Assessment of Surface Water Quality in the Krynka River Basin Using Fluorescence Spectroscopy Methods
Abstract
1. Introduction
2. Materials and Methods
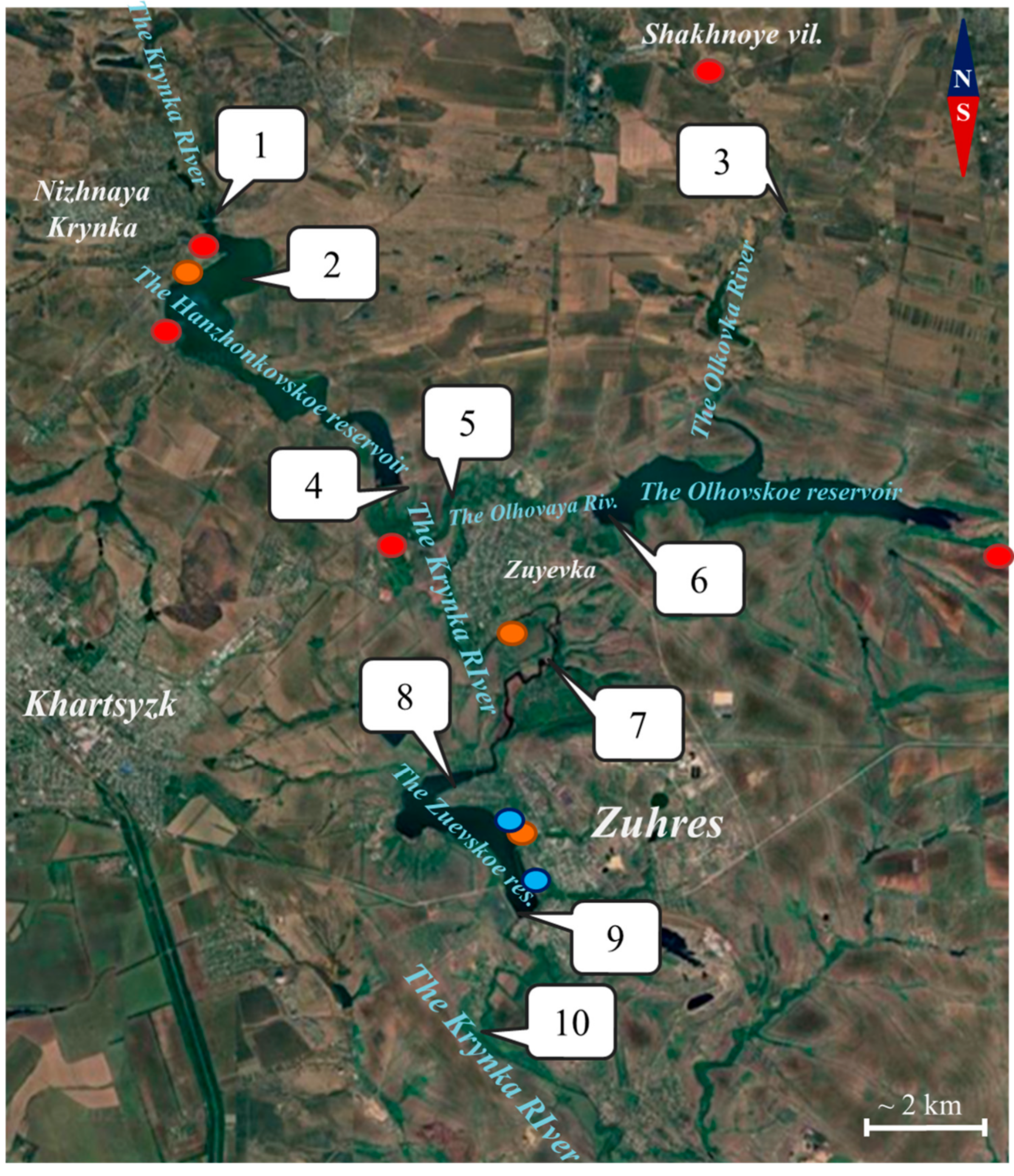
2.1. Monitoring Points and Research Objects
2.2. Determination of the Physicochemical Composition of Water Samples
2.3. Water Sampling and Determination of Phytoplankton Species Composition
2.4. Spectrophotometric Determination of Photopigment Content
2.5. Methodology of Fluorimetric Research
2.6. Data Analysis
3. Results
3.1. Physicochemical Composition of Water Samples
3.2. Species Composition of Phytoplankton in the Krynka River Basin
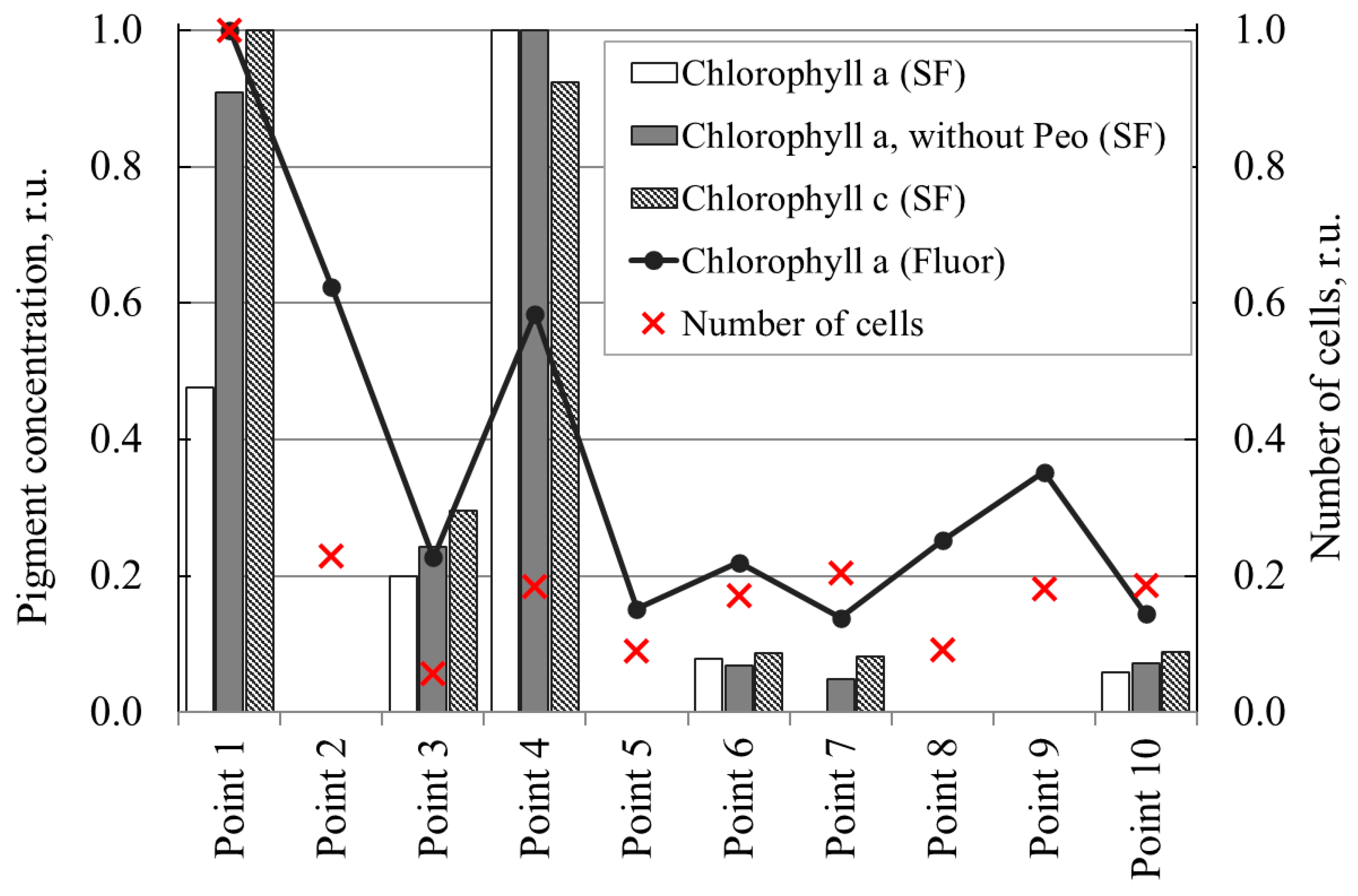
3.3. Spectrophotometric Determination of Photopigment Composition of Water Samples
3.4. Analysis of Photosynthetic Activity of Natural Phytoplankton of the Kalmius River
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SAAs | surfactants |
| COD | chemical oxygen demand |
| MPC | maximum permissible concentrations |
| SCWPI | specific Combinatorial Water Pollution Index |
References
- Panov, B.S.; Dudik, A.M.; Shevchenko, O.A.; Matlak, E.S. On pollution of the biosphere in industrial areas: The example of the Donets coal Basin. Int. J. Coal Geol. 1999, 40, 199–210. [Google Scholar] [CrossRef]
- Udod, V.M.; Zhukova, E.G. Regional-ecological approach to the assessment of possible aftermath of pollution of water basin of the Kalmius River. J. Water Chem. Technol. 2015, 37, 48–50. [Google Scholar] [CrossRef]
- Nazarenko, O.V. Hydrochemical features of rivers in coal mining areas (a study of East Donbass). In Proceedings of the 17th International Multidisciplinary Scientific Geoconference SGEM 2017, Albena, Bulgaria, 29 June–5 July 2017; Volume 17, pp. 371–378. [Google Scholar]
- Bespalova, S.V.; Romanchuk, S.M.; Chufitskiy, S.V.; Perebeinos, V.V.; Gotin, B.A. Fluorimetric Analysis of the Impact of Coal Sludge Pollution on Phytoplankton. Biophysics 2020, 65, 850–857. [Google Scholar] [CrossRef]
- Vorovka, V.P. Anthropogenic aqua landscapes of the sea of Azov. Sci. Bull. Kherson State Univ. Ser. Geogr. Sci. 2021, 14, 30–38. [Google Scholar]
- Selifonova, J.P. Functioning of the Sea of Azov ecosystem. Inland Water Biol. 2008, 1, 199–203. [Google Scholar] [CrossRef]
- Neverova-Dziopak, E.; Dan, O. Assessment of a Metallurgical Plant Impact on the Sea of Azov. Geomat. Environ. Eng. 2022, 16, 5–29. [Google Scholar] [CrossRef]
- Glukhov, A.Z.; Safonov, A.I. Phytomonitoring concept for anthropogenically disturbed regions. Scientific research of the SCO countries: Synergy and integration. In Proceedings of the International Conference, Beijing, China, 14 February 2024; pp. 96–104. [Google Scholar]
- Zinicovscaia, I.I.; Safonov, A.I.; Yushin, N.S.; Nespirnyi, V.N.; Germonova, E.A. Phytomonitoring in Donbass for Identifying New Geochemical Anomalies. Russ. J. Gen. Chem. 2024, 94, 3472–3482. [Google Scholar] [CrossRef]
- Nespirnyi, V.; Safonov, A. The importance of principal component analysis for environmental biodiagnostics of Donbass. E3S Web Conf. 2024, 555, 01007. [Google Scholar] [CrossRef]
- Safonov, A. Assessing landscape disturbance in Donbass using phytomonitoring data. In Proceedings of the International Conference on Advance in Energy, Ecology and Agriculture (AEEA2024), Khujand, Tajikistan, 24–26 June 2024; EDP Sciences: Les Ulis, France; p. 1031. [Google Scholar]
- Gnatyuk, N.Y. Systematic structure of the Krynka river basin flora. Ind. Bot. 2012, 12, 75–80. [Google Scholar]
- Essa, D.I.; Elshobary, M.E.; Attiah, A.M.; Salem, Z.E.; Keshta, A.E.; Edokpayi, J.N. Assessing phytoplankton populations and their relation to water parameters as early alerts and biological indicators of the aquatic pollution. Ecol. Indic. 2024, 159, 111721. [Google Scholar] [CrossRef]
- Du, W.; Wang, J.; Zhao, X.; Liang, E.; He, J.; Kong, L.; Cai, P.; Xu, N. Algal or bacterial community: Who can be an effective indicator of the impact of reclaimed water recharge in an urban river. Water Res. 2023, 247, 120821. [Google Scholar] [CrossRef]
- Xiaofeng, L.; Georgakakos, A.P. Chlorophyll a estimation in lakes using multi-parameter sonde data. Water Res. 2021, 205, 117661. [Google Scholar]
- Zhao, Z. Contrasting the assembly of phytoplankton and zooplankton communities in a polluted semi-closed sea: Effects of marine compartments and environmental selection. Environ. Pollut. 2021, 285, 117256. [Google Scholar] [CrossRef]
- Cai, G. Temporal shifts in the phytoplankton network in a large eutrophic shallow freshwater lake subjected to major environmental changes due to human interventions. Water Res. 2024, 261, 122054. [Google Scholar] [CrossRef]
- Barnett, M.L.; Kemp, A.E.; Hickman, A.E.; Purdie, D.A. Environmental controls on the interannual variability in chlorophyll and phytoplankton community structure within the seasonal sub surface chlorophyll maximum in the western English Channel. Cont. Shelf Res. 2024, 277, 105253. [Google Scholar] [CrossRef]
- Choi, C.J.; Berges, J.A.; Young, E.B. Rapid effects of diverse toxic water pollutants on chlorophyll a fluorescence: Variable responses among freshwater microalgae. Water Res. 2012, 46, 2615–2626. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, J.; Kwon, I.; Song, H.; Kim, B.; Kim, T.; Lee, C.; Yoon, S.J.; Noh, J.; Hong, S.; et al. Ecotoxicological effects of suspended sediments on marine microalgae using flow cytometry and pulse-amplitude modulation (PAM) fluorometry. Mar. Pollut. Bull. 2024, 208, 116968. [Google Scholar] [CrossRef]
- Antal, T.K.; Volgusheva, A.A.; Drozdenko, T.V.; Konyukhov, I.V.; Khruschev, S.S.; Chervitsov, R.N.; Plyusnina, T.Y.; Riznichenko, G.Y.; Rubin, A.B. Analysis of chlorophyll fluorescence induction curves (OJIP transients) of phytoplankton under conditions of high photosynthetic activity. J. Appl. Phycol. 2025, 37, 837–884. [Google Scholar] [CrossRef]
- Bähr, S.; van der Meij, S.E.T.; Santoro, E.P.; Benzoni, F. Imaging PAM fluorometry reveals stable photosynthetic efficiency in multibiont symbioses on coral reefs. Front. Mar. Sci. 2025, 12, 1568287. [Google Scholar] [CrossRef]
- Lysenko, V.; Rajput, V.D.; Singh, R.K.; Guo, Y.; Kosolapov, A.; Usova, E.; Varduny, T.; Chalenko, E.; Yadronova, O.; Dmitriev, P.; et al. Chlorophyll fluorometry in evaluating photosynthetic performance: Key limitations, possibilities, perspectives and alternatives. Physiol. Mol. Biol. Plants 2022, 28, 2041–2056. [Google Scholar] [CrossRef]
- Rousso, B.Z.; Bertone, E.; Stewart, R.A.; Rinke, K.; Hamilton, D.P. Light-induced fluorescence quenching leads to errors in sensor measurements of phytoplankton chlorophyll and phycocyanin. Water Res. 2021, 198, 117133. [Google Scholar] [CrossRef] [PubMed]
- Russian Ministry of Health. SanPin 2.1.4.1074-01. Drinking Water Hygienic Requirements for Water Quality of Centralized Drinking Water Supply Systems. Quality Control (Instead SanPin 2.1.4.559-96); Russian Ministry of Health: Moscow, Russia, 2001; No. 24; 44p.
- Moshkova, N.A.; Hollerbach, M.M. (Eds.) Key to Freshwater Algae of the USSR; Nauka: Leningrad, USSR, 1986. [Google Scholar]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophyll a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Ritchie, R.J. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- Vieira, N.; Amat, F. Variation in chlorophylla concentrations and Margalef’s index of pigment diversity in two solar salt ponds, Aveiro, Portugal. Int. J. Salt Lake Res. 1993, 2, 41–45. [Google Scholar] [CrossRef]
- Antal, T.; Konyukhov, I.; Volgusheva, A.; Plyusnina, T.; Khruschev, S.; Kukarskikh, G.; Goryachev, S.; Rubin, A. Chlorophyll fluorescence induction and relaxation system for the continuous monitoring of photosynthetic capacity in photobioreactors. Physiol. Plant. 2018, 165, 476–486. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Vredenberg, W.; Durchan, M.; Prášil, O. The analysis of PS II photochemical activity using single and multi-turnover excitations. J. Photochem. Photobiol. B 2012, 107, 45–54. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Govindjee, G. Polyphasic chlorophyll a fluorescent transient in plants and cyanobacteria. Photochem. Photobiol. 1999, 61, 32–42. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J.; Govindjee, G. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Arsad, S.; Putra, K.T.; Latifah, N.; Kadim, M.K.; Musa, M. Epiphytic microalgae community as aquatic bioindicator in Brantas River, East Java, Indonesia. Biodiversitas 2021, 22, 2961–2971. [Google Scholar] [CrossRef]
- Cochero, J.; Licursi, M.; Gómez, N. Changes in the epipelic diatom assemblage in nutrient rich streams due to the variations of simultaneous stressors. Limnologica 2015, 51, 15–23. [Google Scholar] [CrossRef]
- Çelekli, A.; Toudjani, A.A.; Lekesiz, H.Ö.; Çetin, T. Ecological quality assessment of running waters in the North Aegean catchment with diatom metrics and multivariate approach. Limnologica 2018, 73, 20–27. [Google Scholar] [CrossRef]
- Park, J.; Bergey, E.A.; Han, T.; Pandey, L.K. Diatoms as indicators of environmental health on Korean islands. Aquat. Toxicol. 2020, 227, 105594. [Google Scholar] [CrossRef] [PubMed]
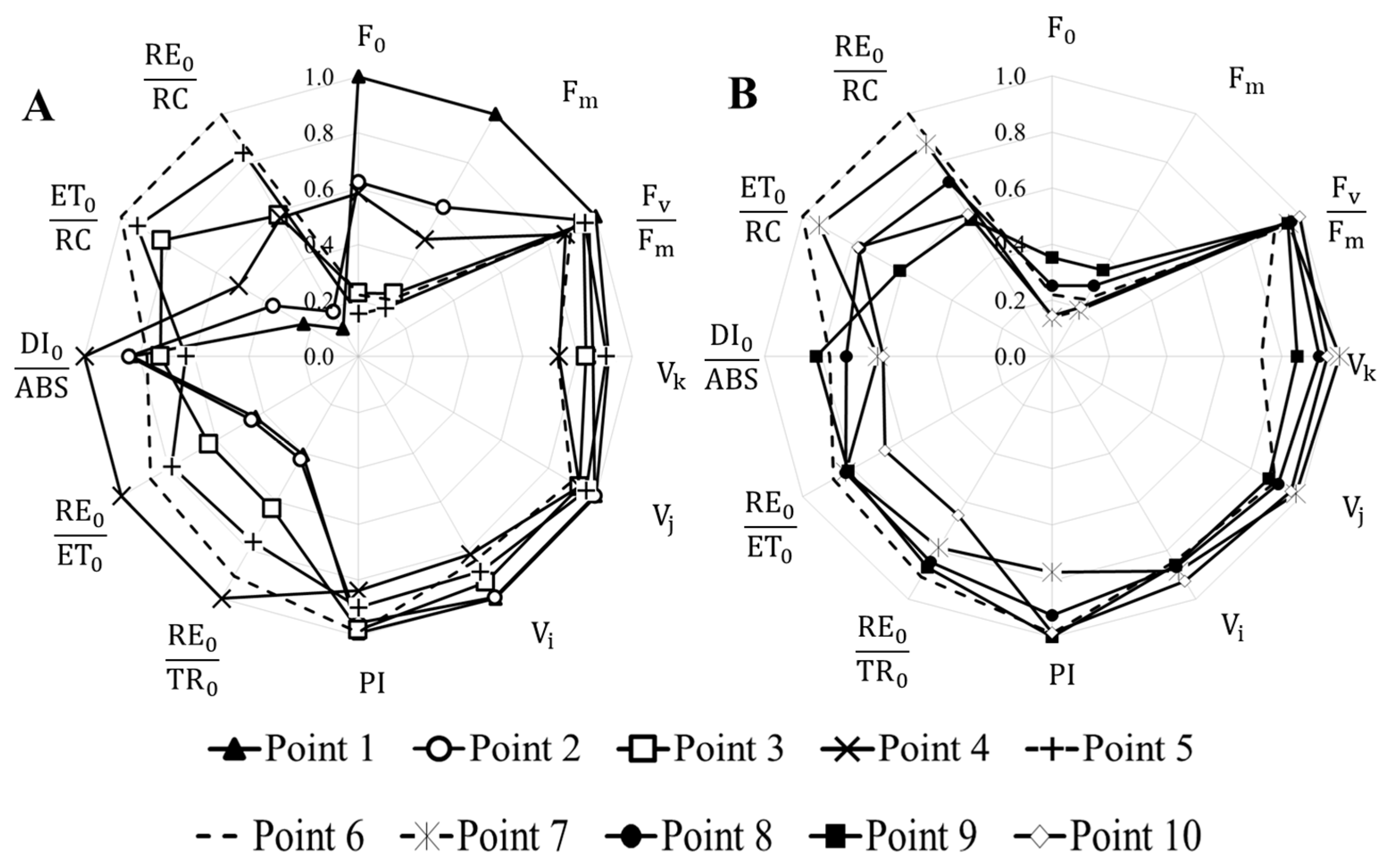
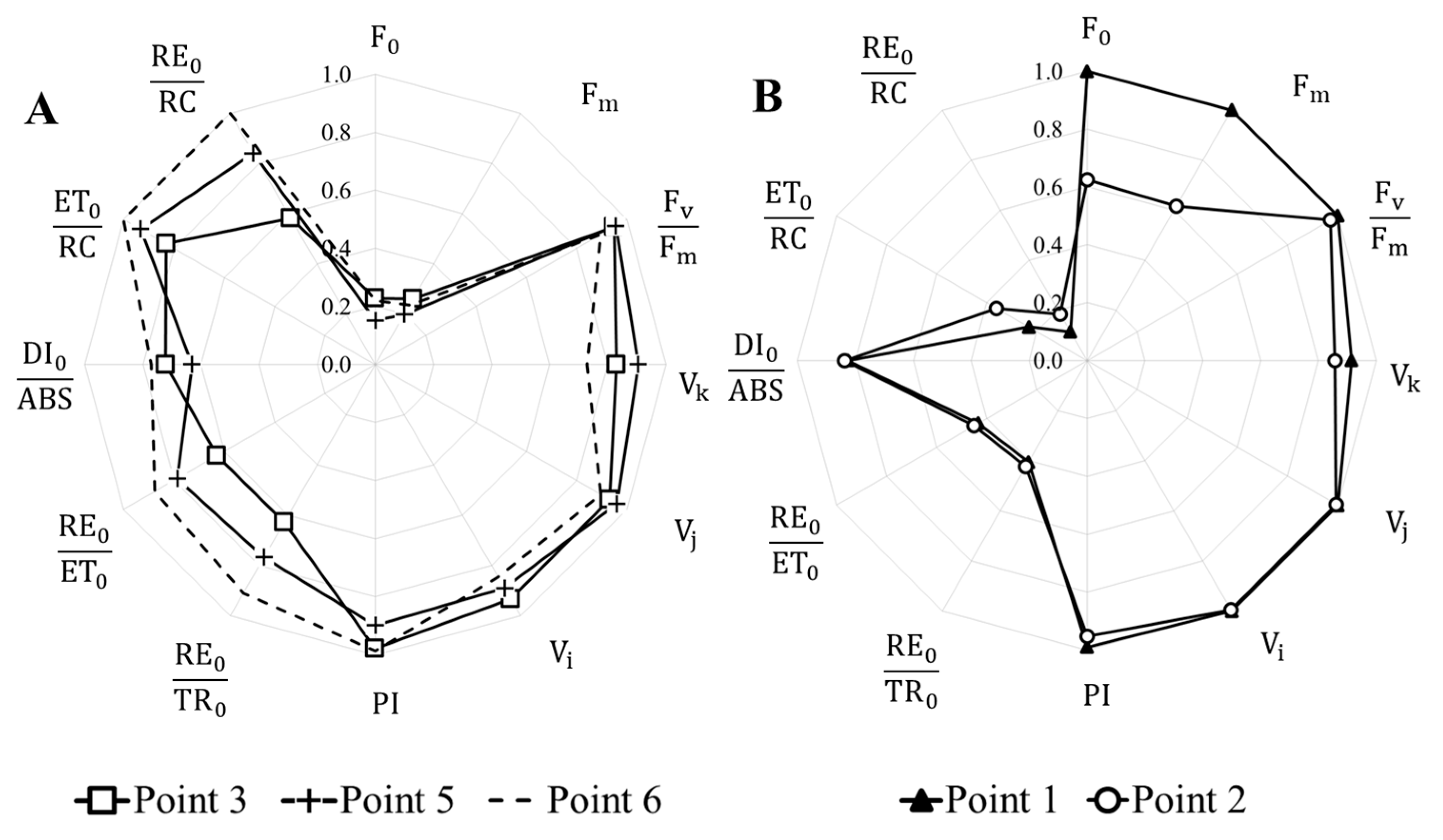
| F;0 | Minimal fluorescence |
| Fm | Maximum fluorescence |
| Fv Fm−1 | Maximum quantum fluorescence yield |
| Vj | Variable fluorescence at J-phase, reflects the proportion of closed reaction centers in photosystem I |
| Vi | Variable fluorescence at I-phase, reflects the ability of photosystem I acceptors to oxidize a plastoquinone pool |
| Vk | Variable fluorescence at 0.3 ms |
| PI | Total photosynthetic activity performance index |
| RE0 ET0−1 | Efficiency of electron transfer from plastoquinone to photosystem I acceptors |
| ET0 ABS−1 | Quantum yield of electron transport between primary quinone and plastoquinone pool |
| RE0 ABS−1 | Quantum yield of electron transport between primary quinone and to photosystem I acceptors |
| DI0 ABS−1 | Quantum yield of energy dissipation in photosystem II antennae |
| RE0 RC−1 | The flux of electrons transferred between primary quinone and to photosystem I acceptors |
| ET0 RC−1 | The flux of electrons transferred between primary quinone and plastoquinone pool |
| TR0 RC−1 | Maximum trapped exciton flux per active photosystem II |
| DI0 RC−1 | The flux of dissipated energy |
| Indicators, mg L−1 | MPC | Point 1 | Point 2 | Point 3 | Point 4 | Point 5 | Point 6 | Point 7 | Point 8 | Point 9 | Point 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anionic surfactants | 0.5 | 0.53 | 0.34 | 0.22 | 0.39 | 0.11 | 0.10 | 0.26 | 0.20 | 0.37 | 0.26 |
| Sulfates | 500 | 658 | 645 | 757 | 656 | 766 | 664 | 773 | 863 | 934 | 815 |
| Chlorides | 350 | 998 | 907 | 922 | 810 | 589 | 570 | 643 | 757 | 826 | 865 |
| Phenol | 0.1 | 0.56 | 0.17 | 0.18 | 0.17 | 0.11 | 0.11 | 0.12 | 0.16 | 0.16 | 0.23 |
| Ammonium | 1.5 | 0.62 | <0.1 | <0.1 | 0.77 | <0.1 | <0.1 | 0.19 | <0.1 | <0.1 | 1.87 |
| Chemical oxygen demand | 15 | 42.8 | 29.7 | 13.4 | 35.5 | 15.7 | 18.4 | 20.9 | 26.4 | 28.4 | 24.1 |
| Manganese | 0.1 | 0.20 | 0.04 | <0.02 | 0.03 | 0.02 | <0.02 | <0.02 | <0.02 | 0.02 | 0.21 |
| Division | Class | Order | Family | Genus | Species | Identified to Genus |
|---|---|---|---|---|---|---|
| Bacillariophyta | 4 | 10 | 11 | 15 | 13 | 10 |
| Chlorophyta | 2 | 3 | 5 | 13 | 19 | 5 |
| Cyanobacteria | 1 | 4 | 4 | 4 | 4 | 3 |
| Euglenozoa | 1 | 1 | 2 | 3 | 3 | 3 |
| Total | 8 | 18 | 22 | 35 | 39 | 21 |
| Pigments, µg L−1 | Chlorophyll a | Chlorophyll a (Without Pheophytin) | Pheophytin | Chlorophyll b | Chlorophyll c1 and c2 | Cyanobacterial Carotenoids | Diatom Carotenoids | Pigment Index (D430/D664) |
|---|---|---|---|---|---|---|---|---|
| Point 1 | 22.79 | 53.25 | 52.20 | 0.00 | 244.56 | 13.54 | 33.86 | 2.26 |
| Point 3 | 9.56 | 14.24 | 8.08 | 0.00 | 72.20 | 4.44 | 11.10 | 2.48 |
| Point 4 | 47.82 | 58.62 | 18.83 | 0.00 | 225.78 | 21.91 | 54.77 | 1.92 |
| Point 6 | 3.73 | 4.01 | 0.51 | 0.00 | 21.21 | 2.52 | 6.31 | 2.57 |
| Point 7 | 0.00 | 2.83 | 6.79 | 0.00 | 19.91 | 1.79 | 4.47 | 3.33 |
| Point 10 | 2.83 | 4.25 | 2.45 | 0.00 | 21.77 | 1.67 | 4.18 | 2.50 |
| Chlorophyll a (Fluor) | Number | Chlorophyll a (SF) | Chlorophyll a, Without Pheo (SF) | Chlorophyll c (SF) | |
|---|---|---|---|---|---|
| Chlorophyll a (Fluor) | 0.826 | 0.660 | 0.890 | 0.934 | |
| Number | 0.826 | 0.230 | 0.571 | 0.654 | |
| Chlorophyll a (SF) | 0.660 | 0.230 | 0.926 | 0.873 | |
| Chlorophyll a, without Pheo (SF) | 0.890 | 0.571 | 0.926 | 0.992 | |
| Chlorophyll c (SF) | 0.934 | 0.654 | 0.873 | 0.992 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chufitskiy, S.; Romanchuk, S.; Meskhi, B.; Olshevskaya, A.; Shevchenko, V.; Odabashyan, M.; Teplyakova, S.; Vershinina, A.; Savenkov, D. Assessment of Surface Water Quality in the Krynka River Basin Using Fluorescence Spectroscopy Methods. Plants 2025, 14, 2014. https://doi.org/10.3390/plants14132014
Chufitskiy S, Romanchuk S, Meskhi B, Olshevskaya A, Shevchenko V, Odabashyan M, Teplyakova S, Vershinina A, Savenkov D. Assessment of Surface Water Quality in the Krynka River Basin Using Fluorescence Spectroscopy Methods. Plants. 2025; 14(13):2014. https://doi.org/10.3390/plants14132014
Chicago/Turabian StyleChufitskiy, Sergey, Sergey Romanchuk, Besarion Meskhi, Anastasiya Olshevskaya, Victoria Shevchenko, Mary Odabashyan, Svetlana Teplyakova, Anna Vershinina, and Dmitry Savenkov. 2025. "Assessment of Surface Water Quality in the Krynka River Basin Using Fluorescence Spectroscopy Methods" Plants 14, no. 13: 2014. https://doi.org/10.3390/plants14132014
APA StyleChufitskiy, S., Romanchuk, S., Meskhi, B., Olshevskaya, A., Shevchenko, V., Odabashyan, M., Teplyakova, S., Vershinina, A., & Savenkov, D. (2025). Assessment of Surface Water Quality in the Krynka River Basin Using Fluorescence Spectroscopy Methods. Plants, 14(13), 2014. https://doi.org/10.3390/plants14132014








