1. Introduction
The study of the flora in Teleorman County is an essential initiative in understanding and preserving the environment [
1]. The county, as an administrative and geographical unit, is home to a surprising diversity of plant species, each with its own characteristics. The aim of this paper is to explore in depth the peculiarities of the flora, highlighting its importance in preserving biodiversity and supporting the sustainable development of the region [
2]. The main purpose of the study of flora is to provide essential data and information for the development, implementation, and management of projects and programs for environmental protection and natural resource management. The study of flora is of major significance, as it is an integral part of the field of environmental engineering, and its main objectives are the conservation of biodiversity, since each plant species is a vital element of the local ecosystem, contributing to its balance and functioning. The registration and documentation of plant species, especially rare or threatened ones, allows the identification of important areas and the development of conservation strategies for their protection [
3,
4,
5,
6,
7,
8,
9]. This ensures that local species do not become extinct and that genetic diversity remains available for the future. The assessment of biodiversity and environmental status through the study of flora supports environmental engineering processes through which elements of local biodiversity can be determined and the monitoring of the state of ecosystems [
5,
10,
11,
12,
13,
14]. This includes identifying the plant species existing in the researched area and assessing their health and diversity. The data obtained can provide insight into changes in the ecosystem and help identify environmental problems, such as habitat degradation or biodiversity loss. Contemporary urban ecosystems are ecosystems with microevolutionary genesis and processes that include a diverse spectrum of different taxonomic groups of plant and animal organisms occupying certain ecological niches of the EU [
4]. The study of protected areas recognizes and incorporates the heterogeneity of landscape mosaics and involves the analysis of context and external threats [
5,
6,
7,
8,
9]. The trends and relevant aspects related to the research of the flora spectrum at present are highlighted in various works that constitute a topic of interest due to the topicality of the works that is ensured by the use of working methods in the fields of botany, phytosociology, forest management, and dendroclimatology that capture a broader spectrum in terms of the dynamics of the development of flora and vegetation over time [
11,
12]. In the literature, it is observed that the emphasis has been placed on the methods of obtaining and processing data [
11]. Research into the knowledge of plant types has significant implications and multiple benefits for environmental conservation, society, and the development of science. It makes important contributions to the understanding, protection, and sustainable use of natural resources [
15,
16,
17,
18,
19]. Knowledge of the floristic composition and structure of vegetation in natural ecosystems is a key element in the conservation and sustainable management of biodiversity and the protection of species and their habitat. Cârlan mentions that vegetation monitoring requires a knowledge of the physiological processes underlying plant development and growth so that the decision-making process in the context of infrastructure management is carried out exhaustively [
14].
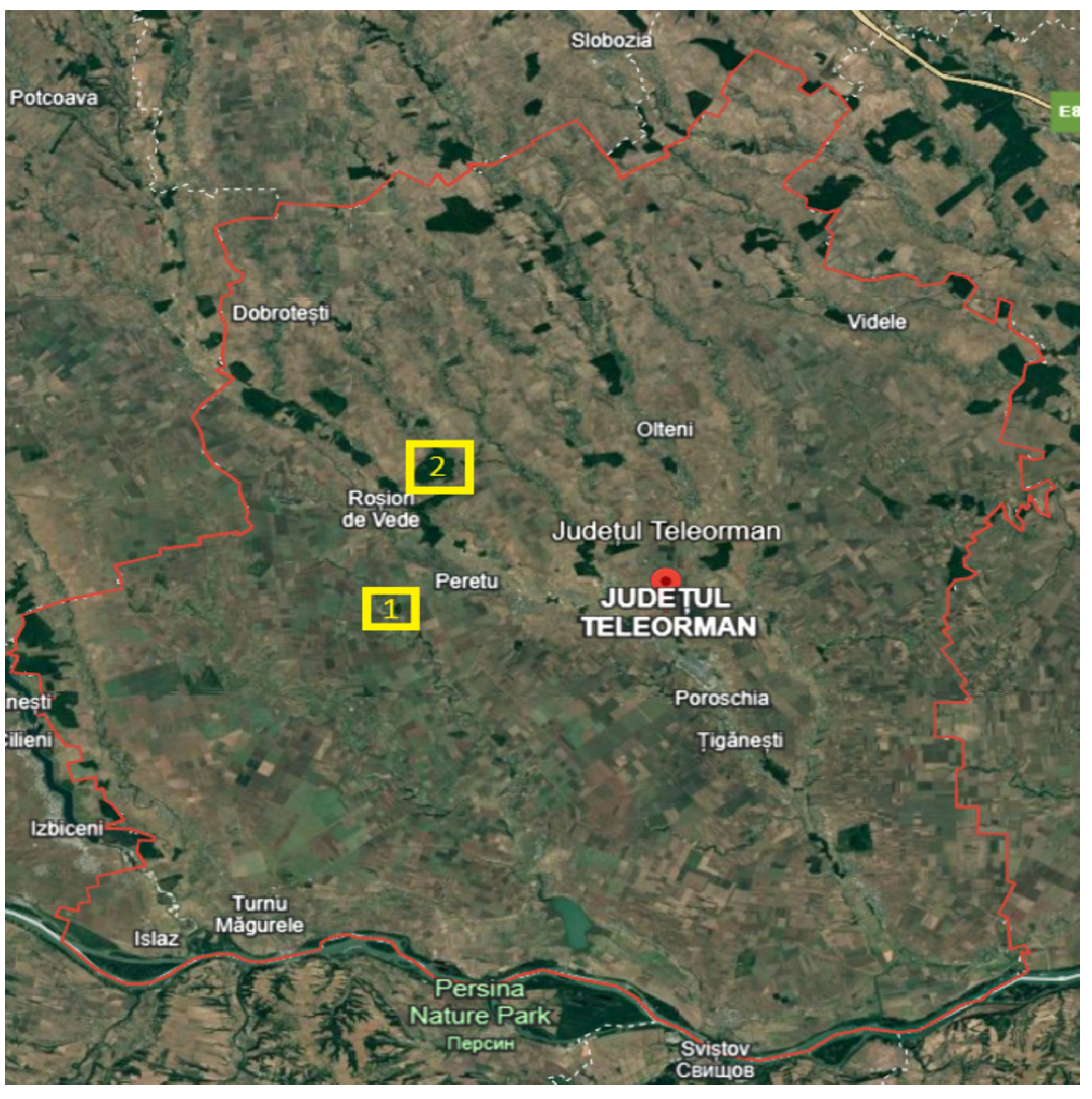
In the researched area there are protected areas such as the Troianul Forest with forest funds, and good management of this area constitutes a continuity of their existence. Forestry aims to return to the natural structures of forest ecosystems, with their self-preserving and self-regulating properties [
11,
12,
20,
21]. In the current stages of research of the flora spectra, as well as in the research area, the natural areas protected by the forest are identified. In this work, the described flora is an integral part of two natural areas: Troianul Forest and Vedere Teleorman Forest, being part of a national ensemble, the Natura 2000 Site (
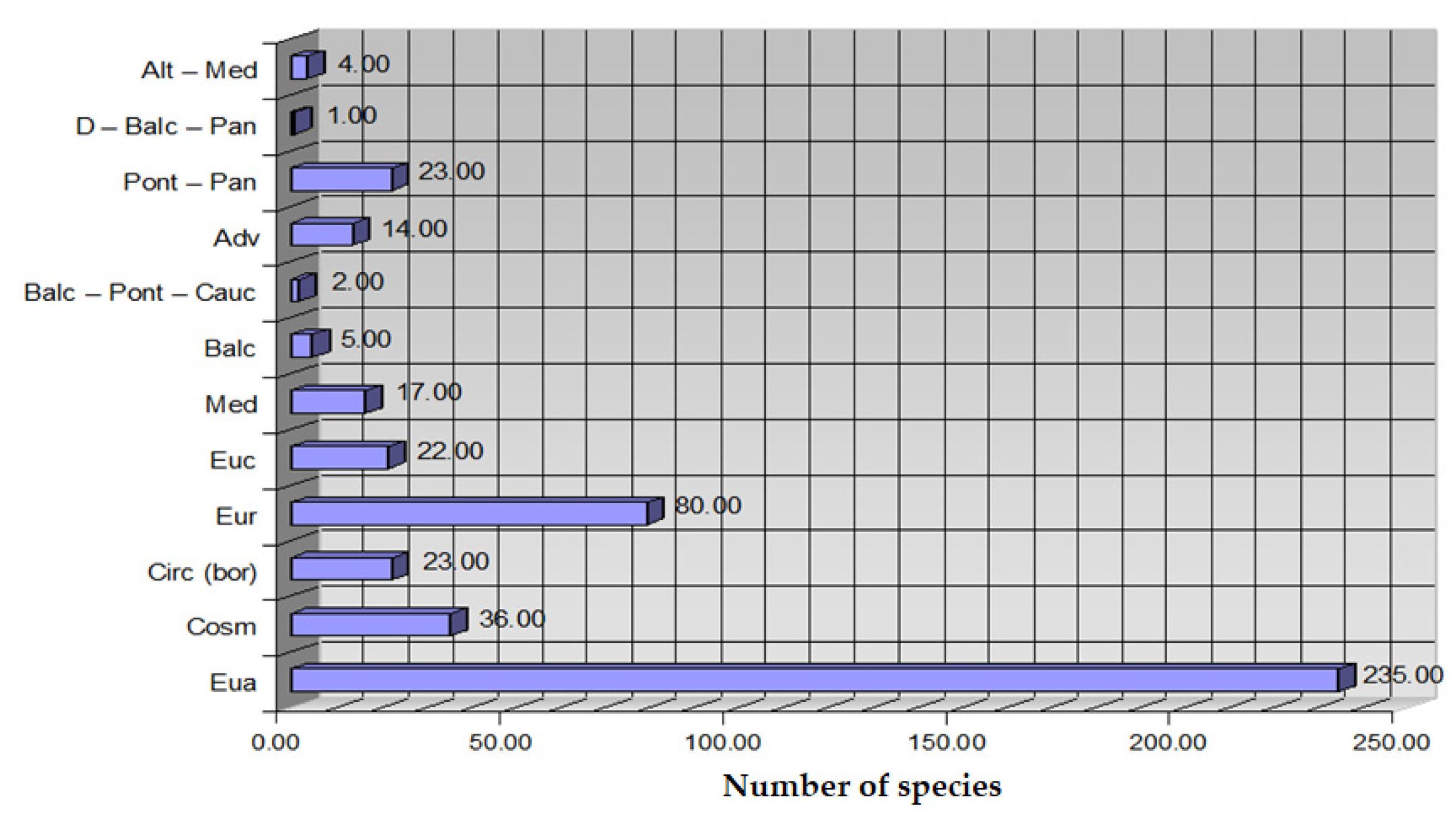
Figure 1) [
22]. The protected areas under study are an integral part of the extensive study of the flora in the entire county, which was also carried out in unprotected areas with increased interest in the flora of these protected areas.
They are intended for the conservation of ecosystems and endangered plants, representing the most widespread tool used in conservation efforts [
17,
23]. The work is intended to be a lever in the scientific support of research, and in the field of biodiversity conservation, bioform analysis is carried out for this purpose. The notion of biodiversity conservation defines a protected area as “a geographically defined area designated or managed for the purpose of achieving specific conservation objectives” [
3,
8,
15,
16,
17,
18,
19]. Studying current research, it is observed that in most protected areas there are many conflicts related to land use, especially in protected areas of flora and fauna. We consider that the incidence of their occurrence is high in developing countries [
24,
25,
26]. Romania’s flora, an integral part of the natural framework, offers a high potential for biodiversity, which has determined that over 23% of the country’s surface is under one type or another of protection [
27,
28,
29,
30,
31,
32]. The research included the identification of the plant species existing in the area and their diversity with the help of establishing the points in the areas of the county by the transect method. Through the study of the flora, the local biodiversity can be evaluated, and the plant species existing in the area and their diversity can be identified. Data obtained by studying the spectrum of biological forms conclude that the geomorphology of the territory, the anthropogenic impact produced on the territory, and the category of land use can provide information about ecosystem changes and help identify environmental problems, such as habitat degradation or biodiversity loss [
32,
33,
34,
35,
36]. This paper aims at a detailed analysis of the flora of Teleorman County by evaluating the diversity of species, the spectrum of bioforms, and their relationship with the main climatic factors: humidity, temperature, and soil reaction. The main objectives are to determine the structure of the flora, to identify the influence of environmental factors on the distribution of species, and to apply the transect method and fractal analysis to understand the complexity and dynamics of local ecosystems. Based on these considerations, the present paper aims to achieve the following main objectives: (1) the detailed characterization of the flora in Teleorman County, (2) the identification and analysis of the distribution of plant bioforms, and (3) the evaluation of the influence of the main climatic factors—such as humidity, temperature, and soil reaction—on the vegetation structure. To achieve these objectives, the research uses a mixed methodological approach, which combines fractal analysis, useful in determining the complexity and spatial distribution of vegetation, with the transect method, which allows the systematic collection of data along representative ecological gradients. Through this approach, this study contributes both to the scientific substantiation of the relationship between climate and the organization of local vegetation, as well as to the development of applicable solutions in the field of biodiversity conservation and sustainable management of regional ecosystems.
3. Materials and Methods
The chosen method of analysis was based on transects in different chosen areas from which plant species were subsequently sampled using systematic determinants. The advantage of the method is that instead of square squares, very elongated squares were used, and the counting of each tracked species was performed on the fly at 3 m from left to right on lengths of 500 m (2500 m2 in total). Going through different layers through layered sampling, we found that there are different levels of soil moisture, temperature, and reactivity. The research methodology was chosen by the author of this study based on the scientific validity of the Ellenberg ecological values and fractal analysis, internationally recognized for the evaluation of the distribution of vegetation in relation to environmental factors. This study was applied in areas of Teleorman County in Romania, such as Troianul Forest, Vedea Forest, Suhaia Pond, pasture areas, and urban areas. The county is a lowland region in the south of the country, characterized by a temperate continental climate and significant anthropogenic influences. The research was carried out between April and August 2022, covering the main growing season to allow for full observation and recording of floristic diversity. These study methods were chosen because of their ability to provide a detailed, quantitative, and comparable assessment of the distribution of species in relation to major environmental factors (temperature, humidity, and soil reaction).
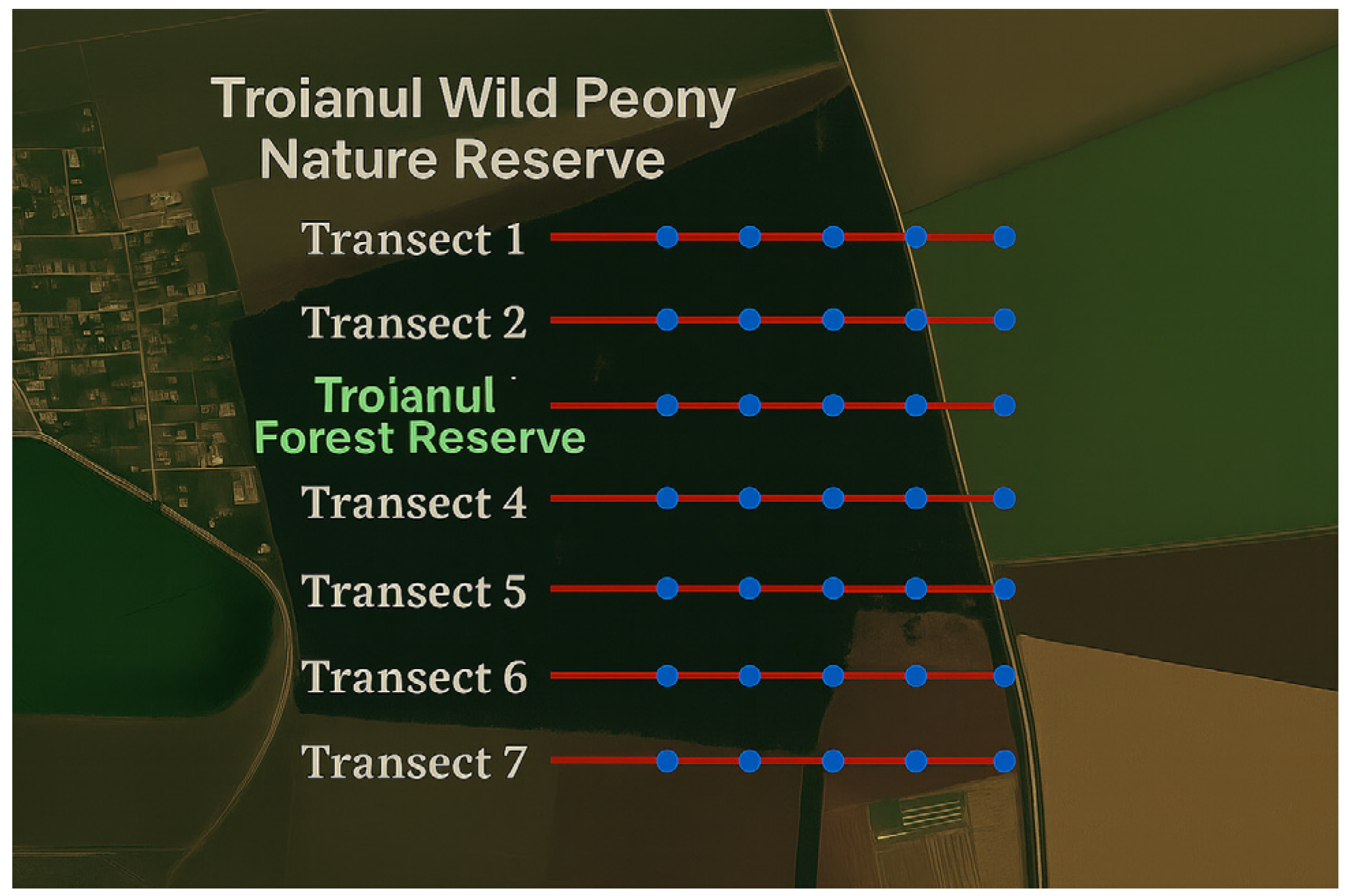
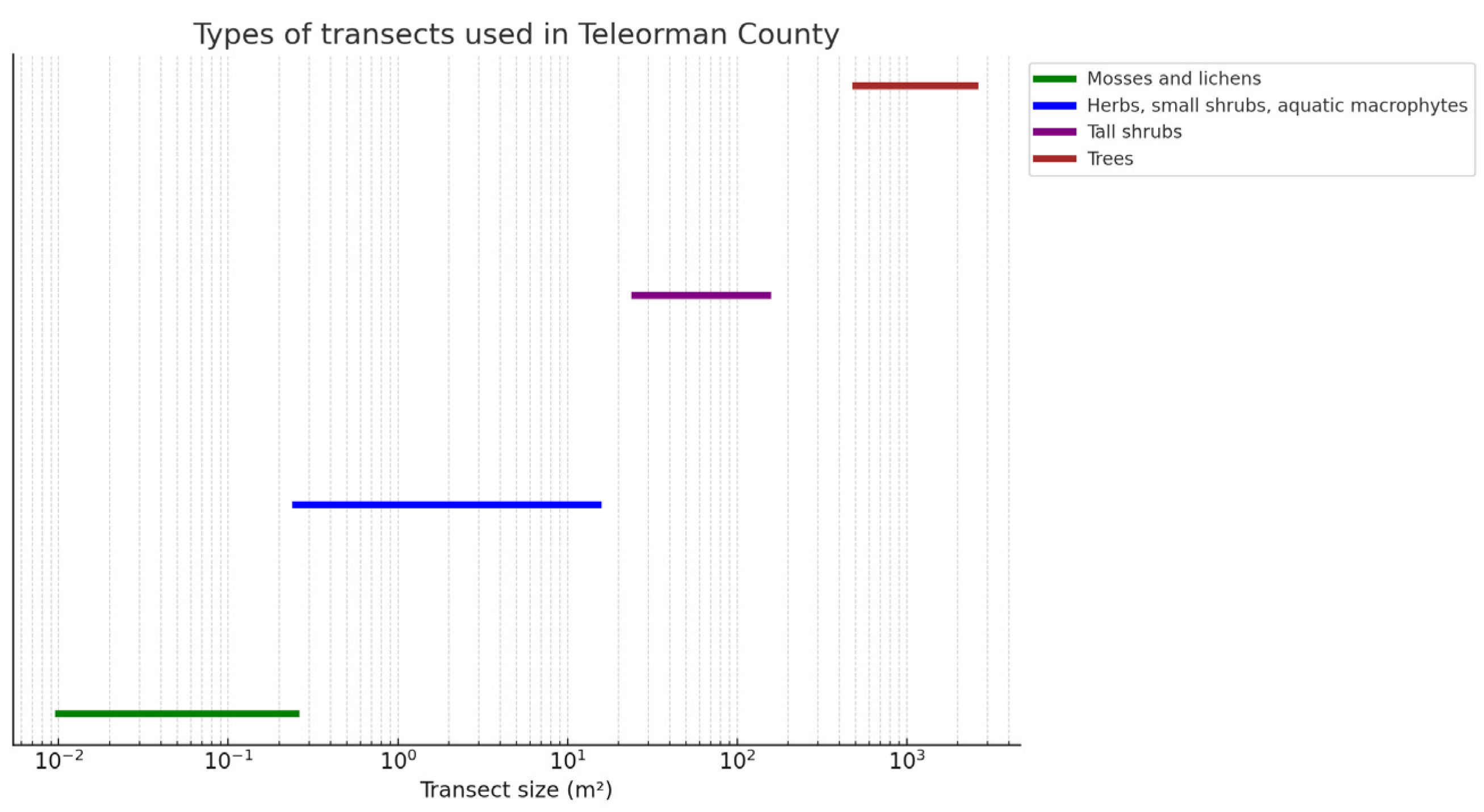
Figure 14 shows the positioning of the transects that were placed along the main lines in one of the studied areas, the Troianul Forest Reserve, proceeding exactly with the other areas that are part of the research. The technique used to determine the plant community for mosses and lichens consisted of changing the dimensions used from transects of 0.01–0.25 m
2 to transects of 0.25–15 m
2 for grasses, small shrubs, and aquatic macrophytes; 25–150 m
2 for tall shrubs; and 500–2500 m
2 for trees, as shown in
Figure 15. The sketch and analysis of the flora in Teleorman County were made based on field observations made in the summer and autumn of 2022.
The transect method was used for the systematic investigation of the flora in Teleorman County by establishing straight lines (transects) drawn on the ground in various types of habitats (forests, meadow areas, agricultural lands, meadows, etc.), along which direct observations and records of the plant species encountered were made. These transects were distributed in such a way as to reflect local ecological variations (soil, exposure, altitude, and humidity) and to allow a balanced sampling of vegetation. The data collected on the transect segments were then analyzed from the point of view of spatial distribution and correlated with fractal analysis, which provided a quantitative interpretation of the degree of aggregation, dispersion, or fragmentation of the identified species. The correlation between the two methods is innovative because transects provide a standardized empirical framework for observations, while fractal analysis turns these data into numerical indicators of ecological complexity. This combined approach allows not only to describe the floristic composition but also to highlight the self-organized structures and natural adaptation strategies of plants according to environmental conditions. The novelty of this method lies in the integrated application of two complementary techniques—one specific to environmental engineering and one mathematical—to obtain a deeper and more rigorous picture of the relationships between flora and environmental factors in a specific territory, contributing to the advancement of research in the field of environmental engineering and biodiversity conservation.
In order to validate the identification of the species, direct field observations were used, supplemented by taxonomic determinations made with the help of reference floristic works, such as “Flora of Romania” and specialized atlases. The correct identification of taxa was supported by consulting updated botanical works and, where necessary, by checking the nomenclature in international databases such as Euro + Med PlantBase and The Plant List. For measurements, such as the analysis of ecological affinities (humidity, temperature, soil reaction), standardized ecological scales (e.g., Ellenberg) and correlations with the climatic and edaphic data available at the regional level were applied. The values obtained were compared with the specialized literature to ensure the consistency and ecological validity of the interpretations. This double validation—botanical and environmental engineering—allowed the solid foundation of subsequent analyses on bioforms, distribution, and relationships with environmental factors.
The application of the Ellenberg ecological scale in the field involved the association of each identified species with the estimated ecological values for the main environmental variables: humidity (F), temperature (T), soil reaction/pH (R), nutrient content (N), and luminosity (L). These values are not measured directly in the field but are assigned on the basis of standardized tables developed by Heinz Ellenberg and subsequently supplemented by other European authors, in which each plant species is classified according to its ecological preferences. In the field, we collected the plants, made the list of plant species present on a certain transect, and then, in the analysis, assigned to each plant species the values of the corresponding Ellenberg scale. The average of these values for each factor allows the indirect estimation of the prevailing environmental conditions in that area. Thus, the method provided a quick and efficient assessment of habitats based on floral composition, without the need for direct measurement of environmental factors.
Steps to obtain ecological values based on the Ellenberg scale are as follows:
Transects were drawn in the field (
Figure 14) in various habitat types of representative of Teleorman County (e.g., forest, meadow, marginal agricultural lands). Direct observations were made on each transect, and the plant species present were recorded.
- 2.
Identification of plant taxa
The species were determined based on morphological characteristics, using specialized flora works, such as “Flora of Romania”, and regional botanical atlases. In ambiguous cases, additional taxonomic determinations were made by consulting the literature and databases (Euro + Med PlantBase, The Plant List).
- 3.
Associating each species with Ellenberg ecological values
For each species identified, the corresponding ecological values were extracted from the standardized Ellenberg tables for the following factors:
F—humidity;
T—temperature;
R—soil reaction (pH).
- 4.
Calculation of averages on each environmental factor
After assigning the values of each species, the arithmetic means for each factor (F, T, R) were calculated, weighted with the frequency of occurrence of the species on the transects. These average values indirectly reflect the environmental conditions in the studied areas.
- 5.
Interpreting the results
The mean values obtained were then classified in the descriptive classes (e.g., mesophyll, mesoxerophil, micromesotherm, weak acid–neutrophil, etc.), according to the standard interpretations in the literature. This step allowed the drafting of ecological conclusions on moisture, temperature, and soil reaction in the analyzed areas.
The application of fractal analysis in this study provides an innovative tool for investigating plant biodiversity through its ability to reflect the complexity and spatial organization of species distribution. This method allows the mathematical quantification of the degree of fragmentation, density, and dispersion of vegetation, thus being able to capture how environmental factors—such as temperature, humidity, and soil pH—directly influence the organization of ecosystems. The calculated fractal size highlights the self-organized structure of vegetation, providing an overview of the ecological relationships between plants and their habitat. In addition, the transect method, used to collect floristic data in the field, ensures systematic sampling of different types of habitats, contributing to the identification of the real distribution of species in relation to ecological variability. The identification of the species was carried out through direct observations and consultation of specialized regional flowers, corroborated taxonomic checks to ensure the accuracy of the data. This integrative approach supports not only the precision of the analysis but also the originality of the scientific approach, offering a complex and rigorous perspective on the local flora and its relations with the environment.
Calculation of Ellenberg ecological values:
To assess the relationship between species and local ecological conditions, the Ellenberg ecological values, recognized at the European level for characterizing plant preferences over environmental factors, were applied. Thus, each identified species was associated with the corresponding numerical values for moisture (F), temperature (T), and soil reaction (R), based on standardized tables in the literature. Subsequently, the weighted average of each ecological factor was calculated, depending on the frequency of occurrence of species on transects. The applied formula is as follows:
where:
Xi = Ellenberg value for the species,
fi = the recurrence of the occurrence of the species on transects,
n = total number of species,
= weighted average of the analyzed factor.
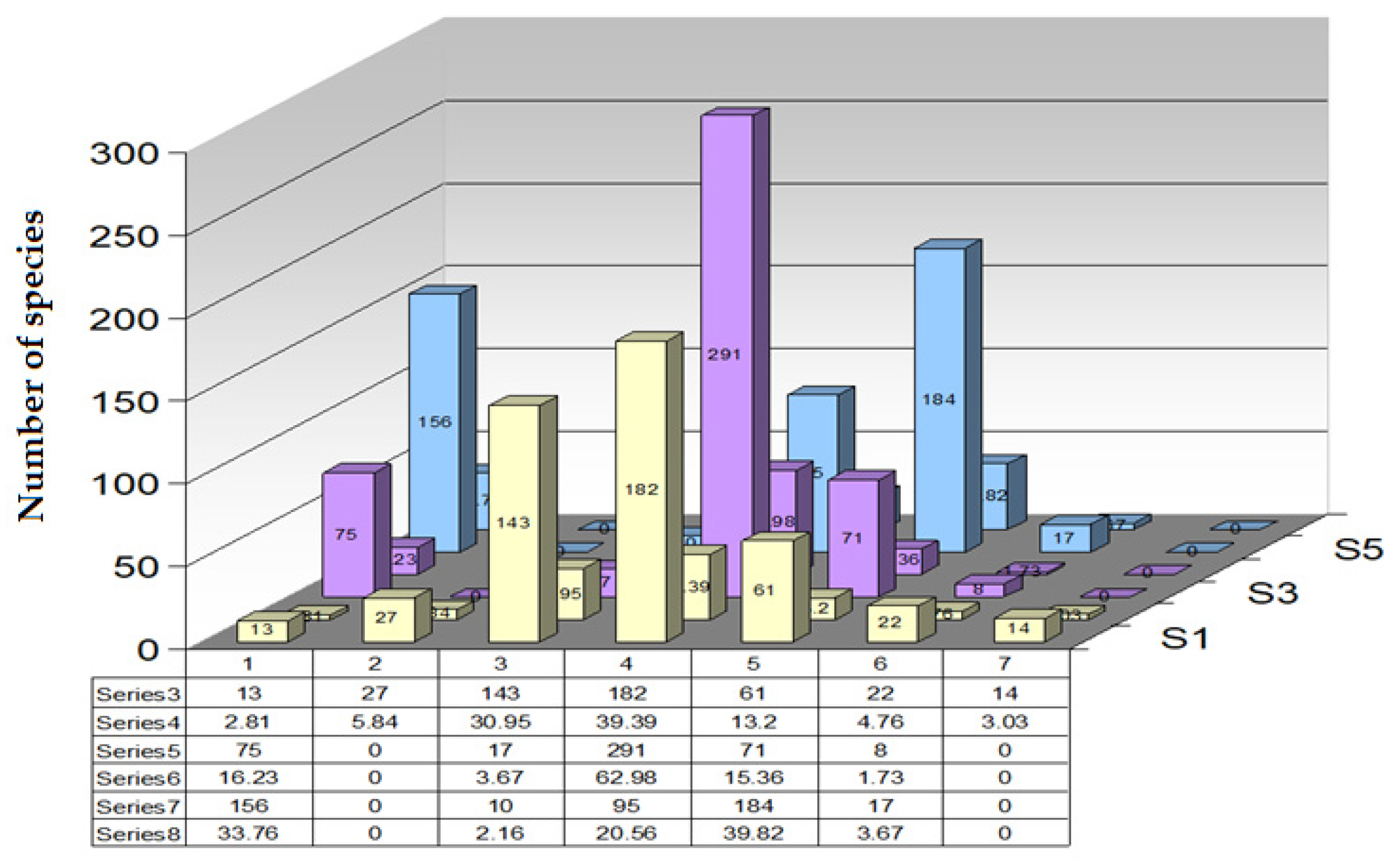
The following 5 dominant species have been identified on a transect:
Plantago lanceolata—F = 5, T = 6, R = 5, frequency: 20 occurrences;
Taraxacum officinale—F = 6, T = 6, R = 6, frequency: 15 occurrences;
Achillea millefolium—F = 4, T = 5, R = 6, frequency: 10 occurrences;
Poa pratensis—F = 6, T = 6, R = 6, frequency: 8 occurrences;
Stellaria media—F = 5, T = 7, R = 7, frequency: 7 occurrences.
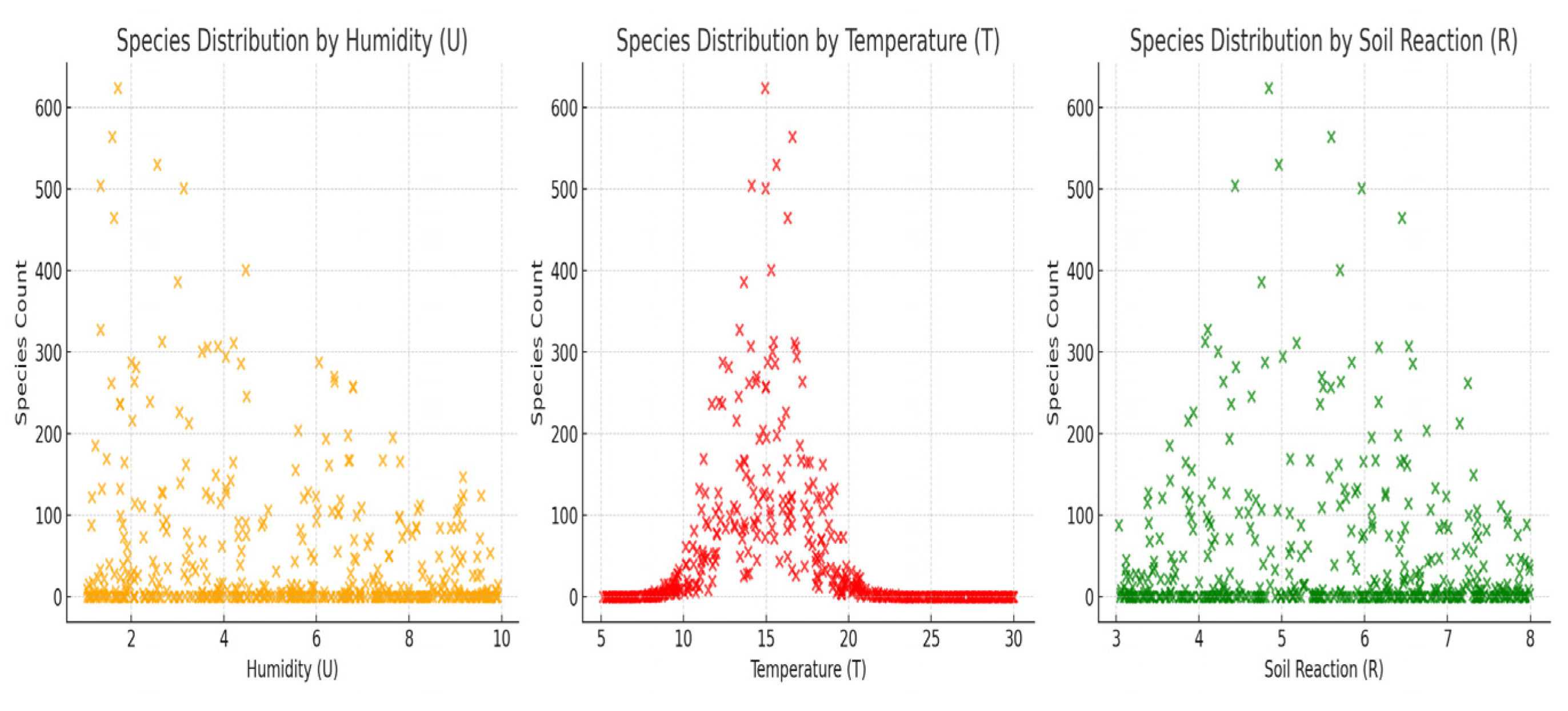
Moisture calculation (F):
For the temperature, the same type of calculation and the soil reaction were applied. The resulting mean for all transects in the county was humidity (F) ≈ 5.08, temperature (T) ≈ 6.08, and soil reaction (R) ≈ 5.92.
These aggregate values confirm the predominance of a mesothermic climate, with moderate humidity and weakly acidic–neutral soils. Soil reaction bioform analysis is a technique used to assess and understand the distribution of plant species in a given ecosystem. This method is based on observing plant species’ preferences for certain soil characteristics, such as pH (acidity or alkalinity), chemical composition, texture, or other properties. The basic steps taken for the analysis of soil reaction bioforms are collecting soil data from the selected transects by collecting samples, recording detailed information about each soil sample, including pH, nutrient content, and relevant characteristics, identifying plant species, noting transects and identified species, classifying species according to soil preferences, analyzing data with the help of statistical analysis and making diagrams, and interpreting results. Full sets of buffers were used, which included solutions for calibration at multiple pH values, such as 4.01, 7.00, and 10.01. These kits provided a wider range of solutions to check and calibrate the pH meter and accurately identify soil reaction as well as the nutritional support characteristics of plants [
43]. For the classification of bioforms according to the moisture index, the plants were weighed before and after drying, going through the following steps: collecting the plants from the transects and weighing them carefully to record their initial weight. Drying the plants at a constant temperature (at 70–80 °C) for several hours to remove all the water, weighing the dried plants again, and calculating the difference in weight to determine the water content. The classification of bioforms according to the temperature index was performed by using existing climate data, such as monthly or annual average temperatures, to identify temperature patterns in the study area. The correlation of climate data with the distribution and behavior of plant species was arranged to determine their thermal preferences. In the analysis of the climatic indices, the Annual Average Temperature Index (MAT), the Annual Thermal Variation Index (AAT), or the Seasonal Temperature Index (TSI) were taken into account to assess the thermal environment of the studied area and the identified plant species. The integration of the three methods—transects, fractal analysis, and the Ellenberg scale [
44,
45]—allowed a complementary and interdisciplinary approach, providing a complex ecological diagnosis that correlates the spatial distribution of vegetation with ecological determinants.
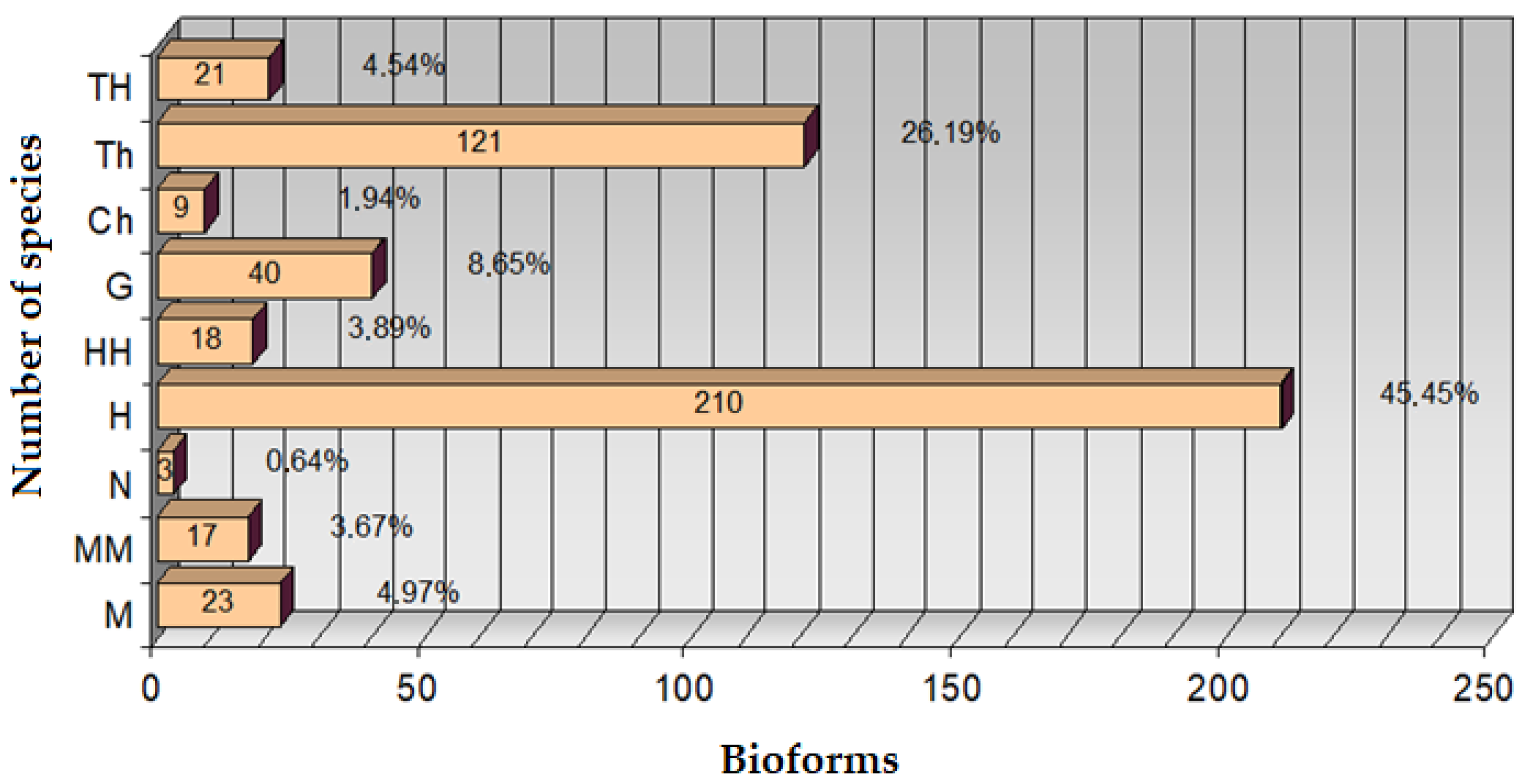
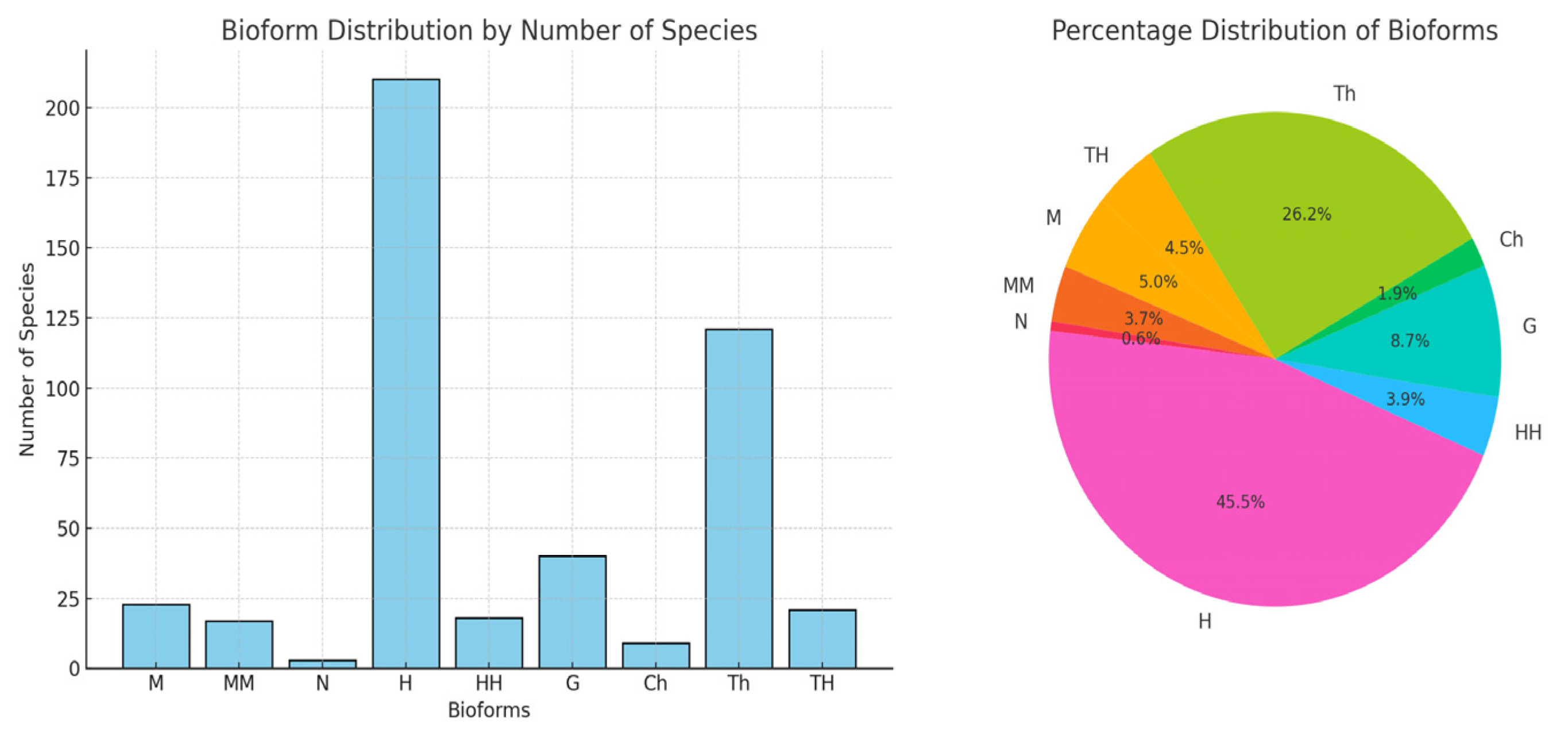
The floral ecosystem in Teleorman is strongly influenced by the thermal factor, which explains the predominance of bioforms adapted to heat stress (hemicryptophytes, therophytes) and the irregular distribution of species, typical for ecologically changing areas. The vulnerability to global warming → flora has a high sensitivity to temperature variations. The results obtained in Teleorman County—where hemicryptophytes (45.45%) and therophytes (26.19%) predominate, and the most frequent Ellenberg values indicate a moderate mesothermic climate (F ≈ 5.08; T ≈ 6.08; R ≈ 5.92)—partially align with, but also contrast with, other research conducted in adjacent or ecologically different regions:
To highlight the ecological specificity of Teleorman County, the results obtained in this study—particularly the average Ellenberg values (F = 5.08; T = 6.08; R = 5.92) and the structure of life forms (hemicryptophytes—45.45%; therophytes—26.19%)—were compared with real data published from other regions of Romania: the Oltenia Plain, the Getic Subcarpathians, and the Danube Delta.
In the case of the Oltenia Plain, the study conducted by Vlăduț, Nikolova, and Licurici (2017) [
46] reveals an increased influence of intensive agriculture and a slightly higher pluviometric regime (F ≈ 5.9), which favors a higher proportion of
therophytes (over 35%) and indicates significant anthropogenic pressure on natural ecosystems. Temperatures are similar to those recorded in Teleorman (T ≈ 6.2), although the soils are slightly more acidic.
In the Getic Subcarpathians, according to the research by Neniu and Vlăduț (2017) [
46], the vegetation is adapted to a significantly more humid (F ≈ 6.7) and cooler climate (T ≈ 5.5), which supports the development of
phanerophytes and
chamaephytes—life forms typical of mountainous and moist areas. This type of vegetation is also tolerant of acidic soils, in contrast to the eurionic flora of Teleorman.
In the Danube Delta, the study by Schneider-Binder (2018) [
47] on aquatic macrophyte communities in the Gorgova–Isac–Uzlina area reveals a dominant presence of hydrophytes, with very high Ellenberg values for moisture (F ≈ 7.5) and a slightly alkaline soil reaction (R ≈ 6.5). These data reflect edaphic and climatic conditions that are entirely different from those found in the southern lowlands of Romania.
Therefore, the comparative analysis confirms that the flora of Teleorman County develops within an intermediate ecological context, characterized by a mesothermal climate, moderate humidity, and slightly acidic to neutral soils (
Figure 16). This ecological configuration supports an adaptive biodiversity, where perennial and annual species coexist and are capable of withstanding seasonal thermal stress and moderate anthropogenic influence. Moreover, the integrated application of the fractal–transect method and Ellenberg values within a unified methodological framework gives this study significant scientific value, complementing the national biodiversity map and providing useful data for conservation strategies in the southern lowland regions of Romania.
The forest of Teleorman County is distinguished by a unique ecological configuration at the national level, the result of the interaction between a mesothermic climate, moderate humidity, and soils with a weak acid–neutral reaction. The present study, through the combined application of fractal analysis and the transect method, highlights not only a high floristic diversity with 462 identified species but also spatial tendencies of self-organization of vegetation clearly correlated with local climatic variables. This integrated approach is rarely found in the Romanian literature, and the calculated fractal dimension offers an innovative perspective on the relationship between bioforms and the environment. Compared to other studied regions (e.g., Getic Subcarpathians, Danube Delta), Teleorman is distinguished by its ecological balance and high adaptability of species, which makes this study a valuable methodological landmark and an original contribution to the assessment of biodiversity in lowland areas subject to climatic and anthropogenic pressures.
3.1. International Connections and Comparative Relevance
The results obtained by using the Ellenberg ecological values allow a direct correlation with studies carried out in other European regions. In Germany and Poland, the average values for moisture are between 6 and 6.5, the temperature is between 5.8 and 6.2, and the soil reaction is around 6–6.3 [
48]. In comparison, Teleorman falls in humidity (F = 5.08) as a moderately dry region, with a temperature (T = 6.08) close to the Central European values and a soil with a weak acid–neutral reaction (R = 5.92), reflecting a flora adapted to a mesothermic and balanced climate. French studies on lowland vegetation indicate values similar to those in Teleorman, which reinforces the comparative relevance of the research.
The international importance of this study lies in the integrated application of fractal analysis and Ellenberg values in an area of the Eastern European plain, rarely studied at this level of detail. This approach provides replicable data in a global context and contributes to the completion of European floristic databases, with the potential to become a methodological benchmark in the monitoring of plant biodiversity on a continental scale.
Numerous international research studies support the application of these methodologies in the assessment of plant biodiversity. Tichý, L., Chytrý, M., et al. (2022) [
49] used Ellenberg indicators for the analysis of plant communities in Germany’s temperate zones, emphasizing the response of species to climatic variations. Grabherr, G., Gottfried, M., and Pauli, H. (2010) [
50] integrated Ellenberg values into studies on the effects of climate change on alpine vegetation in Austria. In Poland, Szymura, M. and Szymura, T. H. (2016) [
51] demonstrated the validity of this method for investigating forest and wetland flora, obtaining data comparable to those in southern Romania. Perring, M. P., Bernhardt-Römermann, M., and Baeten, L. (2018) [
52] applied fractal analysis in fragmented agricultural landscapes in France, highlighting the irregular pattern of species distribution in relation to anthropogenic stressors.
An integrated application of fractal analysis and Ellenberg values in an Eastern European plain area is rarely studied at this level of detail. This approach provides replicable data in a global context and contributes to the completion of European floristic databases, with the potential to become a methodological benchmark in the monitoring of plant biodiversity on a continental scale.
3.2. Comparison Between the Teleorman Study and International Research
This study carried out in Teleorman County is methodologically and thematically aligned with recent floristic research in Central and Western Europe. Similar to the analysis by Tichý et al. (2022) [
49], from Germany, who used Ellenberg values to assess plant communities’ reactions to climate change, the Teleorman study applies the same indicators to determine the influence of soil moisture, temperature, and pH on flora. The values obtained in Teleorman (F = 5.08; T = 6.08; R = 5.92) are placed in the vicinity of those reported by Tichý (F ≈ 6.2; T ≈ 5.8; R ≈ 6.3), which demonstrates similarities in vegetation structure between German mesothermic zones and southern Romania.
In Austria, Grabherr et al. (2010) [
50] highlighted the adaptability of the Ellenberg indicators to alpine ecosystems, and in Poland, Szymura and Szymura (2016) [
51] validated this method for the assessment of forest and riparian flora. However, the Teleorman study makes a unique contribution, as it applies the same methods in an Eastern European lowland region, where the flora is subject to different edaphic and climatic conditions—in particular a lower rainfall regime and a stronger anthropogenic influence.
Moreover, the application of fractal analysis in plant landscapes in France by Perring et al. (2018) [
52] allowed the detection of irregular patterns of species distribution in relation to anthropogenic stress. The Teleorman study takes this approach and adapts it to the lowland environment, demonstrating that temperature is the main factor that determines the complexity and structure of the local vegetation. Thus, the integration of this method in the analysis of the Romanian flora represents a rare and valuable methodological contribution, comparable to the most recent international research trends.
The methodology used combines fractal analysis with Ellenberg values in an Eastern European context poorly represented in the global literature. Detailed floristic traces for 462 species help complement global databases on species distribution and ecological conditions, which are valuable for climate and conservation research at a continental scale. The integration of the fractal dimension for the description of the spatial organization of vegetation is less used in Southeast Europe, and its application on real data in this study can serve as a replicable model for other temperate ecosystems. The results obtained allow international comparability by using a standardized methodology (Ellenberg values), allowing correlation with studies from Germany, Austria, Poland, or France. The conclusions highlight that temperature is the main determinant of the structure of bioforms—a valuable observation in the context of current climate change, providing benchmarks for predictive models in other regions with similar characteristics.