Fine-Scale Environmental Heterogeneity Drives Intra- and Inter-Site Variation in Taraxacum officinale Flowering Phenology
Abstract
1. Introduction
2. Results
2.1. Intra-Site Variation: Differences Among Quadrat-Level Phenology Within Sites
2.2. Inter-Site Variation: Phenological Differences Among Sites
3. Discussion
4. Materials and Methods
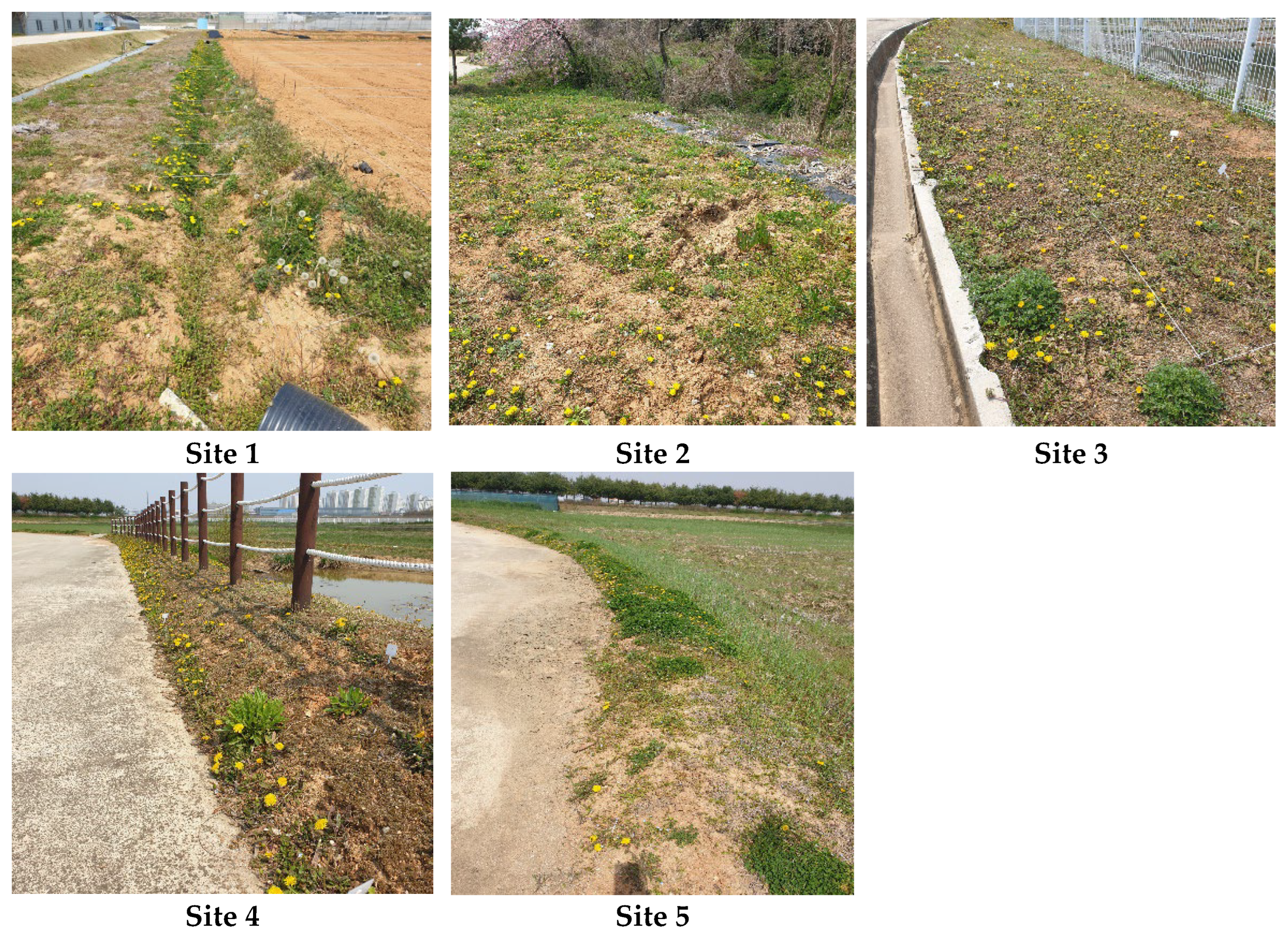
4.1. Study Area and Experimental Design
4.2. Phenological Observation
4.3. Data Analysis
4.3.1. Modeling Flowering Phenology Using Nlstimedist
4.3.2. Quadrat-Level Modeling (Intra-Site Variation)
4.3.3. Site-Level Modeling (Inter-Site Variation)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Menzel, A.; Fabian, P. Growing season extended in Europe. Nature 1999, 397, 659. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Parmesan, C. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob. Change Biol. 2007, 13, 1860–1872. [Google Scholar] [CrossRef]
- Forrest, J.; Miller-Rushing, A.J. Toward a synthetic understanding of the role of phenology in ecology and evolution. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3101–3112. [Google Scholar] [CrossRef]
- Thackeray, S.J.; Henrys, P.A.; Hemming, D.; Bell, J.R.; Botham, M.S.; Burthe, S.; Helaouet, P.; Johns, D.G.; Jones, I.D.; Leech, D.I.; et al. Phenological sensitivity to climate across taxa and trophic levels. Nature 2016, 535, 241–245. [Google Scholar] [CrossRef]
- Vitasse, Y.; Signarbieux, C.; Fu, Y.H. Global warming leads to more uniform spring phenology across elevations. Proc. Natl. Acad. Sci. USA 2018, 115, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Roslin, T.; Antão, L.; Hällfors, M.; Meyke, E.; Lo, C.; Tikhonov, G.; Delgado, M.d.M.; Gurarie, E.; Abadonova, M.; Abduraimov, O.; et al. Phenological shifts of abiotic events, producers and consumers across a continent. Nat. Clim. Change 2021, 11, 241–248. [Google Scholar] [CrossRef]
- Inouye, D.W. Climate change and phenology. WIREs Clim. Change 2022, 13, e764. [Google Scholar] [CrossRef]
- Leathers, K.; Herbst, D.; de Mendoza, G.; Ruhi, A. Climate change is poised to alter mountain stream ecosystem processes via organismal phenological shifts. Proc. Natl. Acad. Sci. USA 2024, 121, e2310513121. [Google Scholar] [CrossRef]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Change Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.R.K.; Thomson, J.D. An examination of synchrony between insect emergence and flowering in Rocky Mountain meadows. Ecol. Monogr. 2011, 81, 469–491. [Google Scholar] [CrossRef]
- Sherry, R.A.; Zhou, X.; Gu, S.; Arnone, J.A.; Schimel, D.S.; Verburg, P.S.; Wallace, L.L.; Luo, Y. Divergence of Reproductive Phenology under Climate Warming. Ecology 2007, 104, 198–202. [Google Scholar] [CrossRef]
- Iler, A.M.; CaraDonna, P.J.; Forrest, J.R.K.; Post, E. Demographic Consequences of Phenological Shifts in Response to Climate Change. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 221–245. [Google Scholar] [CrossRef]
- Liu, F.Y.; Gao, C.J.; Chen, M.; Tang, G.Y.; Sun, Y.; Li, K. The Impacts of Flowering Phenology on the Reproductive Success of the Narrow Endemic Nouelia insignis Franch (Asteraceae). Ecol. Evol. 2021, 11, 9396–9409. [Google Scholar] [CrossRef]
- Pareja-Bonilla, D.; Ortiz, P.L.; Morellato, L.P.C.; Arista, M. Functional Traits Predict Changes in Floral Phenology under Climate Change in a Highly Diverse Mediterranean Community. Funct. Ecol. 2025. [Google Scholar] [CrossRef]
- Fitter, A.H.; Fitter, R.S.R. Rapid Changes in Flowering Time in British Plants. Science 2002, 296, 1689–1691. [Google Scholar] [CrossRef]
- Faidiga, A.S.; Oliver, M.G.; Budke, J.M.; Kalisz, S. Shifts in flowering phenology in response to spring temperatures in eastern Tennessee. Am. J. Bot. 2023, 110, e16203. [Google Scholar] [CrossRef]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant-pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef]
- Kudo, G.; Ida, T.Y. Early onset of spring increases the phenological mismatch between plants and pollinators. Ecology 2013, 94, 2311–2320. [Google Scholar] [CrossRef]
- Gehrmann, F.; Hänninen, H.; Liu, C.; Saarinen, T. Phenological responses to small-scale spatial variation in snowmelt timing reveal compensatory and conservative strategies in subarctic-alpine plants. Polar Biol. 2018, 41, 453–468. [Google Scholar] [CrossRef]
- Olliff-Yang, R.L.; Ackerly, D.D. Topographic heterogeneity lengthens the duration of pollinator resources. Ecol. Evol. 2020, 10, 9301–9312. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.J.; Givnish, T.J. Fine-scale environmental heterogeneity and spatial niche partitioning among spring-flowering forest herbs. Am. J. Bot. 2021, 108, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Nepal, S.; Trunschke, J.; Ren, Z.-X.; Burgess, K.S.; Wang, H. Flowering phenology differs among wet and dry sub-alpine meadows in southwestern China. AoB Plants 2024, 16, plae002. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; She, X.; Gao, X.; Huang, Y.; Zeng, Y.; Ding, C.; Fu, D.; Shao, J.; Li, Y. Spatiotemporal variation of spring phenology and the corresponding scale effects and uncertainties: A case study in southwestern China. Int. J. Appl. Earth Obs. Geoinf. 2024, 135, 104294. [Google Scholar] [CrossRef]
- Hassan, T.; Gulzar, R.; Hamid, M.; Ahmad, R.; Waza, S.A.; Khuroo, A.A. Plant phenology shifts under climate warming: A systematic review of recent scientific literature. Environ. Monit. Assess. 2024, 196, 36. [Google Scholar] [CrossRef]
- Solbrig, O.T. The population biology of dandelions: These common weeds provide experimental evidence for a new model to explain the distribution of plants. Am. Sci. 1971, 59, 686–694. [Google Scholar]
- Ogawa, K.; Mototani, I. Land-use selection by dandelions in the Tokyo metropolitan area, Japan. Ecol. Res. 1991, 6, 233–246. [Google Scholar] [CrossRef]
- Gray, E.; McGehee, E.M.; Carlisle, D.F. Seasonal Variation in Flowering of Common Dandelion. Weed Sci. 1973, 21, 230–232. [Google Scholar] [CrossRef]
- Yoshie, F. Latitudinal variation in sensitivity of flower bud formation to high temperature in Japanese Taraxacum officinale. J. Plant Res. 2014, 127, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Szabó, B.; Vincze, E.; Czúcz, B. Flowering phenological changes in relation to climate change in Hungary. Int. J. Biometeorol. 2016, 60, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Pisman, M.; Bonte, D.; de la Peña, E. Urbanization alters plastic responses in the common dandelion Taraxacum officinale. Ecol. Evol. 2020, 10, 4082–4090. [Google Scholar] [CrossRef] [PubMed]
- Woudstra, Y.; Kraaiveld, R.; Jorritsma, A.; Vijverberg, K.; Ivanovic, S.; Erkens, R.; Huber, H.; Gravendeel, B.; Verhoeven, K.J.F. Some like it hot: Adaptation to the urban heat island in common dandelion. Evol. Lett. 2024, 8, 881–892. [Google Scholar] [CrossRef]
- Verduijn, M.; Van Dijk, P.; Van Damme, J. The role of tetraploids in the sexual–asexual cycle in dandelions (Taraxacum). Heredity 2004, 93, 390–398. [Google Scholar] [CrossRef]
- Katal, N.; Rzanny, M.; Mäder, P.; Römermann, C.; Wittich, H.C.; Boho, D.; Musavi, T.; Wäldchen, J. Bridging the gap: How to adopt opportunistic plant observations for phenology monitoring. Front. Plant Sci. 2023, 14, 1150956. [Google Scholar] [CrossRef]
- Kalvāne, G.; Kalvāns, A.; Ģērmanis, A. Long-term phenological data set of multi-taxonomic groups, agrarian activities, and abiotic parameters from Latvia, northern Europe. Earth Syst. Sci. Data 2021, 13, 4621–4633. [Google Scholar] [CrossRef]
- CaraDonna, P.J.; Iler, A.M.; Inouye, D.W. Shifts in flowering phenology reshape a subalpine plant community. Proc. Natl. Acad. Sci. USA 2014, 111, 4916–4921. [Google Scholar] [CrossRef]
- Vitasse, Y.; Baumgarten, F.; Zohner, C.M.; Kaewthongrach, R.; Fu, Y.H.; Walde, M.G.; Moser, B. Impact of microclimatic conditions and resource availability on spring and autumn phenology of temperate tree seedlings. New Phytol. 2021, 232, 537–550. [Google Scholar] [CrossRef]
- Lorer, E.; Verheyen, K.; Blondeel, H.; De Pauw, K.; Sanczuk, P.; De Frenne, P.; Landuyt, D. Forest understorey flowering phenology responses to experimental warming and illumination. New Phytol. 2023, 241, 1476–1491. [Google Scholar] [CrossRef] [PubMed]
- Plos, C.; Hensen, I.; Korell, L.; Auge, H.; Römermann, C. Plant species phenology differs between climate and land-use scenarios and relates to plant functional traits. Ecol. Evol. 2024, 14, e11441. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.D.; Ahas, R.; Aasa, A. Onset of spring starting earlier across the Northern Hemisphere. Glob. Change Biol. 2006, 12, 343–351. [Google Scholar] [CrossRef]
- Zohner, C.M.; Mo, L.; Renner, S.S. Global warming reduces leaf-out and flowering synchrony among individuals. eLife 2018, 7, e40214. [Google Scholar] [CrossRef]
- Diez, J.M.; Ibáñez, I.; Miller-Rushing, A.J.; Mazer, S.J.; Crimmins, T.M.; Crimmins, M.A.; Bertelsen, C.D.; Inouye, D.W. Forecasting phenology: From species variability to community patterns. Ecol. Lett. 2012, 15, 545–553. [Google Scholar] [CrossRef]
- Fridley, J.D. Extended leaf phenology and the autumn niche in deciduous forest invasions. Nature 2012, 485, 359–362. [Google Scholar] [CrossRef]
- Kamsurya, M.Y.; Ala, A.; Musa, Y.; Rafiuddin. The effect of micro climate on the flowering phenology of forest clove plants (Zyzygium obtusifolium L.). IOP Conf. Ser. Earth Environ. Sci. 2023, 1134, 012031. [Google Scholar] [CrossRef]
- Ramirez-Parada, T.H.; Park, I.W.; Record, S.; Davis, C.C.; Mazer, S.J. Scaling flowering onset and duration responses among species predicts phenological community reassembly under warming. Ecosphere 2025, 16, e70070. [Google Scholar] [CrossRef]
- Takeno, K. Stress-induced flowering: The third category of flowering response. J. Exp. Bot. 2016, 67, 4925–4934. [Google Scholar] [CrossRef]
- Liang, M.; Xiao, S.; Cai, J.; Ow, D.W. OXIDATIVE STRESS 3 regulates drought-induced flowering through APETALA 1. Biochem. Biophys. Res. Commun. 2019, 519, 585–590. [Google Scholar] [CrossRef]
- Castillioni, K.; Newman, G.S.; Souza, L.; Iler, A.M. Effects of drought on grassland phenology depend on functional types. New Phytol. 2022, 236, 1558–1571. [Google Scholar] [CrossRef]
- Rafferty, N.E.; Ives, A.R. Effects of experimental shifts in flowering phenology on plant–pollinator interactions. Ecol. Lett. 2011, 14, 69–74. [Google Scholar] [CrossRef]
- Chuine, I.; Régnière, J. Process-based models of phenology for plants and animals. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 159–182. [Google Scholar] [CrossRef]
- Wheeler, K.I.; Dietze, M.C.; LeBauer, D.; Peters, J.A.; Richardson, A.D.; Ross, A.A.; Thomas, R.Q.; Zhu, K.; Bhat, U.; Munch, S.; et al. Predicting Spring Phenology in Deciduous Broadleaf Forests: NEON Phenology Forecasting Community Challenge. Agric. For. Meteorol. 2024, 345, 109810. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Yan, Z.; Moon, M.; Yang, D.; Meng, L.; Bucher, S.F.; Wang, J.; Song, G.; Guo, Z.; et al. Exploring the role of biotic factors in regulating the spatial variability in land surface phenology across four temperate forest sites. New Phytol. 2024, 242, 1965–1980. [Google Scholar] [CrossRef]
- Di Napoli, A.; Zucchetti, P. A comprehensive review of the benefits of Taraxacum officinale on human health. Health. Bull. Natl. Res. Cent. 2021, 45, 110. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Franco, M. The Time Distribution of Biological Phenomena—Illustrated with the London Marathon. PeerJ Prepr. 2018, 6, e27223v1. [Google Scholar]
- Steer, N.C.; Ramsay, P.M.; Franco, M. NLSTIMEDIST: An R package for the biologically meaningful quantification of unimodal phenology distributions. Methods Ecol. Evol. 2019, 10, 1700–1707. [Google Scholar] [CrossRef]



| Site | Onset (DOY) | Peak (DOY) | End (DOY) | Duration (Days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | CV (%) | Range | Mean ± SD | CV (%) | Range | Mean ± SD | CV (%) | Range | Mean ± SD | CV (%) | Range | |
| S1 | 92.3 ± 5.2 | 5.64 | 83.7~96.8 | 107.1 ± 3.0 | 2.82 | 101.8~109.3 | 129.2 ± 11.6 | 8.98 | 120.1~145.4 | 36.9 ± 16.5 | 44.74 | 25.2~61.7 |
| S2 | 89.0 ± 1.8 | 2.04 | 87.6~92.2 | 102.0 ± 3.7 | 3.58 | 99.0~108.3 | 118.9 ± 4.6 | 3.83 | 111.8~123.5 | 29.9 ± 4.5 | 15.07 | 23.2~35.8 |
| S3 | 88.7 ± 0.7 | 0.74 | 88.1~89.8 | 97.2 ± 0.5 | 0.51 | 96.8~98.0 | 105.0 ± 0.9 | 0.87 | 104.1~106.3 | 16.3 ± 0.7 | 4.48 | 15.7~17.5 |
| S4 | 90.4 ± 1.6 | 1.75 | 87.6~91.7 | 99.0 ± 1.0 | 0.99 | 97.3~99.9 | 109.7 ± 2.1 | 1.87 | 107.4~111.8 | 19.3 ± 2.3 | 11.99 | 15.8~21.1 |
| S5 | 89.0 ± 1.1 | 1.25 | 87.5~90.3 | 100.7 ± 2.1 | 2.11 | 98.9~104.0 | 125 ± 13.4 | 10.62 | 110.8~141.8 | 37.0 ± 14.2 | 38.53 | 20.5~53.6 |
| Site | Onset (DOY) | Peak (DOY) | End (DOY) | Duration (Days) |
|---|---|---|---|---|
| S1 | 92.2 | 107.9 | 127.9 | 35.7 |
| S2 | 87.4 | 102.6 | 120.3 | 32.9 |
| S3 | 88.6 | 97.2 | 104.8 | 16.2 |
| S4 | 90.8 | 99.4 | 109.0 | 18.2 |
| S5 | 88.9 | 100.5 | 125.8 | 36.9 |
| Mean (SD) | 89.6 (1.8) | 101.5 (3.7) | 117.6 (9.1) | 28.0 (9.2) |
| CV (%) | 2.0 | 3.6 | 7.7 | 32.9 |
| Flowering Phenology | Maximum Intra-Site Difference (Days) | Maximum Inter-Site Difference (Days) | CaraDonna et al., 2014 (Days/Decade) [40] | Szabó et al., 2016 (Days/Decade) [34] | Lorer et al., 2023 (Days/°C) [42] | Pareja-Bonilla et al., 2025 (Days/Decade) [19] |
|---|---|---|---|---|---|---|
| Onset | 13.1 | 4.8 | −6.4 | −3.4~−3.9 | −7.1 | −4.5 |
| Peak | 9.3 | 10.7 | −5.3 (spring) −3.3 (summer) | - | - | - |
| End | 31.0 | 23.1 | +3.1 | - | - | −2.3 |
| Duration | 36.5 | 20.7 | +8.9 | - | - | +2.6 |
| Number of species | 1 (T. officinale) | 1 (T. officinale) | 60 species | 1 (T. officinale) | 10 herbaceous species | 269 species |
| Region | South Korea | South Korea | Calorado Rocky Mountains, USA | Hungary | Belgium, France (experiment) | Spain (Mediterranean region) |
| Warming magnitude | - | - | +0.4 °C per decade (summer air temperature increase) | Observational regression | +3 °C experimental warming | Elevation/latitude gradient |
| Site | Landform Type | Slope Direction | Soil Water Content ± SD (%) | Soil EC (ds/m) | Soil Temperature (℃) |
|---|---|---|---|---|---|
| Site 1 | Upland field ridge with drainage ditch | Mixed (SW and EN) | 20.8 ± 5.59 ab (CV = 26.9) | 0.431 ± 0.040 ab (CV = 9.35) | 22.9 ± 0.84 ab (CV = 03.68) |
| Site 2 | Upland field adjacent to forest edge | Flat | 22.5 ± 2.37 ab (CV = 10.6) | 0.583 ± 0.062 c (CV = 10.50) | 22.5 ± 2.18 a (CV = 9.66) |
| Site 3 | Maintained law of Zoysia japonica near artificial reservoir | W | 20.9 ± 1.40 ab (CV = 6.68) | 0.383 ± 0.058 a (CV = 15.00) | 24.1 ± 0.34 bc (CV = 1.42) |
| Site 4 | Maintained law of Zoysia japonica on roadside ridge | W | 19.7 ± 1.97 a (CV = 10.00) | 0.457 ± 0.071 b (CV = 15.60) | 24.3 ± 0.56 c (CV = 2.31) |
| Site 5 | Paddy field ridge | Mixed (SW and EN) | 24.0 ± 4.69 b (CV = 19.50) | 0.463 ± 0.067 b (CV = 14.40) | 24.2 ± 0.48 c (CV = 2.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-H.; Oh, Y.-J. Fine-Scale Environmental Heterogeneity Drives Intra- and Inter-Site Variation in Taraxacum officinale Flowering Phenology. Plants 2025, 14, 2211. https://doi.org/10.3390/plants14142211
Kim M-H, Oh Y-J. Fine-Scale Environmental Heterogeneity Drives Intra- and Inter-Site Variation in Taraxacum officinale Flowering Phenology. Plants. 2025; 14(14):2211. https://doi.org/10.3390/plants14142211
Chicago/Turabian StyleKim, Myung-Hyun, and Young-Ju Oh. 2025. "Fine-Scale Environmental Heterogeneity Drives Intra- and Inter-Site Variation in Taraxacum officinale Flowering Phenology" Plants 14, no. 14: 2211. https://doi.org/10.3390/plants14142211
APA StyleKim, M.-H., & Oh, Y.-J. (2025). Fine-Scale Environmental Heterogeneity Drives Intra- and Inter-Site Variation in Taraxacum officinale Flowering Phenology. Plants, 14(14), 2211. https://doi.org/10.3390/plants14142211






