Abstract
Among various plants, corn is the primary host damaged by Spodoptera frugiperda J. E. Smith (Lepidoptera: Noctuidae). After long-term regional colonization, its larvae feed on sweet waxy corn and fresh corn for extended periods. A question arises: Does long-term feeding on different corn varieties affect their rhythms? Currently, there are no reports addressing these issues. To facilitate the formulation of effective prevention and control measures, Zhengdan 958 and Zhenghuangnuo were selected as representative varieties of normal and sweet waxy corn, respectively, for laboratory experiments. S. frugiperda were fed the leaves of these two corn types over nine consecutive generations, thereby establishing distinct S. frugiperda strains associated with each corn variety. Additionally, a strain fed an artificial diet served as the control group. Through a comparative analysis of the emergence, movement, nutritional foraging, dormancy, mating, and oviposition behaviors of adult fall armyworms from different populations, differences in the six behavioral peak times among the strains were identified. RT-qPCR analysis indicated significant differences in the expression levels of four circadian clock genes across different populations and tissues of the fall armyworm. Feeding on different host plants influenced the expression of circadian clock genes and their associated behavioral rhythms. Our study showed that sweet corn is more conducive to pupation, mating, and oviposition. Because of these differences in adult insect rhythms, sweet corn may have an impact on the reproduction of fall armyworms in the Huang–Huai–Hai corn-planting region.
1. Introduction
Throughout the extensive evolutionary journey of insects, the cycles of day and night, seasonal variations, and other terrestrial changes have led to the development of rhythmic behaviors, commonly referred to as biological clocks [1,2]. In moths, the primary rhythmic activities during developmental stages include pupation, eclosion, mating, and oviposition. Additionally, the behavioral rhythms exhibited by different insect species vary significantly. Even within the same species, the consistency of behavioral rhythms is influenced by numerous external factors. These variations in insect behavioral rhythms reflect their adaptations to diverse environments and contribute to the extensive variety within moth species [3,4].
The fall armyworm (FAW), Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), is a significant migratory pest that affects crops globally and poses a considerable risk to food security in China [5]. Current research on the biology and rhythmic patterns of FAW primarily investigates mating, oviposition, hatching, adult lifespan, and how various feeding conditions impact its growth and development [6,7,8,9]. Significant advancements have also been made in understanding the interactions between FAW and its host plants. Studies have demonstrated that the selectivity and fitness of FAW in relation to different hosts, along with oviposition preferences and the influence of feeding on various host plants, considerably affect its growth and development [10,11,12,13,14].
Although FAW has an extensive range of hosts in the Huang–Huai–Hai region of China, it predominantly inflicts damage on maize. Trap cropping is a behavioral management strategy where a preferred host plant attracts pests away from the main crop; this method utilizes the host-finding behavior of insects to decrease pest pressure [15]. S. frugiperda feeding on different host plants may experience alterations in their circadian rhythms, and even minor variations in these rhythms can influence gene expression among individuals and impact a range of reproductive behaviors [16,17]. Studies have shown that geographical and host differences can affect the growth, development, and reproduction of S. frugiperda [18,19]. Understanding how these changes might influence the FAW’s ovarian development and subsequent reproductive success is crucial for developing effective control strategies. In this study, we aimed to investigate the ovarian development of FAW raised on different host plants and assess any potential variations in their circadian rhythms that could impact their reproductive behaviors. This study investigates sweet maize’s potential as a trap crop for S. frugiperda under agroecosystem conditions of the summer maize production area in the Huang–Huai–Hai region of China, which will provide valuable insights into the biology of FAW and contribute to the development of more targeted and sustainable pest management practices.
2. Materials and Methods
2.1. Insects
The initial insects of S. frugiperda comprised 3 to 5 instar larvae collected from maize fields in Minggang Town, Xinyang, Henan Province, in June 2019. These larvae were reared continuously over 3 generations using an artificial diet in a climate-controlled environment (PQX-280A-3H artificial climate box, Ningbo Laifu Technology Co., Ltd., Ningbo, China) maintained at a temperature of (27 ± 1) °C, with a photoperiod of 14 h of light and 10 h of dark, and a relative humidity of (75 ± 5)%. Additionally, two maize strains were reared for 9 generations on fresh corn leaves. Following pupation of the various larval strains, male and female pupae were separated into different containers, while the control group consisted of adults of S. frugiperda raised on an artificial diet in the laboratory.
2.2. Observation of Adult Behavior and Rhythm
The pupae of S. frugiperda were categorized by gender, and those ready for eclosion were chosen and placed in 50 mL insect boxes (dimensions: bottom × upper bottom × height = 28 mm × 38 mm × 30 mm) [20]. Each box contained two pupae, which were organized neatly, and an infrared camera was used for video recording, with observations made every hour.
The eclosion of the fall armyworm was conducted with a pairing ratio of female to male moths of 1:1, referenced by Helicoverpa armigera Hübner density protocols to minimize crowding effects and behavioral artifacts [21]. Transparent packaging boxes measuring 10 cm on each side were used, with one pair per box. A total of 11 treatments were set up, each repeated three times, resulting in 33 pairs. To provide nutrition for the adults, a cotton ball soaked in 10% sucrose water was placed inside each box, adult behavior was observed and recorded every 20 min using the infrared camera, and observers were blinded during behavioral recording.
2.3. Differentiation of Behavioral Characteristics
The activities of adult S. frugiperda were categorized into five distinct types. 1. Movement: Adults either crawl or fly within the insect box, with activity lasting over 3 s. 2. Nutritional Supplementation: Adults feed on sucrose water placed on defatted cotton. 3. Resting: Adults remain stationary in the insect box (excluding feeding, mating, and oviposition). 4. Mating: The females and males align their abdomens closely, forming a V-shape or type I configuration. 5. Oviposition: The tip of the adult’s abdomen moves as it lays eggs. Additionally, the eclosion and behavioral patterns of S. frugiperda reared for one generation on corn leaves were observed and documented, and these were compared to those of a strain reared for nine generations on the same diet.
2.4. Determination of Ovarian Development
Pupae that exhibited a normal appearance and consistent size were chosen and placed into a dipping box. Following eclosion, no nutritional supplementation was provided. At 8:30 a.m. on the second day post-eclosion, females were randomly selected for dissection to assess ovarian development. During dissection, the wings of the female moths were first removed, and scales were collected in a wax plate, secured with an insect needle. Using tweezers, the membrane from the 8th internode of the tail was carefully torn to the midline of the dorsal side, and the body wall was anchored on both sides using an insect needle. The ovary was extracted with a hook needle and weighed on an analytical balance (Mettler Toledo ME204T), recorded as M0. It was then placed in a wax plate containing 5% pentanediol for observation and photography with a super-depth-of-field microscope (Moticam Pro S5 Lite). The ovarian duct’s length was measured using Image J 1.52a (https://javadoc.io/doc/net.imagej/ij/1.52a, 19 June 2025) after dissection and imaging under a binocular stereomicroscope (Shimadzu Kalnew Stereo Microscope (20X/40X)). The fat body was removed and placed into a fresh culture dish. Excess water and the free fat body were absorbed with filter paper and weighed, denoted as M1. The weight of the fat body was determined by calculating the following difference: M0–M1. Ten individuals were dissected for each treatment, with three repetitions conducted.
2.5. In Vitro Expression of Rhythm-Related Genes
The male and female adults of S. frugiperda after 3 days of eclosion were taken, and the RNA of different parts was extracted at 8:00 a.m. to prepare cDNA libraries. For generating the first-strand cDNA, 2 µg of RNA of female and male S. frugiperda adults was reverse-transcribed in a 20 μL reaction volume using the PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time). To confirm the single-amplicon product for qRT-PCR and the bio-specificity for S. frugiperda, gradient PCR was performed for all primer pairs and optimized for a 60 °C annealing temperature for all primer pairs. PCR amplicon products were run on 1.5% 1xTAE agarose gel electrophoresis and Sanger-sequenced (Biological Engineering (Shanghai) Co., Ltd., Shanghai, China).
qRT-PCR was performed using the SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) (TaKaRa) according to the manufacturer’s protocol, in the StepOne Plus Real-time PCR system (Applied Biosystems, Waltham, MA, USA), with the following PCR-cycling conditions: 95 °C for 10 s, 40 cycles of 95 °C for 30 s, and 60 °C for 30 s with a concentration of 200 nM of each primer pair. The mean threshold cycle (Ct) was measured by three replicates of each gene to test sample contamination and dimer formation, and nuclease-free water as the template was included in all qRT-PCR experiments as the negative control. The primer design of four circadian clock genes like Period (Per), Cryptochrome (Cry), Cycle (Cyc), and Clock (Clk) in S. frugiperda was established [22]. The 2-ΔΔ Ct method [23] was used to calculate the relative expression levels of individual genes. The actin gene (unigene 62750) was chosen as the endogenous reference gene in the qRT-PCR experiment as previously demonstrated. All primers pairs for qRT-PCR are listed in Table 1.

Table 1.
Information on circadian clock gene primers.
2.6. Data Analysis
The experimental data were statistically analyzed using SPSS 22.0. One-way analysis of variance was used. The difference between the same gender was tested by Tukey’s method. The significant difference between male and female was tested using the t-test.
3. Results
3.1. The Effect of Different Maize Cultivars on the Emergence Rhythm of the First Generation of the S. frugiperda Strain
The emergence times of various S. frugiperda strains varied, yet a distinct peak was observed. For the first generation of the Zhengdan 958 strain and the artificial diet feed strain, the emergence peak occurred between 0:00 and 1:00, while the first generation of the Zhenghuangnuo strain peaked from 23:00 to 0:00. The emergence peak of the Zhenghuangnuo strain occurred earlier than that of the grain corn strain. All three strains exhibited their emergence peaks during the dark period.
The emergence duration for each strain was further recorded. The Zhengdan 958 strain and the artificial diet feed strain exhibited emergence periods of approximately two hours, centered around their respective peaks. The Zhenghuangnuo strain, on the other hand, displayed a slightly longer emergence period, spanning nearly three hours around its peak. The grain corn strain showed the most variable emergence times, with individuals emerging over a broader window of several hours, primarily during the dark period. The synchronization of emergence within strains, especially during the dark phase, suggests the potential for a reduced predation risk and increased mating opportunities (Figure 1).
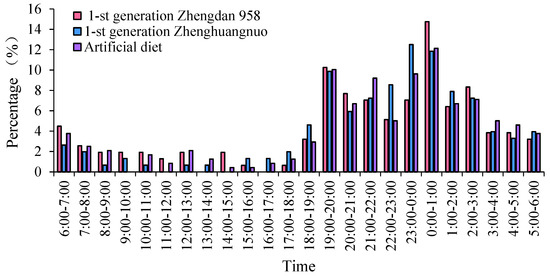
Figure 1.
The adult emergence rhythm of the first-generation population of S. frugiperda. Data in the figure are mean ± SE. Different capital and lowercase letters above the bars indicate extremely significant (p < 0.01) and significant differences (p < 0.05) in the different strains of S. frugiperda adults (ANOVA and Duncan’s new multiple range test), respectively.
3.2. The Impact of Different Maize Cultivars on the Activity Patterns of FAW Adults in the First-Generation Strain
During the light–dark cycle, different strains of the fall armyworm exhibit distinct activity patterns, accompanied by significant periods of rest. The activity levels of all three strains significantly decrease after 8:00. However, the activity of the first generation of Zhengdan 958 and Zhenghuangnuo strains significantly increases at 18:00 in the evening, while the artificially fed strain reaches its peak activity at 19:00. During the dark period, each strain shows a pattern of gradually increasing activity followed by a decline, with the artificially fed strain exhibiting a more gradual decrease. Among all strains, the highest frequency of activity occurs between 00:00 and 01:00. Before the commencement of the dark period, the time at which adult activity begins to rise varies among the three strains: the Zhengdan 958 and Zhenghuangnuo strains begin to rise 2 h before the dark period, while the artificially fed strain rises 1 h before. The resting behavior of all the adult strains is inversely proportional to the amount of activity, with higher frequencies during the light period and lower frequencies during the dark period (Figure 2C).
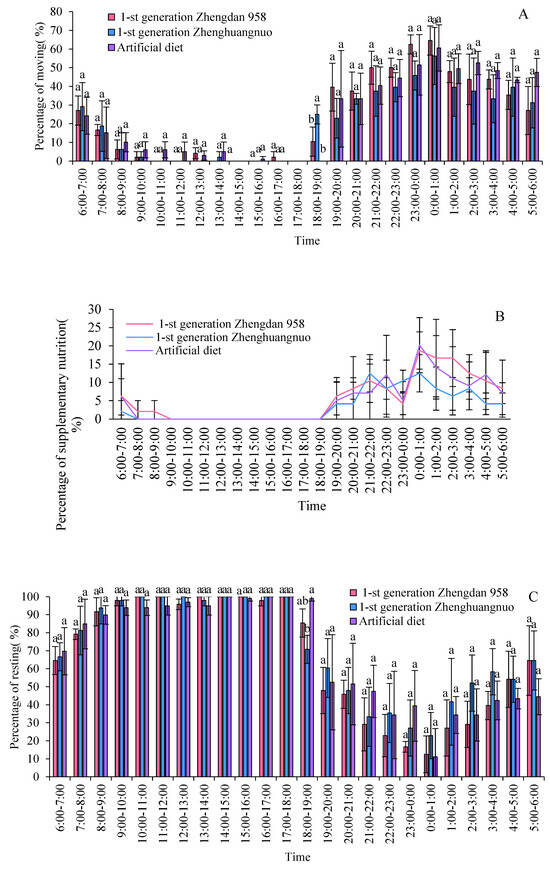
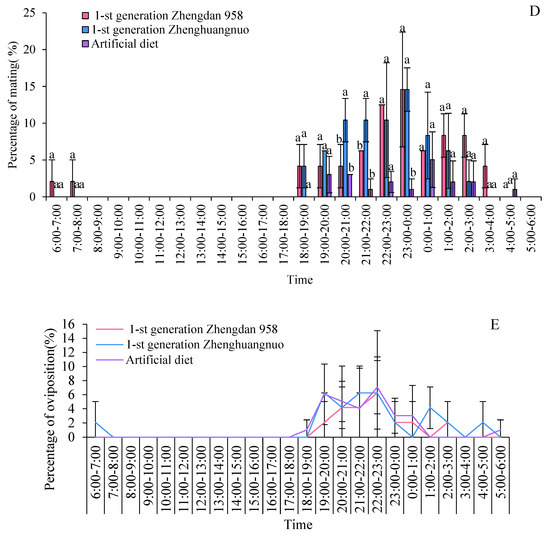
Figure 2.
Rhythms of emergence, movement, resting, mating, and oviposition for different strains of S. frugiperda adults in the first generation. (A–C) Emergence, movement, and resting rhythms of the first-generation S. frugiperda strains, respectively. (D,E) Mating and oviposition rhythms of different S. frugiperda strains in the first generation, respectively. The different lowercase letters above the column represent the significance of differences between strains.
Supplementary nutritional behavior among adult S. frugiperda species primarily occurred in darkness, with minimal activity during the light hours. The peak for supplementary feeding was between 00:00 and 01:00 for both the first generation of the Zhengdan 958 strain and the artificial diet feed strain, whereas the Zhenghuangnuo strain peaked at 21:00 to 22:00 and again at 00:00 to 01:00.
Additionally, the frequency of supplementary feeding was notably higher in the Zhengdan 958 strain compared to the other two strains, suggesting potential differences in nutritional requirements or feeding habits among these variants. For all strains, the amount of food consumed during the supplementary feeding periods was consistent across replicates, indicating a reliable and reproducible feeding pattern.
All three strains exhibited a distinct peak in mating behavior: the Zhengdan 958 and Zhenghuangnuo strains peaked between 23:00 and 00:00, while the artificial diet strain peaked between 00:00 and 01:00. Spawning peaks were observed as follows: the first-generation Zhengdan 958 strain had one peak (22:00 to 23:00), the first-generation Zhenghuangnuo strain had two peaks (21:00 to 22:00 and 22:00 to 23:00), and the artificial diet strain also had two peaks (19:00 to 20:00 and 22:00 to 23:00). The highest spawning frequency for each strain was recorded between 22:00 and 23:00 (Figure 2D).
In addition, the ovarian development of the insects was monitored, revealing that all strains reached the maximum ovarian maturity during their respective spawning peaks. The ovarian development index for each strain correlated positively with their spawning frequencies, indicating a strong relationship between ovarian maturity and reproductive activity.
3.3. Impact of Various Maize Cultivars on the Mating Duration of Adult S. frugiperda from a First-Generation Strain
The mating duration for S. frugiperda across the three strains was at least 60 min. Specifically, the first generation of Zhengdan 958 averaged 206.18 ± 90.43 min, the first generation of Zhenghuangnuo averaged 189.90 ± 116.23 min, and the artificial diet strain averaged 83.35 ± 52.32 min. The mating times for both the first-generation Zhengdan 958 and Zhenghuangnuo strains were significantly longer than that of the artificial diet strain (p = 0.002) (Figure 3).
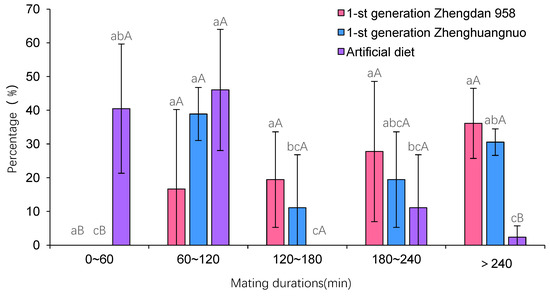
Figure 3.
The mating duration of S. frugiperda adults in the first-generation population. Data in the figure are mean ± SE. Different lowercase and capital letters above the bars indicate significant differences (p < 0.05) and extreme significant (p < 0.01) in the different strains of S. frugiperda adults respectively (ANOVA and Duncan’s new multiple range test).
Across the three strains, the Zhengdan 958 strain exhibited an average copulation frequency of 1.67 ± 0.71 times per female, while the Zhenghuangnuo strain averaged 1.58 ± 0.65 times per female. The artificial diet strain showed a slightly lower frequency at 1.42 ± 0.50 times per female. However, no statistically significant differences were observed among the strains in terms of copulation frequency (p = 0.347) (Figure 3).
3.4. The Impact of Various Maize Cultivars on the Expression of Rhythm Genes in the First Generation of S. frugiperda Adults
The expression levels of the four circadian clock genes varied among different strains. In comparison to the females of the artificial diet strain, per gene expression was reduced in the antennae, head, flight muscle, and ovary of the first generation of the Zhengdan 958 strain, while it increased in the leg. For the first generation of the Zhenghuangnuo strain, per gene expression decreased in the antennae, head, and flight muscle but rose in the leg and ovary. clk gene expression was lower in the antennae, leg, and ovary of the first generation of the Zhengdan 958 strain, but higher in the head and flight muscle. In the first generation of the Zhenghuangnuo strain, clk expression decreased in the antennae and ovary, while it increased in the head, leg, and flight muscle. cry gene expression diminished in the antennae, leg, and flight muscle of the first generation of the Zhengdan 958 strain, while it increased in the head and ovary. Similarly, in the first generation of the Zhenghuangnuo strain, cry gene expression decreased in the antennae and leg, but increased in the head, flight muscle, and ovary. cry gene expression rose in the antennae, head, and ovary of the first generation of the Zhengdan 958 strain and also increased in the head and ovary. In contrast, the expression level of cry in the antennae and ovaries of the first generation of the Zhenghuangnuo strain increased, while it decreased in the head, leg, and flight muscle.
In comparison to the male of the artificial diet strain, the expression of the per gene was reduced in the antenna and head of the first generation of the Zhengdan 958 strain, while it was elevated in the leg, flight muscle, and testis. Conversely, the expression level rose across all regions in the first generation of the Zhenghuangnuo strain. For the clk gene, expression decreased in the antennae of the first-generation Zhengdan 958 strain but increased in the head, leg, flight muscle, and testis. In the first generation of the Zhenghuangnuo strain, the expression of clk reduced in both the antennae and testis but was heightened in the head, leg, and flight muscle. The cry gene exhibited an increase in expression throughout all parts of the first-generation Zhengdan 958 strain. Similarly, in the first generation of the Zhenghuangnuo strain, cry expression rose in the antennae, leg, flight muscle, and testis, while it dropped in the head. The cyc gene’s expression increased in the antenna and head of the first-generation Zhengdan 958 strain but decreased in the leg, flight muscle, and testis. In contrast, the expression of cyc was heightened in the antennae of the first-generation Zhenghuangnuo strain, yet it was reduced in the head, leg, flight muscle, and testis.
When comparing the artificial diet strain to the first generation of both the Zhengdan 958 and Zhenghuangnuo strains, variations in circadian gene expression levels were observed, with significant differences in certain genes across the strains. Notably, cry gene expression in the ovary of the first-generation Zhenghuangnuo strain was found to be 11.39 times greater than that of the artificial diet strain. In the ovaries of the first-generation Zhengdan 958 and Zhenghuangnuo strains, cyc gene expression was 12.94 and 19.08 times higher, respectively, than in the artificial diet strain, although most expression levels fluctuated between 0.5 and 2 times (Figure 4).
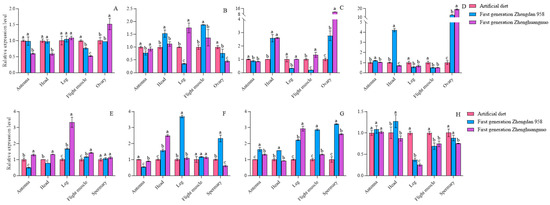
Figure 4.
The relative levels of circadian clock gene expression in adult individuals of the first generation of the S. frugiperda strain. Data in the figure are mean ± SE. Different lowercase letters above the bars indicate significant differences (p < 0.05) in the different strains of S. frugiperda adults (ANOVA and Duncan’s new multiple range test), respectively. The expression levels of Per, Clk, Cry, and Cyc in adult females are labeled (A–D), whereas in adult males, they are indicated by (E–H).
3.5. Impact of Various Maize Cultivars on Emergence Timing of S. frugiperda
Different strains of S. frugiperda exhibited varying emergence times, although a clear peak was noted. The emergence peaks for the Zhengdan 958 strain, Zhenghuangnuo strain, and artificial diet feed strain occurred between 0:00 and 1:00, 22:00 and 23:00, and 0:00 and 1:00, respectively. All three strains peaked during the dark period, but the Zhengdan 958 and Zhenghuangnuo strains showed a more concentrated emergence, while the artificial diet strain had a more scattered emergence. Notably, the emergence peak of the Zhenghuangnuo strain was 2 h earlier than that of both the Zhengdan 958 strain and the artificial diet strain (Figure 5).
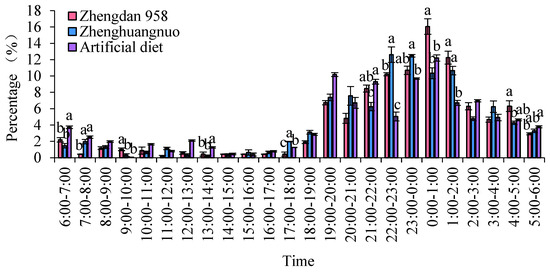
Figure 5.
The adult emergence pattern of S. frugiperda. Data in the figure are mean ± SE. Different lowercase letters above the bars indicate significant differences (p < 0.05) in the different strains of S. frugiperda adults (ANOVA and Duncan’s new multiple range test).
3.6. Eclosion Patterns of Male and Female Adults Across Different Strains
The eclosion peaks of S. frugiperda varied among different strains, with females emerging earlier than males. In two maize strains, the eclosion of females occurred before that of males. Specifically, in the Zhengdan 958 strain, the eclosion peaks for males and females occurred between 18:00 and 19:00 and from 00:00 to 01:00, respectively. During the time frame of 18:00 to 01:00, the eclosion rates were 81.26% for males and 46.80% for females. For the Zhenghuangnuo strain, the peak eclosion times were 19:00 to 20:00 and 22:00 to 23:00 for males, followed by 23:00 to 00:00 and 01:00 to 02:00 for females. The eclosion percentages between 19:00 and 23:00 were 48.53% for males and 18.87% for females. In the artificial diet strain, the peaks occurred from 19:00 to 20:00 and from 21:00 to 22:00 for males, with females emerging from 19:00 to 20:00 and 00:00 to 01:00. The eclosion rates during 19:00 to 22:00 were 29.91% for males and 22.13% for female adults (Figure 6).
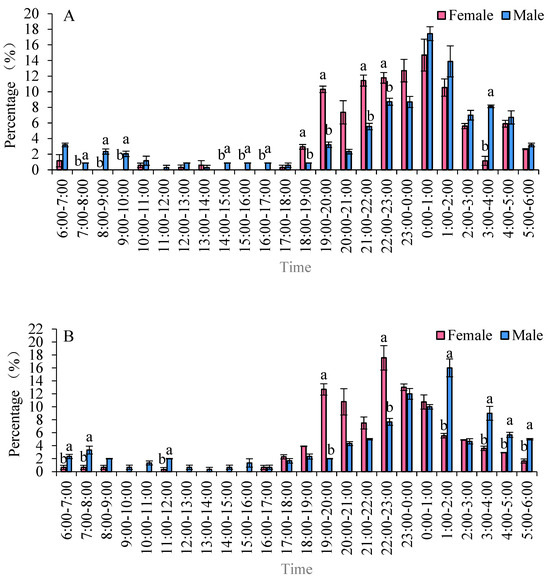
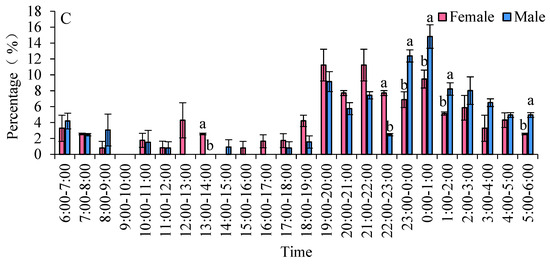
Figure 6.
The emergence patterns of adult S. frugiperda across various strains. (A) Zhengdan 958 strain; (B) Zhenghuangnuo strain; (C) artificial diet feed strain. Data in the figure are mean ± SE. Lowercase letters above the bars indicate significant differences (p < 0.05) in the male and female adults (ANOVA and Duncan’s new multiple range test).
3.7. Impact of Various Maize Cultivars on Circadian Activity Patterns of S. frugiperda Adults
Different strains of S. frugiperda exhibited a resting phase during light periods and an active phase during dark periods. The activity levels of all three strains significantly declined, starting at 8:00, and rose notably after 19:00. However, the Zhengdan 958 strain displayed a pronounced peak of activity between 7:00 and 8:00, marking the highest activity time of the day. The Zhenghuangnuo strain’s activity frequency initially increased slowly before stabilizing during the dark period. In contrast, the artificial diet feed strain’s activity frequency showed a gradual rise followed by a decrease in the dark period, peaking between 0:00 and 1:00. All three strains began to show increased activity before the onset of darkness, although the timings varied: Zhengdan 958 at 2 h, Zhenghuangnuo at 3 h, and the artificial diet strain at 1 h before the lights went out. The resting behavior of the adults in these strains was inversely related to their activity patterns, revealing higher and lower resting frequencies during light and dark periods, respectively.
Supplementary feeding behaviors in adult S. frugiperda predominantly occurred during dark periods, with negligible activity in light periods. The peaks for supplementary feeding were from 3:00 to 5:00 for the Zhengdan 958 strain, from 3:00 to 4:00 for the Zhenghuangnuo strain, and from 0:00 to 1:00 for the artificial diet feed strain.
Both the Zhengdan 958 and Zhenghuangnuo strains exhibited two mating peaks, whereas the artificial diet feed strain showed a single noticeable peak. The mating peaks for the Zhengdan 958 strain occurred between 20:00 and 21:00 and between 2:00 and 3:00, accounting for 10.10 and 6.06% of total mating events, respectively. The Zhenghuangnuo strain peaked at 18:00–19:00 and 2:00–3:00, representing 5.05 and 9.09%, respectively. The artificial diet feed strain’s mating peak was from 0:00 to 1:00, making up 5.05%. The mating patterns of the strains reared on maize were more concentrated, while those of the artificial diet feed strain were more dispersed.
In the Zhengdan 958, Zhenghuangnuo, and artificial diet feed strains, there were three (from 21:00 to 22:00, 00:00 to 01:00, and 02:00 to 03:00), two (from 21:00 to 22:00 and 01:00 to 02:00), and two spawning peaks (from 19:00 to 20:00 and 22:00 to 23:00), respectively. The spawning peaks for the two maize strains occurred approximately two hours later than those of the artificial diet feed strain (Figure 7).
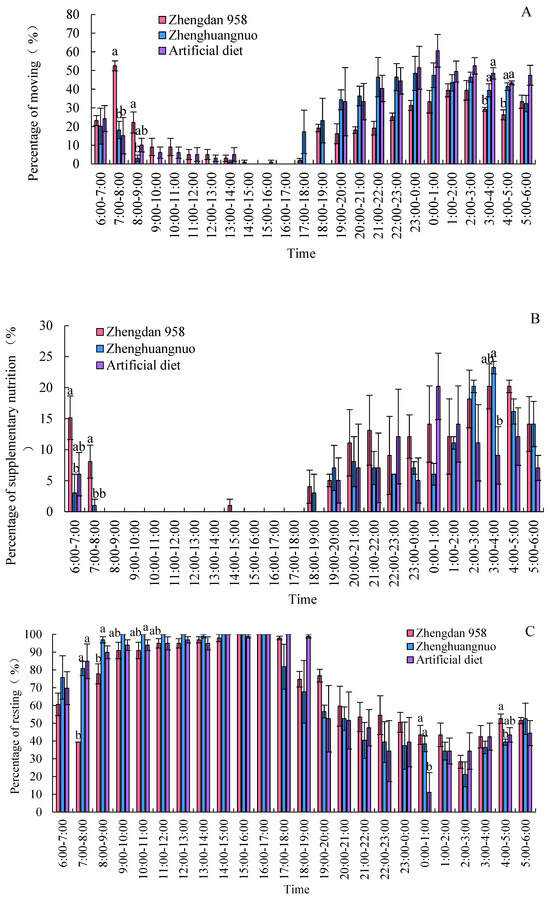
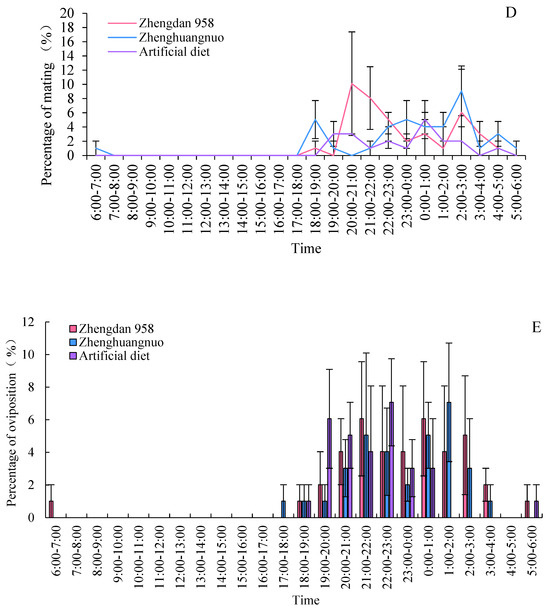
Figure 7.
Behavior rhythm of S. frugiperda adults. (A–C) Emergence, movement, and resting rhythms of S. frugiperda strains, respectively. (D,E) Mating and oviposition rhythms of different S. frugiperda strains, respectively. Data in figure are mean ± SE. Different lowercase letters above bars indicate significant differences (p < 0.05) in the different strains of S. frugiperda adults (ANOVA and Duncan’s new multiple range test).
3.8. Effects of Different Maize Cultivars on Mating Duration of Adult S. frugiperda
The average mating duration for S. frugiperda across the three strains was at least 60 min. Specifically, the average mating times were 143.73 ± 15.53, 1286.45 ± 20.66, and 70.87 ± 2.45 min for the Zhengdan 958, Zhenghuangnuo, and artificial diet strains, respectively. The mating duration for the Zhenghuangnuo strain was significantly longer than those of the Zhengdan 958 and artificial diet strains (p = 0.02) (Figure 8).
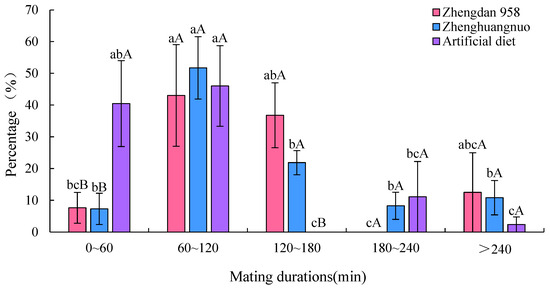
Figure 8.
Mating duration times of S. frugiperda adults. The lowercase letters in the diagram indicate the significance of differences in mating time within the same strain, while the uppercase letters denote the significance of differences in the percentage of mating time among various strains during the same interval.
3.9. Daily Time Allocation of Adult Behavior of S. frugiperda
The adult S. frugiperda exhibited the highest proportion of resting behavior among the various strains, which was significantly greater than that of other behaviors (p < 0.05). This was followed by moving behavior, which was also significantly more prevalent than supplementary nutrition, mating, and oviposition behaviors (p < 0.05). Specifically, the resting behavior proportions for the Zhengdan 958, Zhenghuangnuo, and artificial diet strains were 69.74, 68.77, and 68.86%, respectively. The three strains showed limited mating and oviposition behaviors, and there were no significant differences in the distribution of various behaviors across the strains (Figure 9).
Figure 9.
The time allocation of behavior of S. frugiperda adults. The lowercase letters above the bars indicate significant differences in the proportion of time allocation of each behavior among the same strain.
3.10. Effects of Different Maize Cultivars on Ovarian Development of S. frugiperda
After nine generations of continuous feeding on two different cultivars of corn, the newly emerged female moths were examined through ovarian dissection. The ovary weights were 13.80, 22.10, and 17.40; the fat body weights were 12.20, 13.80, and 6.7; the fat body contents % were 35.22, 43.36, and 31.54%; and the ovarian tube lengths were 39.95, 41.98, and 35.66 for Zhengdan 958, Zhenghuangnuo, and the artificial diet, respectively. The results indicated that the corn cultivars did not significantly influence the ovarian development stage of S. frugiperda. When fed corn leaves and an artificial diet, both strains of S. frugiperda displayed an ovarian development grade of I (the transparent milky stage). However, the strain of sweet corn exhibited a superior ovarian tube length compared with the other two strains. Conversely, the fat body weight and fat body content of the sweet and normal corn strains were significantly heavier than those of the artificial diet strain (p < 0.05) (Table 2, Figure 10).

Table 2.
Effects of different maize cultivars on ovarian development of S. frugiperda.
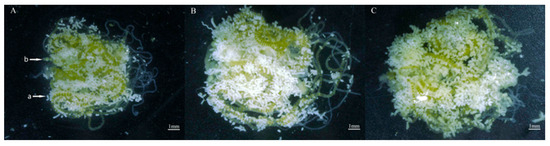
Figure 10.
Ovaries of S. frugiperda reared on two types of corn leaves and artificial diet. (A) Zhengdan 958 strain; (B) Zhenghuangnuo strain; (C) artificial diet feed strain. a: fat body; b: eggs. Bar = 1 mm.
3.11. Effects of Different Maize Cultivars on the Expression of Rhythm Genes in Different Tissues of S. frugiperda Adults In Vitro, as Studied by RT-qPCR
The expression levels of four circadian clock genes in various strains and tissues of S. frugiperda were examined using RT-qPCR. Significant differences were observed in the expression of per, clk, cry, and cyc genes across different strains and tissues. In the artificial diet strain, the per gene exhibited the highest expression in flight muscle, followed by the head and antennae. The clk and cry genes showed their highest expression in the antennae, while the cyc gene was predominantly expressed in the flight muscle. For male insects, the per gene had peak expression in the head, with the clk gene also highest in the antennae. The cry and cyc genes were most highly expressed in the antennae, followed by the head.
In the Zhengdan 958 strain, the per gene also showed the highest expression in the flight muscle, followed by the head and antennae. The clk gene had the highest expression in the antennae, while the cry gene was most abundant in the antennae as well, followed by the head. The cyc gene peaked in the flight muscle, followed by the antennae and head. Additionally, the per gene’s expression was highest in the testis, followed by the head. The clk gene had the highest expression in the antennae, the cry gene was most expressed in the testis, and the cyc gene peaked in the foot.
In the Zhenghuangnuo strain, the per gene exhibited the highest expression in flight muscles, followed closely by the head. The clk, cry, and cyc genes showed their peak expression levels in the antennae. For males, the expression of per, clk, and cry genes was also highest in the antennae, while the cyc gene had the highest expression in the head, followed by the antennae. Across the three strains of S. frugiperda, the four circadian clock genes were predominantly expressed in the antennae and head. The expression patterns of these circadian clock genes in females were largely consistent across different strains. In males, the expression patterns of the artificial diet and Zhenghuangnuo strains were similar, differing from those observed in the Zhengdan 958 strain (Figure 11).
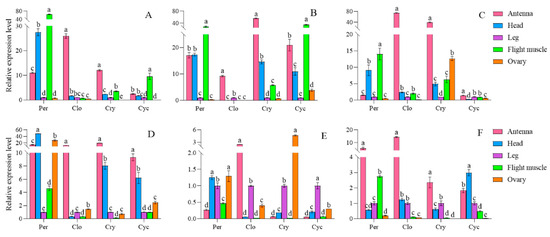
Figure 11.
Relative expression levels of circadian clock genes in different tissues of S. frugiperda strains. (A–F). Relative expression levels of circadian clock genes (clk, cry, and cyc genes) in female and male adults of artificial diet, Zhengdan 958, and Zhenghuangnuo strains, respectively. The lowercase letters above the bars indicate the significance of differences in gene expression in different tissues.
The expression levels of the four circadian clock genes varied across different strains and anatomical regions. In comparison to females from the artificial diet strain, per gene expression in the antennae, head, and feet of the Zhengdan 958 strain was higher and lower in the flight muscles and ovaries, respectively. For the Zhenghuangnuo strain, expression levels of the per gene decreased in the antennae, head, flight muscles, and ovaries but increased in the leg. The clk gene showed increased expression in the antennae and leg of the Zhengdan 958 strain, while it decreased in the head, flight muscle, and ovary. However, in the Zhenghuangnuo strain, clk gene expression was elevated in the antennae but reduced in the head, leg, flight muscle, and ovary. The expression levels of both cry and cyc genes in the Zhengdan 958 and Zhenghuangnuo strains decreased in the antennae, head, leg, and flight muscle, while they increased in the ovary.
When compared to males from the artificial diet strain, per gene expression in the antennae and head of the Zhengdan 958 strain decreased but increased in the feet, flight muscle, and testis. In the Zhenghuangnuo strain, expression levels were elevated in the antennae, leg, and flight muscles, while they decreased in the head and testis. clk gene expression rose across all tissues in the Zhengdan 958 strain. In the Zhenghuangnuo strain, it increased in the antennae, head, and leg, while it decreased in the flight muscle and testis. The levels of cry and cyc genes were reduced in the antennae and head of the Zhengdan 958 strain, yet they increased in the legs, flight muscles, and testes. In the Zhenghuangnuo strain, the expression was enhanced in the antennae, head, leg, and flight muscle, while it decreased in the testis (Figure 12).
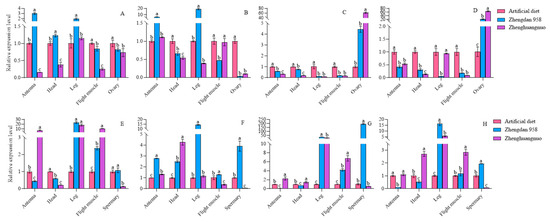
Figure 12.
The relative expression of circadian clock genes in adults of the S. frugiperda strain. The expression levels of Per, Clk, Cry, and Cyc in female adults are represented by (A–D), while those in male adults are represented by (E–H), respectively. The lowercase letters above the bars indicate the significance of differences in gene expression in different strains.
4. Discussion
The majority of insects display distinct circadian rhythms in their emergence behavior. Nevertheless, there are significant variations in the eclosion patterns across different insect species. Typically, Lepidopteran insects emerge at night, but certain species have peak emergence times during the day. For example, Lymantria dispar asiatica Vnukovskij’s peak activity is between 7:00 and 10:00 [24], whereas that of L. xylina occurs between 10:00 and 12:00 and again from 14:00 to 16:00 [25]. The peak activity of Cydia pomonella Linnaeus is at 8:00–11:00 and 9:00–13:00 [26], while that of Plutella xylostella Linnaeus is at 6:00 and 10:00 [27]. Iragoides fasciata Moore, on the other hand, primarily emerges in the afternoon, specifically between 14:00 and 19:00 [28].
The eclosion rhythm of a single insect species can be influenced by various external factors, with different photoperiod treatments resulting in altered rhythms. For example, Antheraea pernyi Guérin-Meneville exhibited changes in its eclosion rhythm under varying photoperiods, with continuous light treatment showing no distinct periodicity [29]. P. xylostella subjected to photoperiod reversal lacked a regular emergence pattern [27], and H. armigera also displayed a reversed rhythm, primarily emerging during the day [30]. Additionally, plant secondary metabolites can impact insect rhythms; for instance, the methanol extract from indica rice affects serotonin levels in S. frugiperda, subsequently altering its behavioral and intestinal peristalsis rhythms [31].
Among the three strains, the emergence of female adults of S. frugiperda occurred earlier than that of males, aligning with observations in S. litura [32] and Spodoptera exigua Hübner [33]. However, in the cases of L. dispar asiatica, L. xylina, C. pomonella, P. xylostella, and I. fasciata, males preceded females in emergence [20,21,22,23,24,25,26,27,28]. This suggests that there were notable variations in the eclosion patterns among different Lepidopteran species.
The behavioral rhythms across the three strains varied, but resting was the predominant behavior observed. During the light period, all strains primarily rested with minimal activity, similar to observations in Spodoptera litura Fabricius [32] and Athetis dissimilis Hampson [34]. Although behavioral rhythms differ among insects, time allocation is primarily aimed at reducing energy expenditure [35].
S. frugiperda feeding on different corn cultivars over multiple generations resulted in variations in the ovarian development of newly emerged females. Those fed on the artificial diet exhibited a greater ovarian weight and length compared to those fed two types of corn leaves. S. frugiperda adults that consumed Zhenghuangnuo leaves had a significantly higher fat body weight and content than those fed on Zhengdan958 and the artificial diet. This suggests that the higher sugar content in Zhenghuangnuo leaves provides more nutrients conducive to fat accumulation during the larval stage. The artificial diet, rich in various nutrients and vitamins, supports ovarian development, leading to a superior ovary weight and length compared to the corn leaf strains. S. frugiperda adults exhibited different behavioral rhythms when feeding on various hosts, particularly in mating behavior, as noted in studies of S. litura [32] and Chilo suppressalis Walker [36,37]. Pashley identified differences in the mating rhythms between maize-type and rice-type S. frugiperda, which showed a preference for mating with their respective types [38]. These findings emphasize that the host plants on which insects feed can significantly influence their behavioral rhythms [33]. Recognizing these differences in the behavioral rhythms of pests on various host plants is essential for effective forecasting, prevention, control, and sexual trapping.
Xie discovered that the HoPer gene in Holotrichia oblita Faldermann exhibited high expression levels in the head and antennae, with female adults showing the highest expression in the antennae, while male adults had the highest expression in the head [39]. In Bombyx mori Linnaeus, the cry gene was predominantly expressed in the head antennae and flight muscles [40]. Ji investigated the expression of four circadian clock genes in Mythimna separata Walker, revealing that these genes were primarily expressed in the antennae and head, with varying expression patterns across different tissues [41]. The findings of this study indicated distinct expression levels of the four circadian clock genes in various strains of S. frugiperda adults across different tissues, predominantly in the antennae and head, aligning with previous results. Unlike Ji’s study, which showed similar expression patterns for the same genes in male and female adults of M. separata, this study found variations in expression patterns between genders, consistent with Xie’s findings. In H. armigera, the cry1 gene was found to be highly expressed in the abdomen and less so in the antennae, while the cry2 gene showed high expression in the thorax and low expression in the head [42]. In C. suppressalis, the cry1 gene had high expression in the antennae, and the cry2 gene was significantly expressed in the abdomen, followed by the head, with lower levels in the leg [43]. The silkworm exhibited high expression of the cry1 gene in its head and antennae, while the cry2 gene was more highly expressed in the head and flight muscle [40], indicating that the expression patterns of circadian clock genes vary across species.
Sweet and normal maize were used for the continuous rearing of S. frugiperda over nine generations. The expression levels of circadian clock genes in the two corn cultivars significantly differed from those in the artificial diet strain. The four circadian clock genes exhibited a decreasing trend in expression levels within the tissues of the two corn strains compared to the artificial diet strain, whereas the expression levels in male insects of the two strains showed an increasing trend. The expression patterns of the cry and cyc genes in the two maize strains were similar to those of the artificial diet strain. After multiple generations of continuous feeding, the expression differences in biological clock genes between the two maize strains and the artificial diet strain considerably increased compared to the first generation, indicating that prolonged feeding on different hosts amplifies the expression differences in insect biological clock genes. Dres and Mallet found that the same herbivorous insect can adapt to various host plants, with differing internal physiology, external morphology, and behavior among host strains potentially leading to reproductive isolation and the formation of distinct host strains within the same region [44]. Feeding on diverse hosts can also result in changes to insect-reproduction-related genes. Yao identified variations in the expression of the vitellogenin gene in S. frugiperda when feeding on different hosts [45], and similar differences were noted in S. litura [46]. This suggests that herbivorous insects feeding on various host plants can influence the expression of numerous genes, thereby impacting their behavior, reproduction, and other life processes. The results of this study demonstrate that S. frugiperda feeding on different host plants leads to variations in various behavioral rhythms. Further research has indicated that there are differences in the expression of circadian clock genes among adults of S. frugiperda from different host strains. This necessitates an additional investigation into the mechanisms behind these differences and highlights the importance of considering the influence of different hosts when predicting and implementing comprehensive pest control measures.
5. Conclusions
Interest in trap cropping, a traditional pest management tool, has increased significantly in recent years [47]. Attracting the major invasive pests- S. frugiperda by growing sweet and grain corn at the same time is a novel idea. In the Huang–Huai–Hai region of China, grain corn is mainly grown, and sweet and sticky corn can be used as a lure plant. Our results demonstrate that this dual-cropping approach might effectively lure S. frugiperda away from the main maize, thereby reducing pest pressure and the need for chemical pesticides. This method not only preserves the environment but also supports sustainable agriculture [48]. Future research should focus on optimizing trap crop placement, variety selection, and timing to maximize its effectiveness. Overall, sweet corn as a trap crop presents a promising alternative to conventional pest management strategies. The initial generation of S. frugiperda was cultivated using leaves from normal corn and sweet waxy corn. Compared to the strain fed an artificial diet, the behavioral rhythms of the three strains differed; however, they all exhibited activity during the resting dark period within the light phase. The RT-qPCR results are consistent with the behavioral rhythm findings, revealing variations in circadian clock gene expression among the three strains. After nine generations of continuous feeding on different maize leaf cultivars, behavioral rhythm differences persisted among the strains, though the time allocation for each behavior remained consistent, with resting behavior occupying the majority of the time. RT-qPCR analysis indicated that the expression levels of four circadian clock genes varied across different tissues in adult S. frugiperda, with the highest expression primarily found in the antennae and head. Significant differences in circadian clock gene expression were observed between the two maize cultivar strains and the artificial diet strain. In female insects from the two maize strains, the expression of the four circadian clock genes showed a decline compared to that in the artificial diet strain, while male insects from the two maize strains exhibited increased expression. The expression patterns of the cry and cyc genes in the two maize strains were similar to those in the artificial diet strain.
After the establishment of FAW, it will feed on corn for a long time, and using its rhythm difference will provide a scientific basis for field pest control. The results of this study emphasize the importance of understanding the feeding rhythms of the FAW in corn fields. By recognizing and exploiting these rhythms, farmers and pest control experts can develop more effective strategies to manage and mitigate the damage caused by this pest. Additionally, the findings contribute to the broader field of entomology by adding to our understanding of insect behavior and its ecological implications. Future research could further explore the potential of using rhythm differences in combination with other biological, cultural, or chemical control methods to enhance the overall effectiveness of pest management programs. Our findings suggest that the dietary environment, particularly the type of maize leaf consumed, can significantly influence the circadian clock gene expression and behavioral rhythms of S. frugiperda. The persistence of behavioral rhythm differences across generations highlights the potential for adaptive changes in the insects’ circadian systems in response to dietary cues. The tissue-specific expression patterns of circadian clock genes indicate that these genes may play different roles in different parts of the insect body, with the antennae and head potentially being key regions for circadian regulation. The observed sex-specific differences in circadian clock gene expression between the maize strains and the artificial diet strain suggest that dietary effects on circadian rhythms may also be sex-dependent. Overall, our study provides insights into the interplay between the dietary environment and circadian rhythms in insects, which may have implications for understanding the ecological and evolutionary consequences of dietary specialization in insects.
Our findings underscore the complexity of circadian regulation in insects and highlight the need for further research to fully elucidate the mechanisms underlying these interactions. Additionally, the observed sex-specific differences in circadian clock gene expression suggest that gender should be considered as a factor in studies examining the effects of the diet on circadian rhythms.
Author Contributions
C.T., X.Y., S.W. and H.F.: conceptualization. C.T., J.Z., G.L. and J.H.: experiment. C.T. and J.Z.: data processing and graphing. C.T.: writing the original draft. X.Y. and H.F.: review and editing. All authors contributed to the article and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the National Key Research and Development Plan (2021YFD1400701), the Program of Zhongyuan Leading Talents for Scientific and Technological Innovation (244200510036), the Independent Innovation Project of the Henan Academy of Agricultural Sciences (2025ZC52); Scientific and Technological Breakthrough Foundation of Henan Province (232103810016; 222102240057), the National Industrial Technology System (CARS-27), the Henan Province Joint Fund (235101610045), and the Central Plains Scholars Program (254000510002).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We extend our profound gratitude to the editors and reviewers for their meticulous evaluation and constructive suggestions. We are particularly thankful to the Academic Editor, for his exceptional expertise and crucial guidance in the enhancement of our research paradigm and his dedicated efforts in refining our perspectives.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pittendrigh, C.S. Temporal organization: Reflections of a Darwinian clock-watcher. Annu. Rev. Physiol. 1993, 55, 16–54. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Molecular neurobiology and genetics of circadian rhythms in mammals. Annu. Rev. Neurosci. 1995, 18, 531–553. [Google Scholar] [CrossRef]
- Wu, S.H.; Xiang, Q.; Xue, F.S. Circadian behavioral rhythms in insects. Jiangxi Plant Prot. 2006, 4, 147–157. [Google Scholar]
- Tu, X.Y.; Chen, Y.S. Circadian Behavioral Rhythms in Moths. Biol. Disaster Sci. 2013, 36, 18–21. [Google Scholar]
- Guo, J.F.; He, K.L.; Wang, Z.Y. Biological characteristics, trend of fall armyworm Spodoptera frugiperda, and the strategy for management of the pest. J. Appl. Entomol. 2019, 56, 361–369. [Google Scholar]
- Lu, Z.H.; He, S.Q.; Yan, N.S.; Zhao, W.J.; Yao, W.F.; Chen, Y.P.; Yang, T.; Jiang, Y.Y.; Gui, F.R. Effects of temperatures on the development and reproduction of fall armyworm (Spodoptera frugiperda Smith). Plant Protect. 2019, 45, 27–31+53. [Google Scholar]
- Zhang, H.M.; Yin, Y.Q.; Zhao, X.Q.; Li, X.Y.; Wang, Y.; Liu, Y.; Chen, F.S.; Chen, A.D. The growth and development characteristics of Spodoptera frugipetda under different temperature conditions. J. Environ. Entomol. 2020, 42, 52–59. [Google Scholar]
- Meng, L.H.; Jiang, X.F.; Li, P.; Xia, J.X.; Zhang, T.Q.; Cheng, Y.X.; Zhang, L. Comparison of bisexual life tables of Spodoptera frugiperda in different photoperiods. Plant Protect. 2022, 48, 63–73. [Google Scholar]
- Zhang, L.Y.; Wang, F.; Wan, X.S.; Xu, J.; Ye, H. Reproductive behavior and circadian rhythms of Spodoptera frugiperda. J. Environ. Entomol. 2022, 44, 509–522. [Google Scholar]
- Li, D.Y.; Zhi, J.R.; Zhang, T.; Ye, J.Q.; Yu, Y.C.; Hu, C.X. Preference of Spodoptera frugiperda to four host plants. Plant Protect. 2019, 45, 50–54. [Google Scholar]
- Fang, M.; Yao, L.; Tang, Q.F.; Li, G.T.; Jiang, X.C. Feeding adaptability of fall armyworm Spodoptera frugiperda to several weeds. J. Plant Prot. 2020, 47, 1055–1061. [Google Scholar]
- Li, D.Y.; Zhi, J.R.; Zhang, T.; Ye, J.Q.; Liang, Y.J. Effects of different host plants on the development and reproduction of Spodoptera frugiperda. J. Environ. Entomol. 2020, 42, 311–317. [Google Scholar]
- Liu, H.; Zhang, Y.; Chen, J.L. Feeding preference and adaptability of Spodoptera frugiperda (Lepidoptera: Noctuidae) on different wheat cultivars in relation to leaf biochemical contents. Acta Entomol. Sin. 2021, 64, 230–239. [Google Scholar]
- Zhang, Y.H.; Zhang, Z.; Liu, J.; Jiang, Y.Y.; Li, X.R.; Ba, T.X.; Chen, Z.Y.; Lin, P.J.; Huang, H.J. Oviposition and feeding preference of Spodoptera frugiperda to gramineous weeds. Plant Protect. 2021, 47, 117–122+147. [Google Scholar]
- Badenes-Pérez, F.R.; Hokkanen, H.M.T. Advances in trap cropping. Arthropod-Plant Interact 2024, 18, 1147–1149. [Google Scholar] [CrossRef]
- Hall, J.C.; Rosbash, M. Genes and biological rhythms. Trends Genet. 1987, 3, 185–191. [Google Scholar] [CrossRef]
- Petersen, G.; Hall, J.C.; Rosbash, M. The period gene of Drosophila carries species-specific behavioral instructions. EMBO J. 1988, 7, 3939–3947. [Google Scholar] [CrossRef]
- Murua, M.G.; Vera, M.T.; Abraham, S.; Juarez, M.L.; Prieto, S.; Head, G.P.; Willink, E. Fitness and mating compatibility of Spodoptera frugiperda (lepidoptera: Noctuidae) populations from different host plant species and regions in argentina. Ann. Entomol. Soc. Am. 2008, 101, 639–649. [Google Scholar] [CrossRef]
- Cruz-Esteban, S.; Rojas, J.C.; Malo, E.A. Calling Behavior, Copulation Time, and Reproductive Compatibility of Corn-Strain Fall Armyworm (Lepidoptera: Noctuidae) From Populations in Mexico. Environ. Entomol. 2017, 46, 901–906. [Google Scholar] [CrossRef]
- Liu, J.; Tallat, M.; Wang, G.S.; Li, Z.; Li, G.P.; Zhao, X.C.; Feng, H.Q. Courtship Behavior of Adult Spodoptera frugiperda (Lepidoptera: Noctuidae) Observed Using Track 3D Trajectory Tracking. Insects 2024, 15, 824. [Google Scholar] [CrossRef]
- Cunningham, J.P.; Jallow, M.F.A.; Wright, D.J.; Zalucki, M.P. Learning in host selection in Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Anim. Behav. 1998, 55, 227–234. [Google Scholar] [CrossRef][Green Version]
- Wu, C.X.; Yang, M.F.; Yao, M.M.; Zeng, Z.H. Diurnal activity rhythm and time budgets of adult behavior of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) after paired. J. Plant Protect. 2016, 43, 979–985. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xue, Y.; Jiang, D.; Jiang, H.; Yang, L.N.; Yan, S.C. Circadian Rhythm of Emergence and Reproduction of the Asian Gypsy Moth (Lymantria dispar asiatica, AGM). J. Northeast For. Univ. 2017, 45, 111–116. [Google Scholar]
- Zuo, C.; Lin, J.C.; Zhang, J.F.; Zhang, Z.H.; Wang, J.D.; Wang, R. Observation of emerging and reproductive behavior rhythm of Lymantria xylina. Agric. Sci. J. Yanbian 2020, 42, 37–43. [Google Scholar]
- Wei, Y.H.; Luo, J.C.; Liu, Y.Y.; Zhou, Z.X.; Zhang, D.W. Circadian rhythm of adult eclosion, oviposition and hatching in the codling moth, Cydia pomonella. Plant Prot. 2014, 40, 143–146. [Google Scholar]
- Tian, H.J.; Xu, R.B.; Chen, Y.; Chen, Y.X.; Lin, S.; Yu, Y.; Yang, G. Effect of photoperiod on the timing of eclosion in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). J. Appl. Entomol. 2022, 59, 75–83. [Google Scholar]
- Huang, A.P.; Bao, Q.; Wang, Y.J.; Zhou, L.Y.; Zeng, Z. Diurnal Rhythms of Eclosion in Iragoides fasciata Moore (Lepidoptera: Eucleidae). J. Tea Commun. 2016, 43, 28–30. [Google Scholar]
- Truman, J.W. The role of the brain in the ecdysisrhythm of silkmoths: Comparison with the photoperiodictermination of diapause. In Biochronometry; Menaker, M., Ed.; National Academy of Sciences: Washington, DC, USA, 1971; pp. 483–504. [Google Scholar]
- Sun, X.T.; Xu, R.B.; Ge, S.S.; Fu, X.W.; Zhao, X.C.; Wu, K.M. Effects of photoperiod on the eclosion, reproduction and flight performance of Helicoverpa armigera (Lepidoptera: Noctuidae). J. Environ. Entomol. 2019, 41, 1045–1056. [Google Scholar]
- Oyarzabal, A.E.; Alquicira, M.J.; Zuniga, R.B.; Arreola, R.J.L.; Guevara, F.P.; Lara, F.C.O.; Escamilla, C.E.G. Effect of Azadirachta indica A. Juss (Meliaceae) on the serotonin rhythm of Spodoptera frugiperda (Lepidoptera: Noctuidae). Chronobiol. Int. 2021, 38, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.X.; Yang, M.F.; Zeng, Z.H.; Yao, M.M.; Liao, Q.R. Diurnal rhythm of reproductive behavior of cotton leafworm Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) feeding on different hosts. J. Plant Protect. 2015, 42, 210–216. [Google Scholar]
- Li, J.X.; Li, J.; Cheng, W.X.; Liu, Y.L.; Liu, H.; Wang, J.J. The Bionomics of Adult Beet Armyworm, Spodoptera exigua (Hübner). Chin. Agric. Sci. Bulletin 2008, 5, 318–322. [Google Scholar]
- Dong, S.Q.; Tian, C.H.; Guo, X.R.; Yuan, X.X.; Tang, J.R.; Wang, X.H.; Zhao, M. Adult activity and hatching rhythms of Athetis dissimilis (Lepidoptera, Noctuidae). J. Appl. Entomol. 2021, 58, 398–407. [Google Scholar]
- Yang, M.F.; Yang, D.X.; Xu, J.; Liu, J.F.; Wu, C.X. Diurnal rhythm of adult behavior of the rice water weevil, Lissorhoptrus oryzophilus (Coleoptera: Curculionidae). Acta Entomol. Sin. 2013, 56, 952–959. [Google Scholar]
- Quan, W.L. Host Plant-Associated Population Divergence and Endogenous Mechanism of Mating Rhythm Variation in Chilo suppressalis. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2017. [Google Scholar]
- Sun, L.J.; Dai, H.G.; Yi, W.X.; Lu, Y.Q. The adult emergence rhythm and mating rhythm of rice host population and water-oats host population of the rice stem borer, Chilo suppressalis. Insects Knowl. 2002, 39, 421–423. [Google Scholar]
- Pashley, D.P.; Hammond, A.M.; Hardy, T.N. Reproductive Isolating Mechanisms in Fall Armyworm Host Strains (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1992, 4, 400–405. [Google Scholar] [CrossRef]
- Xie, M.H.; Zhong, Y.Z.; Lin, L.L.; Zhang, G.L.; Su, W.H.; Ni, W.L.; Chen, H.L. Cloning and expression profiling of circadian clock gene HoPer in Holotrichia oblita (Coleoptera: Melolonthidae). J. Environ. Entomol. 2024, 46, 471–479. [Google Scholar]
- Liang, H. Cloning and Functional Identification of Cry Genefrom Bombyx mori. Master’s Thesis, Soochow University, Suzhou, China, 2011. [Google Scholar]
- Ji, J.Y. Cloning, Expression and Functional Analysis of Clock Gene in Mythimna separata (Walker). Master’s Thesis, Northwest A&F University, Xianyang, China, 2017. [Google Scholar]
- Yan, S.; Ni, H.; Li, H.T.; Zhang, J.; Liu, X.X.; Zhang, Q.W. Molecular cloning, characterization, and mRNA expression of two Cryptochrome genes in Helicoverpa armigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 2013, 106, 450–462. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.L. Cloning and Expression Profile Analysis of Cryptochrome1 and Cryptochrome2 Genes in Chilo suppressalis. J. Anhui Sci. Technol. Univ. 2014, 28, 29–33. [Google Scholar]
- Drès, M.; Malle, J. Host races in plant-feeding insects and their importance in sympatric speciation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2002, 357, 471–492. [Google Scholar] [CrossRef]
- Yao, L. Effects of Two Host Plants on Fecundity and Vitellogenin Gene Expression of Spodoptera frugiperda. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2022. [Google Scholar]
- Xin, M.Y. Effects of Two Host Plants of Fecundity and Vitellogenin Gene Expression of Spodoptera litura Fabricius. Master’s Thesis, Yangzhou University, Yangzhou, China, 2016. [Google Scholar]
- Shelton, A.M.; Badenes-Perez, F.R. Concepts and applications of trap cropping in pest management. Annu. Rev. Entomol. 2006, 51, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Badenes-Perez, F.R. Plant–Insect Interactions: Host Plant Resistance, Biological Control, and Pollination. Plants 2025, 14, 1488. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).