Identification and Functional Characterization of a Geraniol Synthase UrGES from Uncaria rhynchophylla
Abstract
1. Introduction
2. Results
2.1. Physicochemical Analysis of UrGES Protein
2.2. Identification and Phylogenetic Analysis of UrGES
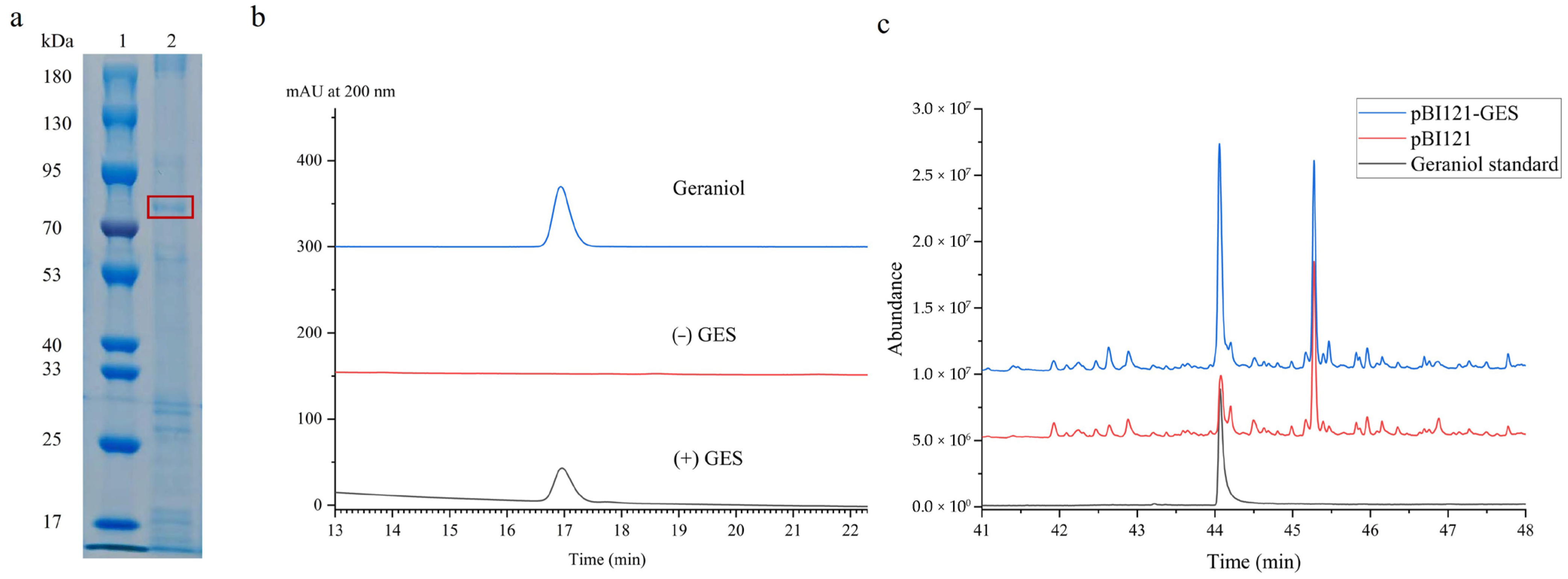
2.3. Heterologous Overexpression and Functional Characterization of Recombinant UrGES Protein
2.4. Functional Analysis of UrGES In Vivo
2.5. Tissue Expression Pattern of UrGES
2.6. MeJA Promotes TIA Accumulation and UrGES Upregulation
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Total RNA Extraction and cDNA Synthesis
4.3. Molecular Cloning and Plasmid Construction for UrGES
4.4. Bioinformatics Analysis of UrGES Protein
4.5. Heterologous Overexpression and Purification of UrGES
4.6. Catalytic Activity Assay of UrGES Protein
4.7. Transient Heterologous Expression Assay of UrGES in N. benthamiana Leaves
4.8. Expression Pattern of UrGES Gene
4.9. Analysis of U. rhynchophylla Alkaloid Content After MeJA Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ndagijimana, A.; Wang, X.; Pan, G.; Zhang, F.; Feng, H.; Olaleye, O. A review on indole alkaloids isolated from Uncaria rhynchophylla and their pharmacological studies. Fitoterapia 2013, 86, 35–47. [Google Scholar] [CrossRef]
- Guo, Q.; Ma, X.; Wei, S.; Qiu, D.; Wilson, I.W.; Wu, P.; Tang, Q.; Liu, L.; Dong, S.; Zu, W. De novo transcriptome sequencing and digital gene expression analysis predict biosynthetic pathway of rhynchophylline and isorhynchophylline from Uncaria rhynchophylla, a non-model plant with potent anti-alzheimer’s properties. BMC Genom. 2014, 15, 676. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Wang, X.-M.; Ye, C.-Y.; Su, H.-F.; Tian, Q. The Main Alkaloids in Uncaria rhynchophylla and Their Anti-Alzheimer’s Disease Mechanism Determined by a Network Pharmacology Approach. Int. J. Mol. Sci. 2021, 22, 3612. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Chen, F.; Sun, J.; Simpkins, J.; Yuan, D. Isolation and Identification of Twelve Metabolites of Isocorynoxeine in Rat Urine and their Neuroprotective Activities in HT22 Cell Assay. Planta Medica 2014, 81, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-R.; Kim, M.J.; Lee, J.A.; Roh, S.-S.; Fan, Y.-C. Effect of Uncaria rhynchophylla against Thioacetamide-Induced Acute Liver Injury in Rat. Can. J. Gastroenterol. Hepatol. 2021, 2021, 5581816. [Google Scholar] [CrossRef]
- Kang, M.; Fu, R.; Zhang, P.; Lou, S.; Yang, X.; Chen, Y.; Ma, T.; Zhang, Y.; Xi, Z.; Liu, J. A chromosome-level Camptotheca acuminata genome assembly provides insights into the evolutionary origin of camptothecin biosynthesis. Nat. Commun. 2021, 12, 3531. [Google Scholar] [CrossRef]
- Hao, X.; Wang, C.; Zhou, W.; Ruan, Q.; Xie, C.; Yang, Y.; Xiao, C.; Cai, Y.; Wang, J.; Wang, Y.; et al. OpNAC1 transcription factor regulates the biosynthesis of the anticancer drug camptothecin by targeting loganic acid O-methyltransferase in Ophiorrhiza pumila. J. Integr. Plant Biol. 2023, 65, 133–149. [Google Scholar] [CrossRef]
- Yan, L.; Wang, X.; Liu, H.; Tian, Y.; Lian, J.; Yang, R.; Hao, S.; Wang, X.; Yang, S.; Li, Q.; et al. The Genome of Dendrobium officinale Illuminates the Biology of the Important Traditional Chinese Orchid Herb. Mol. Plant 2015, 8, 922–934. [Google Scholar] [CrossRef]
- Guo, E.; Yuan, M.; Xu, L.; Ren, Q.; Wang, Z.; Li, Z.; Wu, Z.; Liu, W.; Zhao, Y.; Feng, F.; et al. Identification of three key enzymes involved in the biosynthesis of tetracyclic oxindole alkaloids in Uncaria rhynchophylla. Bioorganic Chem. 2023, 136, 106545. [Google Scholar] [CrossRef]
- Jiang, C.-x.; Yu, J.-x.; Fei, X.; Pan, X.-j.; Zhu, N.-n.; Lin, C.-l.; Zhou, D.; Zhu, H.-r.; Qi, Y.; Wu, Z.-g. Gene coexpression networks allow the discovery of two strictosidine synthases underlying monoterpene indole alkaloid biosynthesis in Uncaria rhynchophylla. Int. J. Biol. Macromol. 2023, 226, 1360–1373. [Google Scholar] [CrossRef]
- Li, X.; Han, H.-q.; Wei, Y.-l.; Hu, T.; Qiang, W.; Wang, X.-h.; Zhang, M.-s. Phytochrome interacting factor 3 mediates low light signaling to regulate isorhynchophylline biosynthesis in Uncaria rhynchophylla. Sci. Rep. 2024, 14, 25032. [Google Scholar] [CrossRef]
- Shao, Y.; Mu, D.; Pan, L.; Lu, Z.; Zhou, Y.; Zhao, H.; Wilson, I.W.; Lu, Y.; Zhu, L.; Zhang, Y.; et al. Genome-wide identification of Uncaria rhynchophylla bHLH transcription factors and in-vitro validation of UrbHLH1 through interaction with terpenoid indole alkaloid synthesis pathway members. Curr. Plant Biol. 2024, 38, 100330. [Google Scholar] [CrossRef]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Miettinen, K.; Claudel, P.; Burlat, V.; Guirimand, G.; Courdavault, V.; Papon, N.; Meyer, S.; Godet, S.; St-Pierre, B.; et al. Characterization of the plastidial geraniol synthase from Madagascar periwinkle which initiates the monoterpenoid branch of the alkaloid pathway in internal phloem associated parenchyma. Phytochemistry 2013, 85, 36–43. [Google Scholar] [CrossRef]
- Sun, P.; Schuurink, R.C.; Caissard, J.-C.; Hugueney, P.; Baudino, S. My Way: Noncanonical Biosynthesis Pathways for Plant Volatiles. Trends Plant Sci. 2016, 21, 884–894. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Jiang, H.; Xu, S.; Xu, Y.; Liu, Z.; Luo, Y. Functional characterization of Camptotheca acuminata 7-deoxyloganetic acid synthases and 7-deoxyloganetic acid glucosyltransferases involved in camptothecin biosynthesis. Plant Physiol. Biochem. 2025, 218, 109305. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, Y.; Yang, L.; Mu, D.; Wilson, I.W.; Zhang, Y.; Zhu, L.; Liu, X.; Luo, L.; He, J.; et al. Genome-wide identification of GATA transcription factor family and the effect of different light quality on the accumulation of terpenoid indole alkaloids in Uncaria rhynchophylla. Plant Mol. Biol. 2024, 114, 15. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.; Yu, F.; Li, X.; Jin, J.; Zhou, Y.; Wang, Q.; Jing, T.; Wan, X.; Schwab, W.; et al. A geraniol synthase regulates plant defense via alternative splicing in tea plants. Hortic. Res. 2023, 10, uhad184. [Google Scholar] [CrossRef]
- Jin, J.; Kim, M.J.; Dhandapani, S.; Tjhang, J.G.; Yin, J.-L.; Wong, L.; Sarojam, R.; Chua, N.-H.; Jang, I.-C. The floral transcriptome of ylang ylang (Cananga odorata var. fruticosa) uncovers biosynthetic pathways for volatile organic compounds and a multifunctional and novel sesquiterpene synthase. J. Exp. Bot. 2015, 66, 3959–3975. [Google Scholar] [CrossRef]
- Chen, F.; Li, W.; Jiang, L.; Pu, X.; Yang, Y.; Zhang, G.; Luo, Y. Functional characterization of a geraniol synthase-encoding gene from Camptotheca acuminata and its application in production of geraniol in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2016, 43, 1281–1292. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Shen, S.; Yin, X.; Klee, H.; Zhang, B.; Chen, K. Transcription factor CitERF71 activates the terpene synthase gene CitTPS16 involved in the synthesis of E-geraniol in sweet orange fruit. J. Exp. Bot. 2017, 68, 4929–4938. [Google Scholar] [CrossRef]
- Hou, J.; Wu, Y.; Lei, L.; Wang, Y.; Ling, Q.; Zhang, J.; Zhao, J.; Jin, Z.; Zhang, H. Identification and functional analysis of a deduced geraniol synthase from Camphora officinarum. Physiol. Mol. Biol. Plants 2024, 30, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Magnard, J.-L.; Roccia, A.; Caissard, J.-C.; Vergne, P.; Sun, P.; Hecquet, R.; Dubois, A.; Hibrand-Saint Oyant, L.; Jullien, F.; Nicolè, F.; et al. Biosynthesis of monoterpene scent compounds in roses. Science 2015, 349, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Wan, L.; Shao, Y.; Pan, L.; Liu, X.; Wilson, I.W.; Qing, Z.; Zhou, Y.; Lu, Y.; He, Y.; et al. The Genome of Medicinal Plant Uncaria rhynchophylla Provides New Insights into Monoterpenoid Indole Alkaloids Metabolism and its molecular regulatory mechanism. Mol. Hortic. 2025; in press. [Google Scholar]
- Jiang, S.-Y.; Jin, J.; Sarojam, R.; Ramachandran, S.; Alba, M. A Comprehensive Survey on the Terpene Synthase Gene Family Provides New Insight into Its Evolutionary Patterns. Genome Biol. Evol. 2019, 11, 2078–2098. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Horbowicz, M.; Wiczkowski, W.; Góraj-Koniarska, J.; Miyamoto, K.; Ueda, J.; Saniewski, M. Effect of Methyl Jasmonate on the Terpene Trilactones, Flavonoids, and Phenolic Acids in Ginkgo biloba L. Leaves: Relevance to Leaf Senescence. Molecules 2021, 26, 4682. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, S.; Wang, H.; Chen, Z.; Sun, M.; Sun, L.; Gong, L.; Li, Y.; Jiang, H. Intervention of Uncaria and Its Components on Liver Lipid Metabolism in Spontaneously Hypertensive Rats. Front. Pharmacol. 2020, 11, 910. [Google Scholar] [CrossRef]
- Yang, W.; Ip, S.-P.; Liu, L.; Xian, Y.-F.; Lin, Z.-X. Uncaria rhynchophylla and its Major Constituents on Central Nervous System: A Review on Their Pharmacological Actions. Curr. Vasc. Pharmacol. 2020, 18, 346–357. [Google Scholar] [CrossRef]
- Xie, L.; Wang, T.; Lin, S.; Lu, Z.; Wang, Y.; Shen, Z.; Cheng, Y.; Shen, A.; Peng, J.; Chu, J. Uncaria Rhynchophylla attenuates angiotensin II-induced myocardial fibrosis via suppression of the RhoA/ROCK1 pathway. Biomed. Pharmacother. 2022, 146, 112607. [Google Scholar] [CrossRef]
- Salim, V.; Jones, A.D.; DellaPenna, D. Camptotheca acuminata 10-hydroxycamptothecin O-methyltransferase: An alkaloid biosynthetic enzyme co-opted from flavonoid metabolism. Plant J. 2018, 95, 112–125. [Google Scholar] [CrossRef]
- Eng, J.G.M.; Shahsavarani, M.; Smith, D.P.; Hájíček, J.; De Luca, V.; Qu, Y. A Catharanthus roseus Fe(II)/α-ketoglutarate-dependent dioxygenase catalyzes a redox-neutral reaction responsible for vindolinine biosynthesis. Nat. Commun. 2022, 13, 3335. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Yang, Y.; Xiao, C.; Ruan, Q.; Li, P.; Zhou, Q.; Sheng, M.; Hao, X.; Kai, G. Comprehensive analysis of OpHD-ZIP transcription factors related to the regulation of camptothecin biosynthesis in Ophiorrhiza pumila. Int. J. Biol. Macromol. 2023, 242, 124910. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Tang, M.; Jin, L.; Mi, X.; Chen, H.; Zhu, J.; Liu, S.; Wei, C. A monoterpene synthase gene cluster of tea plant (Camellia sinensis) potentially involved in constitutive and herbivore-induced terpene formation. Plant Physiol. Biochem. 2022, 184, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kuo, Y.-W.; Chuang, Y.-C.; Yang, Y.-P.; Huang, L.-M.; Jeng, M.-F.; Chen, W.-H.; Chen, H.-H. Terpene Synthase-b and Terpene Synthase-e/f Genes Produce Monoterpenes for Phalaenopsis bellina Floral Scent. Front. Plant Sci. 2021, 12, 700958. [Google Scholar] [CrossRef]
- Jia, Q.; Brown, R.; Köllner, T.G.; Fu, J.; Chen, X.; Wong, G.K.-S.; Gershenzon, J.; Peters, R.J.; Chen, F. Origin and early evolution of the plant terpene synthase family. Proc. Natl. Acad. Sci. USA 2022, 119, e2100361119. [Google Scholar] [CrossRef]
- Bohlmann, J.; Meyer-Gauen, G.; Croteau, R. Plant terpenoid synthases: Molecular biology and phylogenetic analysis. Proc. Natl. Acad. Sci. USA 1998, 95, 4126–4133. [Google Scholar] [CrossRef]
- Gao, F.; Liu, B.; Li, M.; Gao, X.; Fang, Q.; Liu, C.; Ding, H.; Wang, L.; Gao, X. Identification and characterization of terpene synthase genes accounting for volatile terpene emissions in flowers of Freesia x hybrida. J. Exp. Bot. 2018, 69, 4249–4265. [Google Scholar] [CrossRef]
- Tholl, D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 2006, 9, 297–304. [Google Scholar] [CrossRef]
- Fan, W.-L.; Wen, C.-H.; Ma, L.-T.; Ho, C.-L.; Tung, G.-S.; Tien, C.-C.; Chu, F.-H. Monoterpene synthases contribute to the volatile production in tana (Zanthoxylum ailanthoides) through indigenous cultivation practices. Plant Physiol. Biochem. 2023, 202, 107969. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, Z.; Silva, J.A.T.d.; He, C.; Wang, H.; Si, C.; Zhang, M.; Zeng, D.; Duan, J. Functional Characterization of a Dendrobium officinale Geraniol Synthase DoGES1 Involved in Floral Scent Formation. Int. J. Mol. Sci. 2020, 21, 7005. [Google Scholar] [CrossRef]
- Luo, F.; Zhou, Q.; Chen, F.; Liu, X.; Chiu, T.Y.; Zhu, G.Y.; Huang, A.C. Divergent multifunctional P450s-empowered biosynthesis of bioactive tripterifordin and cryptic atiserenoids in Aconitum implies convergent evolution. Nat. Commun. 2025, 16, 5857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Guo, Y.; Shi, X.; Yang, Y.; Chen, J.; Zhang, Q.; Sun, M. Overexpression of LiTPS2 from a cultivar of lily (Lilium ‘Siberia’) enhances the monoterpenoids content in tobacco flowers. Plant Physiol. Biochem. 2020, 151, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.M.; Gershenzon, J.; Bohlmann, J.r. Induction of Volatile Terpene Biosynthesis and Diurnal Emission by Methyl Jasmonate in Foliage of Norway Spruce. Plant Physiol. 2003, 132, 1586–1599. [Google Scholar] [CrossRef]
- Wei, S. Methyl jasmonic acid induced expression pattern of terpenoid indole alkaloid pathway genes in Catharanthus roseus seedlings. Plant Growth Regul. 2010, 61, 243–251. [Google Scholar] [CrossRef]
- van der Fits, L.; Memelink, J. ORCA3, a Jasmonate-Responsive Transcriptional Regulator of Plant Primary and Secondary Metabolism. Science 2000, 289, 295–297. [Google Scholar] [CrossRef]
- Liu, W.; Chen, R.; Chen, M.; Zhang, H.; Peng, M.; Yang, C.; Ming, X.; Lan, X.; Liao, Z. Tryptophan decarboxylase plays an important role in ajmalicine biosynthesis in Rauvolfia verticillata. Planta 2012, 236, 239–250. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473. [Google Scholar] [CrossRef]
- Shao, Y.; Mu, D.; Pan, L.; Wilson, I.W.; Zheng, Y.; Zhu, L.; Lu, Z.; Wan, L.; Fu, J.; Wei, S.; et al. Optimization of Isolation and Transformation of Protoplasts from Uncaria rhynchophylla and Its Application to Transient Gene Expression Analysis. Int. J. Mol. Sci. 2023, 24, 3633. [Google Scholar] [CrossRef]
- Guo, G.; Yu, T.; Zhang, H.; Chen, M.; Dong, W.; Zhang, S.; Tang, X.; Liu, L.; Heng, W.; Zhu, L.; et al. Evidence That PbrSAUR72 Contributes to Iron Deficiency Tolerance in Pears by Facilitating Iron Absorption. Plants 2023, 12, 2173. [Google Scholar] [CrossRef]
- Wang, R.; Lei, S.; Du, Z.; Yue, L.; Zhang, X.; Yang, G.; Wang, M.; Tan, J.; Xu, Y. ‘Shine Muscat’ Grape Fruit Quality Evaluation in Different Mature Periods of Six Areas in the South of China. Mol. Plant Breed. 2024; early access. [Google Scholar]




| Primer Name | Sequences (5′ to 3′) | Usage |
|---|---|---|
| UrGES-F | ATGGCTTGCACAAGTAACGT | Gene cloning |
| UrGES-R | TCAAGTAATTATAGGAGTGAAAAA | |
| pET32a-UrGES-F | GCCATGGCTGATATCGGATCCATGGCTTGCACAAGTAACG | Prokaryotic expression |
| pET32a-UrGES-R | TTGTCGACGGAGCTCGAATTCTCAAGTAATTATAGGAGTGAAAAACAGAG | |
| pBI121-UrGES-F | ACGGGGGACTCTAGAGGATCCATGGCTTGCACAAGTAACG | Transient expression |
| pBI121-UrGES-R | GGACTGACCACCCGGGGATCCAGTAATTATAGGAGTGAAAAACAGAGC | |
| UrGES-qRT-F | AGGTCCAATCACTTCATCA | qRT-PCR |
| UrGES-qRT-R | GCATTCAAGCGGTCTATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Chen, W.; Li, L.; Mu, D.; Wilson, I.W.; Huang, X.; Xiang, Y.; Zhu, L.; Pan, L.; Qiu, D.; et al. Identification and Functional Characterization of a Geraniol Synthase UrGES from Uncaria rhynchophylla. Plants 2025, 14, 2273. https://doi.org/10.3390/plants14152273
Liu X, Chen W, Li L, Mu D, Wilson IW, Huang X, Xiang Y, Zhu L, Pan L, Qiu D, et al. Identification and Functional Characterization of a Geraniol Synthase UrGES from Uncaria rhynchophylla. Plants. 2025; 14(15):2273. https://doi.org/10.3390/plants14152273
Chicago/Turabian StyleLiu, Xinghui, Wenqiang Chen, Linxuan Li, Detian Mu, Iain W. Wilson, Xueshuang Huang, Yahui Xiang, Lina Zhu, Limei Pan, Deyou Qiu, and et al. 2025. "Identification and Functional Characterization of a Geraniol Synthase UrGES from Uncaria rhynchophylla" Plants 14, no. 15: 2273. https://doi.org/10.3390/plants14152273
APA StyleLiu, X., Chen, W., Li, L., Mu, D., Wilson, I. W., Huang, X., Xiang, Y., Zhu, L., Pan, L., Qiu, D., & Tang, Q. (2025). Identification and Functional Characterization of a Geraniol Synthase UrGES from Uncaria rhynchophylla. Plants, 14(15), 2273. https://doi.org/10.3390/plants14152273







