Effects of Biochar Amendment on Potassium Supply Capacity and Potassium Accumulation in Soybean Across Diverse Soils
Abstract
1. Introduction
2. Results
2.1. Soil Properties
2.2. Soil Potassium Availability
2.3. Potassium Adsorption State in Soil
2.4. Soil Potassium Q/I Curve and Parameters
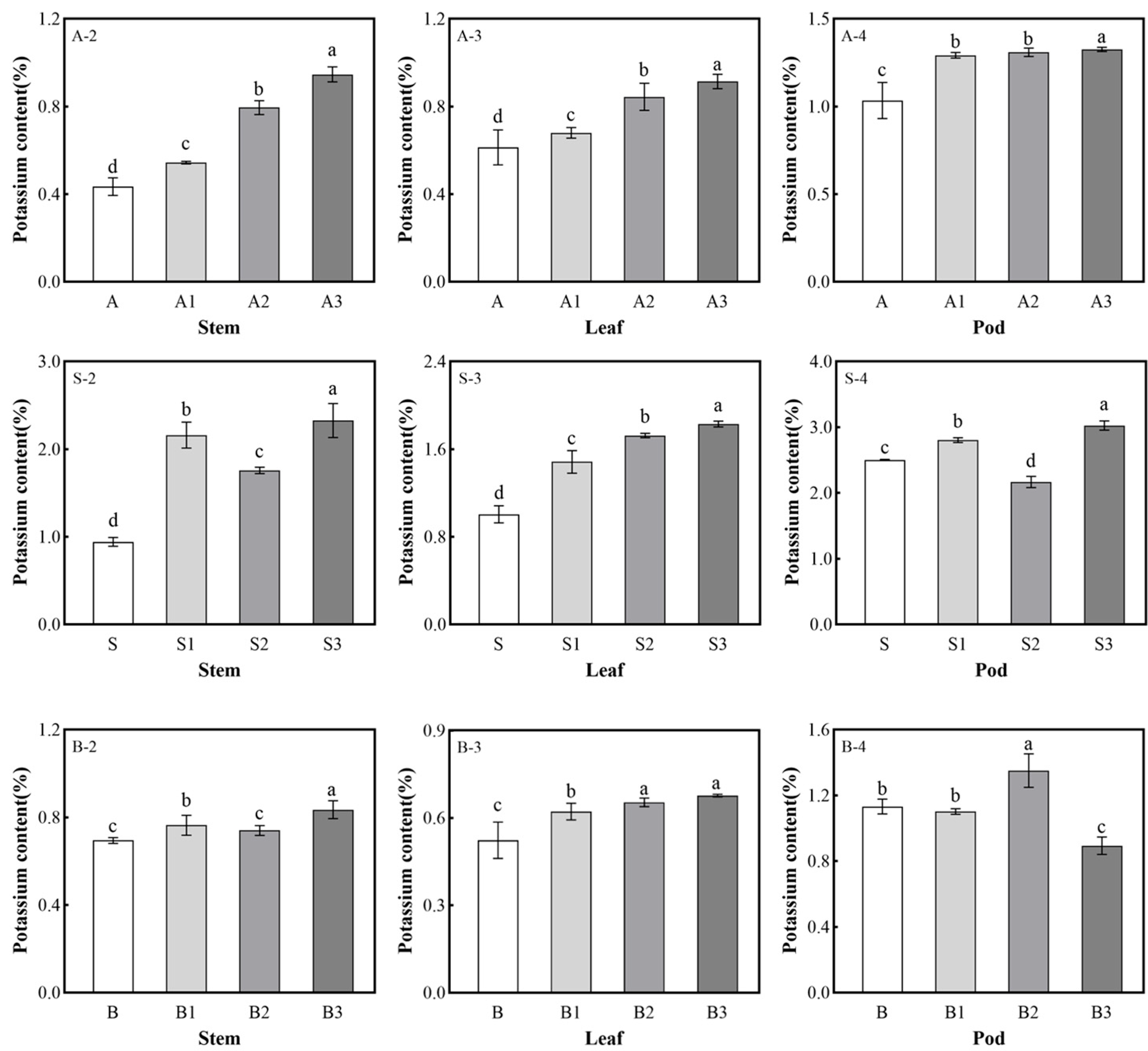
2.5. Potassium Content in Soybean Plants
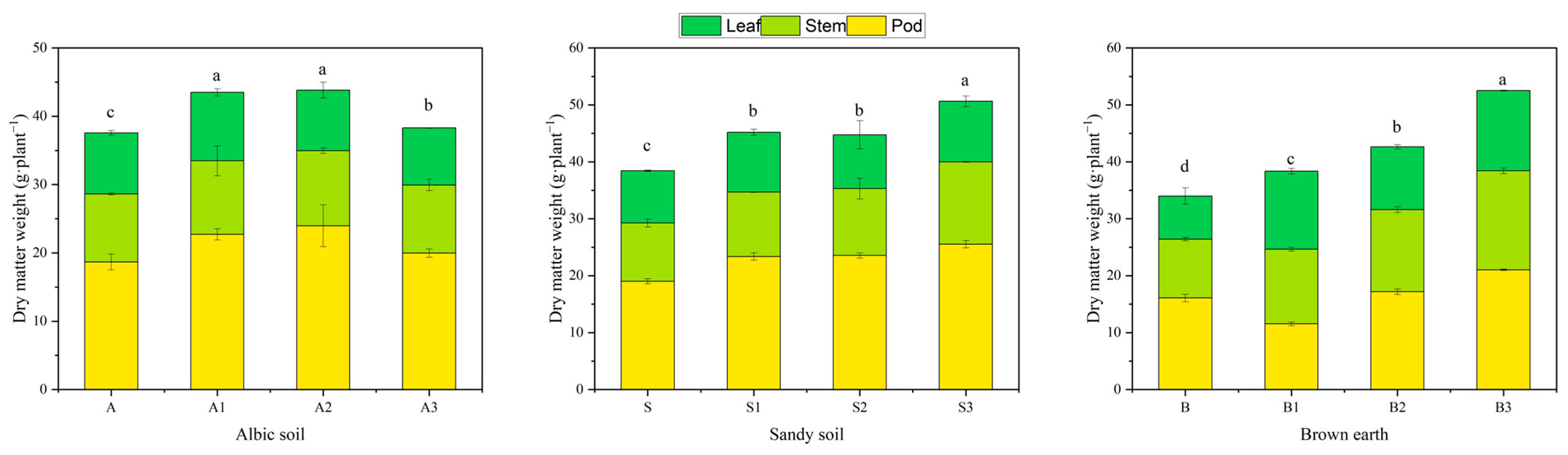
2.6. Dry Matter Accumulation in Soybean Plants
2.7. Accumulation of Potassium in Soybean Plants
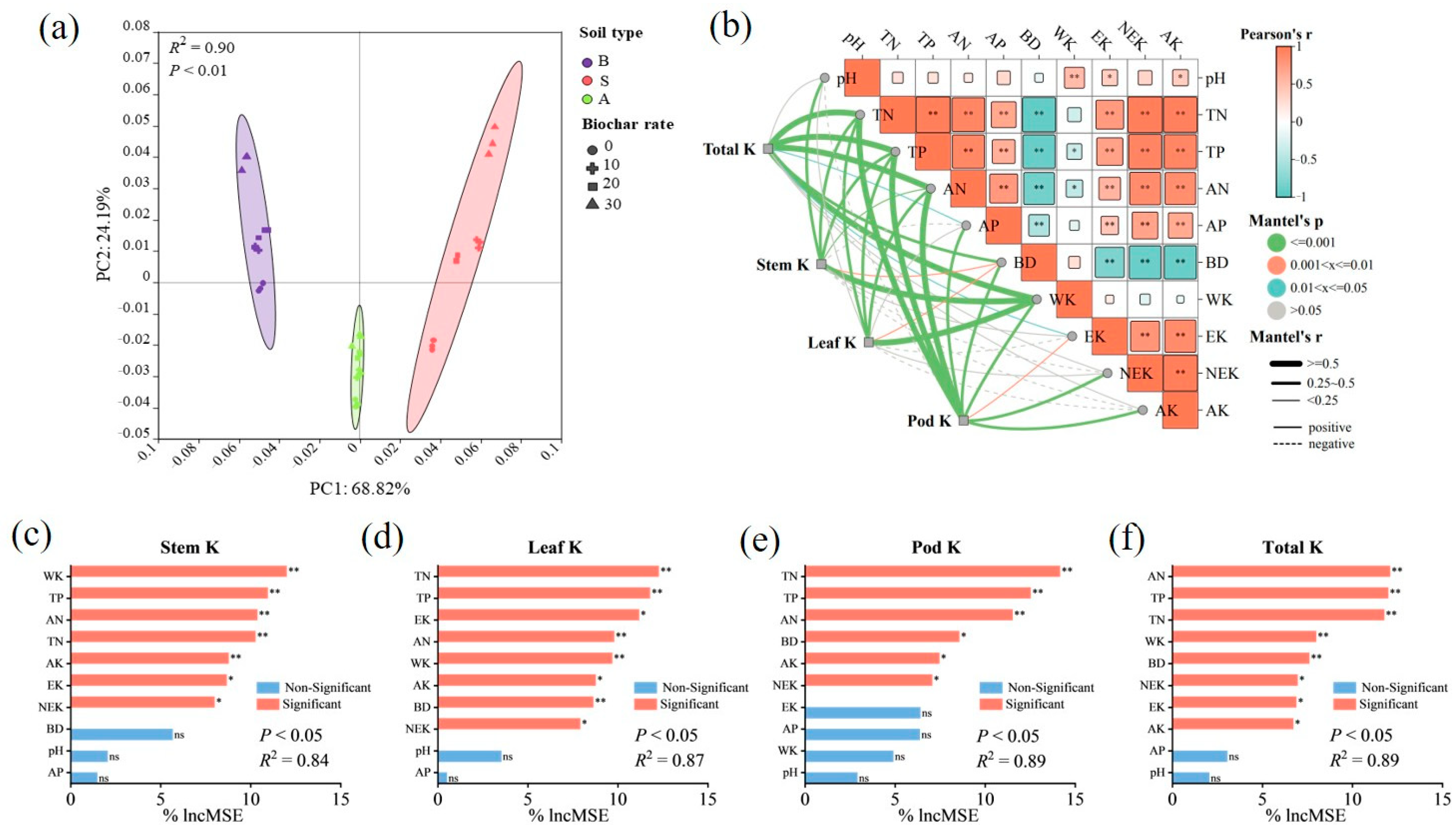
2.8. Regulation of Potassium Accumulation in Soybean Plants by Biochar
2.9. Yield
3. Discussion
3.1. Potassium Availability
3.2. Potassium Supply Capacity
3.3. Potassium Accumulation
3.4. Crop Yield
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Design
4.3. Analysis of Soil and Soybean Samples
4.4. Soil Potassium Adsorption Sites
4.5. Quantity–Intensity (Q/I) Isotherms
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
Correction Statement
References
- Carciochi, W.D.; Dobermann, A.; Cafaro La Menza, N.; Brouder, S.M.; Donough, C.R.; Heuschele, D.J.; Oberthür, T.; Sandaña, P.; Shehu, B.M.; Pereira, J.T.S.; et al. Quantifying potassium requirement and removal across crop species. Field Crops Res. 2025, 322, 109717. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Y.; Zhang, H.; Jin, H.; He, L.; Wang, H.; Lu, P.; Miao, C.; Yu, J.; Ding, X. Low-Potassium Fruits and Vegetables: Research Progress and Prospects. Plants 2024, 13, 1893. [Google Scholar] [CrossRef]
- Thenveettil, N.; Reddy, K.N.; Reddy, K.R. Effects of Potassium Nutrition on Corn (Zea mays L.) Physiology and Growth for Modeling. Agriculture 2024, 14, 968. [Google Scholar] [CrossRef]
- Sparks, D.L. Potassium dynamics in soils. In Advances in Soil Science; Springer: New York, NY, USA, 1987; pp. 1–63. [Google Scholar]
- Li, Y.; Zheng, J.; Wu, Q.; Gong, X.; Zhang, Z.; Chen, Y.; Chen, T.; Siddique, K.H.M.; Chi, D. Zeolite increases paddy soil potassium fixation, partial factor productivity, and potassium balance under alternate wetting and drying irrigation. Agric. Water Manag. 2022, 260, 107294. [Google Scholar] [CrossRef]
- Ji, H.J.; Zhang, H.Z.; Zhang, W.L.; Tian, C.Y. Farmland potassium balance in China: A review. Chin. J. Eco-Agric. 2017, 25, 920–930. [Google Scholar] [CrossRef]
- Liu, Y.F.; Wang, P.; Feng, D.Y.; Liu, X.; Han, Z.; Dai, T.; Zhang, S.T.; Chen, W.Q. Illustrating China’s journey to balance, circular, and secure potassium cycles in the last three decades. Resour. Conserv. Recycl. 2024, 202, 107378. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, N.; Sun, X.; Niu, R. Development history of China’s potash fertilizer import and export trade and analysis on the situation in recent years. Phosphate Compd. Fertil. 2020, 35, 1–7. [Google Scholar]
- Chai, R.S.; Huang, J.; Liu, K.L.; Zhang, H.M.; Luo, L.C.; Zhang, C.C. Importance of rice straw potassium resource distribution and its return to soil potassium balance in China. J. Plant Nutr. Fert. 2022, 28, 1745–1754. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Chen, W.; Meng, J.; Han, X.R.; Lan, Y.; Zhang, W.M. Past, present, and future of biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef]
- Cui, X.; Li, X.; Wang, J.; Wang, X.; Yu, F.; Yang, G.; Xu, S.; Cheng, Z.; Yang, Q.; Yan, B.; et al. A review on the production of nutrient-enriched biochar: Insights from the evolution of nitrogen, phosphorus, and potassium. Crit. Rev. Environ. Sci. Technol. 2025, 55, 885–903. [Google Scholar] [CrossRef]
- Xiu, L.; Gu, W.; Sun, Y.; Wu, D.; Wang, Y.; Zhang, H.; Zhang, W.; Chen, W. The fate and supply capacity of potassium in biochar used in agriculture. Sci. Total Environ. 2023, 902, 165969. [Google Scholar] [CrossRef]
- Li, N. Effects of Long-Term Fertilization on Potassium Supply Characteristics and Availability in Brown Soil. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 2012. [Google Scholar]
- Goulding, K.W.T. Potassium fixation and release. In Methodology in Soil Research; International Potash Institute: Baden bei Wien, Austria, 1987; pp. 137–154. [Google Scholar]
- Munson, R.D. Potassium in Agriculture; Fan, Q.Z.; Zheng, W.Q., Translators; Science Press: Beijing, China, 1995; pp. 160–161. [Google Scholar]
- Kirkman, J.H.; Basker, A.; Saurapaneni, A. Potassium in the soils of New Zealand: A review. N. Z. J. Agric. Res. 1994, 37, 207–227. [Google Scholar] [CrossRef]
- Bilias, F.; Barbayiannis, N. Potassium availability: An approach using thermodynamic parameters derived from quantity-intensity relationships. Geoderma 2019, 338, 355–364. [Google Scholar] [CrossRef]
- Islam, A.; Karim, S.A.; Solaiman, A.; Islam, S.; Saleque, A. Eight-year long potassium fertilization effects on quantity/intensity relationship of soil potassium under double rice cropping. Soil Tillage Res. 2017, 169, 99–117. [Google Scholar] [CrossRef]
- Beckett, P.H.T. Studies on soil potassium: I. Confirmation of the ratio law: Measurement of potassium potential. J. Soil Sci. 1964, 15, 1–8. [Google Scholar] [CrossRef]
- Beckett, P.H.T. Studies on soil potassium: II. The “immediate” Q/I relations of labile potassium in the soil. J. Soil Sci. 1964, 15, 9–23. [Google Scholar] [CrossRef]
- Bilias, F.; Kalderis, D.; Richardson, C.; Barbayiannis, N.; Gasparatos, D. Biochar application as a soil potassium management strategy: A review. Sci. Total Environ. 2023, 858, 159782. [Google Scholar] [CrossRef]
- Bao, Z.; Dai, W.; Su, X.; Liu, Z.; An, Z.; Sun, Q.; Jing, H.; Lin, L.; Chen, Y.; Meng, J. Long-term biochar application promoted soil aggregate-associated potassium availability and maize potassium uptake. GCB Bioenergy 2024, 16, e13134. [Google Scholar] [CrossRef]
- Dong, M.; Jiang, M.; He, L.; Zhang, Z.; Gustave, W.; Vithanage, M.; Niazi, N.K.; Chen, B.; Zhang, X.; Wang, H.; et al. Challenges in safe environmental applications of biochar: Identifying risks and unintended consequences. Biochar 2025, 7, 12. [Google Scholar] [CrossRef]
- Sun, Q.; Meng, J.; Lan, Y.; Shi, G.; Yang, X.; Cao, D.; Chen, W.; Han, X. Long-term effects of biochar amendment on soil aggregate stability and biological binding agents in brown earth. Catena 2021, 205, 105460. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Jiao, X.; Jiang, H.; Liu, Y.; Wang, X.; Ma, C. The Fate and Challenges of the Main Nutrients in Returned Straw: A Basic Review. Agronomy 2024, 14, 698. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, X.; Fan, X.; Xia, Y.; Zhang, F.; Liu, D.; Wu, M.; Zhang, Z. Long-term straw returning increase the capacity and intensity of soil potassium supply in a rice and wheat rotation system. J. Plant Nutr. Fert. 2022, 28, 589–597. [Google Scholar] [CrossRef]
- Zhou, J.M. (Ed.) Dictionary of Soil Science; Science Press: Beijing, China, 2013; pp. 433–437. [Google Scholar]
- Safar, M.; Lin, B.J.; Chen, W.H.; Langauer, D.; Chang, J.S.; Raclavska, H.; Pétrissans, A.; Rousset, P.; Pétrissans, M. Catalytic effects of potassium on biomass pyrolysis, combustion and torrefaction. Appl. Energy 2019, 235, 346–355. [Google Scholar] [CrossRef]
- Wei, X.; Yi, Y.; Yuan, X.; Liu, Y.; Wan, Q.; Gou, G.; Zhang, Y.; Zhou, G.; Song, Y. Intrinsic carbon structure modification overcomes the challenge of potassium bond chemistry. Energy Environ. Sci. 2024, 17, 2968–3003. [Google Scholar] [CrossRef]
- Mulet, J.M.; Porcel, R.; Yenush, L. Modulation of potassium transport to increase abiotic stress tolerance in plants. J. Exp. Bot. 2023, 74, 5989–6005. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.P. Regulation of potassium ion absorption in higher plants. Plant Physiol. Commun. 1989, 4, 1–7. [Google Scholar]
- Mao, P.P.; Zhao, Y.Y. Advances in potassium absorption, transport and stress response in plants. Bull. Biol. 2008, 8, 11–13. [Google Scholar]
- Leigh, R.A.; Wyn Jones, R.G. A hypothesis relating critical potassium concentrations for growth to the distribution and function of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Bons, H.K.; Sharma, A. Impact of foliar sprays of potassium, calcium, and boron on fruit setting behavior, yield, and quality attributes in fruit crops: A review. J. Plant Nutr. 2023, 46, 3232–3246. [Google Scholar] [CrossRef]
- Ahmed, A.; Aftab, S.; Hussain, S.; Cheema, H.N.; Liu, W.; Yang, F.; Yang, W. Nutrient Accumulation and Distribution Assessment in Response to Potassium Application under Maize–Soybean Intercropping System. Agronomy 2020, 10, 725. [Google Scholar] [CrossRef]
- Ohyama, T.; Takayama, K.; Akagi, A.; Saito, A.; Higuchi, K.; Sato, T. Development of an N-Free Culture Solution for Cultivation of Nodulated Soybean with Less pH Fluctuation by the Addition of Potassium Bicarbonate. Agriculture 2023, 13, 739. [Google Scholar] [CrossRef]
- Santos, M.G.B.d.; Paiva, A.B.; Viana, R.d.S.R.; Jindo, K.; Figueiredo, C.C.d. Biochar as a Feedstock for Sustainable Fertilizers: Recent Advances and Perspectives. Agriculture 2025, 15, 894. [Google Scholar] [CrossRef]
- Li, N.; Han, X.R.; Yang, J.F.; Liu, N.; Li, Q.N.; Fang, D.W. Effects of long-term fertilization on the availability of K adsorbed by clay minerals and profile distribution in brown soil. Plant Nutr. Fert. Sci. 2012, 18, 1412–1417. [Google Scholar]
- Rupa, T.R.; Srivastava, S.; Swarup, A.; Singh, D. Potassium supplying power of a Typic Ustochrept profile using quantity/intensity technique in a long-term fertilized plot. J. Agric. Sci. 2001, 137, 195–203. [Google Scholar] [CrossRef]
- Moore, W.J. Physical Chemistry; Prentice Hall: Englewood Cliffs, NJ, USA, 1972. [Google Scholar]
- Sparks, D.L.; Liebhardt, W.C. Effect of long-term lime and potassium application on quantity-intensity (Q/I) relationships in sandy soil. Soil Sci. Soc. Am. J. 1981, 45, 786–790. [Google Scholar] [CrossRef]


| Total Nitrogen (g·kg−1) | Total Phosphorus (g·kg−1) | Available Nitrogen (mg·kg−1) | Available Phosphorus (mg·kg−1) | Soil pH | Bulk Density (g·cm−3) | |
|---|---|---|---|---|---|---|
| Albic soil | ||||||
| A | 0.83 ± 0.01b | 0.28 ± 0.03b | 52.67 ± 1.15c | 11.70 ± 2.40b | 5.66 ± 0.24b | 1.34 ± 0.01a |
| A1 | 0.87 ± 0.01a | 0.39 ± 0.01a | 57.00 ± 1.00a | 14.57 ± 1.21a | 6.53 ± 0.12a | 1.28 ± 0.02b |
| A2 | 0.96 ± 0.01a | 0.38 ± 0.01a | 54.67 ± 0.58b | 15.43 ± 1.62a | 6.66 ± 0.41a | 1.26 ± 0.07b |
| A3 | 0.99 ± 0.02a | 0.38 ± 0.04a | 58.67 ± 0.58a | 12.77 ± 2.14b | 6.92 ± 0.21a | 1.16 ± 0.04c |
| Sandy soil | ||||||
| S | 0.32 ± 0.01b | 0.09 ± 0.01b | 27.67 ± 0.18b | 13.57 ± 0.45a | 7.96 ± 0.32a | 1.55 ± 0.03a |
| S1 | 0.37 ± 0.01a | 0.12 ± 0.01a | 26.67 ± 0.23c | 13.40 ± 0.69a | 7.06 ± 0.28c | 1.52 ± 0.02a |
| S2 | 0.4 ± 0.02a | 0.11 ± 0.01a | 28.33 ± 0.58a | 13.40 ± 0.69a | 7.30 ± 0.34b | 1.45 ± 0.04b |
| S3 | 0.48 ± 0.02a | 0.12 ± 0.00a | 27.67 ± 0.41b | 13.43 ± 1.11a | 8.14 ± 0.13a | 1.39 ± 0.08c |
| Brown soil | ||||||
| B | 1.15 ± 0.02b | 0.52 ± 0.00b | 87.00 ± 0.00b | 21.43 ± 0.72b | 7.30 ± 0.60b | 1.25 ± 0.01a |
| B1 | 1.16 ± 0.01a | 0.55 ± 0.00a | 93.02 ± 0.21a | 22.27 ± 1.42a | 7.97 ± 0.25a | 1.20 ± 0.01a |
| B2 | 1.26 ± 0.00a | 0.58 ± 0.02a | 80.00 ± 0.33c | 22.50 ± 1.10a | 8.17 ± 0.03a | 1.13 ± 0.03b |
| B3 | 1.26 ± 0.00a | 0.57 ± 0.01a | 88.33 ± 0.58b | 22.87 ± 1.16a | 8.13 ± 0.01a | 1.15 ± 0.02b |
| Available Potassium (mg·kg−1) | Water-Soluble Potassium (mg·kg−1) | Exchangeable Potassium (mg·kg−1) | Non-Exchangeable Potassium (mg·kg−1) | |
| Albic soil | ||||
| A | 367.24 ± 0.47d | 15.21 ± 3.90b | 48.71 ± 1.06d | 303.33 ± 2.29b |
| A1 | 378.19 ± 6.94c | 17.56 ± 2.40b | 57.25 ± 1.60c | 303.38 ± 12.73b |
| A2 | 427.55 ± 0.52b | 19.80 ± 1.07b | 66.69 ± 2.18b | 341.06 ± 0.89a |
| A3 | 458.48 ± 8.17a | 27.73 ± 2.89a | 77.03 ± 1.78a | 353.73 ± 15.77a |
| Sandy soil | ||||
| S | 272.88 ± 0.11d | 34.59 ± 3.13b | 15.10 ± 2.84d | 223.19 ± 0.45c |
| S1 | 289.72 ± 3.10c | 35.16 ± 0.70b | 24.79 ± 0.66c | 229.78 ± 10.24b |
| S2 | 313.68 ± 0.60b | 37.46 ± 1.70ab | 49.01 ± 4.31b | 227.22 ± 2.68bc |
| S3 | 439.08 ± 2.60a | 47.75 ± 9.70a | 78.60 ± 3.15a | 312.73 ± 2.07a |
| Brown soil | ||||
| B | 1269.42 ± 5.28d | 17.36 ± 2.51b | 56.87 ± 0.61b | 1195.2 ± 10.63c |
| B1 | 1381.50 ± 8.18c | 20.54 ± 4.99b | 65.00 ± 3.59b | 1295.96 ± 16.24b |
| B2 | 1421.76 ± 8.87b | 23.93 ± 2.59b | 83.79 ± 4.38a | 1314.04 ± 18.01b |
| B3 | 1763.37 ± 12.12a | 38.92 ± 6.84a | 85.43 ± 5.29a | 1639.02 ± 22.43a |
| p-Site Potassium (mg·kg−1) | e-Site Potassium (mg·kg−1) | i-Site Potassium (mg·kg−1) |
|---|---|---|
| Albic soil | ||
| 40.02 ± 2.24d | 44.07 ± 1.08a | 267.95 ± 2.12d |
| 46.69 ± 3.60c | 35.79 ± 0.67b | 278.15 ± 3.79c |
| 57.88 ± 2.57b | 37.26 ± 2.08b | 312.61 ± 0.46b |
| 65.03 ± 2.05a | 44.00 ± 2.34a | 321.73 ± 2.67a |
| Sandy soil | ||
| 35.80 ± 1.66a | 19.99 ± 0.93a | 182.49 ± 3.75d |
| 36.50 ± 1.66a | 21.15 ± 1.71a | 200.26 ± 3.73c |
| 40.03 ± 0.63a | 22.02 ± 5.54a | 214.18 ± 5.70b |
| 38.70 ± 3.13a | 22.47 ± 1.04a | 330.16 ± 5.79a |
| Brown soil | ||
| 47.80 ± 1.51b | 57.66 ± 3.52b | 1146.60 ± 4.00d |
| 53.12 ± 3.18b | 80.80 ± 3.93a | 1227.04 ± 4.56c |
| 71.89 ± 3.18a | 84.09 ± 3.81a | 1241.84 ± 2.43b |
| 72.84 ± 0.66a | 87.66 ± 4.92a | 1563.95 ± 5.44a |
| AR0 | ΔK | Kl | Kx | PBC | |
|---|---|---|---|---|---|
| Albic soil | |||||
| A | 1.81 | 0.13 | 0.14 | 0.0096 | 70.38 |
| A1 | 2.29 | 0.14 | 0.17 | 0.0246 | 62.13 |
| A2 | 3.41 | 0.16 | 0.20 | 0.0358 | 48.40 |
| A3 | 3.56 | 0.16 | 0.23 | 0.0687 | 46.00 |
| Sandy soil | |||||
| S | 1.29 | 0.04 | 0.21 | 0.1616 | 34.61 |
| S1 | 2.09 | 0.06 | 0.20 | 0.1358 | 30.20 |
| S2 | 3.06 | 0.07 | 0.21 | 0.1346 | 24.39 |
| S3 | 3.07 | 0.07 | 0.28 | 0.2038 | 23.49 |
| Brown soil | |||||
| B | 1.91 | 0.13 | 0.17 | 0.0416 | 67.63 |
| B1 | 2.18 | 0.16 | 0.2 | 0.0409 | 72.05 |
| B2 | 2.54 | 0.18 | 0.23 | 0.0488 | 70.10 |
| B3 | 2.68 | 0.17 | 0.29 | 0.1148 | 64.90 |
| Potassium Accumulation in Pods (mg·plant−1) | Potassium Accumulation in Stem (mg·plant−1) | Potassium Accumulation in Leaves (mg·plant−1) | Potassium Accumulation in Soybeans (mg·plant−1) | |
|---|---|---|---|---|
| Albic soil | ||||
| A | 192.38 ± 0.24d | 42.99 ± 2.64d | 54.35 ± 9.92c | 289.72 ± 1.04d |
| A1 | 293.65 ± 3.44b | 58.47 ± 3.44c | 67.95 ± 3.12b | 420.07 ± 0.56c |
| A2 | 313.71 ± 1.60a | 71.54 ± 4.88b | 74.63 ± 6.88a | 459.88 ± 3.20a |
| A3 | 264.6 ± 0.24c | 94.16 ± 4.4a | 76.21 ± 1.20a | 434.97 ± 4.32b |
| Sandy soil | ||||
| S | 466.83 ± 4.64d | 96.32 ± 5.12d | 87.95 ± 4.64d | 651.10 ± 3.84d |
| S1 | 654.72 ± 0.88b | 244.28 ± 3.68b | 155.68 ± 2.64c | 1054.68 ± 2.8b |
| S2 | 510.35 ± 3.12c | 206.36 ± 7.12c | 163.01 ± 4.64b | 879.72 ± 0.32c |
| S3 | 772.08 ± 9.2a | 336.56 ± 4.24a | 194.53 ± 4.4a | 1303.17 ± 5.44a |
| Brown soil | ||||
| B | 181.68 ± 4.24b | 71.74 ± 5.20d | 38.75 ± 1.20d | 292.17 ± 2.48d |
| B1 | 127.03 ± 3.36c | 99.94 ± 3.84c | 84.88 ± 0.72c | 311.85 ± 2.24c |
| B2 | 232.04 ± 0.88a | 106.49 ± 2.32b | 72.02 ± 7.04b | 410.55 ± 1.12b |
| B3 | 187.84 ± 0.02b | 144.77 ± 5.36a | 95.39 ± 4.32a | 428.01 ± 1.92a |
| Treatment | Main Stem Seed Number (Seed·Plant−1) | Main Stem Seed Weight (g·Plant−1) | Branch Seed Number (Seed·Plant−1) | Branch Seed Weight (g·Plant−1) | Total Seed Weight (g·Plant−1) |
|---|---|---|---|---|---|
| Albic soil | |||||
| A | 23.67 ± 1.25b | 3.86 ± 0.48c | 23.67 ± 2.36c | 7.31 ± 0.22c | 11.17 ± 0.47c |
| A1 | 34.00 ± 3.74a | 7.97 ± 0.87a | 45.33 ± 3.4b | 8.92 ± 1.14b | 16.88 ± 0.60b |
| A2 | 38.67 ± 2.87a | 8.06 ± 0.74a | 44.67 ± 3.3b | 9.09 ± 1.43b | 17.15 ± 2.04b |
| A3 | 29.00 ± 1.63b | 6.71 ± 0.55b | 55.33 ± 2.87a | 14.62 ± 1.49a | 21.33 ± 1.91a |
| Sandy soil | |||||
| S | 23.33 ± 2.05d | 4.92 ± 0.89d | 41.00 ± 1.63b | 9.45 ± 0.66b | 14.37 ± 1.37d |
| S1 | 47.67 ± 1.25b | 9.92 ± 0.39b | 54.00 ± 0.82a | 11.20 ± 0.26a | 21.12 ± 0.65a |
| S2 | 43.00 ± 0.82c | 10.40 ± 0.18c | 56.33 ± 4.50a | 10.41 ± 1.13a | 20.81 ± 1.31b |
| S3 | 54.33 ± 1.25a | 11.07 ± 0.17a | 43.33 ± 2.49b | 7.71 ± 0.77c | 18.78 ± 0.63c |
| Brown soil | |||||
| B | 29.00 ± 1.63c | 6.90 ± 0.38c | 42.33 ± 2.05b | 9.68 ± 0.87b | 16.58 ± 1.23b |
| B1 | 37.67 ± 2.05b | 8.93 ± 0.65b | 50.33 ± 2.87a | 12.81 ± 1.37a | 21.74 ± 1.68a |
| B2 | 46.00 ± 3.56a | 11.48 ± 1.03a | 36.00 ± 2.45c | 9.24 ± 0.16b | 20.71 ± 1.18a |
| B3 | 32.33 ± 1.70b | 5.56 ± 0.76c | 24.00 ± 2.16d | 4.12 ± 0.35c | 9.67 ± 0.41c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiu, L.; Sun, Y.; Han, X. Effects of Biochar Amendment on Potassium Supply Capacity and Potassium Accumulation in Soybean Across Diverse Soils. Plants 2025, 14, 1959. https://doi.org/10.3390/plants14131959
Xiu L, Sun Y, Han X. Effects of Biochar Amendment on Potassium Supply Capacity and Potassium Accumulation in Soybean Across Diverse Soils. Plants. 2025; 14(13):1959. https://doi.org/10.3390/plants14131959
Chicago/Turabian StyleXiu, Liqun, Yuanyuan Sun, and Xiaori Han. 2025. "Effects of Biochar Amendment on Potassium Supply Capacity and Potassium Accumulation in Soybean Across Diverse Soils" Plants 14, no. 13: 1959. https://doi.org/10.3390/plants14131959
APA StyleXiu, L., Sun, Y., & Han, X. (2025). Effects of Biochar Amendment on Potassium Supply Capacity and Potassium Accumulation in Soybean Across Diverse Soils. Plants, 14(13), 1959. https://doi.org/10.3390/plants14131959





