Plant Diversity and Microbial Community Drive Ecosystem Multifunctionality in Castanopsis hystrix Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Plot Design and Soil Sampling
2.3. Sampling Analysis
2.4. Evaluating Ecosystem Multifunctionality
2.5. Statistical Analysis
3. Results
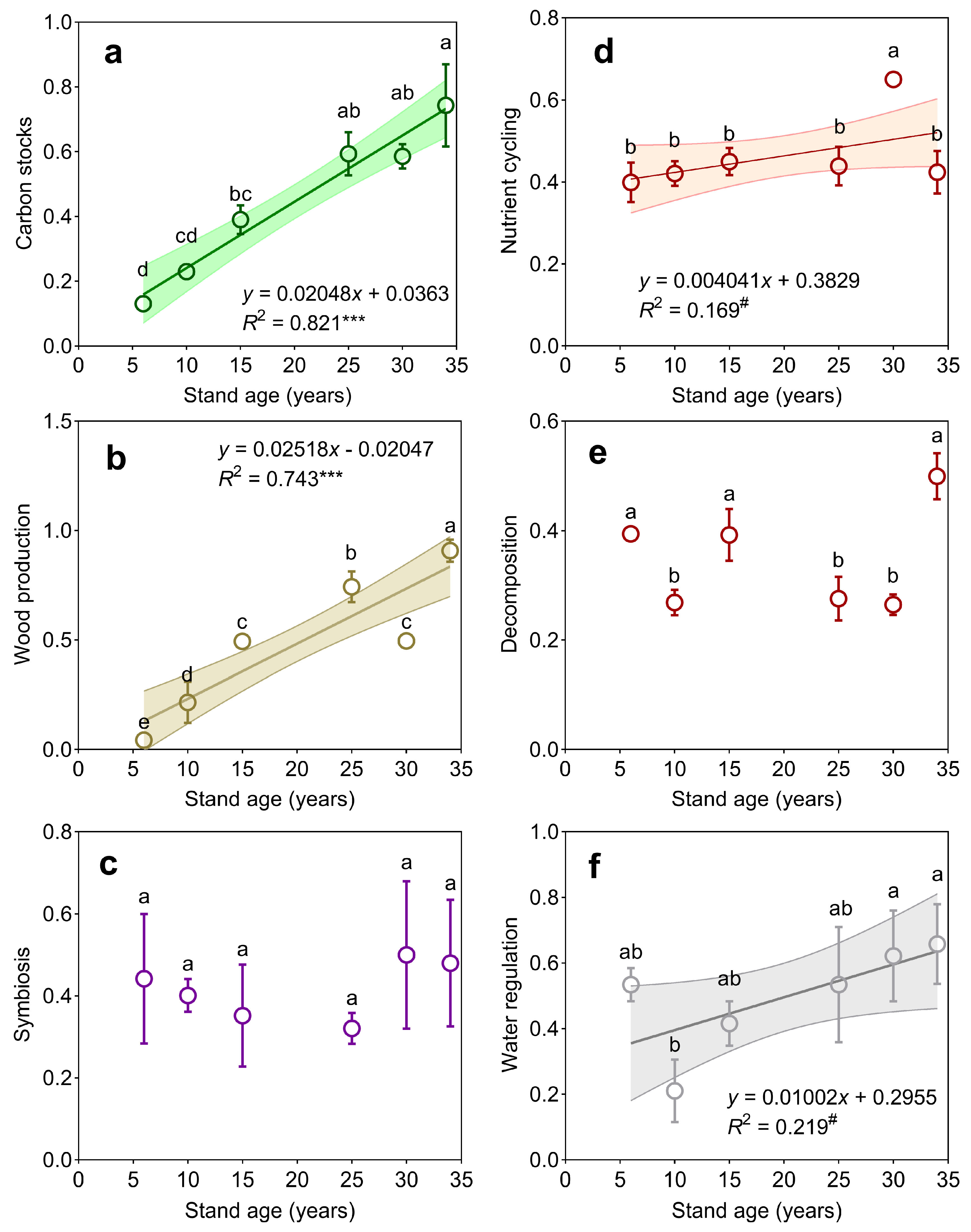
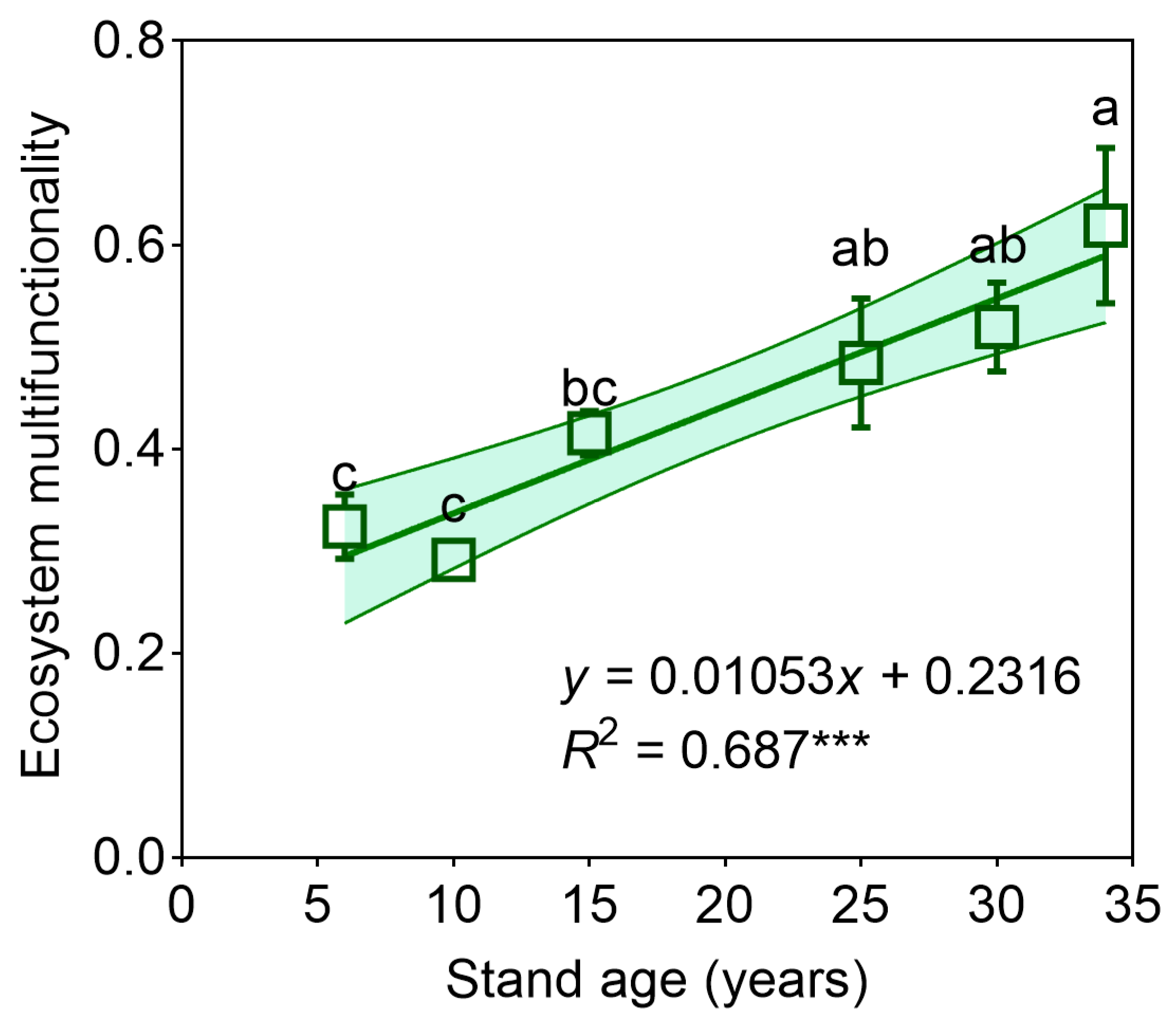
3.1. Individual Ecosystem Functions and Multifunctionality
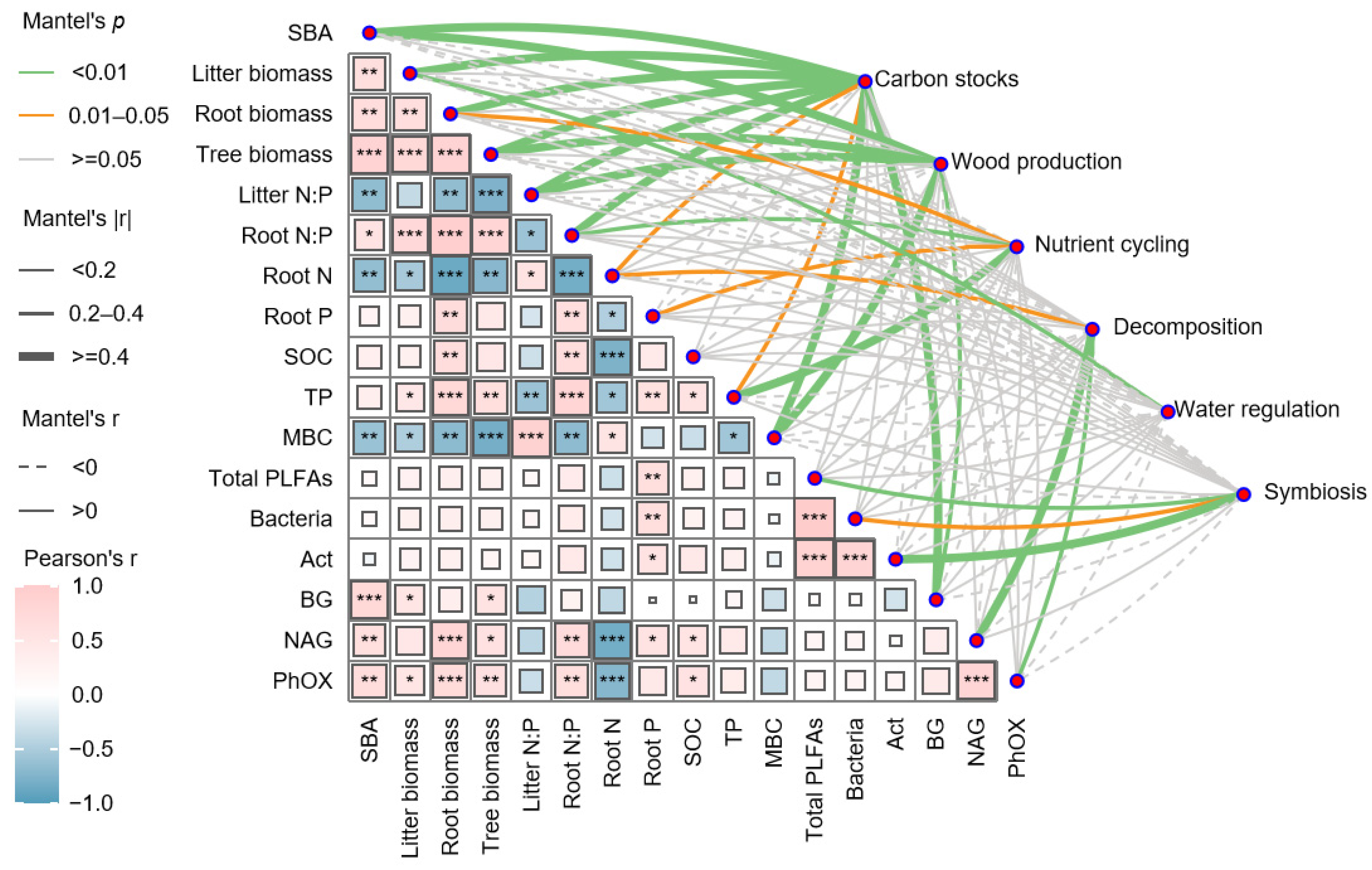
3.2. Relationships Among Ecosystem Functions
3.3. Factors Influencing Individual Ecosystem Functions and Multifunctionality
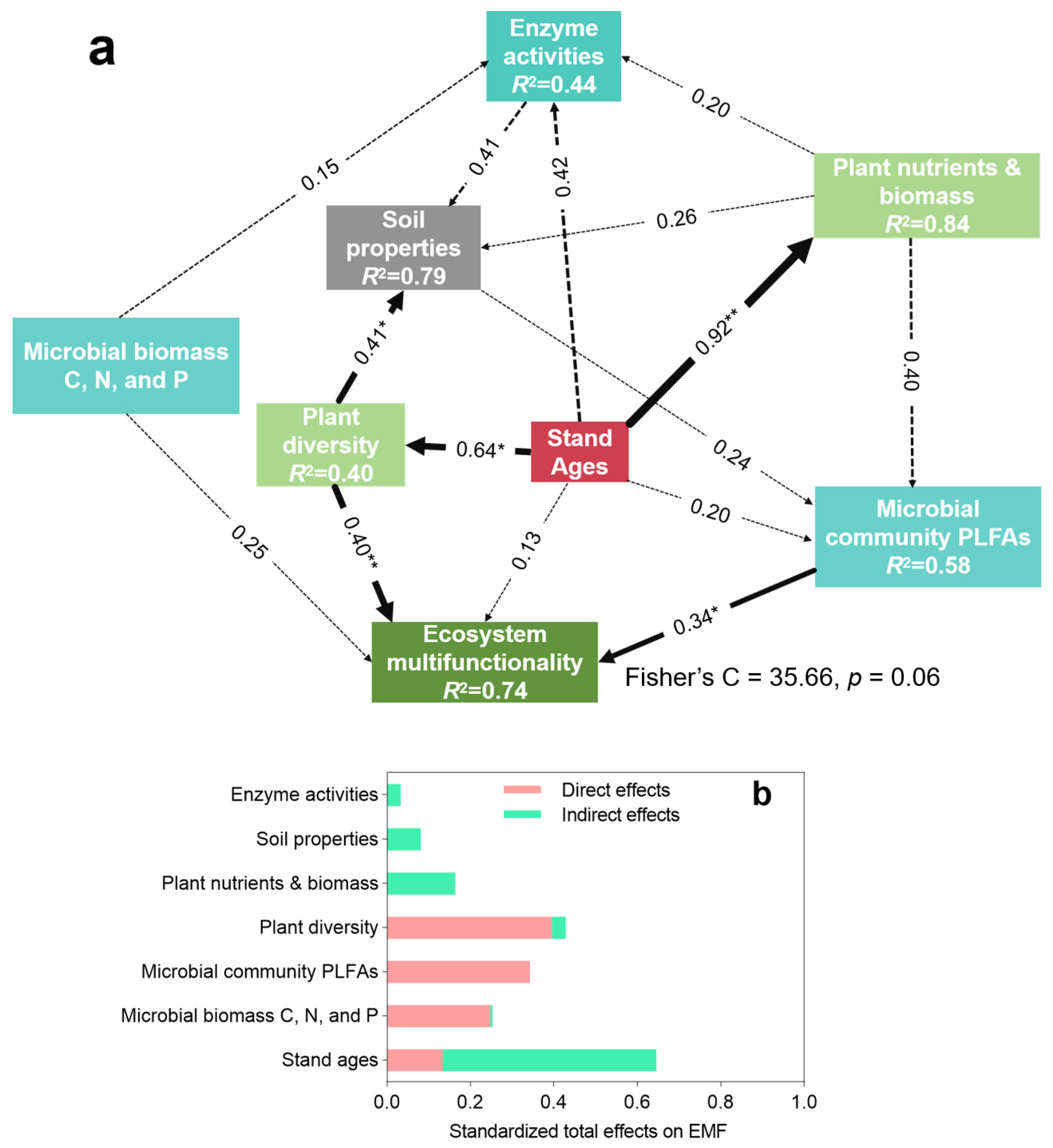
3.4. Contribution of Plant Diversity and Microbial Characteristics to Ecosystem Multifunctionality
4. Discussion
4.1. Shifts in Individual Functions and Ecosystem Multifunctionality with Forest Stand Age
4.2. Plant Diversity and Microbial Communities Drive Ecosystem Multifunctionality
4.3. Managing Forests to Drive Multiple Ecosystem Services and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Byrnes, J.E.K.; Gamfeldt, L.; Isbell, F.; Jonathan, S.L.; John, N.G.; Andy, H.; Bradley, J.; Cardinale, D.U.H.; Laura, E.; Dee, J.E.D. Investigating the relationship between biodiversity and ecosystem multifunctionality: Challenges and solutions. Methods Ecol. Evol. 2014, 5, 111–124. [Google Scholar] [CrossRef]
- Manning, P.; van der Plas, F.; Soliveres, S.; Eric, A.; Fernando, T.; Maestre, G.M.; Mark, J.W.; Markus, F. Redefining ecosystem multifunctionality. Nature Ecol. Evol. 2018, 2, 427–436. [Google Scholar] [CrossRef]
- Hua, F.; Bruijnzeel, L.A.; Meli, P.; Martin, A.; Zhang, J.; Nakagawa, S.; Miao, X.; Wang, W.; McEvoy, C.; Peña-Arancibia, J.L.; et al. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, 839–844. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Lucas-Borja, M.E.; Wang, Z.; Li, X.; Huang, Z. Microbial diversity regulates ecosystem multifunctionality during natural secondary succession. J. Appl. Ecol. 2021, 58, 2833–2842. [Google Scholar] [CrossRef]
- Wang, J.; Shi, X.; Lucas-Borja, M.E.; Lam, S.K.; Wang, Z.; Huang, Z. Plants, soil properties and microbes directly and positively drive ecosystem multifunctionality in a plantation chronosequence. Land Degrad. Dev. 2022, 33, 3049–3057. [Google Scholar] [CrossRef]
- Li, F.; Shi, Z.; Liu, S.; Xu, G.; Zhang, M.; Cao, X.; Chen, M.; Chen, J.; Xing, H.; Gong, S. Soil properties and plant diversity co-regulate ecosystem multifunctionality of subalpine primary dark coniferous forest on the eastern Qinghai-Tibetan Plateau. Plant Soil 2023, 23, 6222. [Google Scholar] [CrossRef]
- Selvaraj, S.; Duraisamy, V.; Huang, Z.; Guo, F.; Ma, X. Influence of long-term successive rotations and stand age of Chinese fir (Cunninghamia lanceolata) plantations on soil properties. Geoderma 2017, 306, 127–134. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, H.; Ouyang, S.; Chen, L.; Fang, X.; Peng, C.; Liu, S.; Xiao, W.; Xiang, W. Ecosystem service multifunctionality of Chinese fir plantations differing in stand age and implications for sustainable management. Sci. Total Environ. 2021, 788, 147791. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Delgado-Baquerizo, M. Plant diversity and soil stoichiometry regulates the changes in multifunctionality during pine temperate forest secondary succession. Sci. Total Environ. 2019, 697, 134204. [Google Scholar] [CrossRef]
- Jönsson, M.; Snäll, T.; Leverkus, A.B. Ecosystem service multifunctionality of low-productivity forests and implications for conservation and management. J. Appl. Ecol. 2020, 57, 695–706. [Google Scholar] [CrossRef]
- Walker, L.R.; Wardle, D.A.; Bardgett, R.D.; Clarkson, B.D. The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 2010, 98, 725–736. [Google Scholar] [CrossRef]
- Yu, G.R.; Chen, Z.; Piao, S.L.; Peng, C.H.; Philippe, C.; Wang, Q.; Li, X.; Zhu, X. High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsoon region. Proc. Natl. Acad. Sci. USA 2014, 111, 4910–4915. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.M.; Liu, S.R.; Wang, H.; Hu, Z.; Li, Z.; You, Y. Changes of soil microbial biomass carbon and community composition through mixing nitrogen-fixing species with Eucalyptus urophylla in subtropical China. Soil Biol. Biochem. 2014, 73, 42–48. [Google Scholar] [CrossRef]
- Hooper, D.U.; Vitousek, P.M. Effects of plant composition and diversity on nutrient cycling. Ecol. Monogr. 1998, 68, 121–149. [Google Scholar] [CrossRef]
- Garland, G.; Banerjee, S.; Edlinger, A.; Emily, M.O.; Chantal, H.; Raphaël, W.; Laurent, P.; Fernando, T.M.; van der Marcel, G.A.H. A closer look at the functions behind ecosystem multifunctionality: A review. J. Ecol. 2021, 109, 600–613. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wu, G.; Lie, Z.; Sheng, H.; Aguila, L.C.R.; Khan, M.S.; Liu, X.; Zhou, S.; Wu, T.; et al. Mixed plantations do not necessarily provide higher ecosystem multifunctionality than monoculture plantations. Sci. Total Environ. 2024, 914, 170156. [Google Scholar] [CrossRef]
- Huang, W.; Song, B.; Liu, Y.; Liu, J.; Wang, X. Multifunctional evaluation of spruce-fir forest based on different thinning intensities. Forests 2024, 15, 1703. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, H.; Lei, X.; Zeng, J.; Lan, J.; Guo, X.; Gao, D.; Liu, X.; Zhang, H. Impact of management measures on multiple ecosystem function trade-offs and their dynamics in subtropic Pinus massoniana plantations. Forests 2024, 15, 1224. [Google Scholar] [CrossRef]
- Sheng, H.; Long, F.; Li, X.; Haider, F.U.; Shi, Z.; Xian, L.; Meng, C.; Li, H. Thinning improves large diameter timber cultivation but undermines ecosystem multifunctionality in the short term. Forests 2025, 16, 134. [Google Scholar] [CrossRef]
- Jonsson, M.; Bengtsson, J.; Gamfeldt, L.; Jon, M.; Snäll, T. Levels of forest ecosystem services depend on specific mixtures of commercial tree species. Nat. Plants 2019, 5, 141–147. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Hedo, J.; Cerdá, A.; Candel-Pérez, D.; Viñegla, B. Unravelling the importance of forest age stand and forest structure driving microbiological soil properties, enzymatic activities and soil nutrients content in Mediterranean Spanish black pine (Pinus nigra Ar. ssp. salzmannii) Forest. Sci. Total Environ. 2016, 562, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nature Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, G.; Lie, Z.; Aguila, L.C.R.; Khan, M.S.; Luo, H.; Liu, X.; Liu, J. Microbial community variation in rhizosphere and non-rhizosphere soils of Castanopsis hystrix plantations across stand ages. J. For. Res. 2025, 36, 82. [Google Scholar] [CrossRef]
- Li, X.; Wu, T.; Wu, G.; Aguila, L.C.R.; Liu, X.; Liu, Y.; Cheng, Y.; Jiang, F.; Lie, Z.; Liu, J. Increasing stand age increases N deficiency but alleviates relative P limitations in Castanopsis hystrix plantations in southern China. Land Degrad. Dev. 2024, 35, 2173–2183. [Google Scholar] [CrossRef]
- Wu, G.; Li, X.; Zhou, S.; Liu, X.; Lie, Z.; Aguila, L.C.R.; Xu, W.; Liu, J. Soil organic carbon sources exhibit different patterns with stand age in rhizosphere and non-rhizosphere soils. CATENA 2025, 248, 108579. [Google Scholar] [CrossRef]
- Hurlbert, S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Li, X.; Aguila, L.C.R.; Wu, D.; Lie, Z.; Xu, W.; Tang, X.; Liu, J. Carbon sequestration and storage capacity of Chinese fir at different stand ages. Sci. Total Environ. 2023, 904, 166962. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Niu, S.; Tian, D.; Wu, Q.; Gao, X.; Schellenberg, M.P.; Han, G. Diversity of plant and soil microbes mediates the response of ecosystem multifunctionality to grazing disturbance. Sci. Total Environ. 2021, 776, 145730. [Google Scholar] [CrossRef]
- Tebenkova, D.N.; Lukina, N.V.; Chumachenko, S.I.; Danilova, M.A.; Kuznetsova, A.I.; Gornov, A.V.; Shevchenko, N.E.; Kataev, A.D.; Gagarin, Y.N. Multifunctionality and biodiversity of forest ecosystems. Contemp. Probl. Ecol. 2020, 13, 709–719. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Z.; Ma, Y.; Fu, S.L.; Chen, H.Y.H. Increased litterfall contributes to carbon and nitrogen accumulation following cessation of anthropogenic disturbances in degraded forests. For. Ecol. Manag. 2019, 432, 832–839. [Google Scholar] [CrossRef]
- Zhou, S.Y.D.; Li, X.; Josep, P.; David, T.T.; Roy, N.; Liu, X.; Lie, Z.; Huang, F.Y.; Yan, J.H.; Zhu, D.; et al. Soil and litter microbiomes as joint drivers of ecosystem multifunctionality in a 60-year-old forest plantation. J. Environ. Manag. 2025, 380, 124900. [Google Scholar] [CrossRef] [PubMed]
- Kermavnar, J.; Vilhar, U. Canopy precipitation interception in urban forests in relation to stand structure. Urban Ecosyst. 2017, 20, 1373–1387. [Google Scholar] [CrossRef]
- Cavaleri, M.A.; Sack, L. Comparative water use of native and invasive plants at multiple scales: A global meta-analysis. Ecology 2010, 91, 2705–2715. [Google Scholar] [CrossRef]
- Felton, A.; Nilsson, U.; Sonesson, J.; Annika, M.F.; Jean-Michel, R.; Thomas, R.; Martin, A.; Johan, B.; Christer, B.; Johanna, B.; et al. Replacing monocultures with mixed-species stands: Ecosystem service implications of two production forest alternatives in Sweden. Ambio 2016, 45, 124–139. [Google Scholar] [CrossRef]
- Liu, W.W.; Liu, M.C.; Li, W.H.; Zeng, W.; Qu, Y. Influence of ginseng cultivation under larch plantations on plant diversity and soil properties in Liaoning Province, Northeast China. J. Mount. Sci. 2016, 13, 1598–1608. [Google Scholar] [CrossRef]
- Xie, H.T.; Tang, Y.; Yu, M.K.; Wang, G.G. The effects of afforestation tree species mixing on soil organic carbon stock, nutrients accumulation, and understory vegetation diversity on reclaimed coastal lands in Eastern China. Glob. Ecol. Conserv. 2021, 26, e01478. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Li, J.J.; Zheng, J.; Liu, J.; Lin, W.; Wu, C. Soil microbial community structure and catabolic activity are significantly degenerated in successive rotations of Chinese fir plantations. Sci. Rep. 2017, 7, 6691. [Google Scholar] [CrossRef]
- Chen, W.Q.; Wang, J.Y.; Chen, X.; Meng, Z.; Xu, R.; Duoji, D.; Zhang, J.; He, J.; Wang, Z.; Chen, J.; et al. Soil microbial network complexity predicts ecosystem function along elevation gradients on the Tibetan Plateau. Soil Biol. Biochem. 2022, 172, 108766. [Google Scholar] [CrossRef]
- Ouyang, S.; Xiang, W.H.; Wang, X.P.; Xiao, W.F.; Chen, L.; Li, S.; Sun, H.; Deng, X.; David, I.F.; Zeng, L.; et al. Effects of stand age, richness and density on productivity in subtropical forests in China. J. Ecol. 2019, 107, 2266–2277. [Google Scholar] [CrossRef]
- Zhou, S.; Lie, Z.; Liu, X.; Zhu, Y.; Josep, P.; Roy, N.; Su, X.; Liu, Z.; Chu, G.; Meng, Z.; et al. Distinct patterns of soil bacterial and fungal community assemblages in subtropical forest ecosystems under warming. Global Change Biol. 2023, 29, 1501–1513. [Google Scholar] [CrossRef]
- Chen, H.Y.H.; Biswas, S.R.; Sobey, T.M.; Brassard, B.W.; Bartels, S.F. Reclamation strategies for mined forest soils and overstory drive understorey vegetation. J. Appl. Ecol. 2018, 55, 926–936. [Google Scholar] [CrossRef]
- Fanin, N.; Gundale, M.J.; Farrell, M.; Ciobanu, M.; Baldock, J.A.; Nilsson, M.C.; Kardol, P.; Wardle, D.A. Consistent effects of biodiversity loss on multifunctionality across contrasting ecosystems. Nature Ecol. Evol. 2018, 2, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Mahaut, L.; Fort, F.; Violle, C.; Freschet, G. Multiple facets of diversity effects on plant productivity: Species richness, functional diversity, species identity and intraspecific competition. Funct. Ecol. 2020, 34, 287–298. [Google Scholar] [CrossRef]
- Haddad, N.M.; Crutsinger, G.M.; Gross, K.; Haarstad, J.; Tilman, D. Plant diversity and the stability of foodwebs. Ecol. Lett. 2011, 14, 42–46. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, S.Y.; Sun, M.M.; Yi, X.Y.; Duan, G.L.; Ye, M.; Michael, R.G.; Zhu, Y.G. Adaptive expression of phage auxiliary metabolic genes in paddy soils and their contribution toward global carbon sequestration. Proc. Natl. Acad. Sci. USA 2024, 121, e2419798121. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Eiko, E.K.; van der Marcel, G.A.H. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Giraldo-Charria, D.L.; Escobedo, F.J.; Clerici, N.; Quesada, B. Urban forests mitigate extreme heat exposure in a vulnerable tropical city. Urban Clim. 2025, 59, 102311. [Google Scholar] [CrossRef]
- Yao, H.; Li, Z.; Geisen, S.; Qiao, Z.; Breed, M.F.; Sun, X. Degree of urbanization and vegetation type shape soil biodiversity in city parks. Sci. Total Environ. 2023, 899, 166437. [Google Scholar] [CrossRef]
- Barrico, L.; Castro, H.; Coutinho, A.P.; Gonçalves, M.T.; Freitas, H.; Castro, P. Plant and microbial biodiversity in urban forests and public gardens: Insights for cities’ sustainable development. Urban For. Urban Green. 2018, 29, 19–27. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, J.; Wang, Q.; He, X.; Zheng, H. Stand structural characteristics determine ecosystems multifunctionality of urban forests in Changchun City, Northeast China. Urban For. Urban Green. 2025, 104, 128647. [Google Scholar] [CrossRef]
- Allan, E.; Manning, P.; Alt, F.; Julia, B.; Stefan, B.; Nico, B.; Stefan, B.; Fabrice, G.; Norbert, H.; Valentin, H.K.; et al. Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 2015, 18, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.A.; Wood, S.A.; Bardgett, R.D.; Helaina, I.J.B.; Michael, B.; Till, E.; Susan, J.G.; Ellen, K.; Peter, M.; Heikki, S.; et al. Discontinuity in the responses of ecosystem processes and multifunctionality to altered soil community composition. Proc. Natl. Acad. Sci. USA 2014, 111, 14478–14483. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, H.; Shahzad, B.; Long, F.; Haider, F.U.; Li, X.; Xian, L.; Huang, C.; Ma, Y.; Li, H. Plant Diversity and Microbial Community Drive Ecosystem Multifunctionality in Castanopsis hystrix Plantations. Plants 2025, 14, 1973. https://doi.org/10.3390/plants14131973
Sheng H, Shahzad B, Long F, Haider FU, Li X, Xian L, Huang C, Ma Y, Li H. Plant Diversity and Microbial Community Drive Ecosystem Multifunctionality in Castanopsis hystrix Plantations. Plants. 2025; 14(13):1973. https://doi.org/10.3390/plants14131973
Chicago/Turabian StyleSheng, Han, Babar Shahzad, Fengling Long, Fasih Ullah Haider, Xu Li, Lihua Xian, Cheng Huang, Yuhua Ma, and Hui Li. 2025. "Plant Diversity and Microbial Community Drive Ecosystem Multifunctionality in Castanopsis hystrix Plantations" Plants 14, no. 13: 1973. https://doi.org/10.3390/plants14131973
APA StyleSheng, H., Shahzad, B., Long, F., Haider, F. U., Li, X., Xian, L., Huang, C., Ma, Y., & Li, H. (2025). Plant Diversity and Microbial Community Drive Ecosystem Multifunctionality in Castanopsis hystrix Plantations. Plants, 14(13), 1973. https://doi.org/10.3390/plants14131973









