Molecular Insights into the Diversification and Biogeographic History of Six Astragalus L. Sections in the Turkish Flora
Abstract
1. Introduction
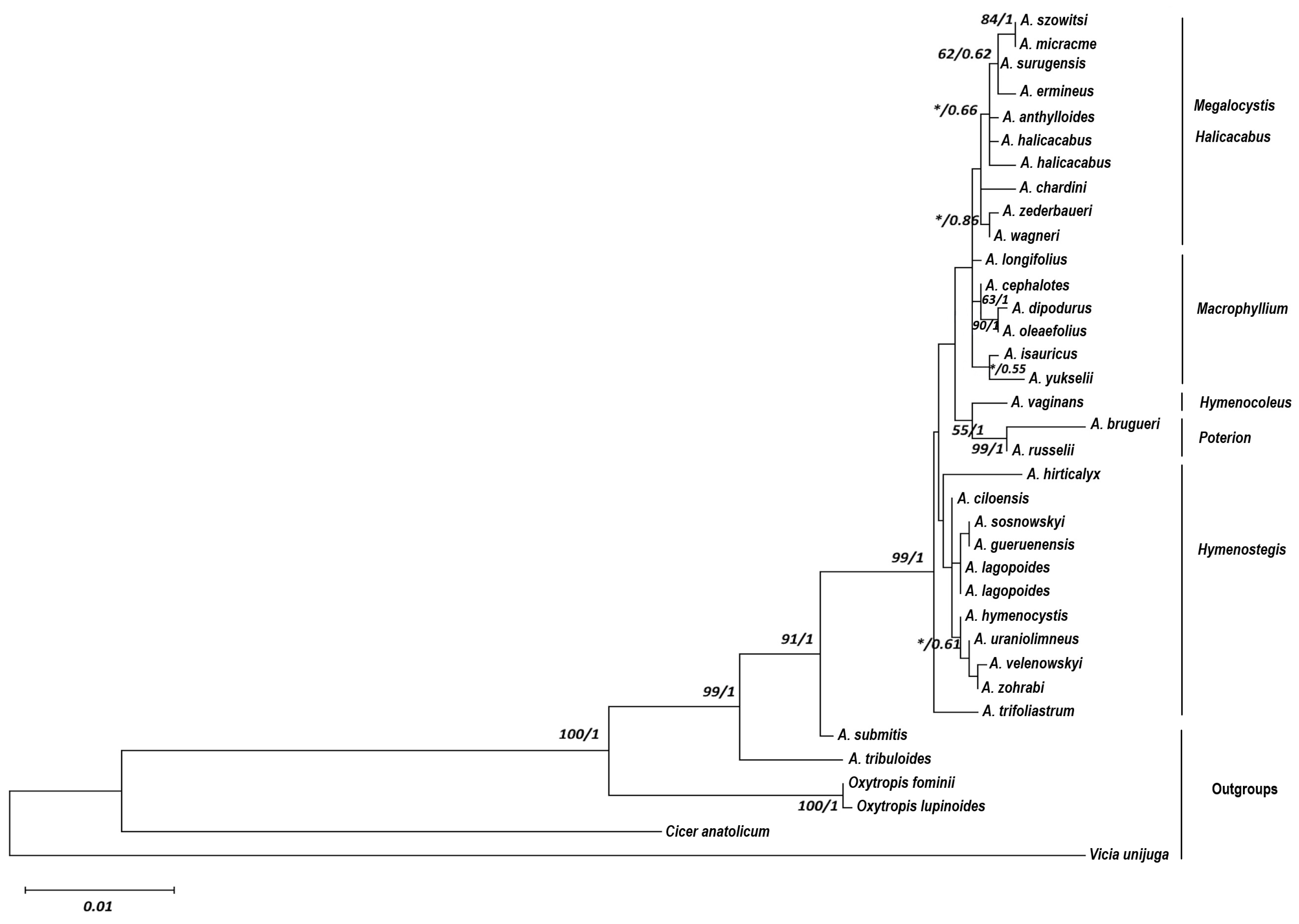
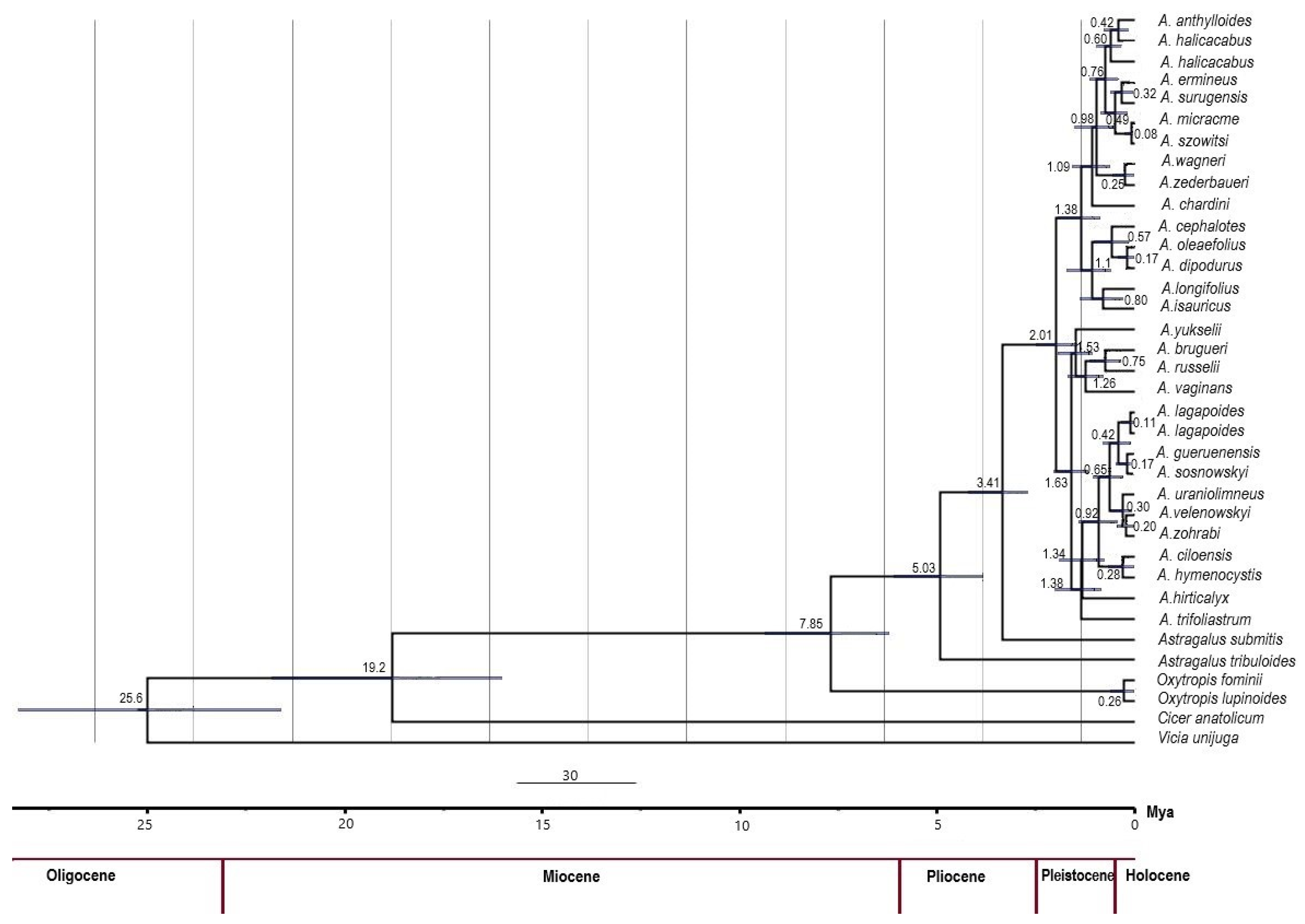
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. DNA Extraction, Amplification, and Sequencing
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NCBI | National Center for Biotechnology Information |
| MEGA | Molecular Evolutionary Genetic Analysis |
| BEAST | Bayesian Evolutionary Analysis Sampling Trees |
| RASP | Reconstruction of Ancestral State in Phylogenies |
| S-DIVA | Statistical DIVA |
| MCMC | Markov Chain Monte Carlo |
| AIC | Akaike Information Criterion |
Appendix A
| Section | Sample Number (SK) | Species Name | Location (Town/Province) | GenBank Accession Numbers |
|---|---|---|---|---|
| Halicacabus | 2541, 2617 | A. anthylloides * | Ankara/Ayaş | PV897526, PV902929, PV902899 |
| 2558 | A. anthylloides | Sivas/Divriği | ||
| 2567 | A. chardini * | Erzurum/Oltu | PV897528, PV902930, PV902900 | |
| 2571 | A. chardini | Erzurum/Horasan | ||
| 2578 | A. halicacabus * | Van/Muradiye | PV897532, PV902931, PV902901 | |
| 2585 | A. halicacabus | Van/Pirreşit Dağı | ||
| 2701 | A. halicacabus | Ağrı/Murat Vadisi | ||
| 2704, 2713 | A. halicacabus | Muş/Malazgirt | ||
| 2706 | A. halicacabus * | Van/Bahçesaray | PV897538, PV902932, PV902902 | |
| 2709 | A. halicacabus | Van/Muradiye | ||
| 2572 | A. wagneri * | Ağrı/Doğubeyazıt | PV897547, PV902933, PV902903 | |
| 2543 | A. zederbaueri * | Konya/Altınapa Barajı | PV897549, PV902927, PV902897 | |
| 2547 | A. zederbaueri | Konya/Beyşehir Yolu | ||
| 2548 | A. zederbaueri | Konya/Ermenek | ||
| 2697 | A. surugensis * | Ş.Urfa/Hilvan Yolu | PV897541, PV902928, PV902898 | |
| Megalocystis | 2597, 2698 | A. micracme * | Hakkari/Çukurca | PV897524, PV902936, PV902906 |
| 2627 | A. ermineus * | Van/Gevaş | PV897531, PV902935, PV902905 | |
| 2734 | A. szowitsii * | Ağrı/Doğubeyazıt | PV897542, PV902934, PV902904 | |
| Poterion | 2519, 2538 | A. russelli * | Ş.Urfa/Ceylanpınarı | PV897521, PV902938, PV902908 |
| 2536 | A. russelli | Ş.Urfa/Hilvan | ||
| 2702 | A. brugueri | Şırnak/Cizre | ||
| 2705 | A. brugueri * | Şırnak/Kumçatı | PV897522, PV902937, PV902907 | |
| 2710 | A. brugueri | Şırnak/Nusaybin | ||
| Hymenostegis | 2589 | A. zohrabi | Van/Erçek | |
| 2590 | A. zohrabi | Ağrı/Gevaş | ||
| 2626 | A. zohrabi * | Van/Bahçesaray | PV897550, PV902955, PV902925 | |
| 2712 | A. velenowskyii * | Van/Çaldıran | PV897546, PV902954, PV902924 | |
| 2632 | A. velenowskyii | Van/Erciş | ||
| 2591 | A. hymenocystis | Van/Muradiye | ||
| 2635 | A. hymenocystis * | Van/Tendürek | PV897534, PV902948, PV902918 | |
| 2752, 2765 | A. hymenocystis | Van/Gürpınar | ||
| 2599, 2600 | A. hirticalyx | Van/Hoşap | ||
| 2601 | A. hirticalyx * | Van/Erciş | PV897533, PV902949, PV902919 | |
| 2569 | A. lagopoides | Van/Muradiye | ||
| 2635 | A. lagopoides | Van/Erciş | ||
| 2576, 2581 | A. lagopoides | Van/Bendimahi | ||
| 2588 | A. lagopoides * | Van/Altındere | PV897536, PV902946, PV902916 | |
| 2618 | A. lagopoides * | Nevşehir/Ürgüp | PV897527, PV902945, PV902915 | |
| 2616 | A. lagopoides | Van/Güzeldere | ||
| 2622 | A. lagopoides | Van/Yukarınarlı | ||
| 2646 | A. lagopoides | Ağrı/Çaldıran | ||
| 2654 | A. lagopoides | Van/Horasan | ||
| 2761 | A. lagopoides | Van/Sugeçer Köyü | ||
| 2779 | A. lagopoides | Sivas/Gürün | ||
| 2783 | A. lagopoides | Ağrı/Doğubeyazıt | ||
| 2794 | A. lagopoides | Ardahan | ||
| 2798 | A. lagopoides | Erzurum/Oltu | ||
| 2584 | A. sosnowskii | Van/Adaklı Köyü | ||
| 2588, 2632, 2633 | A. sosnowskii | Van/Erciş | ||
| 2592 | A. sosnowskii * | Van/Erçek | PV897540, PV902950, PV902920 | |
| 2593 | A. sosnowskii | Van/Özalp | ||
| 2660, 2667 | A. sosnowskii | Erzurum/Oltu | ||
| 2609, 2650 | A. trifoliastrum | Van/Güzeldere | ||
| 2781 | A. trifoliastrum * | Van/Kurubaş | PV897543, PV902951, PV902921 | |
| 2652 | A. ciloensis * | Van/Gürpınar | PV897529, PV902947, PV902917 | |
| 2527, 2575, 2622, 2659 | A. gueruenensis * | Sivas/Gürün | PV897525, PV902956, PV902926 | |
| 2577, 2582, 2599, 2747 | A. uraniolimneus | Van/Muradiye | ||
| 2749, 2797 | A. uraniolimneus * | Van/Hoşap | PV897544, PV902952, PV902922 | |
| Macrophyllium | 2625 | A. dipodurus * | Konya/Karasınır | PV897530, PV902940, PV902910 |
| 2736 | A. dipodurus | G.Antep/Arat Dağı | ||
| 2620 | A. yukselii * | Konya/Hadim | PV897548, PV902944, PV902914 | |
| 2542 | A. oleaefolius * | Aksaray/Taptuk emre Köyü | PV897539, PV902943, PV902913 | |
| 2557 | A. oleaefolius | Sivas/Şarkışla | ||
| 2657 | A. oleaefolius | Erzurum/İspir | ||
| 2735 | A. oleaefolius | G.Antep/Nizip | ||
| 2738 | A. oleaefolius | Sivas/Gemerek | ||
| 2598 | A. longifolius | Hakkari/Çukurca | ||
| 2587, 2647, 2659, 2661 | A. longifolius * | Van/Hasanabdal | PV897537, PV902942, PV902912 | |
| 2549 | A. isauricus * | Konya/Taşkent | PV897535, PV902941, PV902911 | |
| 2670, 2793 | A. cephalotes * | Artvin/Şavşat | PV897523, PV902939, PV902909 | |
| 2733, 2795 | A. cephalotes | Artvin/Yusufeli | ||
| Hymenocoleus | 2672 | A. vaginans | K.Maraş/Çağlayancerit | |
| 2776 | A. vaginans * | Niğde/Ulukışla | PV897545, PV902953, PV902923 |
References
- Maassoumi, A.A. A Checklist of Astragalus in the World: New Grouping, New Changes, and Additional Species with Augmented Data; Research Institute of Forests and Rangelands: Tehran, Iran, 2022; pp. 1–563. [Google Scholar]
- Dinçman, G.E.; Aytaç, Z.; Çalış, İ. Turkish Astragalus Species: Botanical Aspects, Secondary Metabolites, and Biotransformation. Planta Medica 2025, 91, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Zarre, S.; Azani, N. Perspectives in taxonomy and phylogeny of the genus Astragalus (Fabaceae): A review. Prog. Biol. Sci. 2013, 3, 1–6. [Google Scholar]
- Chamberlain, D.F.; Matthews, V.A. Astragalus L. In Flora of Turkey and the East Aegean Islands; Davis, P.H., Ed.; Edinburgh University Press: Edinburgh, UK, 1970; Volume 3, pp. 49–254. [Google Scholar]
- Davis, P.H.; Mill, R.R.; Tan, K. Flora of Turkey and the East Aegean Islands (Supplement); Edinburgh University Press: Edinburgh, UK, 1988; Volume 10, p. 590. [Google Scholar]
- Aytac, Z. Astragalus L. In Flora of Turkey and the East Aegean Islands (Supplement); Güner, A., Ozhatay, N., Ekim, T., Buser, K.H.C., Eds.; Edinburgh University Press: Edinburgh, UK, 2020; Volume 11. [Google Scholar]
- Hamzaoğlu, E. Astragalus askaleensis (sek. Adiaspastus, Fabaceae), Türkiye’den yeni bir tür. Türler Habitatlar 2020, 1, 114–123. [Google Scholar]
- Hamzaoğlu, E. Astragalus nallihanicus (sect. Caprini, Fabaceae), a new species from Türkiye. Acta Bot. Croat. 2024, 83, 26–31. [Google Scholar] [CrossRef]
- Ozhatay, N.; Kultur, S.; Gurdal, B. Check-list of additional taxa to the supplement of flora of Turkey X. Istanb. J. Pharm. 2022, 52, 226–249. [Google Scholar]
- Bizim Bitkiler. Available online: http://www.bizimbitkiler.org.tr (accessed on 5 April 2025).
- Aytaç, Z.; Duman, H.; Akan, H. Astragalus Türkiye Bitkileri Listesi (Damarlı Bitkiler); Güner, A., Aslan, S., Ekim, T., Vural, M., Babaç, M.T., Eds.; Nezahat Gökyiğit Botanik Bahçesi ve Flora Araştırmaları Derneği Yayını: İstanbul, Türkiye, 2012; pp. 427–456. [Google Scholar]
- Taeb, F.; Uzunhisarcikli, M.E. Astragalus argentophyllus (Fabaceae), a new species from south Anatolia, Turkey. Ann. Bot. Fenn. 2012, 49, 259–262. [Google Scholar] [CrossRef]
- Dinç, M.; Aytaç, Z.; Doğu, S. A new species of Astragalus (Fabaceae) from Turkey. Turk. J. Bot. 2013, 37, 841–846. [Google Scholar] [CrossRef]
- Çeçen, Ö.; Aytaç, Z.; Mısırdalı, H. Astragalus unalii (Fabaceae), a new species from Turkey. Turk. J. Bot. 2016, 40, 81–86. [Google Scholar] [CrossRef]
- Karaman Erkul, S.; Aytaç, Z. Astragalus yukselii (Leguminosae), a new species from Turkey. Turk. J. Bot. 2013, 37, 836–840. [Google Scholar] [CrossRef]
- Karaman Erkul, S.; Aytaç, Z.; Ekici, M. Synopsis of sect. Hymenocoleus, sect. Hymenostegis and sect. Macrophyllium belong to Astragalus (Fabaceae) in Turkey. Turk. J. Bot. 2016, 40, 412–418. [Google Scholar] [CrossRef]
- Ilcim, A.; Behçet, L. Astragalus topalanense (Fabaceae), a new species from Turkey. Turk. J. Bot. 2016, 40, 74–80. [Google Scholar] [CrossRef]
- Tunçkol, B.; Aytaç, Z.; Aksoy, N.; Fişne, A. Astragalus bartinense (Fabaceae), a new species from Turkey. Acta Bot. Croat. 2020, 79, 131–136. [Google Scholar] [CrossRef]
- Dönmez, A.A.; Aydın Uğurlu, Z. Astragalus ihsancalisii (Fabaceae), a new species from Erzurum Province, Eastern Turkey. Willdenowia 2018, 48, 399–404. [Google Scholar] [CrossRef]
- Aytaç, Z.; Çeçen, Ö.; Fişne, A. Astragalus sertavulensis (sect. Onobrychoidei/Fabaceae), a new species from Turkey. Nord. J. Bot. 2020, 38, njb-02829. [Google Scholar] [CrossRef]
- Duman, H.; Aytaç, Z.; Özbek, F. Astragalus aybarsii a new species of sect. Onobrychoidei DC. (Fabaceae) from Turkey. Turk. J. Bot. 2020, 44, 661–669. [Google Scholar] [CrossRef]
- Uzun, A.; Aytaç, Z.; Tülücü, F. Astragalus nurhakdagensis (sect. Hololeuce Bunge/Fabaceae), a new species from Turkey. Turk. J. Bot. 2021, 45, 573–586. [Google Scholar] [CrossRef]
- Karaman Erkul, S.; Duman, H.; Ateş, M.A. Astragalus oksutdagensis (Fabaceae), a new species from Turkey. Nord. J. Bot. 2022, 3, njb-03237. [Google Scholar] [CrossRef]
- Karaman, S.; Duman, H.; Evran, A.H.; Aytaç, Z. A new record for the flora of Turkey: Astragalus schmalhausenii Bunge (Fabaceae). Herb. Turcicum 2023, 4, 18–21. [Google Scholar]
- Fırat, M. Astragalus nordizensis (Fabaceae), a new species from Van province (Türkiye) belonging to section Hymenostegis. Nord. J. Bot. 2024, 2, 04172. [Google Scholar]
- Fırat, M. Astragalus miksensis Fırat (Fabaceae), a new species in section Hymenostegis from Van province, Türkiye. Phytotaxa 2024, 641, 149–160. [Google Scholar] [CrossRef]
- Wojciechowski, M.F.; Sanderson, M.J.; Hu, J.M. Evidence on the monophyly of Astragalus (Fabaceae) and its major subgroups based on nuclear ribosomal DNA ITS and chloroplast DNA trnL intron data. Syst. Bot. 1999, 24, 409–437. [Google Scholar] [CrossRef]
- Sanderson, M.J. Phylogenetic relationships within North American Astragalus L. (Fabaceae). Syst. Bot. 1991, 16, 414–430. [Google Scholar] [CrossRef]
- Kazempour Osaloo, S.; Massoumi, A.A.; Murakami, N. Molecular systematics of the genus Astragalus L. (Fabaceae): Phylogenetic analyses of nuclear ribosomal DNA internal transcribed spacers and chloroplast gene ndhF sequences. Plant Syst. Evol. 2003, 242, 1–32. [Google Scholar] [CrossRef]
- Saarela, J.M.; Rai, H.S.; Doyle, J.A.; Endress, P.K.; Mathews, S.; Marchant, A.D.; Briggs, B.G.; Graham, S.W. Hydatellaceae identified as a new branch near the base of the angiosperm phylogenetic tree. Nature 2007, 446, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jiang, B. Species identification in complex groups of medicinal plants based on DNA barcoding: A case study on Astragalus spp. (Fabaceae) from southwest China. Conserv. Genet. Resour. 2020, 12, 469–478. [Google Scholar] [CrossRef]
- Kress, W.J.; Wurdack, K.J.; Zimmer, E.A.; Weigt, L.A.; Janzen, D.H. Use of DNA barcodes to identify flowering plants. Proc. Natl. Acad. Sci. USA 2005, 102, 8369–8374. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yao, H.; Han, J.; Liu, C.; Song, J. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef] [PubMed]
- China Plant BOL Plant Group. Comparative analysis of a large data set indicates that internal transcribed spacer (ITS) should be incorporated in to the core barcode for seed plants. Proc. Natl. Acad. Sci. USA 2011, 108, 19641–19646. [Google Scholar] [CrossRef] [PubMed]
- Kress, W.J. Plant DNA barcodes: Applications today and in the future. J. Syst. Evol. 2017, 55, 291–307. [Google Scholar] [CrossRef]
- Wojciechowski, M.F. Astragalus (Fabaceae): A molecular phylogenetic perspective. Brittonia 2005, 57, 382–396. [Google Scholar] [CrossRef]
- Sanderson, M.J.; Wojciechowski, M.F. Diversification rates in a temperate legume clade: Are there “So many species” of Astragalus (Fabaceae)? Am. J. Bot. 1996, 83, 1488–1502. [Google Scholar] [CrossRef]
- Dong, T.T.; Ma, X.Q.; Clarke, C.; Song, Z.H.; Ji, Z.N.; Lo, C.K.; Tsim, K.W. Phylogeny of Astragalus in China: Molecular evidence from the DNA sequences of 5S rRNA spacer, ITS, and 18S rRNA. J. Agric. Food Chem. 2003, 51, 6709–6714. [Google Scholar] [CrossRef] [PubMed]
- Dizkirici, A.; Ekici, M.; Kaya, Z. Comparative molecular phylogenetics of Astragalus L. sections from Turkey with New World Astragalus species using nrDNA ITS sequences. Plant Syst. Evol. 2014, 300, 163–175. [Google Scholar] [CrossRef]
- Abd El-Ghani, M.M.; El-Sayed, A.S.; Moubarak, A.; Rashad, R.; Nosier, H.; Khattab, A. Biosystematic study on some Egyptian species of Astragalus L. (Fabaceae). Agriculture 2021, 11, 125. [Google Scholar] [CrossRef]
- Tian, C.; Li, X.; Wu, Z.; Li, Z.; Hou, X.; Li, F.Y. Characterization and comparative analysis of complete chloroplast genomes of three species from the genus Astragalus (Leguminosae). Front. Genet. 2021, 12, 705482. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Duan, L.; Liu, P.; Liu, J.; Chang, Z.; Wen, J. Chloroplast phylogenomics and character evolution of eastern Asian Astragalus (Leguminosae): Tackling the phylogenetic structure of the largest genus of flowering plants in Asia. Mol. Phylogenetics Evol. 2021, 156, 107025. [Google Scholar] [CrossRef] [PubMed]
- Khal, L.H.; Tahir, N.A.R.; Abdul-Razaq, R.T. Molecular variation in some taxa of genus Astragalus L. (Fabaceae) in the Iraqi Kurdistan Region. Horticulturae 2023, 9, 1110. [Google Scholar] [CrossRef]
- Moghaddam, M.; Wojciechowski, M.F.; Kazempour-Osaloo, S. Characterization and comparative analysis of the complete plastid genomes of four Astragalus species. PLoS ONE 2023, 18, e0286083. [Google Scholar] [CrossRef] [PubMed]
- Gielly, L.; Taberlet, P. A phylogeny of the European gentians inferred from chloroplast trnL (UAA) intron sequences. Bot. J. Linn. Soc. 1996, 120, 57–75. [Google Scholar] [CrossRef]
- Wang, X.R.; Tsumura, Y.; Yoshimaru, H.; Nagasaka, K.; Szmidt, A.E. Phylogenetic relationships of Eurasian pines (Pinus, Pinaceae) based on chloroplast rbcL, matK, rpl20-rps18 spacer, and tnV intron sequences. Am. J. Bot. 1999, 86, 17421753. [Google Scholar] [CrossRef]
- Ohsako, T.; Ohnishi, O. Intra- and interspecific phylogeny of wild Fagopyrum (Polygonaceae) species based on nucleotide sequences of noncoding regions in chloroplast DNA. Am. J. Bot. 2000, 87, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Mummenhoff, K.; Bruggemann, H.; Bowman, J.L. Chloroplast DNA phylogeny and biogeography of Lepidium (Brassicaceae). Am. J. Bot. 2001, 88, 2051–2063. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Lickey, E.B.; Beck, J.T.; Farmer, S.B.; Liu, W.; Miller, J.; Siripun, K.C.; Winder, C.T.; Schilling, E.E.; Small, R.L. The Tortoise and the Hare II: Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am. J. Bot. 2005, 92, 142–166. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Kazempour, O.S.; Maassoumi, A.A.; Rastegar, P.E. Molecular phylogeny of selected Old World Astragalus (Fabaceae): Incongruence among chloroplast trnL-F, ndhF and nuclear ribosomal DNA ITS sequences. Nord. J. Bot. 2009, 27, 425–436. [Google Scholar] [CrossRef]
- Neuhaus, H.; Link, G. The chloroplast tRNA Lys (UUU) gene from mustard (Sinapis alba) contains a class II intron potentially coding for a maturase-related polypeptide. Curr. Genet. 1987, 11, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Karaman Erkul, S.; Maassoumi, A.A.; Rahiminejad, M.R.; Blattner, F.R. Astragalus trifoliastrum (Fabaceae), a revived species for the flora of Turkey. Nord. J. Bot. 2015, 33, 532–539. [Google Scholar] [CrossRef]
- Zarre, M.S.; Podlech, D. Problems in the Taxonomy of Tragacanthic Astragalus. Sendtnera 1997, 4, 243–250. [Google Scholar]
- Karaman Erkul, S.; Bagheri, A.; Maassoumi, A.A.; Rahiminejad, M.R. Notes on Astragalus sect. Hymenostegis (Fabaceae) from Turkey. Turk. J. Bot. 2015, 39, 205–207. [Google Scholar] [CrossRef]
- Massoumi, A.A. The Genus Astragalus in Iran; Research Institute of Forests and Rangelands: Tehran, Iran, 2005; Volume 2, p. 389. [Google Scholar]
- Podlech, D. Contributions to the knowledge of the genus Astragalus L. (Leguminosae) VII-X. Sendtnera 2001, 7, 163–201. [Google Scholar]
- Bagheri, A.; Ghahremaninejad, F.; Maassoumi, A.A. Additions to Astragalus sect. Hymenostegis (Fabaceae) in Iran. Arch. SID 2011, 17, 15–19. [Google Scholar]
- Podlech, D.; Zarre, S. A Taxonomic Revision of the Genus Astragalus L. (Leguminosae) in the Old World 1–3; Naturhistorisches Museum Press: Vienna, Austria, 2013; p. 2117. [Google Scholar]
- Safar Naderi, K.; Osaloo Kazempour, S.; Maassoumi, A.A.; Zarre, S. Molecular Phylogeny of Astragalus section Anthylloidei (Fabaceae) infereed from nrDNA ITS and plastid rpl32-trnL sequence data. Turk. J. Bot. 2014, 38, 637–652. [Google Scholar] [CrossRef]
- Zarre, S. Hair micromorphology and its phylogenetic application in thorny species of Astragalus (Fabaceae). Bot. J. Linn. Soc. 2003, 143, 323–330. [Google Scholar] [CrossRef]
- Tietz, S. Revision of Astragalus L. Sect. Campylanthus Bunge, Sect. Microphysa Bunge and Sect. Poterion Bunge; Cabi Digital Library: Wallingford, UK, 1998. [Google Scholar]
- Bagheri, A.; Maassoumi, A.A.; Rahiminejad, M.R.; Brassac, J.; Blattner, F.R. Molecular phylogeny and divergence times of Astragalus section Hymenostegis: An analysis of a rapidly diversifying species group in Fabaceae. Sci. Rep. 2017, 7, 14033. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Yu, Y.; Pfosser, M.; Wetschnig, W. Inferences of biogeographical histories within subfamily Hyacinthoideae using S-DIVA and Bayesian binary MCMC analysis implemented in RASP (Reconstruct Ancestral State in Phylogenies). Ann. Bot. 2012, 109, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.W.; Stenøien, H.K.; Grundmann, M.; Russell, S.J.; Koch, M.A.; Schneider, H.; Vogel, J.C. The importance of Anatolian mountains as the cradle of global diversity in Arabis alpina, a key arctic–alpine species. Ann. Bot. 2011, 108, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, R. Back to the suture: The distribution of intraspecific genetic diversity in and around Anatolia. Int. J. Mol. Sci. 2011, 12, 4080–4103. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bogle, A.L.; Klein, A.S. Interspecific relationships and genetic divergence of the disjunct genus Liquidambar (Hamamelidaceae) inferred from DNA sequences of plastid gene matK. Rhodora 1997, 99, 229–240. [Google Scholar]
- Hsiao, C.; Chatterton, N.J.; Asay, K.H.; Jensen, K.B. Molecular phylogeny of the Pooideae (Poaceae) based on nuclear rDNA (ITS) sequences. Theor. Appl. Genet. 1995, 90, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Chamberlain, B.; Thayer, D. Finch TV, Version 1.4.0. 2004. Available online: http://www.geospiza.com/Products/finchtv.shtml (accessed on 4 May 2025).
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Resour. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11, molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with beauty and the beast 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Maassoumi, A.A.; Brassac, J.; Blattner, F.R. Dated Phylogeny of Astragalus Section Stereothrix (Fabaceae) and Allied Taxa in the Hypoglottis Clade. Biology 2023, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree–Tree Figure Drawing Tool; Version 1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar]
- Yu, Y.; Blair, C.; He, X. RASP 4: Ancestral state reconstruction tool for multiple genes and characters. Mol. Biol. Evol. 2020, 37, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J. Trees within trees: Genes and species, molecules and morphology. Syst. Biol. 1997, 46, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Maddison, W.P. Gene trees in species trees. Syst. Biol. 1997, 46, 523–536. [Google Scholar] [CrossRef]
- Soltis, D.E.; Kuzoff, R.K. Discordance between nuclear and chloroplast phylogenies in the Heuchera group (Saxifragaceae). Evolution 1995, 49, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, R.; De Castro, O.; Del Guacchio, E.; Caputo, P. Genetic diversity and origin of the rare, narrow endemic Asperula crassifolia (Rubiaceae). Plant Syst. Evol. 2019, 305, 181–192. [Google Scholar] [CrossRef]




| cp DNA | ITS (ITS1 + 5.8S + ITS2) | |||
|---|---|---|---|---|
| trn L5′-L3′ | trn L3′-F(GAA) | matK | ||
| Number of species | 30 | 30 | 30 | 30 |
| Total length (bp) | 520 | 145 | 1225 | 615 |
| GC content (%) | 30.5 | 34.1 | 31 | 53.9 |
| Conserved sites | 471 | 119 | 1188 | 553 |
| Variable sites | 20 | 15 | 23 | 49 |
| Parsimony informative sites | 12 | 15 | 7 | 49 |
| Transitional pairs | 40.14 | 43.87 | 42.27 | 60 |
| Transversional pairs | 59.86 | 56.13 | 57.73 | 30 |
| Transition/transversion (tr/tv) (R)ratio | 0.58 | 0.95 | 0.63 | 1.48 |
| Molecular diversity | 0.04 | 0.028 | 0.017 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ateş, M.A.; Karaman, S.; Aytaç, Z.; Kaya, Z. Molecular Insights into the Diversification and Biogeographic History of Six Astragalus L. Sections in the Turkish Flora. Plants 2025, 14, 2226. https://doi.org/10.3390/plants14142226
Ateş MA, Karaman S, Aytaç Z, Kaya Z. Molecular Insights into the Diversification and Biogeographic History of Six Astragalus L. Sections in the Turkish Flora. Plants. 2025; 14(14):2226. https://doi.org/10.3390/plants14142226
Chicago/Turabian StyleAteş, Mevlüde Alev, Seher Karaman, Zeki Aytaç, and Zeki Kaya. 2025. "Molecular Insights into the Diversification and Biogeographic History of Six Astragalus L. Sections in the Turkish Flora" Plants 14, no. 14: 2226. https://doi.org/10.3390/plants14142226
APA StyleAteş, M. A., Karaman, S., Aytaç, Z., & Kaya, Z. (2025). Molecular Insights into the Diversification and Biogeographic History of Six Astragalus L. Sections in the Turkish Flora. Plants, 14(14), 2226. https://doi.org/10.3390/plants14142226







