Wheat Straw Biochar Amendment Increases Salinity Stress Tolerance in Alfalfa Seedlings by Modulating Physiological and Biochemical Responses
Abstract
1. Introduction
2. Materials and Methods
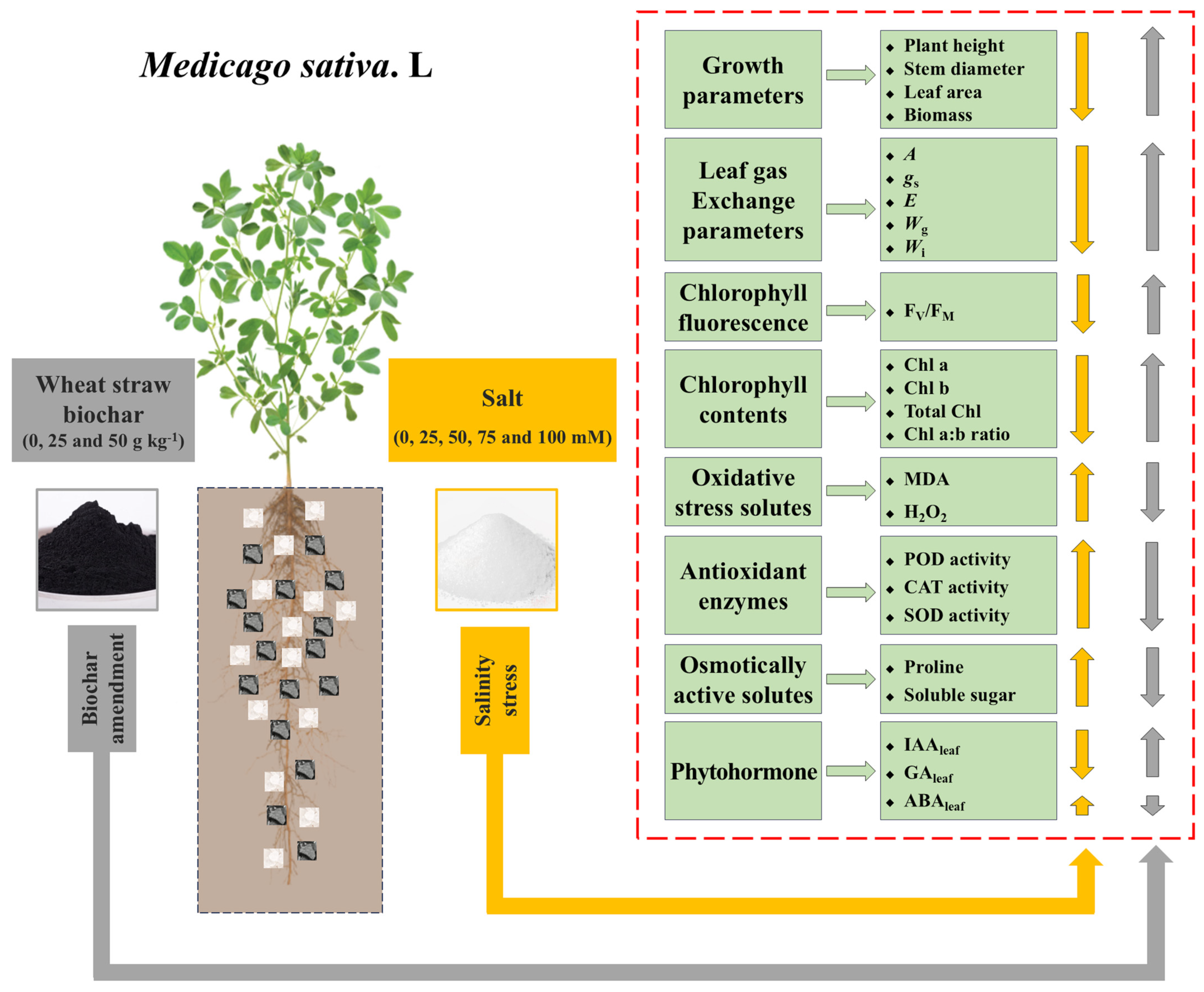
2.1. Study Site and Experimental Design
2.2. Leaf Gas Exchange Measurements
2.3. Chlorophyll Fluorescence Measurements
2.4. Chlorophyll Content Measurements
2.5. Oxidative Stress Solutes Measurements
2.6. Antioxidant Enzyme Activity Measurements
2.7. Osmotically Active Solute Measurements
2.8. Phytohormone Measurements
2.9. Growth and Biomass Measurements
2.10. Statistical Analysis
3. Results
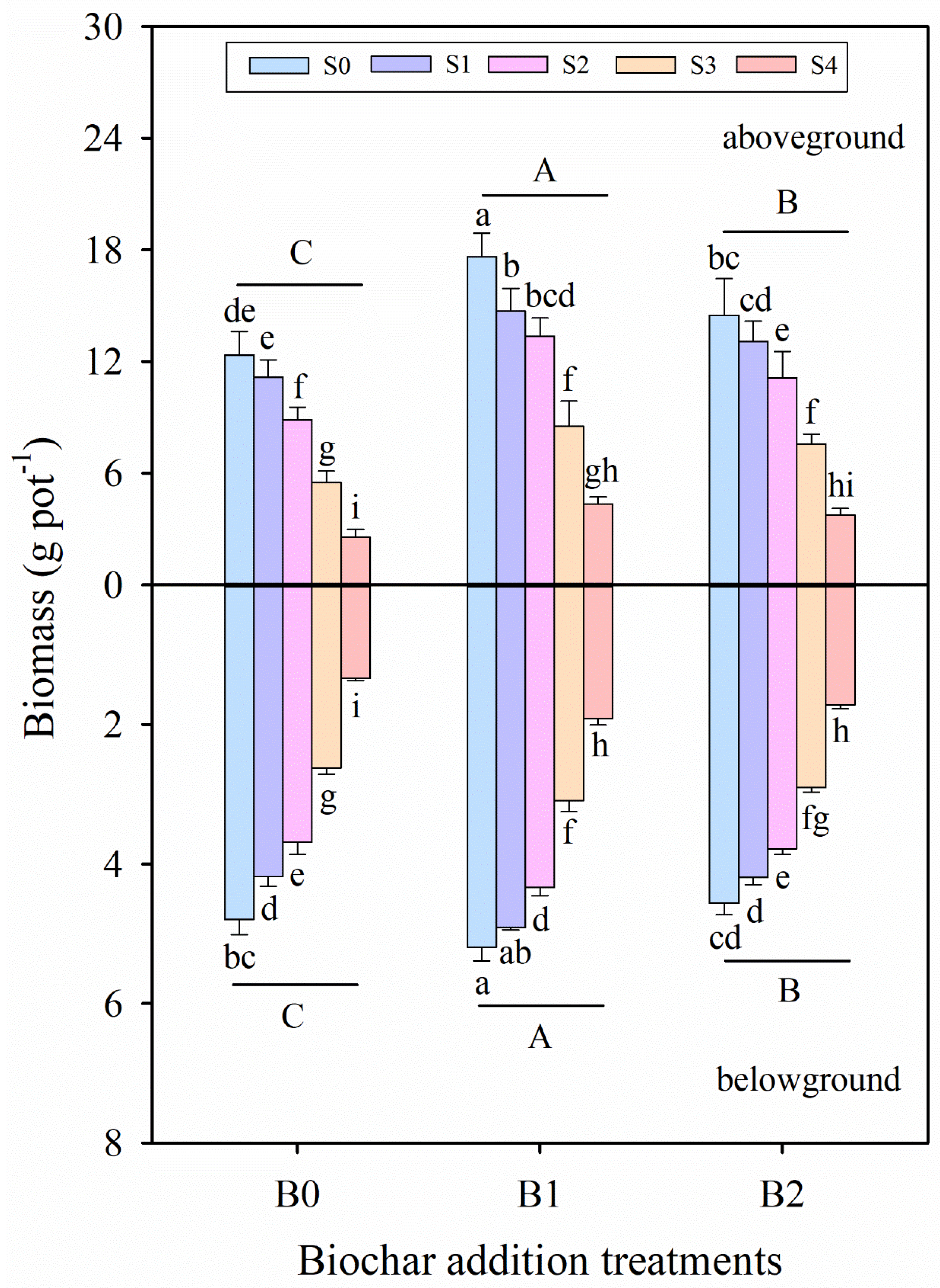
3.1. Effects of Wheat Straw Biochar Addition on Plant Growth Parameters Under Salinity Stress
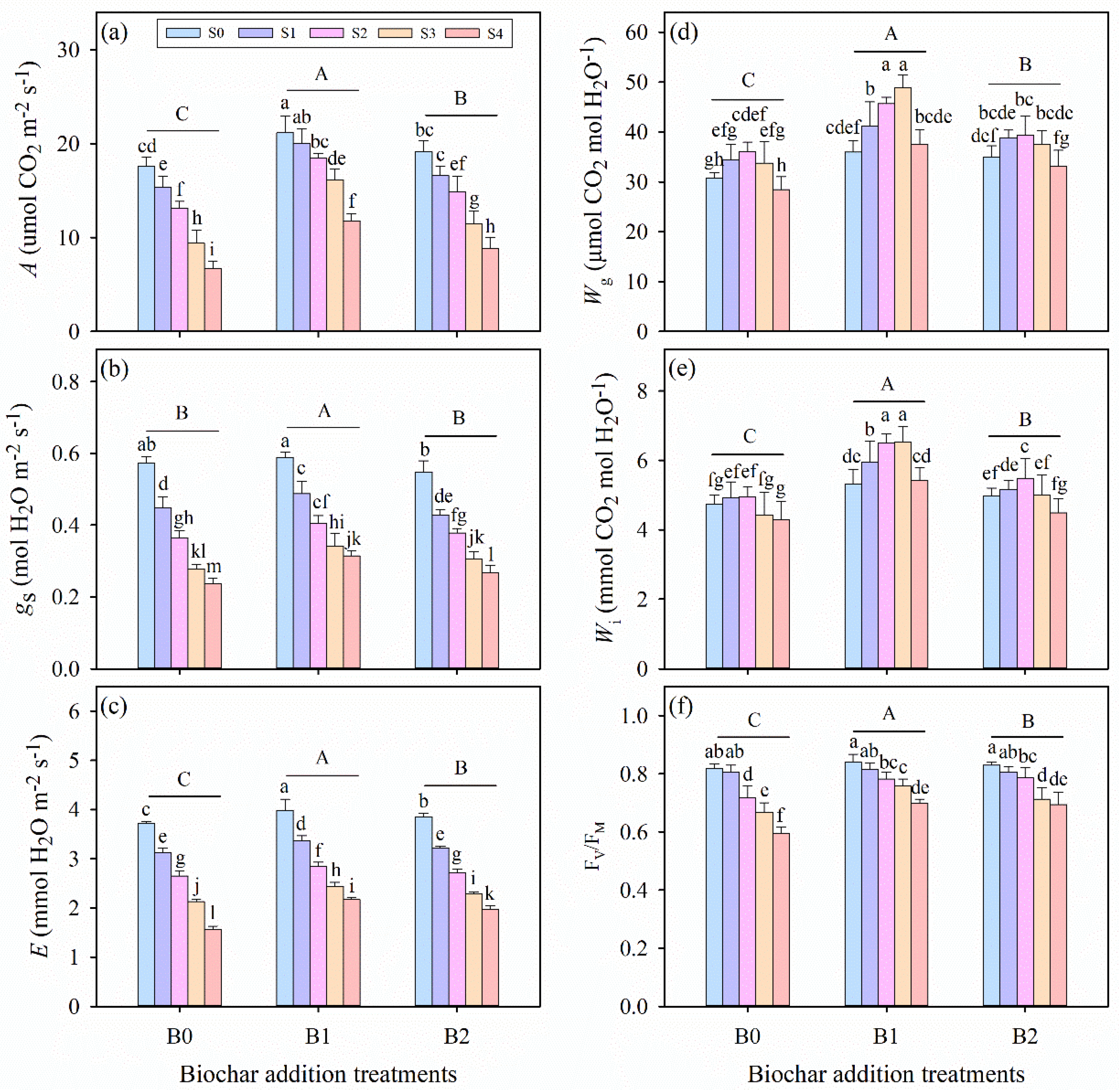
3.2. Effects of Wheat Straw Biochar Addition on Leaf Gas Exchange Parameters Under Salinity Stress
3.3. Effects of Wheat Straw Biochar Addition on Chlorophyll Fluorescence Under Salinity Stress
3.4. Effects of Wheat Straw Biochar Addition on Chlorophyll Content Under Salinity Stress
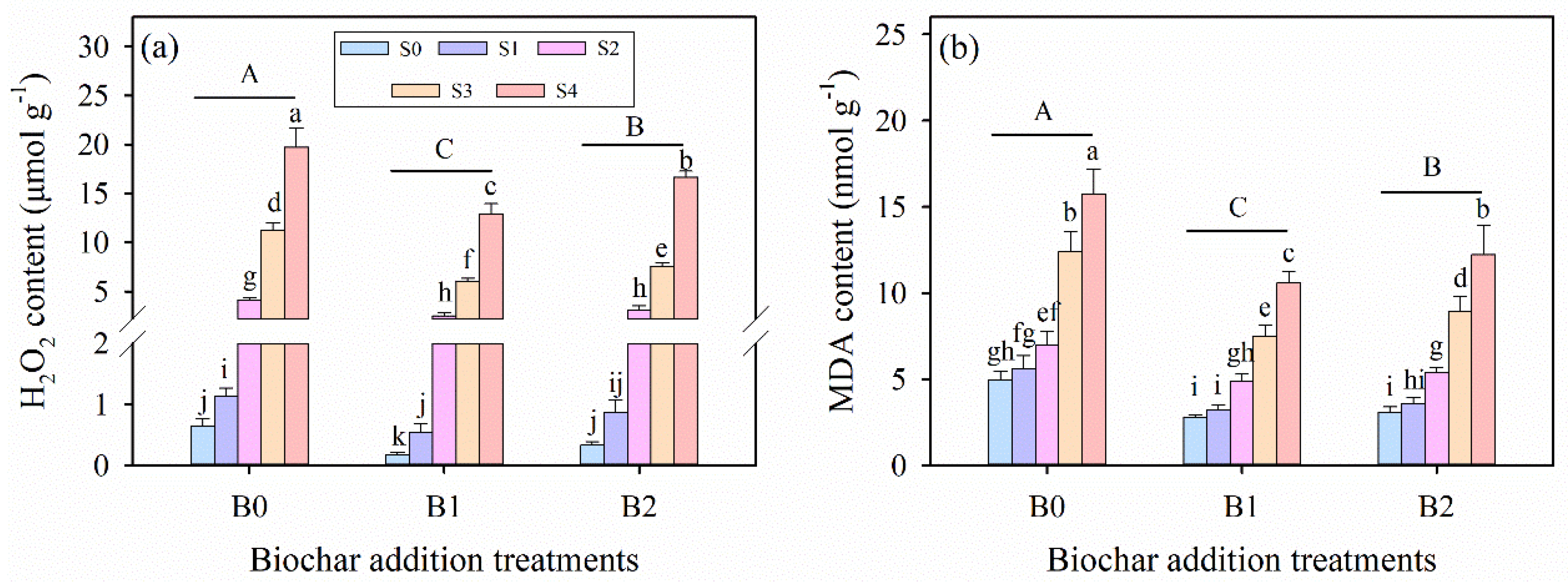
3.5. Effects of Wheat Straw Biochar Addition on Oxidative Stress Solutes Under Salinity Stress
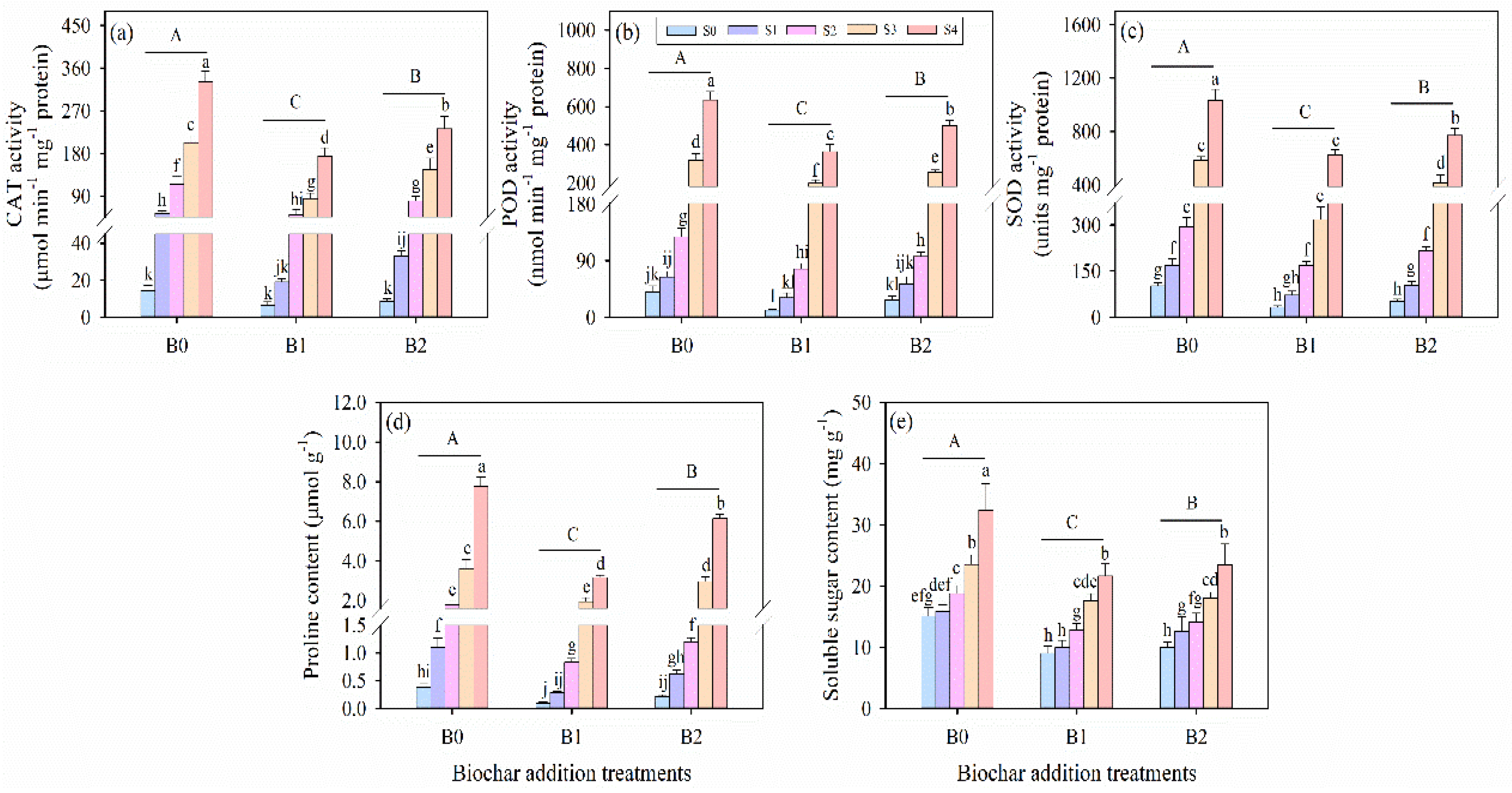
3.6. Effects of Wheat Straw Biochar Addition on Antioxidant Enzyme Activity Under Salinity Stress
3.7. Effects of Wheat Straw Biochar Addition on Osmotically Active Solutes Under Salinity Stress
3.8. Effects of Wheat Straw Biochar Addition on Phytohormone Under Salinity Stress
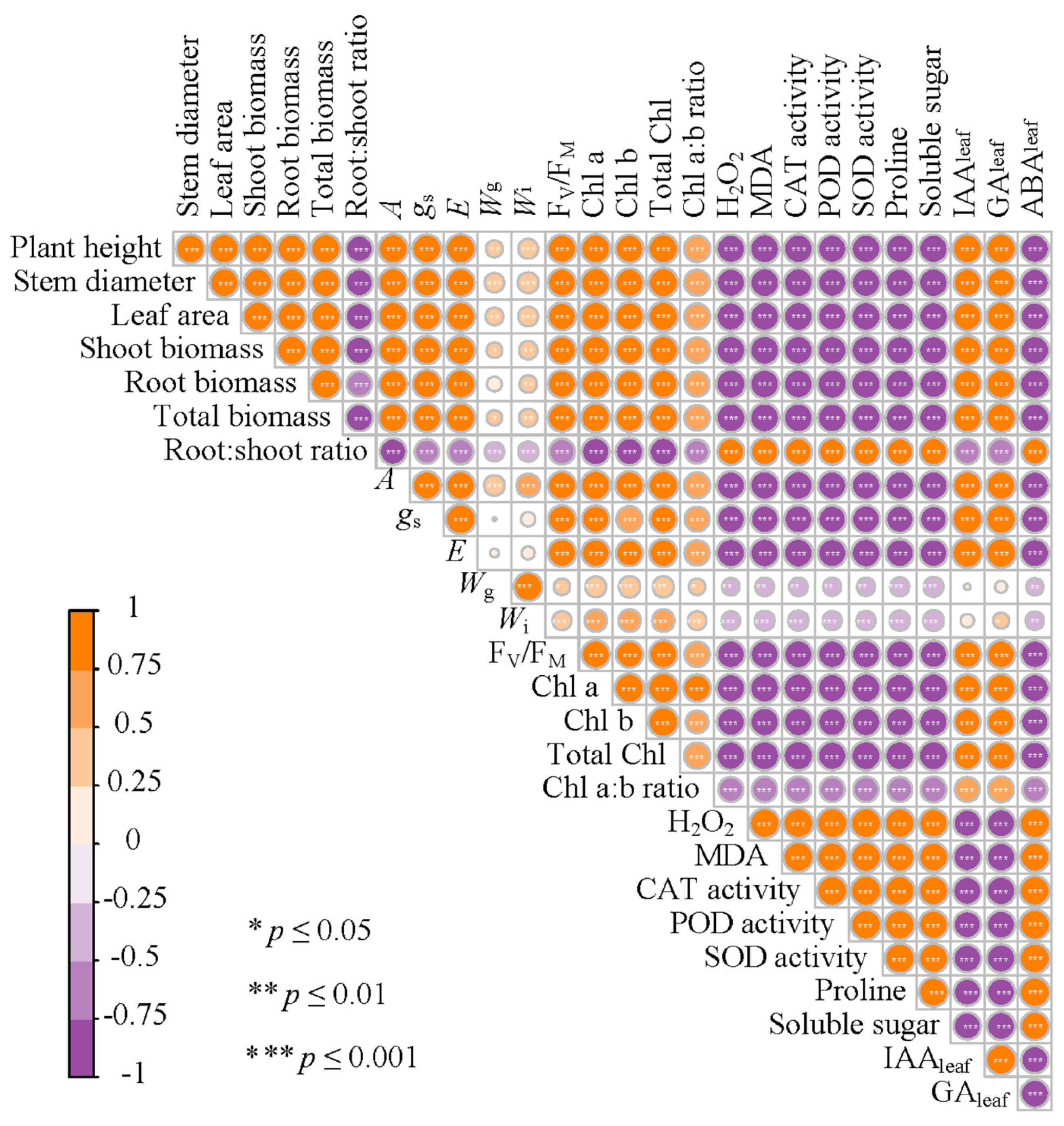
3.9. The Correlation Between Different Physiological, Biochemical, and Plant Traits
4. Discussion
4.1. Effects of Wheat Straw Biochar Amendment on the Growth and Photosynthetic Characteristics of Alfalfa Under Salinity Stress
4.2. Effects of Wheat Straw Biochar Amendment on the Physiological and Biochemical Indices of Alfalfa Under Salinity Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Herrera, R.M.; Sánchez-Hernández, C.V.; Palmeros-Suárez, P.A.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Meza-Canales, I.D.; Becerril-Espinosa, A. Seaweed extract improves growth and productivity of tomato plants under salinity stress. Agronomy 2022, 12, 2495. [Google Scholar] [CrossRef]
- Ahmad, I.; Zhu, G.; Zhou, G.; Younas, M.U.; Suliman, M.S.E.; Liu, J.; Zhu, Y.M.; Salih, E.G.I. Integrated approaches for increasing plant yield under salt stress. Front. Plant Sci. 2023, 14, 1215343. [Google Scholar] [CrossRef]
- Li, J.; Pu, L.; Han, M.; Zhu, M.; Zhang, R.; Xiang, Y. Soil salinization research in China: Advances and prospects. J. Geogr. Sci. 2014, 24, 943–960. [Google Scholar] [CrossRef]
- Singh, A. Soil salinity: A global threat to sustainable development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Kamal, M.Z.U.; Sarker, U.; Roy, S.K.; Alam, M.S.; Azam, M.G.; Miah, M.Y.; Hossain, N.; Ercisli, S.; Alamri, S. Manure-biochar compost mitigates the soil salinity stress in tomato plants by modulating the osmoregulatory mechanism, photosynthetic pigments, and ionic homeostasis. Sci. Rep. 2024, 14, 21929. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Naveed, M.; Zahir, Z.A.; Liu, F. Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct. Plant Biol. 2015, 42, 770–781. [Google Scholar] [CrossRef]
- Abid, M.; Zhang, Y.J.; Li, Z.; Bai, D.F.; Zhong, Y.P.; Fang, J.B. Effect of salt stress on growth, physiological and biochemical characters of four kiwifruit genotypes. Sci. Hortic. 2020, 271, 109473. [Google Scholar] [CrossRef]
- Shabbir, A.; Saqib, M.; Murtaza, G.; Abbas, G.; Imran, M.; Rizwan, M.; Naeem, M.A.; Ali, S.; Javeed, H.M.R. Biochar mitigates arsenic-induced human health risks and phytotoxicity in quinoa under saline conditions by modulating ionic and oxidative stress responses. Environ. Pollut. 2021, 287, 117348. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant. 2019, 168, 256–277. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, Y.; Zhang, M.; Qiu, Y.; Zhou, H.; Li, M. Current perspectives on improving soybean performance on saline-alkaline lands. New Crops 2025, 100079. [Google Scholar] [CrossRef]
- Hafez, Y.; Elkohby, W.; Mazrou, Y.S.; Ghazy, M.; Elgamal, A.; Abdelaal, K. Alleviating the detrimental impacts of salt stress on morpho-physiological and yield characters of rice plants (Oryza sativa L.) using Actosol, Nano-Zn and Nano-Si. Fresenius Environ. Bull. 2020, 29, 6882–6897. [Google Scholar]
- Waqas, M.; Yaning, C.; Iqbal, H.; Shareef, M.; ur Rehman, H.; Bilal, H.M. Synergistic consequences of salinity and potassium deficiency in quinoa: Linking with stomatal patterning, ionic relations and oxidative metabolism. Plant Physiol. Biochem. 2021, 159, 17–27. [Google Scholar] [CrossRef]
- Wei, X.; Su, X.; Cao, P.; Liu, X.; Chang, W.; Li, M.; Zhang, X.; Liu, Z. Structure of spinach photosystem II-LHCII supercomplex at 3.2 Å resolution. Nature 2016, 534, 69–74. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Biochar alleviates fluoride toxicity and oxidative stress in safflower (Carthamus tinctorius L.) seedlings. Chemosphere 2019, 223, 406–415. [Google Scholar] [CrossRef]
- Hou, J.; Liu, X.; Zhang, J.; Wei, Z.; Ma, Y.; Wan, H.; Liu, J.; Cui, B.; Zong, Y.; Chen, Y.; et al. Combined application of biochar and partial root-zone drying irrigation improves water relations and water use efficiency of cotton plants under salt stress. Agric. Water Manag. 2023, 290, 108584. [Google Scholar] [CrossRef]
- Gullap, M.K.; Karabacak, T.; Severoglu, S.; Kurt, A.N.; Ekinci, M.; Turan, M.; Aktas, H.; Yildirim, E. Biochar derived from olive oil pomace mitigates salt stress on seedling growth of forage pea. Front. Plant Sci. 2024, 15, 1398846. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Beritognolo, I.; Muleo, R.; Piazzai, M.; Sabatti, M.; Mugnozza, G.S.; Kuzminsky, E. Leaf morphological plasticity and stomatal conductance in three Populus alba L. genotypes subjected to salt stress. Environ. Exp. Bot. 2009, 66, 381–388. [Google Scholar] [CrossRef]
- Yuan, J.; Cao, H.; Qin, W.; Yang, S.; Zhang, D.; Zhu, L.; Song, H.; Zhang, Q. Genomic and modern biotechnological strategies for enhancing salt tolerance in crops. New Crops 2024, 2, 100057. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd_Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 2015, 158, 61–68. [Google Scholar] [CrossRef]
- Hagemann, N.; Joseph, S.; Schmidt, H.P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W.; et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, M.N.; Zhang, K.; Luo, T.; Zhu, K.; Hu, L. The application of biochar alleviated the adverse effects of drought on the growth, physiology, yield and quality of rapeseed through regulation of soil status and nutrients availability. Ind. Crops Prod. 2021, 171, 113878. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, J.; Liu, X.; Ma, Y.; Wei, Z.; Wan, H.; Liu, F. Effect of biochar addition and reduced irrigation regimes on growth, physiology and water use efficiency of cotton plants under salt stress. Ind. Crops Prod. 2023, 198, 116702. [Google Scholar] [CrossRef]
- Laird, D.A.; Brown, R.C.; Amonette, J.E.; Lehmann, J. Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod. Biorefining 2009, 3, 547–562. [Google Scholar] [CrossRef]
- Khedulkar, A.P.; Pandit, B.; Dang, D.V.; Doong, R.A. Agricultural waste to real worth biochar as a sustainable material for supercapacitor. Sci. Total Environ. 2023, 869, 161441. [Google Scholar] [CrossRef]
- Hou, R.; Zhang, J.; Fu, Q.; Li, T.; Gao, S.; Wang, R.; Zhao, S.; Zhu, B. The boom era of emerging contaminants: A review of remediating agricultural soils by biochar. Sci. Total Environ. 2024, 931, 172899. [Google Scholar] [CrossRef]
- Bamminger, C.; Poll, C.; Sixt, C.; Högy, P.; Wüst, D.; Kandeler, E.; Marhan, S. Short-term response of soil microorganisms to biochar addition in a temperate agroecosystem under soil warming. Agric. Ecosyst. Environ. 2016, 233, 308–317. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Jiang, Y.; Wang, J.; Cai, X.; Wen, Z.; Qiu, Z.; Qiao, G. Tobacco straw biochar improved the growth of Chinese cherry (Prunus pseudocerasus) via altering plant physiology and shifting the rhizosphere bacterial community. Sci. Hortic. 2022, 303, 111244. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, W.; Zhao, W.; Xu, L.; Wang, M.; Jian, J.; Chen, X.; Wang, E.; Yan, J. Effects of biochar application on soil properties and the growth of Melissa officinalis L. under salt stress. Sci. Hortic. 2024, 338, 113704. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Al-Wabel, M.I.; Ok, Y.S.; Al-Harbi, A.; Wahb-Allah, M.; El-Naggar, A.H.; Ahmad, M.; Al-Faraj, A.; Al-Omran, A. Conocarpus biochar induces changes in soil nutrient availability and tomato growth under saline irrigation. Pedosphere 2016, 26, 27–38. [Google Scholar] [CrossRef]
- Kapoor, A.; Sharma, R.; Kumar, A.; Sepehya, S. Biochar as a means to improve soil fertility and crop productivity: A review. J. Plant Nutr. 2022, 45, 2380–2388. [Google Scholar] [CrossRef]
- Shi, X.; Zhao, Y.; Xu, M.; Ma, L.; Adams, J.M.; Shi, Y. Insights into plant–microbe interactions in the rhizosphere to promote sustainable agriculture in the new crops era. New Crops 2024, 1, 100004. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Zhang, L.; Zheng, Y.; Liu, X.; Zhang, Y. The critical role of biochar to mitigate the adverse impacts of drought and salinity stress in plants. Front. Plant Sci. 2023, 14, 1163451. [Google Scholar] [CrossRef]
- Baigorri, R.; San Francisco, S.; Urrutia, Ó.; García-Mina, J.M. Biochar-Ca and biochar-Al/-Fe-mediated phosphate exchange capacity are main drivers of the different biochar effects on plants in acidic and alkaline soils. Agronomy 2020, 10, 968. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Naeem, M.A.; Khalid, M.; Aon, M.; Abbas, G.; Tahir, M.; Amjad, M.; Murtaza, B.; Ynag, A.; Akhtar, S.S. Effect of wheat and rice straw biochar produced at different temperatures on maize growth and nutrient dynamics of a calcareous soil. Arch. Agron. Soil Sci. 2017, 63, 2048–2061. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Campion, L.; Bekchanova, M.; Malina, R.; Kuppens, T. The costs and benefits of biochar production and use: A systematic review. J. Clean. Prod. 2023, 408, 137138. [Google Scholar] [CrossRef]
- Zhong, S.Z.; Liu, X.J.; Ouyang, J.H.; Tu, X.J.; Song, W.Z.; Cao, W.; Tao, Q.B.; Sun, J. Effects of biochar and phosphorus fertilizer combination on the physiological growth characteristics of alfalfa in saline-alkali soil of the Yellow River Delta. Chin. J. Grassl. 2024, 46, 35–45. [Google Scholar] [CrossRef]
- Fan, J.W.; Chen, M.; Tian, F.; Yao, R.; Qin, N.N.; Wu, W.H.; Turner, N.C.; Li, F.M.; Du, Y.L. Root morphology, exudate patterns, and mycorrhizal symbiosis are determinants to improve phosphorus acquisition in alfalfa. J. Exp. Bot. 2025, eraf107. [Google Scholar] [CrossRef]
- Noori, F.; Etesami, H.; Zarini, H.N.; Khoshkholgh-Sima, N.A.; Salekdeh, G.H.; Alishahi, F. Mining alfalfa (Medicago sativa L.) nodules for salinity tolerant non-rhizobial bacteria to improve growth of alfalfa under salinity stress. Ecotoxicol. Environ. Saf. 2018, 162, 129–138. [Google Scholar] [CrossRef]
- Ghafoor, A.Z.; Javed, H.H.; Karim, H.; Studnicki, M.; Ali, I.; Yue, H.; Xiao, P.; Asghar, M.A.; Brock, C.; Wu, Y. Biological nitrogen fixation for sustainable agriculture development under climate change–new insights from a meta-analysis. J. Agron. Crop Sci. 2024, 210, e12754. [Google Scholar] [CrossRef]
- Fougère, F.; Le Rudulier, D.; Streeter, J.G. Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.). Plant Physiol. 1991, 96, 1228–1236. [Google Scholar] [CrossRef]
- Al-Farsi, S.M.; Nawaz, A.; Rehman, A.U.; Nadaf, S.K.; Al-Sadi, A.M.; Siddique, K.H.M.; Farooq, M. Effects, tolerance mechanisms and management of salt stress in lucerne (Medicago sativa). Crop Pasture Sci. 2020, 71, 411–428. [Google Scholar] [CrossRef]
- Saifullah; Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef]
- Sultan, H.; Li, Y.; Ahmed, W.; Yixue, M.; Shah, A.; Faizan, M.; Ahmad, A.; Abbas, H.M.M.; Nie, L.; Khan, M.N. Biochar and nano biochar: Enhancing salt resilience in plants and soil while mitigating greenhouse gas emissions: A comprehensive review. J. Environ. Manag. 2024, 355, 120448. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Xu, Y.; Meng, B.; Loik, M.E.; Ma, J.Y.; Sun, W. Nitrogen addition increases the sensitivity of photosynthesis to drought and re-watering differentially in C3 versus C4 grass species. Front. Plant Sci. 2019, 10, 815. [Google Scholar] [CrossRef] [PubMed]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C. Water stress tolerance of wheat (Triticum aestivum L.): Variations in hydrogen peroxide accumulation and antioxidant activity in tolerant and susceptible genotypes. J. Agron. Crop Sci. 2001, 186, 63–70. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 2017, 137, 64–70. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Zhao, D.; Mackown, C.T.; Starks, P.J.; Kindiger, B.K. Rapid analysis of nonstructural carbohydrate components in grass forage using microplate enzymatic assays. Crop Sci. 2010, 50, 1537–1545. [Google Scholar] [CrossRef]
- Koshita, Y.; Takahara, T.; Ogata, T.; Goto, A. Involvement of endogenous plant hormones (IAA, ABA, GAs) in leaves and flower bud formation of satsuma mandarin (Citrus unshiu Marc.). Sci. Hortic. 1999, 79, 185–194. [Google Scholar] [CrossRef]
- Jabborova, D.; Abdrakhmanov, T.; Jabbarov, Z.; Abdullaev, S.; Azimov, A.; Mohamed, I.; AlHarbi, M.; Abu-Elsaoud, A.; Elkelish, A. Biochar improves the growth and physiological traits of alfalfa, amaranth and maize grown under salt stress. PeerJ 2023, 11, e15684. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef]
- El-Nahhas, N.; AlKahtani, M.D.; Abdelaal, K.A.; Al Husnain, L.; AlGwaiz, H.I.; Hafez, Y.M.; Attia, K.A.; El-Esawi, M.A.; Ibrahim, M.F.M.; Elkelish, A. Biochar and jasmonic acid application attenuates antioxidative systems and improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water. Plant Physiol. Biochem. 2021, 166, 807–817. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, Y.; Hettenhausen, C.; Lu, C.; Shen, G.; Zhang, C.; Li, J.; Song, J.; Lin, H.; Wu, J. Comparative analysis of alfalfa (Medicago sativa L.) leaf transcriptomes reveals genotype-specific salt tolerance mechanisms. BMC Plant Biol. 2018, 18, 1–14. [Google Scholar] [CrossRef]
- Chen, Z.; Niu, J.; Guo, Z.; Sui, X.; Xu, N.; Kareem, H.A.; Hassan, M.U.; Yan, M.; Zhang, Q.; Cui, J.; et al. Graphene enhances photosynthesis and the antioxidative defense system and alleviates salinity and alkalinity stresses in alfalfa (Medicago sativa L.) by regulating gene expression. Environ. Sci. Nano 2021, 8, 2731–2748. [Google Scholar] [CrossRef]
- Niu, J.; Chen, Z.; Guo, Z.; Xu, N.; Sui, X.; Roy, M.; Kareem, H.A.; Hassan, M.U.; Cui, J.; Wang, Q. Exogenous melatonin promotes the growth of alfalfa (Medicago sativa L.) under NaCl stress through multiple pathways. Ecotoxicol. Environ. Saf. 2022, 242, 113938. [Google Scholar] [CrossRef]
- Chen, J.; Tang, L.; Guo, W.; Wang, D.; Sun, Y.; Guo, C. Oxalic acid secretion alleviates saline-alkali stress in alfalfa by improving photosynthetic characteristics and antioxidant activity. Plant Physiol. Biochem. 2024, 208, 108475. [Google Scholar] [CrossRef]
- Guo, S.; Wang, X.; Li, X.; Ma, Y.; Yang, J.; Fu, B.; Li, S. Melatonin and calcium synergistically improve salt tolerance in alfalfa (Medicago sativa. L). Ind. Crops Prod. 2025, 224, 120322. [Google Scholar] [CrossRef]
- Murtaza, G.; Usman, M.; Iqbal, J.; Tahir, M.N.; Elshikh, M.S.; Alkahtani, J.; Toleikienė, M.; Iqbal, R.; Akram, M.I.; Gruda, N.S. The impact of biochar addition on morpho-physiological characteristics, yield and water use efficiency of tomato plants under drought and salinity stress. BMC Plant Biol. 2024, 24, 356. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, Z.; Zhao, M.; Hao, J.; Liu, J.; Yan, Y.; Sun, P.; Jia, Y.; Ge, G. Hydrothermal biochar enhances the photosynthetic efficiency and yield of alfalfa by optimizing soil chemical properties and stimulating the activity of microbial communities. Sci. Rep. 2024, 14, 31420. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, X.; Chen, L.; Wang, Z.; Xia, Y.; Zhang, Y.; Wang, H.; Luo, X.; Xing, B. Enhanced growth of halophyte plants in biochar-amended coastal soil: Roles of nutrient availability and rhizosphere microbial modulation. Plant Cell Environ. 2018, 41, 517–532. [Google Scholar] [CrossRef]
- Steduto, P.; Albrizio, R.; Giorio, P.; Sorrentino, G. Gas-exchange response and stomatal and non-stomatal limitations to carbon assimilation of sunflower under salinity. Environ. Exp. Bot. 2000, 44, 243–255. [Google Scholar] [CrossRef]
- Sarabi, B.; Fresneau, C.; Ghaderi, N.; Bolandnazar, S.; Streb, P.; Badeck, F.W.; Citerne, S.; Tangama, M.; David, A.; Ghashghaie, J. Stomatal and non-stomatal limitations are responsible in down-regulation of photosynthesis in melon plants grown under the saline condition: Application of carbon isotope discrimination as a reliable proxy. Plant Physiol. Biochem. 2019, 141, 1–19. [Google Scholar] [CrossRef]
- Liang, K.; Chen, Y.; Hou, J.; Yan, F.; Liu, F. ABA-mediated stomatal response modulates the effects of drought, salinity and combined stress on tomato plants grown under elevated CO2. Environ. Exp. Bot. 2024, 223, 105797. [Google Scholar] [CrossRef]
- Hedrich, R.; Shabala, S. Stomata in a saline world. Curr. Opin. Plant Biol. 2018, 46, 87–95. [Google Scholar] [CrossRef]
- Li, P.; Zhu, Y.; Song, X.; Song, F. Negative effects of long-term moderate salinity and short-term drought stress on the photosynthetic performance of Hybrid Pennisetum. Plant Physiol. Biochem. 2020, 155, 93–104. [Google Scholar] [CrossRef]
- Liu, X.; Elzenga, J.T.M.; Venema, J.H.; Tiedge, K.J. Thriving in a salty future: Morpho-anatomical, physiological and molecular adaptations to salt stress in alfalfa (Medicago sativa L.) and other crops. Ann. Bot. 2024, 134, 1113–1130. [Google Scholar] [CrossRef]
- Bethke, P.C.; Drew, M.C. Stomatal and nonstomatal components to inhibition of photosynthesis in leaves of Capsicum annuum during progressive exposure to NaCl salinity. Plant Physiol. 1992, 99, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Oukarroum, A.; Bussotti, F.; Goltsev, V.; Kalaji, H.M. Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ. Exp. Bot. 2015, 109, 80–88. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, B.; Nagpal, S.; Kumar, A.; Tiwari, S.; Nair, R.M. Plant growth-promoting rhizobacteria: Salt stress alleviators to improve crop productivity for sustainable agriculture development. Front. Plant Sci. 2023, 13, 1101862. [Google Scholar] [CrossRef]
- Wen, Y.; Shi, F.; Zhang, B.; Li, K.; Chang, W.; Fan, X.; Dai, C.L.; Song, F. Rhizophagus irregularis and biochar can synergistically improve the physiological characteristics of saline-alkali resistance of switchgrass. Physiol. Plant. 2024, 176, e14367. [Google Scholar] [CrossRef]
- Al-Farsi, S.M.; Al-Sadi, A.M.; Ullah, A.; Rehman, A.; Farooq, M. Integration of seed priming with nano-sized chitosan-proline and biochar application improves salt tolerance in differentially responding genotypes of alfalfa (Medicago sativa). Crop Pasture Sci. 2025, 76, CP24222. [Google Scholar] [CrossRef]
- Hotta, Y.; Tanaka, T.; Takaoka, H.; Takeuchi, Y.; Konnai, M. New physiological effects of 5-aminolevulinic acid in plants: The increase of photosynthesis, chlorophyll content, and plant growth. Biosci. Biotechnol. Biochem. 1997, 61, 2025–2028. [Google Scholar] [CrossRef]
- Xu, N.; Chen, Z.; Niu, J.; Niu, K.; Khan, Z. Effects of exogenous 5-aminolevulinic acid (5-ALA) on alfalfa (Medicago sativa L.) under NaCl-induced salinity stress. J. Soil Sci. Plant Nutr. 2025, 25, 478–494. [Google Scholar] [CrossRef]
- Guo, L.; Bornø, M.L.; Niu, W.; Liu, F. Biochar amendment improves shoot biomass of tomato seedlings and sustains water relations and leaf gas exchange rates under different irrigation and nitrogen regimes. Agric. Water Manag. 2021, 245, 106580. [Google Scholar] [CrossRef]
- Ramzan, M.; Jamshaid, T.; Ali, L.; Dawar, K.; Saba, R.; Jamshaid, U.; Fahad, S.; Salmen, S.H.; Ansari, M.J.; Danish, S.; et al. Modulation of sunflower growth via regulation of antioxidants, oil content and gas exchange by arbuscular mycorrhizal fungi and quantum dot biochar under chromium stress. BMC Plant Biol. 2023, 23, 629. [Google Scholar] [CrossRef]
- Farooq, M.; Rehman, A.; Al-Alawi, A.K.; Al-Busaidi, W.M.; Lee, D.J. Integrated use of seed priming and biochar improves salt tolerance in cowpea. Sci. Hortic. 2020, 272, 109507. [Google Scholar] [CrossRef]
- Singh, B.K.; Sharma, S.R.; Singh, B. Antioxidant enzymes in cabbage: Variability and inheritance of superoxide dismutase, peroxidase and catalase. Sci. Hortic. 2010, 124, 9–13. [Google Scholar] [CrossRef]
- Xiong, X.; Wei, Y.Q.; Liu, M.H.; Liu, N.; Zhang, Y.J. Localized and systemic abilities of arbuscular mycorrhizal fungi to control growth, antioxidant defenses, and the nutrient uptake of alfalfa under uniform and non-uniform salt stress. Plant Soil 2024, 1–19. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, P.; Wang, B.; Li, H.; Li, S.; Zhang, H.; Haider, F.U.; Li, X. Harnessing the role of rhizo-bacteria to mitigate salinity stress in rice (Orzya sativa); Focus on antioxidant defense system, photosynthesis response, and rhizosphere microbial diversity. Rhizosphere 2025, 33, 101043. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Ciura, J.; Kruk, J. Phytohormones as targets for improving plant productivity and stress tolerance. J. Plant Physiol. 2018, 229, 32–40. [Google Scholar] [CrossRef]
- Ali, J.; Mukarram, M.; Ojo, J.; Dawam, N.; Riyazuddin, R.; Ghramh, H.A.; Khan, K.A.; Chen, R.; Kurjak, D.; Bayram, A. Harnessing phytohormones: Advancing plant growth and defence strategies for sustainable agriculture. Physiol. Plantarum 2024, 176, e14307. [Google Scholar] [CrossRef]
- Xu, C.Y.; Hosseini-Bai, S.; Hao, Y.; Rachaputi, R.C.; Wang, H.; Xu, Z.; Wallace, H. Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environ. Sci. Pollut. Res. 2015, 22, 6112–6125. [Google Scholar] [CrossRef]
- Huang, K.; Li, M.; Li, R.; Rasul, F.; Shahzad, S.; Wu, C.; Shao, J.; Huang, G.; Li, R.; Alamri, S.; et al. Soil acidification and salinity: The importance of biochar application to agricultural soils. Front. Plant Sci. 2023, 14, 1206820. [Google Scholar] [CrossRef]
- Liu, M.; Ke, X.; Liu, X.; Fan, X.; Xu, Y.; Li, L.; Solaiman, Z.M.; Pan, G. The effects of biochar soil amendment on rice growth may vary greatly with rice genotypes. Sci. Total Environ. 2022, 810, 152223. [Google Scholar] [CrossRef]
- Amin, A.E.E.A.Z. Effects of saline water on soil properties and red radish growth in saline soil as a function of co-applying wood chips biochar with chemical fertilizers. BMC Plant Biol. 2023, 23, 382. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xia, J.; Yang, H.; Liu, J.; Shao, P. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Abrar, M.M.; Naeem, M.A.; Siddiqui, M.H.; Ali, H.M.; Li, Y.; Ahmed, K.; Sun, N.; Xu, M. Biochar increases salt tolerance and grain yield of quinoa on saline-sodic soil: Multivariate comparison of physiological and oxidative stress attributes. J. Soils Sediments 2022, 22, 1446–1459. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yang, Q.; Xu, H.; Shen, G.; Chen, Q. Optimizing Biochar Application Rates to improve soil properties and crop growth in saline–alkali soil. Sustainability 2024, 16, 2523. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Zia-ur-Rehman, M.; Qayyum, M.F.; Ok, Y.S.; Murtaza, G. Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ. Sci. Pollut. Res. 2018, 25, 25668–25680. [Google Scholar] [CrossRef]
- Sun, J.; He, F.; Shao, H.; Zhang, Z.; Xu, G. Effects of biochar application on Suaeda salsa growth and saline soil properties. Environ. Earth Sci. 2016, 75, 1–6. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, H.; Shi, W.; Zhou, M.; Ma, X. Effect of biochar on nitrogen use efficiency, grain yield and amino acid content of wheat cultivated on saline soil. Plant Soil Environ. 2019, 65, 83–89. [Google Scholar] [CrossRef]
- She, D.; Sun, X.; Gamareldawla, A.H.D.; Nazar, E.A.; Hu, W.; Edith, K.; Yu, S. Benefits of soil biochar amendments to tomato growth under saline water irrigation. Sci. Rep. 2018, 8, 14743. [Google Scholar] [CrossRef]
- Lin, X.; Xie, Z.; Zheng, J.; Liu, Q.; Bei, Q.; Zhu, J. Effects of biochar application on greenhouse gas emissions, carbon sequestration and crop growth in coastal saline soil. Eur. J. Soil Sci. 2015, 66, 329–338. [Google Scholar] [CrossRef]
- Abulaiti, A.; She, D.; Liu, Z.; Sun, X.; Wang, H. Application of biochar and polyacrylamide to revitalize coastal saline soil quality to improve rice growth. Environ. Sci. Pollut. Res. 2023, 30, 18731–18747. [Google Scholar] [CrossRef]
- El-Sayed, M.E.; Hazman, M.; Abd El-Rady, A.G.; Almas, L.; McFarland, M.; Shams El Din, A.; Burian, S. Biochar reduces the adverse effect of saline water on soil properties and wheat production profitability. Agriculture 2021, 11, 1112. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, J.; Yao, R.; Chen, X.; Wang, X. Biochar and fulvic acid amendments mitigate negative effects of coastal saline soil and improve crop yields in a three year field trial. Sci. Rep. 2020, 10, 8946. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Lin, Q.; Li, G.; Zhao, X.; Chen, H. Biochar Amends saline soil and enhances maize growth: Three-year field experiment findings. Agronomy 2023, 13, 1111. [Google Scholar] [CrossRef]
- Zonayet, M.; Paul, A.K.; Faisal-E-Alam, M.; Syfullah, K.; Castanho, R.A.; Meyer, D. Impact of biochar as a soil conditioner to improve the soil properties of saline soil and productivity of tomato. Sustainability 2023, 15, 4832. [Google Scholar] [CrossRef]


| Factor | Biochar | B0-Soil | B1-Soil | B2-Soil |
|---|---|---|---|---|
| Total C, % | 57.59 | 1.93 | 7.30 | 18.95 |
| Total N, % | 1.33 | 0.04 | 0.39 | 0.67 |
| Available N, ppm | 102.87 | 62.45 | 77.71 | 82.75 |
| Available P, ppm | 108.45 | 40.67 | 68.63 | 77.42 |
| Available K, ppm | 529.76 | 114.58 | 229.91 | 351.40 |
| pH | 8.87 | 0.31 | 0.82 | 1.05 |
| EC, dS m−1 | 2.23 | 309.01 | 817.33 | 1017.39 |
| CEC, cmol + kg−1 | 12.08 | 5.44 | 7.76 | 9.44 |
| Surface area, m2 g−1 | 24.59 | - | - | - |
| Total ash, % | 30.87 | - | - | - |
| Volatile matter, % | 18.82 | - | - | - |
| C stability, % | 89.96 | - | - | - |
| Treatment | Plant Height | Stem Diameter | Leaf Area | Total Biomass | Root/Shoot Ratio | |
|---|---|---|---|---|---|---|
| (cm) | (mm) | (cm2) | (g pot−1) | (None) | ||
| B0 | S0 | 24.64 ± 1.57 cd | 1.85 ± 0.02 bc | 1079.31 ± 77.21 de | 17.14 ± 1.66 d | 0.39 ± 0.03 de |
| S1 | 23.29 ± 1.20 cde | 1.79 ± 0.03 cd | 989.07 ± 83.86 ef | 15.34 ± 1.14 e | 0.38 ± 0.03 efg | |
| S2 | 19.19 ± 1.10 f | 1.72 ± 0.03 ef | 853.82 ± 58.99 g | 12.56 ± 0.86 f | 0.42 ± 0.05 cd | |
| S3 | 13.84 ± 1.24 i | 1.61 ± 0.06 g | 685.34 ± 47.65 h | 8.11 ± 0.61 h | 0.48 ± 0.08 b | |
| S4 | 11.53 ± 1.18 j | 1.47 ± 0.07 h | 507.83 ± 43.70 i | 3.89 ± 0.45 j | 0.53 ± 0.08 a | |
| B1 | S0 | 32.32 ± 1.96 a | 1.93 ± 0.05 a | 1387.96 ± 46.99 a | 23.23 ± 1.58 a | 0.30 ± 0.03 j |
| S1 | 28.37 ± 0.82 b | 1.92 ± 0.05 a | 1284.07 ± 54.51 ab | 19.61 ± 1.25 b | 0.32 ± 0.02 hi | |
| S2 | 24.19 ± 0.98 cd | 1.87 ± 0.02 ab | 1104.20 ± 53.12 cde | 17.69 ± 1.02 cd | 0.33 ± 0.04 gh | |
| S3 | 21.46 ± 1.64 e | 1.79 ± 0.03 cd | 981.35 ± 74.44 ef | 11.61 ± 1.69 fg | 0.37 ± 0.03 efg | |
| S4 | 16.45 ± 0.83 gh | 1.71 ± 0.04 ef | 714.24 ± 58.23 h | 6.29 ± 0.49 i | 0.44 ± 0.05 bc | |
| B2 | S0 | 27.76 ± 1.23 b | 1.90 ± 0.05 ab | 1220.01 ± 89.17 bc | 19.06 ± 1.81 bc | 0.32 ± 0.06 gh |
| S1 | 25.42 ± 1.47 c | 1.85 ± 0.05 b | 1154.83 ± 74.67 cd | 17.28 ± 0.88 d | 0.33 ± 0.04 fgh | |
| S2 | 22.67 ± 1.52 de | 1.76 ± 0.06 de | 993.72 ± 64.86 ef | 14.86 ± 1.45 e | 0.34 ± 0.03 fgh | |
| S3 | 16.95 ± 0.95 g | 1.70 ± 0.02 f | 880.57 ± 52.69 fg | 10.46 ± 0.56 g | 0.39 ± 0.04 de | |
| S4 | 14.24 ± 0.76 hi | 1.62 ± 0.04 g | 643.92 ± 61.85 h | 5.47 ± 0.48 i | 0.46 ± 0.01 bc | |
| Significance | ||||||
| Salinity stress (S) | F = 314.95 *** | F = 102.54 *** | F = 211.44 *** | F = 383.06 *** | F = 27.31 *** | |
| Biochar (B) | F = 143.42 *** | F = 175.24 *** | F = 112.23 *** | F = 84.52 *** | F = 28.77 *** | |
| S × B | F = 2.16 * | F = 2.47 ** | F = 0.76 n.s | F = 2.46 * | F = 0.32 n.s | |
| Salinity Stress (S) | Biochar (B) | S × B | |||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | |
| Shoot biomass | 4 | 274.71 | <0.001 | 2 | 72.76 | <0.001 | 8 | 2.21 | <0.05 |
| Root biomass | 4 | 318.95 | <0.001 | 2 | 28.83 | <0.001 | 8 | 2.18 | <0.05 |
| A | 4 | 165.81 | <0.001 | 2 | 129.04 | <0.001 | 8 | 2.02 | <0.05 |
| gs | 4 | 423.67 | <0.001 | 2 | 34.21 | <0.001 | 8 | 2.57 | <0.01 |
| E | 4 | 1107.54 | <0.001 | 2 | 82.39 | <0.001 | 8 | 4.81 | <0.001 |
| Wg | 4 | 21.16 | <0.001 | 2 | 63.31 | <0.001 | 8 | 3.10 | <0.01 |
| Wi | 4 | 9.39 | <0.001 | 2 | 55.75 | <0.001 | 8 | 2.33 | <0.05 |
| FV/FM | 4 | 318.77 | <0.001 | 2 | 20.48 | <0.001 | 8 | 2.73 | <0.01 |
| Chl a content | 4 | 144.54 | <0.001 | 2 | 116.23 | <0.001 | 8 | 0.96 | n.s |
| Chl b content | 4 | 88.41 | <0.001 | 2 | 66.93 | <0.001 | 8 | 1.10 | n.s |
| Total Chl content | 4 | 151.11 | <0.001 | 2 | 119.78 | <0.001 | 8 | 2.06 | <0.05 |
| Chl a:b ratio | 4 | 13.02 | <0.001 | 2 | 12.27 | <0.001 | 8 | 0.61 | n.s |
| H2O2 content | 4 | 1498.89 | <0.001 | 2 | 121.72 | <0.001 | 8 | 24.72 | <0.001 |
| MDA content | 4 | 346.54 | <0.001 | 2 | 112.68 | <0.001 | 8 | 4.54 | <0.001 |
| CAT activity | 4 | 681.76 | <0.001 | 2 | 186.34 | <0.001 | 8 | 24.28 | <0.001 |
| POD activity | 4 | 1368.53 | <0.001 | 2 | 149.13 | <0.001 | 8 | 30.61 | <0.001 |
| SOD activity | 4 | 1115.06 | <0.001 | 2 | 191.94 | <0.001 | 8 | 20.64 | <0.001 |
| Proline content | 4 | 136.76 | <0.001 | 2 | 87.84 | <0.001 | 8 | 2.09 | <0.05 |
| Soluble sugar content | 4 | 1661.12 | <0.001 | 2 | 395.89 | <0.001 | 8 | 87.17 | <0.001 |
| IAAleaf content | 4 | 662.06 | <0.001 | 2 | 74.16 | <0.001 | 8 | 3.16 | <0.01 |
| GAleaf content | 4 | 813.47 | <0.001 | 2 | 144.74 | <0.001 | 8 | 2.55 | <0.01 |
| ABAleaf content | 4 | 4830.93 | <0.001 | 2 | 244.09 | <0.001 | 8 | 60.65 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, S.; Hou, P.; Zheng, C.; Yang, X.; Tao, Q.; Sun, J. Wheat Straw Biochar Amendment Increases Salinity Stress Tolerance in Alfalfa Seedlings by Modulating Physiological and Biochemical Responses. Plants 2025, 14, 1954. https://doi.org/10.3390/plants14131954
Zhong S, Hou P, Zheng C, Yang X, Tao Q, Sun J. Wheat Straw Biochar Amendment Increases Salinity Stress Tolerance in Alfalfa Seedlings by Modulating Physiological and Biochemical Responses. Plants. 2025; 14(13):1954. https://doi.org/10.3390/plants14131954
Chicago/Turabian StyleZhong, Shangzhi, Pengxin Hou, Congcong Zheng, Xuechen Yang, Qibo Tao, and Juan Sun. 2025. "Wheat Straw Biochar Amendment Increases Salinity Stress Tolerance in Alfalfa Seedlings by Modulating Physiological and Biochemical Responses" Plants 14, no. 13: 1954. https://doi.org/10.3390/plants14131954
APA StyleZhong, S., Hou, P., Zheng, C., Yang, X., Tao, Q., & Sun, J. (2025). Wheat Straw Biochar Amendment Increases Salinity Stress Tolerance in Alfalfa Seedlings by Modulating Physiological and Biochemical Responses. Plants, 14(13), 1954. https://doi.org/10.3390/plants14131954






