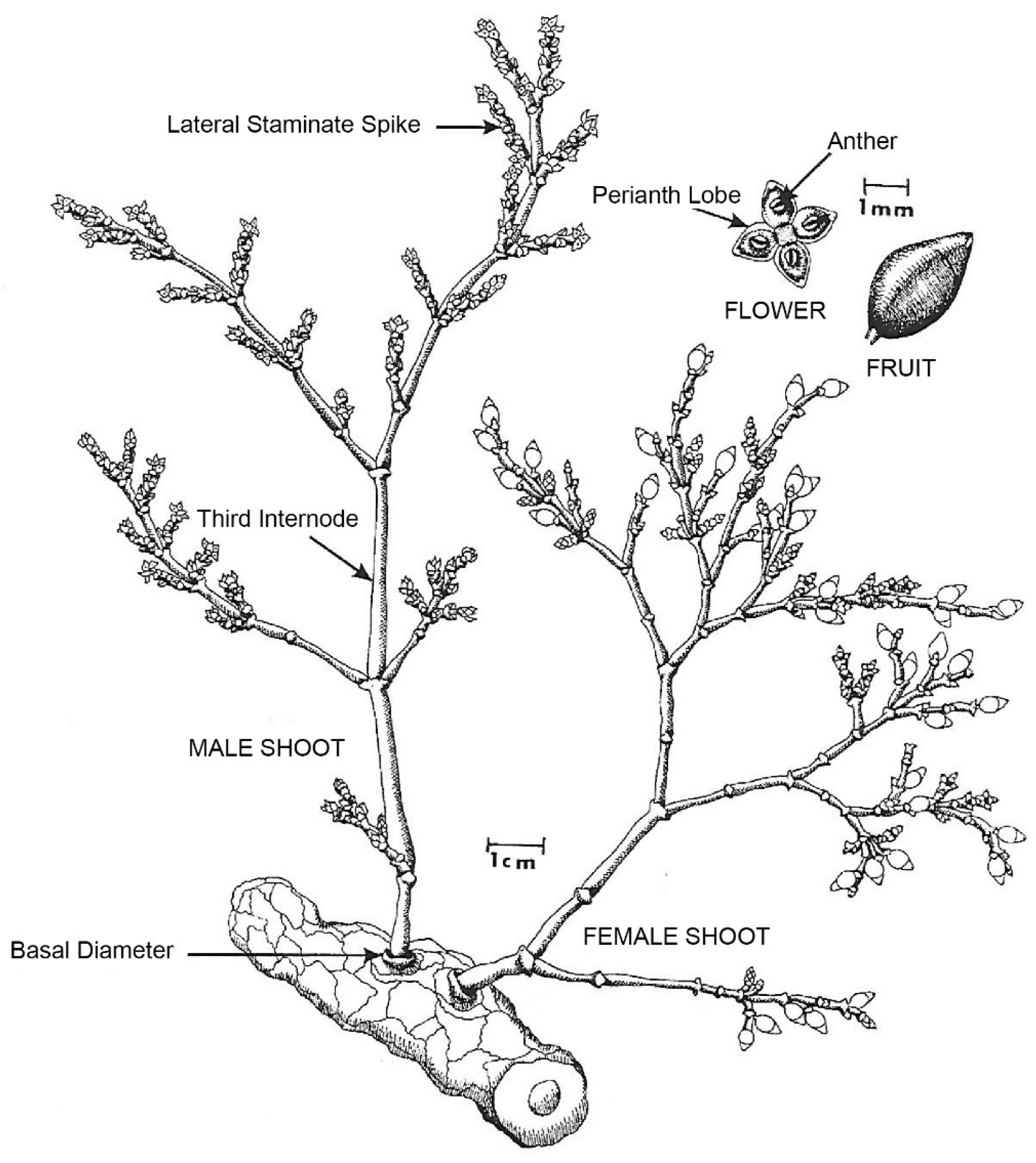
Dwarf Mistletoes (Arceuthobium, Viscaceae) of North America: Classification Systems, Phylogenetic Relationships, and Taxonomic Characteristics
Abstract
1. Introduction
| Species/Subspecies | Section | Description (yr) | Country a | Reference(s) |
|---|---|---|---|---|
| Dept., Prov., or State | ||||
| A. abietinum | Campylopoda | 1872 | Engelmann in Gray [70] | |
| subsp. abietinum | 2019 | USA—CA. | Mathiasen and Kenaley [61] | |
| subsp. grandae | 2020 | USA—CA; OR; WA. | Kenaley [44] | |
| subsp. magnificae | 2019 | USA—CA. | Mathiasen and Kenaley [61] | |
| subsp. mathiasenii | 2020 | USA—AZ; NV; UT. MEX—CH; DG. | Kenaley [44] | |
| subsp. wiensii | 2009 | USA—CA; OR. | Mathiasen and Daugherty [71] | |
| A.abietis-religiosae | Americana | 1923 | MEX—DF; HI; JA; MC; NL; PU; TA; TL. | Heil [72]; Hawksworth and Wiens [1] |
| A. americanum | Americana | 1850 | CAN—AB; BC; MB; ON; SK. USA—CA; CO; ID; MT; NV; OR; UT; WA; WY. | Engelmann in Gray [73]; Hawksworth and Wiens [1] |
| A. apachecum | Campylopoda | 1970 | USA—AZ; NM. MEX—CU. | Hawksworth and Wiens [74]; Kenaley et al. [75] |
| A. bicarinatum | Pusilla | 1912 | DOM—AZ; BH; JU; ST; VE. HTI—OU. | Urban [76]; Hawksworth and Wiens [1] |
| A. blumeri | Campylopoda | 1913 | USA—AZ. MEX—CH; DG; NL; SO. | Nelson [77]; Kenaley et al. [75] |
| A. californicum | Campylopoda | 1970 | USA—CA. | Hawksworth and Wiens [74]; Kenaley et al. [75] |
| A. campylopodum | Campylopoda | 1850 | USA—CA; ID; NV; OR; WA. MEX—BN. | Engelmann in Gray [73]; Mathiasen and Kenaley [39]; Kenaley and Mathiasen [40] |
| A. cyanocarpum | Campylopoda | 1906 | USA—CA; CO; ID; OR; MT; NV; UT; WY. | Rydberg [78]; Coulter and Nelson [79]; Kenaley et al. [75] |
| A. douglasii | Minuta | 1878 | CAN—BC. USA—AZ; CA; CO; ID; MT; NM; NV; OR; TX; UT; WA; WY. MEX-CH; CU; DG; NL. | Engelmann in Wheeler [80]; Hawksworth and Wiens [1] |
| A. divaricatum | Minuta | 1878 | USA—AZ; CA; CO; NM; NV; UT; TX. MEX—BN. | Engelmann in Wheeler [80]; Mathiasen et al. [81] |
| A. gillii | Rubra | 1964 | USA—AZ; NM. MEX—CH; DG; SI; SO. | Hawksworth and Wiens [35]; Kenaley and Mathiasen [54] |
| A. globosum | Globosa | 1965 | Hawksworth and Wiens [35] | |
| subsp. aureum | 2008 | MEX—CS. GTM—AV; BV; CM; QC; ZA. | Mathiasen [53] | |
| subsp. globosum | 2008 | MEX—CH; DG; JA; SO. | Hawksworth and Wiens [35]; Mathiasen [53] | |
| subsp. grandicaule | 2008 | MEX—DF; GE; HD; JA; MC; MR; MX; OA; PU; TL; VC. GTM—CM; HU; SM; TO. HND—LE. | Mathiasen [53] | |
| subsp. petersonii | 2008 | MEX—CS; OA. | Mathiasen [53] | |
| A. guatemalense | Penda | 1970 | MEX—CS; OA. GTM—HU; TO. | Hawksworth and Wiens [74] |
| A. hondurense | Vaginata | 1970 | Hawksworth and Wiens [74]) | |
| subsp. hawksworthii | 1994 | BLZ—CD; HND—OL. | Wiens and Shaw [82]1; Mathiasen [36] | |
| subsp. hondurense | 1970 | MEX—CS; OA; HND—CR; EP; FM; LE; NIC—NS. | Hawksworth and Wiens [74]; Mathiasen [36] | |
| A. laricis | Campylopoda | 1906 | CAN—BC. USA—ID; MT; OR; WA. | Piper [83]; St. John [84]; Mathiasen and Kenaley [42] |
| A. littorum | Campylopoda | 1992 | USA—CA. | Hawksworth et al. [85]; Mathiasen and Kenaley [86] |
| A. microcarpum | Campylopoda | 1878 | Engelmann in Wheeler [80]; Hawksworth and Wiens [74] | |
| subsp. aristatae | 2009 | USA—AZ. | Scott and Mathiasen [37] | |
| subsp. microcarpum | 1878 | USA—AZ; NM. | Engelmann in Wheeler [80]; Hawksworth and Wiens [74] | |
| A. monticola | Campylopoda | 1992 | USA—CA; OR. | Hawksworth et al. [85]; Kenaley et al. [75] |
| A. nigrum | Rubra | 1965 | MEX—CS; DG; GJ; HD; MC; OA; PU; VC; ZA. | Hawksworth and Wiens [35]; Hawksworth and Wiens [48]; Kenaley and Mathiasen [54] |
| A. occidentale | Campylopoda | 1878 | USA—CA. | Engelmann in Wheeler [80]; Mathiasen and Kenaley [86] |
| A. pendens | Penda | 1980 | MEX—PU; SL; VC. | Hawksworth and Wiens [46]; Mathiasen and Daugherty [87] |
| A. pusillum | Pusilla | 1872 | CAN—MB; NB; NL; NS; ON; PE; QC; SK. USA—CT; ME; MA; MI; MN; NH; NJ; NY; PA; RI; VT; WI. | Peck [88]; Hawksworth and Wiens [1] |
| A. rubrum | Rubra | 1965 | MEX—DG; OA; SI. | Hawksworth and Wiens [35]; Mathiasen et al. [52] |
| A. siskiyouense | Campylopoda | 1992 | USA—CA; OR. | Hawksworth et al. [85]; Mathiasen and Kenaley [86] |
| A. strictum | Vaginata | 1965 | MEX—DG. | Hawksworth and Wiens [35] |
| A. tsugense | Campylopoda | 1903 | Rosendahl [89]; Jones [90] | |
| subsp. amabilae | 2007 | USA—OR, WA. | Mathiasen and Daugherty [91]; Mathiasen and Kenaley [43] | |
| subsp. contortae | 2003 | CAN—BC. USA—WA. | Wass and Mathiasen [92]; Mathiasen and Kenaley [43] | |
| subsp. mertensianae | 1992 | USA—CA; OR. | Hawksworth et al. [85]; Mathiasen and Kenaley [43] | |
| subsp. tsugense | 1903 | CAN—BC. USA—AK; CA; OR; WA. | Rosendahl [89]; Jones [90]; Mathiasen and Kenaley [43] | |
| A. vaginatum | Vaginata | 1825 | Berchtold and Presl [93] | |
| subsp. cryptopodum | 1965 | USA—AZ; CO; NM; TX; UT. MEX—CH; CU; SO. | Hawksworth and Wiens [35]; Hawksworth and Wiens [1] | |
| subsp. durangense | 1965 | MEX—DG; JA; SI. | Hawksworth and Wiens [35]; Hawksworth and Wiens [1] | |
| subsp. vaginatum | 1825 | MEX—CH, CU; DF; DG; HD; JA; MX; NA; NL; OA; PU; QE; SI; TA; VC; ZA. | Berchtold and Presl [93]; Hawksworth and Wiens [1] | |
| A. verticilliflorum | Americana | 1880 | MEX—DG. | Engelmann [94]; Hawksworth and Wiens [1] |
| A. yecorense | Rubra | 1989 | MEX—CH; DG; SO. | Hawksworth and Wiens [48] |
2. Species and Subspecies Concepts in Arceuthobium
3. Taxonomic History and Classification of N. American Arceuthobium
3.1. Early Taxonomy: Early 1800s to 1935
3.2. The Host-Form Classification System: 1935–Early 1960s
3.3. Expansion of Species Number and Competing Classification Systems: 1965–Early 2000s
| A. Hawksworth and Wiens [1], excluding Old World taxa. | B. Nickrent et al. [3], excluding Old World taxa |
|
|
3.4. Resolving Species and Subspecies: Early 2000s–Present
4. Phylogenetics of N. American Arceuthobium
5. Taxonomic Characteristics of N. American Arceuthobium
5.1. Section Americana
| Character | Arceuthobium abietis-religiosae a | Arceuthobium americanum b | Arceuthobium verticilliflorum a |
|---|---|---|---|
| Plant color | Olive-green, black variegations with age | Olive-green, yellow–green, yellow | Yellow, yellow–green, purplish |
| Branching habit | Limited verticillate, minus secondary branching | Verticillate, plus secondary branching | Verticillate, minus secondary branching, shoots basal |
| Plant height | ca. 10.0 [---, 16.0] | ca. 5.0-9.0 [---, 30.0] | ca. 7.0 [---, 11.0] |
| Basal diameter | 4.0 [2.0–10.0] | 1,5 [1.0, 3.0] | 3.6 [2.5, 5.0] |
| Third internode length | 15.4 [8.0–24.0] | 12.1 [6.0, 23.0] | 3.0 [2.0, 7.0] |
| Third internode width | 2.8 [1.0–4.0] | 1.2 [1.0, 2.0] | 3.2 [2.5, 4.5] |
| Staminate flowers | |||
| Anthesis | March–April?; Sept.–Oct.? | Early April–early June | March–April |
| Perianth lobes | 3-merous, occasionally 4-merous | 3-merous, occasionally 4-merous | 4-merous, occasionally 3-merous |
| Diameter | --- [2.0, 4.0] | ca. 2.0 [---, ---] | 4.0 [3.5, 4.5] |
| Petal length | ca. 1.5 [---, ---] | ca. 1.1 [---, ---] | ca. 1.0 [---, ---] |
| Petal width | ca. 1.5 [---, ---] | ca. 1.0 [---, ---] | ca. 0.8 [---, ---] |
| Anther diameter | 0.4 [---, ---] | 0.6 [---, ---] | 1.0 [---, ---] |
| Fruit arrangement | ? | Verticillate on short, reflexed pedicels | Verticillate on short, straight pedicels |
| Fruit length | ca. 3.5 [---, ---] | 4.0 [3.5, 4.5] | ca. 15.0 [---, ---] |
| Fruit width | ca. 2.0 [---, ---] | 2.0 [1.5, 2.5] | ca. 10.0 [---, ---] |
| Seed length | ca. 2.2 [---, ---] | ca. 2.4 [---, ---] | ca. 11.0 [---. ---] |
| Seed width | ca. 1.0 [---, ---] | ca. 1.1 [---, ---] | ca. 6.0 [---, ---] |
| Seed dispersal | Sept.?, Oct. or November? | Late Aug.–Sept. | Sept.–Oct. |
| Host susceptibility c | |||
| Principal | Abies religiosa var. religiosa, var. emarginata; A. vejari | Pinus banksiana; P. contorta var. contorta, var. latifolia, var. murrayana | Pinus arizonica; P. cooperi; P. durangensis; P. engelmannii |
| Secondary | Pinus ponderosa var. scopulorum | ||
| Occasional | Pinus albicaulis; P. flexilis; P. jeffreyi; P. ponderosa var. ponderosa | ||
| Rare | Picea engelmannii; P. glauca; P. mariana; P pungens; Pinus aristata; Pseudotsuga menziesii |
5.2. Section Minuta and Section Penda
| Character | Section Minuta | Section Penda | ||
|---|---|---|---|---|
| Arceuthobium divaricatum a | Arceuthobium douglasii b | Arceuthobium guatamalense c | Arceuthobium pendens d | |
| Plant color | Olive-green, green, dark brown | Green-brown (olive) | Green to purple | Light green; base dark green to dark brown with age |
| Plant height | 2.0 [---, 8.0] | --- [1.0, 3.0 systemic; ---, 7.0 non-systemic] | ||
| Female | 10.9 [5.2, 22.7] | 16.0 [7.0, 26.0] | ||
| Male | 11.7 [5.7, 30.4] | 23.0 [16.0, 32] | ||
| Basal diameter | 1.0 [1.0, 1.5] | --- [2.0, 2.5] | 2.0 [1.5, 3.5] | |
| Female | 2.6 [1.3, 5.0] | |||
| Male | 2.5 [1.6, 4.9] | |||
| Third internode length | 3.6 [2.0, 6.0] | 11.4 [8.0, 15.0] | 16.0 [12.0, 20.0] | |
| Female | 11.6 [5.8, 21.9] | |||
| Male | 11.7 [5.5, 21.8] | |||
| Third internode width | ca. 1.0 [---, ---] | 1.7 [1.5, 2.0] | 1.5 [1.0, 2.0] | |
| Female | 1.9 [1.2, 3.2] | |||
| Male | 1.9 [1.2, 3.1] | |||
| Staminate flower—perianth lobes | 3-merous | 3-merous; occasionally 2-, 4-merous | 2- or 3-merous | 3-merous; rarely 4-merous |
| Anthesis | Mid-July–late Sept. | Early March–late June | (May? -) Aug.–early Sept. | July–Sept. |
| Fruit length | 4.4 [3.2, 5.3] | 4.0 [3.5, 4.5] | --- [3.5, 4.0] | 3.4 [2.6, 4.1] |
| Fruit width | 2.6 [1.9, 3.5] | --- [1.5, 2.0] | --- [1.5, 2.0] | 1.8 [1.4, 2.2] |
| Seed length | 2.2 [1.6, 3.1] | 2.4 [---, ---] | ca. 2.0 [---, ----] | 2.3 [1.9, 2.7] |
| Seed width | 1.1 [0.8, 1.3] | 1.1 [---, ---] | ca. 0.8 [---, ---] | 0.9 [0.7, 1.1] |
| Seed dispersal | Late Aug.–late Oct. | Late Aug.–late Sept. | Sept. | June–Sept., early Oct. |
| Character | Section Minuta | Section Penda | ||
|---|---|---|---|---|
| Arceuthobium divaricatum | Arceuthobium douglasii | Arceuthobium guatemalense | Arceuthobium pendens | |
| Systemic infection | No report | Common | Common | Occasional |
| Witches’ broom | Rare | Common | Common | Common |
| Host susceptibility a | ||||
| Principal | Pinus californiarum subsp. californiarum, subsp. fallax; P. cembroides; P. discolor; P. edulis; P. monophyla; P. quadrifolia | Pseudotsuga menziesii | Pinus ayacahuite | Pinus discolor; P. orizabensis (P. cembroides subsp. orizabensis) |
| Secondary | Abies lasiocarpa var. lasiocarpa | |||
| Occasional | Abies amabilis; A. lasiocarpa var. arizonica | |||
| Rare | Abies concolor; A. grandis; A. bifolia; Picea engelmannii; P. pungens. | |||
| Sympatric | Yes | No | ||
5.3. Section Globosa
5.4. Section Pusilla
5.5. Section Rubra
| Character | Arceuthobium gillii a | Arceuthobium nigrum a | Arceuthobium rubrum b | Arceuthobium yecorense c |
|---|---|---|---|---|
| Plant color | Green-brown | Dark brown–green, dark brown, black | Reddish brown, brown–red, dark brown, dark red, nearly black | Yellow–green, brown |
| Plant height | ca. 10.0 [---. 18.0] | ca. 12.0 [---, 17.0] | ||
| Female | 14.2 [7.9, 28.4] | 24.3 [10.3, 53.5] | ||
| Male | 12.3 [7.3, 21.6] | 19.6 [9.3, 37.2] | ||
| Basal diameter | 2.4 [2.0, 3.0] | 3.0 [2.0, 5.0] | ||
| Female | 5.3 [2.9, 8.5] | 7.8 [4.1, 13.1] | ||
| Male | 4.0 [2.4, 6.5] | 7.0 [4.4, 12.5] | ||
| Third internode length | 6.9 [4.0, 12.0] | 15.0 [10.0, 21.0] | ||
| Female | 13.8 [7.8, 22.3] | 16.5 [11.8, 31.8] | ||
| Male | 10.6 [6.1, 16.7] | 16.8 [11.6, 28.7] | ||
| Third internode width | 2.3 [2.0, 3.0] | 2.4 [2.0, 4.0] | ||
| Female | 4.2 [2.6, 6.6] | 5.5 [4.4, 9.6] | ||
| Male | 3.2 [2.1, 5.0] | 4.9 [4.0, 7.8] | ||
| Staminate flowers | ||||
| Anthesis | Mar.–Apr. | Sept.–Jan. | Early July–early to mid-Sept. | June? |
| Color | Green | Red | --- | --- |
| Spike length | 15.3 [6.9, 25.6] | 20.6 [8.1, 33.3] | --- [---, ---] | --- [---, ---] |
| Diameter | --- [1.0, 1.5] | |||
| Diameter 3-merous | 2.8 [2.1, 3.5] | 3.2 [2.7, 4.0] | --- [---, ---] | --- [---, ---] |
| Diameter 4-merous | 3.6 [3.1, 4.5] | 4.8 [3.6, 5.3] | --- [---, ---] | --- [---, ---] |
| Fruit color/glaucous | Proximal portion glaucous, whitish blue | Proximal portion glaucous | Shiny red | ? |
| Fruit length | 5.8 [4.6, 7.2] | 6.9 [5.2, 8.8] | ca. 3.5 [---, ---] | --- [---, ---] |
| Fruit width | 3.6 [2.8, 4.4] | 4.1 [3.4, 5.0] | ca. 2.0 [---, ---] | --- [---, ---] |
| Seed length | 2.8 [2.0, 3.3] | 3.1 (2.7, 3.9] | ca. 2.0 [---, ---] | --- [---, ---] |
| Seed width | 1.4 [1.1, 1.6] | 1.5 [1.3, 1.9] | ca. 1.0 [---, ---] | --- [---, ---] |
| Seed dispersal | Oct. | Sept.–Oct. | Mid-July–early Oct. | Sept.–Oct.? |
| Character | Arceuthobium gillii | Arceuthobium nigrum | Arceuthobium rubrum | Arceuthobium yecorense |
|---|---|---|---|---|
| Host susceptibility a | ||||
| Principal | Pinus herrerai; P. leiophylla, var. chihuahuana (P. chihuahuana); P. lumholtzii | Pinus lawsonii; P. leiophylla var. leiophylla, var. chihuahuana (P. chihuahuana); P. lumholtzii; P. patula; P. pseudostrobus subsp. oaxacana (P. oaxacana); P. teocote | Pinus cooperi; P. devoniana (P. michoacana); P. durangensis; P. engelmannii; P. herrerai; P. lawsonii; P. pseudostrobus; P. teocote | Pinus durangensis; P. herrerai; P. leiophylla, var. chihuahuana (P. chihuahuana); P. lumholtzii |
| Secondary | Pinus engelmannii | |||
| Occasional | Pinus montezumae; P. pseudostrobus | P. pseudostrobus subsp. oaxacana (P. oaxacana) | ||
| Rare | Pinus arizonica var. arizonica; P. cooperi | |||
| Sympatry | A. yecorense | A. rubrum | A. nigrum | A. gillii |
5.6. Section Vaginata
| Character | Arceuthobium hondurense subsp. | Arceuthobium | Arceuthobium vaginatum subsp. | |||
|---|---|---|---|---|---|---|
| hawksworthii a | hondurense b | strictum c | cryptopodum c | durangense c | vaginatum c | |
| Plant color | Green–brown, yellow–green, light green | Olive-brown, grayish green | Pale yellow, brownish | Predominately orange or reddish brown, occasionally nearly black | Bright orange, reddish orange | Dark brown, black, rarely reddish |
| Branching habit | Flabellate | Flabellate | Predominately unbranched | Flabellate | Flabellate | Flabellate |
| Plant height | 16.0 [9.0, 34.0] | 14.0 [---, 21.0] | ca. 7.0 [---, 13.0 | ca. 10.0 [---, 27.0] | ca. 20.0-30.0 [---, 50.0] | ca. 20.0 [---, 55.0] |
| Basal diameter | 3.8 [2.0, 8.0] | 5.0 [3.0, 9.0] | 3.1 [2.5, 4.0] | 4.0 [2.0, 4.5] | 6.0 [4.0, 8.0] | 7.0 [4.0, 20.0] |
| Third internode length | 12.0 [6.0, 21.0] | 9.1 [7.0, 12.0] | 3.6. [1.0, 8.0] | 7.8 [4.0, 16.0] | 17.9 [9.0, 22.0] | 17.4 [5.0, 30.0] |
| Third internode width | 2.8 [1.7, 4.7] | 3.2 [2.5, 4.0] | 2.3 [1.5, 3.5] | 3.1 [2.0, 4.5] | 4.5 [3.5, 6.0] | 5.0 [2.5, 8.5] |
| Staminate flowers | ||||||
| Anthesis | Dec.–Mar. | Aug.–Nov. | Late July–Oct. | May–June | April | Mar.–April |
| Perianth lobes | 3-merous, occasionally 4-merous, rarely 2-merous; internally dark red to green | 3-merous, occasionally 2- or 4-merous; internally dark red | 3- or 4-merous, upwards of 7-merous | 3-merous, occasionally 4-merous | 3-merous, occasionally 4-merous | 3-merous, occasionally 4-merous |
| Flower diameter | 2.8 [2.0-3.6] | ca. 2.5 [---, ---] | ca. 3.0 [---, ---] | 2.7 [2.5, 3.0] | ca. 2.5 [---, ---] | ca. 3.5 [---, ---] |
| Anther diameter | ca. 0.8 [---, ---] | --- [---, ---] | --- [---, ---] | 0.5 [---, ---] | --- [---, ---] | 0.6 [---, ---] |
| Fruit length | 4.6 [3.9, 5.2] | ca. 5.5 [---, ---] | ca. 4.0 [---, ---] | 5.0 [4.5, 5.5] | ca. 7.0 [---, ---] | ca. 5.5 [---, ---] |
| Fruit width | 2.9 [2.3, 3.8] | ca. 3.0 [---, ---] | ca. 2.5 [---, ---] | 2.5 [2.0, 3.0] | ca. 3.5 [---, --] | ca. 3.5 [---, ---] |
| Seed length | 3.0 [2.6, 3.4] | ca. 3.1 [---, ---] | ca. 2.5 [---, ---] | ca. 2.7 [---, ---] | ca. 4.0 [---, ---] | --- [---, ---] |
| Seed width | 1.3 [1.0, 1.6] | ca. 1.5 [---, ---] | ca. 1.0 [---, ---] | ca. 1.1 [---, ---] | ca. 1.5 [---, ---] | --- [---, ---] |
| Seed dispersal | Nov.–Jan. | Sept.–Oct. | Mid-Sept.–Oct. | Late July–early Aug. | Mid-July–Sept. | Aug. |
| Character | Arceuthobium hondurense subsp. | Arceuthobium | Arceuthobium vaginatum subsp. | |||
|---|---|---|---|---|---|---|
| hawksworthii | hondurense | strictum | cryptopodum | durangense | vaginatum | |
| Host susceptibility | ||||||
| Principal | Pinus caribeae var. hondurensis; Pinus oocarpa? | Pinus maximinoi?; P. oocarpa; Pinus tecunumanii (P. oocarpa var. ochoterenai) | P. leiophylla var. chihuahuana (P. chihuahuana) | Pinus arizonica; P. durangensis; P. englemannii; P. ponderosa var. scopulorum | Pinus cooperi; P. devoniana (P. michoacana); P. douglasiana; P. durangensis; P. engelmannii; P. pseudostrobus; P. montezumae | Pinus arizonica; P. cooperi; P. durangensis; P. engelmannii; P. hartwegii; P. lawsonii; P. montenzumae; P. patula; P. rudis |
| Secondary | Pinus tecunumanii (P. oocarpa var. ochoterenai)? | Pinus cooperi | Pinus herrerai | P. teocote | ||
| Occasional | Pinus teocote | Pinus aristata; P. contorta var. latifolia | Pinus oocarpa? | |||
| Rare | Pinus engelmannii | Pinus flexilis; P. strobiformis | P. culminicola | |||
| Sympatry | None | None | None | None | None | None |
5.7. Section Campylopoda
5.7.1. Arceuthobium campylopodum-occidentale Complex
| Character | Arceuthobium campylopodum | Arceuthobium littorum | Arceuthobium occidentale | Arceuthobium siskiyouense |
|---|---|---|---|---|
| Host susceptibility a | ||||
| Principal | Pinus coulteri; P. jeffreyi; P. ponderosa var. ponderosa, var. scopulorum | Pinus muricata; P. radiata | Pinus sabiniana | Pinus attenuata; P. jeffreyi |
| Secondary | Pinus attenuata | Pinus attenuata; P. coulteri | ||
| Occasional | Pinus contorta var. latifoloia, var. murrayana; P. sabiniana | Pinus contorta var. bolanderi | Pinus jeffreyi; P. ponderosa var. ponderosa | Pinus contorta var. contorta |
| Rare | Pinus ponderosa var. ponderosa | |||
| Sympatry | A. occidentale; A. siskiyouense | None | A. campylopodum | A. campylopodum |
| Character | Arceuthobium campylopodum | Arceuthobium littorum | Arceuthobium occidentale | Arceuthobium siskiyouense |
|---|---|---|---|---|
| Plant color | Olive-green, yellow | Brown–green, dark green, yellow–brown | Yellow, yellow–green, straw (males often more yellow than females) | Dark brown, brown–green, red–brown |
| Plant glaucous | Occasionally glaucous | Occasionally glaucous | Often glaucous | Seldom glaucous |
| Plant height | ||||
| Female | 10.4 [3.9, 22.3] | 10.3 [5.1, 18.7] | 10.6 [4.9, 23.2] | 9.1 [4.8, 14.8] |
| Male | 9.7 [3.6, 21.6] | 10.5 [5.4, 22.8] | 10.1 [4.7, 15.6] | 8.2 [4.8, 15.2] |
| Basal diameter | ||||
| Female | 3.4 [1.7, 6.9] | 3.9 [2.4, 6.9] | 3.2 [1.7, 6.0] | 3.0 [1.9, 5.7] |
| Male | 3.2 [1.8, 6.8] | 3.5 [2.5, 5.8] | 3.0 [1.8, 5.4] | 3.1 [1.8, 6.1] |
| Third internode width | ||||
| Female | 2.5 [1.6, 3.7] | 2.6 [1.9, 3.7] | 2.2 [1.3, 3.5] | 2.0 [1.3, 3.1] |
| Male | 2.5 [1.4, 3.6] | 2.7 [1.8, 3.6] | 2.2 [1.4, 3.1] | 2.1 [1.5, 2.9] |
| Staminate flower | ||||
| Anthesis (peak) | Mid-Aug.–late Sept. (late Aug.–mid-Sept.) | Late Aug.–mid-Oct. (mid- to late Sept.) | Oct.–Nov., Dec. (mid-Oct.–mid-Nov.) | Late July–Sept. (mid-Aug.) |
| Spike Length | 12.7 [3.7, 41.0] | 20.6 [6.1, 55.9] | 13.9 [6.2, 33.9] | 11.8 [6.1, 18.3] |
| Spike Width | 3.0 [2.3, 4.2] | 3.4 [2.1, 4.2] | 2.9 [2.2, 3.9] | 2.0 [1.5, 2.6] |
| Diameter 3-merous | 3.1 | 3.5 | 3.0 | 3.2 |
| Diameter 4-merous | 4.2 | 5.2 | 4.1 | 4.5 |
| Diameter 5-merous | None | 5.7 | Rare (n= 1) | None |
| Petal length | 1.6 [0.9, 2.4] | 1.9 [1.0, 2.6] | 1.5 [1.0, 2.3] | 1.5 [0.9, 2.2] |
| Petal width | 1.4 [0.7, 2.4] | 1.6 [0.8, 2.3] | 1.3 [0.9, 2.2] | 1.5 [0.8, 2.1] |
| Anther distance from tip | 0.6 [0.2, 1.1] | 0.9 [0.4, 1.4] | 0.6 [0.2, 1.0] | 0.8 [0.5, 1.1] |
| Fruit color | Light green | Dark green to red | Light green | Light green |
| Fruit glaucous | Lightly glaucous | Lightly glaucous | Highly glaucous | Lightly glaucous |
5.7.2. White Pine Dwarf Mistletoes
| Character | Arceuthobium apachecum | Arceuthobium blumeri | Arceuthobium californicum | Arceuthobium cyanocarpum | Arceuthobium monticola |
|---|---|---|---|---|---|
| Plant color | Yellow–green, green, reddish; males often yellow | Gray, straw, or light green | Green, yellow–green, or yellow; old plants often brown at base | Green, yellow–green, reddish, or near purple | Dark brown to reddish brown, yellow–green |
| Plant height | |||||
| Female | 5.1 [2.6. 10.4] | 10.0 [2.7, 18.4] | 9.9 [5.8, 14.9] | 3.6 [1.7, 6.7] | 8.5 [6.1, 13.4] |
| Male | 3.7 [1.2, 10.6] | 9.8 [2.5, 18.4] | 8.1 [3.8, 14.4] | 2.8 [0.8, 6.3] | 7.8 [4.4, 12.6] |
| Basal diameter | |||||
| Female | 2.1 [1.4, 4.1] | 2.8 [1.6, 4.6] | 3.0 [1.9, 3.8] | 2.0 [1.0, 9.5] | 2.9 [2.1, 3.8] |
| Male | 1.9 [1.1, 3.2] | 2.7 [1.9, 4.1] | 2.7 [1.8, 4.0] | 1.8 [1.0, 3.1] | 2.8 [2.0, 3.5] |
| Third internode length | |||||
| Female | 8.2 [2.5, 17.4] | 11.3 [3.9, 22.1] | 11.5 [7.6, 20.1] | 6.5 [2.3, 11.8] | 11.5 [6.9, 23.0] |
| Male | 5.9 [1.9, 14.0] | 10.9 [4.6, 21.6] | 10.1 [4.4, 17.9] | 5.2 [1.8, 10.3] | 9.9 [4.8, 17.0] |
| Third internode width | |||||
| Female | 1.6 [1.1, 2.4] | 2.0 [1.4, 2.7] | 1.9 [1.5, 2.5] | 1.5 [1.1, 2.3] | 1.7 [1.3, 2.4] |
| Male | 1.6 [1.0, 2.2] | 2.1 [1.6, 2.8] | 1.7 [1.3, 2.4] | 1.5 [0.9, 2.2] | 1.7 [1.3, 2.4] |
| Staminate flowers | |||||
| Anthesis (peak) | Late July–mid-Sept. (mid-Aug.) | Late July–late Aug. (early Aug.) | Mid-June–late July (late June–early July) | Early July–mid-Sept. (mid-July–early Sept.) | Late July–early Sept. (early to mid-Aug.) |
| Spike length | 9.3 [4.5, 19.3] | 13.7 [5.5, 27.8] | 8.7 [4.1, 14.8] | 5.8 [1.6, 11.7] | 8.6 [5.0, 14.2] |
| Spike width | 2.6 [1.4, 4.0] | 2.6 [1.7, 4.1] | 1.8 [1.1, 2.1] | 2.5 [1.1, 3.9] | 1.4 [1.1, 1.8] |
| Diameter 3-merous | 2.7 [1.9, 3.7] | 3.0 [2.4, 4.0] | 2.6 [1.9, 3.4] | 2.6 [1.9, 3.5] | 2.5 [2.0, 3.1] |
| Diameter 4-merous | 3.1 [2.0, 4.4] | 4.1 [2.6, 5.3] | 3.5 [2.5, 4.2] | 2.8 [1.8, 3.8] | 3.6 [2.8, 4.6] |
| Fruit glaucous | Lightly glaucous | Lightly glaucous | Lightly glaucous | Moderate to highly glaucous | Highly glaucous |
| Fruit length | 4.1 [3.0, 5.7] | 4.7 [1.4, 5.9] | 5.1 [4.3, 6.0] | 3.5 [2.5, 4.5] | 4.7 [4.0, 5.6] |
| Fruit width | 2.9 [2.0, 4.0] | 2.8 [1.9, 3.4] | 3.1 [2.5, 3.8] | 2.4 [1.7, 3.2] | 3.0 [2.4, 3.5] |
| Seed length | 2.1 [1.3, 2.9] | 2.2 [1.9, 2.8] | 2.7 [2.0, 4.3] | 1.9 [1.3, 2.5] | 2.5 [1.8, 3.2] |
| Seed dispersal (peak) | Mid-Aug.–Oct. (mid-Sept.) | Mid-Aug.–early Oct. (mid-Sept.) | Mid-Sept.–mid-Oct., rarely early Nov. (early Oct.) | Mid-Aug.–late Sept. (early Sept.) | Late Aug.–early Oct. (early to mid-Sept.) |
| Character | Arceuthobium apachecum | Arceuthobium blumeri | Arceuthobium californicum | Arceuthobium cyanocarpum | Arceuthobium monticola |
|---|---|---|---|---|---|
| Host susceptibility a | |||||
| Principal | Pinus strobiformis | Pinus strobiformis | Pinus lambertiana | Pinus albicaulis; P. aristata; P. flexilis; P. longaeva | Pinus monticola |
| Secondary | Pinus monticola | Picea breweriana | |||
| Occasional | Pinus balfouriana subsp. balfouriana; P. lambertiana? | Pinus lambertiana | |||
| Rare | Pinus contorta var. latifolia; P. ponderosa var. scopulorum; Tsuga mertensiana | ||||
| Sympatry | None | None | None | None | None |
5.7.3. Hemlock and Larch Dwarf Mistletoes
| Character | Arceuthobium tsugense subsp. | |||
|---|---|---|---|---|
| amabilae | contortae | mertensianae | tsugense | |
| Host susceptibility a | ||||
| Principal | Abies amabilis; A. procera; Tsuga mertensiana | Pinus contorta var. contorta | Tsuga mertensiana | Tsuga heterophylla |
| Secondary | Abies lasiocarp | Picea breweriana | ||
| Occasional | Tsuga heterophylla | Tsuga heterophylla | Pinus monticola; Tsuga heterophylla | Abies amabilis; A. procera; Pinus contorta var. contorta; Tsuga mertensiana |
| Rare | Abies grandis; Pinus monticola | Pinus monticola | Abies grandis; Picea engelmannii; P. sitchensis; Pinus monticola; Pseudotsuga menziesii | |
| Sympatry | subsp. mertensianae | subsp. tsugense | subsp. tsugense | subsp. contortae; subsp. mertensianae |
5.7.4. The Fir Dwarf Mistletoes
| Character | Arceuthobium abietinum subsp. | ||||
|---|---|---|---|---|---|
| abietinum b | grandae c | magnificae b | mathiasenii c | wiensii d | |
| Plant color | Yellow, yellow–green | Yellow, yellow–green, green–brown | Yellow, yellow–red–brown, red | Blue–green, yellow–brown, brown, red | Brown–green, red–brown, red |
| Plant height | |||||
| Female | 13.2 [7.4, 24.5] | 11.5 [6.3, 18.6] | 12.2 [6.8, 25.1] | 9.5 [3.1, 17.2] | 9.5 [3.8, 16.1] |
| Male | 12.7 [5.2, 20.5] | 11.3 [5.6, 20.8] | 11.9 [6.2, 19.7] | 9.3 [3.2, 16.5] | 8.9 [3.5, 15.7] |
| Third internode width | |||||
| Female | 2.4 [1.4, 3.6] | 2.0 [1.4, 3.3] | 2.2 [1.6, 4.1] | 2.2 [1.5, 3.3] | 1.9 [1.3, 2.9] |
| Male | 2.4 [1.3, 3.7] | 2.0 [1.3, 3.3] | 2.2 [1.6, 3.2] | 2.2 [1.5, 3.1] | 1.9 [1.4, 2.9] |
| Staminate flowers | |||||
| Spike length | 10.4 [5.1, 16.5] | 9.9 [4.6, 19.8] | 9.4 [3.9, 19.2] | 11.3 [4.9, 26.3] | 8.7 [3.5, 17.0] |
| Spike width | 2.1 [1.4, 2.8] | 2.0 [1.3, 2.8] | 2.0 [1.3, 3.1] | 2.4 [1.6, 3.1] | 1.5 [1.2, 2.0] |
| Diameter 3-merous | 2.8 [2.1, 3.5] | 2.7 [2.1, 3.7] | 2.6 [2.0, 3.7] | 3.1 [2.0, 3.9] | 2.4 [2.0, 2.9] |
| Diameter 4-merous | 3.7 [2.7, 4.8] | 3.7 [2.7, 4.8] | 3.8 [2.6, 5.0] | 4.1 [2.8, 5.2] | 3.2 [2.6, 3.8] |
| Petal length | 1.4 [0.9, 2.0] | 1.4 [1.0, 2.0] | 1.4 [0.8, 2.0] | 1.5 [1.0, 2.0] | 1.2 [0.8, 1.9] |
| Petal width | 1.2 [0.8, 1.6] | 1.2 [0.8, 1.6] | 1.2 [0.7, 1.7] | 1.4 [0.7, 2.0] | 1.0 [0.8, 1.4] |
| Fruit length | 4.9 [3.5, 6.1] | 4.7 [1.3, 6.2] | 4.7 [3.4, 5.9] | 4.7 [3.7, 6.0] | 4.2 [3.1, 5.0] |
| Seed dispersal (peak) | Early Sept.–early Oct. (mid-Sept.) | Late Aug.–early Oct. (mid-Sept.) | Mid-Sept.–late Oct. (late Sept.) | Late Aug.–late Sept. (early Sept.) | Early Sept.–mid-Oct. (late Sept.–early Oct.) |
| Host susceptibility a | |||||
| Principal | Abies lowiana | Abies grandis; A. grandis × A. concolor | Abies magnifica | Abies concolor; A. durangensis | Abies magnifica; Picea breweriana |
| Secondary | |||||
| Occasional | Abies lasiocarpa | Picea mexicana | Abies grandis × concolor | ||
| Rare | Abies amabilis; Pinus contorta var. murrayana; P. lambertiana; P. monticola | Picea engelmannii; Pinus lambertiana | Pinus strobiformis | Pinus monticola | |
| Sympatry | subsp. magnificae | subsp. magnificae | subsp. grandae | None | None |
5.7.5. Western Spruce Dwarf Mistletoes
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawksworth, F.G.; Wiens, D. Dwarf Mistletoes: Biology, Pathology, and Systematics; Agricultural Handbook 709; USDA Forest Service: Washington, DC, USA, 1996; 410p.
- Nickrent, D.L.; Schuette, K.P.; Starr, E.M. A Molecular Phylogeny of Arceuthobium Based upon rDNA Internal Transcribed Spacer Sequences. Am. J. Bot. 1994, 81, 1149–1160. [Google Scholar] [CrossRef]
- Nickrent, D.L.; García, M.A.; Martín, M.P.; Mathiasen, R.L. A Phylogeny of All Species of Arceuthobium (Viscaceae) using Nuclear and Chloroplast DNA sequences. Am. J. Bot. 2004, 91, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Nickrent, D.L.; García, M.A. On the Brink of Holoparasitism: Plastome Evolution in Dwarf Mistletoes (Arceuthobium, Viscaceae). J. Mol. Evol. 2009, 68, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.J.; Leonard, O.A. Physiological Aspects of Parasitism in Mistletoes (Arceuthobium and Phoradendron). I. The Carbohydrate Nutrition of Mistletoe. Plant Physiol. 1964, 39, 996–1007. [Google Scholar] [CrossRef]
- Hull, R.J.; Leonard, O.A. Physiological Aspects of Parasitism in Mistletoes (Arceuthobium and Phoradendron. II. The Photosynthetic Capacity of Mistletoe. Am. J. Bot. 1964, 39, 1008–1017. [Google Scholar] [CrossRef]
- Fisher, J.T. Water Relations of Mistletoes and Their Hosts. In The Biology of Mistletoes; Calder, M., Bernhard, P., Eds.; Academic Press: Sydney, Australia, 1983; pp. 161–184. [Google Scholar]
- Sala, A.; Carey, E.V.; Callaway, R.M. Dwarf Mistletoe Affects Whole-tree Water Relations of Douglas Fir Western Larch Primarily through Changes in Leaf to Sapwood Ratios. Oecologia 2001, 126, 42–52. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Woodruff, D.R.; Shaw, D.C. Integrated Responses of Hydraulic Architecture, Water and Carbon Relations of Western Hemlock to Dwarf Mistletoe Infection. Plant Cell Environ. 2004, 27, 937–946. [Google Scholar] [CrossRef]
- Bickford, C.P.; Kolb, T.E.; Geils, B.W. Host Physiological Condition Regulates Parasitic Plant Performance: Arceuthobium vaginatum subsp. cryptopodum on Pinus ponderosa. Oecologia 2005, 146, 179–189. [Google Scholar] [CrossRef]
- Marias, D.E.; Meinzer, F.C.; Woodruff, D.R.; Shaw, D.C.; Voelker, S.L.; Brooks, J.R.; Lachenbruch, B.; Falk, K.; McKay, J. Impacts of Dwarf Mistletoe on the Physiology of Host Tsuga heterophylla Trees as Recorded in Tree-ring C and O Stable Isotopes. Tree Physiol. 2014, 34, 595–607. [Google Scholar] [CrossRef]
- Weir, J.R. Mistletoe Injury to Conifers in the Northwest; USDA Bull. 360; USDA: Washington, DC, USA, 1916; 39p.
- Korstian, C.F.; Long, W.H. The Western Yellow Pine Mistletoe: Effects on Growth and Suggestions for Control; USDA Bull. 1112; USDA: Washington, DC, USA, 1922; 35p. [CrossRef]
- Gill, L.S. Arceuthobium in the United States. Conn. Acad. Arts Sci. Trans. 1935, 32, 111–245. [Google Scholar]
- Kuijt, J. Dwarf Mistletoes. Bot. Rev. 1955, 21, 569–627. [Google Scholar] [CrossRef]
- Pierce, W.R. Dwarf mistletoe and Its Effects upon the Larch and Douglas-Fir of Western Montana; Univ. Montana Bull. 10 [Forestry Series]; Montana State University Printing Department: Missoula, MT, USA, 1960; 38p. [Google Scholar]
- Hawksworth, F.G.; Wiens, D. Biology and Classification of Dwarf Mistletoes (Arceuthobium); Agric. Handb. 401; USDA For. Serv.: Washington, DC, USA, 1972; 234p.
- Drummond, D.B. Timber Loss Estimates for the Coniferous Forests of the United States Due to Dwarf Mistletoes; USDA For. Serv. Rep 83-2; For. Pest Mgmt., Methods Applications Group: Ft. Collins, CO, USA, 1982. [Google Scholar] [CrossRef]
- Sterner, T.E.; Davidson, A.G. Forest Insect and Disease Conditions in Canada; Canada For. Serv.: Ottawa, ON, Canada, 1982; 46p.
- Hawksworth, F.G.; Shaw, C.G., III. Damage and Loss Caused by Dwarf Mistletoes in Coniferous Forests of Western North America. In Plant Diseases: Infection, Damage, and Loss; Wood, R.K.S., Jellis, G.J., Eds.; Blackwell Scientific: Oxford, England, UK, 1984; pp. 285–297. [Google Scholar]
- Brandt, J.P.; Brett, R.D.; Knowles, K.R.; Sproule, A. Distribution of Severe Dwarf Mistletoe Damage in West-central Canada; Special report1188-741913; Natural Resources Canada: Edmonton, AB, Canada, 1998.
- Geils, B.W.; Hawksworth, F.G. Damage, effects, and importance of dwarf mistletoes. In Mistletoes of North American Conifers; Geils, B.W., Tovar, J.C., Moody, B., Eds.; Gen. Tech. Rep. RM-GTR-98; USA For. Serv.: Washington, DC, USA, 2002; pp. 57–65. [Google Scholar] [CrossRef]
- Parker, T.J.; Clancy, K.M.; Mathiasen, R.L. Interactions among Fire, Insects and Pathogens in Coniferous Forests of the Interior Western United States and Canada. Agric. For. Entomol. 2006, 8, 167–189. [Google Scholar] [CrossRef]
- Cibrián, T.D.; Alvorado, R.D.; García, D.S.E. Enfermedades Forestales en México/Forest Diseases in México; Universidad Autónoma Chapingo: Chapingo, Mexico; CONAFOR-SEMARNAT: Mexico City, Mexico; USDA Forest Service: Washington, DC, USA; NRCAN Forest Service: Ottawa, ON, Canada; Comisión Forestal de America del Norte, COFAN, FAO: Rome, Italy, 2007; 587p.
- Mathiasen, R.L.; Nickrent, D.L.; Shaw, D.C.; Watson, D.M. Mistletoes: Pathology, Systematics, Ecology, and Management. Plant Dis. 2008, 92, 988–1006. [Google Scholar] [CrossRef]
- González-Elizondo, M.; Flores-Villegas, M.Y.; Álvarez-Zagoya, R.; González-Elizondo, M.S.; Márquez-Linares, M.A.; Quiñonez-Barraza, S.; Howell, B.E.; Mathiasen, R.L. Effects of Mexican Dwarf Mistletoe (Arceuthobium vaginatum subsp. vaginatum) on the Growth of Pinus cooperi in Durango, Mexico—A Case Study. For. Path. 2019, 49, e12473. [Google Scholar] [CrossRef]
- Logan, B.A.; Reblin, J.S.; Zonana, D.M.; Dunlavey, R.F.; Hricko, C.R.; Hall, A.W.; Schmiege, S.C.; Butschek, R.A.; Duran, K.L.; Emery, R.J.N.; et al. Impact of Eastern Dwarf Mistletoe (Arceuthobium pusillum) on Host White Spruce (Picea glauca) Development, Growth and Performance across Multiple Scales. Physiol. Plant. 2013, 147, 502–513. [Google Scholar] [CrossRef]
- Kolb, T.E.; Fettig, C.J.; Ayres, M.P.; Bentz, B.J.; Hicke, J.A.; Mathiasen, R.; Stewart, J.E.; Aaron, S.; Weed, A.S. Observed and Anticipated Impacts of Drought on Forest Insects and Diseases in the United States. For. Ecol. Manag. 2016, 380, 321–334. [Google Scholar] [CrossRef]
- Shaw, D.C.; Agne, M.C. Fire and Dwarf Mistletoe (Viscaceae: Arceuthobium species) in Western North America: Contrasting Arceuthobium tsugense and Arceuthobium americanum. Botany 2017, 95, 231–246. [Google Scholar] [CrossRef]
- Tamudo, E.; Camarero, J.J.; Sangüesa-Barreda, G.; Anadón, J.D. Dwarf Mistletoe and Drought Contribute to Growth Decline, Dieback and Mortality of Junipers. Forests 2021, 12, 1199. [Google Scholar] [CrossRef]
- Tinnin, R.O.; Hawksworth, F.G.; Knutson, D.M. Witches’ Broom Formation in Conifers Infected by Arceuthobium spp.: An Example of Parasitic Impact Upon Community Dynamics. Am. Midl. Nat. 1982, 107, 351–359. [Google Scholar] [CrossRef]
- Mathiasen, R.L. Dwarf Mistletoes in Forest Canopies. Northw. Sci. 1996, 70, 61–71. [Google Scholar]
- Shaw, C.; Watson, D.A.; Mathiasen, R.L. Comparison of Dwarf Mistletoes (Arceuthobium spp., Viscaceae) in the Western United States with Mistletoes (Amyema spp., Loranthaceae) in Australia–Ecological Analogs and Reciprocal Models for Ecosystem Management. Aust. J. Bot. 2004, 52, 481–498. [Google Scholar] [CrossRef]
- Kenaley, S.C.; Mathiasen, R.L.; Harner, E.J. Mortality Associated with a Bark Beetle Outbreak in Dwarf Mistletoe-infested Ponderosa Pine Stands in Northern Arizona. West. J. Appl. For. 2008, 22, 113–120. [Google Scholar] [CrossRef]
- Hawksworth, F.G.; Wiens, D. Arceuthobium in Mexico. Brittonia 1965, 17, 213–238. [Google Scholar] [CrossRef]
- Mathiasen, R.L. A New Combination for Hawksworth’s Dwarf Mistletoe (Viscaceae). Novon 2007, 17, 217–221. [Google Scholar] [CrossRef]
- Scott, J.M.; Mathiasen, R.L. Bristlecone Pine Dwarf Mistletoe: Arceuthobium microcarpum subsp. aristatae (Viscaceae), a New Subspecies of Western Spruce Dwarf Mistletoe from Northern Arizona. J. Bot. Res. Inst. Texas 2009, 3, 13–22. [Google Scholar]
- Schneider, A.C.; Sanders, K.M.; Lee, K.; Idec, J.H.; Kenaley, S.C.; Mathiasen, R.L. Plastome and Nuclear Phylogeny of Dwarf Mistletoes (Arceuthobium: Viscaceae). Syst. Bot. 2021, 46, 389–402. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Kenaley, S.C. The Classification of Dwarf Mistletoes (Arceuthobium spp., Viscaceae) in Section Campylopoda, Series Campylopoda. Botany 2021, 100, 1–31. [Google Scholar] [CrossRef]
- Kenaley, S.C.; Mathiasen, R.L. Perspectives on the Reclassification of Taxa in the Arceuthobium campylopodum Complex (Viscaceae). Botany 2024, 102, 72–97. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Elizondo, M.S.G.; Elizondo, M.G.; Howell, B.E.; Enriquez, I.L.L.; Scott, J.; Flores, J.A.T. Distribution of Dwarf Mistletoes (Arceuthobium spp., Viscaceae) in Durango, Mexico. Madroño 2008, 55, 161–169. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Kenaley, S.C. A Morphometric Analysis of Arceuthobium campylopodum, A. laricis, and A. tsugense (Viscaceae). Phytologia 2015, 97, 200–218. [Google Scholar]
- Mathiasen, R.L.; Kenaley, S.C. Arceuthobium tsugense (Viscaceae): Four Subspecies with Contrasting Morphologies and Host Distributions. J. Bot. Res. Inst. Texas 2017, 11, 363–390. [Google Scholar] [CrossRef]
- Kenaley, S.C. New Subspecies of Fir Dwarf mistletoe (Arceuthobium abietinum, Viscaceae) from the Western United States and Northern Mexico. J. Bot. Res. Inst. Texas 2020, 14, 27–45. [Google Scholar] [CrossRef]
- Hawksworth, F.G.; Wiens, D. Arceuthobium (Viscaceae) in Mexico and Guatemala: Additions and Range Extensions. Brittonia 1977, 29, 411–418. [Google Scholar] [CrossRef]
- Hawksworth, F.G.; Wiens, D. A New Species of Arceuthobium (Viscaceae) from Central Mexico. Brittonia 1980, 32, 348–352. [Google Scholar] [CrossRef]
- Hawksworth, F.G.; Wiens, D. Biology and Classification of Arceuthobium: An Update. In Biology of Dwarf Mistletoes: Proceedings of the Symposium; Gen. Tech. Rep. RM-111; USDA For. Serv.: Washington, DC, USA, 1984; pp. 2–17. [Google Scholar]
- Hawksworth, F.G.; Wiens, D. Two New Species, Nomenclatural Changes, and Range Extensions in Mexican Arceuthobium (Viscaceae). Phytologia 1989, 66, 5–11. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Parks, C.G.; Geil, B.W.; Beatty, J.S. Notes on the Distribution, Host Range, Plant Size, Phenology, and Sex Ratio of Two Rare Dwarf Mistletoes from Central America: Arceuthobium hawksworthii and A. hondurense. Phytologia 1998, 84, 154–164. [Google Scholar]
- Mathiasen, R.; Parks, C.; Nickrent, D.; Beatty, J.; Geils, B. Status of Dwarf Mistletoes in Central America. In Proceedings of the Forty-Eighth Annual Western International Forest Disease Work Conference, Waikoloa, HI, USA, 14–18 August 2000; pp. 78–92. [Google Scholar]
- Mathiasen, R.L.; Melgar, J.; Beatty, J.; Parks, C.; Nickrent, D.L.; Sesnie, S.; Daugherty, C.; Howell, B.; Garnett, G. New Distributions and Hosts for Mistletoes Parasitizing Pines in Southern Mexico and Central America. Madroño 2003, 50, 115–121. [Google Scholar]
- Mathiasen, R.L.; Daugherty, C.M.; Reif, B.P. Arceuthobium rubrum (Viscaceae) in Mexico. Madroño 2009, 56, 99–103. [Google Scholar] [CrossRef]
- Mathiasen, R.L. New Combinations for Arceuthobium aureum (Viscaceae) in Mexico and Central America. Novon 2008, 18, 501–507. [Google Scholar] [CrossRef]
- Kenaley, S.C.; Mathiasen, R.L. Arceuthobium gillii and A. nigrum revisited: Distribution, Morphology, and rDNA-ITS Analysis. J. Bot. Res. Inst. Texas 2013, 7, 311–322. [Google Scholar]
- Mathiasen, R.; Sediles, A.; Sesnie, S. First Report of Arceuthobium hondurense and Struthanthus deppeanus in Nicaragua. Plant Dis. 2006, 90, 1458. [Google Scholar] [CrossRef] [PubMed]
- Norton, D.A.; Carpenter, M.A. Mistletoes as Parasites: Host Specificity and Speciation. Tree 1998, 13, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Kuijt, J. A Systematic Study of Branching Patterns in Dwarf Mistletoe (Arceuthobium). Mem. Torrey Bot. Club 1970, 22, 1–38. [Google Scholar]
- Baker, F.A.; French, D.W. Note on the Biology of Arceuthobium pusillum. Can. J. For. Res. 1979, 9, 544–545. [Google Scholar] [CrossRef]
- Atsatt, P.R. Host-parasite Interactions in Higher Plants. In Physiological Plant Physiology III, Encyclopedia of Plant Physiology; Lange, O.L., Nobel, P.S., Osmond, C., Ziegler, H.C., Eds.; Springer: Berlin, Germany, 1983; Volume 12, pp. 519–531. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Kenaley, S.C. The Classification of California Viscaceae: An Alternative Perspective. Madroño 2016, 63, 8–33. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Kenaley, S.C. A Morphological Comparison of Arceuthobium abietinum and A. campylopodum (Viscaceae) and Nomenclatural Changes for A. abietinum. J. Bot. Res. Inst. Texas 2019, 13, 83–101. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Kenaley, S.C.; Scott, J.M. Arceuthobium microcarpum (Viscaceae): Morphological Evidence for Continued Species Recognition and Discrimination from Arceuthobium campylopodum. Phytologia 2018, 100, 71–90. [Google Scholar]
- Nickrent, D.L. Molecular systematics. In Dwarf mistletoes: Biology, Pathology, and Systematics; Hawksworth, F.G., Wiens, D., Eds.; Agric. Handb. 709; USDA For. Serv.: Washington, DC, USA, 1996; pp. 155–172. [Google Scholar]
- Kuijt, J. Review of Biology and Classification of Dwarf Mistletoes (Arceuthobium) by Hawksworth, F.G., and Wiens, D. Madroño 1973, 22, 34–35. [Google Scholar]
- Kuijt, J. Viscaceae. In The Jepson Manual: Vascular Plants of California; Baldwin, B.G., Goldman, G.D., Keil, D.K., Patterson, R., Rosatii, T.J., Wilken, D.H., Eds.; University of California Press: Berkeley, CA, USA, 2012; pp. 600–603. [Google Scholar]
- Nickrent, D.L. Justification for Subspecies in Arceuthobium campylopodum (Viscaceae). Phytoneuron 2012, 51, 1–11. [Google Scholar]
- Nickrent, D.L. Viscaceae Batch–Christmas Mistletoe Family. In Flora of North America; Flora of North America Editorial Committee, Ed.; Oxford University Press: New York, NY, USA, 2016; Volume 12, pp. 422–440. [Google Scholar]
- Nickrent, D.L. Genetic Polymorphism in the Morphologically Reduced Dwarf Mistletoes (Arceuthobium, Viscaceae): An Electrophoretic Study. Am. J. Bot. 1986, 73, 1492–1502. [Google Scholar] [CrossRef]
- Reif, B.P.; Mathiasen, R.L.; Kenaley, S.C.; Allan, G.J. Genetic Structure and Morphological Differentiation of Three Western North American Dwarf Mistletoes (Arceuthobium: Viscaceae). Syst. Bot. 2015, 40, 191–207. [Google Scholar] [CrossRef]
- Gray, A. Six hundred and forty-first meeting. February 13, 1872. Adjourned Stated Meeting; Botanical Contributions. Proc. Am. Acad. Arts Sci. 1872, 8, 401. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Daugherty, C.M. Arceuthobium abietinum subspecies wiensii, a New Subspecies of Fir Dwarf Mistletoe (Viscaceae) from Northern California and Southern Oregon. Madroño 2009, 56, 118–126. [Google Scholar] [CrossRef]
- Heil, H. Die Bedeutung des Haustoriums von Arceuthobium. Centralbl. Bakteriol. Protozool. Para-Sitenk. u. Infektionskr. Abt. 2 1923, 59, 26–55. [Google Scholar]
- Gray, A. Plantae Lindheimerianae. Part II. Boston J. Nat. Hist. 1850, 6, 141–240. [Google Scholar]
- Hawksworth, F.G.; Wiens, D. New Taxa and Nomenclatural Changes in Arceuthobium (Viscaceae). Brittonia 1970, 22, 265–269. [Google Scholar] [CrossRef]
- Kenaley, S.C.; Mathiasen, R.L.; Daugherty, C.M. Morphological Evidence for Continued Species Recognition among White Pine Dwarf Mistletoes (Viscaceae): Arceuthobium apachecum, A. blumeri, A. californicum, A. cyanocarpum, and A. monticola. J. Bot. Res. Inst. Texas 2016, 10, 361–383. [Google Scholar]
- Urban, I. Symbolae Antillanae: Fundamenta Florae Indiae Occidentalis; Borntrager, F.: Berlin, Germany; Klinchsieck, P.: Paris, France, 1912; Volume 7, pp. 204–205. [Google Scholar]
- Nelson, A. Contributions from the Rocky Mountain Herbarium. XIII. Bot. Gaz. 1913, 56, 65. [Google Scholar] [CrossRef]
- Rydberg, P.A. Flora of Colorado; Bull. Colorado Agric. Exp. Sta. 100; Agric. Exp. Sta. Colorado Agric. College: Ft. Collins, CO, USA, 1906; p. 101. [Google Scholar]
- Coulter, J.M.; Nelson, A. New Manual of Botany of the Central Rocky Mountains (Vascular Plants); American Book Company: New York, NY, USA, 1909; p. 146. [Google Scholar] [CrossRef]
- Wheeler, G.M. Report upon United States Geological Surveys West of the One hundredth Meridian; Volume VI: Botany; U.S. Government Printing Office: Washington, DC, USA, 1878; pp. 253–254.
- Mathiasen, R.L.; Kenaley, S.C.; Daugherty, C.M. A Morphometric Analysis of Arceuthobium campylopodum and Arceuthobium divaricatum (Viscaceae). Aliso 2016, 34, 9–23. [Google Scholar] [CrossRef]
- Wiens, D.; Shaw III, C.G. Arceuthobium hawksworthii (Viscaceae), a New Species of Dwarf Mistletoe from Belize. J. Idaho Acad. Sci. 1994, 30, 25–32. [Google Scholar]
- Piper, C.V. Flora of the State of Washington; Government Printing Office: Washington, DC, USA, 1906; Volume 11. [CrossRef]
- St. John, H. Flora of Southeast Washington and of Adjacent Idaho; Students Book Corporation: Pullman, WA, USA, 1937; p. 115. [Google Scholar]
- Hawksworth, F.G.; Wiens, D.; Nickrent, D.L. New Western North American Taxa of Arceuthobium (Viscaceae). Novon 1992, 2, 204–211. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Kenaley, S.C. A Morphometric Analysis of Dwarf Mistletoes in the Arceuthobium campylopodum-occidentale Complex (Viscaceae). Madroño 2015, 62, 1–20. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Daugherty, C.M. Additional Taxonomic Studies of Arceuthobium pendens (Viscaceae): A Rare Dwarf Mistletoe from Central Mexico. Madroño 2006, 53, 69–71. [Google Scholar] [CrossRef]
- Peck, C.H. Report of the Second Class, in the Second Department (Botany). Trans. Albany Inst. 1872, 7, 191. [Google Scholar]
- Rosendahl, C.O. A New Species of Razoumofskya. Minn. Bot. Stud. 1903, 3, 271–273. [Google Scholar]
- Jones, G.N. A Botanical Survey of the Olympic Peninsula; Univ. Wash. Publ. Biol.: Seattle, WA, USA, 1936; Volume 5, p. 139. [Google Scholar]
- Mathiasen, R.L.; Daugherty, C.M. Arceuthobium tsugense subsp. amabilae, a New Subspecies of Hemlock Dwarf Mistletoe (Viscaceae) from Oregon. Novon 2007, 17, 222–227. [Google Scholar] [CrossRef]
- Wass, E.F.; Mathiasen, R.L. A New Subspecies of Hemlock Dwarf Mistletoe (Arceuthobium tsugense subsp. contortae, Viscaceae) from British Columbia and Washington. Novon 2003, 13, 268–276. [Google Scholar] [CrossRef]
- Berchtold, F.; Presl, J.S. O Přirozenosti Rostlin Aneb Rostlinář; K.W. Enders: Prague, Czech Republic, 1825; Volume 2, p. 28. [Google Scholar]
- Engelmann, G. Loranthaceae. In Geological Survey of California, Botany; Watson, S., Ed.; John Wilson and Son, University Press: Cambridge, MA, USA, 1880; Volume 2, pp. 104–107. [Google Scholar]
- de Queiroz, K. Species Concepts and Species Delimitation. Syst. Biol. 2007, 56, 879–886. [Google Scholar] [CrossRef]
- Mathiasen, R.L. Taxonomic Studies of Dwarf Mistletoes (Arceuthobium spp.) Parasitizing Pinus strobiformis. Great Basin Natur. 1982, 42, 120–127. [Google Scholar]
- Tiehm, A. New Combinations for the Flora of Nevada, U.S.A. J. Bot. Res. Inst. Tex. 2023, 17, 21–29. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. (Eds.) International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017; Regnum Vegetabile 159; Koeltz Botanical Books: Glashütten, Germany, 2017. [Google Scholar] [CrossRef]
- Soltis, P.S.; Soltis, D.E. The Role of Hybridization in Plant Speciation. Annu. Rev. Plant Biol. 2009, 60, 561–588. [Google Scholar] [CrossRef] [PubMed]
- Alix, K.; Gérard, P.R.; Schwarzacher, T.; Heslop-Harrison, J.S.P. Polyploidy and Interspecific Hybridization: Partners for Adaptation, Speciation and Evolution in Plants. Ann. Bot. 2017, 120, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, G.F. Enumeratio Plantarum et Seminum Hort Botanici Mosquensis; Hortus Mosquensis: Moscow, Russia, 1808; 8p. [Google Scholar]
- Marchall von Bieberstein, F.A. Flora Taurico Caucasica Exhibens Stirpes Phaenogamas, in Chersoneso Taurica et Regionibus Caucasicis Sponte Crescentes; Typis Academicis: Charkouiae [Kharkov], Ukraine, 1819; 654p. [Google Scholar]
- New England Botanical Club. On the Rule of Botanical Nomenclature Adopted by the Vienna Congress. Rhodora 1907, 9, 29–55. [Google Scholar]
- Cambridge Botanical Congress. International Botanical Congress Cambridge (England). Nomenclature: Proposals by British Botanists; Wyman & Sons, Ltd.: London, UK, 1930; pp. 97–109. [Google Scholar]
- Munz, P.A. A Manual of Southern California Botany; J.W. Stacey: San Francisco, CA, USA, 1935; 642p. [Google Scholar]
- Hawksworth, F.G.; Graham, D.P. Dwarf Mistletoes on Spruce in the Western United States. Northwest Sci. 1963, 37, 31–38. [Google Scholar]
- Kuijt, J. The Distribution of Dwarf Mistletoes, Arceuthobium, in California. Madroño 1960, 15, 129–139. [Google Scholar]
- Hawksworth, F.G. Observations of Arceuthobium vaginatum in Mexico. Madroño 1961, 16, 31–32. [Google Scholar]
- Standley, P.C. Trees and Shrubs of Mexico; Government Printing Office: Washington, DC, USA, 1922; Volume 23. [CrossRef]
- Hawksworth, F.G.; Wiens, D. A New Species of Arceuthobium from Arizona. Brittonia 1964, 16, 54–57. [Google Scholar] [CrossRef]
- Hawksworth, F.G.; Lightle, P.C.; Scharpf, R.F. Arceuthobium in Baja California, Mexico. Southw. Naturalist 1968, 13, 101. [Google Scholar] [CrossRef]
- Hawksworth, F.G.; Wiens, D. Biology and Taxonomy of the Dwarf Mistletoes. Annu. Rev. Phytopathol. 1970, 8, 325–350. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Hawksworth, F.G. Taxonomy and Effects of Dwarf Mistletoe on Bristlecone Pine on the San Francisco Peaks, Arizona; Res. Pap. RM-224; USDA For. Serv.: Ft. Collins, CO, USA, 1980; 10p.
- Hawksworth, F.G. Taxonomy of Hemlock Dwarf Mistletoe. In Proceedings of the 34th Western International Forest Disease Work Conference, Juneau, AK, USA, 8–11 September 1986; pp. 45–46. [Google Scholar]
- Hawksworth, F.G. Paleobotany and Evolution of the Dwarf Mistletoes (Arceuthobium). In Proceedings of the Fourth International Symposium on Parasitic Plants; Weber, H.C., Forstreuter, W., Eds.; Philipps-Universität Marburg: Marburg, Germany, 1987; pp. 309–316. [Google Scholar]
- Hawksworth, F.G. Taxonomia y Distribucion de Arceuthobium en Mexico y Centro America. Publica cion Especial 60; Instituto Nacional de Investigaciones Forestales y Agropecurias: Mexico City, Mexico, 1991; pp. 559–591. [Google Scholar]
- Nickrent, D.L.; Stell, A.L. Electrophoretic Evidence for Genetic Differentiation in Two Host Races of Hemlock Dwarf Mistletoe (Arceuthobium tsugense). Biochem. Syst. Ecol. 1990, 18, 267–280. [Google Scholar] [CrossRef]
- Nickrent, D.L.; Butler, T.L. Allozymic Relationships of Arceuthobium campylopodum and Allies in California. Biochem. Syst. Ecol. 1990, 18, 253–265. [Google Scholar] [CrossRef]
- Nickrent, D.L.; Butler, T.L. Genetic Relationships in Arceuthobium monticola and A. siskiyouense (Viscaceae): New dwarf mistletoe species from California and Oregon. Biochem. Syst. Ecol. 1991, 19, 305–313. [Google Scholar] [CrossRef]
- Hawksworth, F.G.; Wiens, D. Viscaceae. In The Jepson Manual: Higher Plants of California; Hickman, J.C., Ed.; University of California Press: Berkeley, CA, USA, 1993; pp. 1092–1097. [Google Scholar]
- Mathiasen, R.L. Comparative Susceptibility of Conifers to Knobcone Pine Dwarf Mistletoe. West. N. Am. Nat. 2009, 69, 42–48. [Google Scholar] [CrossRef]
- Mathiasen, R.L. Morphological Comparisons of White Fir and Red Fir Dwarf Mistletoes in the Sierra Nevada and Southern Cascade Mountains. Madroño 2011, 58, 101–105. [Google Scholar] [CrossRef]
- Mathiasen, R.L. Susceptibility of Red Fir and White Fir to Fir Dwarf Mistletoe (Arceuthobium abietinum) in California. For. Path. 2019, 49, e12516. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Daugherty, C.M. Susceptibility of Conifers to Western Hemlock Dwarf Mistletoe in the Cascade Range of Washington and Oregon. W. J. Appl. For. 2005, 20, 94–100. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Daugherty, C.M. Distribution of Red fir and Noble fir in Oregon Based on Dwarf Mistletoe Host specificity. Northw. Sci. 2008, 82, 108–119. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Daugherty, C.M. Additional Morphological Measurements of Arceuthobium siskiyouense and A. monticola (Viscaceae). J. Bot. Res. Inst. Texas 2009, 3, 741–749. [Google Scholar]
- Mathiasen, R.L.; Daugherty, C.M. Additional Morphological Measurements of Arceuthobium littorum and A. occidentale (Viscaceae). Phytologia 2013, 95, 70–78. [Google Scholar]
- Kuijt, J. Measurements and Taxonomy in Arceuthobium (Viscaceae). Phytologia 2016, 98, 186–189. [Google Scholar]
- Mathiasen, R.L.; Hawksworth, F.G.; Edminster, C.B. Effects of Dwarf Mistletoe on Spruce in the White Mountains, Arizona. Great Basin Nat. 1986, 46, 685–689. [Google Scholar]
- Crawford, D.J.; Hawksworth, F.G. Flavonoid Chemistry of Arceuthobium (Viscaceae). Brittonia 1979, 31, 212–216. [Google Scholar] [CrossRef]
- Luckow, M. Species Concepts: Assumptions, Methods, and Applications. Syst. Bot. 1995, 20, 589–605. [Google Scholar] [CrossRef]
- Baldwin, B.G.; Sanderson, M.J.; Porter, J.M.; Wojciechowski, M.F.; Campbell, C.S.; Donoghue, M.J. The ITS Region of Nuclear Ribosomal DNA: A Valuable Source of Evidence on Angiosperm Phylogeny. Ann. Mo. Bot. Gard. 1995, 82, 247–277. [Google Scholar] [CrossRef]
- Taberlet, P.; Coissac, E.; Pompanon, F.; Gielly, L.; Miquel, C.; Valentini, A.; Vermat, T.; Corthier, G.; Brochmann, C.; Willerslev, E. Power and Limitations of the Chloroplast trnL (UAA) intron for Plant DNA barcoding. Nucleic Acids Res. 2007, 35, e14. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Shafer, H.L.; Leonard, O.R.; Kovach, M.J.; Schorr, M.; Morris, A.B. Chloroplast DNA Sequence Utility for the Lowest Phylogenetic and Phylogeographic Inferences in Angiosperms: The Tortoise and the Hare IV. Am. J. Bot. 2014, 101, 1987–2004. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, B.G. Roles of Modern Plant Systematics in Discovery and Conservation of Fine-scale Biodiversity. Madroño 2000, 47, 219–229. [Google Scholar]
- Álvarez, I.; Wendel, J.F. Ribosomal ITS Sequences and Plant Phylogenetic Inference. Mol. Phylogenet. Evol. 2004, 29, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Nieto Feliner, G.; Rosselló, J.A. Better the Devil You Know? Guidelines for Insightful Utilization of nrDNA ITS in Species-level Evolutionary Studies in Plants. Mol. Phylogenet. Evol. 2007, 44, 911–919. [Google Scholar] [CrossRef]
- Nosil, P.; Feder, J.L. Genomic Divergence during Speciation: Causes and Consequences. Phil. Trans. R. Soc. B. 2012, 367, 332–342. [Google Scholar] [CrossRef]
- Jerome, C.A.; Ford, B.A. The Discovery of Three Genetic Races of the Dwarf Mistletoe Arceuthobium americanum (Viscaceae) Provides Insight into the Evolution of Parasitic Angiosperms. Mol. Ecol. 2002, 11, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A Robust Simple Genotyping-by-sequencing (GBS) Approach for High Diversity Species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef] [PubMed]
- Torkamaneh, D.; Laroche, J.; Belzile, F. Genome-wide SNP Calling from Genotyping by Sequencing (GBS) Data: A Comparison of Seven Pipelines and Two Sequencing Technologies. PLoS ONE 2016, 11, e0161333. [Google Scholar] [CrossRef]
- Baena-Díaz, F.; Ramírez-Barahona, S.; Ornelas, J.F. Hybridization and Differential Introgression Associated with Environmental Shifts in a Mistletoe Species Complex. Sci. Rep. 2018, 8, 5591. [Google Scholar] [CrossRef]
- Fernández-Mazuecos, M.; Mellers, G.; Vigalondo, B.; Llorenç, S.; Vargas, P.; Glover, B.J. Resolving Recent Plant Radiations: Power and Robustness of Genotyping-by-sequencing. System. Biol. 2018, 67, 250–268. [Google Scholar] [CrossRef]
- Paetzold, C.; Wood, K.R.; Eaton, D.A.; Wagner, W.L.; Appelhans, M.S. Phylogeny of Hawaiian Melicope (Rutaceae): RAD-seq Resolves Species Relationships and Reveals Ancient Introgression. Front. Plant Sci. 2019, 10, 467762. [Google Scholar] [CrossRef]
- Thorogood, C.J.; Rumsey, F.J.; Hiscock, S.J. Host-specific Races in the Holoparasitic Angiosperm Orobanche minor: Implications for Speciation in Parasitic Plants. Ann. Bot. 2009, 103, 1005–1014. [Google Scholar] [CrossRef]
- Okubamichael, D.Y.; Griffiths, M.E.; Ward, D. Host Specificity in Parasitic Plants-perspectives from Mistletoes. AoB Plants 2017, 8, plw069. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Loftis, L. Douglas-fir Dwarf Mistletoe Parasitizing Pacific Silver Fir in Northern California. Great Basin Nat. 1987, 47, 161–162. [Google Scholar]
- Mathiasen, R.L. Comparative Susceptibility of Corkbark Fir and Douglas-fir to Douglas-fir Dwarf Mistletoe. For. Sci. 1984, 30, 842–847. [Google Scholar]
- Chazaro, B.M.; Olivia, R.H. Loranthaceae del centro de Veracruz y zona limitrofe de Puebla. I. Cact. Suc. Mex. 1987, 32, 55–60. [Google Scholar]
- Beatty, J.S.; Mathiasen, R.L.; Parks, C.G. Additional Populations of Arceuthobium hondurense Discovered in Honduras. Phytologia 1998, 85, 268–270. [Google Scholar]
- Mathiasen, R.; Beatty, J.; Melgar, J. First Report of Arceuthobium hondurense on Pinus tecunumanii. Plant Dis. 2000, 84, 372. [Google Scholar] [CrossRef]
- Mathiasen, R.; Nickrent, D.; Parks, C.; Beatty, J.; Sesnie, S. First Report of Arceuthobium hondurense in Mexico. Plant Dis. 2001, 85, 444. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, R.; Howell, B.; Melgar, J. First Report of Arceuthobium hawksworthii in Honduras. Plant Dis. 2002, 86, 815. [Google Scholar] [CrossRef]
- Mathiasen, R.; Nickrent, D.; Daugherty, C. First Report of Arceuthobium hondurense in Oaxaca, Mexico. Plant Dis. 2002, 86, 72. [Google Scholar] [CrossRef]
- Mathiasen, R.; Melgar, J. First Report of Arceuthobium hondurense from Department El Paraiso, Honduras. Plant Dis. 2006, 90, 685. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Daugherty, C.M. First Report of Limber Pine Dwarf Mistletoe (Arceuthobium cyanocarpum) on Sugar Pine (Pinus lambertiana) from California. Plant Dis. 2009, 94, 134. [Google Scholar] [CrossRef]
- Mathiasen, R.L. Comparative Susceptibility of Conifers to Larch Dwarf Mistletoe Based on Natural Infection in the Pacific Northwest. For. Sci. 1998, 44, 559–568. [Google Scholar]
- Mathiasen, R.L.; Hawksworth, F.G. Dwarf Mistletoes on Western White Pine and Whitebark Pine in Northern California and Southern Oregon. For. Sci. 1988, 34, 429–440. [Google Scholar] [CrossRef]
- Wass, E.F. Ecological Study of Shore Pine Stands Infested with Dwarf Mistletoe on Southeastern Vancouver Island; Report BC-X-142; Canada Dept. Environ., Pacific Forestry Res. Centre: Victoria, BC, Canada, 1976; 33p. [Google Scholar]
- Shaw, C.G. Mountain Hemlock is an Occasional Host for Hemlock Dwarf Mistletoe in Alaska. Plant Dis. 1982, 66, 852–854. [Google Scholar] [CrossRef]
A. Gill [14]—United States
| B. Hawksworth and Wiens [17], excluding Old World taxa. Subgenus Arceuthobium New World species
Section Vaginata
Series Campylopoda
|
Subgenus Vaginata Hawksw. & Wiens
|
| Character | Arceuthobium globosum subsp. | |||
|---|---|---|---|---|
| aureum | globosum | grandicaule | petersonii | |
| Plant color | Yellow (golden) | Yellow | Yellow–green | Yellow–brown |
| Plant height | ||||
| Female | 14.2 [8.2, 21.6] | 19.6 [8.0, 44.7] | 37.5 [17.0, 66.0] | 27.2 [18.1, 46.2] |
| Male | 15.4 [8.1, 31.4] | 21.0 [11.3, 42.3] | 44.7 [17.1, 93.3] | 32.6 [19.6, 46.8] |
| Basal diameter | ||||
| Female | 5.7 [2.8, 13.3] | 12.7 [4.2, 25.6] | 17.5 [6.0, 40.1] | 10.4 [6.6, 20.1] |
| Male | 5.5 [3.0, 13.4] | 11.3 [4.5, 31.6] | 16.1 [6.0, 37.1] | 10.2 [4.2, 22.9.] |
| Third internode length | ||||
| Female | 16.9 [10.8, 30.2] | 19.9 [4.2, 35.0] | 33.7 [15.0, 56.2] | 23.1 [9.4, 34.1] |
| Male | 18.0 [9.7, 29.3] | 20.0 [9.7, 45.6] | 32.8 [15.0, 54.2] | 25.7 [13.8, 38.3] |
| Third internode width | ||||
| Female | 3.5 [1.8, 7.3] | 7.3 [3.0, 18.8] | 11.6 [4.0, 25.5] | 6.4 [4.3, 16.7] |
| Male | 3.2 [1.9, 6.4] | 6.2 [2.7, 19.9] | 10.4 [4.0, 31.4] | 5.6 [3.4, 13.7] |
| Staminate flowers | ||||
| Anthesis (peak) | Mid-Feb.–June (no apparent peak) | Mar.–April (Mar.) | Jan.–May (Mar.–Apr.) | Mid-Aug.–early Oct. (mid-Sept.). |
| Staminate spike width | 1.2 [0.8, 1.2] | 2.1 [1.8, 2.3] | 2.2 [1.9, 2.4] | 1.3 [0.8, 1.4] |
| Diameter 3-merous | 2.2 [1.9, 2.6] | 3.1 [2.5, 4.0] | 3.1 [2.1, 4.0] | 2.2 [1.9, 2.4] |
| Diameter 4-merous | 2.9 [3.1, 4.4] | 4.2 [3.1, 5.6] | 4.2 [3.0, 5.8] | 3.0 [2.6, 3.5] |
| Fruit length | 4.1 [3.6, 4.6] | 6.7 [5.3, 8.2] | 7.0 [5.6, 8.2] | 5.1 [4.5, 5.7] |
| Fruit width | 2.7 [2.3, 3.0] | 4.1 [2.9, 5.2] | 4.1 [3.1, 5.2] | 3.4 [2.9, 4.0] |
| Seed dispersal (peak) | Aug.–early Oct. (no apparent peak) | ? (June–July) | Mid-Aug.–Sept. (?) | Mid-Aug.–early Oct. (mid-Sept.) |
| Witches’ brooms | Common | Rare | Common | Common |
| Host(s) | ||||
| Principal | Pinus maximinoi; P. montezumae; P. oocarpa?; P. pseudostrobus subsp. oaxacana (P. oaxacana) | Pinus cooperi; P. durangensis; P. engelmannii | Pinus douglasiana; P. devoniana (P. michoacana); P. durangensis; P. hartwegii (P. rudis); P. lawsonii; P. maximinoi; P. montezumae; P. patula; P. pringlei; P. pseudostrobus; P. teocote | Pinus maximinoi; P. montezumae; P. oocarpa; P. patula (P. oocarpa var. ochoterenai)?; P. pseudostrobus subsp. oaxacana (P. oaxacana)? |
| Secondary | Pinus devoniana (P. michoacana) | |||
| Occasional | Pinus arizonica | - | ||
| Rare | Pinus teocote | |||
| Distribution | Chiapas, MEX; GTM | Northwest MEX | Central and southern MEX; GTM; HND | Chiapas, MEX |
| Sympatry | subsp. grandicaule (Chiapas, MEX) | None | subsp. aureum (Chiapas, MEX) | ? |
| Character | Arceuthobium bicarinatum | Arceuthobium pusillum |
|---|---|---|
| Plant color | Dark brownish red | Green, brown |
| Plant height | 10. 0 [---, 17.0] | 1.0 [ ---, 3.0] |
| Basal diameter | 3.0 [2.0, 4.0] | 1.0 [---, ---] |
| Third internode length | 10.5 [6.0, 14.0] | 1.9 [1.0, 4.0] |
| Third internode width | 2.0 [1.5, 4.0] | 1.0 [0.5, 1.5] |
| Anthesis | Sept. | Apr.–May |
| Apical nodes sterile on pistillate plants | Yes | No |
| Fruit length | 4.0 [---, ---] | 3.0 [---, ---] |
| Fruit width | 2.0 [---, ---] | 1.5 [1.3, 1.8] |
| Seed length | 2.5 [---, ---] | 2.0 [---, ---] |
| Seed width | 1.2 [---, ---] | 0.9 [---, ---] |
| Seed dispersal | Late Aug.–Sept. | Sept.–early Oct. |
| Systemic infection | ? | Frequent |
| Witches’ brooms | Frequent | Frequent |
| Host susceptibility | ||
| Principal | Pinus occidentalis | Picea mariana; P. glauca; P. rubens |
| Secondary | ||
| Occasional | Larix laricina | |
| Rare | Abies balsamea; Pinus banksiana; P. resinosa; P. strobus | |
| Distribution | Hispaniola (DMN, HTI) | North Central–Northeastern USA and South-central—Eastern CAN, including maritime provinces |
| Sympatric | No | No |
| Character | Arceuthobium laricis a | Arceuthobium tsugense subsp. mertensianae b |
|---|---|---|
| Plant color | Yellow–green, brown–green, occasionally purple–red | Green, green–brown, yellow–green |
| Plant height | ||
| Female | 5.3 [3.1, 9.8] | 6.1 [3.0, 11.6] |
| Male | 4.7 [2.1, 8.6] | 5.7 [2.7, 9.8] |
| Basal diameter | ||
| Female | 2.4 [1.4, 4.1] | 2.2 [1.4, 3.7] |
| Male | 2.1 [1.4, 5.6] | 1.9 [1.0, 3.3] |
| Third internode length | ||
| Female | 8.5 [3.8, 15.6] | 9.8 [4.0, 19.0] |
| Male | 7.5 [2.6, 11.6] | 8.0 [2.0, 16.0] |
| Third internode width | ||
| Female | 1.7 [1.2, 2.4] | 1.4 [1.0, 2.4] |
| Male | 1.7 [1.3, 2.5] | 1.3 [0.7, 1.9] |
| Staminate flower | ||
| Anthesis | Mid-July to mid-Aug.–late August to mid-Sept. | Mid-Aug.–late Sept. |
| Spike length | 10.1 [5.5, 18.1] | 6.9 [2.0, 14.0] |
| Spike width | 2.6 [2.0, 3.1] | 1.2 [0.7, 1.4] |
| Diameter 3-merous | 2.7 [2.0, 3.4] | 2.4 [1.9, 2.9] |
| Diameter 4-merous | 3.7 [3.0, 4.8] | 3.1 [2.4, 3.8] |
| Petal lobe length | 1.4 [0.9, 1.8] | 1.1 [0.7, 1.5] |
| Petal lobe width | 1.2 [0.8, 1.6] | 1.0 [0.7, 1.3] |
| Anther diameter | 0.5 [0.3, 0.8] | 0.4 [0.3, 0.7] |
| Anther distance from tip | 0.5 [0.2, 0.8] | 0.4 [0.2, 0.7] |
| Fruit length | 4.3 [3.5, 5.5] | 4.4 [3.3, 5.5] |
| Fruit width | 3.0 [2.1, 3.7] | 2.6 [1.8, 3.5] |
| Seed length | 2.4 [1.7, 3.2] | 2.6 [1.8, 3.5] |
| Seed width | 1.2 [0.7, 1.3] | 1.1 [0.8, 1.4] |
| Seed dispersal (peak) | Late Aug.–early Oct. (Sept.) | Late Sept.–early Nov. (mid-Oct.) |
| Host susceptibility c | ||
| Principal | Larix occidentalis; | Tsuga mertensiana |
| Secondary | Tsuga mertensiana Pinus contorta var. latifolia | Picea breweriana |
| Occasional | Abies lasiocarpa; A. bifolia; Pinus ponderosa var. ponderosa | Pinus monticola; Tsuga heterophylla |
| Rare | Abies grandis; Picea engelmannii; Pinus albicaulis; P. monticola | |
| Sympatric | None; the southernmost distribution of A. laricis extends into central Oregon whereas the northern distribution of A. tsugense subsp. mertensianae terminates in south-central Oregon. | |
| Character | Arceuthobium tsugense subsp. | |||
|---|---|---|---|---|
| amabiliae | contortae | mertensianae | tsugense | |
| Plant color | ||||
| Female | Green, green–brown | Green–brown | Green, green–brown | Yellow–green, purple |
| Male | Green–brown, yellow–green, green | Green–brown | Yellow–green, green–brown | Yellow–green |
| Plant height | ||||
| Female | 10.6 [4.6, 18.5] | 6.6 [4.0, 9.5] | 6.1 [3.0, 11.6] | 8.0 [3.8, 13.7] |
| Male | 9.4 [4.1, 17.9] | 5.6 [3.2, 10.8] | 5.7 [2.7, 9.8] | 7.8 [3.4, 16.1] |
| Basal diameter | ||||
| Female | 3.4 [1.3, 5.8] | 3.3 [1.8, 5.0] | 2.2 [1.4, 3.7] | 2.7 [1.3, 5.5] |
| Male | 3.1 [1.8, 4.7] | 2.8 [1.7, 4.7] | 1.9 [1.0, 3.3] | 2.6 [1.3, 5.0] |
| Third internode length | ||||
| Female | 15.0 [7.0, 28.0] | 10.7 [5.3, 16.4] | 9.8 [4.0, 19.0] | 12.3 [6.0, 22.0] |
| Male | 12.6 [6.0, 22.0] | 9.2 [5.8, 15.7] | 8.0 [2.0, 16.0] | 11.8 [4.5, 23.0] |
| Third internode width | ||||
| Female | 2.0 [1.0, 3.0] | 1.7 [1.3, 2.5] | 1.4 [1.0, 2.4] | 1.6 [1.0, 3.1] |
| Male | 1.9 [1.1, 3.0] | 1.8 [1.1, 2.5] | 1.3 [0.7, 1.9] | 1.6 [0.8, 3.0] |
| Staminate flowers | ||||
| Anthesis | Mid-July–mid-Sept. (late July–mid-Aug.) | Mid-July–early Oct. (late July–mid-Aug.) | Mid-Aug.–late Sept. (early Sept.; 1 wk later than subsp. tsugense) | Late July–late Sept. (mid-Aug.; 1 wk earlier than subsp. mertensianae) |
| Spike length | 9.5 [3.0, 22.0] | 12.6 [5.0, 22.0] | 6.9 [2.0, 14.0] | 10.8 [5.0, 27.4] |
| Spike width | 1.3 [1.0, 2.1] | 3.4 [2.0, 5.5] | 1.2 [0.7, 1.4] | 1.6 [0.9, 5.0] |
| Diameter 3-merous | 3.2 [2.3, 4.9] | --- | 2.4 [1.9, 2.9] | 3.1 [2.2, 4.7] |
| Diameter 4-merous | 3.7 [2.6, 5.1] | --- | 3.1 [2.4, 3.8] | 3.8 [2.5, 5.5] |
| 3- and 4-merous a | 3.4 [2.3, 5.1] | 4.3 [2.8, 5.9] | 2.7 [1.9, 3.8] | 3.5 [2.2, 5.5] |
| Petal length | 1.4 [1.0, 1.9] | 1.7 [1.2, 2.8] | 1.1 [0.7, 1.5] | 1.5 [1.0, 2.1] |
| Petal width | 1.2 [0.8, 1.8] | 1.4 [1.0, 2.3] | 1.0 [0.7, 1.3] | 1.2 [0.8, 2.1] |
| Fruit length | 4.7 [3.2, 6.0] | 4.6 [3.3, 5.5] | 3.8 [2.8, 4.8] | 4.4 [3.3, 5.5] |
| Seed length | 3.0 [2.3, 3.8] | 2.5 [1.8, 3.0] | 2.8 [2.2, 3.4] | 2.6 [1.8, 3.5] |
| Seed width | 1.2 [0.8, 1.5] | 1.4 [1.0. 1.7] | 1.1 [0.8, 1.4] | 1.1 [0.8, 1.4] |
| Seed dispersal (peak) | Early Sept.–late Oct. (late Sept.–early Oct.; 2 wks earlier than subsp. tsugense) | Mid-Sept.–early Nov. (mid-Sept.–mid-Oct.; 1 wk earlier than subsp. tsugense) | Mid-Aug.–early Oct. (mid-Sept.; 2 wks earlier than subsp. tsugense) | Late Sept.–early Nov. (mid-Oct.; 2 wks later subsp. amabilae and subsp. mertensianae) |
| Character | Arceuthobium microcarpum subsp. aristatae | Arceuthobium microcarpum subsp. microcarpum |
|---|---|---|
| Plant color | Light green, green–brown, purple | Light green, green–brown, blue-green |
| Plant height | ||
| Female | 3.6 [1.4, 7.0] | 6.9 [2.0, 15.7] |
| Male | 2.7 [0.8, 7.0] | 6.0 [1.8, 14.9] |
| Basal diameter | ||
| Female | 1.8 [0.6, 3.0] | 2.0 [0.8, 3.8] |
| Male | 1.8 1.0, 3.0] | 1.9 [0.8, 3.4] |
| Anthesis (peak) | Mid-July–early Sept. (mid-Sept.; 1-2 wks. earlier than subsp. microcarpum) | Mid-July–late Sept. (late Aug.–mid-Sept.; 1-2 wks. later than subsp. aristatae) |
| Staminate flower diameter | 2.5 [1.8, 4.0] | 2.4 [1.6, 3.1] |
| Fruit length | 3.3 [3.5–5.1] | 3.5 [3.4 [3.4–5.2] |
| Fruit width | 2.1 [1.7–2.9] | 2.2 [1.9–3.1] |
| Seed length | 2.4 [1.5–3.4] | 2.4 [1.3–3.4] |
| Seed dispersal (peak) | Sept. (Sept.) | Sept.–Oct. (Sept.) |
| Host susceptibility a | ||
| Principal | Pinus aristata | Picea engelmannii; P. pungens |
| Secondary | Picea engelmannii | |
| Occasional | ||
| Rare | Abies lasiocarpa var. arizonica; Pinus strobiformis | Abies lasiocarpa var. arizonica; Pinus strobiformis |
| Distribution | Limited to high elevation; USA—AZ (Kendrick Peak and San Francisco Peaks) | Limited to high elevation; USA—AZ and NM |
| Sympatry | subsp. microcarpum; San Francisco Peaks, AZ, USA | subsp. aristatae; San Francisco Peaks, AZ, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenaley, S.C.; Mathiasen, R.L. Dwarf Mistletoes (Arceuthobium, Viscaceae) of North America: Classification Systems, Phylogenetic Relationships, and Taxonomic Characteristics. Plants 2025, 14, 2051. https://doi.org/10.3390/plants14132051
Kenaley SC, Mathiasen RL. Dwarf Mistletoes (Arceuthobium, Viscaceae) of North America: Classification Systems, Phylogenetic Relationships, and Taxonomic Characteristics. Plants. 2025; 14(13):2051. https://doi.org/10.3390/plants14132051
Chicago/Turabian StyleKenaley, Shawn C., and Robert L. Mathiasen. 2025. "Dwarf Mistletoes (Arceuthobium, Viscaceae) of North America: Classification Systems, Phylogenetic Relationships, and Taxonomic Characteristics" Plants 14, no. 13: 2051. https://doi.org/10.3390/plants14132051
APA StyleKenaley, S. C., & Mathiasen, R. L. (2025). Dwarf Mistletoes (Arceuthobium, Viscaceae) of North America: Classification Systems, Phylogenetic Relationships, and Taxonomic Characteristics. Plants, 14(13), 2051. https://doi.org/10.3390/plants14132051





