Climatic Adaptability Changes in Leaf Functional Traits of Old Pinus tabulaeformis in Loess Plateau
Abstract
1. Introduction
2. Results
2.1. Characteristics and Variation in Leaf Variation of Leaf Functional Traits in Different Regions
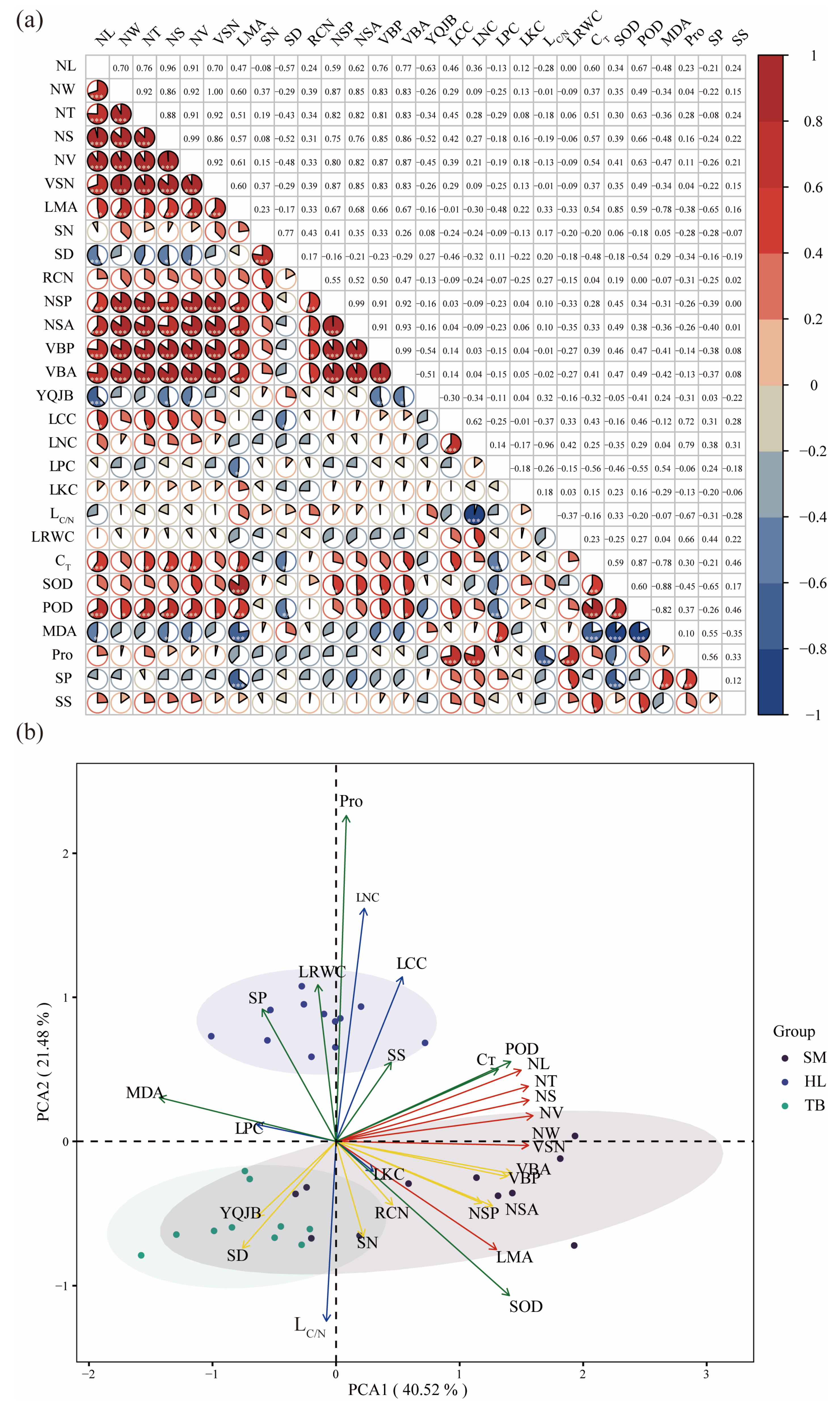
2.2. Correlation of Leaf Functional Traits
2.3. Phylogenetic Signal Analysis
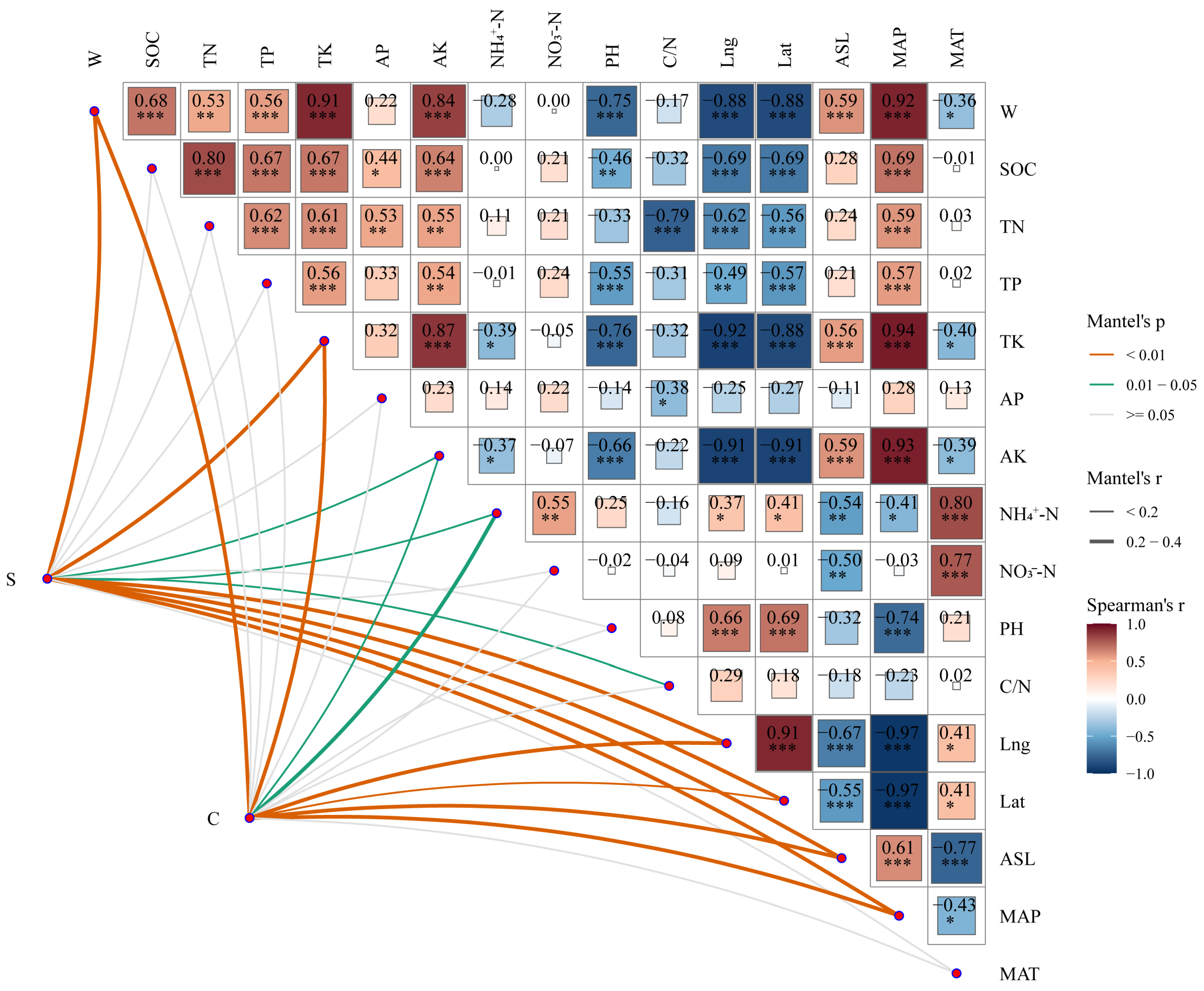
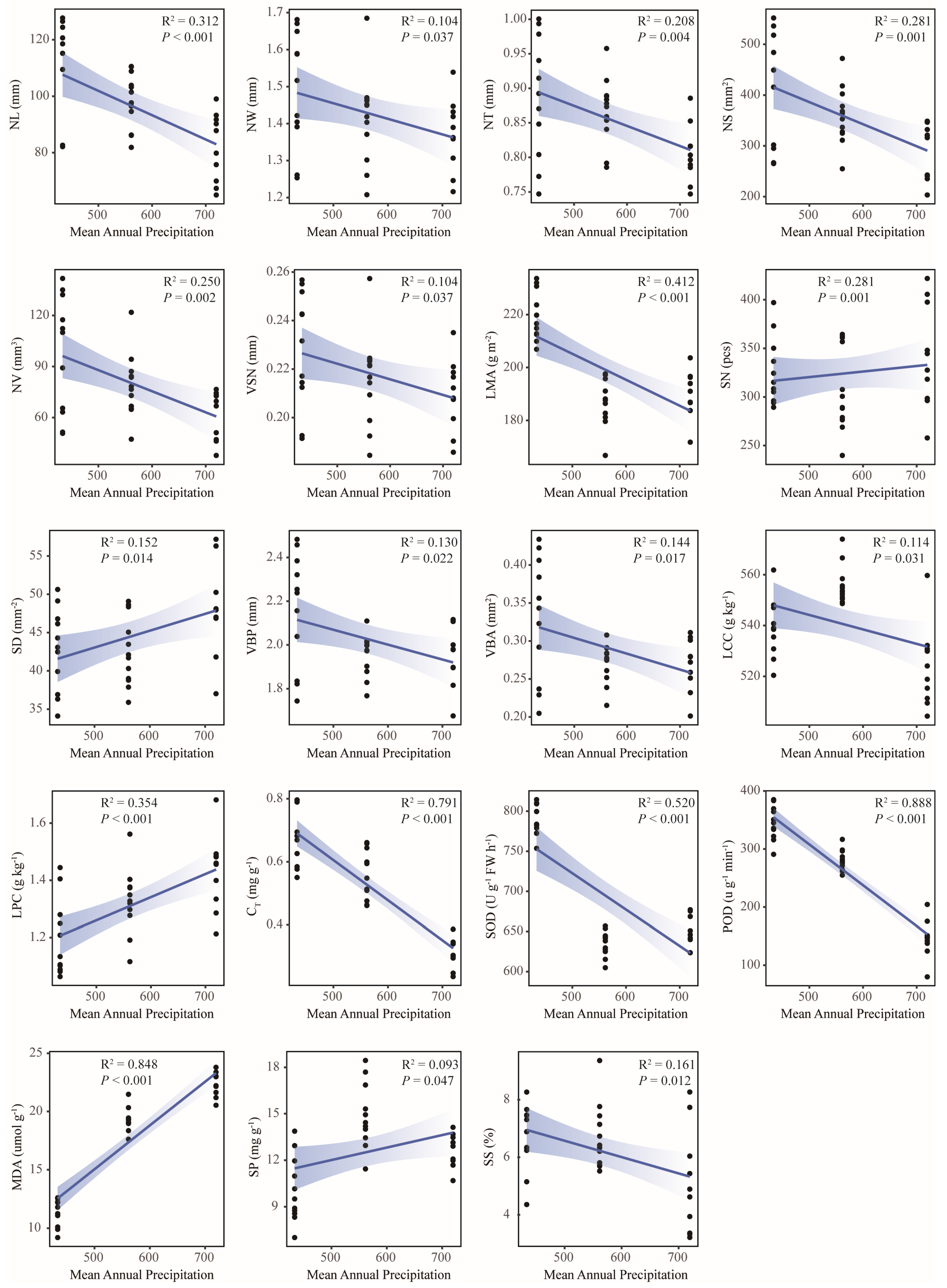
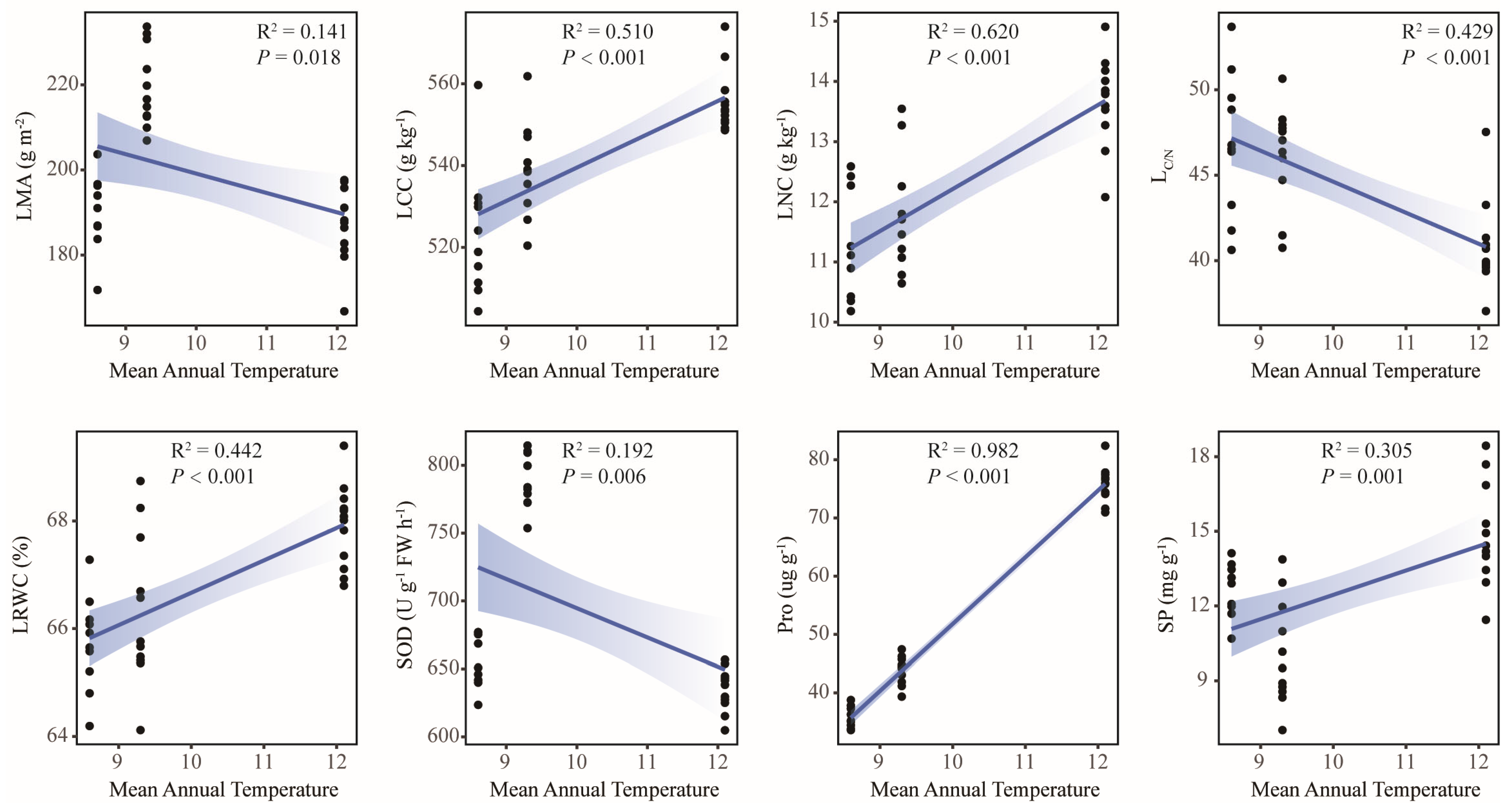
2.4. Environmental Driving Factors of Leaf Functional Traits of Old Tree
2.5. CSR Survival Strategies
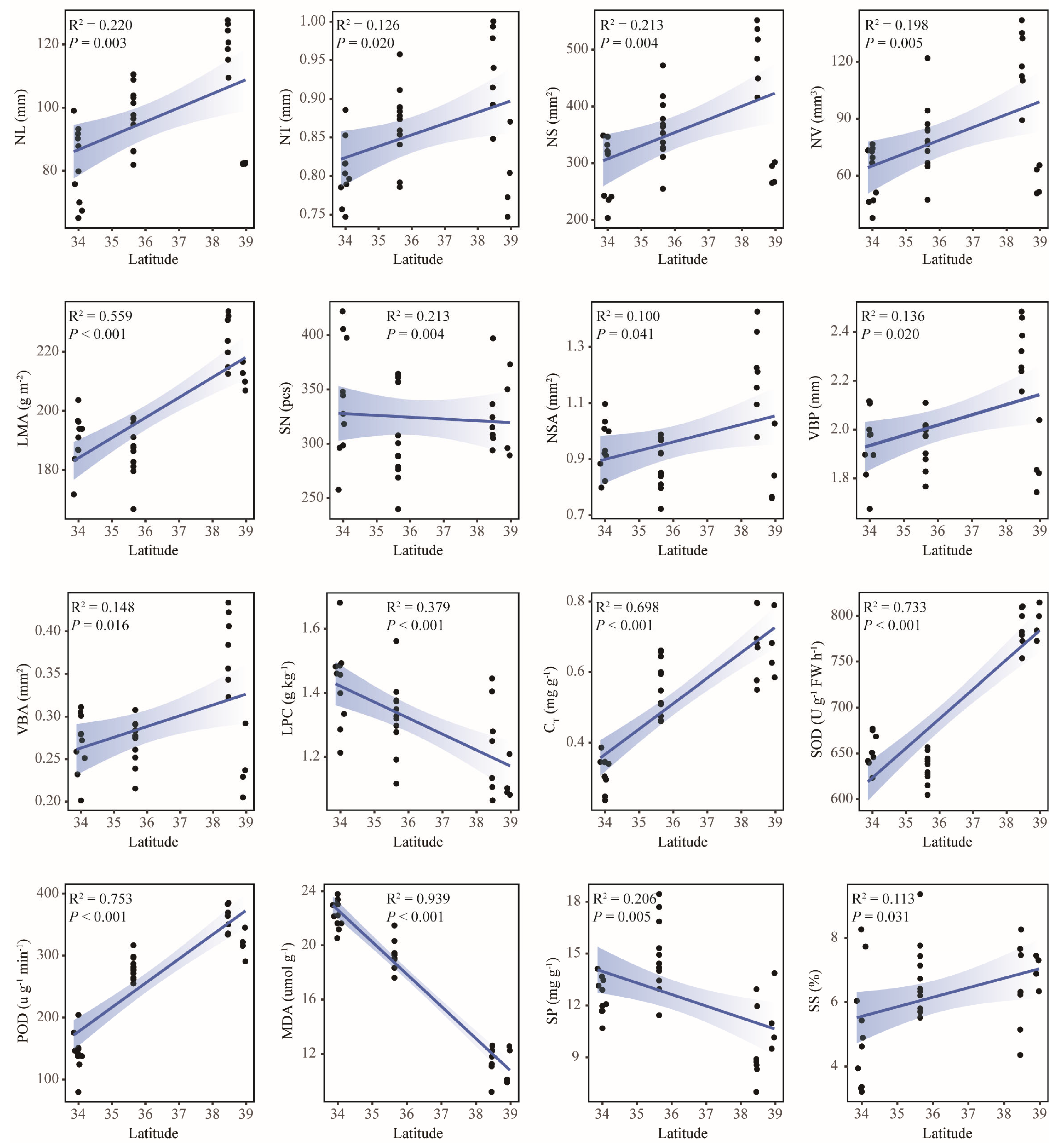
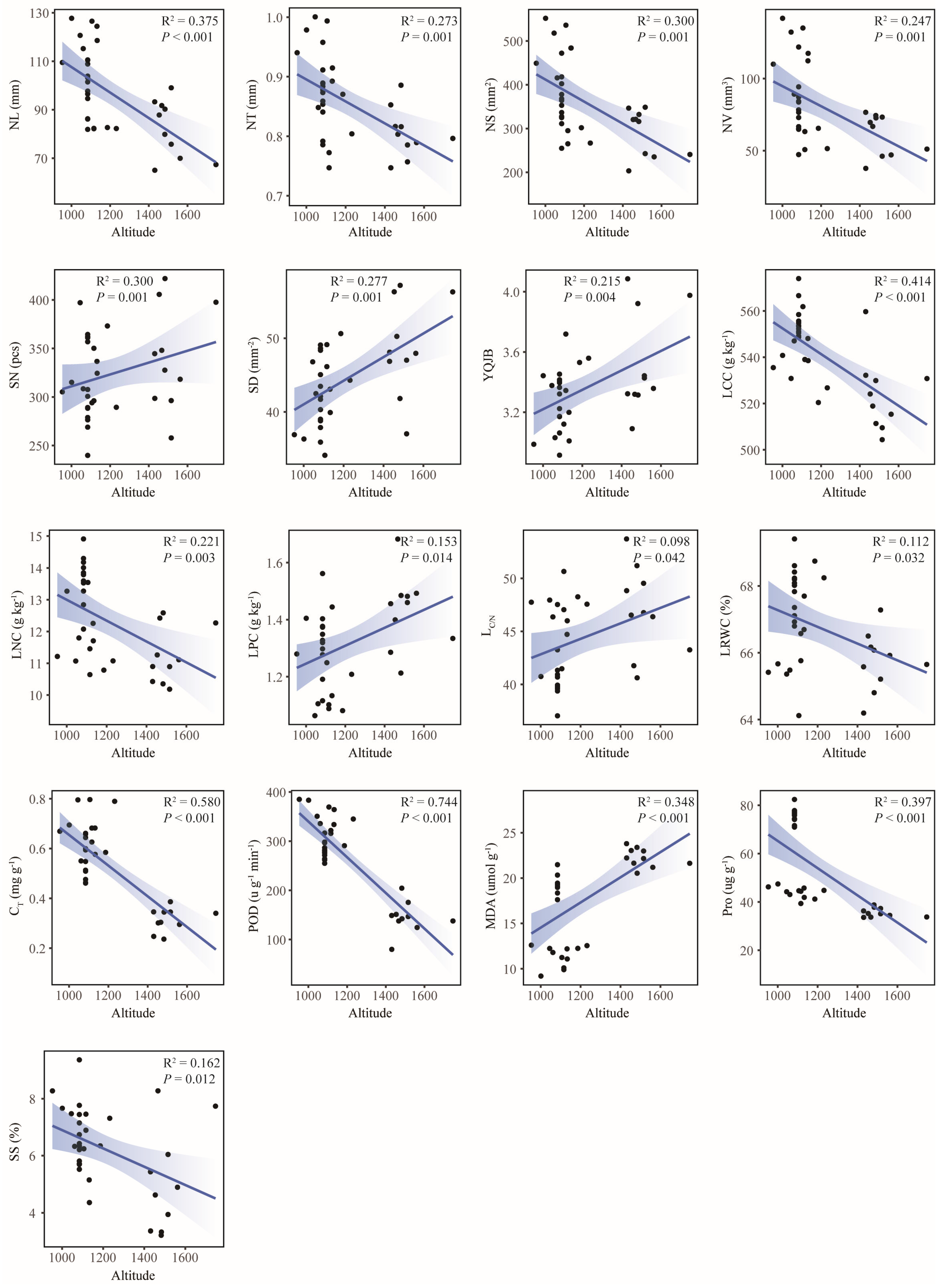
2.6. Responses of Leaf Functional Traits to Geographical and Climatic Factors
3. Discussion
3.1. Characteristics of Leaf Functional Traits of Old P. tabuliformis
3.2. Geo-Climate Model for Leaf Functional Traits
3.3. The Main Driving Factors and Action Models of Leaf Functional Traits
4. Materials and Methods
4.1. Study Site and Experimental Design
4.2. Analysis of Soil Physical and Chemical Properties
4.3. Determination of Leaf Functional Traits
4.4. Construction of Phylogenetic Tree
4.5. Quantification of the Competition-Stress-Tolerant-Weed Type (CSR) Strategy
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Piovesan, G.; Cannon, C.H.; Liu, J.; Munné-Bosch, S. Ancient trees: Irreplaceable conservation resource for ecosystem restoration. Trends Ecol. Evol. 2022, 37, 1025–1028. [Google Scholar] [CrossRef]
- Büntgen, U.; Krusic, P.J.; Piermattei, A.; Coomes, D.A.; Esper, J.; Myglan, V.S.; Kirdyanov, A.V.; Camarero, J.J.; Crivellaro, A.; Körner, C. Limited capacity of tree growth to mitigate the global greenhouse effect under predicted warming. Nat. Commun. 2019, 10, 2171. [Google Scholar] [CrossRef] [PubMed]
- Camarero, J.J.; García-Ruiz, J.M.; Sangüesa-Barreda, G.; Galván, J.D.; Alla, A.Q.; Sanjuán, Y.; Beguería, S.; Gutiérrez, E. Recent and Intense Dynamics in a Formerly Static Pyrenean Treeline. Arct. Antarct. Alp. Res. 2015, 47, 773–783. [Google Scholar] [CrossRef]
- Lindenmayer, D.B. Conserving large old trees as small natural features. Biol. Conserv. 2017, 211, 51–59. [Google Scholar] [CrossRef]
- Cannon, C.H.; Piovesan, G.; Munné-Bosch, S. Old and ancient trees are life history lottery winners and vital evolutionary resources for long-term adaptive capacity. Nat. Plants 2022, 8, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.B.; Laurance, W.F.; Franklin, J.F. Global Decline in Large Old Trees. Science 2012, 338, 1305–1306. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive shifts in forest dynamics in a changing world. Science 2020, 368, eaaz9463. [Google Scholar] [CrossRef]
- Fu, Q.; Qiu, E.; Zhang, Y.; Huang, L.; Wang, H.; Jiang, S. Discussion of the Distribution Pattern and Driving Factors of 2 Large Old Tree Resources in Beijing. Forests 2022, 13, 1500. [Google Scholar] [CrossRef]
- Xie, C.; Jim, C. Safeguarding China’s irreplaceable natural legacy: Combating the illicit trade of old trees. Environ. Conserv. 2025, 52, 57–59. [Google Scholar] [CrossRef]
- Islam, T.; Hamid, M.; Nawchoo, I.A.; Khuroo, A.A. Leaf functional traits vary among growth forms and vegetation zones in the Himalaya. Sci. Total Environ. 2024, 906, 167274. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, G.; Bricca, A.; Chelli, S.; Petruzzellis, F.; Acosta, A.T.R.; Bacaro, G.; Beccari, E.; Bernardo, L.; Bonari, G.; Bolpagni, R.; et al. Intraspecific variability of leaf form and function across habitat types. Ecol. Lett. 2024, 27, e14396. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, R.; Yang, L.; Tan, T.; Li, H.; Liu, M. Elevation gradient distribution of indices of tree population in a montane forest: The role of leaf traits and the environment. For. Ecosyst. 2022, 9, 100012. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.; Harrison, S.P.; Prentice, I.C. Leaf morphological traits as adaptations to multiple climate gradients. J. Ecol. 2022, 110, 1344–1355. [Google Scholar] [CrossRef]
- Santos, K.R.; Pereira, M.P.; Ferreira, A.C.G.; de Almeida Rodrigues, L.C.; de Castro, E.M.; Corrêa, F.F.; Pereira, F.J. Typha domingensis Pers. growth responses to leaf anatomy and photosynthesis as influenced by phosphorus. Aquat. Bot. 2015, 122, 47–53. [Google Scholar] [CrossRef]
- Pan, S.; Wang, X.; Yan, Z.; Wu, J.; Guo, L.; Peng, Z.; Wu, Y.; Li, J.; Wang, B.; Su, Y.; et al. Leaf stomatal configuration and photosynthetic traits jointly affect leaf water use efficiency in forests along climate gradients. New Phytol. 2024, 244, 1250–1262. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, H.; Sun, T.; Li, D.; Wang, X.; Mo, W.; Wang, R.; Zhang, S. Variation in woody leaf anatomical traits along the altitudinal gradient in Taibai Mountain, China. Glob. Ecol. Conserv. 2021, 26, e01523. [Google Scholar] [CrossRef]
- Cai, Q.; Ji, C.; Yan, Z.; Jiang, X.; Fang, J. Anatomical responses of leaf and stem of Arabidopsis thaliana to nitrogen and phosphorus addition. J. Plant Res. 2017, 130, 1035–1045. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, X.; Wang, S.; Feng, S.; Ning, Z.; Wang, R.; Chen, X.; Zhao, H.; Chen, M. Untangling the influence of abiotic and biotic factors on leaf C, N, and P stoichiometry along a desert-grassland transition zone in northern China. Sci. Total Environ. 2023, 884, 163902. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J.; Liu, W.; Yuan, Y.; Hu, L.; Cai, Q. Linkages of plant and soil C:N:P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Zhu, D.; Hui, D.; Wang, M.; Yang, Q.; Yu, S. Light and competition alter leaf stoichiometry of introduced species and native mangrove species. Sci. Total Environ. 2020, 738, 140301. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zuo, X.; Yue, P.; Zhao, S.; Guo, X.; Li, X.; Medina-Roldán, E. Increased Precipitation Shapes Relationship between Biochemical and Functional Traits of Stipa glareosa in Grass-Dominated Rather than Shrub-Dominated Community in a Desert Steppe. Plants 2020, 9, 1463. [Google Scholar] [CrossRef]
- Su, Y.; Cui, B.; Luo, Y.; Wang, J.; Wang, X.; Ouyang, Z.; Wang, X. Leaf Functional Traits Vary in Urban Environments: Influences of Leaf Age, Land-Use Type, and Urban–Rural Gradient. Front. Ecol. Evol. 2021, 9, 681959. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Guo, S.; Li, Z.; He, M.; Zhang, Y.; Li, G.; Han, X.; Yang, G. Divergent patterns and drivers of leaf functional traits of Robinia pseudoacacia and Pinus tabulaeformis plantations along a precipitation gradient in the Loess plateau, China. J. Environ. Manag. 2023, 348, 119318. [Google Scholar] [CrossRef] [PubMed]
- Pasques, O.; Munné-Bosch, S. Ancient trees are essential elements for high-mountain forest conservation: Linking the longevity of trees to their ecological function. Proc. Natl. Acad. Sci. USA 2024, 121, e2317866121. [Google Scholar] [CrossRef]
- Yan, P.; He, N.; Yu, K.; Xu, L.; Van Meerbeek, K. Integrating multiple plant functional traits to predict ecosystem productivity. Commun. Biol. 2023, 6, 239. [Google Scholar] [CrossRef]
- Chen, X.; Xie, J.; Wu, Q.; Zhang, H.; Li, Y. Climate and soil explain contrasting intraspecific trait variability of widespread species over a large environmental gradient. Glob. Ecol. Conserv. 2024, 56, e03338. [Google Scholar] [CrossRef]
- Ren, L.; Huang, Y.; Pan, Y.; Xiang, X.; Huo, J.; Meng, D.; Wang, Y.; Yu, C. Differential Investment Strategies in Leaf Economic Traits Across Climate Regions Worldwide. Front. Plant Sci. 2022, 13, 798035. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Villar, R.; Pérez-Ramos, I.M.; Quero, J.L.; Matías, L.; Poorter, L.; Marañón, T. Relationships between leaf mass per area and nutrient concentrations in 98 Mediterranean woody species are determined by phylogeny, habitat and leaf habit. Trees 2018, 32, 497–510. [Google Scholar] [CrossRef]
- Joswig, J.S.; Wirth, C.; Schuman, M.C.; Kattge, J.; Reu, B.; Wright, I.J.; Sippel, S.D.; Rüger, N.; Richter, R.; Schaepman, M.E.; et al. Climatic and soil factors explain the two-dimensional spectrum of global plant trait variation. Nat. Ecol. Evol. 2022, 6, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K.; Mishra, A.; Gupta, R.; Garkoti, S.C. Leaf and tree age-related changes in leaf ecophysiological traits, nutrient, and adaptive strategies of Alnus nepalensis in the central Himalaya. J. Biosci. 2024, 49, 24. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef]
- Gao, X.; Dai, J.; Shahzad, K.; Wang, H.; Tao, Z.; Alatalo, J.M. Association of spring phenological traits with phylogeny and adaptation to native climate in temperate plant species in Northeast China. Ecol. Indic. 2022, 143, 109381. [Google Scholar] [CrossRef]
- An, N.; Lu, N.; Fu, B.; Wang, M.; He, N. Distinct Responses of Leaf Traits to Environment and Phylogeny Between Herbaceous and Woody Angiosperm Species in China. Front. Plant Sci. 2021, 12, 799401. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, L.; Yin, M.; Guo, W. Phylogenetic relationship and soil salinity shape intraspecific trait variability of Phragmites australis in the Yellow River Delta. Front. Mar. Sci. 2022, 9, 980695. [Google Scholar] [CrossRef]
- Sun, J.; Li, G.; Zhang, Y.; Qin, W.; Wang, M. Identification of priority areas for afforestation in the Loess Plateau region of China. Ecol. Indic. 2022, 140, 108998. [Google Scholar] [CrossRef]
- Su, J.; Xiao, S.; Peng, X.; Che, C.; Zhao, P. Responses of radial growth to climate change for two dominant artificial coniferous trees. Dendrochronologia 2024, 83, 126163. [Google Scholar] [CrossRef]
- Stotz, G.C.; Salgado-Luarte, C.; Escobedo, V.M.; Valladares, F.; Gianoli, E. Global trends in phenotypic plasticity of plants. Ecol. Lett. 2021, 24, 2267–2281. [Google Scholar] [CrossRef]
- Yang, Y.; Kang, L.; Zhao, J.; Qi, N.; Li, R.; Wen, Z.; Kassout, J.; Peng, C.; Lin, G.; Zheng, H. Quantifying Leaf Trait Covariations and Their Relationships with Plant Adaptation Strategies along an Aridity Gradient. Biology 2021, 10, 1066. [Google Scholar] [CrossRef]
- Tian, M.; Yu, G.; He, N.; Hou, J. Leaf morphological and anatomical traits from tropical to temperate coniferous forests: Mechanisms and influencing factors. Sci. Rep. 2016, 6, 19703. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C.; John, G.P.; Poorter, H.; Mason, C.M.; Mendez-Alonzo, R.; Donovan, L.A. How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. J. Exp. Bot. 2013, 64, 4053–4080. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, J.; OuYang, F.; Wang, J.; Song, L.; Kong, L.; Zhang, H. Instrinsic relationship among needle morphology, anatomy, gas exchanges and tree growth across 17 Picea species. New For. 2021, 52, 509–535. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Xu, L.; Chen, Z.; He, N. Variation in leaf morphological, stomatal, and anatomical traits and their relationships in temperate and subtropical forests. Sci. Rep. 2019, 9, 5803. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wang, L.; Lei, M.; Dang, H.; Quan, J.; Tian, T.; Chai, Y.; Yue, M. The relationships between leaf economics and hydraulic traits of woody plants depend on water availability. Sci. Total Environ. 2018, 621, 245–252. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Zhu, J.; Wang, X.; Chen, K.; Yuan, Y.; Yang, X.; Mo, W.; Wang, R.; Zhang, S. Strong conservatism in leaf anatomical traits and their multidimensional relationships with leaf economic traits in grasslands under different stressful environments. Ecol. Process. 2024, 13, 71. [Google Scholar] [CrossRef]
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, H.; He, Z.; Ma, D.; Sun, W.; Xu, X.; Tian, Q. Effects of Drought Stress on Leaf Functional Traits and Biomass Characteristics of Atriplex canescens. Plants 2024, 13, 2006. [Google Scholar] [CrossRef]
- Paine, C.E.T.; Amissah, L.; Auge, H.; Baraloto, C.; Baruffol, M.; Bourland, N.; Bruelheide, H.; Daïnou, K.; de Gouvenain, R.C.; Doucet, J.-L.; et al. Globally, functional traits are weak predictors of juvenile tree growth, and we do not know why. J. Ecol. 2015, 103, 978–989. [Google Scholar] [CrossRef]
- Fortunel, C.; Stahl, C.; Heuret, P.; Nicolini, E.; Baraloto, C. Disentangling the effects of environment and ontogeny on tree functional dimensions for congeneric species in tropical forests. New Phytol. 2020, 226, 385–395. [Google Scholar] [CrossRef]
- Dong, X.; Shi, L.; Bao, S.; Ren, Y.; Fu, H.; You, Y.; Li, Q.; Chen, Z. Comprehensive evaluation of freezing tolerance in prickly ash and its correlation with ecological and geographical origin factors. Sci. Rep. 2024, 14, 26301. [Google Scholar] [CrossRef]
- Huang, W.-D.; He, Y.-Z.; Wang, H.-H.; Zhu, Y.-Z. Leaf Physiological Responses of Three Psammophytes to Combined Effects of Warming and Precipitation Reduction in Horqin Sandy Land, Northeast China. Front. Plant Sci. 2022, 12, 785653. [Google Scholar] [CrossRef] [PubMed]
- Ling-Ling, S.; Qing, T.; Guang, L.; Zong-Xing, L.; Xiaoying, L.; Juan, G.; Yuchen, L.; Qiao, C.; Yue, Z. Variation in characteristics of leaf functional traits of alpine vegetation in the Three-River Headwaters Region, China. Ecol. Indic. 2022, 145, 109557. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Ma, S.; He, F.; Tian, D.; Zou, D.; Yan, Z.; Yang, Y.; Zhou, T.; Huang, K.; Shen, H.; Fang, J. Variations and determinants of carbon content in plants: A global synthesis. Biogeosciences 2018, 15, 693–702. [Google Scholar] [CrossRef]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, Q.; Ge, W.; Liu, Q.; Kong, D.; Yin, H. Coordination of leaf and root economic space in alpine coniferous forests on the Tibetan Plateau. Plant Soil 2024, 496, 555–568. [Google Scholar] [CrossRef]
- Rosner, S.; Hannrup, B. Resin canal traits relevant for constitutive resistance of Norway spruce against bark beetles: Environmental and genetic variability. For. Ecol. Manag. 2004, 200, 77–87. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Wu, B.; Liang, Y.; Wang, G.; Lin, X.; Yang, J.; Cheng, Q.; Wang, J. Leaf stoichiometry of potassium, calcium, and magnesium in tropical plants: Responses to climatic and geographical variations—A case study from Hainan Island. Land Degrad. Dev. 2024, 35, 2591–2601. [Google Scholar] [CrossRef]
- Blomberg, S.P.; Garland, T., Jr.; Ives, A.R. Testing for Phylogenetic Signal in Comparative Data: Behavioral Traits are More Labile. Evolution 2003, 57, 717–745. [Google Scholar] [CrossRef]
- Chai, Y.; Dang, H.; Yue, M.; Xu, J.; Zhang, L.; Quan, J.; Guo, Y.; Li, T.; Wang, L.; Wang, M.; et al. The role of intraspecific trait variability and soil properties in community assembly during forest secondary succession. Ecosphere 2019, 10, e02940. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, W.; Chen, L.; Yang, L.; Chen, H.Y.H.; Luo, Y. Soil Water Availability Drives Changes in Community Traits Along a Hydrothermal Gradient in Loess Plateau Grasslands. Rangel. Ecol. Manag. 2020, 73, 276–284. [Google Scholar] [CrossRef]
- Wang, J.; Wen, X. Limiting resource and leaf functional traits jointly determine distribution patterns of leaf intrinsic water use efficiency along aridity gradients. Front. Plant Sci. 2022, 13, 909603. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, K.; Li, J.; Zhao, G.; Shangguan, Z. Effect of soil moisture and atmospheric humidity on both plant productivity and diversity of native grasslands across the Loess Plateau, China. Ecol. Eng. 2016, 94, 525–531. [Google Scholar] [CrossRef]
- Wang, J.; Wen, X.; Lyu, S.; Guo, Q. Transitioninmulti-dimensionalleaftraitsandtheircontrolsonwaterusestrategiesofco-occurringspecies along asoillimiting-resourcegradient. Ecol. Indic. 2021, 128, 107838. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, Q.; Tian, D.; Ji, C.; Shen, H.; Fan, D.; Wang, X.; Fang, J. Nitrogen addition promotes conservative resource-use strategies via aggravating phosphorus limitation of evergreen trees in subtropical forest. Sci. Total Environ. 2023, 889, 164047. [Google Scholar] [CrossRef]
- Zou, Y.; Li, B.; Peñuelas, J.; Sardans, J.; Yu, H.; Chen, X.; Deng, X.; Cheng, D.; Zhong, Q. Response of functional traits in Machilus pauhoi to nitrogen addition is influenced by differences of provenances. For. Ecol. Manag. 2022, 513, 120207. [Google Scholar] [CrossRef]
- Zhou, X.; Xin, J.; Huang, X.; Li, H.; Li, F.; Song, W. Linking Leaf Functional Traits with Soil and Climate Factors in Forest Ecosystems in China. Plants 2022, 11, 3545. [Google Scholar] [CrossRef]
- Shao, S.; Li, G.; Wang, J.; Wang, Y.; Qu, M.; Zhao, H.; Zhu, W.; Li, J. Temperature and soil attributes drive the regional variation in leaf anatomical traits of Populus euphratica. Glob. Ecol. Conserv. 2024, 54, e03107. [Google Scholar] [CrossRef]
- Xie, G.; Wang, Y.; Chen, Z.; Ji, Y.; Lu, Y.; Liang, Y.; Zhou, R.; Tao, J. Response of Plant Leaf Traits to Environmental Factors in Climax Communities at Varying Latitudes in Karst Regions. Plants 2025, 14, 183. [Google Scholar] [CrossRef]
- Chelli, S.; Ottaviani, G.; Simonetti, E.; Wellstein, C.; Canullo, R.; Carnicelli, S.; Andreetta, A.; Puletti, N.; Bartha, S.; Cervellini, M.; et al. Climate is the main driver of clonal and bud bank traits in Italian forest understories. Perspect. Plant Ecol. Evol. Syst. 2019, 40, 125478. [Google Scholar] [CrossRef]
- Gong, H.; Cui, Q.; Gao, J. Latitudinal, soil and climate effects on key leaf traits in northeastern China. Glob. Ecol. Conserv. 2020, 22, e00904. [Google Scholar] [CrossRef]
- Zhao, Z. Theory and Technology of Ancient Tree Protection; Science Press: Beijing, China, 2021. [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Shi, Y.; Yu, X.; Wang, X.; Zhang, J. The effects of stand structure on specific needle area in closed-canopy Chinese pine plantations. J. For. Res. 2013, 18, 445–453. [Google Scholar] [CrossRef]
- Lai, M.; Dong, L.; Su, R.; Zhang, L.; Jia, T.; Chen, T.; Yi, M. Needle functional features in contrasting yield phenotypes of slash pine at three locations in southern China. Ind. Crops Prod. 2023, 206, 117613. [Google Scholar] [CrossRef]
- Gadallah, M.A.A. Effect of water stress, abscisic acid and proline on cotton plants. J. Arid. Environ. 1995, 30, 315–325. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: II. Purification and Quantitative Relationship with Water-soluble Protein in Seedlings 1 2. Plant Physiol. 1977, 59, 315–318. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Kuć, J. Lignification as a mechanism for induced systemic resistance in cucumber. Physiol. Plant Pathol. 1982, 20, 61–71. [Google Scholar] [CrossRef]
- Dipierro, S.; De Leonardis, S. The ascorbate system and lipid peroxidation in stored potato (Solanum tuberosum L.) tubers. J. Exp. Bot. 1997, 48, 779–783. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Snyder, J.C.; Desborough, S.L. Rapid estimation of potato tuber total protein content with coomassie brilliant blue G-250. Theor. Appl. Genet. 1978, 52, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.P.; Tabatabai, M.A. Colorimetric determination of reducing sugars in soils. Soil Biol. Biochem. 1994, 26, 473–477. [Google Scholar] [CrossRef]
- Wu, W.; He, K.; Di, H.; Niu, S.; Ma, Y.; Zhang, Z.; Li, Y. Genetic structure and geographic system of geographical population of Pinus tabuliformis mountain range based on SSR in Shanxi Province of northern China. J. Beijing For. Univ. 2018, 40, 51–59. [Google Scholar] [CrossRef]
- Wang, J.; Guo, S.; Zhang, Y.; Zhang, F.; Yun, Y.; Zhang, G. Transcriptome Analysis and Novel EST-SSR Marker Development for Pinus tabuliformis Seedlings from Four Provenances. Forests 2023, 14, 1810. [Google Scholar] [CrossRef]
- Yang, B.; Niu, S.; El-Kassaby, Y.A.; Li, W. Monitoring genetic diversity across Pinus tabuliformis seed orchard generations using SSR markers. Can. J. For. Res. 2021, 51, 1534–1540. [Google Scholar] [CrossRef]
- Hulce, D.; Li, X.; Snyder-Leiby, T.; Johathan Liu, C.S. GeneMarker® Genotyping Software: Tools to Increase the Statistical Power of DNA Fragment Analysis. J. Biomol. Tech. 2011, 22, S35–S36. [Google Scholar] [PubMed Central]
- Peakall, R.; Smouse, P.E. Genalex 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Pierce, S.; Negreiros, D.; Cerabolini, B.E.L.; Kattge, J.; Díaz, S.; Kleyer, M.; Shipley, B.; Wright, S.J.; Soudzilovskaia, N.A.; Onipchenko, V.G.; et al. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Funct. Ecol. 2017, 31, 444–457. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix. 2017. Available online: https://github.com/taiyun/corrplot (accessed on 1 January 2025).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Revell, L.J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, R. Vegan: Community Ecology Package, Version 1.8-1. 2006. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 January 2025).
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P. Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]


| Category | Traits | Unit | Mean | SD | Min | Max | CV(%) |
|---|---|---|---|---|---|---|---|
| Morphological traits | NL | mm | 96.16 | 17.26 | 65.05 | 127.70 | 17.95 |
| NW | mm | 1.43 | 0.13 | 1.21 | 1.69 | 9.40 | |
| NT | mm | 0.86 | 0.07 | 0.75 | 1.00 | 8.17 | |
| NS | mm2 | 357.15 | 91.57 | 203.28 | 551.91 | 25.64 | |
| NV | mm3 | 79.63 | 27.51 | 37.75 | 141.76 | 34.55 | |
| VSN | mm | 0.22 | 0.02 | 0.18 | 0.26 | 9.40 | |
| LMA | g m−2 | 198.65 | 17.33 | 166.78 | 233.67 | 8.72 | |
| Anatomical traits | SN | pcs | 324.25 | 44.70 | 239.80 | 421.96 | 13.79 |
| SD | pcs mm−2 | 44.51 | 6.04 | 34.10 | 57.19 | 13.57 | |
| RCN | pcs | 9.84 | 1.22 | 7.80 | 13.77 | 12.35 | |
| NSP | mm | 3.81 | 0.32 | 3.27 | 4.69 | 8.39 | |
| NSA | mm2 | 0.97 | 0.17 | 0.72 | 1.43 | 17.28 | |
| VBP | mm | 2.02 | 0.20 | 1.68 | 2.48 | 9.83 | |
| VBA | mm2 | 0.29 | 0.06 | 0.20 | 0.43 | 20.14 | |
| YQJB | 3.36 | 0.27 | 2.91 | 4.09 | 8.09 | ||
| Chemical traits | LCC | g kg−1 | 540.38 | 17.49 | 504.37 | 573.99 | 3.24 |
| LNC | g kg−1 | 12.28 | 1.37 | 10.18 | 14.91 | 11.14 | |
| LPC | g kg−1 | 1.31 | 0.15 | 1.06 | 1.68 | 11.65 | |
| LKC | g kg−1 | 7.59 | 1.07 | 5.53 | 9.64 | 14.02 | |
| LC/N | 44.42 | 4.23 | 37.05 | 53.69 | 9.52 | ||
| Physiological traits | LRWC | % | 66.73 | 1.38 | 64.12 | 69.40 | 2.07 |
| CT | mg g−1 | 0.52 | 0.16 | 0.24 | 0.80 | 31.69 | |
| SOD | U g−1 FWh−1 | 692.32 | 71.85 | 604.81 | 814.49 | 10.38 | |
| POD | u g−1 min−1 | 260.95 | 86.31 | 80.00 | 385.08 | 33.07 | |
| MDA | umol g−1 | 17.55 | 4.71 | 9.19 | 23.80 | 26.85 | |
| Pro | ug g−1 | 52.99 | 18.00 | 33.60 | 82.40 | 33.97 | |
| SP | mg g−1 | 12.55 | 2.67 | 7.01 | 18.44 | 21.28 | |
| SS | % | 6.20 | 1.52 | 3.21 | 9.37 | 24.53 |
| Leaf Functional Traits | Blomberg’s K | Pagel’s λ | |||
|---|---|---|---|---|---|
| K | p-Value | λ | p-Value | ||
| Morphological traits | NL | 0.79 | 0.84 | 0.00 | 1.00 |
| NW | 0.79 | 0.83 | 0.00 | 1.00 | |
| NT | 0.80 | 1.00 | 0.00 | 1.00 | |
| NS | 0.78 | 1.00 | 0.00 | 1.00 | |
| NV | 0.78 | 1.00 | 0.00 | 1.00 | |
| VSN | 0.79 | 1.00 | 0.00 | 1.00 | |
| LMA | 0.89 | 0.67 | 0.00 | 1.00 | |
| Anatomical traits | SN | 1.10 | 0.37 | 1.00 | 0.83 |
| SD | 0.86 | 0.68 | 0.00 | 1.00 | |
| RCN | 1.21 | 0.16 | 1.00 | 0.56 | |
| NSP | 0.99 | 0.53 | 0.00 | 1.00 | |
| NSA | 0.96 | 0.68 | 0.00 | 1.00 | |
| VBP | 0.86 | 0.67 | 0.00 | 1.00 | |
| VBA | 0.85 | 1.00 | 0.00 | 1.00 | |
| YQJB | 0.89 | 0.64 | 0.00 | 1.00 | |
| Chemical traits | LCC | 1.08 | 0.34 | 0.83 | 0.90 |
| LNC | 1.20 | 0.33 | 1.00 | 0.57 | |
| LPC | 0.78 | 1.00 | 0.00 | 1.00 | |
| LKC | 0.88 | 0.66 | 0.00 | 1.00 | |
| LC/N | 1.22 | 0.32 | 1.00 | 0.55 | |
| Physiological traits | LRWC | 1.18 | 0.14 | 1.00 | 0.61 |
| CT | 0.78 | 1.00 | 0.00 | 1.00 | |
| SOD | 0.90 | 0.67 | 0.00 | 1.00 | |
| POD | 0.79 | 1.00 | 0.00 | 1.00 | |
| MDA | 0.79 | 1.00 | 0.00 | 1.00 | |
| Pro | 1.20 | 0.35 | 1.00 | 0.58 | |
| SP | 1.06 | 0.72 | 0.00 | 1.00 | |
| SS | 0.85 | 0.68 | 0.00 | 1.00 | |
| Site Name | S (%) | C (%) | R (%) |
|---|---|---|---|
| SM | 89.75 b | 10.25 a | 0.00 |
| HL | 89.92 b | 10.08 a | 0.00 |
| TB | 92.32 a | 7.68 b | 0.00 |
| Site Name | Lng | Lat | ASL (m) | MAP (mm) | MAT (°C) |
|---|---|---|---|---|---|
| SM | E 110°34′3″ | N 38°27′9″ | 1098 | 433.4 | 9.3 |
| HL | E 109°41′37″ | N 35°38′26″ | 1084 | 561.4 | 12.1 |
| TB | E 107°8′35″ | N 34°6′24″ | 1508 | 719.6 | 8.6 |
| Category | Traits | Abbreviation | Unit | Functional Significance |
|---|---|---|---|---|
| Morphological traits | needle length | NL | mm | The longitudinal and radial growth of needles. |
| needle width | NW | mm | ||
| needle thickness | NT | mm | ||
| needle surface area | NS | mm2 | Reflect the drought-resistant ability of plants. | |
| needle volume | NV | mm3 | ||
| the ratio of NV to NS | VSN | mm | ||
| leaf mass per area | LMA | g m−2 | Reflect plant tradeoff strategy. | |
| Anatomical traits | stomata number | SN | pcs | Reflect the plant’s ability to breathe and transpiration. |
| stomatal density | SD | pcs mm−2 | ||
| resin canal number | RCN | pcs | Reflect the transport capacity of secondary metabolites. | |
| needle section perimeter | NSP | mm | Reflect the growth of water and nutrient-carrying tissue of coniferous leaves. | |
| needle section area | NSA | mm2 | ||
| vascular bundle perimeter | VBP | mm | ||
| vascular bundle area | VBA | mm2 | ||
| the ratio of NSA to VBA | YQJB | Photosynthesis and material transport capacity. | ||
| Chemical traits | contents of carbon | LCC | g kg−1 | Comprehensive parameters to characterize plant growth and development. |
| contents of nitrogen | LNC | g kg−1 | ||
| contents of phosphorus | LPC | g kg−1 | ||
| contents of potassium | LKC | g kg−1 | ||
| carbon nitrogen ratio | LC/N | Reflect plant nutrient use efficiency. | ||
| Physiological traits | leaf water content | LRWC | % | Reflect the photosynthetic status of trees. |
| total chlorophyll content | CT | mg g−1 | ||
| superoxide dismutase activities | SOD | U g−1 FWh−1 | Important protective enzymes in plants to remove reactive oxygen species. | |
| peroxidase activities | POD | u g−1 min−1 | ||
| contents of malondialdehyde | MDA | umol g−1 | Measuring the damage to the cell membrane of trees. | |
| contents of proline | Pro | ug g−1 | Important osmoregulatory substances. | |
| soluble protein | SP | mg g−1 | ||
| soluble sugar | SS | % |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Feng, Z.; Zhao, Z. Climatic Adaptability Changes in Leaf Functional Traits of Old Pinus tabulaeformis in Loess Plateau. Plants 2025, 14, 2128. https://doi.org/10.3390/plants14142128
Lei Y, Feng Z, Zhao Z. Climatic Adaptability Changes in Leaf Functional Traits of Old Pinus tabulaeformis in Loess Plateau. Plants. 2025; 14(14):2128. https://doi.org/10.3390/plants14142128
Chicago/Turabian StyleLei, Yuting, Zimao Feng, and Zhong Zhao. 2025. "Climatic Adaptability Changes in Leaf Functional Traits of Old Pinus tabulaeformis in Loess Plateau" Plants 14, no. 14: 2128. https://doi.org/10.3390/plants14142128
APA StyleLei, Y., Feng, Z., & Zhao, Z. (2025). Climatic Adaptability Changes in Leaf Functional Traits of Old Pinus tabulaeformis in Loess Plateau. Plants, 14(14), 2128. https://doi.org/10.3390/plants14142128





