Celosia argentea: Towards a Sustainable Betalain Source—A Critical Review and Future Prospects
Abstract
1. Introduction
2. Literature Search Strategy
3. Celosia argentea as a Source of Betalains
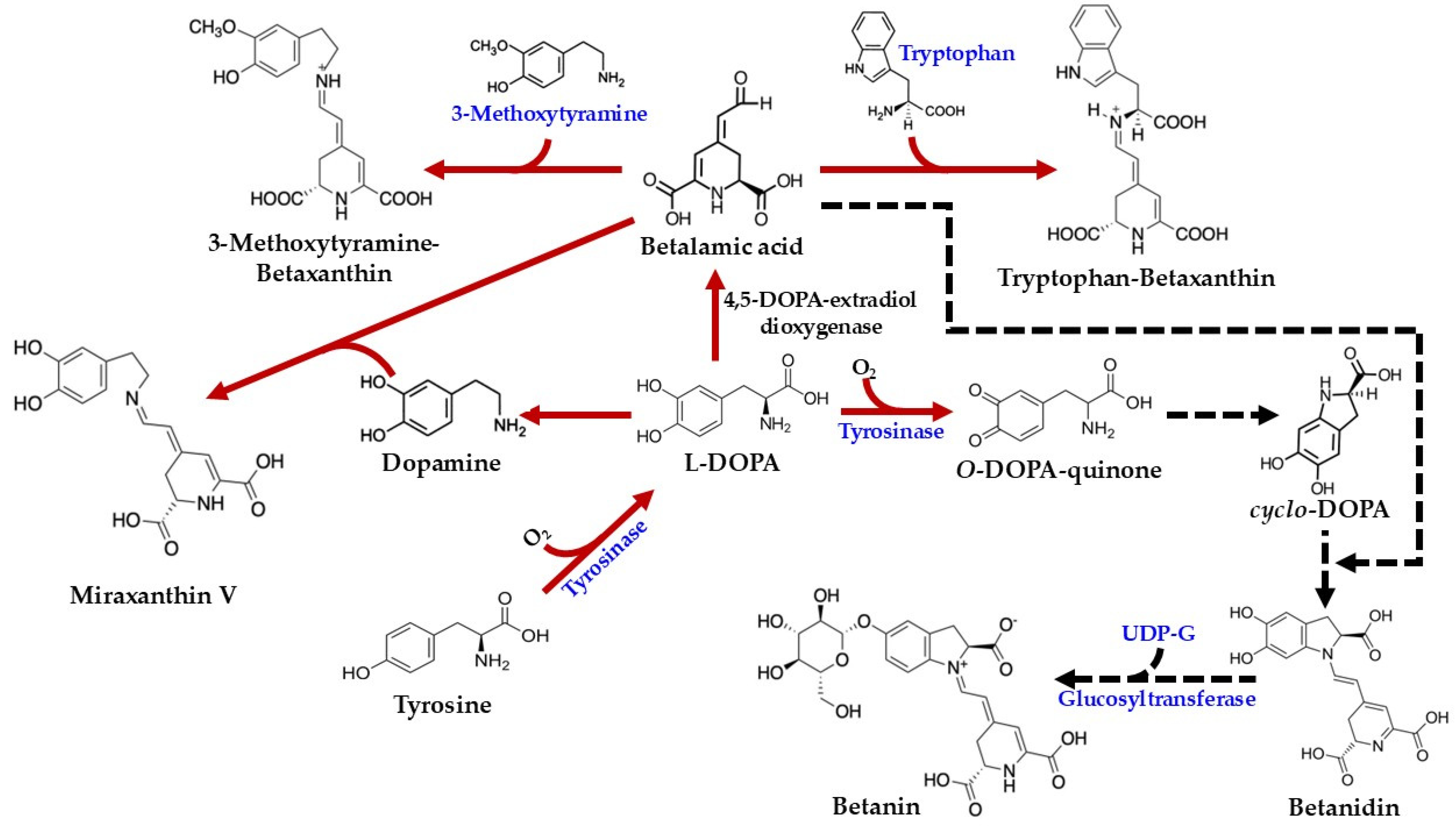
4. Biosynthesis Pathway of Betalains in C. argentea
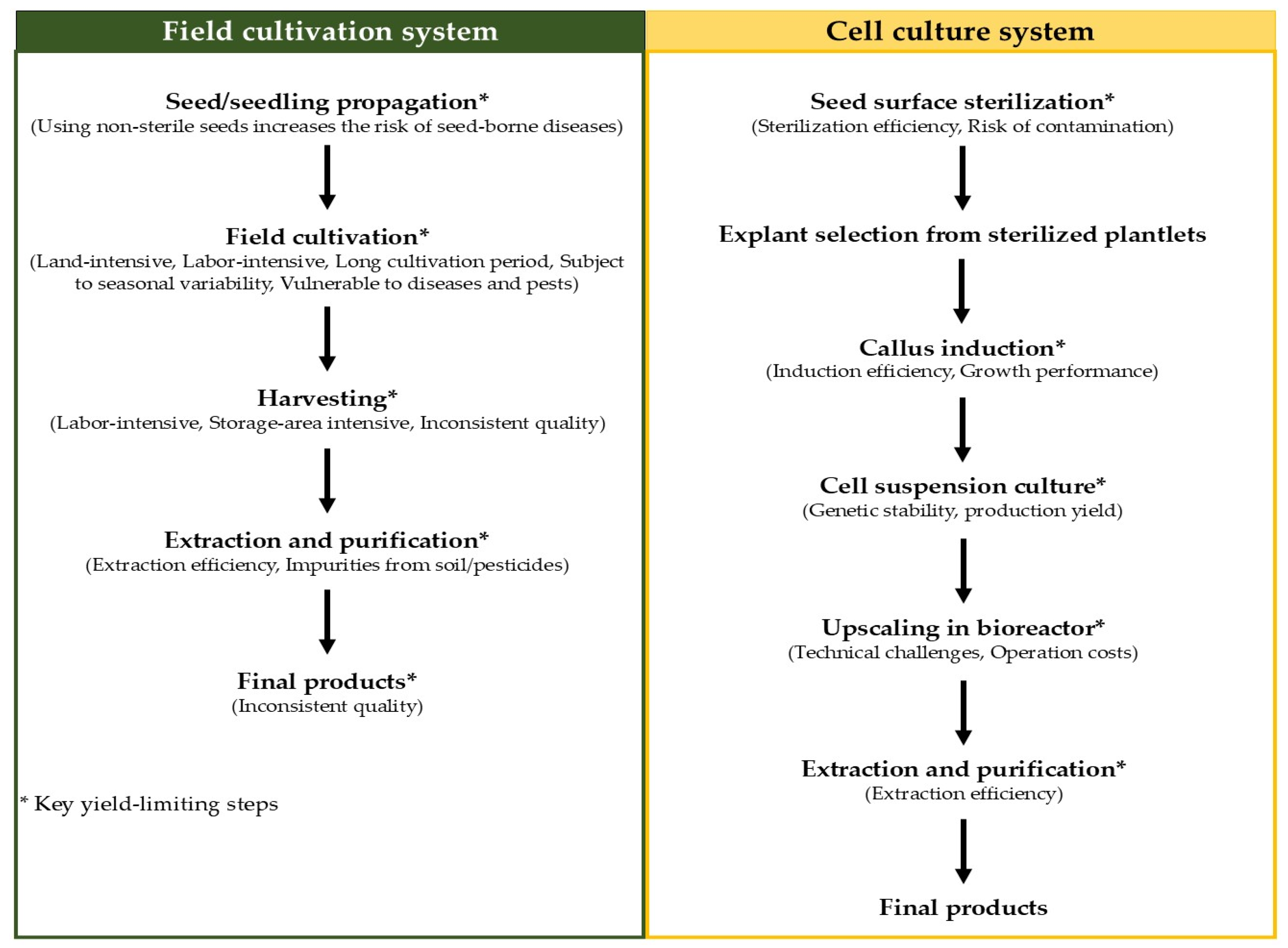
5. Production of Betalains from C. argentea
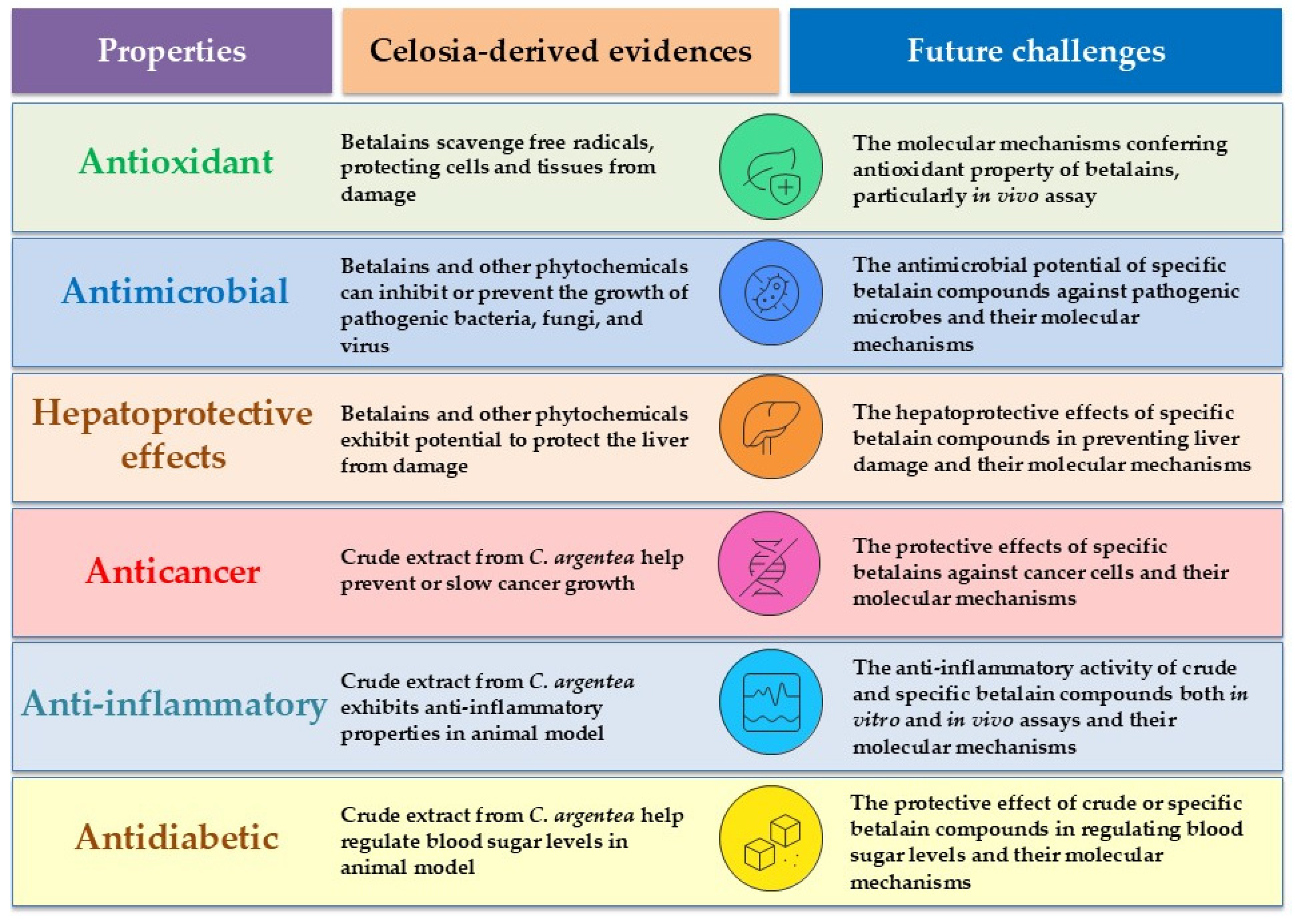
6. Biological Properties of C. argentea Betalains
6.1. Antioxidant Activity
6.2. Antimicrobial Activity
6.3. Hepatoprotective Effect
6.4. Other Pharmacological Properties
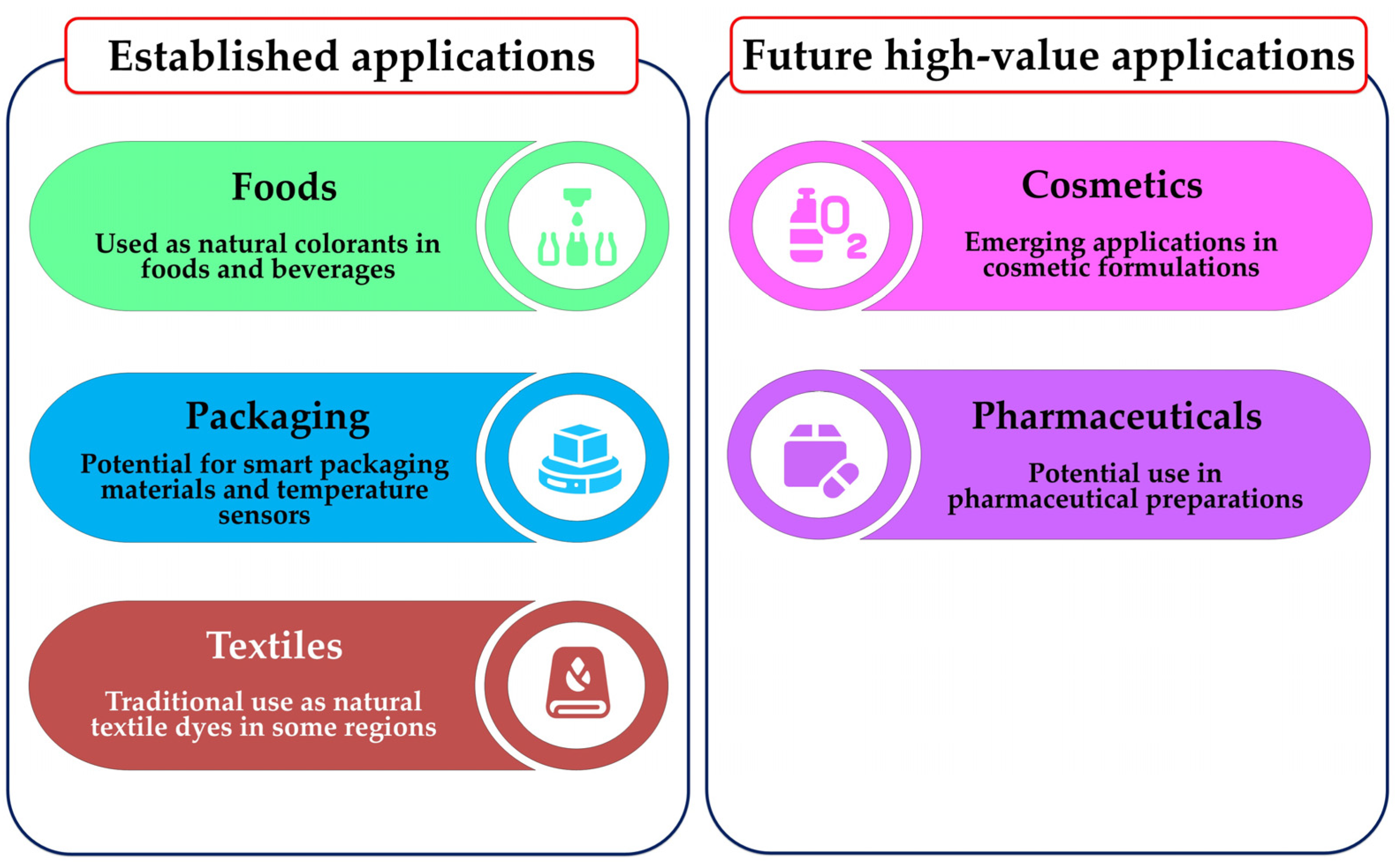
7. Applications of Betalains
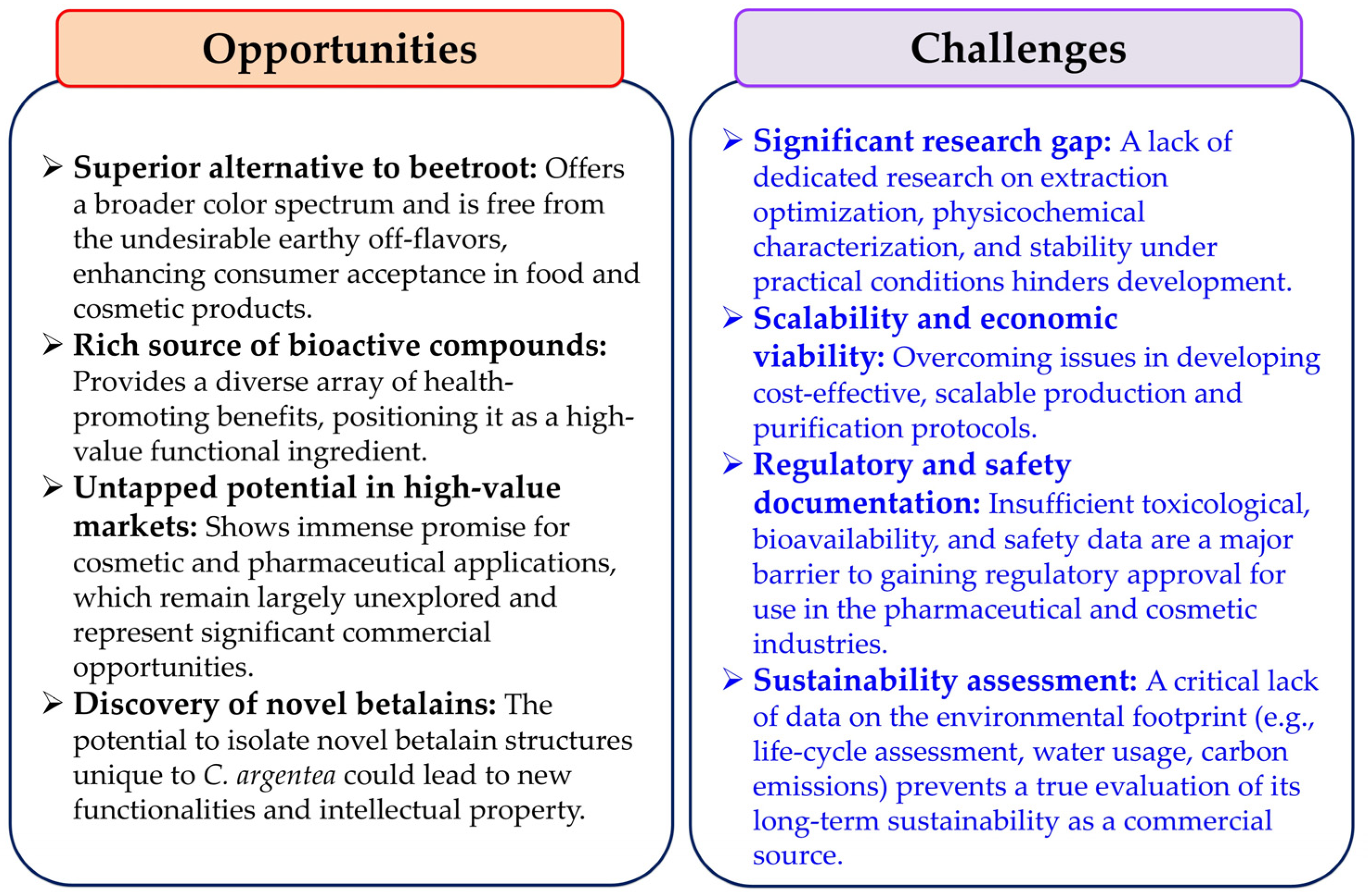
8. Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schliemann, W.; Cai, Y.; Degenkolb, T.; Schmidt, J.; Corke, H. Betalains of Celosia argentea. Phytochemistry 2001, 58, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Surse, S.N.; Shrivastava, B.; Sharma, P.; Gide, P.S.; Attar, S. Celosia cristata: Potent pharmacotherapeutic herb—A review. Int. J. Pharm. Phytopharm. Res. 2014, 3, 444–446. [Google Scholar]
- Nidavani, R.B.; Mahalakshmi, A.M.; Shalawadi, M. Towards a better understanding of an updated of ethnopharmacology of Celosia argentea L. Int. J. Pharm. Pharm. Sci. 2013, 5, 54–59. [Google Scholar]
- Miguel, M.G. Betalains in some species of the Amaranthaceae family: A review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef]
- Tang, Y.; Xin, H.L.; Guo, M.L. Review on research of the phytochemistry and pharmacological activities of Celosia argentea. Braz. J. Pharmacogn. 2016, 26, 787–796. [Google Scholar] [CrossRef]
- Palada, M.C.; Crossman, S.M.A. Evaluation of tropical leaf vegetables in the Virgin Islands. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 388–393. [Google Scholar]
- Lock, M.; Grubben, G.J.H.; Denton, O.A. Plant resources of tropical Africa 2. Vegetables. Kew Bull. 2004, 59, 650. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Schliemann, W.; Corke, H. Chemical stability and colorant properties of betaxanthin pigments from Celosia argentea. J. Agric. Food Chem. 2001, 49, 4429–4435. [Google Scholar] [CrossRef]
- Rub, R.A.; Pati, M.J.; Siddiqui, A.A.; Moghe, A.S.; Shaikh, N.N. Characterization of anticancer principles of Celosia argentea (Amaranthaceae). Pharmacogn. Res. 2016, 8, 97–104. [Google Scholar] [CrossRef]
- Wu, Q.B.; Wang, Y.; Liang, L.; Jiang, Q.; Guo, M.L.; Zhang, J.J. Novel triterpenoid saponins from the seeds of Celosia argentea L. Nat. Prod. Res. 2013, 27, 1353–1360. [Google Scholar] [CrossRef]
- Showkat, S.; Rafiq, A.; Richa, R.; Sidique, Q.; Hussain, A.; Lohani, U.C.; Bhat, O.; Kumar, S. Stability enhancement of betalain pigment extracted from Celosia cristata L. flower through copigmentation and degradation kinetics during storage. Food Chem. X 2025, 26, 102312. [Google Scholar] [CrossRef]
- Zhang, H.C.; Zhang, T.T.; Du, B.; Cheng, D.Y.; Li, Z.G. Chemical constituents of Celosia cristata L. Chin. Trad. Pat. Med. 2014, 36, 122–125. [Google Scholar]
- Zheng, X.L.; Wei, J.H.; Sun, W.; Li, R.T.; Liu, S.B.; Dai, H.F. Ethnobotanical study on medicinal plants around Limu Mountains of Hainan Island, China. J. Ethnopharmacol. 2013, 148, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Sun, Z.L.; Guo, M.L.; Wang, Y.; Zhang, G. Two new compounds from Semen celosiae and their protective effects against CCl4-induced hepatotoxicity. Nat. Prod. Res. 2011, 25, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Rub, R.A.; Patil, M.J.; Shaikh, N.N.; Haikh, T.; Ahmed, J.; Siddiqui, A.A. Immunomodulatory profile of Celosia argentea–Activity of isolated compounds I and II. Int. J. Adv. Biotechnol. Res. 2015, 6, 270–277. [Google Scholar]
- Shen, S.; Ding, X.; Ouyang, M.A.; Wu, Z.J.; Xie, L.H. A new phenolic glycoside and cytotoxic constituents from Celosia argentea. J. Asian Nat. Prod. Res. 2010, 12, 821–827. [Google Scholar] [CrossRef]
- Bhujbal, S.S.; Chitlange, S.S.; Suralkar, A.A.; Shinde, D.B.; Patil, M.J. Anti-inflammatory activity of an isolated flavonoid fraction from Celosia argentea Linn. J. Med. Plant Res. 2008, 2, 52–54. [Google Scholar]
- Suzuki, H.; Morita, H.; Iwasaki, S.; Kobayashi, J. New antimitotic bicyclic peptides, celogentins D-H and J, from the seeds of Celosia argentea. Tetrahedron 2003, 59, 5307–5315. [Google Scholar] [CrossRef]
- Sun, Z.L.; Wang, Y.; Guo, M.L.; Li, Y.X. Two new hepaprotective saponins from Semen celosiae. Fitoterapia 2010, 81, 375–380. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Y.; Guo, M. Triterpenoid saponins from the seeds of Celosia argentea and their anti-inflammatory and antitumor activities. Chem. Pharm. Bull. 2011, 59, 666–671. [Google Scholar] [CrossRef]
- Pang, X.; Yan, H.X.; Wang, Z.F.; Fan, M.X.; Zhao, Y.; Fu, X.T.; Xiong, C.Q.; Zhang, J.; Ma, B.P.; Guo, H.Z. New oleanane-type triterpenoid saponins isolated from the seeds of Celosia argentea. J. Asian Nat. Prod. Res. 2014, 16, 240–247. [Google Scholar] [CrossRef]
- Morita, H.; Shimbo, K.; Shigemori, H.; Kobayashi, J. Antimitotic activity of moroidin, a bicyclic peptide from the seeds of Celosia argentea. Bioorg. Med. Chem. Lett. 2000, 10, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Suzuki, H.; Shimbo, K.; Takeya, K.; Morita, H. Celogentins A–C, new antimitotic bicyclic peptides from the seeds of Celosia argentea. J. Org. Chem. 2001, 66, 6626–6633. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Morita, H.; Shiro, M.; Kobayashi, J.I. Celogentin K, a new cyclic peptide from the seeds of Celosia argentea and X-ray structure of moroidin. Tetrahedron 2004, 60, 2489–2495. [Google Scholar] [CrossRef]
- Morita, H.; Suzuki, H.; Kobayashi, J. Celogenamide A, a new cyclic peptide from the seeds of Celosia argentea. J. Nat. Prod. 2004, 67, 1628–1630. [Google Scholar] [CrossRef]
- Zheng, Q.H.; Cui, X.; Zhou, P.; Li, S.L. A comparative study of fatty acids and inorganic elements in Semen celosiae and cockscomb. J. Chin. Med. Mat. 1995, 18, 466–467. [Google Scholar]
- Lin, W.Q.; Chen, Z.; Liu, J.Q. The chemical constituents of Perilla frutescens (L.) Britt. var. acute (Thunb.) and Celosia argentea L. seeds grown in Fujian province. Chin. Acad. Med. Mag. Org. 2002, 57–59. [Google Scholar]
- Fu, H.Z.; Meng, X.Y.; Li, S.S.; Wu, L.J. Study on the chemical constituents of Semen celosiae. Chin. Trad. Herb. Drugs 1992, 23, 344–345. [Google Scholar]
- Xue, Q.; Guo, M.; Zhang, G. Study of chemical constituents of Semen celosiae. Pharm. Care Res. 2006, 6, 345–347. [Google Scholar] [CrossRef]
- Odukoya, O.A.; Inya-Agha, S.I.; Segun, F.I.; Sodifiya, M.O.; Ilori, O.O. Antioxidant activities of selected Nigerian green leafy vegetables. Am. J. Food Technol. 2007, 2, 169–175. [Google Scholar] [CrossRef]
- Molehin, O.R.; Adefegha, S.A.; Oboh, G.; Saliu, J.A.; Athayde, M.L.; Boligon, A.A. Comparative study on the phenolic content, antioxidant properties and HPLC fingerprinting of three varieties of Celosia species. J. Food Biochem. 2014, 38, 575–583. [Google Scholar] [CrossRef]
- Wiart, C.; Mogana, S.; Khalifah, S.; Mahan, M.; Ismail, S.; Buckle, M.; Narayana, A.K.; Sulaiman, M. Antimicrobial screening of plants used for traditional medicine in the state of Perak, Peninsular Malaysia. Fitoterapia 2004, 75, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Vetrichelvan, T.; Jegadeesan, M.; Devi, B.A.U. Anti-diabetic activity of alcoholic extract of Celosia argentea Linn. seeds in rats. Biol. Pharm. Bull. 2002, 25, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Fujii, H.; Hase, K.; Ohnishi, Y.; Sakukawa, R.; Kadota, S.; Namba, T.; Saiki, I. Anti-metastatic and immunomodulating properties of the water extract from Celosia argentea seeds. Biol. Pharm. Bull. 1998, 21, 1154–1159. [Google Scholar] [CrossRef]
- Hase, K.; Kadota, S.; Basnet, P.; Takahashi, T.; Namba, T. Hepatoprotective effects of traditional medicines. Isolation of the active constituent from seeds of Celosia argentea. Phytother. Res. 1996, 10, 387–392. [Google Scholar] [CrossRef]
- Roon, T.S.A.; Klanrit, P.; Klanrit, P.; Thanonkeo, P.; Apiraksakorn, J.; Thanonkeo, S.; Klanrit, P. Establishment of betalain-producing cell line and optimization of pigment production in cell suspension cultures of Celosia argentea var. plumosa. Plants 2024, 13, 3225. [Google Scholar] [CrossRef]
- Mueangnak, K.; Kitwetcharoen, H.; Thanonkeo, S.; Klanrit, P.; Apiraksakorn, J.; Klanrit, P.; Klanrit, P.; Thanonkeo, P. Enhancing betalains production and antioxidant activity in Celosia argentea cell suspension cultures using biotic and abiotic elicitors. Sci. Rep. 2025, 15, 376. [Google Scholar] [CrossRef]
- Sidique, Q.; Shahi, N.C.; Lohani, U.C.; Kumar, A.; Prakash, O. Studies on the effect of extraction methods and process parameters on betalain content obtained from Celosia cristata L. flowers. Pharma Innov. J. 2023, 12, 1094–1098. [Google Scholar]
- Guadarrama-Flores, B.; Rodríguez-Monroy, M.; Cruz-Sosa, F.; García-Carmona, F.; Gandía-Herrero, F. Production of dihydroxylated betalains and dopamine in cell suspension cultures of Celosia argentea var. plumosa. J. Agric. Food Chem. 2015, 63, 2741–2749. [Google Scholar] [CrossRef]
- Thorat, B.R. Review on Celosia argentea L. plant. Res. J. Pharmacogn. Phytochem. 2018, 10, 109–119. [Google Scholar] [CrossRef]
- Divya, B.J.; Jyothi Sravani, M.; Hari Chandana, J.; Sumana, T.; Thyagaraju, K. Phytochemical and phytotherapeutic activities of Celosia argentea: A review. World J. Pharm. Pharmaceu. Sci. 2019, 8, 488–505. [Google Scholar] [CrossRef]
- Khan, M.I.; Harsha, P.S.C.S.; Chauhan, A.S.; Vijayendra, S.V.N.; Asha, M.R.; Giridhar, P. Betalains rich Rivina humilis L. berry extract as natural colorant in product (fruit spread and RTS beverage) development. J. Food Sci. Technol. 2015, 52, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Polturak, G.; Aharoni, A. “La Vie en Rose”: Biosynthesis, sources, and applications of betalain pigments. Mol. Plant 2018, 11, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Carreón-Hidalgo, J.P.; Franco-Vásquez, D.C.; Gómez-Linton, D.R.; Pérez-Flores, L.J. Betalain plant sources, biosynthesis, extraction, stability enhancement methods, bioactivity, and applications. Food Res. Int. 2022, 151, 110821. [Google Scholar] [CrossRef] [PubMed]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Production of betalains in plant cell and organ cultures: A review. Plant Cell Tissue Organ Cult. 2024, 158, 28. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; García-Carmona, F. Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 2013, 18, 334–343. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Abderrabba, M. Chemical and antioxidant properties of betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar] [CrossRef]
- Khan, M.I. Stabilization of betalains: A review. Food Chem. 2016, 197, 1280–1285. [Google Scholar] [CrossRef]
- Warhade, M.I.; Badere, R.S. Isolation of callus lines of Celosia cristata L. with variation in betalain content. J. Indian Bot. Soc. 2015, 94, 89–96. [Google Scholar]
- Warhade, M.I.; Badere, R.S. Fusarium oxysporum cell elicitor enhances betalain content in the cell suspension culture of Celosia cristata. Physiol. Mol. Biol. Plants 2018, 24, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Lystvan, K.; Kumorkiewicz, A.; Szneler, E.; Wybraniec, S. Study on betalains in Celosia cristata Linn. callus culture and identification of new malonylated amaranthins. J. Agric. Food Chem. 2018, 66, 3870–3879. [Google Scholar] [CrossRef] [PubMed]
- Mastuti, R.; Munawarti, A.; Siswanto, D. Effect of growth regulators on betalain profile in callus culture of Celosia sp. IOP Conf. Ser. Earth Environ. Sci. 2021, 743, 012042. [Google Scholar] [CrossRef]
- Coimbra, P.P.S.; Silva-E-Silva, A.C.A.G.D.; Antonio, A.D.S.; Pereira, H.M.G.; Veiga-Junior, V.F.d.; Felzenszwalb, I.; Araujo-Lima, C.F.; Teodoro, A.J. Antioxidant capacity, antitumor activity and metabolomic profile of a beetroot peel flour. Metabolites 2023, 13, 277. [Google Scholar] [CrossRef]
- Xie, F.; Hua, Q.; Chen, C.; Zhang, L.; Zhang, Z.; Chen, J.; Zhang, R.; Zhao, J.; Hu, G.; Zhao, J.; et al. Transcriptomics-based identification and characterization of glucosyltransferases involved in betalain biosynthesis in Hylocereus megalanthus. Plant Physiol. Biochem. 2020, 152, 112–124. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chiu, Y.C.; Tsao, N.W.; Chou, Y.L.; Tan, C.M.; Chiang, Y.H.; Liao, P.C.; Lee, Y.C.; Hsieh, L.C.; Wang, S.Y.; et al. Elucidation of the core betalain biosynthesis pathway in Amaranthus tricolor. Sci. Rep. 2021, 11, 6086. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. The biosynthetic pathways for shikimate and aromatic amino acids in Arabidopsis thaliana. Arab. Book 2010, 8, e0132. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Chen, N.; Yu, Z.H.; Xiao, X.G. Cytosolic and nuclear colocalization of betalain biosynthetic enzymes in tobacco suggests that betalains are synthesized in the cytoplasm and/or nucleus of betalainic plant cells. Front. Plant Sci. 2017, 8, 831. [Google Scholar] [CrossRef]
- Sunnadeniya, R.; Bean, A.; Brown, M.; Akhavan, N.; Hatlestad, G.; Gonzalez, A.; Symonds, V.V.; Lloyd, A. Tyrosine hydroxylation in betalain pigment biosynthesis is performed by cytochrome P450 enzymes in beets (Beta vulgaris). PLoS ONE 2016, 11, e0149417. [Google Scholar] [CrossRef]
- Sasaki, N.; Aabe, Y.; Goda, Y.; Adachi, T.; Kasahara, K.; Ozeki, Y. Detection of DOPA 4,5-dioxygenase (DOD) activity using recombinant protein prepared from Escherichia coli cells harboring cDNA encoding DOD from Mirabilis jalapa. Plant Cell Physiol. 2009, 50, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Dreiding, A.S. Biosynthesis of betalains. On the cleavage of aromatic ring during enzymatic transformation of DOPA into betalamic acid. Helv. Chim. Acta 1972, 55, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Adachi, T.; Koda, T.; Ozeki, Y. Detection of UDP glucose: Cyclo-DOPA 5-O-glucosyltransferase activity in four o’clocks (Mirabilis jalapa L). FEBS Lett. 2004, 568, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Wada, K.; Koda, T.; Kasahara, K.; Adachi, T.; Ozeki, Y. Isolation and characterization of cDNAs encoding an enzyme with glucosyltransferase activity for cyclo-DOPA from four o’clocks and feather cockscombs. Plant Cell Physiol. 2005, 46, 666–670. [Google Scholar] [CrossRef]
- Schippers, R.R. African Indigenous Vegetables. An Overview of the Cultivated Species; Natural Resources Institute The University of Greenwich and the ACP-EU Technical Centre for Agricultural and Rural Co-Operation: London, UK, 2000; pp. 16–23. [Google Scholar]
- Law-Ogbomo, K.E.; Ekunwe, P.A. Growth and herbage yield of Celosia argentea as influenced by plant density and NPK fertilization in degraded ultisol. Trop. Subtrop. Agroecosyst. 2011, 14, 251–260. [Google Scholar]
- Odulana, D.A.; Salau, A.W.; Odeyemi, O.M.; Makinde, E.A. Effects of variety, plant population and age at harvest on plant regrowth, shoot yield and nutritional composition of Celosia (Celosia argentea). Nigerian J. Hort. Sci. 2023, 27, 74–84. [Google Scholar]
- De Laporte, A.V.; Ripplinger, D.G. Economic viability of energy beets (Beta vulgaris) as advanced biofuel feedstocks. Ind. Crops Prod. 2018, 111, 254–260. [Google Scholar] [CrossRef]
- Mia, M.N.; Rashid, M.H.A. Yield and quality performance of beetroot (Beta vulgaris L.) as influenced by organic manure management. Fund. Appl. Agric. 2023, 8, 377–389. [Google Scholar] [CrossRef]
- Then, K.H. Planting density of red pitaya (Hylocereus polyrhizus) to achieve optimum yield under Malaysia weather condition. Int. J. Agric. Inno. Res. 2017, 6, 354–358. [Google Scholar]
- Sibut, H.C.P.; Faleiro, F.G.; Oliveira, J.D.S.; da Luz, A.L.; Neto, D.C.; Junqueira, N.T.V. Yield capacity of six superior pitaya genotypes under edaphoclimatic conditions of the federal district. Rev. Bras. Frutic. 2023, 45, e-025. [Google Scholar] [CrossRef]
- Thiyajai, P.; Koyama, T. Binary ethanol-water solvents affect betalain contents and health-promoting properties of red Celosia argentea inflorescence extracts. Int. Food Res. J. 2022, 29, 67–77. [Google Scholar] [CrossRef]
- Talukder, S.; Mendiratta, S.K.; Biswas, A.K.; Sen, A.R.; Devadason, I.P.; Chand, S.; Ahmad, T.; Kumar, D.; Agrawal, R.; Tarafdar, A. Natural dye-based sensor for monitoring temperature variation in storage for chicken patties. Food Biosci. 2024, 60, 104425. [Google Scholar] [CrossRef]
- Nemzer, B.; Pietrzkowski, Z.; Spórna, A.; Stalica, P.; Thresher, W.; Michałowski, T.; Wybraniec, S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011, 127, 42–53. [Google Scholar] [CrossRef]
- Hernández-Aguirre, O.A.; Muro, C.; Hernández-Acosta, E.; Alvarado, Y.; Díaz-Nava, M.D.C. Extraction and stabilization of betalains from beetroot (Beta vulgaris) wastes using deep eutectic solvents. Molecules 2021, 26, 6342. [Google Scholar] [CrossRef]
- Fernando, G.S.N.; Sergeeva, N.N.; Frutos, M.J.; Marshall, L.J.; Boesch, C. Novel approach for purification of major betalains using flash chromatography and comparison of radical scavenging and antioxidant activities. Food Chem. 2022, 385, 132632. [Google Scholar] [CrossRef]
- Cheok, A.; Xu, Y.; Zhang, Z.; Caton, P.W.; Rodriquez-Mateos, A. Betalain-rich dragon fruit (pitaya) consumption improves vascular function in men and women: A double-blind, randomized controlled crossover trial. Am. J. Clin. Nutr. 2022, 115, 1418–1431. [Google Scholar] [CrossRef]
- Khoo, H.E.; He, X.; Tang, Y.; Li, Z.; Li, C.; Zeng, Y.; Tang, J.; Sun, J. Betacyanins and anthocyanins in pulp and peel of red pitaya (Hylocereus polyrhizus cv. Jindu), inhibition of oxidative stress, lipid reducing, and cytotoxic effects. Front. Nutr. 2022, 9, 894438. [Google Scholar] [CrossRef]
- Goenaga, R.; Marrero, A.; Pérez, D. Yield and fruit quality traits of dragon fruit cultivars grown in Puerto Rico. HortTechnology 2020, 30, 803–808. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, V.; Thamaraiselvi, S.P.; Uma, D. Studies on extraction of betalain pigments by different solvents and assessing antioxidant activity of Bougainvillea spectabilis and Celosia argentea flowers. Madras Agric. J. 2019, 106, 104–108. [Google Scholar] [CrossRef]
- Yun, S.M.; Choi, B.H.; Ku, H.O.; Lee, M.H.; Nam, H.M.; Lee, K.J.; Park, S.W.; Jang, H.J.; Son, S.W. Antimicrobial activities of the flower extract of Celosia cristata L. Planta Medica 2008, 74, PA31. Planta Medica 2008, 74, PA31. [Google Scholar] [CrossRef]
- Sridevi, A.; Vijayalakshmi, M.; Narasimha, G. Antibacterial activity of some higher plant floral petals. Nat. Prod. Indian J. 2011, 7, 168–170. [Google Scholar]
- Rao, S.; Ekanath, M.; Chandrakala, G.; Rao, D.H.; Venkatesh, R.; Kiran, P. Evaluation of pharmacological activity of selected flowers used in Bathukamma-State Festival of Telangana. Am. J. Phytomed. Clin. Ther. 2015, 3, 666–678. [Google Scholar]
- Subba, B.; Basnet, P. Antimicrobial and antioxidant activity of some indigenous plants of Nepal. J. Pharmac. Phytochem. 2014, 3, 62–67. [Google Scholar]
- Okpako, E.; Ajibesin, K.K. Antimicrobial Activity of Celosia argentea L. Amaranthaceae. Am. J. Res. Commun. 2015, 3, 123–133. [Google Scholar]
- Shahzadi, T.; Zaib, M.; Riaz, T.; Shehzadi, S.; Abbasi, M.A.; Shahid, M. Synthesis of eco-friendly cobalt nanoparticles using Celosia argentea plant extract and their efficacy studies as antioxidant, antibacterial, hemolytic and catalytical agent. Arab. J. Sci. Eng. 2019, 44, 6435–6444. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, L. The chemical composition and pharmaceutical effect of celosia cristata: A review on nutritional aspect. Int. J. Innov. Sci. Technol. 2021, 6, 218–223. [Google Scholar]
- Balasubrahmanyam, A.; Baranwal, V.K.; Lodha, M.L.; Varma, A.; Kapoor, H.C. Purification and properties of growth stage-dependent antiviral proteins from the leaves of Celosia cristata. Plant Sci. 2000, 154, 13–21. [Google Scholar] [CrossRef]
- Begam, M.; Narwal, S.; Roy, S.; Kumar, S.; Lodha, M.L.; Kapoor, H.C. An antiviral protein having deoxyribonuclease and ribonuclease activity from leaves of the post-flowering stage of Celosia cristata. Biochemistry 2006, 71, S44–S48. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Hwang, J.W.; Sung, S.H.; Jeon, Y.J.; Jeong, J.H.; Jeon, B.T.; Moon, S.H.; Park, P.J. Antioxidant activity and protective effect of extract of Celosia cristata L. flower on tert-butyl hydroperoxide-induced oxidative hepatotoxicity. Food Chem. 2015, 168, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.J.; Shin, Y.S.; Park, G.N.; Chang, K.S. Inhibition of hepatitis C virus (HCV) genotype 2a RNA replication by extracts of medicinal herbs with anti-cholesterol effects. J. Med. Plants Res. 2013, 7, 1089–1099. [Google Scholar] [CrossRef]
- Singh, S.; Mazumder, A.; Pentela, B. Phytochemical evaluation and pharmacological potential of Celosia cristata L. Allelopath. J. 2024, 63, 19–30. [Google Scholar] [CrossRef]
- Sharma, P.; Vidyasagar, G.; Singh, S.; Ghule, S.; Kumar, B. Antidiarrhoeal activity of leaf extract of Celosia argentea in experimentally induced diarrhoea in rats. J. Adv. Pharm. Technol. Res. 2010, 1, 41–48. [Google Scholar] [CrossRef]
- Devhare, S.V.; Nirmal, S.A.; Rub, R.A.; Dhasade, V.V.; Zaware, B.B.; Mandal, S.C. Immunomodulating activity of Celosia argentea Linn aerial parts. Lat. Am. J. Pharm. 2011, 30, 168–171. [Google Scholar]
- Huang, Z.Q.; Cheng, Q.L.; Li, H.L.; Huang, C. The study for apoptosis of human cervical cancer HeLa cell induced by Celosin A from Celosiae Semen and its mechanisms. Acta Pharmacol. Sin. 2013, 34, 7. [Google Scholar]
- Cheng, Q.L.; Li, H.L.; Huang, Z.Q. Study on apoptosis of HepG2 cell induced by Celosin A from Celosia Semen and its mechanism. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 200–204. [Google Scholar]
- Rub, R.A.; Patil, M.J.; Ghorpade, P.; Siddiqui, A. Evaluation of antioxidant potential of Celosia argentea extracts. Pharmacogn. J. 2013, 5, 140–141. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I. By-product recovery of Opuntia spp. peels: Betalainic and phenolic profiles and bioactive properties. Ind. Crops Prod. 2017, 107, 353–359. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, P.M.; Guerrero-Rubio, A.; Henarejos-Escudero, P.; García-Carmona, F.; Gandía-Herrero, F. Health-promoting potential of betalains in vivo and their relevance as functional ingredients: A review. Trends Food Sci. Technol. 2022, 122, 66–82. [Google Scholar] [CrossRef]
- de Mejia, E.G.; Zhang, Q.; Penta, K.; Eroglu, A.; Lila, M.A. The colors of health: Chemistry, bioactivity, and market demand for colorful foods and natural food sources of colorants. Ann. Rev. Food Sci. Technol. 2020, 11, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Morales, P.; Ferreira, I.C. Natural Food Additives: Quo Vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Khan, M.I. Plant betalains: Safety, antioxidant activity, clinical efficacy, and bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 316–330. [Google Scholar] [CrossRef]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef]
- Polturak, G.; Grossman, N.; Vela-Corcia, D.; Dong, Y.; Nudel, A.; Pliner, M.; Levy, M.; Rogachev, I.; Aharoni, A. Engineered gray mold resistance, antioxidant capacity, and pigmentation in betalain-producing crops and ornamentals. Proc. Natl. Acad. Sci. USA 2017, 114, 9062–9067. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shin, Y.C.; Byun, S.M.; Jo, J.S.; Cho, S.J. Evaluation of red pigment of cockscomb flower in model food systems as a natural food colorant. Korean J. Food Sci. Technol. 1986, 18, 388. [Google Scholar]
- Lee, S.Y.; Cho, S.J.; Lee, K.A.; Byun, P.H.; Byun, S.M. Red pigment of the Korean cockscomb flower: Color stability of the red pigment. Korean J. Food Sci. Technol. 1989, 21, 446–452. [Google Scholar]
- Saikia, A.; Goswami, S.; Gohain, S.; Bora, A. Plant-based natural colourants for food, textile and health. In Agri Start-Ups and Agribusiness—Innovation for Fostering the Future of Agriculture; Brillion Publishing: New Delhi, India, 2021. [Google Scholar]
- Pavokovic, D.; Krsnik-Rasol, M. Complex biochemistry and biotechnological production of betalains. Food Technol. Biotechnol. 2011, 49, 145–155. [Google Scholar]

| Bioactive Compound | Chemical | Analytical Technique * | Plant Part | Reference |
|---|---|---|---|---|
| Saponins | Celosin A, Celosin B, Celosin C, Celosin D, Celosin E, Celosin F, Celosin G, Celosin I, Celosin II, Celosin H, Celosin I, Celosin J, Cristatain | NMR, HPLC-ELSD | Seed | [10,14,19,20,21] |
| Polyphenols | Lutin, Epigallocatechin, Gallic acid, Caffeic acid, Rosmarinic acid, Quercetin, 4-O-β-d-apifuranosyl-(1→2)-β-d-glucopyranosyl-2-hydroxy-6-methoxyacetophenone | HPLC | Leaf | [16,31] |
| Peptides | Moroidin, Celogentins A, Celogentins B, Celogentins C, Celogentins D, Celogentins E, Celogentins F, Celogentins G, Celogentins H, Celogentins J, Celogentins K, Celogenamide A | NMR, MS/MS, CD spectra | Seed | [18,22,23,24,25] |
| Amino acids | Glycine, Alanine, Arginine, Lysine, Glutamic acid, Valine, Methionine, Isoleucine, Phenylalanine, Serine, Tyrosine, Proline, Leucine, Histidine, Aspartic acid, Cysteine, Cytine, Threonine, Ornithine | Amino acid analyzer | Seed, Leaf | [26,27] |
| Fatty acids | Arachic acid, Arachidonic acid, Linolenic acid, Hexadecanoic acid, Palmitoleic acid, Octadecanoic acid, Octadecanoic monoenoic acid, Oleinic acid, Linoleic acid | GC | Seed | [26,27] |
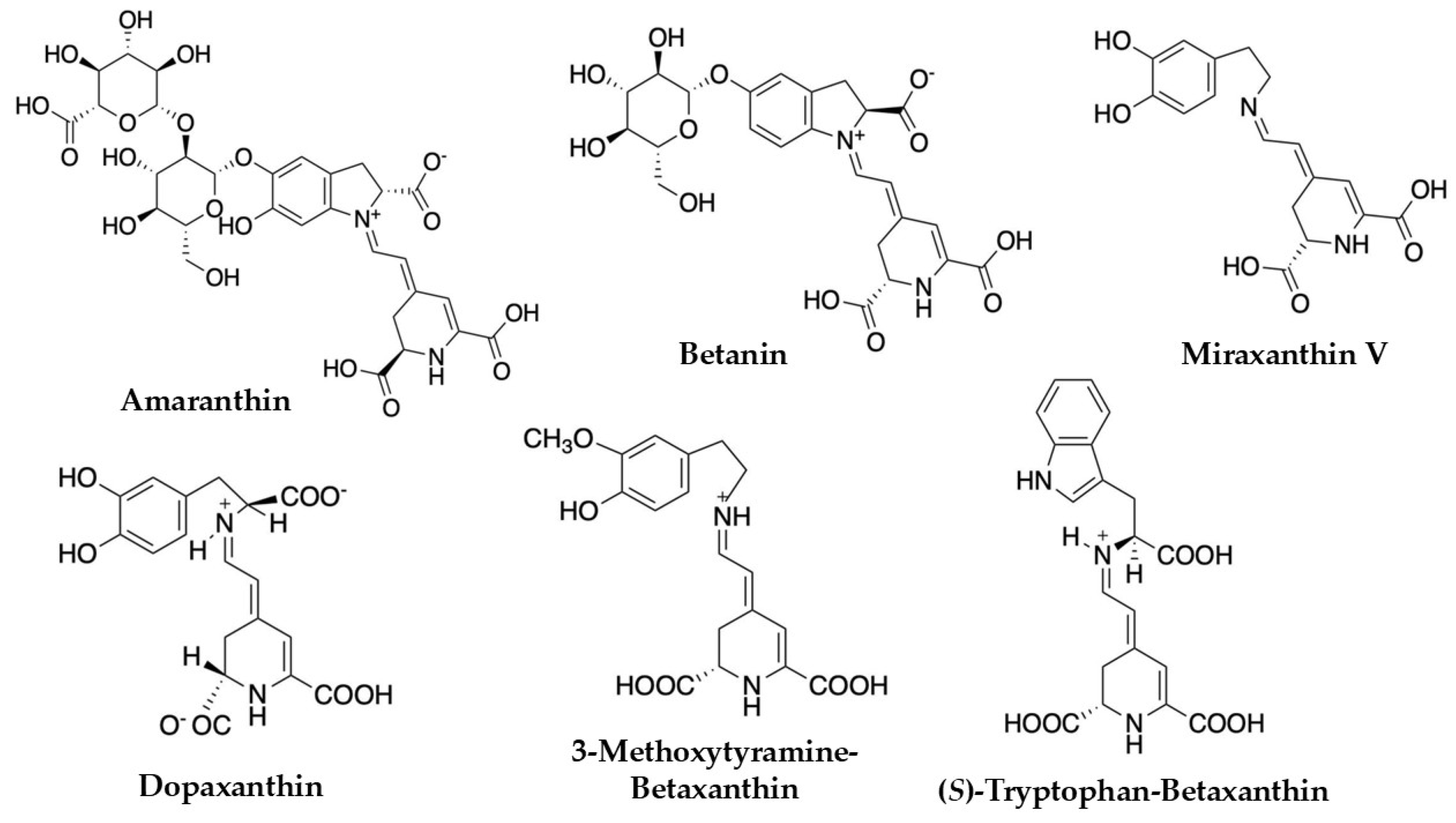
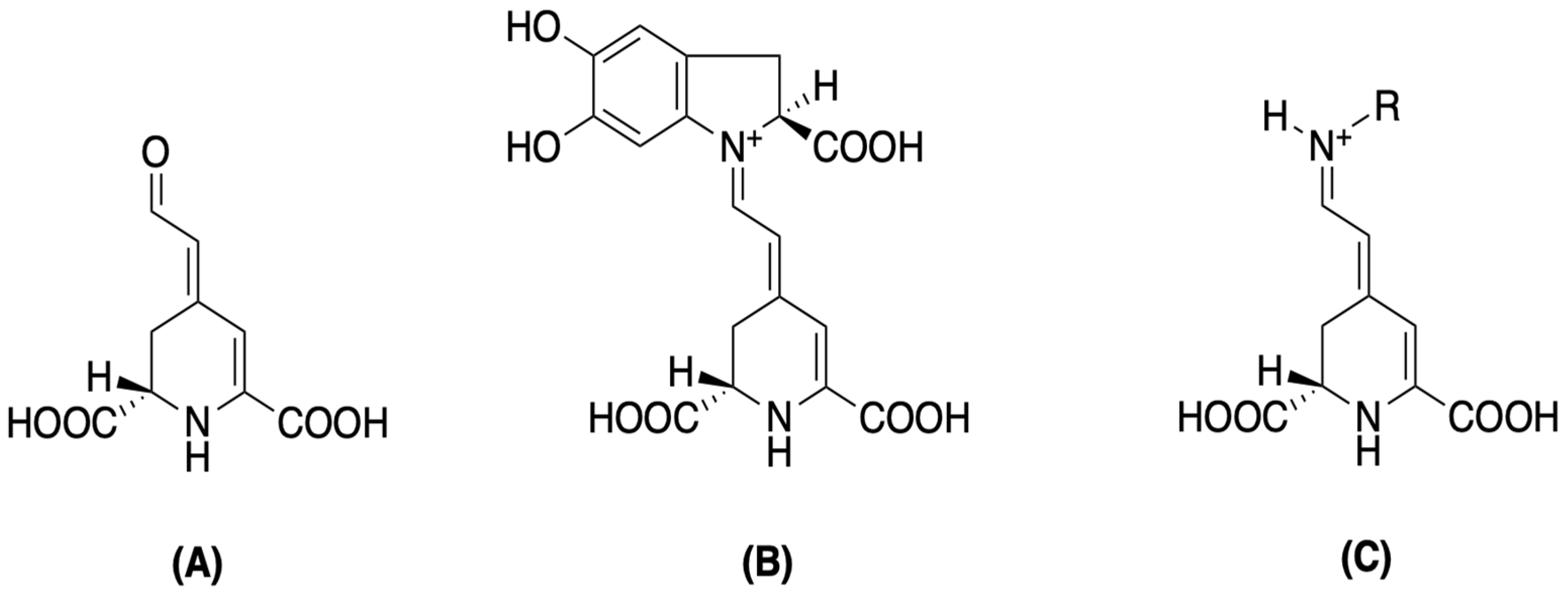
| Betalains | Betaxanthins (Indicaxanthin, Dopaxanthin), Betacyanins (Betanin, Gomphrenin, Amaranthin, and Bougainvillein) | Spectrophotometry | Leaf, Inflorescence | [1,36,37] |
| Minerals | K, Ca, Mg, Na, Fe, Mn, Cu, Zn, S, Si, Ti, Cd, Hg, Cr, Mo, Pb | AA | Seed, Leaf | [26,27] |
| Others | Β-Sitosterol, Stigmasterol, β-Carotene, Ascorbic acid | - | Seed, Leaf | [28,29] |
| Parameter | Celosia argentea | Beta vulgaris | Hylocereus spp. |
|---|---|---|---|
| Biomass yield (t/ha) | 6.83–39.00 [66,67,68] | 15.00–62.30 [69,70] | 2.97–41.55 [71,72] |
| Betalain content (mg/g DW) | 1.40–14.91 [11,37,38,73,74] | 2.40–12.60 [70,75,76,77] | 1.40–35.12 [78,79] |
| Life-cycle (months) * | 3 [37] | 3 [70] | 12–15 [80] |
| Water requirement | n.a. | Moderate; requires regular irrigation; 8–10 irrigations [70] | Low; drought-tolerant [71,72] |
| Primary plant part used | Inflorescences, Leaves | Root | Fruit pulp and peel |
| Characteristic | Field Cultivation System | Ref. | Cell Culture System | Ref. |
|---|---|---|---|---|
| Biomass yield | 6.83–39.00 t/ha * | [66,67,68] | 16.45–20.30 g/L ** | [36,37] |
| Betalain content | 1.40–14.91 mg/g DW | [11,37,38,73,74] | 1.84–42.08 mg/g DW | [36,37,39] |
| Betalain productivity | 0.03 mg/g·day | [37] | 0.10–0.42 mg/g·day | [36,37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klanrit, P.; Thanonkeo, S.; Klanrit, P.; Klanrit, P.; Mueangnak, K.; Thanonkeo, P. Celosia argentea: Towards a Sustainable Betalain Source—A Critical Review and Future Prospects. Plants 2025, 14, 1940. https://doi.org/10.3390/plants14131940
Klanrit P, Thanonkeo S, Klanrit P, Klanrit P, Mueangnak K, Thanonkeo P. Celosia argentea: Towards a Sustainable Betalain Source—A Critical Review and Future Prospects. Plants. 2025; 14(13):1940. https://doi.org/10.3390/plants14131940
Chicago/Turabian StyleKlanrit, Preekamol, Sudarat Thanonkeo, Poramaporn Klanrit, Poramate Klanrit, Kanchanok Mueangnak, and Pornthap Thanonkeo. 2025. "Celosia argentea: Towards a Sustainable Betalain Source—A Critical Review and Future Prospects" Plants 14, no. 13: 1940. https://doi.org/10.3390/plants14131940
APA StyleKlanrit, P., Thanonkeo, S., Klanrit, P., Klanrit, P., Mueangnak, K., & Thanonkeo, P. (2025). Celosia argentea: Towards a Sustainable Betalain Source—A Critical Review and Future Prospects. Plants, 14(13), 1940. https://doi.org/10.3390/plants14131940