Phylogenetic and Structural Insights into Melatonin Receptors in Plants: Case Study in Capsicum chinense Jacq
Abstract
1. Introduction
2. Results
2.1. Identification of Melatonin Receptor Candidates
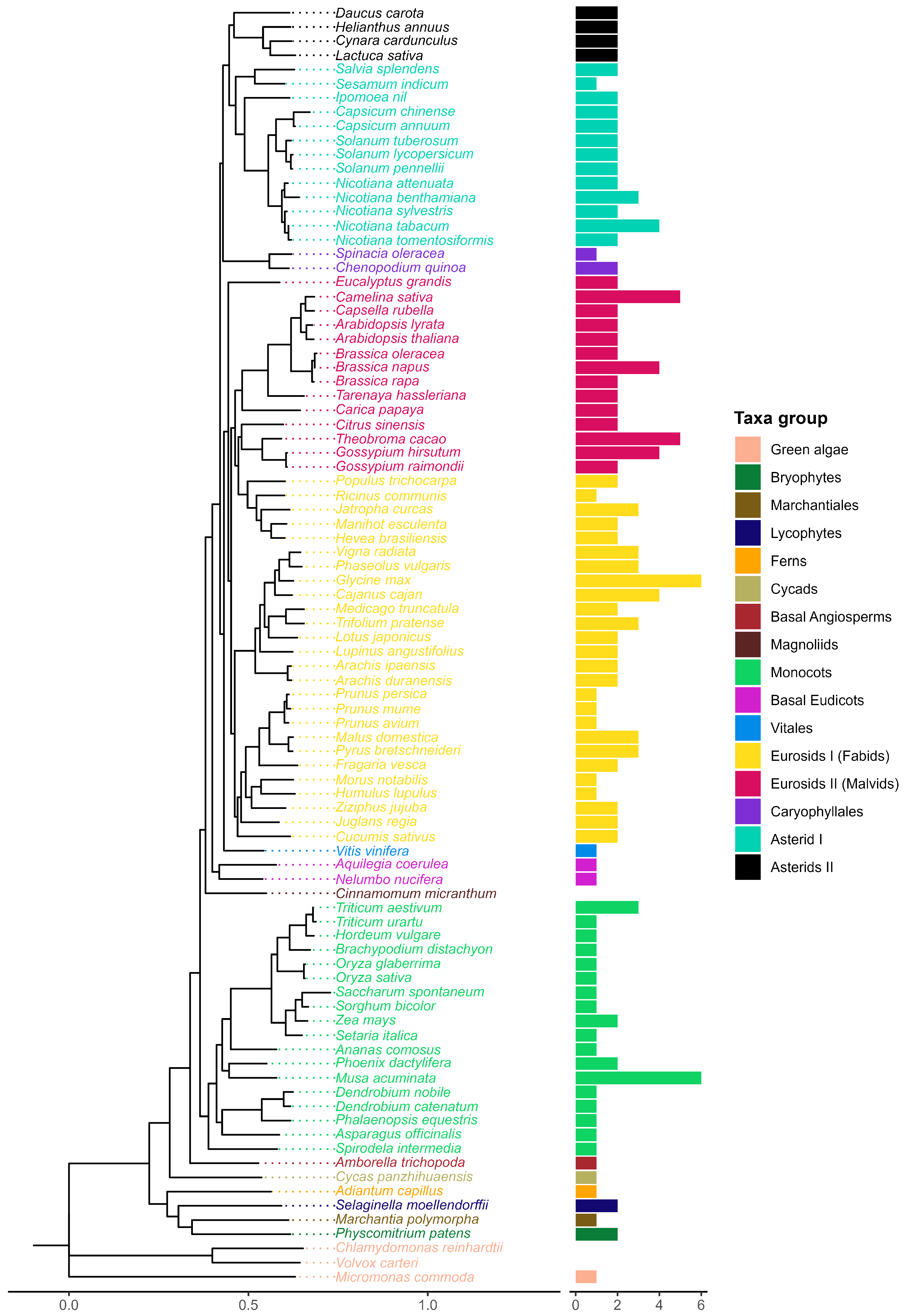
2.2. Phylogeny of Plant Melatonin Receptors

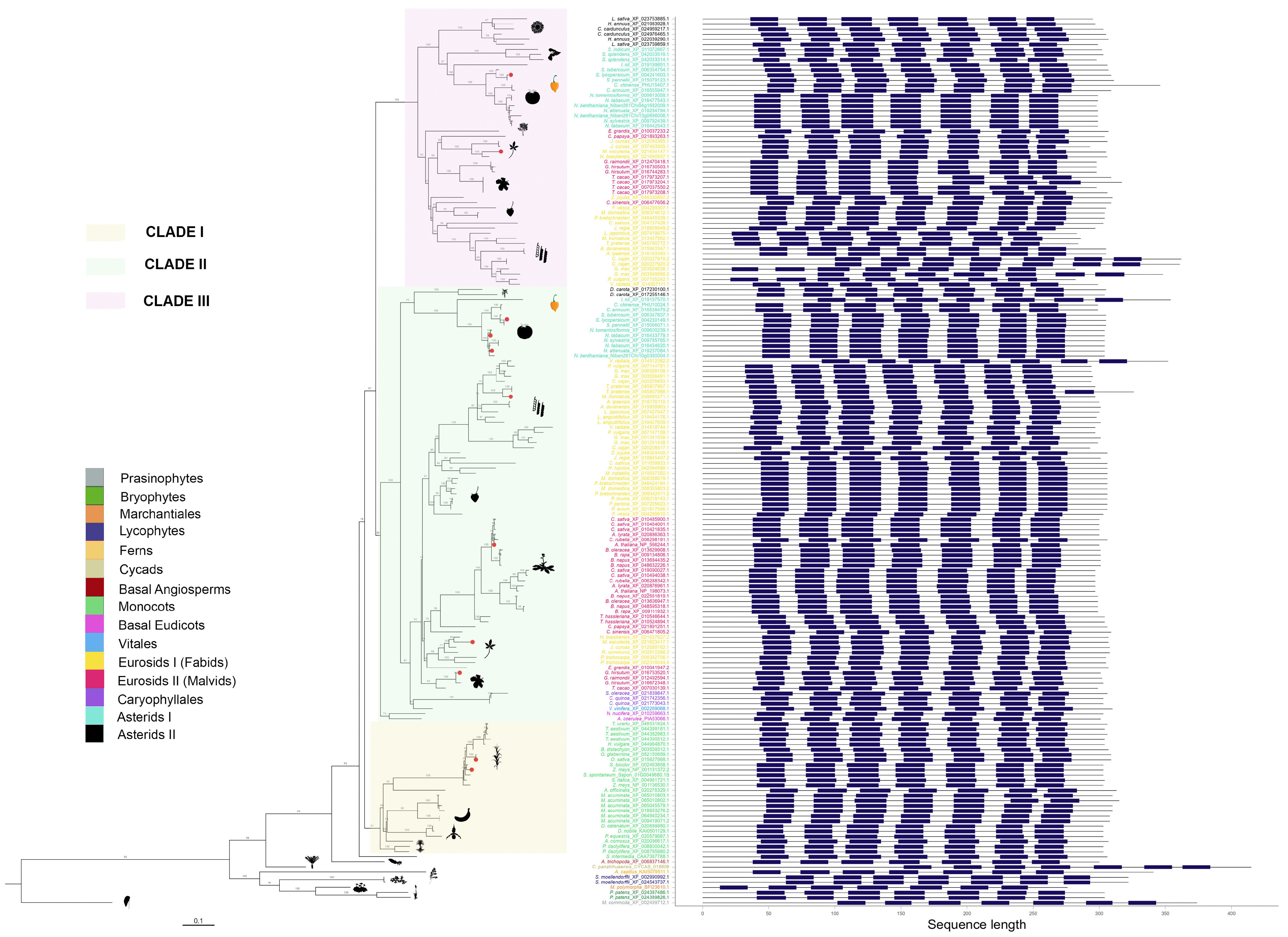
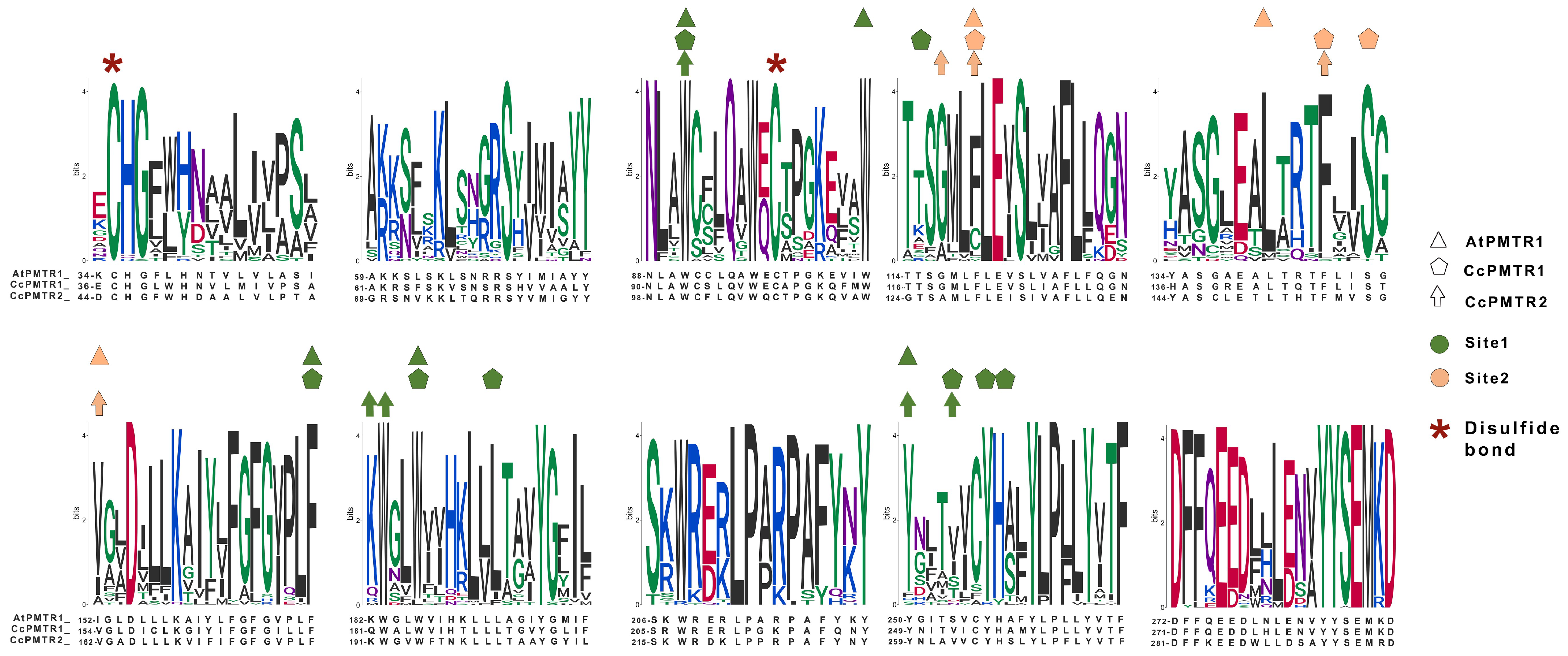
2.3. PMTR Protein Family: Motif Analysis
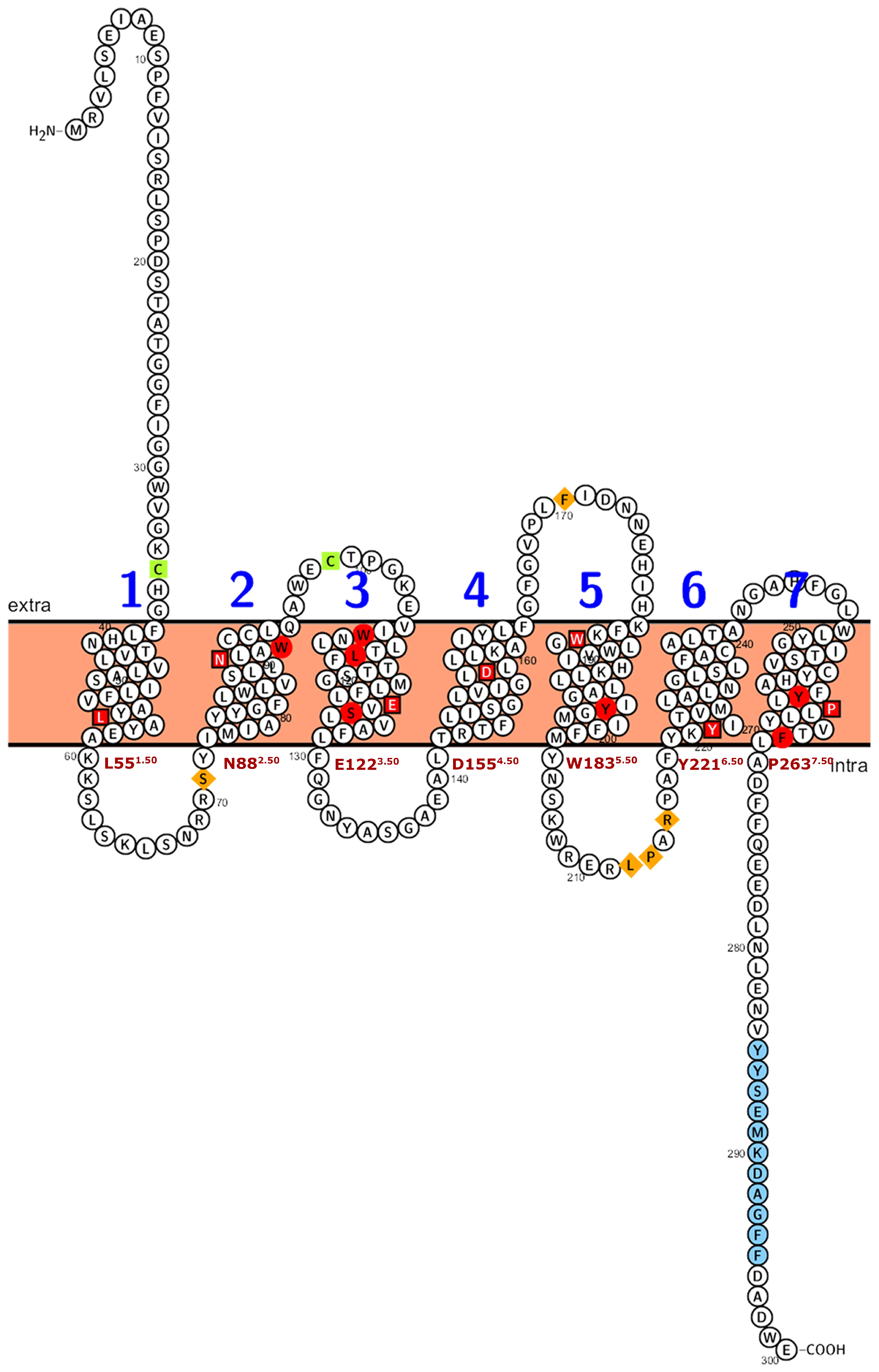
2.4. Subcellular Localization
2.5. Post-Translational Modifications
2.6. Molecular Docking Predictions for Melatonin Binding to PMTRs

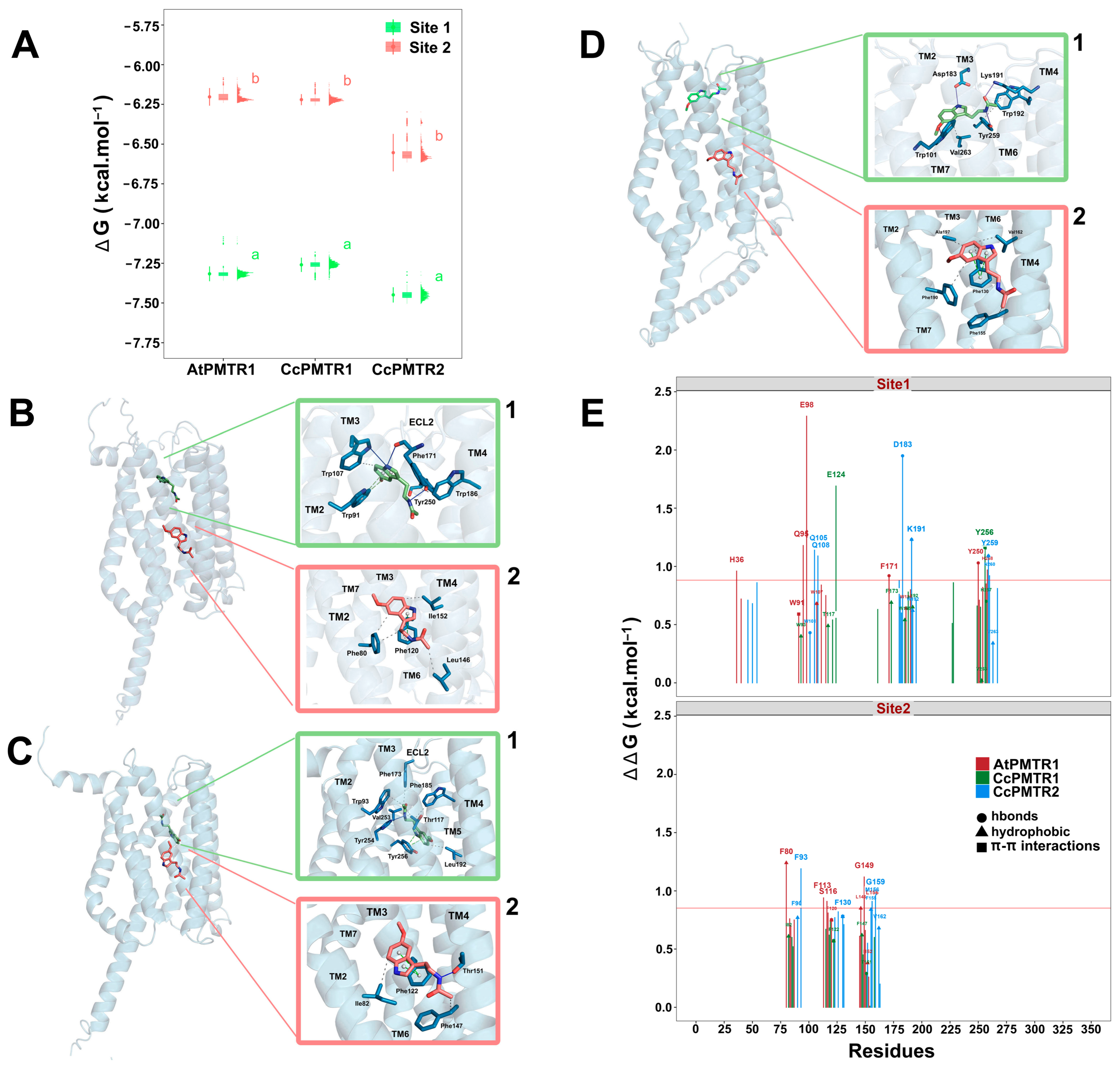
2.7. Tunnel and Ligand Transport Analysis

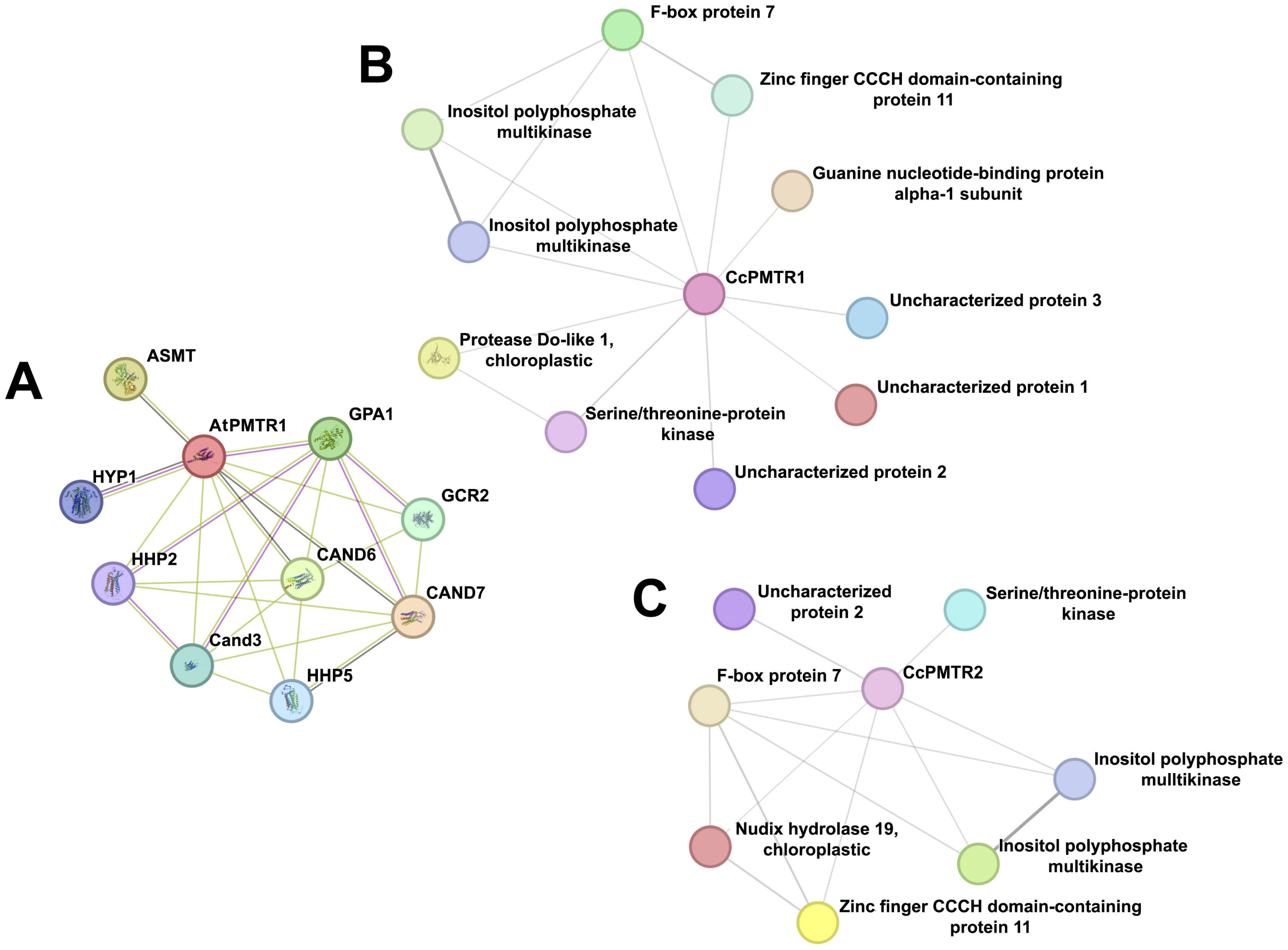
2.8. Protein–Protein Interaction
3. Discussion
3.1. Origin of PMTRs
3.2. Subcellular Localization
3.3. Post-Translational Modifications
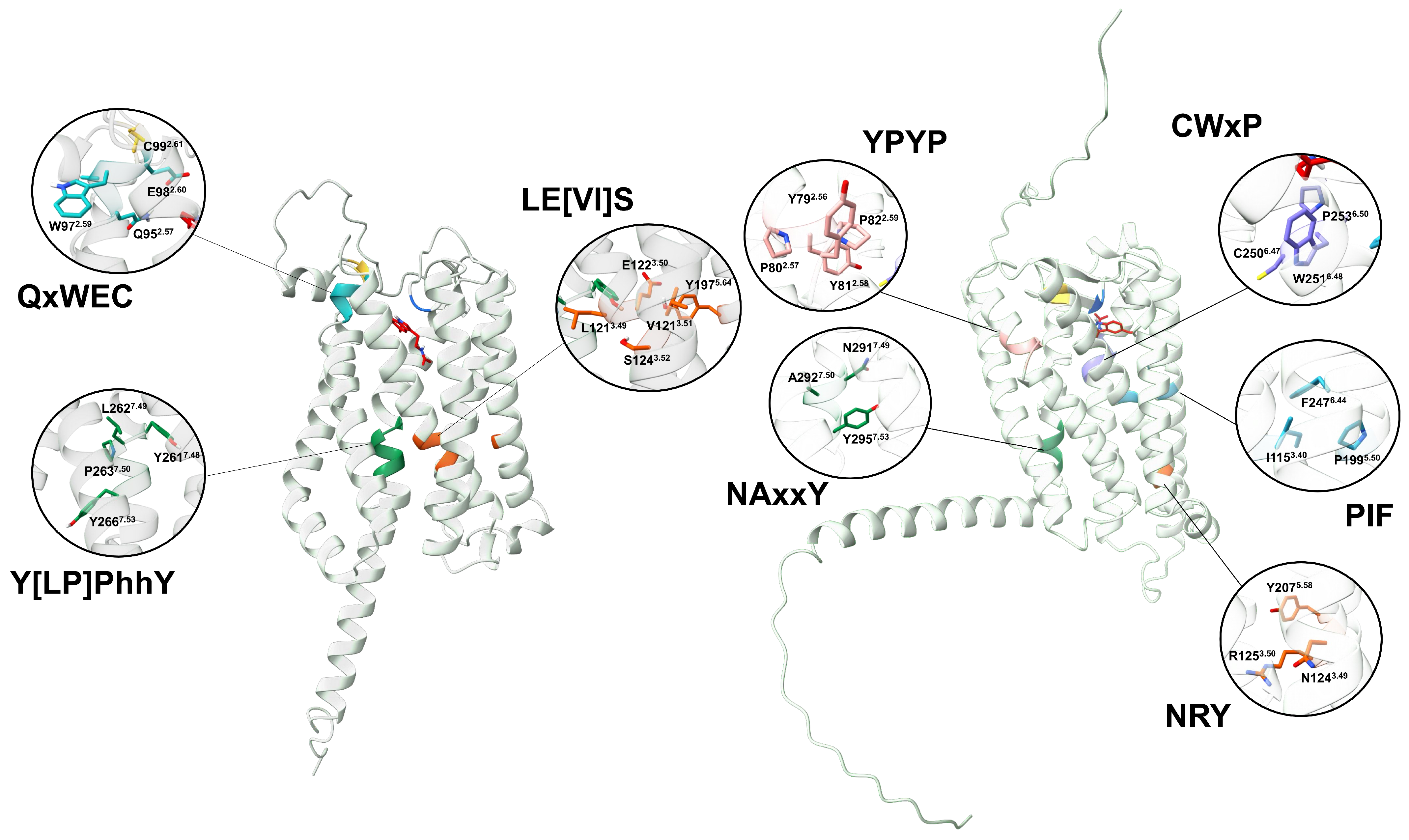
3.4. Structural Basis of Melatonin Binding and Receptor Activation
3.5. Tunnel and Transport
3.6. Protein–Protein Interactions String Network
3.7. Similarities and Divergence in MTRs from Plants and Animals
4. Materials and Methods
4.1. Identification of Melatonin Receptor Candidates
4.2. Data Download
4.3. Phylogenetic Analysis
4.4. Topology Prediction and Conserved Motifs
4.5. Subcellular Localization and Signal Peptide Prediction
4.6. Post-Translational Modifications
4.7. Molecular Docking
4.8. Docking Site Refinement
4.9. Tunnel and Ligand Transport Analysis
4.10. Protein–Protein Interaction Networks
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Caspi, Y.; Pantazopoulou, C.K.; Prompers, J.J.; Pieterse, C.; Hulshoff Pol, H.; Kajala, K. Why did glutamate, GABA, and melatonin become intercellular signalling molecules in plants? eLife 2023, 12, e83361. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wei, J.; Peng, Z.; Ma, W.; Yang, Q.; Song, Z.; Sun, W.; Yang, W.; Yuan, L.; Xu, X.; et al. Daily rhythms of phytomelatonin signaling modulate diurnal stomatal closure via regulating reactive oxygen species dynamics in Arabidopsis. J. Pineal Res. 2020, 68, e12640. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tanveer, M.; Wang, H.; Arnao, M.B. Melatonin as a key regulator in seed germination under abiotic stress. J. Pineal Res. 2024, 76, e12937. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Ahmad, S.; Shen, W. Melatonin-Mediated Molecular Responses in Plants: Enhancing Stress Tolerance and Mitigating Environmental Challenges in Cereal Crop Production. Int. J. Mol. Sci. 2024, 25, 4551. [Google Scholar] [CrossRef]
- Khan, D.; Cai, N.; Zhu, W.; Li, L.; Guan, M.; Pu, X.; Chen, Q. The role of phytomelatonin receptor 1-mediated signaling in plant growth and stress response. Front. Plant Sci. 2023, 14, 1142753. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.; Zhang, J.; Shan, C.; Rengel, Z.; Song, Z.; Chen, Q. Phytomelatonin receptor PMTR 1-mediated signaling regulates stomatal closure in Arab. thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Lee, H.Y.; Back, K. The phytomelatonin receptor (PMRT1) Arabidopsis Cand2 is not a bona fide G protein–coupled melatonin receptor. Melatonin Res. 2020, 3, 177–186. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Jing, S.; Jia, C.; Li, H.; Li, C.; He, Q.; Zhang, N.; Guo, Y. Integration of Phytomelatonin Signaling With Jasmonic Acid in Wound-induced Adventitious Root Regeneration. Adv. Sci. 2025, 12, 2413485. [Google Scholar] [CrossRef]
- Yu, R.; Zuo, T.; Diao, P.; Fu, J.; Fan, Y.; Wang, Y.; Zhao, Q.; Ma, X.; Lu, W.; Li, A.; et al. Melatonin Enhances Seed Germination and Seedling Growth of Medicago sativa Under Salinity via a Putative Melatonin Receptor MsPMTR1. Front. Plant Sci. 2021, 12, 702875. [Google Scholar] [CrossRef]
- Ebisawa, T.; Karne, S.; Lerner, M.R.; Reppert, S.M. Expression cloning of a high-affinity melatonin receptor from Xenopus dermal melanophores. Proc. Natl. Acad. Sci. USA 1994, 91, 6133–6137. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Ji, N.; Jiang, L.; Zhou, Y.; Feng, X.; Li, J.; Zeng, X.; Wang, J.; Shen, Y.Q.; Chen, Q. GPCRs identified on mitochondrial membranes: New therapeutic targets for diseases. J. Pharm. Anal. 2025, 101178. [Google Scholar] [CrossRef]
- Okamoto, H.H.; Miyauchi, H.; Inoue, A.; Raimondi, F.; Tsujimoto, H.; Kusakizako, T.; Shihoya, W.; Yamashita, K.; Suno, R.; Nomura, N.; et al. Cryo-EM structure of the human MT1–Gi signaling complex. Nat. Struct. Mol. Biol. 2021, 28, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.C.; Stauch, B.; McCorvy, J.D.; Han, G.W.; Patel, N.; Huang, X.P.; Batyuk, A.; Gati, C.; Slocum, S.T.; Li, C.; et al. XFEL structures of the human MT2 melatonin receptor reveal the basis of subtype selectivity. Nature 2019, 569, 289–292. [Google Scholar] [CrossRef]
- Stauch, B.; Johansson, L.C.; McCorvy, J.D.; Patel, N.; Han, G.W.; Huang, X.P.; Gati, C.; Batyuk, A.; Slocum, S.T.; Ishchenko, A.; et al. Structural basis of ligand recognition at the human MT1 melatonin receptor. Nature 2019, 569, 284–288. [Google Scholar] [CrossRef]
- Stein, R.M.; Kang, H.J.; McCorvy, J.D.; Glatfelter, G.C.; Jones, A.J.; Che, T.; Slocum, S.; Huang, X.P.; Savych, O.; Moroz, Y.S.; et al. Virtual discovery of melatonin receptor ligands to modulate circadian rhythms. Nature 2020, 579, 609–614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moriyama, E.N.; Strope, P.K.; Opiyo, S.O.; Chen, Z.; Jones, A.M. Mining the Arabidopsis thaliana genome for highly-divergent seven transmembrane receptors. Genome Biol. 2006, 7, R96. [Google Scholar] [CrossRef]
- Gookin, T.E.; Kim, J.; Assmann, S.M. Whole proteome identification of plant candidate G-protein coupled receptors in Arabidopsis, rice, and poplar: Computational prediction and in-vivo protein coupling. Genome Biol. 2008, 9, R120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, M.; Sheng, T.; Liang, J.; Ali, Q.; Gu, Q.; Wu, H.; Chen, J.; Liu, J.; Gao, X. Melatonin and Its Homologs Induce Immune Responses via Receptors trP47363-trP13076 in Nicotiana benthamiana. Front. Plant Sci. 2021, 12, 691835. [Google Scholar] [CrossRef]
- Bai, Y.; Wei, Y.; Yin, H.; Hu, W.; Cheng, X.; Guo, J.; Dong, Y.; Zheng, L.; Xie, H.; Zeng, H.; et al. PP2C1 fine-tunes melatonin biosynthesis and phytomelatonin receptor PMTR1 binding to melatonin in cassava. J. Pineal Res. 2022, 73, e12804. [Google Scholar] [CrossRef]
- Wang, L.F.; Lu, K.K.; Li, T.T.; Zhang, Y.; Guo, J.X.; Song, R.F.; Liu, W.C. Maize PHYTOMELATONIN RECEPTOR1 functions in plant tolerance to osmotic and drought stress. J. Exp. Bot. 2022, 73, 5961–5973. [Google Scholar] [CrossRef] [PubMed]
- Barman, D.; Kumar, M.N.; Dalal, M.; Khan, F.N.; Yadav, J.; Nagar, S.; Santosh Kumar, V.V.; Singh, M.P.; Sathee, L.; Gopala Krishnan, S.; et al. Identification of rice melatonin receptor OsPMTR and its comparative Silico Anal. Arab. AtCAND2 Recept. S. Afr. J. Bot. 2023, 162, 813–829. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, M.; Wu, Z.; Wang, S.; Fan, Y.; Ni, K.; Lu, X.; Liu, X.; Liu, M.; Chen, W.; et al. Melatonin receptor, GhCAND2-D5 Motiv. Responding NaCl Signal. Cotton. Plant Physiol. Biochem. 2023, 203, 108001. [Google Scholar] [CrossRef]
- Burgos-Valencia, E.; García-Laynes, F.; Echevarría-Machado, I.; Medina-Lara, F.; Monforte-González, M.; Narváez-Zapata, J.; Martínez-Estévez, M. Differential Expression of Genes Related to Fruit Development and Capsaicinoids Synthesis in Habanero Pepper Plants Grown in Contrasting Soil Types. Phyton-Int. J. Exp. Bot. 2024, 93, 151–183. [Google Scholar] [CrossRef]
- Ruiz-Lau, N.; Medina-Lara, F.; Minero-García, Y.; Zamudio-Moreno, E.; Guzmán-Antonio, A.; Echevarría-Machado, I.; Martínez-Estévez, M. Water Deficit Affects the Accumulation of Capsaicinoids in Fruits of Capsicum chinense Jacq. HortScience 2011, 46, 487–492. [Google Scholar] [CrossRef]
- León-García, F.; García-Laynes, F.; Estrada-Tapia, G.; Monforte-González, M.; Martínez-Estevez, M.; Echevarría-Machado, I. In Silico Analysis of Glutamate Receptors in Capsicum chinense: Structure, Evolution, and Molecular Interactions. Plants 2024, 13, 812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef]
- Bernhofer, M.; Rost, B. TMbed: Transmembrane proteins predicted through language model embeddings. BMC Bioinform. 2022, 23, 326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf 2016: An improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ballesteros, J.A.; Weinstein, H. [19] Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. In Receptor Molecular Biology; Academic Press: Cambridge, MA, USA, 1995; Volume 25, pp. 366–428. [Google Scholar] [CrossRef]
- Omasits, U.; Ahrens, C.H.; Müller, S.; Wollscheid, B. Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 2014, 30, 884–886. [Google Scholar] [CrossRef]
- Tremblay, B.J.-M. Universalmotif: An R package for biological motif analysis. J. Open Source Softw. 2024, 9, 7012. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. AMDock: A versatile graphical tool for assisting molecular docking with Autodock Vina and Autodock4. Biol. Direct 2020, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Prakash, A.; Jeffryes, M.; Bateman, A.; Finn, R.D. The HMMER Web Server for Protein Sequence Similarity Search. Curr. Protoc. Bioinform. 2017, 60, 3.15.1–3.15.23. [Google Scholar] [CrossRef]
- Yin, X.; Bai, Y.L.; Gong, C.; Song, W.; Wu, Y.; Ye, T.; Feng, Y.Q. The phytomelatonin receptor PMTR1 regulates seed development and germination by modulating abscisic acid homeostasis in Arabidopsis thaliana. J. Pineal Res. 2022, 72, e12797. [Google Scholar] [CrossRef]
- Pan, J.; Wang, H.; Chen, W.; You, Q.; Li, X.; Yu, D. Phytomelatonin inhibits seed germination by regulating germination-related hormone signaling in Arabidopsis. Plant Signal. Behav. 2021, 16, 1970447. [Google Scholar] [CrossRef]
- Lv, Y.; Pan, J.; Wang, H.; Reiter, R.J.; Li, X.; Mou, Z.; Zhang, J.; Yao, Z.; Zhao, D.; Yu, D. Melatonin inhibits seed germination by crosstalk with abscisic acid, gibberellin, and auxin in Arabidopsis. J. Pineal Res. 2021, 70, e12736. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, H.y.; Liu, J.; Zhang, C.Y. Melatonin facilitates the coordination of cell growth and lipid accumulation in nitrogen-stressed Chlamydomonas Reinhardtii Biodiesel Prod. Algal Res. 2020, 46, 101786. [Google Scholar] [CrossRef]
- Tal, O.; Malkiel, H.; Sinam, B.; Harel, O.; Haim, A. Melatonin regulates antioxidative mechanisms in microalgae Chlamydomonas reinhardtii (Volvocales, Chlorophyceae). Phycologia 2015, 54, 292–298. [Google Scholar] [CrossRef]
- Tal, O.; Haim, A.; Harel, O.; Gerchman, Y. Melatonin as an antioxidant and its semi-lunar rhythm in green macroalga Ulva sp. J. Exp. Bot. 2011, 62, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Zhang, C.; Xu, S.; Li, X.; Zhou, S.; Zhou, J. Melatonin application enhances the remediation of cadmium-contaminated soils by Cinnamomum Camphora. Sci. Total Environ. 2025, 968, 178912. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Peng, Z.; Ma, W.; Zhang, S.; Hou, S.; Wei, J.; Dong, S.; Yu, X.; Song, Y.; Gao, W.; et al. Melatonin functions in priming of stomatal immunity in Panax notoginseng and Arabidopsis thaliana. Plant Physiol. 2021, 187, 2837–2851. [Google Scholar] [CrossRef]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals—An Overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef]
- Calebiro, D.; Koszegi, Z.; Lanoiselée, Y.; Miljus, T.; O’Brien, S. G protein-coupled receptor-G protein interactions: A single-molecule perspective. Physiol. Rev. 2021, 101, 857–906. [Google Scholar] [CrossRef]
- Zheng, X.; Tan, D.X.; Allan, A.C.; Zuo, B.; Zhao, Y.; Reiter, R.J.; Wang, L.; Wang, Z.; Guo, Y.; Zhou, J.; et al. Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci. Rep. 2017, 7, 41236. [Google Scholar] [CrossRef]
- Ye, T.; Yin, X.; Yu, L.; Zheng, S.J.; Cai, W.J.; Wu, Y.; Feng, Y.Q. Metabolic analysis of the melatonin biosynthesis pathway using chemical labeling coupled with liquid chromatography-mass spectrometry. J. Pineal Res. 2019, 66, e12531. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef]
- Wang, T.; Nakagawa, S.; Miyake, T.; Setsu, G.; Kunisue, S.; Goto, K.; Hirasawa, A.; Okamura, H.; Yamaguchi, Y.; Doi, M. Identification and functional characterisation of N-linked glycosylation of the orphan G protein-coupled receptor Gpr176. Sci. Rep. 2020, 10, 4429. [Google Scholar] [CrossRef]
- Govorovska, K.A.; Markowska, M.; Kai, L.; Pazin, M.; Gerdin, M.J.; Masana, M.I.; Dubocovich, M.L. Role of N-linked glycosylation on ligand binding and cellular expression of hMT1 and hMT2 melatonin receptors. FASEB J. 2006, 20, A250. [Google Scholar] [CrossRef]
- Venkatakrishnan, A.J.; Deupi, X.; Lebon, G.; Tate, C.G.; Schertler, G.F.; Babu, M.M. Molecular signatures of G-protein-coupled receptors. Nature 2013, 494, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Adams, W.; Pollock, J.; Witt-Enderby, P.A. C-terminal domains within human MT1 and MT2 melatonin receptors are involved in internalization processes. J. Pineal Res. 2008, 45, 212–218. [Google Scholar] [CrossRef]
- Cui, W.; Dong, J.; Wang, S.; Vogel, H.; Zou, R.; Yuan, S. Molecular basis of ligand selectivity for melatonin receptors. RSC Adv. 2023, 13, 4422–4430. [Google Scholar] [CrossRef]
- de Lima Menezes, G.; Sales Bezerra, K.; Nobre Oliveira, J.I.; Fontenele Araújo, J.; Soares Galvão, D.; Alves da Silva, R.; Vogel Saivish, M.; Laino Fulco, U. Quantum mechanics insights into melatonin and analogs binding to melatonin MT1 and MT2 receptors. Sci. Rep. 2024, 14, 10922. [Google Scholar] [CrossRef]
- Palczewski, K.; Kumasaka, T.; Hori, T.; Behnke, C.A.; Motoshima, H.; Fox, B.A.; Trong, I.L.; Teller, D.C.; Okada, T.; Stenkamp, R.E.; et al. Crystal Structure of Rhodopsin: A G Protein-Coupled Receptor. Science 2000, 289, 739–745. [Google Scholar] [CrossRef]
- Patel, N.; Huang, X.P.; Grandner, J.M.; Johansson, L.C.; Stauch, B.; McCorvy, J.D.; Liu, Y.; Roth, B.; Katritch, V. Structure-based discovery of potent and selective melatonin receptor agonists. eLife 2020, 9, e53779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cecon, E.; Boutin, J.A.; Jockers, R. Molecular Characterization and Pharmacology of Melatonin Receptors in Animals. Receptors 2023, 2, 127–147. [Google Scholar] [CrossRef]
- Okamoto, H.H.; Cecon, E.; Nureki, O.; Rivara, S.; Jockers, R. Melatonin receptor structure and signaling. J. Pineal Res. 2024, 76, e12952. [Google Scholar] [CrossRef]
- Bradford, W.; Buckholz, A.; Morton, J.; Price, C.; Jones, A.M.; Urano, D. Eukaryotic G Protein Signaling Evolved to Require G Protein–Coupled Receptors for Activation. Sci. Signal. 2013, 6, ra37. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Dong, D.; Guo, L.; Dong, X.; Leng, J.; Zhao, B.; Guo, Y.D.; Zhang, N. Research Advances in Heterotrimeric G-Protein ? Subunits and Uncanonical G-Protein Coupled Receptors in Plants. Int. J. Mol. Sci. 2021, 22, 8678. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, N.; Pandey, S. Heterotrimeric G-Protein Interactions Are Conserved Despite Regulatory Element Loss in Some Plants. Plant Physiol. 2020, 184, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Booe, J.M.; Pioszak, A.A. Structural insights into ligand recognition and selectivity for classes A, B, and C GPCRs. Eur. J. Pharmacol. 2015, 763, 196–205. [Google Scholar] [CrossRef]
- Elisi, G.M.; Scalvini, L.; Lodola, A.; Mor, M.; Rivara, S. Free-Energy Simulations Support a Lipophilic Binding Route for Melatonin Receptors. J. Chem. Inf. Model. 2022, 62, 210–222. [Google Scholar] [CrossRef]
- Dror, R.O.; Pan, A.C.; Arlow, D.H.; Borhani, D.W.; Maragakis, P.; Shan, Y.; Xu, H.; Shaw, D.E. Pathway and mechanism of drug binding to G-protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 13118–13123. [Google Scholar] [CrossRef]
- Stauch, B.; Johansson, L.C.; Cherezov, V. Structural insights into melatonin receptors. FEBS J. 2020, 287, 1496–1510. [Google Scholar] [CrossRef]
- Bedini, A.; Elisi, G.M.; Fanini, F.; Retini, M.; Scalvini, L.; Pasquini, S.; Contri, C.; Varani, K.; Spadoni, G.; Mor, M.; et al. Binding and unbinding of potent melatonin receptor ligands: Mechanistic simulations and experimental evidence. J. Pineal Res. 2024, 76, e12941. [Google Scholar] [CrossRef]
- Ma, M.; Wang, W.; Fei, Y.; Cheng, H.Y.; Song, B.; Zhou, Z.; Zhao, Y.; Zhang, X.; Li, L.; Chen, S.; et al. A surface-receptor-coupled G protein regulates plant immunity through nuclear protein kinases. Cell Host Microbe 2022, 30, 1602–1614.e5. [Google Scholar] [CrossRef]
- Kuznetsova, K.; Efremova, E.; Dodueva, I.; Lebedeva, M.; Lutova, L. Functional Modules in the Meristems: “Tinkering” in Action. Plants 2023, 12, 3661. [Google Scholar] [CrossRef]
- Gao, Y.; Zeng, Q.; Guo, J.; Cheng, J.; Ellis, B.E.; Chen, J.G. Genetic characterization reveals no role for the reported ABA receptor, GCR2, in ABA control of seed germination and early seedling development in Arabidopsis. Plant J. 2007, 52, 1001–1013. [Google Scholar] [CrossRef]
- Tahir, J.; Brendolise, C.; Hoyte, S.; Lucas, M.; Thomson, S.; Hoeata, K.; McKenzie, C.; Wotton, A.; Funnell, K.; Morgan, E.; et al. QTL Mapping for Resistance to Cankers Induced by Pseudomonas syringae pv. actinidiae (Psa) in a Tetraploid Actinidia chinensis Kiwifruit Population. Pathogens 2020, 9, 967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Francis, S.M.; Gas, M.; Daugeron, M.C.; Bravo, J.; Séraphin, B. Rbg1–Tma46 dimer structure reveals new functional domains and their role in polysome recruitment. Nucleic Acids Res. 2012, 40, 11100–11114. [Google Scholar] [CrossRef]
- Garcia-Marcos, M. Heterotrimeric G protein signaling without GPCRs: The Gα-binding-and-activating (GBA) motif. J. Biol. Chem. 2024, 300, 105756. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, Y.; Wu, J.; Zhou, X.; Gao, H. Heterotrimeric G-proteins: Multi-dimensional regulation in plant growth, development and abiotic stress responses. Stress Biol. 2025, 5, 3. [Google Scholar] [CrossRef]
- Chen, J.G.; Jones, A.M. AtRGS1 Function in Arabidopsis thaliana. In Regulators of G-Protein Signaling, Part A; Academic Press: Cambridge, MA, USA, 2004; Volume 389, pp. 338–350. [Google Scholar] [CrossRef]
- Johnston, C.A.; Taylor, J.P.; Gao, Y.; Kimple, A.J.; Grigston, J.C.; Chen, J.G.; Siderovski, D.P.; Jones, A.M.; Willard, F.S. GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 17317–17322. [Google Scholar] [CrossRef]
- Ponting, C.P. Evidence for PDZ domains in bacteria, yeast, and plants. Protein Sci. 1997, 6, 464–468. [Google Scholar] [CrossRef]
- Guillaume, J.L.; Daulat, A.M.; Maurice, P.; Levoye, A.; Migaud, M.; Brydon, L.; Malpaux, B.; Borg-Capra, C.; Jockers, R. The PDZ Protein Mupp1 Promotes Gi Coupling and Signaling of the Mt1 Melatonin Receptor. J. Biol. Chem. 2008, 283, 16762–16771. [Google Scholar] [CrossRef]
- Tse, L.H.; Wong, Y.H. Modeling the Heterodimer Interfaces of Melatonin Receptors. Front. Cell. Neurosci. 2021, 15, 725296. [Google Scholar] [CrossRef]
- Ayoub, M.A.; Levoye, A.; Delagrange, P.; Jockers, R. Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol. Pharmacol. 2004, 66, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Dupré, C.; Legros, C.; Boutin, J.A. Functionality of Melatonin Receptors: Internalization. Methods Mol. Biol. 2022, 2550, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Maurice, P.; Daulat, A.M.; Turecek, R.; Ivankova-Susankova, K.; Zamponi, F.; Kamal, M.; Clement, N.; Guillaume, J.; Bettler, B.; Galès, C.; et al. Molecular organization and dynamics of the melatonin MT1 receptor/RGS20/Gi protein complex reveal asymmetry of receptor dimers for RGS and Gi coupling. EMBO J. 2010, 29, 3646–3659. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H. An Alternative Mode of GPCR Transactivation: Activation of GPCRs by Adhesion GPCRs. Int. J. Mol. Sci. 2025, 26, 552. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Lami, K.; Prucker, I.; Stolze, S.C.; Strauß, A.; Langenbach, K.; Kamleitner, M.; Belay, Y.Z.; Ritter, K.; Furkert, D.; et al. NUDIX Hydrolases Target Specific Inositol Pyrophosphates and Regulate Phosphate and Iron Homeostasis, and the Expression of Defense Genes in Arabidopsis. BioRxiv 2024. [Google Scholar] [CrossRef]
- McCombe, C.L.; Wegner, A.; Wirtz, L.; Zamora, C.S.; Casanova, F.; Aditya, S.; Greenwood, J.R.; de Paula, S.; England, E.; Shang, S.; et al. Plant pathogenic fungi hijack phosphate signaling with conserved enzymatic effectors. Science 2025, 387, 955–962. [Google Scholar] [CrossRef]
- Domínguez-May, Á.; Carrillo-Pech, M.; Barredo-Pool, F.A.; Martínez-Estévez, M.; Us-Camas, R.Y.; Moreno-Valenzuela, O.A.; Echevarría-Machado, I. A Novel Effect for Glycine on Root System Growth of Habanero Pepper. J. Am. Soc. Hortic. Sci. 2013, 138, 433–442. [Google Scholar] [CrossRef]
- Serralta-Interian, A.; Miranda-Ham, M.; Echevarría-Machado, I. Stimulation of root growth and enhanced nitrogenous metabolite content in habanero pepper (Capsicum chinense Jacq.) treated with a d-amino acid mixture. Theor. Exp. Plant Physiol. 2020, 32, 31–47. [Google Scholar] [CrossRef]
- Celis-Arámburo, T.; Carrillo-Pech, M.; Castro-Concha, L.A.; Miranda-Ham, M.; Martínez-Estévez, M.; Echevarría-Machado, I. Exogenous nitrate induces root branching and inhibits primary root growth in Capsicum Chinense Jacq. Plant Physiol. Biochem. 2011, 49, 1456–1464. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Ruiz-Lau, N.; Velarde-Buendía, A.; Echevarría-Machado, I.; Pottosin, I.; Martínez-Estévez, M. Natural variation in primary root growth and K+ retention in roots of habanero pepper (Capsicum chinense) under salt stress. Funct. Plant Biol. 2016, 43, 1114–1125. [Google Scholar] [CrossRef]
- Lizama-Gasca, M.G.; Estrada-Tapia, G.; Escalante-Magaña, C.A.; Martínez-Estévez, M.; Zepeda-Jazo, I.; Medina-Lara, F.; Echevarría-Machado, I. Cloning and Molecular Characterization of CcNRT2.1/CcNAR2, a Putative Inducible High Affinity Nitrate Transport System in Capsicum chinense Jacq. Roots. Trop. Plant Biol. 2020, 13, 73–90. [Google Scholar] [CrossRef]
- Zoghbi-Rodríguez, N.M.; Gamboa-Tuz, S.D.; Pereira-Santana, A.; Rodríguez-Zapata, L.C.; Sánchez-Teyer, L.F.; Echevarría-Machado, I. Phylogenomic and Microsynteny Analysis Provides Evidence of Genome Arrangements of High-Affinity Nitrate Transporter Gene Families of Plants. Int. J. Mol. Sci. 2021, 22, 13036. [Google Scholar] [CrossRef]
- Meneses-Lazo, R.; Garruña, R.; Echevarría-Machado, I.; Alvarado-López, C.; Villanueva-Couoh, E.; García-Maldonado, J.; Cristóbal-Alejo, J.; Meneses-Lazo, R.; Garruña, R.; Echevarría-Machado, I.; et al. Growth, chlorophyll fluorescence and gas exchange of pepper (Capsicum chinense Jacq.) plants in response to uptake and partitioning of nutrients. Chil. J. Agric. Res. 2020, 80, 585–597. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, Q.; Guo, Q.; Teng, M.; Gong, Q.; Li, X.; Du, Y.; Liu, Z.; Tao, Y. Structural basis of the ligand binding and signaling mechanism of melatonin receptors. Nat. Commun. 2022, 13, 454. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.M.; Rasmussen, S.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Clement, N.; Renault, N.; Guillaume, J.L.; Cecon, E.; Journé, A.S.; Laurent, X.; Tadagaki, K.; Cogé, F.; Gohier, A.; Delagrange, P.; et al. Importance of the second extracellular loop for melatonin MT1 receptor function and absence of melatonin binding in GPR50. Br. J. Pharmacol. 2018, 175, 3281–3297. [Google Scholar] [CrossRef]
- Levoye, A.; Dam, J.; Ayoub, M.A.; Guillaume, J.; Couturier, C.; Delagrange, P.; Jockers, R. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J. 2006, 25, 3012–3023. [Google Scholar] [CrossRef]
- Li, Y.; Lv, Y.; Bian, C.; You, X.; Shi, Q. Molecular evolution of melatonin receptor genes (Mtnr) Vertebr. Its Shedding Light mtnr1c. Gene 2021, 769, 145256. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Y.; Li, X.; Zhang, L.; Rengel, Z. Phytomelatonin: Biosynthesis, Signaling, and Functions. Annu. Rev. Plant Biol. 2025, 76, 171–195. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of Phylogenies after Removing Divergent and Ambiguously Aligned Blocks from Protein Sequence Alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef]
- Mistry, J.; Finn, R.D.; Eddy, S.R.; Bateman, A.; Punta, M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013, 41, e121. [Google Scholar] [CrossRef]
- Winter, D.J. rentrez: An R package for the NCBI eUtils API. PeerJ Prepr. 2017, 9, 520–526. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.i.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castresana, J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; the UGENE team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Wang, L.G.; Lam, T.T.Y.; Xu, S.; Dai, Z.; Zhou, L.; Feng, T.; Guo, P.; Dunn, C.W.; Jones, B.R.; Bradley, T.; et al. Treeio: An R Package for Phylogenetic Tree Input and Output with Richly Annotated and Associated Data. Mol. Biol. Evol. 2020, 37, 599–603. [Google Scholar] [CrossRef]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T.Y. ggtree: An r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Xu, S.; Dai, Z.; Guo, P.; Fu, X.; Liu, S.; Zhou, L.; Tang, W.; Feng, T.; Chen, M.; Zhan, L.; et al. ggtreeExtra: Compact Visualization of Richly Annotated Phylogenetic Data. Mol. Biol. Evol. 2021, 38, 4039–4042. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. STAG: Species Tree Inference from All Genes. BioRxiv 2018. [Google Scholar] [CrossRef]
- Käll, L.; Krogh, A.; Sonnhammer, E.L.L. A Combined Transmembrane Topology and Signal Peptide Prediction Method. J. Mol. Biol. 2004, 338, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Armenteros, J.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. BioRxiv 2022, 487609. [Google Scholar] [CrossRef]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. In Proceedings of the International Conference on Intelligent Systems for Molecular Biology, Palo Alto, CA, USA, 14–17 August 1994; Volume 2, pp. 28–36. [Google Scholar]
- Gupta, S.; Stamatoyannopoulos, J.A.; Bailey, T.L.; Noble, W.S. Quantifying similarity between motifs. Genome Biol. 2007, 8, R24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Nystrom, S.L.; McKay, D.J. Memes: A motif analysis environment in R using tools from the MEME Suite. PLoS Comput. Biol. 2021, 17, e1008991. [Google Scholar] [CrossRef]
- Quast, J.P.; Schuster, D.; Picotti, P. protti: An R package for comprehensive data analysis of peptide- and protein-centric bottom-up proteomics data. Bioinform. Adv. 2022, 2, vbab041. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Yariv, B.; Yariv, E.; Kessel, A.; Masrati, G.; Chorin, A.B.; Martz, E.; Mayrose, I.; Pupko, T.; Ben-Tal, N. Using evolutionary data to make sense of macromolecules with a “face-lifted” ConSurf. Protein Sci. A Publ. Protein Soc. 2023, 32, e4582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pupko, T.; Bell, R.E.; Mayrose, I.; Glaser, F.; Ben-Tal, N. Rate4Site: An algorithmic tool for the identification of functional regions in proteins by surface mapping of evolutionary determinants within their homologues. Bioinformatics 2002, 18, S71–S77. [Google Scholar] [CrossRef]
- Gillani, M.; Pollastri, G. Protein subcellular localization prediction tools. Comput. Struct. Biotechnol. J. 2024, 23, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.S.; Loaiza, C.D.; Kaundal, R. Plant-mSubP: A computational framework for the prediction of single- and multi-target protein subcellular localization using integrated machine-learning approaches. AoB PLANTS 2020, 12, plz068. [Google Scholar] [CrossRef] [PubMed]
- Sperschneider, J.; Catanzariti, A.M.; DeBoer, K.; Petre, B.; Gardiner, D.M.; Singh, K.B.; Dodds, P.N.; Taylor, J.M. LOCALIZER: Subcellular localization prediction of both plant and effector proteins in the plant cell. Sci. Rep. 2017, 7, 44598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Almagro Armenteros, J.; Sønderby, C.K.; Sønderby, S.; Nielsen, H.; Winther, O. DeepLoc: Prediction of protein subcellular localization using deep learning. Bioinformatics 2017, 33, 3387–3395. [Google Scholar] [CrossRef]
- Duhan, N.; Kaundal, R. AtSubP-2.0: An integrated web server for the annotation of Arabidopsis proteome subcellular localization using deep learning. Plant Genome 2025, 18, e20536. [Google Scholar] [CrossRef]
- Teufel, F.; Almagro Armenteros, J.; Johansen, A.R.; Gíslason, M.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Wang, D.; Liu, D.; Yuchi, J.; He, F.; Jiang, Y.; Cai, S.; Li, J.; Xu, D. MusiteDeep: A deep-learning based webserver for protein post-translational modification site prediction and visualization. Nucleic Acids Res. 2020, 48, W140–W146. [Google Scholar] [CrossRef]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites1. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. A Publ. Protein Soc. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harris, R.; Olson, A.J.; Goodsell, D.S. Automated prediction of ligand-binding sites in proteins. Proteins Struct. Funct. Bioinform. 2008, 70, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Krause, F.; Voigt, K.; Di Ventura, B.; Öztürk, M.A. ReverseDock: A web server for blind docking of a single ligand to multiple protein targets using AutoDock Vina. Front. Mol. Biosci. 2023, 10, 1243970. [Google Scholar] [CrossRef]
- Hahsler, M.; Piekenbrock, M.; Doran, D. dbscan: Fast Density-Based Clustering with R. J. Stat. Softw. 2019, 91, 1–30. [Google Scholar] [CrossRef]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M. cluster: Cluster Analysis Basics and Extensions. CRAN 2025. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Sun, T.; Chen, Y.; Wen, Y.; Zhu, Z.; Li, M. PremPLI: A machine learning model for predicting the effects of missense mutations on protein-ligand interactions. Commun. Biol. 2021, 4, 1311. [Google Scholar] [CrossRef]
- Filipovič, J.; Vávra, O.; Plhák, J.; Bednář, D.; Marques, S.; Brezovský, J.; Matyska, L.; Damborský, J. CaverDock: A Novel Method for the Fast Analysis of Ligand Transport. IEEE/ACM Trans. Comput. Biol. Bioinform. 2020, 17, 1625–1638. [Google Scholar] [CrossRef]
- Jurcik, A.; Bednar, D.; Byska, J.; Marques, S.M.; Furmanova, K.; Daniel, L.; Kokkonen, P.; Brezovsky, J.; Strnad, O.; Stourac, J.; et al. CAVER Analyst 2.0: Analysis and visualization of channels and tunnels in protein structures and molecular dynamics trajectories. Bioinformatics 2018, 34, 3586–3588. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.; Pyysalo, S.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Gustavsen, J.A.; Pai, S.; Isserlin, R.; Demchak, B.; Pico, A.R. RCy3: Network biology using Cytoscape from within R. F1000Research 2019, 8, 1774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo-Castiñeira, A.; Valdés-Tresanco, M.E.; Estrada-Tapia, G.; Monforte-González, M.; Martínez-Estévez, M.; Echevarría-Machado, I. Phylogenetic and Structural Insights into Melatonin Receptors in Plants: Case Study in Capsicum chinense Jacq. Plants 2025, 14, 1952. https://doi.org/10.3390/plants14131952
Toledo-Castiñeira A, Valdés-Tresanco ME, Estrada-Tapia G, Monforte-González M, Martínez-Estévez M, Echevarría-Machado I. Phylogenetic and Structural Insights into Melatonin Receptors in Plants: Case Study in Capsicum chinense Jacq. Plants. 2025; 14(13):1952. https://doi.org/10.3390/plants14131952
Chicago/Turabian StyleToledo-Castiñeira, Adrian, Mario E. Valdés-Tresanco, Georgina Estrada-Tapia, Miriam Monforte-González, Manuel Martínez-Estévez, and Ileana Echevarría-Machado. 2025. "Phylogenetic and Structural Insights into Melatonin Receptors in Plants: Case Study in Capsicum chinense Jacq" Plants 14, no. 13: 1952. https://doi.org/10.3390/plants14131952
APA StyleToledo-Castiñeira, A., Valdés-Tresanco, M. E., Estrada-Tapia, G., Monforte-González, M., Martínez-Estévez, M., & Echevarría-Machado, I. (2025). Phylogenetic and Structural Insights into Melatonin Receptors in Plants: Case Study in Capsicum chinense Jacq. Plants, 14(13), 1952. https://doi.org/10.3390/plants14131952







