Impact of a Soil Cyanobacteria Consortium-Based Bioinoculant on Tomato Growth, Yield, and Fruit Quality
Abstract
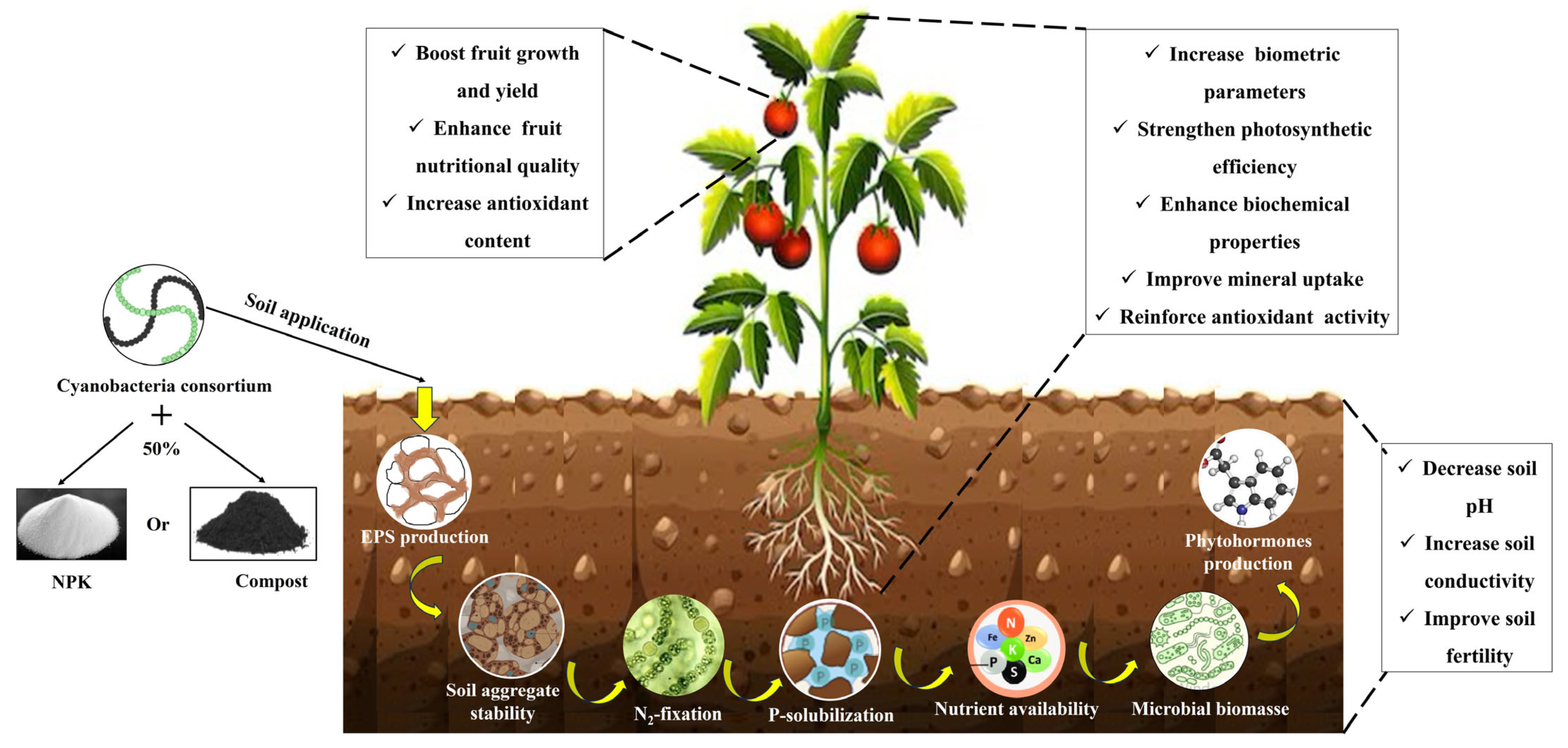
1. Introduction
2. Results
2.1. Growth and Physiological Properties of Cyanobacterial Strains
2.2. Cyanobacterial Biochemical Composition
2.3. Effect of Cyanobacterial Bioinoculants on Soil Fertility
2.4. Effects of Cyanobacterial Bioinoculants on Tomato Growth
2.5. Effect of Cyanobacterial Bioinoculants on Fruit Agronomic Parameters and Tomato Yield
2.6. Effect of Cyanobacterial Bioinoculants on Tomato Biochemical Composition
2.7. Effect of Cyanobacterial Bioinoculants on Tomato Physiological Properties
2.8. Effect of Cyanobacterial Bioinoculants on Tomato Mineral Properties
2.9. Effect of Cyanobacterial Bioinoculants on Tomato Fruit Quality
2.9.1. Effect on Fruit Biochemical Parameters
2.9.2. Effect of Cyanobacterial Bioinoculants on Physicochemical Parameters of Tomato Fruits
2.10. Correlation Analysis of Growth, Biochemical, and Physiological Traits in Tomato Shoots and Fruit Quality
3. Discussion
4. Materials and Methods
4.1. Cyanobacterial Strains and Culture Conditions
4.2. Physiological Traits of Cyanobacterial Strains
4.3. Cyanobacterial Biomass Production and Characterization
4.4. Tomato Pot Experiments and Bioassay
4.4.1. Soil Sampling and Physicochemical Analysis
4.4.2. Conventional Fertilizers
4.4.3. Experimental Design
- C−, control, non-bioinoculated, and non-amended soil.
- C+min, positive control, soil fertilized by a full dose of NPK (3.5 g NPK/kg soil).
- C+org, positive control, soil fertilized by a full dose of compost (50 g/kg soil).
- AN, 10 g of A. cylindrica (AN) (2 g fresh biomass/kg soil).
- NO, 10 g of N. punctiforme (NO) (2 g fresh biomass/kg soil).
- AN + NO, 10 g of consortium, 5 g of A. cylindrica + 5 g of N. punctiforme (2 g fresh biomass/kg soil).
- AN + NO + 50% NPK, 10 g of consortium + 50% NPK (1.75 g NPK/kg soil).
- AN + NO + 50% Compost, 10 g of consortium + 50% compost (25 g/kg soil).
4.5. Tomato Plant Growth and Yield Parameters Measurement
4.6. Tomato Plant Biochemical and Mineral Analyses
4.7. Tomato Plant Physiological Analyses
4.8. Tomato Fruit Quality Characteristics
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osorio-Reyes, J.G.; Valenzuela-Amaro, H.M.; Pizaña-Aranda, J.J.P.; Ramírez-Gamboa, D.; Meléndez-Sánchez, E.R.; López-Arellanes, M.E.; Castañeda-Antonio, M.D.; Coronado-Apodaca, K.G.; Gomes Araújo, R.; Sosa-Hernández, J.E.; et al. Microalgae-Based Biotechnology as Alternative Biofertilizers for Soil Enhancement and Carbon Footprint Reduction: Advantages and Implications. Mar. Drugs 2023, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, B.; Maddela, N.R.; Venkateswarlu, K.; Megharaj, M. Potential of Microalgae and Cyanobacteria to Improve Soil Health and Agricultural Productivity: A Critical View. Environ. Sci. Adv. 2023, 2, 586–611. [Google Scholar] [CrossRef]
- Sadvakasova, A.K.; Bauenova, M.O.; Kossalbayev, B.D.; Zayadan, B.K.; Huang, Z.; Wang, J.; Balouch, H.; Alharby, H.F.; Chang, J.S.; Allakhverdiev, S.I. Synthetic Algocyanobacterial Consortium as an Alternative to Chemical Fertilizers. Environ. Res. 2023, 233, 116418. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.L.; Weyers, S.L.; Gardner, R.D. Cyanobacteria-Based Soil Amendments in the Soil-Plant System: Effects of Inoculations on Soil Nutrient and Microbial Dynamics under Spring Wheat Growth. Algal Res. 2024, 77, 103326. [Google Scholar] [CrossRef]
- Ben-Laouane, R.; Ait-El-Mokhtar, M.; Anli, M.; Boutasknit, A.; Ait Rahou, Y.; Raklami, A.; Oufdou, K.; Wahbi, S.; Meddich, A. Green Compost Combined with Mycorrhizae and Rhizobia: A Strategy for Improving Alfalfa Growth and Yield Under Field Conditions. Gesunde Pflanz. 2021, 73, 193–207. [Google Scholar] [CrossRef]
- Chabili, A.; Minaoui, F.; Hakkoum, Z.; Douma, M.; Meddich, A.; Loudiki, M. A Comprehensive Review of Microalgae and Cyanobacteria-Based Biostimulants for Agriculture Uses. Plants 2024, 13, 159. [Google Scholar] [CrossRef]
- Hakkoum, Z.; Minaoui, F.; Chabili, A.; Douma, M.; Mouhri, K.; Loudiki, M. Biofertilizing Effect of Soil Cyanobacterium Anabaena cylindrica–Based Formulations on Wheat Growth, Physiology, and Soil Fertility. Agriculture 2025, 15, 189. [Google Scholar] [CrossRef]
- Jose, S.; Malla, M.A.; Renuka, N.; Bux, F.; Kumari, S. Cyanobacteria-Green Microalgae Consortia Enhance Soil Fertility and Plant Growth by Shaping the Native Soil Microbiome of Capsicum annuum. Rhizosphere 2024, 30, 100892. [Google Scholar] [CrossRef]
- Minaoui, F.; Hakkoum, Z.; Chabili, A.; Douma, M.; Mouhri, K.; Loudiki, M. Biostimulant Effect of Green Soil Microalgae Chlorella vulgaris Suspensions on Germination and Growth of Wheat (Triticum aestivum Var. Achtar) and Soil Fertility. Algal Res. 2024, 82, 103655. [Google Scholar] [CrossRef]
- Massey, M.S.; Davis, J.G. Beyond Soil Inoculation: Cyanobacteria as a Fertilizer Replacement. Nitrogen 2023, 4, 253–262. [Google Scholar] [CrossRef]
- Chabili, A.; Minaoui, F.; Hakkoum, Z.; Douma, M.; Meddich, A.; Loudiki, M. Effects of Extraction Methods on the Plant Biostimulant Activity of the Soil Microalga Chlorella vulgaris. J. Appl. Phycol. 2024, 36, 3301–3314. [Google Scholar] [CrossRef]
- Jose, S.; Renuka, N.; Ratha, S.K.; Kumari, S.; Bux, F. Bioprospecting of Microalgae from Agricultural Fields and Developing Consortia for Sustainable Agriculture. Algal Res. 2024, 78, 103428. [Google Scholar] [CrossRef]
- Kholssi, R.; Marks, E.A.N.; Miñón, J.; Montero, O.; Lorentz, J.F.; Debdoubi, A.; Rad, C. Biofertilizing Effects of Anabaena cylindrica Biomass on the Growth and Nitrogen Uptake of Wheat. Commun. Soil Sci. Plant Anal. 2022, 53, 1216–1225. [Google Scholar] [CrossRef]
- Hakkoum, Z.; Minaoui, F.; Chabili, A.; Douma, M.; Mouhri, K.; Loudiki, M. Effect of Cyanobacterial Suspensions from Soil Nitrogen-Fixing Anabaena cylindrica on Wheat Germination. Plant Biosyst. 2025, 159, 488–499. [Google Scholar] [CrossRef]
- Ismail, G.A.; Abo-Hamad, S.A. Effect of Different Anabaena variabilis (Kütz) Treatments on Some Growth Parameters and Physiological Aspects of Hordeum vulgare L. and Trigonella foenum-graecum L. Egypt. J. Bot. 2017, 57, 507–516. [Google Scholar] [CrossRef]
- Chabili, A.; Hakkoum, Z.; Minaoui, F.; Douma, M.; Meddich, A.; Loudiki, M. Germination Screen of Eco-Extracts from Soil Cyanobacteria and Microalgae for Their Biostimulant Effects on Wheat Seeds Emergence and Vigor. Algal Res. 2025, 89, 104087. [Google Scholar] [CrossRef]
- Prasanna, R.; Hossain, F.; Babu, S.; Bidyarani, N.; Adak, A.; Verma, S.; Shivay, Y.S.; Nain, L. Prospecting Cyanobacterial Formulations as Plant-Growth-Promoting Agents for Maize Hybrids. S. Afr. J. Plant Soil 2015, 32, 199–207. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Johnson, J.M.F.; Gardner, R.D. Soil Inoculations with Anabaena cylindrica Improve Aggregate Stability and Nutrient Dynamics in an Arable Soil and Exhibit Potential for Erosion Control. J. Appl. Phycol. 2021, 33, 3041–3057. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as Multi-Functional Options in Modern Agriculture: Current Trends, Prospects and Challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- Coppens, J.; Grunert, O.; Van Den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; De Gelder, L. The Use of Microalgae as a High-Value Organic Slow-Release Fertilizer Results in Tomatoes with Increased Carotenoid and Sugar Levels. J. Appl. Phycol. 2016, 28, 2367–2377. [Google Scholar] [CrossRef]
- Grzesik, M.; Romanowska-Duda, Z.; Kalaji, H.M. Effectiveness of Cyanobacteria and Green Algae in Enhancing the Photosynthetic Performance and Growth of Willow (Salix viminalis L.) Plants under Limited Synthetic Fertilizers Application. Photosynthetica 2017, 55, 510–521. [Google Scholar] [CrossRef]
- Nivedha, R.M.; Bhardwaj, A.; Prasanna, R.; Bavana, N.; Kokila, V.; Nishanth, S.; Rudra, S.G.; Singh, A.K.; Reddy, K.S.; Shivay, Y.S. Enhancing Fruit Quality and Yield in Tomato through Cyanobacterium Mediated Nutri-Fertigation. Biocatal. Agric. Biotechnol. 2024, 61, 103344. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A Precious Bio-Resource in Agriculture, Ecosystem, and Environmental Sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Belnap, J.; Büdel, B.; Antoninka, A.J.; Barger, N.N.; Chaudhary, V.B.; Darrouzet-Nardi, A.; Eldridge, D.J.; Faist, A.M.; Ferrenberg, S.; et al. What Is a Biocrust? A Refined, Contemporary Definition for a Broadening Research Community. Biol. Rev. 2022, 97, 1768–1785. [Google Scholar] [CrossRef]
- Wu, L.; Dong, J.; Song, J.; Zhu, Y.; Che, S.; Qin, X.; Xu, Y.; Tian, S.; Wang, D.; Tian, P.; et al. The Nitrogen Fixation Characteristics of Terrestrial Nitrogen-Fixing Cyanobacteria and Their Role in Promoting Rice Growth. Agronomy 2025, 15, 62. [Google Scholar] [CrossRef]
- Gheda, S.F.; Ahmed, D.A. Improved Soil Characteristics and Wheat Germination as Influenced by Inoculation of Nostoc kihlmani and Anabaena cylindrica. Rend. Lincei 2015, 26, 121–131. [Google Scholar] [CrossRef]
- Iniesta-pallar, M.; Gordillo-cant, F.M. Applied Sciences Sustaining Rice Production through Biofertilization with. Appl. Sci. 2021, 11, 4628. [Google Scholar] [CrossRef]
- Jose, S.; Renuka, N.; Ratha, S.K.; Kumari, S.; Bux, F. Microalgal Bioinoculants for Sustainable Agriculture and Their Interaction with Biotic and Abiotic Components of the Soil. Pedosphere 2023, 34, 297–314. [Google Scholar] [CrossRef]
- Gautam, K.; Tripathi, J.K.; Pareek, A.; Sharma, D.K. Growth and Secretome Analysis of Possible Synergistic Interaction between Green Algae and Cyanobacteria. J. Biosci. Bioeng. 2019, 127, 213–221. [Google Scholar] [CrossRef]
- Chamizo, S.; Adessi, A.; Mugnai, G.; Simiani, A.; De Philippis, R. Soil Type and Cyanobacteria Species Influence the Macromolecular and Chemical Characteristics of the Polysaccharidic Matrix in Induced Biocrusts. Microb. Ecol. 2019, 78, 482–493. [Google Scholar] [CrossRef]
- Just, B.S.; Marks, E.A.N.; Roquer-Beni, L.; Llenas, L.; Ponsà, S.; Vilaplana, R. Biofertilization Increases Soil Organic Carbon Concentrations: Results of a Meta-Analysis. Int. J. Agric. Sustain. 2024, 22, 2361578. [Google Scholar] [CrossRef]
- Roque, J.; Brito, Â.; Rocha, M.; Pissarra, J.; Nunes, T.; Bessa, M.; Vieira, J.; Vieira, C.P.; Melo, P.; Tamagnini, P. Isolation and Characterization of Soil Cyanobacteria and Microalgae and Evaluation of Their Potential as Plant Biostimulants. Plant Soil 2023, 493, 115–136. [Google Scholar] [CrossRef]
- Dossou, J.; Dossou, J.; Soulé, I.; Montcho, M. Evaluation Des Caractéristiques Physico-Chimiques et Sensorielles de La Purée de Tomate Locale Produite à Petite Échelle Au Bénin Evaluation Des Caractéristiques Physico-Chimiques et Sensorielles de La Purée de Tomate Locale Produite à Petite Échelle Au B. Tropicultura 2007, 25, 119–125. [Google Scholar]
- Gonçalves, A.L. The Use of Microalgae and Cyanobacteria in the Improvement of Agricultural Practices: A Review on Their Biofertilising, Biostimulating and Biopesticide Roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Górka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.P. Algae Biomass: Characteristics and Applications; Springer: Cham, Switzerland, 2018; pp. 103–114. [Google Scholar] [CrossRef]
- El-Ayouty, Y.M.; Ghazal, F.M.; Hassan, A.Z.A.; Abd El-Aal, A.A.M. Effect of Algal Inoculation and Different Water Holding Capacity Levels on Soil Aggregation and Soil Moisture Content of Sandy and Calcareous Soils under Tomato Cultivation Condition. J. Agric. Sci. Mans. Univ. 2004, 29, 2801–2809. [Google Scholar]
- Boutasknit, A.; Anli, M.; Tahiri, A.; Raklami, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Ait Rahou, Y.; Boutaj, H.; Oufdou, K.; Wahbi, S.; et al. Potential Effect of Horse Manure-Green Waste and Olive Pomace-Green Waste Composts on Physiology and Yield Of Garlic (Allium sativum L.) and Soil Fertility. Gesunde Pflanz. 2020, 72, 285–295. [Google Scholar] [CrossRef]
- Jhala, Y.K.; Panpatte, D.G.; Vyas, R.V. Cyanobacteria: Source of Organic Fertilizers for Plant Growth. Microorg. Sustain. 2017, 6, 253–264. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, A.; Singh, M. Cyanobacteria: A Sustainable and Commercial Bio-Resource in Production of Bio-Fertilizer and Bio-Fuel from Waste Waters. Environ. Sustain. Indic. 2019, 3–4, 100008. [Google Scholar] [CrossRef]
- EL-Zawawy, H.A. Effect of Nitrogen-Fixing Cyanobacteria on the Growth of Wheat Crop. J. Agric. Chem. Biotechnol. 2019, 10, 221–225. [Google Scholar] [CrossRef]
- Boopathi, T.; Balamurugan, V.; Gopinath, S.; Sundararaman, M. Characterization of IAA Production by the Mangrove Cyanobacterium Phormidium Sp. MI405019 and Its Influence on Tobacco Seed Germination and Organogenesis. J. Plant Growth Regul. 2013, 32, 758–766. [Google Scholar] [CrossRef]
- Mazhar, S.; Cohen, J.D.; Hasnain, S. Auxin Producing Non-Heterocystous Cyanobacteria and Their Impact on the Growth and Endogenous Auxin Homeostasis of Wheat. J. Basic Microbiol. 2013, 53, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Bona, E.; Todeschini, V.; Cantamessa, S.; Cesaro, P.; Copetta, A.; Lingua, G.; Gamalero, E.; Berta, G.; Massa, N. Combined Bacterial and Mycorrhizal Inocula Improve Tomato Quality at Reduced Fertilization. Sci. Hortic. 2018, 234, 160–165. [Google Scholar] [CrossRef]
- Pereira, I.; Ortega, R.; Barrientos, L.; Moya, M.; Reyes, G.; Kramm, V. Development of a Biofertilizer Based on Filamentous Nitrogen-Fixing Cyanobacteria for Rice Crops in Chile. J. Appl. Phycol. 2009, 21, 135–144. [Google Scholar] [CrossRef]
- Osman, M.E.H.; El-Sheekh, M.M.; El-Naggar, A.H.; Gheda, S.F. Effect of Two Species of Cyanobacteria as Biofertilizers on Some Metabolic Activities, Growth, and Yield of Pea Plant. Biol. Fertil. Soils 2010, 46, 861–875. [Google Scholar] [CrossRef]
- Haroun, S.A.; Hussein, M.H. The Promotive Effect of Algal Biofertilizers on Growth, Protein Pattern and Some Metabolic Activities of Lupinus termis Plants Grown in Siliceous Soil. Asian J. Plant Sci. 2003, 2, 944–951. [Google Scholar] [CrossRef]
- Martínez Lozano, S.; Verde Star, J.; Maiti, R.K.; Oranday, A.; Gaona, H.; Aranda, E.; Rojas, M. Effect of an algae extract and several plant growth regulators on the nutritional value of potato (Solanum tuberosum L. var. gigant). Arch. Latinoam. Nutr. 1999, 49, 166–170. [Google Scholar]
- Fallah Hosseini, Z.; Riahi, H.; Ghorbani Nohooji, M.; Shariatmadari, Z. The Effect of Cyanobacterial Bioelicitors on Total Phenolic Content of Echinacea purpurea L. J. Phycol. Res. 2022, 6, 914–922. [Google Scholar]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Mycorrhizal Stimulation of Leaf Gas Exchange in Relation to Root Colonization, Shoot Size, Leaf Phosphorus and Nitrogen: A Quantitative Analysis of the Literature Using Meta-Regression. Front. Plant Sci. 2016, 7, 1084. [Google Scholar] [CrossRef]
- Mutale-joan, C.; Redouane, B.; Najib, E.; Yassine, K.; Lyamlouli, K.; Laila, S.; Zeroual, Y.; Hicham, E.A. Screening of Microalgae Liquid Extracts for Their Bio Stimulant Properties on Plant Growth, Nutrient Uptake and Metabolite Profile of Solanum lycopersicum L. Sci. Rep. 2020, 10, 2820. [Google Scholar] [CrossRef]
- Ördög, V. Beneficial Effects of Microalgae and Cyanobacteria in Plant/Soil-Systems, with Special Regard to Their Auxin-and Cytokinin-like Activity. In Proceedings of the International Workshop and Training Course on Microalgal Biology and Biotechnology, Mosonmagyaróvár, Hungary, 13–26 June 1999; pp. 13–26. [Google Scholar]
- Karthikeyan, N.; Prasanna, R.; Nain, L.; Kaushik, B.D. Evaluating the Potential of Plant Growth Promoting Cyanobacteria as Inoculants for Wheat. Eur. J. Soil Biol. 2007, 43, 23–30. [Google Scholar] [CrossRef]
- Sahu, D.; Priyadarshani, I.; Rath, B. Cyanobacteria -As Potential Biofertilizer. CIBTech J. Microbiol. 2012, 1, 20–26. [Google Scholar]
- Gashash, E.A.; Osman, N.A.; Alsahli, A.A.; Hewait, H.M.; Asmawi, A.E.; Alshallash, K.S.; El-Taher, A.M.; Azab, E.S.; Abd El-Raouf, H.S.; Ibrahim, M.F.M. Effects of Plant-Growth-Promoting Rhizobacteria (PGPR) and Cyanobacteria on Botanical Characteristics of Tomato (Solanum lycopersicon L.) Plants. Plants 2022, 11, 2732. [Google Scholar] [CrossRef]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional Quality of Ten Leafy Vegetables Harvested at Two Light Intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef]
- Bade, K.K.; Bhati, V.; Singh, V.B. Effect of Organic Manures and Biofertilizers on Growth, Yield and Quality of Chilli (Capsicum annum) Cv. Pusa Jwala. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2545–2552. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Billard, V.; Etienne, P.; Jannin, L.; Garnica, M.; Cruz, F.; Garcia-Mina, J.M.; Yvin, J.C.; Ourry, A. Two Biostimulants Derived from Algae or Humic Acid Induce Similar Responses in the Mineral Content and Gene Expression of Winter Oilseed Rape (Brassica napus L.). J. Plant Growth Regul. 2014, 33, 305–316. [Google Scholar] [CrossRef]
- Liu, X.; Rezaei Rashti, M.; Dougall, A.; Esfandbod, M.; Van Zwieten, L.; Chen, C. Subsoil Application of Compost Improved Sugarcane Yield through Enhanced Supply and Cycling of Soil Labile Organic Carbon and Nitrogen in an Acidic Soil at Tropical Australia. Soil Tillage Res. 2018, 180, 73–81. [Google Scholar] [CrossRef]
- Soussani, F.E.; Boutasknit, A.; Ben-Laouane, R.; Benkirane, R.; Baslam, M.; Meddich, A. Arbuscular Mycorrhizal Fungi and Compost-Based Biostimulants Enhance Fitness, Physiological Responses, Yield, and Quality Traits of Drought-Stressed Tomato Plants. Plants 2023, 12, 1856. [Google Scholar] [CrossRef]
- Hakkoum, Z.; Minaoui, F.; Douma, M.; Mouhri, K.; Loudiki, M. Diversity and Spatial Distribution of Soil Cyanobacteria along an Altitudinal Gradient in Marrakesh Area (Morocco). Appl. Ecol. Environ. Res. 2020, 18, 5527–5545. [Google Scholar] [CrossRef]
- Kotai, J. Instructions for Preparation of Modified Nutrient Solution Z8 for Algae. Nor. Inst. Water Res. 1972, 11, 5. [Google Scholar]
- Komárek, J. Cyanoprokaryota. 3. Heterocytous Genera; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Springer Spektrum: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Mischke, U.; Thackeray, S.; Dunbar, M.; McDonald, C.; Carvalho, L.; de Hoyos, C.; Jarvinen, M.; Laplace-Treyture, C.; Morabito, G.; Skjelbred, B.; et al. WISER Deliverable D3.1-4: Guidance Document on Sampling, Analysis and Counting Standards for Phytoplankton in Lakes; WISER: Tallinn, Estonia, 2012; pp. 1–51. [Google Scholar]
- Monod, J. The Growth of Bacterial Cultures. Annu. Rev. M 1949, 3, 371–394. [Google Scholar] [CrossRef]
- Strieth, D.; Stiefelmaier, J.; Wrabl, B.; Schwing, J.; Schmeckebier, A.; Di Nonno, S.; Muffler, K.; Ulber, R. A New Strategy for a Combined Isolation of EPS and Pigments from Cyanobacteria. J. Appl. Phycol. 2020, 32, 1729–1740. [Google Scholar] [CrossRef]
- Glickmann, E.; Dessaux, Y. A Critical Examination of the Specificity of the Salkowski Reagent for Indolic Compounds Produced by Phytopathogenic Bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef]
- Mazhar, S.; Hasnain, S. Screening of Native Plant Growth Promoting Cyanobacteria and Their Impact on Triticum aestivum Var. Uqab 2000 Growth. Afr. J. Agric. Res. 2011, 6, 3988–3993. [Google Scholar]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A Colorimetric Method for the Determination of Sugars. Nature 1956, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Bahorun, T.; Gressier, B.; Trotin, F.; Brunet, C.; Dine, T.; Luyckx, M.; Vasseur, J.; Cazin, M.; Cazin, J.; Pinkas, M. Oxygen Species Scavenging Activity of Phenolic Extracts from Hawthorn Fresh Plant Organs and Pharmaceutical Preparations. Arzneimittelforschung 1996, 46, 1086–1089. [Google Scholar]
- Haug, R. The Practical Handbook of Compost Engineering, 1st ed.; Lewis Publishers: New York, NY, USA, 1993. [Google Scholar]
- Pequerul, A.; Pérez, C.; Madero, P.; Val, J.; Monge, E. A Rapid Wet Digestion Method for Plant Analysis. In Optimization of Plant Nutrition; Springer: Dordrecht, The Netherlands, 1993; pp. 3–6. [Google Scholar] [CrossRef]
- Rodier, J.; Geoffray, C.; Rodi, L. L’analyse de L’eau: Eaux Naturelles, Eaux Résiduaires, Eau de Mer: Chimie, Physico-Chimie, Bactériologie, Biologie; Dunod: Malakoff, France, 1984. [Google Scholar]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Bennett, A.; Bogobad, L. Complementary Chromatic Adaptation in a Filamentous Blue-Green Alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Chézeau, A.; Turquet, J.; Quod, J.P.; Puiseux-Dao, S. Morphological and Toxicological Variability of Prorocentrum Lima Clones Isolated from Four Locations in the South-West Indian Ocean. Toxicon 2001, 39, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G. Methodes d’Analyses Des Sols: Documents de Travail Tous Droits Reserves; Centre régional de documentation pédagogique: Montpellier, France, 1978. [Google Scholar]
- Olsen, S.; Cole, C.; Watanabe, F.; Dean, L. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954; pp. 18–19.
- Baize, D. Guide des Analyses Courantes en Pédologie; INRA: Paris, France, 1988. [Google Scholar]
- Mobaligh, M.; Hassani, O.S.; Rahou, Y.A.; Fares, K. Impact of the Addition of Sugar Beet Lime Sludge on the Composting of Argan Oil By-Products. Catal. Res. 2023, 3, 17. [Google Scholar] [CrossRef]
- Elalaoui, A.C. Fertilisation Minérale des Cultures: Les Élèments Fertilisants Majeurs (N, P, K). Transf. Technol. Agric 2007, 155, 4. [Google Scholar]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of Liquid Seaweed Extracts on Growth of Tomato Seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Alavoine, F.; Crochon, M.; Fady, C.; Fallot, J.; Moras, P.; Pech, J.C. The Taste Quality of Fruits, Practical Methods of Analysis; Ministry of Agriculture: Paris, France, 1988; p. 50.
- Bremner, J.M. Determination of Nitrogen in Soil by the Kjeldahl Method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Shome, S.; Roy, D.; Bhattacharya, M.K. Arsenic Induced Changes in Growth and Physiological Responses in Vigna radiata Seedling: Effect of Curcumin Interaction. Am. J. Plant Sci. 2014, 5, 3609–3618. [Google Scholar] [CrossRef]
- Strasser, B.J.; Strasser, R.J. Measuring Fast Fluorescence Transients to Address Environmental Questions: The JIP-Test. In Photosynth. from Light to Biosph; KAP Press: Dordrecht, The Netherlands, 1995; pp. 4869–4872. [Google Scholar] [CrossRef]
- Pathy, K. Process for Preparation of Vitamin C and Method for Determination of Vitamin C in Tablets. Surg. Case Stud. Open Access J. 2018, 2, 1–17. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar Applications of a Legume-Derived Protein Hydrolysate Elicit Dose-Dependent Increases of Growth, Leaf Mineral Composition, Yield and Fruit Quality in Two Greenhouse Tomato Cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2024. Available online: https://www.R-project.org/ (accessed on 22 June 2025).
- Wei, T.; Simko, V. R package ‘corrplot’: Visualization of a Correlation Matrix (Version 0.95). 2024. Available online: https://github.com/taiyun/corrplot (accessed on 22 June 2025).
- Kassambara, A. ggcorrplot: Visualization of a Correlation Matrix Using ‘ggplot2’_. R Package Version 0.1.4.1. 2023. Available online: https://CRAN.R-project.org/package=ggcorrplot (accessed on 22 June 2025).
- Galili, T.; O’Callaghan, A.; Sidi, J.; Sievert, C. heatmaply: An R package for creating interactive cluster heatmaps for online publishing. Bioinformatics 2018, 34, 1600–1602. [Google Scholar] [CrossRef]
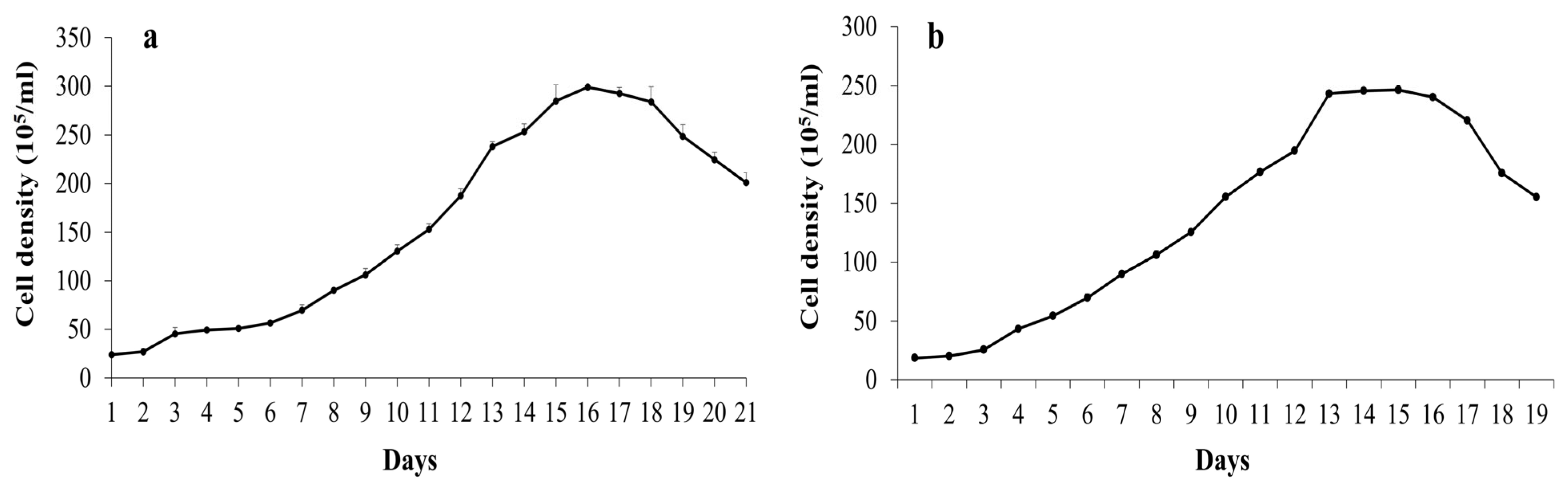
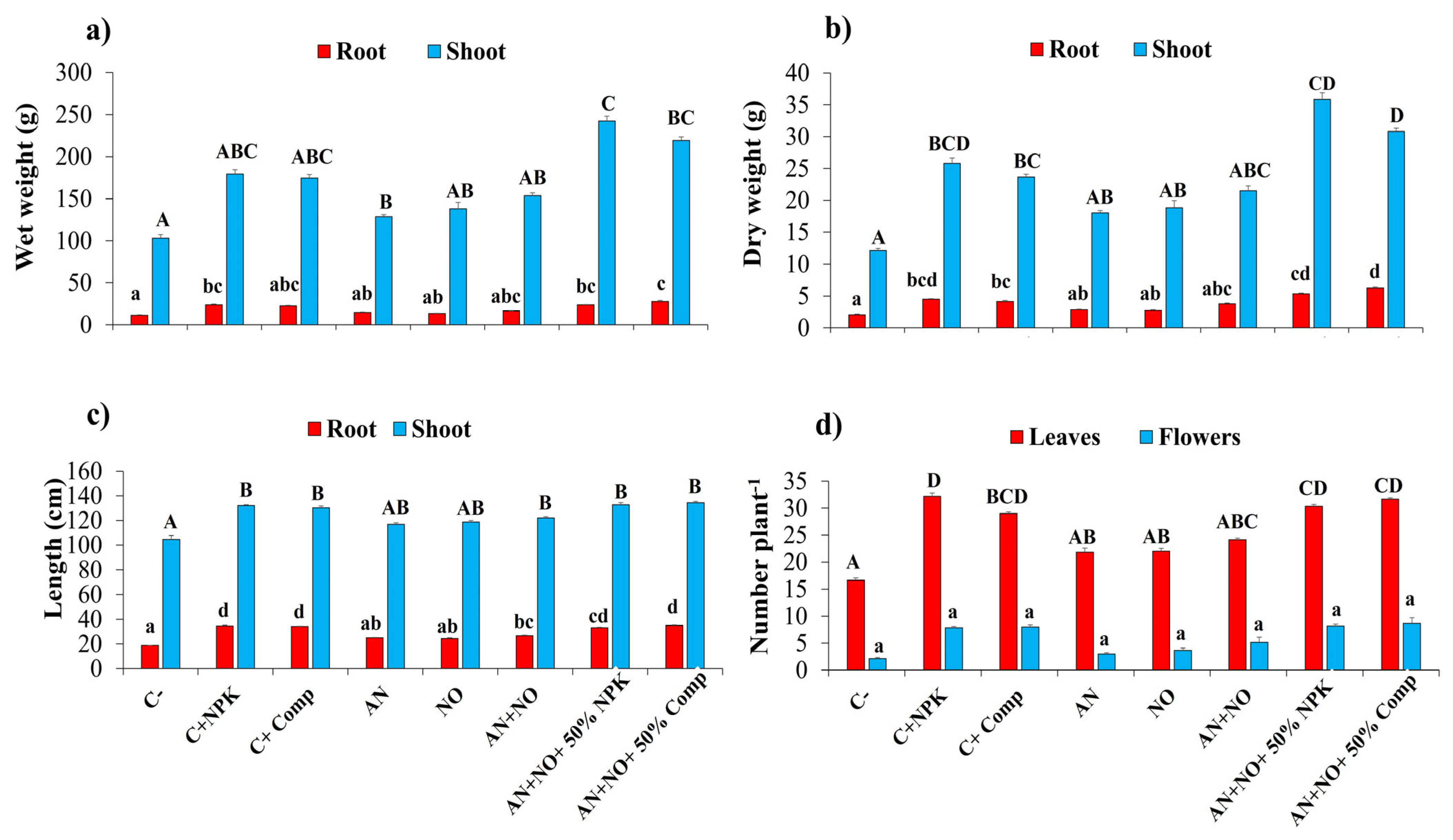
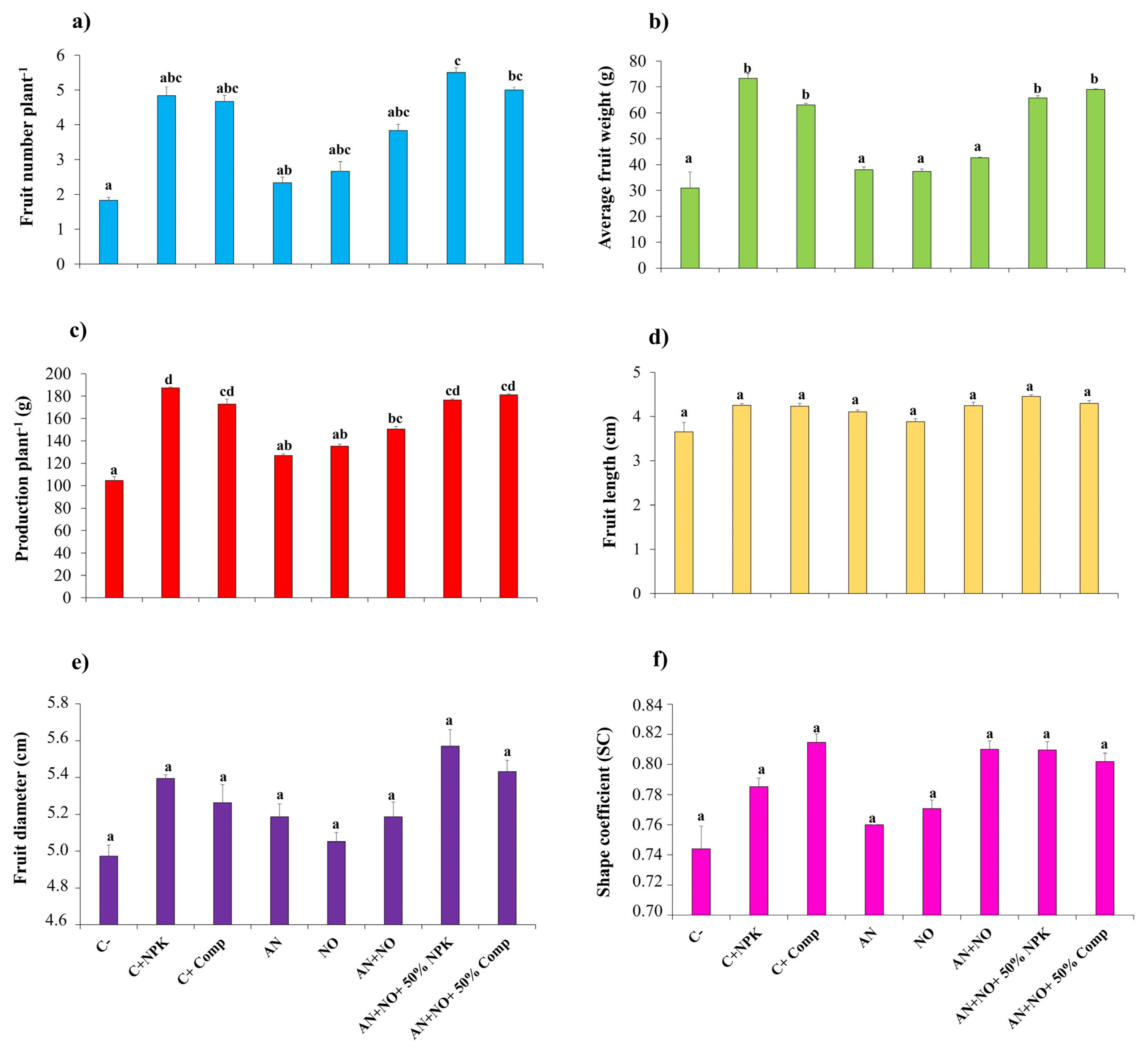


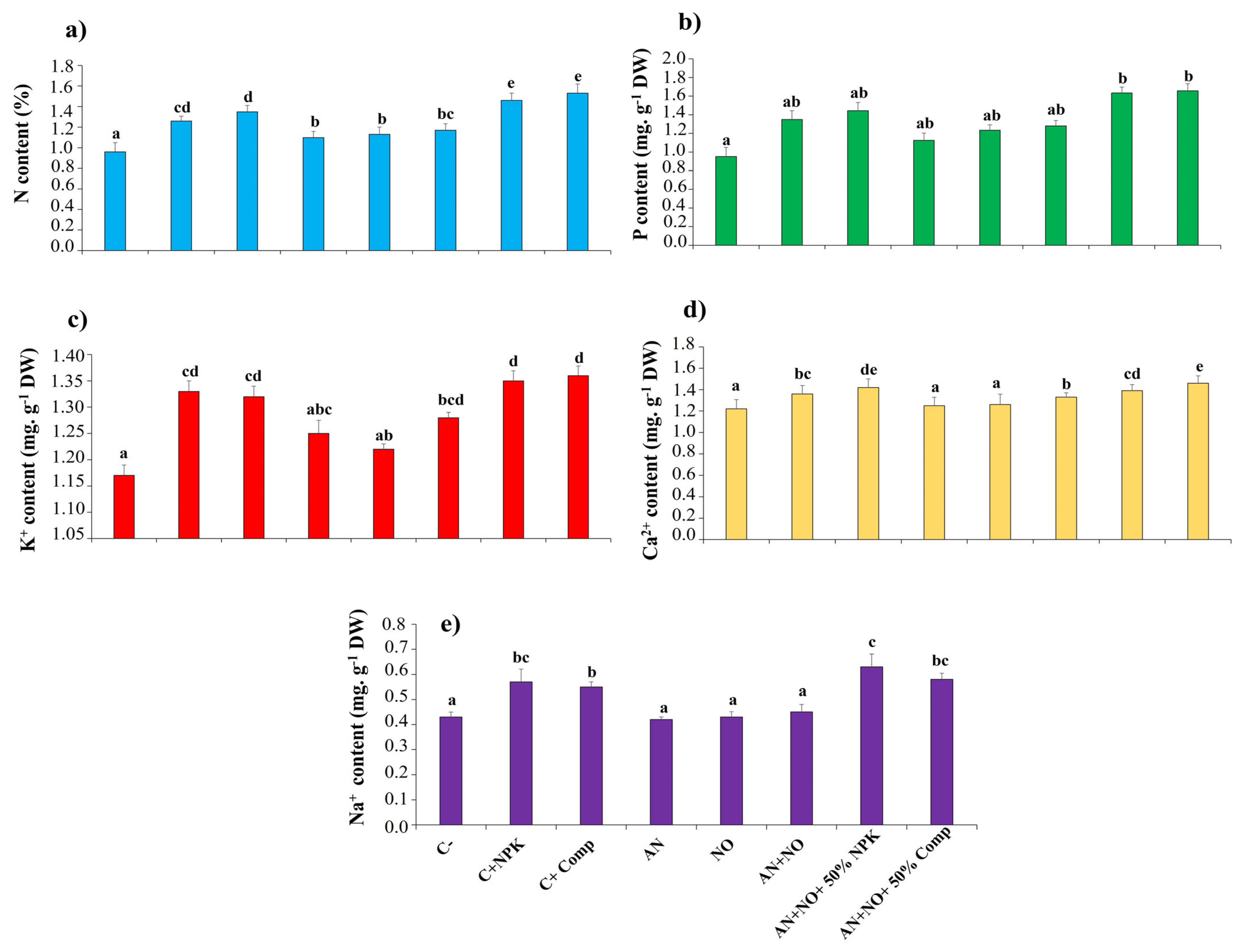

| Parameters | Anabaena cylindrica | Nostoc punctiforme |
|---|---|---|
| Growth rates (µ d−1) | 0.20 ± 0.01 a | 0.17 ± 0.01 b |
| Biomass (g DW L−1) | 0.77 ± 0.00 a | 0.71 ± 0.00 b |
| Chlorophyll a (mg L−1) | 3.64 ± 0.59 a | 3.08 ± 1.03 a |
| IAA production (μg mL−1) | 12.74 ± 0.46 a | 4.16 ± 0.25 b |
| EPS production (mg g−1 DW) | 42.73 ± 1.40 a | 24.67 ± 5.70 a |
| Nitrogen fixation N2 | + | + |
| Phosphate solubilization | + | + |
| Parameters | Anabaena cylindrica | Nostoc punctiforme |
|---|---|---|
| pH | 7.84 ± 0.02 a | 7.75 ± 0.02 a |
| Electrical conductivity (μS cm−1) | 366 ± 20 a | 487 ± 25 a |
| Phycocyanins (mg L−1) | 122.5 ± 3.1 a | 60.8 ± 2.9 b |
| Allophycocyanins (mg L−1) | 60.4 ± 5.4 a | 65 ± 4.8 a |
| Phycoerythrins (mg L−1) | 4.8 ± 0.8 a | 55.4 ± 4.0 b |
| Polyphenols (µg eq gallic acid mL−1) | 102.9 ± 0.5 a | 58.7 ± 4.9 b |
| Flavonoids (µg eq catechin mL−1) | 63.5 ± 0.4 a | 22.90 ± 0.14 b |
| Total carbohydrates (mg g DW−1) | 32.1 ± 1.7 a | 18.8 ± 1.3 b |
| Proteins (mg g DW−1) | 53.7 ± 4.8 a | 85.1 ± 5.3 b |
| Total phosphorus (%) | 0.12 ± 0.02 a | 0.13 ± 0.03 a |
| Total Kjeldahl nitrogen (%) | 2.31 ± 0.02 a | 1.98 ± 0.04 b |
| Total organic carbon (%) | 22.22 ± 0.00 a | 18.33 ± 0.01 b |
| K+ (%) | 0.48 ± 0.01 a | 0.47 ± 0.01 a |
| Ca2+ (%) | 0.29 ± 0.02 a | 0.33 ± 0.02 b |
| Na+ (%) | 0.10 ± 0.01 a | 0.13 ± 0.01 b |
| Treatments | pH | EC (mS cm−1) | TOC (%) | NTK (mg kg−1) | P (mg kg−1) | K+ (mg kg−1) | Na+ (mg kg−1) | Ca2+ (mg kg−1) |
|---|---|---|---|---|---|---|---|---|
| C- | 7.68 ± 0.04 a | 0.48 ± 0.03 c | 0.70 ± 0.03 e | 204.1 ± 5.4 d | 147 ± 9 d | 939 ± 11 d | 247.5 ± 6.4 e | 1095.2 ± 2.7 d |
| C+NPK | 7.15 ± 0.01 c | 1.33 ± 0.03 a | 0.75 ± 0.06 de | 385.6 ± 7.6 a | 327 ± 11 a | 1336 ± 13 a | 317.0 ± 3.9 c | 1194 ± 30 cd |
| C+Comp | 7.23 ± 0.01 bc | 1.30 ± 0.01 a | 1.93 ± 0.04 a | 325 ± 16 abc | 385 ± 17 a | 1301 ± 5 ab | 382.5 ± 3.5 a | 1433 ± 21 a |
| AN | 7.35 ± 0.02 b | 0.73 ± 0.07 b | 1.05 ± 0.03 bc | 279.7 ± 7.6 bcd | 195 ± 11 cd | 1083 ± 31 c | 276 ± 11 d | 1246 ± 22 c |
| NO | 7.30 ± 0.05 bc | 0.82 ± 0.02 b | 1.15 ± 0.09 bc | 257 ± 21 cd | 219 ± 14 bcd | 1041 ± 21 c | 280.8 ± 9.4 d | 1284 ± 21 bc |
| AN + NO | 7.32 ± 0.01 b | 0.88 ± 0.03 b | 1.18 ± 0.02 b | 294.8 ± 5.4 bc | 223 ± 10 bcd | 1103 ± 21 c | 291 ± 11 d | 1305 ± 30 bc |
| AN + NO + 50%NPK | 7.22 ± 0.01 bc | 1.26 ± 0.04 a | 0.93 ± 0.04 cd | 363 ± 11 ab | 287 ± 36 abc | 1282 ± 34 ab | 324.5 ± 8.4 c | 1227 ± 17 c |
| AN + NO + 50% Comp | 7.27 ± 0.02 bc | 1.24 ± 0.04 a | 1.75 ± 0.03 a | 355.3 ± 7.6 ab | 302 ± 37 ab | 1228 ± 10 b | 345.8 ± 3.7 b | 1373 ± 13 ab |
| Treatments | Proteins (mg g−1 FW) | Sugars (mg g−1 FW) | Polyphenols (mg g−1 DW) | Vitamin C (mg 100g−1 FW) |
|---|---|---|---|---|
| C− | 10.25 ± 0.05 a | 32.52 ± 0.01 a | 9.00 ± 0.58 a | 19.28 ± 0.35 a |
| C+NPK | 20.43 ± 0.04 f | 51.93 ± 0.04 ab | 15.33 ± 1.76 de | 30.74 ± 0.09 bcd |
| C+Comp | 23.18 ± 0.02 g | 55.02 ± 0.52 b | 17.34 ± 1.45 e | 32.13 ± 0.84 cd |
| AN | 15.20 ± 0.05 b | 35.11 ± 1.02 a | 9.67 ± 2.19 b | 21.60 ± 0.01 ab |
| NO | 15.39 ± 0.05 b | 47.19 ± 0.06 b | 11.33 ± 1.20 b | 22.63 ± 0.55 ab |
| AN + NO | 16.96 ± 0.01 c | 48.75 ± 0.37 b | 12.35 ± 1.67 ab | 25.42 ± 0.33 abc |
| AN + NO + 50% NPK | 18.41 ± 0.02 d | 49.45 ± 0.97 ab | 16.33 ± 1.20 c | 28.85 ± 0.18 cd |
| AN + NO + 50% Comp | 19.26 ± 0.05 e | 50.57 ± 2.00 ab | 18.32 ± 1.22 c | 30.40 ± 0.36 d |
| Treatments | pH | Total Titratable Acidity (%) | Dry Matter (%) | P (mg g−1) | K+ (mg g−1) | Na+ (mg g−1) | Ca2+ (mg g−1) |
|---|---|---|---|---|---|---|---|
| C− | 4.25 ± 0.00 a | 0.43 ± 0.01 d | 5.21 ± 0.02 a | 2.21 ± 0.02 a | 3.11 ± 0.03 a | 0.78 ± 0.01 d | 1.74 ± 0.02 a |
| C+NPK | 4.29 ± 0.02 a | 0.34 ± 0.00 ab | 6.31 ± 0.04 c | 3.39 ± 0.02 d | 4.00 ± 0.09 bcd | 0.68 ± 0.00 bc | 2.84 ± 0.02 d |
| C+Comp | 4.28 ± 0.04 a | 0.35 ± 0.00 ab | 6.18 ± 0.05 c | 3.77 ± 0.01 e | 4.24 ± 0.07 cde | 0.67 ± 0.02 ab | 2.86 ± 0.02 d |
| AN | 4.26 ± 0.02 a | 0.36 ± 0.00 b | 5.58 ± 0.11 b | 2.30 ± 0.05 a | 3.71 ± 0.03 abc | 0.72 ± 0.01 c | 1.91 ± 0.03 a |
| NO | 4.25 ± 0.00 a | 0.42 ± 0.00 cd | 5.56 ± 0.07 b | 2.62 ± 0.05 b | 3.53 ± 0.18 ab | 0.67 ± 0.01 ab | 2.08 ± 0.00 b |
| AN + NO | 4.24 ± 0.04 a | 0.30 ± 0.00 a | 5.39 ± 0.02 ab | 2.53 ± 0.00 b | 3.60 ± 0.05 abc | 0.64 ± 0.00 ab | 2.51 ± 0.02 c |
| AN + NO + 50%NPK | 4.28 ± 0.07 a | 0.31 ± 0.03 a | 6.14 ± 0.04 c | 3.32 ± 0.00 d | 4.75 ± 0.03 e | 0.62 ± 0.00 a | 2.92 ± 0.04 de |
| AN + NO + 50%Com | 4.27 ± 0.05 a | 0.37 ± 0.02 bc | 6.04 ± 0.04 c | 3.03 ± 0.00 c | 4.60 ± 0.04 de | 0.63 ± 0.01 ab | 3.06 ± 0.00 e |
| Parameters | Results | |
|---|---|---|
| Physical analysis | pH | 7.82 ± 0.02 |
| Electrical conductivity | 0.37 ± 0.02 (mS cm−1) | |
| Mechanical analysis | Clay | 49 (%) |
| Sand | 25.7 (%) | |
| Silt | 25.3 (%) | |
| Soil type | Clay soil | |
| Chemical analysis | Total organic carbon | 0.20 ± 0.04 (%) |
| Available phosphorus | 104.59 ± 0.213 (mg kg−1) | |
| Total nitrogen | 173.88 ± 5.35 (mg kg−1) | |
| Potassium | 795.50 ± 1.77 (mg kg−1) | |
| Calcium | 939.25 ± 16.44 (mg kg−1) | |
| Sodium | 195.00 ± 1.41 (mg kg−1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakkoum, Z.; Minaoui, F.; Tazart, Z.; Chabili, A.; Douma, M.; Mouhri, K.; Loudiki, M. Impact of a Soil Cyanobacteria Consortium-Based Bioinoculant on Tomato Growth, Yield, and Fruit Quality. Plants 2025, 14, 2034. https://doi.org/10.3390/plants14132034
Hakkoum Z, Minaoui F, Tazart Z, Chabili A, Douma M, Mouhri K, Loudiki M. Impact of a Soil Cyanobacteria Consortium-Based Bioinoculant on Tomato Growth, Yield, and Fruit Quality. Plants. 2025; 14(13):2034. https://doi.org/10.3390/plants14132034
Chicago/Turabian StyleHakkoum, Zineb, Farah Minaoui, Zakaria Tazart, Amer Chabili, Mountasser Douma, Khadija Mouhri, and Mohammed Loudiki. 2025. "Impact of a Soil Cyanobacteria Consortium-Based Bioinoculant on Tomato Growth, Yield, and Fruit Quality" Plants 14, no. 13: 2034. https://doi.org/10.3390/plants14132034
APA StyleHakkoum, Z., Minaoui, F., Tazart, Z., Chabili, A., Douma, M., Mouhri, K., & Loudiki, M. (2025). Impact of a Soil Cyanobacteria Consortium-Based Bioinoculant on Tomato Growth, Yield, and Fruit Quality. Plants, 14(13), 2034. https://doi.org/10.3390/plants14132034







