 100, 04001. [Google Scholar] [CrossRef]
- Shahzadi, I.; Mehmood, F.; Ali, Z.; Ahmed, I.; Mirza, B. Chloroplast Genome Sequences of Artemisia maritima and Artemisia absinthium: Comparative Analyses, Mutational Hotspots in Genus Artemisia and Phylogeny in Family Asteraceae. Genomics 2020, 112, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Benito Alonso, J.L.; Coldea, G.; Dick, J.; Erschbamer, B.; Fernández Calzado, M.R.; et al. Continent-Wide Response of Mountain Vegetation to Climate Change. Nat. Clim. Change 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Akhalkatsi, M.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, R.F. Recent Plant Diversity Changes on Europe’s Mountain Summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Brummitt, N.A.; Bachman, S.P.; Griffiths-Lee, J.; Lutz, M.; Moat, J.F.; Farjon, A.; Donaldson, J.S.; Hilton-Taylor, C.; Meagher, T.R.; Albuquerque, S. Green Plants in the Red: A Baseline Global Assessment for the IUCN Sampled Red List Index for Plants. PLoS ONE 2015, 10, e0135152. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-T.; Yi, T.-S.; Gao, L.-M.; Ma, P.-F.; Zhang, T.; Yang, J.-B.; Gitzendanner, M.A.; Fritsch, P.W.; Cai, J.; Luo, Y. Origin of Angiosperms and the Puzzle of the Jurassic Gap. Nat. Plants 2019, 5, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Kobylina, T.; Mukhitdinov, N.; Abidkulova, K.; Kurbatova, N.; Kudrina, N.; Alimkulova, M.; Zaltauskaite, J. Anatomic-Morphological and Phytochemical Study of a Rare Species-Rheum Wittrockii Lundstr. Int. J. Biol. Chem. 2020, 13, 69–79. [Google Scholar] [CrossRef]
- Mohtashami, L.; Amiri, M.S.; Ayati, Z.; Ramezani, M.; Jamialahmadi, T.; Emami, S.A.; Sahebkar, A. Ethnobotanical Uses, Phytochemistry and Pharmacology of Different Rheum Species (Polygonaceae): A Review. In Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health; Barreto, G.E., Sahebkar, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 309–352. ISBN 978-3-030-64872-5. [Google Scholar]
- Dagarova, S.S.; Sitpayeva, G.T.; Pak, J.-H.; Kim, J.S. The Complete Plastid Genome Sequence of Rheum wittrockii (Polygonaceae), Endangered Species of Kazakhstan. Mitochondrial DNA Part B 2017, 2, 516–517. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, R.; Cultrera, N.G.; Díez, C.M.; Baldoni, L.; Rubini, A. Identification of New Polymorphic Regions and Differentiation of Cultivated Olives (Olea europaea L.) Through Plastome Sequence Comparison. BMC Plant Biol. 2010, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Kersten, B.; Faivre Rampant, P.; Mader, M.; Le Paslier, M.-C.; Bounon, R.; Berard, A.; Vettori, C.; Schroeder, H.; Leplé, J.-C.; Fladung, M. Genome Sequences of Populus Tremula Chloroplast and Mitochondrion: Implications for Holistic Poplar Breeding. PLoS ONE 2016, 11, e0147209. [Google Scholar] [CrossRef] [PubMed]
- Kokoreva, I.; Otradnykh, I.; Sedina, I.; Lysenko, V. Rare Plant Species of the Northern Tien Shan (Population, Morphogenesis, Resumption); Monography: Toronto, ON, Canada, 2013. [Google Scholar]
- Hoban, S.; Bruford, M.; Jackson, J.D.; Lopes-Fernandes, M.; Heuertz, M.; Hohenlohe, P.A.; Paz-Vinas, I.; Sjögren-Gulve, P.; Segelbacher, G.; Vernesi, C. Genetic Diversity Targets and Indicators in the CBD Post-2020 Global Biodiversity Framework Must Be Improved. Biol. Conserv. 2020, 248, 108654. [Google Scholar] [CrossRef]
- Flora of Kazakhstan; Academy of Sciences of the Kazakh SSR: Almaty, Kazakhstan, 1956; Volume 1–9.
- Illustrated Identifier of Plants of Kazakhstan; Academy of Sciences of the Kazakh SSR: Almaty, Kazakhstan, 1962; Volume 1–2.
- Opredelitel’ Rastenii Srednei Azii (Key to Identification of the Plants of Central Asia); Fan: Tashkent, Uzbekistan, 1968; Volume 1–10.
- Abdulina, S.A. Spisok Sosudistyh Rastenij Kazahstana (The List of Vascular Plants of Kazakhstan9/); Kamelina, R.V., Ed.; Academy of Sciences of the Republic of Kazakhstan: Almaty, Kazakhstan, 1998; p. 187. [Google Scholar]
- Cherepanov, S.K. Vascular Plants of Russia and Neighboring States (Within the Former USSR); Mir i Semya: St. Petersburg, Russia, 1995. [Google Scholar]
- Rabotnov, T.A. Life Cycle of Perennial Herbaceous Plants in Meadow Cenoses; Proceedings of the Komarov Botanical Institute; Academy of Sciences of the USSR: Almaty, Kazakhstan, 1950; Volume 3, pp. 7–204. [Google Scholar]
- Uranov, A.A.; Smirnova, O.V. Classification and Main Features of Development of Populations of Perennial Plants. Bull. Mosc. Soc. Nat. Dep. Biol. 1969, 74, 119–134. [Google Scholar]
- Rabotnov, T. On Coenopopulations of Perennial Herbaceous Plants in Natural Coenoses. Vegetatio 1969, 19, 87–95. [Google Scholar] [CrossRef]
- Gatsuk, L.; Smirnova, O.; Vorontzova, L.; Zaugolnova, L.; Zhukova, L. Age States of Plants of Various Growth Forms: A Review. J. Ecol. 1980, 68, 675–696. [Google Scholar] [CrossRef]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Being the Collected Papers of C. Raunkiær; The Clarendon Press: Oxford, UK, 1934. [Google Scholar]
- Serebryakov, I.G. Ecological Morphology of Plants; Vyssh. Shk.: Moscow, Russia, 1962. [Google Scholar]
- Prozina, M.N. Botanical Microtechnics; Moscow State University: Moscow, Russia, 1960. [Google Scholar]
- Barykina, R.; Veselova, T.; Devyatov, A. Spravochnik Po Botanicheskoy Mikrotekhnike. In Handbook of Botanical Microtechnology (Fundamentals and Methods); Moscow State University: Moscow, Russia, 2024; p. 313. [Google Scholar]
- Permyakov, A.I. Microtechnics; Moscow State University: Moscow, Russia, 1988; pp. 11–29. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-J.; Yu, W.-B.; Yang, J.-B.; Song, Y.; DePamphilis, C.W.; Yi, T.-S.; Li, D.-Z. GetOrganelle: A Fast and Versatile Toolkit for Accurate de Novo Assembly of Organelle Genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq–Versatile and Accurate Annotation of Organelle Genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 1-4939-9172-8. [Google Scholar]
- Boratyn, G.M.; Camacho, C.; Cooper, P.S.; Coulouris, G.; Fong, A.; Ma, N.; Madden, T.L.; Matten, W.T.; McGinnis, S.D.; Merezhuk, Y. BLAST: A More Efficient Report with Usability Improvements. Nucleic Acids Res. 2013, 41, W29–W33. [Google Scholar] [CrossRef] [PubMed]
- Bi, G.; Luan, X.; Yan, J. ORPA: A Fast and Efficient Phylogenetic Analysis Method for Constructing Genome-Wide Alignments of Organelle Genomes. J. Genet. Genom. 2024, 51, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- GitHub—Rambaut/Figtree: Automatically Exported from Code.Google.Com/p/Figtree. Available online: https://github.com/rambaut/figtree/ (accessed on 10 July 2025).


| Population | Age Group | Number of Individuals | Leaf Length (cm) ± SD | Plant Height (cm) ± SD |
|---|---|---|---|---|
| Pop. 1 | Juvenile (j) | 45 | 1.13 ± 0.32 | 1.37 ± 0.42 |
| Immature (i) | 41 | 1.80 ± 0.26 | 1.80 ± 0.14 | |
| Virginile (v) | 64 | 3.47 ± 0.45 | 2.67 ± 0.25 | |
| Generative (g) | 30 | 8.33 ± 0.58 | 11.00 ± 1.00 | |
| Pop. 2 | Juvenile (j) | 30 | 1.93 ± 0.21 | 0.67 ± 0.15 |
| Immature (i) | 39 | 2.60 ± 0.17 | 1.85 ± 0.07 | |
| Virginile (v) | 52 | 4.30 ± 0.53 | 3.20 ± 0.36 | |
| Generative (g) | 61 | 7.00 ± 3.61 | 12.00 ± 2.00 |
| Population | Age Group | Number of Individuals | Leaf Length (cm) ± SD | Plant Height (cm) ± SD |
|---|---|---|---|---|
| Population 1 | Juvenile (j) | 30 | 1.60 ± 0.66 | 9.00 ± 10.00 |
| Immature (i) | 610 | 1.83 ± 0.15 | 13.33 ± 15.28 | |
| Virginile (v) | 949 | 1.97 ± 0.06 | 25.00 ± 30.00 | |
| Generative (g) | 730 | 2.10 ± 0.36 | 42.33 ± 68.07 |
| Population | Age Group | Number of Individuals | Leaf Length (cm) ± SD | Plant Height (cm) ± SD |
|---|---|---|---|---|
| Pop. 1 | Juvenile (j) | 65 | 6.67 ± 2.08 | 8.00 ± 1.00 |
| Immature (i) | 31 | 10.33 ± 1.53 | 20.00 ± 5.00 | |
| Virginile (v) | 154 | 18.33 ± 1.53 | 23.67 ± 6.03 | |
| Generative (g) | 189 | 31.33 ± 3.21 | 29.67 ± 2.52 | |
| Pop. 2 | Juvenile (j) | 20 | 5.67 ± 1.53 | 11.33 ± 1.53 |
| Immature (i) | 29 | 10.67 ± 2.08 | 12.33 ± 1.53 | |
| Virginile (v) | 33 | 16.17 ± 1.04 | 22.33 ± 2.52 | |
| Generative (g) | 41 | 26.00 ± 3.61 | 30.67 ± 4.04 |
| Population | Age Group | Number of Individuals | Leaf Length (cm) ± SD | Plant Height (cm) ± SD |
|---|---|---|---|---|
| Pop. 1 | Juvenile (j) | 35 | 8.83 ± 0.76 | 15.67 ± 1.15 |
| Immature (i) | 47 | 14.17 ± 1.20 | 30.00 ± 5.00 | |
| Virginile (v) | 74 | 17.83 ± 1.26 | 47.33 ± 7.51 | |
| Generative (g) | 29 | 31.17 ± 3.40 | 83.33 ± 7.64 | |
| Pop. 2 | Juvenile (j) | 29 | 6.67 ± 1.53 | 17.33 ± 2.52 |
| Immature (i) | 33 | 11.33 ± 1.53 | 23.33 ± 1.53 | |
| Virginile (v) | 63 | 19.33 ± 2.08 | 40.67 ± 9.29 | |
| Generative (g) | 38 | 30.67 ± 4.04 | 78.33 ± 7.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadyrova, G.; Taskuzhina, A.; Pozharskiy, A.; Orazbekova, K.; Yanin, K.; Kerimbek, N.; Zhamilova, S.; Kamiyeva, G.; Tanybaeva, A.; Gritsenko, D. Insights into Biological and Ecological Features of Four Rare and Endemic Plants from the Northern Tian Shan (Kazakhstan). Plants 2025, 14, 2305. https://doi.org/10.3390/plants14152305
Sadyrova G, Taskuzhina A, Pozharskiy A, Orazbekova K, Yanin K, Kerimbek N, Zhamilova S, Kamiyeva G, Tanybaeva A, Gritsenko D. Insights into Biological and Ecological Features of Four Rare and Endemic Plants from the Northern Tian Shan (Kazakhstan). Plants. 2025; 14(15):2305. https://doi.org/10.3390/plants14152305
Chicago/Turabian StyleSadyrova, Gulbanu, Aisha Taskuzhina, Alexandr Pozharskiy, Kuralai Orazbekova, Kirill Yanin, Nazym Kerimbek, Saule Zhamilova, Gulzhanat Kamiyeva, Ainur Tanybaeva, and Dilyara Gritsenko. 2025. "Insights into Biological and Ecological Features of Four Rare and Endemic Plants from the Northern Tian Shan (Kazakhstan)" Plants 14, no. 15: 2305. https://doi.org/10.3390/plants14152305
APA StyleSadyrova, G., Taskuzhina, A., Pozharskiy, A., Orazbekova, K., Yanin, K., Kerimbek, N., Zhamilova, S., Kamiyeva, G., Tanybaeva, A., & Gritsenko, D. (2025). Insights into Biological and Ecological Features of Four Rare and Endemic Plants from the Northern Tian Shan (Kazakhstan). Plants, 14(15), 2305. https://doi.org/10.3390/plants14152305






