Pipecolic Acid, a Drought Stress Modulator, Boosts Chlorophyll Assimilation, Photosynthetic Performance, Redox Homeostasis, and Osmotic Adjustment of Drought-Affected Hordeum vulgare L. Seedlings
Abstract
1. Introduction
2. Results
2.1. Vegetative Growth
2.2. Anatomical Study and Stomatal Number
2.3. Chlorophyll Metabolic Pathways Intermediates
2.4. Photosynthetic Pigments
2.5. Photosynthetic Performance
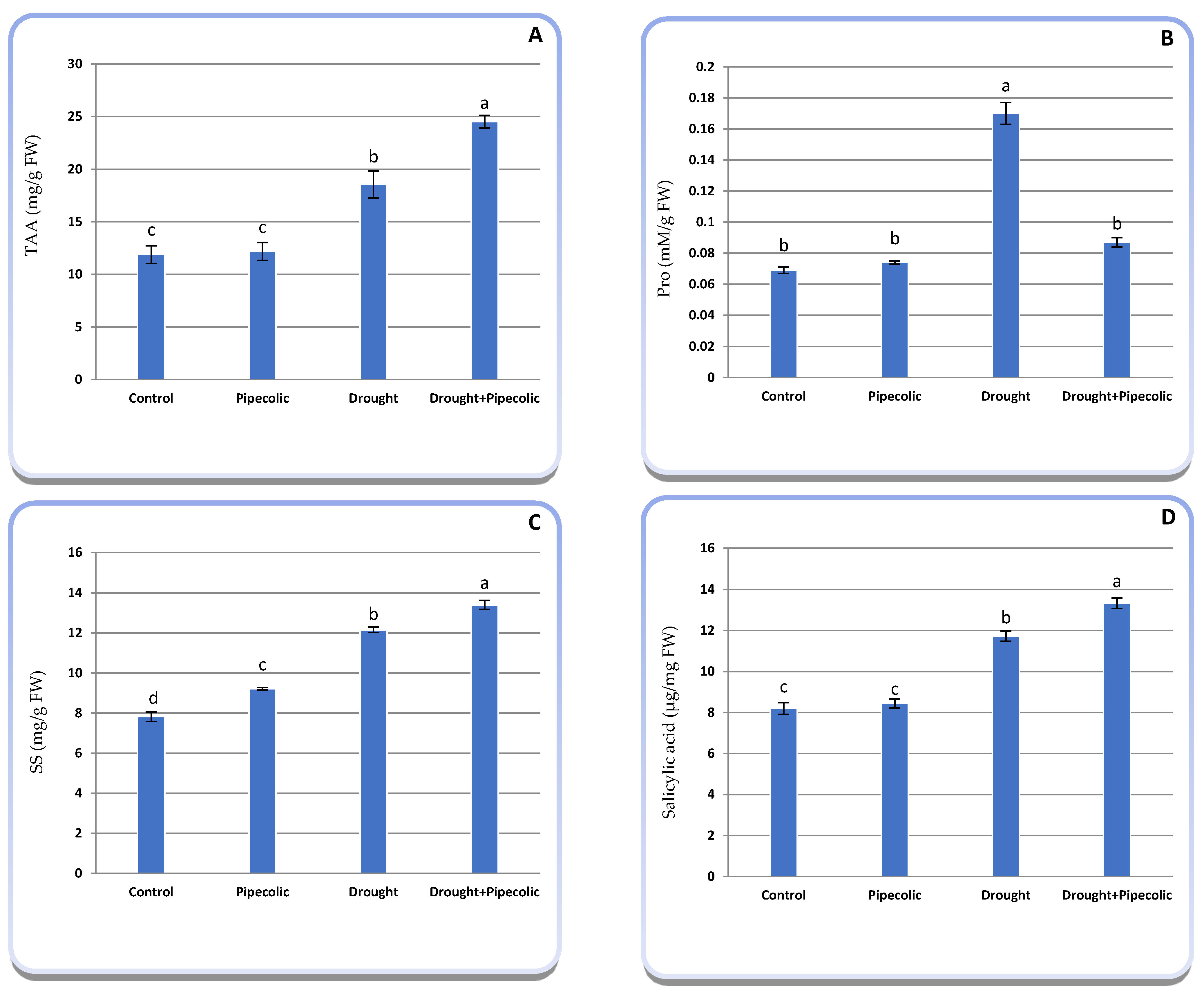
2.6. Osmotically Energetic Molecules
2.7. Water Status and Osmotic Adjustment
2.8. Oxidative Biomarkers
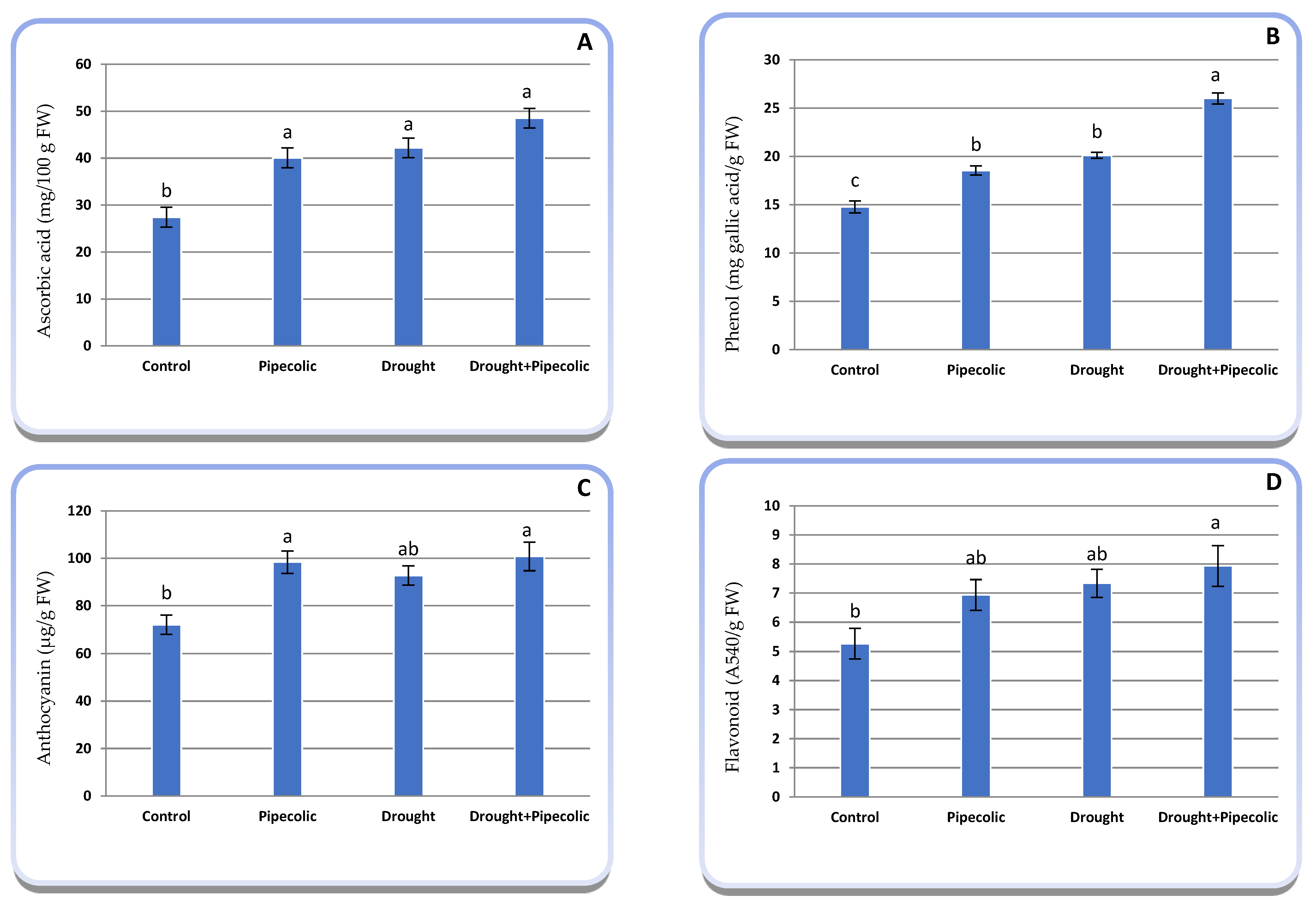
2.9. Non-Enzymatic Antioxidant Metabolites
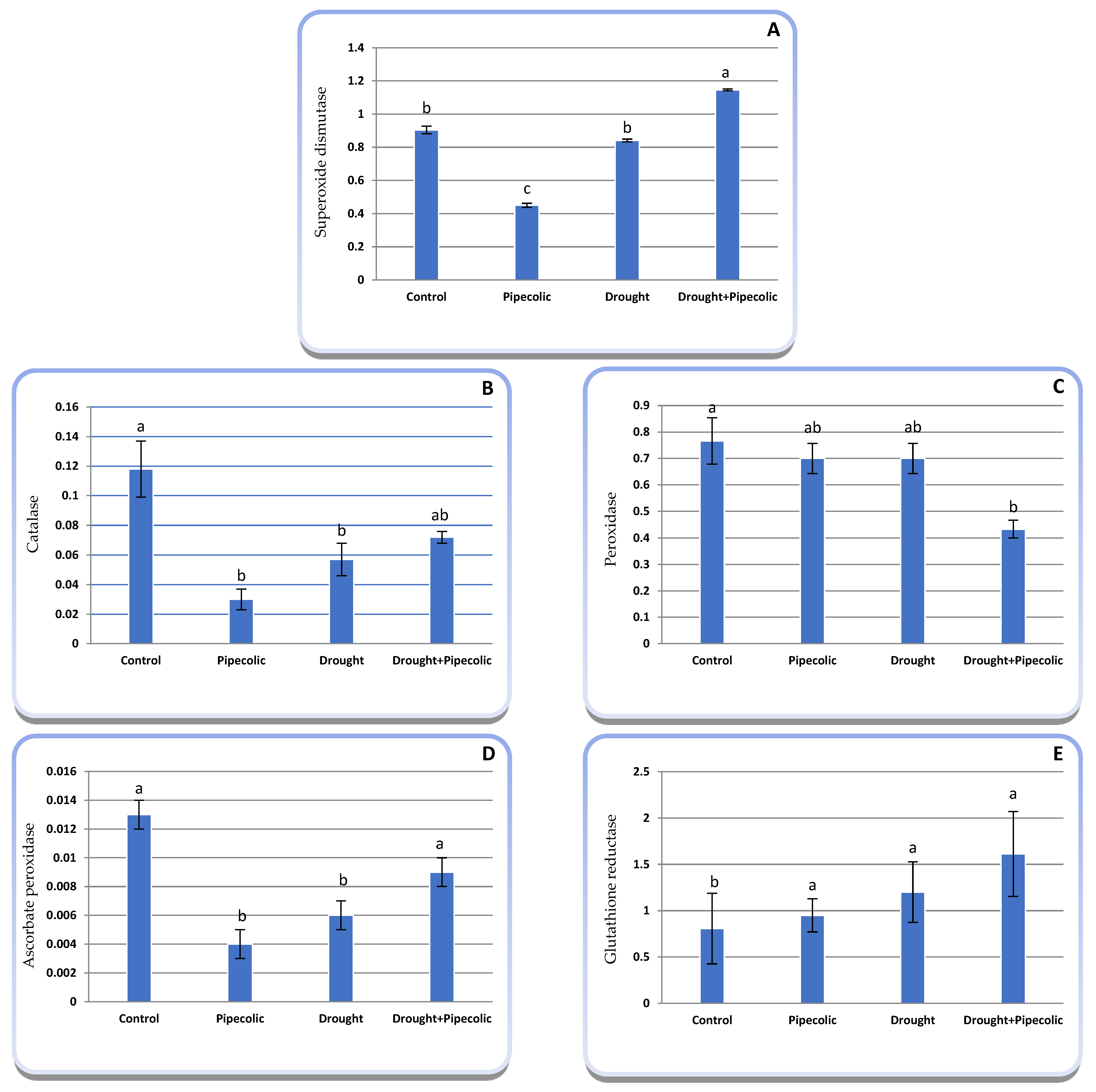
2.10. Antioxidant Enzymes
3. Discussion
4. Materials and Methods
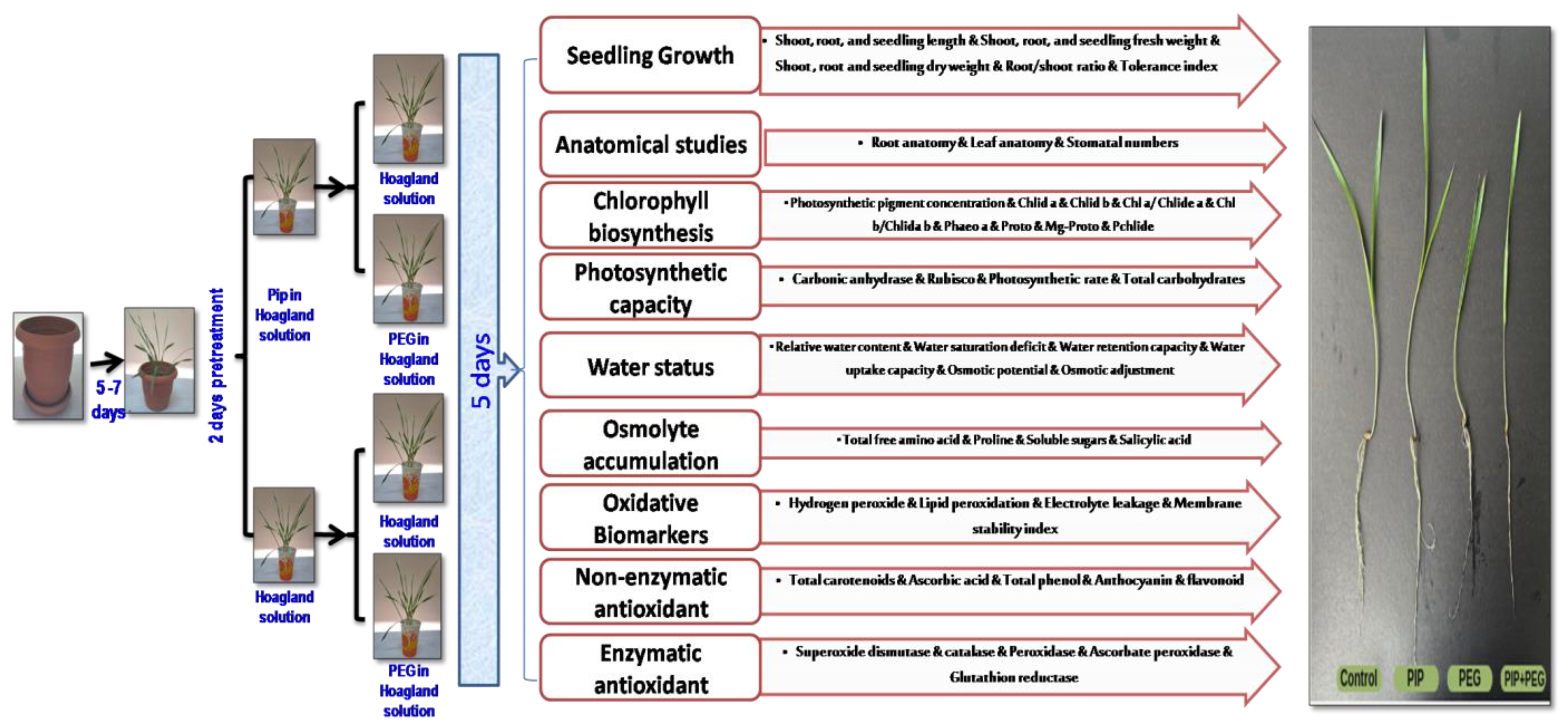
4.1. Plant Materials and Experimental Outline
4.2. Morphological Characteristics
4.3. Leaf and Root Anatomy
4.4. Stomatal Density
4.5. Determination of Chlorophyll Metabolic Intermediates
4.6. Photosynthetic Pigment Concentration
4.7. Photosynthetic Performance Features
4.8. Osmotically Energetic Molecules
4.9. Water Status and Osmotic Adjustment
4.10. Oxidative Biomarkers
4.11. In Situ Hydrogen Peroxide and Superoxide Anions Localization
4.12. Non-Enzymatic Antioxidant Metabolites
4.13. Antioxidant Enzymes Assay
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, A.A.; Wang, Y.F.; Akbar, R.; Alhoqail, W.A. Mechanistic insights and future perspectives of drought stress management in staple crops. Front. Plant Sci. 2025, 16, 1547452. [Google Scholar] [CrossRef] [PubMed]
- Yasin, S.; Zavala-García, F.; Nino-Medina, G.; Rodríguez-Salinas, P.A.; Gutiérrez-Diez, A.; Sinagawa-García, S.R.; Lugo-Cruz, E. Morphological and physiological response of maize (Zea mays L.) to drought stress during reproductive stage. Agronomy 2024, 14, 1718. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Ferioun, M.; Bouhraoua, S.; Belahcen, D.; Zouitane, I.; Srhiouar, N.; Louahlia, S.; ElGhachtouli, N. PGPR consortia enhance growth and yield in barley cultivars subjected to severe drought stress and subsequent recovery. Rhizosphere 2024, 31, 100926. [Google Scholar] [CrossRef]
- Sharma, S.; Bhatt, S.; Yadav, S.K.B.; Poudel, M. A comprehensive review on drought stress in wheat: Causes, mechanism and management practices. Arch. Agric. Environ. Sci. 2025, 10, 164–174. [Google Scholar] [CrossRef]
- Chen, Q.; Nie, T.; Li, Y.; Li, H.; Sun, Y.; Wu, Y.; Zhang, Y.; Wang, M. Optimized phosphorus application under water stress enhances photosynthesis, physiological traits, and yield in soybean during flowering stage. Agronomy 2025, 15, 444. [Google Scholar] [CrossRef]
- Farouk, S.; Omar, M.M. Sweet basil growth, physiological and ultrastructural modification, and oxidative defense system under water deficit and silicon forms treatment. J. Plant Growth Regul. 2020, 39, 1307–1331. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Ghamdi, A.A.M. Sodium nitroprusside application enhances drought tolerance in marjoram herb by promoting chlorophyll biosynthesis and enhancing osmotic adjustment capacity. Arab. J. Geosci. 2021, 14, 430. [Google Scholar] [CrossRef]
- Zoufan, P.; ZareBavani, M.R.; Tousi, S.; Rahnama, A. Effect of exogenous melatonin on improvement of chlorophyll content and photochemical efficiency of PSII in mallow plants (Malva parviflora L.) treated with cadmium. Physiol. Mol. Biol. Plants 2022, 29, 145–157. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef] [PubMed]
- Návarová, H.; Bernsdorff, F.; Döring, A.C.; Zeier, J. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 2012, 24, 5123–5141. [Google Scholar] [CrossRef]
- Kucukkalyon, S.M.; Dinler, B.S. Pipecolic acid priming promotes salt stress tolerance via regulating antioxidant defense system and sugar metabolism in barley plants. Cereal Res. Com. 2025, 1–16. [Google Scholar] [CrossRef]
- Kumari, M.; Sharma, P.; Singh, A. Pipecolic acid: A positive regulator of systemic acquired resistance and plant immunity. BBA Gen. Subj. 2025, 1869, 130808. [Google Scholar] [CrossRef]
- Pérez-García, F.; Peters-Wendisch, P.; Wendisch, V.F. Engineering Corynebacterium glutamicum for fast production of L-lysine and L-pipecolic acid. Appl. Microbiol. Biotech. 2016, 100, 8075–8090. [Google Scholar] [CrossRef] [PubMed]
- Moulin, M.; Deleu, C.; Larher, F.; Bouchereau, A. The lysine ketoglutarate reductase–saccharopine dehydrogenase is involved in the osmo-induced synthesis of pipecolic acid in rapeseed leaf tissues. Plant Physiol. Biochem. 2006, 44, 474–482. [Google Scholar] [CrossRef]
- Caddell, D.F.; Louie, K.; Bowen, B.P.; Sievert, J.A.; Hollingsworth, J.; Dahlberg, J.; Purdom, E.; Northen, T.; Coleman, D. Drought shifts sorghum root metabolite and microbiome profiles and enriches for pipecolic acid. Phytobiomes J. 2023, 7, 449–463. [Google Scholar] [CrossRef]
- Hartmann, M.; Zeier, T.; Bernsdorff, F.; Reichel-Deland, V.; Kim, D.; Hohmann, M.; Scholten, N.; Schuck, S.; Bräutigam, A.; Hölzel, T.; et al. Flavinmonooxygenase-generated N-hydroxy pipecolic acid is a critical element of plant systemic immunity. Cell 2018, 173, 456–469.e16. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Q.; Yang, W.; Ahammed, G.J.; Ding, S.; Chen, X.; Shi, K. A novel role of pipecolic acid biosynthetic pathway in drought tolerance through the antioxidant system in tomato. Antioxidants 2021, 10, 1923. [Google Scholar] [CrossRef]
- Koc, F.N.; Dinler, B.S. Pipecolic acid in plants: Biosynthesis, signaling, and role under stress. Botanica 2022, 28, 4–14. [Google Scholar] [CrossRef]
- FAOSTAT. Population Data. Food and Agricultural Organization of United Nation, Roma. 2024. Available online: http://Faostat.fao.org/download/O/OA/E (accessed on 2 February 2024).
- Talaat, N.B. Drought stress alleviator melatonin reconfigures water-stressed barley (Hordeum vulgare L.) plants’ photosynthetic efficiency, antioxidant capacity, and endogenous phytohormone profile. Int. J. Mol. Sci. 2023, 24, 6228. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, E.L.; Bayih, T. Four Ethiopian barley (H. vulgare) varieties with a range of tolerance to salinity and water stress. Rhizosphere 2024, 29, 100841. [Google Scholar] [CrossRef]
- Kato, N.; Esaka, M. Changes in ascorbate oxidase gene expression and ascorbate levels in cell division and cell elongation in tobacco cells. Physiol. Plant. 1999, 105, 321–329. [Google Scholar] [CrossRef]
- Szafrańska, K.; Reiter, R.J.; Posmyk, M.M. Melatonin improves the photosynthetic apparatus in pea leaves stressed by paraquat via chlorophyll breakdown regulation and its accelerated de novo synhesis. Front. Plant Sci. 2017, 8, 878. [Google Scholar] [CrossRef]
- Hörtensteiner, S.; Kräutler, B. Chlorophyll breakdown in higher plants. Biochim. Biophys. Acta 2011, 1807, 977–988. [Google Scholar] [CrossRef]
- Hosain, M.T.; Rahman, M.S.; Nuruzzaman, M.; Munshi, M.H.; Bari, A.F. Morpho-physiological responses of rice to salicylic acid under drought stress. J. Bangladesh Agric. Univ. 2022, 20, 1–11. [Google Scholar] [CrossRef]
- Zangani, E.; Ansari, A.; Shekari, F.; Andalibi, B.; Afsahi, K.; Mastinu, A. Alleviating the injuries of NacL exposure on respiratory activities, leaf stomatal and antioxidant defense of Silybum marianum L. seedlings by exogenous nitric oxide. J. Plant Growth Regul. 2023, 42, 7731–7748. [Google Scholar] [CrossRef]
- Kumar, V.; Shriram, V.; KaviKishor, P.B.; Jawali, N.; Shitole, M.G. Enhanced proline accumulation and salt stress tolerance of transgenic indica rice by over-expressing P5CSF129A gene. Plant Biotec. Rep. 2010, 4, 37–48. [Google Scholar] [CrossRef]
- Zhang, H.; Pei, Y.; He, Q.; Zhu, W.; Jahangir, M.; Haq, S.; Khan, A.; Chen, R. Salicylic acid-related ribosomal protein CaSLP improves drought and Pst. DC3000 tolerance in pepper. Mol. Hortic. 2023, 3, 6. [Google Scholar] [CrossRef]
- Wang, I.J.; Fan, L.; Loescher, W.; Duan, W.; Lin, G.J.; Cheng, J.S. Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol. 2010, 10, 34–40. [Google Scholar] [CrossRef]
- Raven, J.A. Rubisco: Still the most abundant protein of Earth? New Phytol. 2013, 198, 1–3. [Google Scholar] [CrossRef]
- Sharwood, R.E. Engineering chloroplasts to improve Rubisco catalysis: Prospects for translating improvements into food and fiber crops. New Phytol. 2017, 213, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Semedo, J.N.; Rodrigues, A.P.; Lidon, F.C.; Pais, I.P.; Marques, I.; Gouveia, D.; Armengaud, J.; Silva, M.J.; Martins, S.; Semedo, M.C.; et al. Intrinsicnon-stomatal resilience to drought of the photosynthetic apparatus in Coffea spp. Is strengthened by elevated air (CO2). Tree Physiol. 2021, 41, 708–727. [Google Scholar] [CrossRef]
- Rudenko, N.N.; Ivanov, B.N. Unsolved problems of carbonic anhydrases functioning in photosynthetic cells of higher C3 plants. Biochemistry 2021, 86, 1243–1255. [Google Scholar] [CrossRef]
- Badger, M.R.; Price, G.D. The role of carbonica nhydrase in photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 369–392. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought stress tolerance in plants: Interplay of molecular, biochemical and physiological responses in important development stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Pazarlar, S. Exogenous application of pipecolic acid induces tomatalclosurein Arabidopsis thaliana L. Ege Üniv. Ziraat Fak. Derg. 2024, 61, 143–150. [Google Scholar] [CrossRef]
- Buqori, D.M.A.I.; Bambang, S.; Siswoyo, T.A.; Hariyono, K. Mitigating drought stress by application of drought-tolerant Bacillus spp. Enhanced root architecture, growth, antioxidant and photosynthetic genes expression in sugar cane. Sci. Rep. 2025, 15, 5259. [Google Scholar] [CrossRef]
- Suganthi, S.; Sivagami, K.G.; Maheswari, C.; Manivannan, P. Effect of drought stress on biochemical contents and proline metabolizing enzymes of Pennisetum glaucum L. Innov. Agric. 2021, 4, e32832. [Google Scholar] [CrossRef]
- Spormann, S.; Nadais, P.; Sousa, F.; Pinto, M.; Martins, M.; Sousa, B.; Fidalgo, F.; Soares, C. Accumulation of proline in plants under contaminated soils-A rewe on the same page? Antioxidants 2023, 12, 666. [Google Scholar] [CrossRef]
- Yousefvand, P.; Sohrabi, Y.; Heidari, G.; Weisany, W.; Mastinu, A. Salicylic acid stimulates defense systems in Allium hirtifolium grown under water deficit stress. Molecules 2022, 27, 3083. [Google Scholar] [CrossRef] [PubMed]
- Shuyskaya, E.V.; Rakhmankulova, Z.F.; Toderich, K.N. Role of Proline and Potassium in Adaptation to Salinity in Different Types of Halophytes. In Handbook of Halophytes, from Molecules to Ecosystems Towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer Nature SwitzerlandAG: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Shah, N.A.; Ullah, S.; Nafees, M.; Khan, M.N. Exogenous effect of sugar beet extract on physio-biochemical traits of Hordeum vulgare L. under induced salinity stress. Gesunde Pflanz. 2023, 75, 2655–2667. [Google Scholar] [CrossRef]
- Kostopoulou, Z.; Therios, I.; Roumeliotis, E.; Kanellis, A.K.; Molassiotis, A. Melatonin combined with ascorbic acid provides salt adaptation in Citrus aurantium L. seedlings. Plant Physiol. Biochem. 2015, 86, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Malan, C.; Berner, J.M. Comparative PSII photochemistry of quinoa and maize under mild to severe drought stress. Photosynthetica 2022, 60, 362–371. [Google Scholar] [CrossRef]
- El-Bauome, H.A.; Abdeldaym, E.A.; AbdEl-Hady, M.A.; Darwish, D.B.E.; Alsubeie, M.S.; El-Mogy, M.M.; Basahi, M.A.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; et al. Exogenous proline, methionine, and melatonin stimulate growth, quality, and drought tolerance in cauliflower plants. Agriculture 2022, 12, 1301. [Google Scholar] [CrossRef]
- González-Villagra, J.; Reyes-Díaz, M.M.; Tighe-Neira, R.; Inostroza-Blancheteau, C.; Escobar, A.L.; Bravo, L.A. Salicylic acid improves antioxidant defense system and photosynthetic performance in Aristotelia chilensis plants subjected to moderate drought stress. Plants 2022, 11, 639. [Google Scholar] [CrossRef]
- Shaheen, A.; Akram, S.; Sharif, S.; Rashid, A.; Adnan, A.; Mushtaq, M. Fractionation of Xanthium strumarium L. foliage phenolics, in-vitro antioxidant activities, and in-vivo anti-diabetic potential. Front. Chem. 2023, 11, 1279729. [Google Scholar] [CrossRef]
- Smirnoff, N. Engineering of metabolic pathways using synthetic enzyme complexes. Plant Physiol. 2019, 179, 918–928. [Google Scholar] [CrossRef]
- Simontacchi, M.; Galatro, A.; Ramos-Artuso, F.; Santa-María, G.E. Plant survival in a changing environment: The role of nitric oxide in plant responses to a biotic stress. Front. Plant Sci. 2015, 6, 977. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Huqail, A.A. Sodium nitroprusside application regulates antioxidant capacity, improves phytopharmaceutical production and essential oil yield of marjoram herb under drought. Ind. Crops Prod. 2020, 158, 13034. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Saito, K. Enhancement of oxidative and drought tolerance in Arabidopsis by over accumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signaling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Anwar, F.; Ashraf, M.; Saari, N.; Perveen, R. Ameliorating effects of exogenously applied proline on seed composition, seed oil quality and oil antioxidant activity of maize (Zea mays L.) under drought stress. Int. J. Mol. Sci. 2013, 14, 818–835. [Google Scholar] [CrossRef]
- Foyer, C.H.; Souriau, N.; Perret, S.; Lelandais, M.; Kunert, K.J.; Pruvost, C.; Jouanin, L. Over expression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant physiol. 1995, 109, 1047–1057. [Google Scholar] [CrossRef]
- Gula, E.; Dziurka, M.; Hordyńska, N.; Libik-Konieczny, M. Regulatory effect of pipecolic acid (Pip) on the antioxidant system activity of Mesembryanthemum crystallinum plants exposed to bacterial treatment. Physiol. Plant. 2024, 176, e14583. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Ding, H. Variation in air pollution tolerance index of plans near of steal factory: Implication for landscape-plant species selection for industrial areas. Wseas Trans. Environ. Dev. 2008, 4, 24–32. [Google Scholar]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: Oxford, UK, 1999; p. 322. [Google Scholar]
- Xie, J.; Wang, Z.; Li, Y. Stomatal opening ratio mediates trait coordinating network adaptation to environmental gradients. New Phytol. 2022, 235, 907–922. [Google Scholar] [CrossRef]
- Harpaz-Saad, S.; Azoulay, T.; Arazi, T.; Ben-Yaakov, E.; Mett, A.; Shiboleth, Y.M.; Hörtensteiner, S.; Gidoni, D.; Gal-On, A.; Goldschmidt, E.E.; et al. Chlorophyllase is a rate-limiting enzyme in chlorophyll catabolism and is post translationally regulated. Plant Cell 2007, 19, 1007–1022. [Google Scholar] [CrossRef]
- Radojevič, M.; Bashkin, V.N. Practical Environmental Analysis, 2nd ed.; Chapter 4; RSC Publishing: Cambridge, UK, 2006. [Google Scholar]
- Sarropoulou, V.; Dimassi-Theriou, K.; Therios, I.; Koukourikou-Petridou, M. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry root stock PHL=C (Prunus avium×Prunus cerasus). Plant Physiol. Biochem. 2012, 61, 162–168. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; pp. 350–382. [Google Scholar] [CrossRef]
- Sairam, R.K.; Deshmukh, P.S.; Shukla, D.S. Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J. Agron. Crop Sci. 1997, 178, 171–178. [Google Scholar] [CrossRef]
- Dwivedi, R.S.; Randhawa, N.S. Evaluation of a rapid test for the hidden hunger of zinc in plants. Plant Soil. 1974, 40, 445–451. [Google Scholar] [CrossRef]
- Lu, K.X.; Cao, B.H.; Feng, X.P.; He, Y.; Jiang, D.A. Photosynthetic response of salt-tolerant and sensitive soybean varieties. Photosynthetica 2009, 47, 381–387. [Google Scholar] [CrossRef]
- Sadasivam, S.; Manickam, A.A. Biochemical Method, 3rd ed.; New Age Inter Publishers: New Delhi, India, 2008. [Google Scholar]
- Claussen, W. Proline as a measure of stress in tomato plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Warrier, R.R.; Paul, M.; Vineetha, M.V. Estimation of salicylic acid in Eucalyptus leaves using spectrophotometric methods. Genet. Plant Physiol. 2013, 3, 90–97. [Google Scholar]
- Islam, S.; Mohammad, F. Modulation of growth, photosynthetic efficiency, leaf biochemistry, cell viability and yield of Indian mustard by the application of trehalose. Sci. Hort. 2021, 290, 110527. [Google Scholar] [CrossRef]
- Janardhan, K.V.; Murthy, A.P.; Giriraj, K.; Panchaksharaiah, S. A rapid method for determination of osmotic potential of plant cell-sap. Curr. Sci. 1975, 44, 390–391. [Google Scholar]
- Blum, A. Osmotic adjustment and growth of barley genotypes under drought stress. Crop Sci. 1989, 29, 230–233. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A.J.S. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- MadhavaRao, K.V.; Stresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Mill spaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, J.; Kumar, P. Effects of plant growth regulators and sucrose on post-harvest physiology, membrane stability and vase life of cut spikes of gladiolus. Plant Growth Regul. 2008, 55, 221–229. [Google Scholar] [CrossRef]
- Huang, X.S.; Wang, W.; Zhang, Q.; Liu, J.H. A basic helix-loop-helix transcription factor, Ptrb HLH, of Poncirus trifoliate confers cold tolerance and modulates peroxidase-mediated scavenging of hydrogen peroxide. Plant Physiol. 2013, 162, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Rabino, I.; Mancinelli, A. Light, temperature, and anthocyanins production. J. Plant Physiol. 1986, 81, 922–924. [Google Scholar] [CrossRef]
- Lin, K.H.; Chao, P.Y.; Yang, C.M.; Cheng, W.C.; Lo, H.F.; Chang, T.R. The effects of flooding and drought stresses on the antioxidant constituents in sweet potato leaves. Bot. Stud. 2006, 47, 417–426. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wang, X.R.; Huang, J.L. Principles and Techniques of Plant Physiology and Biochemistry Experiments; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
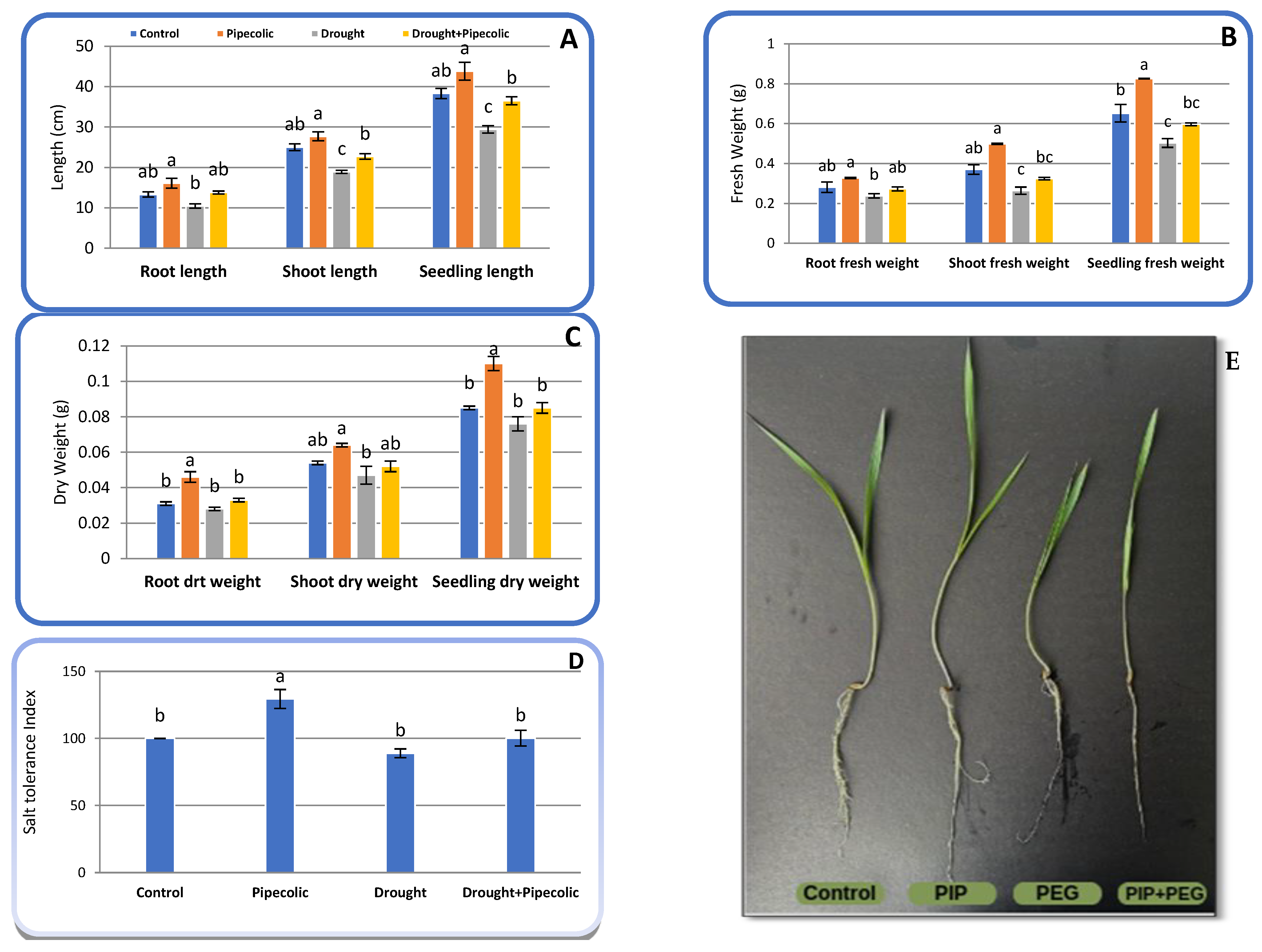
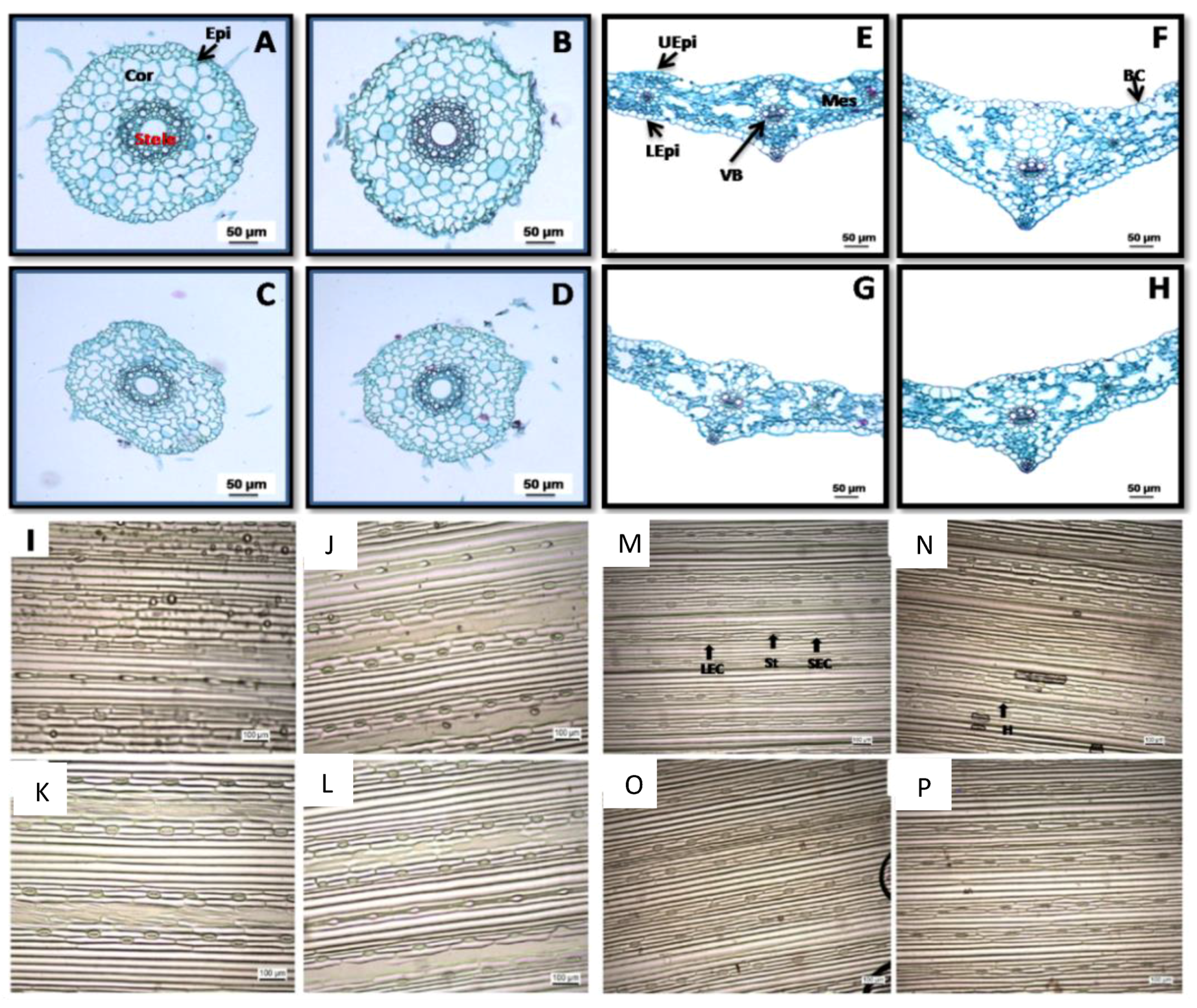
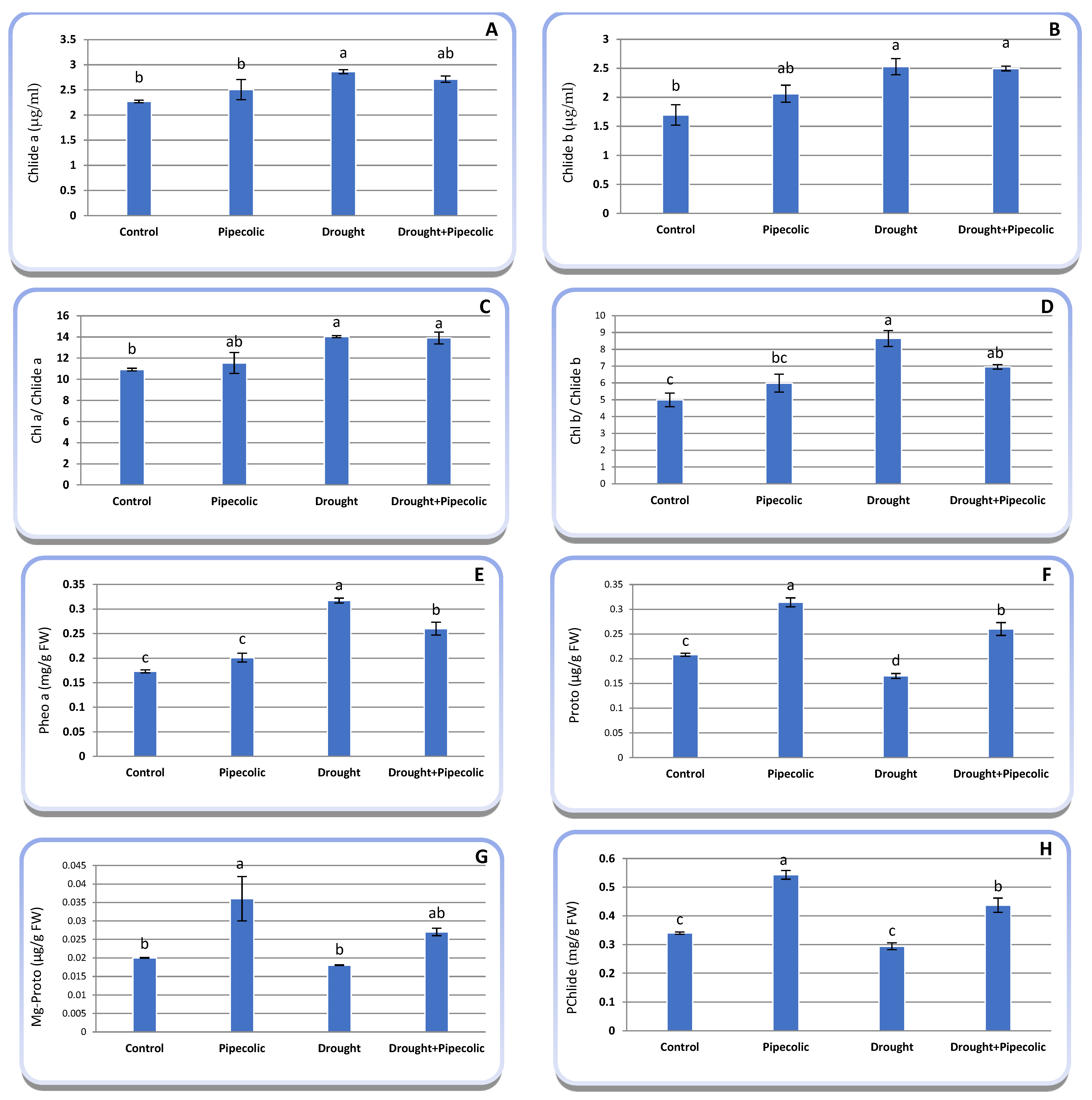

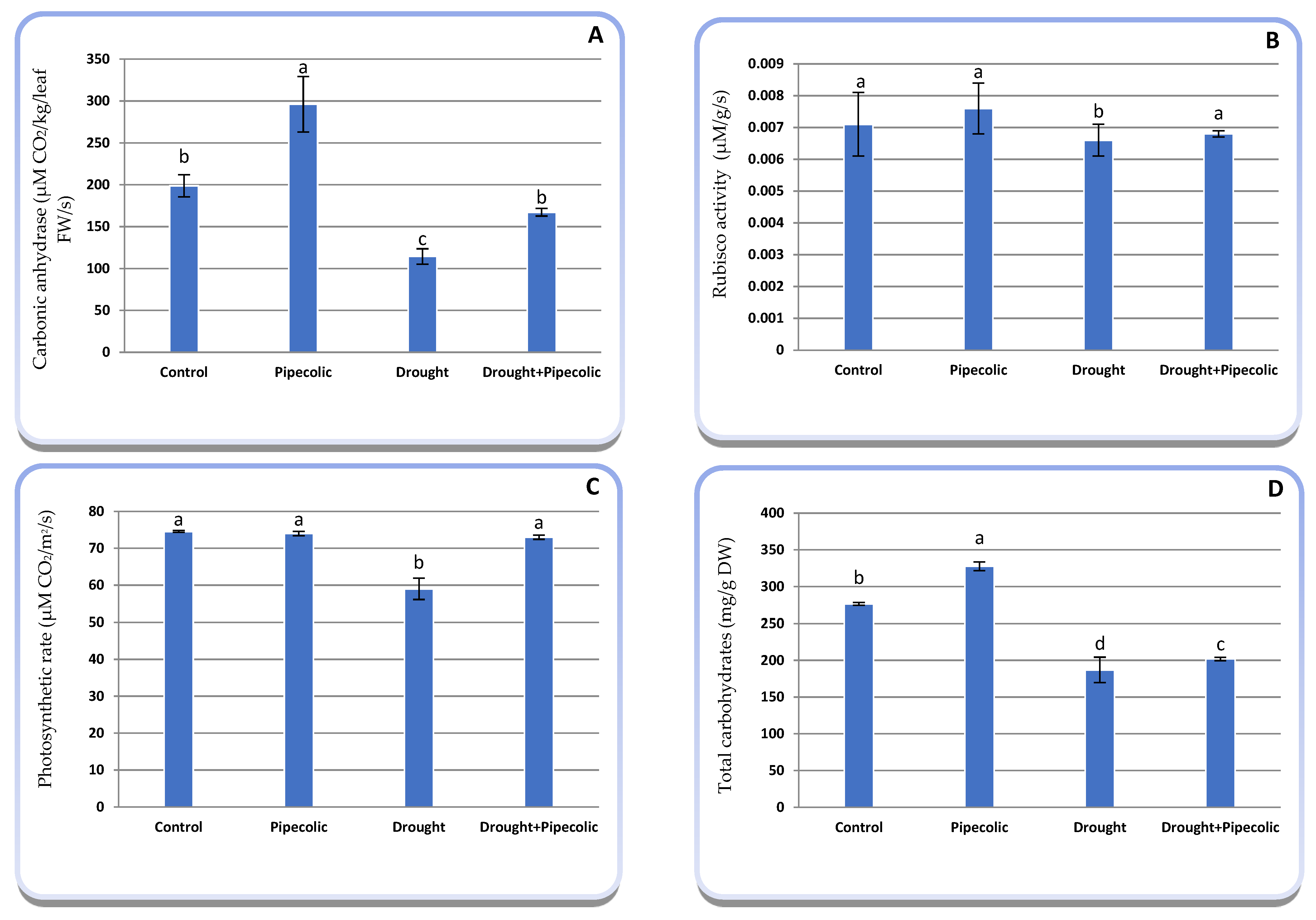


| Treatment | Root | Leaf | Stomatal Density | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermis Thickness (µm) | Cortex Thickness (µm) | Stele Diameter (µm) | Thickness of Vascular Tissues | Leaf Blade Thickness (µm) | Mesophyll Tissue Thickness (µm) | Thickness of Leaf at Midrib Region (µm) | Main Vascular Bundle Dimension (µm) | Metaxylem Vessel Diameter (µm) | ||||
| Length | Width | Adaxial | Abaxial | |||||||||
| Control | 18.75 ± 0.03 a | 137.5 ± 0.33 b | 131.2 ± 2.32 b | 80.25 ± 1.33 b | 143.7 ± 1.04 c | 118.7 ± 1.15 c | 212.5 ± 0.40 c | 56.25 ± 0.60 b | 87.50 ± 0.53 a | 6.87 ± 0.29 b | 72.66 ± 0.44 bc | 64.33 ± 2.66 bc |
| Pipecolic acid | 18.75 ± 0.23 a | 193.7 ± 0.33 a | 150.0 ± 0.46 a | 99.43 ± 1.26 a | 193.7 ± 2.42 a | 150.0 ± 0.55 a | 318.7 ± 1.60 a | 68.75 ± 0.52 a | 75.00 ± 0.46 b | 10.0 ± 0.49 a | 67.83 ± 1.09 c | 57.00 ± 1.04 c |
| Drought | 6.250 ± 0.11 b | 84.30 ± 1.10 d | 80.25 ± 0.02 d | 45.22 ± 0.39 d | 137.5 ± 0.80 c | 106.2 ± 1.75 d | 200.0 ± 0.49 d | 56.25 ± 0.22 b | 75.00 ±0.5 b | 5.70 ± 0.29 b | 102.0 ± 1.52 a | 74.50 ± 1.80 a |
| Drought + Pipecolic | 18.75 ± 0.81 a | 100.0 ± 1.48 c | 87.50 ± 1.09 c | 60.17 ± 0.22 c | 162.5 ± 0.40 b | 131.2 ± 0.60 b | 243.7 ± 2.17 b | 56.25 ± 0.13 b | 75.00 ± 0.24 b | 6.30 ± 0.12 b | 85.60 ± 6.25 b | 68.66 ± 2.84 ab |
| p value | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aktas, N.; Farouk, S.; Al-Ghamdi, A.A.M.; Alenazi, A.S.; AlMalki, M.A.L.; Dinler, B.S. Pipecolic Acid, a Drought Stress Modulator, Boosts Chlorophyll Assimilation, Photosynthetic Performance, Redox Homeostasis, and Osmotic Adjustment of Drought-Affected Hordeum vulgare L. Seedlings. Plants 2025, 14, 1949. https://doi.org/10.3390/plants14131949
Aktas N, Farouk S, Al-Ghamdi AAM, Alenazi AS, AlMalki MAL, Dinler BS. Pipecolic Acid, a Drought Stress Modulator, Boosts Chlorophyll Assimilation, Photosynthetic Performance, Redox Homeostasis, and Osmotic Adjustment of Drought-Affected Hordeum vulgare L. Seedlings. Plants. 2025; 14(13):1949. https://doi.org/10.3390/plants14131949
Chicago/Turabian StyleAktas, Nagihan, Saad Farouk, Amal Ahmed Mohammed Al-Ghamdi, Ahmed S. Alenazi, Mona Abdulaziz Labeed AlMalki, and Burcu Seckin Dinler. 2025. "Pipecolic Acid, a Drought Stress Modulator, Boosts Chlorophyll Assimilation, Photosynthetic Performance, Redox Homeostasis, and Osmotic Adjustment of Drought-Affected Hordeum vulgare L. Seedlings" Plants 14, no. 13: 1949. https://doi.org/10.3390/plants14131949
APA StyleAktas, N., Farouk, S., Al-Ghamdi, A. A. M., Alenazi, A. S., AlMalki, M. A. L., & Dinler, B. S. (2025). Pipecolic Acid, a Drought Stress Modulator, Boosts Chlorophyll Assimilation, Photosynthetic Performance, Redox Homeostasis, and Osmotic Adjustment of Drought-Affected Hordeum vulgare L. Seedlings. Plants, 14(13), 1949. https://doi.org/10.3390/plants14131949







